Abstract
The susceptibility of dogs to experimental inoculation with trophozoites and cysts of human isolates of Giardia duodenalis and the clinical and laboratory profiles of infection of these animals were studied. Two groups (A and B), each comprising three dogs, were inoculated with G. duodenalis trophozoites and cysts, respectively. A third group of two dogs was not inoculated and remained as control. After inoculation feces were collected daily to determine the pre-patent period, by flotation in 33% zinc sulfate solution. Blood samples (5 mL) were collected from animals at 15-day intervals during the 165 days of the experimental period and were used to carry out the hemogram and biochemical evaluation of the levels of total protein, albumin, alanine aminotransferase, gamma glutamyltransferase, urea and creatinine. A prepatent period was observed at 5–6 days post-inoculation (p.i.) in the inoculated dogs, with cysts eliminated for approximately 3 months. No alterations were seen in the clinical parameters evaluated. Anemia was observed at 15 p.i. in the inoculated dogs. The mean eosinophil count of inoculated groups was higher than that of the control (p ≤ 0.05) but none of the biochemical parameters analyzed presented significant differences. The results of this study show that G. duodenalis from human isolates is able to infect dogs with minimal systemic manifestations without producing clinical signs of giardiasis.
Keywords: Giardia, Dogs, Anemia, Blood, Hemogram, Biochemistry
1. Introduction
Giardia is a common and well-known enteric parasite. Giardiasis is considered to be the main non-viral cause of diarrhea in humans worldwide and has been identified as responsible for epidemics both in developed and developing countries (Thompson et al., 2000, Lane and Lloyd, 2002).
Members of the genus are ubiquitous and non-invasive, living and multiplying by asexual reproduction on the lumenal surface of the small intestine in several vertebrate species (Thompson et al., 2000, Thompson, 2004). Of all the species of the genus, Giardia duodenalis (syn. G. intestinalis, G. lamblia) is the only one encountered both in humans and other species, including dogs, cats, bovines, pigs, sheep and equines (Thompson et al., 2000, Thompson, 2004). Transmission occurs through ingestion of cysts eliminated in the feces of infected individuals (Monis and Thompson, 2003). Contagion between dogs occurs through direct animal–animal contact, especially among animals in boarding or breeding kennels, or indirectly from cysts present in the environment, water or food.
Most infected dogs are asymptomatic and when symptoms occur these may be constant or intermittent, including acute or chronic diarrhea, loss of weight despite normal appetite and, more rarely, vomiting and lethargy (Leib and Zajac, 1999). Giardiasis is generally controlled by the healthy immune system but may be fatal in individuals with compromised immunity (Lane and Lloyd, 2002).
Giardiasis has a cosmopolitan distribution with prevalence of approximately 10% in well-treated dogs, 30–50% in puppies and up to 100% in boarding and breeding kennels (Barr and Bowman, 1994). The reported prevalence in dogs varies according to geographical location, differences in definition of the study population, different methods used to determine prevalence and diagnosis, difficulty in the identification of the cysts and intermittent elimination of the parasite (Jacobs et al., 2001).
The G. duodenalis genotypes found in humans also occur in dogs, so that infection may be zoonotic (Van Keulen et al., 1995, Van Keulen et al., 2002). Despite this zoonotic potential being recognized, the conditions necessary for transmission remain unclear (Thompson, 2004). Dog carriers of G. duodenalis could function as reservoirs and act as a potential source of infection for humans and other animals (Anderson et al., 2004, Thompson, 2004). Several G. duodenalis isolates that affect humans may be transmitted experimentally to various animal species, although the specific role of the dog in the epidemiology of human giardiasis still needs to be established (Monis and Thompson, 2003).
The objective of the present study was to determine the susceptibility of dogs to experimental infection with cysts and trophozoites from human isolates of G. duodenalis and observe possible clinical and laboratory alterations in these animals.
2. Materials and methods
Eight cross-bred dogs from the same litter, 5-month-old, of both sexes, were used in the experiments. All the dogs were monitored from birth and maintained in the kennel of the Veterinary Hospital of the Federal University of Uberlândia. They were housed in individual roofed, concrete compartments, cleaned daily with detergent and 1% sodium hypochlorite solution. All the food and water bowls of the dogs were cleaned daily using filtered water as a rinse. Food (commercial dog chow) was provided twice daily, as well as mineral water which was also changed twice daily.
Clinical and laboratory examinations were carried out prior to the experiment to observe the physical conditions of the animals. Fecal samples were collected to look for eggs and larvae of helminths and cysts of G. duodenalis. All the dogs were negative for G. duodenalis and three were positive for Toxocara canis. The animals were treated with anthelmintic drugs in two doses at 21-day intervals (5.0 mg Praziquantel, 14.4 mg Pyrantel and 15 mg/kg Febantel). Additional parasitological exams were performed at 7-day intervals to confirm that they remained free of parasites.
