Abstract
High mobility group box 1 protein (HMGB1) is released by necrotic cells or activated macrophages/monocytes, and functions as a late mediator of lethal systemic and local pulmonary inflammation. Passive immunization with anti-HMGB1 antibodies confers significant protection against lethal endotoxemia, sepsis, and acute lung injury, even when antibodies are administered after the onset of these diseases. In light of observations that three Chinese herbal formulations recommended for treatment of severe acute respiratory syndrome (SARS) specifically inhibited the release of HMGB1 from innate immune cells, we hypothesize that HMGB1 might occupy a pathogenic role in SARS by mediating an injurious pulmonary inflammatory response.
Introduction
Severe acute respiratory syndrome (SARS) has killed more than nine hundreds of people around the world and infected thousands more. It has created international anxiety because of its rapid progression and high mortality. In China, several clinical trials revealed efficacy of traditional Chinese herbal remedies in reducing symptoms and mortality of patients with SARS [1]. High mobility group box 1 protein (HMGB1, formerly known as HMG-1 or amphoterin) has recently been identified as a new proinflammatory cytokine and a late mediator of inflammation, sepsis, and acute lung injury. In light of observations that several Chinese herbal remedies recommended for treatment of SARS specifically inhibited the release of HMGB1 from activated innate immune cells, we hypothesize that HMGB1 might occupy a pathogenic role in SARS by mediating an injurious pulmonary inflammatory response.
HMGB1 as a proinflammatory cytokine
HMGB1 has been for a long time described as a nuclear DNA-binding protein in mammalian cells [2]. Recent evidence has revealed that HMGB1 can be released by necrotic cells or activated innate immune cells (such as macrophages and monocytes), and functions as a proinflammatory cytokine [3], [4], [5]. In response to exogenous stimuli (such as bacterial endotoxin, lipopolysaccharide, LPS) or endogenous cytokines (such as TNF, IL-1β or IFN-γ), cultures of macrophages, monocytes or pituicytes actively release HMGB1 in a time- and dose-dependent manner [3], [6], [7]. The kinetics of HMGB1 release from activated macrophages/monocytes is significantly delayed, with HMGB1 accumulation first detectable at 8 h after stimulation [3], [7]. This delayed release of HMGB1 distinguishes it from TNF and other early mediators of systemic inflammatory responses [5]. Extracellular HMGB1 is able to stimulate the production of TNF and other proinflammatory cytokines from macrophages, endothelial cells or neutrophils, triggering an inflammatory response [8]. In murine models of endotoxemia or sepsis, or patients with sepsis or rheumatoid arthritis [8], [9], [10], serum HMGB1 levels are significantly elevated [3]. Administration of HMGB1 via various (e.g., focal, intraperitoneal, intracerebroventricular, and intratracheal) routes induces marked inflammatory responses in vivo [5], [11], [12]. Taken together, these studies indicate that accumulation of HMGB1 can amplify the cytokine cascade, and mediate injurious inflammatory responses.
The pathogenic role of HMGB1 as a late mediator of lethal systemic inflammation has been defined in animal models of endotoxemia and sepsis using anti-HMGB1 antibodies or inhibitors [2], [3], [13]. Anti-HMGB1 antibodies confer a dose-dependent protection in animal models of sepsis, even when the first dose of anti-HMGB1 antibodies is delayed for 24 h after the onset of sepsis [2]. Administration of a novel anti-inflammatory agent, ethyl pyruvate, dose-dependently inhibits HMGB1 release, and confers significant protection against the lethality of sepsis [14]. In animal models of collagen-induced arthritis, administration of anti-HMGB1 antibodies also confers significant protection [15]. Thus, therapeutic agents capable of inhibiting HMGB1 activities (such as anti-HMGB1 antibodies) or release (such as ethyl pyruvate) may hold potential for the clinical management of sepsis and other inflammation diseases [16].
Role of HMGB1 in acute lung injury
Intratracheal administration of HMGB1 to LPS-resistant C3H/HeJ mice stimulates lung neutrophil accumulation, and increases pulmonary levels of proinflammatory cytokines such as TNF, IL-1β, and MIP-2 [13]. Histological examination of lung tissue reveals evidence of an acute diffuse inflammatory response, with accumulation of neutrophils in the interstitial and intraalveolar areas, interstitial edema, and protein exudation into the alveolar space [13]. In a widely used animal model of acute lung injury induced by intratracheal administration of bacterial endotoxin, administration of anti-HMGB1, either before or after endotoxin treatment, significantly decreases endotoxin-induced neutrophil infiltration, and lung edema [13]. Despite the ameliorative effects of anti-HMGB1 antibodies on the development of lung injury and neutrophil accumulation, this treatment does not affect endotoxin-induced increases in pulmonary levels of TNF, IL-1β, or MIP-2, establishing the important role of endogenous HMGB1 itself as a mediator of acute lung injury [13].