All of the dogs were immunized against adenovirus, coronavirus, distemper, leptospirosis, parainphluenza and parvovirus (Quantum® Dog DA2PPPvLCv, Schering Plough, Omaha, USA), three doses being administered at monthly intervals. They were also vaccinated against rabies (Quantum® Dog R, Schering Plough, Omaha, NE, E.U.A). All the dogs completed vaccination before the experimental period was initiated.
Dogs were divided at random into three groups (A, B and C), groups A and B with three animals and group C with two. Group A was inoculated orally with 3 mL of TYI-S-33 medium with 1.35 × 106 Giardia trophozoites and group B 3mL of TYI-S-33 medium with 1.35 × 103 Giardia cysts. Group C was inoculated 3 mL of TYI-S-33 medium without Giardia and provided a control for the experiment.
The cysts were collected from the feces of naturally infected children attending day care institutions. These children presented symptoms of giardiaisis or chronic elimination of cysts. The cysts were isolated according to the technique of Roberts-Thomson et al. (1976), with adaptations. Trophozoites were obtained from culture maintained in TYI-S-33 medium supplemented with bovine serum (Diamond et al., 1978, Keister, 1983) in the Amebiasis Laboratory of Department of Parasitology of Federal University of Minas Gerais, Brazil and characterized as Portland 1 (ATCC 30888).
After inoculation, feces of dogs were collected daily and examined by flotation in 33% zinc sulfate solution (Faust et al., 1938) to detect cysts and determine the prepatent period. Once cysts appeared in the feces, collections were performed at weekly intervals throughout the 165 days of the experimental period. The cysts were quantified by a technique adapted from that of Roberts-Thomson et al. (1976).
Clinical examinations were carried out on all the dogs, 2 days before inoculation (day 2), on the day of inoculation and thereafter at 7-day intervals at 08:00a.m.–12:00a.m. throughout the 165 days of the experimental period, the animals always being examined in the same sequence. This involved monitoring weight, rectal temperature, femoral pulse and heart rate, cardiac and pulmonary auscultation, and palpation of the lymph nodes and abdomen. The aspect, proportion and consistency of the feces were evaluated twice daily during the cleaning of the individual kennels.
Samples of 5 mL of blood were obtained by atraumatic jugular venipuncture of each animal, using a sterile disposable syringe. Two milliliters of the sample in EDTA were used to carry out the complete hemogram by the conventional technique of Dace and Lewis (1984). Red and white blood cell counts were processed in an automatic cell counter (CELM® CC 510). Hemoglobinometry was carried out by the cyanometahemoglobin method on a CELM® Hb 520 hemoglobinometer and the hematocrit calculated by the microhematocrit method. The hematimetric indices of mean globular volume (MGV), mean globular hemoglobin (MGH) and mean globular hemoglobin concentration (MGHC) were calculated according to Jain (1986).
The differential leukocyte count was carried out on blood films stained by May-Grunwald-Giemsa to establish the percentages of each cell type (relative values) and transform them into absolute numbers (Ferreira Neto et al., 1981).
The remaining 3 mL of without anticoagulant blood was centrifuged at 700 × g during 15 min. The serum obtained was used for the biochemical tests, including total protein, albumin, alanine aminotransferase, gamma glutamyltransferase, urea and creatinine, using the standard commercial kit (Labtest® Diagnóstica S/A) and a COBAS® MIRA/PLUS automatic biochemical analyzer (Roche Diagnostic, Inc., Branchburg, NJ). Blood samples were obtained 2 days before inoculation, on the day of inoculation and at 15-day intervals during the 165 days of the experimental period.
Comparisons between groups A, B and C were carried out based on the days post-inoculation (p.i.). Parameters were analyzed by Mann–Whitney U-test and Student's t-test. Results were considered to be statistically significant at p ≤ 0.05. The GraphPad Prism ® Version 4.00 program (GraphPad Software, Inc.) was used for statistical calculations.
3. Results
Cysts were detected in the feces from the fifth day post-inoculation (p.i.) onwards in most dogs inoculated with cysts and trophozoites, except dog 3 of group A which presented cysts in the feces at 6 p.i.
The dogs of groups A and B presented positive results for G. duodenalis up to 49 p.i., alternation of positive and negative results with variable production of cysts being observed from this day onwards.
The dogs of group A demonstrated negative results between 91 and 105 p.i., thereafter presenting positive results up to 140 p.i. again. Cysts were not detected in the feces starting from 140 p.i. in the dogs of group A and starting from 112 p.i. in group B, with results remaining negative until the end of the experimental period (Fig. 1 ).