Herbal formulations for SARS specifically inhibit HMGB1 release
After the outbreak of the SARS in China, the State Administration of Traditional Chinese Medicine recommended the use of several traditional Chinese herbal remedies for prevention and treatment of SARS [17]. Clinical observations suggest that Chinese herbal remedies are beneficial in the reduction of (i) fever; (ii) air-space shadowing of the chest radiographs; (iii) the use of corticosteroids; and (iv) the death rate of severe SARS patients [1]. To evaluate the immunomodulatory properties of traditional Chinese herbal remedies recommended for SARS, we examined their potential effect on the release of several proinflammatory mediators in cultures of macrophages and human peripheral blood mononuclear cells (HuPBMCs).
Three Chinese herbal formulations were prepared according to the recipes released by the State Administration of traditional Chinese Medicine [17] (Table 1 ). At clinically relevant doses (5–15 μl of extract/ml culture medium), none of these herbal formulations inhibited endotoxin-induced production of TNF or IL-1β by either macrophages or HuPBMCs (data not shown). Two out of three herbal remedies significantly attenuated endotoxin-induced production of nitric oxide (Fig. 1(b) ). Surprisingly, all three Chinese herbal remedies dose-dependently (5–15 μl/ml) suppressed endotoxin-induced HMGB1 release by cultures of both macrophages (Fig. 1(a)), and HuPBMCs (Fig. 1(c)).
Table 1.
Chinese herbal formulations recommended by the State Administration of Traditional Chinese Medicine for the treatment of SARS [17]
| Formulations | Chinese (English) | Dose (g/formulation) |
|---|---|---|
| 1 | Bo He (Peppermint) | 6 |
| Chan Yi (Cicada slough) | 10 | |
| Gan Cao (Raw liquorice) | 5 | |
| Jiang Can (Batryticated silkworm) | 10 | |
| Lian Qiao (Weeping forsythiae capsule) | 10 | |
| Xian Lu Gen (Reed root) | 15 | |
| Yin Hua (Honeysuckle flower) | 10 | |
| 2 | Bai Shu (Bighead atractylodes root) | 6 |
| Cang Zhu (Atractylodes root) | 6 | |
| Fang Feng (Ledebouriella root) | 10 | |
| Guan Zhong (Male fern root) | 6 | |
| Huang Qi (Astragalus root) | 10 | |
| Huo Xiang (Cablin potchouli) | 10 | |
| Sha Shen (Glehia root) | 10 | |
| Yin Hua (Honeysuckle flower) | 10 | |
| 3 | Cang Zhu (Atractylodes root) | 6 |
| Da Qing Ye (Indigowoad leaf) | 10 | |
| Ge Gen (Pueraria root) | 10 | |
| Guan Zhong (Male fern root) | 6 | |
| Huo Xiang (Cablin potchouli) | 10 | |
| Lian Qiao (Weeping forsythiae capsule) | 10 | |
| Peilan (Fortune eupatium) | 10 | |
| Tai Zi Shen (Heterophylly false starwort root) | 15 | |
| Yin Hua (Honeysuckle flower) | 10 | |
| Zi Shu Ye (Perilla leaf) | 6 |
Note: All herbal ingredients were obtained from NY-Tongrentang, Inc. (Flushing, NY, USA), mixed, and boiled in water for 40 min. The water-soluble fraction (recalibrated to a total volume of 100 ml with sterile water) was cleared by centrifugation (4000 rpm, 20 min, 4 °C) and filtration (through 0.2 μm filter), and the clear water-soluble herbal extract was evaluated for the immunomodulatory properties.
Figure 1.

Effects of three traditional Chinese herbal formulations on endotoxin-induced release of nitric oxide and HMGB1. Murine macrophage-like RAW 264.7 cells (panels a, b) or HuUPBMCs (panel c) were stimulated with LPS (500 ng/ml) either alone, or in the presence of herbal formulations (F1, F2, and F3), and levels of HMGB1 and nitric oxide in the culture medium were determined 16 h later by Western blotting analysis or the Griess reaction as previously described [7]. Shown in the bar graphs (panels a, b, and c) are means ± SEM. of three experiments in duplicates (n=6). Student's t-test was performed and a p<0.01 was considered significant (*). Shown in the bottom of panel c is a representative Western blot.
Pathogenic role of HMGB1 in SARS?
An excessive immune response to acute coronavirus infection appears to be the fatal factor in patients who die of SARS [18], [19], [20], [21]. Autopsy of SARS patients revealed signs of diffuse alveolar damage, airspace edema, and bronchiolar fibrin [19], [20], [21]. Our hypothesis is that initial acute coronavirus infection of alveolar endothelial cells or macrophages leads to cell injury via virus-mediated cytolysis, which is accompanied by HMGB1 release from damaged cells (Fig. 2 ). Extracellular HMGB1, as a mediator of acute lung injury [13], subsequently mediates injurious pulmonary inflammatory responses including neutrophil infiltration, derangement of epithelial barrier, lung edema, and lung injury. Collectively, these injurious pulmonary inflammatory responses may consequently lead to respiratory failure, and death. This hypothesis can be tested using anti-HMGB1 antibodies or HMGB1 inhibitors (such as ethyl pyruvate) in future studies. For instance, if immunoassays using anti-HMGB1 antibodies indicate a potential elevation of pulmonary HMGB1 levels in SARS patients, it will support the above hypothesis. Similarly, if HMGB1-specific antibodies or inhibitors attenuate the progression of SARS in animal models or clinical settings, it will support an important role of HMGB1 in the pathogenesis of SARS.
Figure 2.
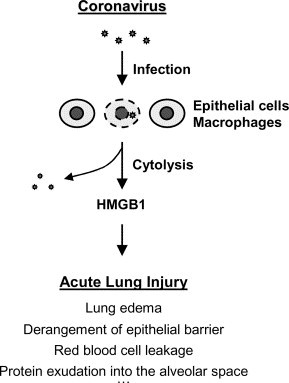
Hypothetical role of HMGB1 in the pathogenesis of SARS. HMGB1 is released from coronavirus infected cells, and then stimulates excessive inflammatory responses.
Acknowledgements
We thank Drs. Ping Wang, Man Yu, and Xiaoling Qiang for critical reading of the manuscript. This research was supported in part by the North Shore-LIJ Research Institute, and the National Institutes of Health (NIH, NIGMS, GM063075).
References
- 1.Jia W., Gao W. Is traditional Chinese medicine useful in the treatment of SARS? Phytother. Res. 2003;17(7):840–841. doi: 10.1002/ptr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H., Ochani M., Li J. Reversing established sepsis with antagonists of endogenous HMGB1. Proc. Natl. Acad. Sci. USA. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H., Bloom O., Zhang M. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 4.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 5.Wang H., Yang H., Czura C.J., Sama A.E., Tracey K.J. HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 2001;164(10):1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 6.Wang H., Vishnubhakat J.M., Bloom O. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126(2):389–392. [PubMed] [Google Scholar]
- 7.Rendon-Mitchell B., Ochani M., Li J. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 2003;170(7):3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 8.Kokkola R., Sundberg E., Ulfgren A.K. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002;46(10):2598–2603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi N., Kawahara K., Yone K. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48(4):971–981. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- 10.Pullerits R., Jonsson I.M., Verdrengh M. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 2003;48(6):1693–1700. doi: 10.1002/art.11028. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Czura C.J., Tracey K.J. HMGB1. In: Thomson A, Lotze MT, editors. The cytokine handbook. 4th ed. Oxford; Academic Press: 2003. pp. 913–923. [Google Scholar]
- 12.Wang H., Yang H., Tracey K.J. Extracellular role of HMGB1 in inflammation and sepsis. J. Int. Med. 2004;255(3):320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 13.Abraham E., Arcaroli J., Carmody A., Wang H., Tracey K.J. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 14.Ulloa L., Ochani M., Yang H. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl. Acad. Sci. USA. 2002;99(19):12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokkola R., Li J., Sundberg E. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48(7):2052–2058. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 16.Wang H., Czura C.J., Tracey K.J. Lipid unites dissipate syndromes of sepsis. Nat. Med. 2004;10(2):124–125. doi: 10.1038/nm0204-124. [DOI] [PubMed] [Google Scholar]
- 17.Herbal supplement. Available from: http://www.china.org.cn/english/features/sars/63785.htm
- 18.Walgate R. Severe immune response kills SARS victims. New Sci. 2003 http://www.newscientist.com/news/news.jsp?id=ns99993693 [Google Scholar]
- 19.Franks T.J., Chong P.Y., Chui P. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol. 2003;34(8):743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Y., Wang H., Shen H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang Z., Zhang L., Zhang S. Pathological study on severe acute respiratory syndrome. Chin. Med. J. (Engl.) 2003;116(7):976–980. [PubMed] [Google Scholar]