Fig. 1.
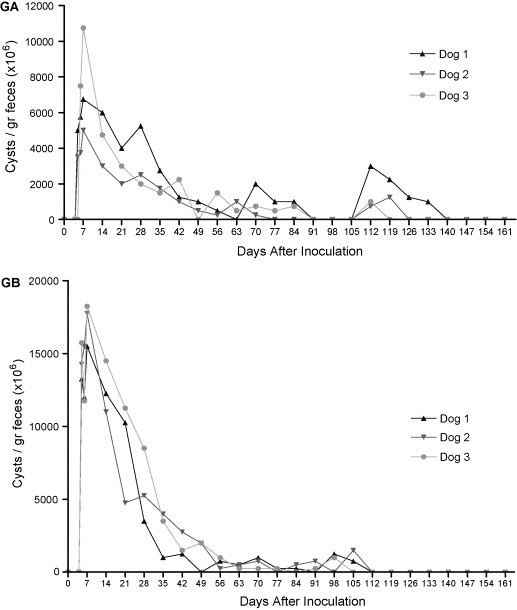
Quantity of Giardia duodenalis cysts identified individually in the feces of inoculated dogs with trophozoites (GA) and of inoculated dogs with cysts (GB), determined through the parasitological examination of zinc sulfate flotation a 33% (Faust et al., 1938).
No alterations were observed in physical parameters analyzed.
Significant differences were observed in values of red blood cell counts, hemoglobin and hematocrit for dogs of group A compared to those of groups B and control (Fig. 2 ), principally at 15 p.i. (p ≤ 0.05). The hematimetric indices of MGV, MGH and MGHC did not present significant differences.
Fig. 2.
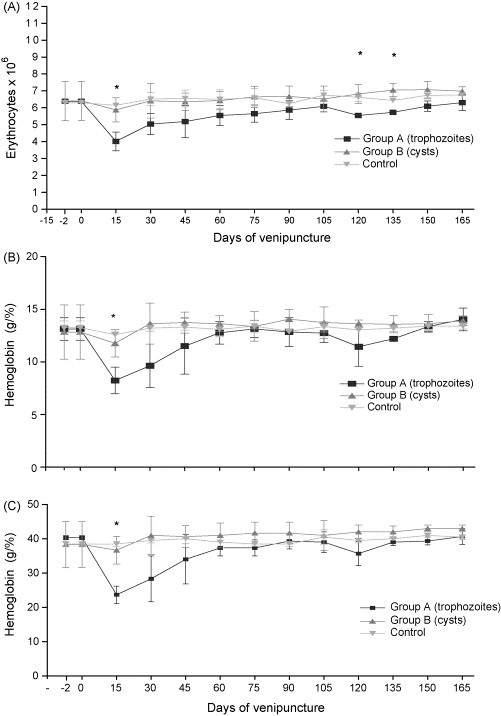
Average values of erythrocytes (A), hemoglobin (B) and hematocrit (C) in control dogs, inoculated dogs with trophozoites and inoculated dogs with cysts of Giardia duodenalis. The symbol (*) represents significant difference between groups (p ≤ 0.05).
The mean values of immature neutrophils of group B were significantly greater than those of the groups A and control at 15, 60, 75 and 165 p.i. (p ≤ 0.05) although no differences were seen for segmented neutrophils (Fig. 3 ).
Fig. 3.
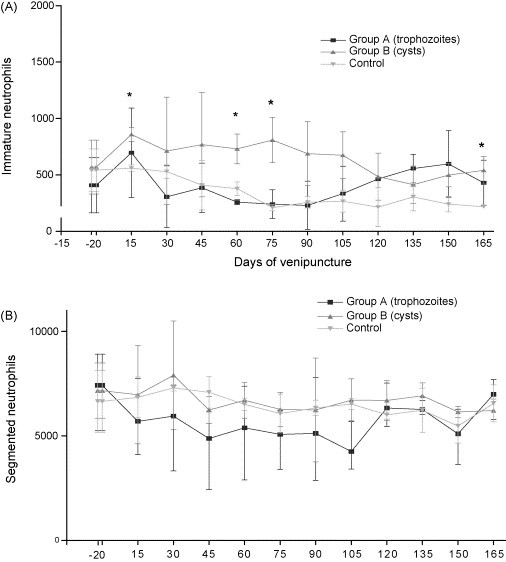
Average values of immature neutrophils (A) and segmented neutrophils (B) in control dogs, inoculated dogs with trophozoites and inoculated dogs with cysts of Giardia duodenalis. The symbol (*) represents significant difference between groups (p ≤ 0.05).
The mean values of eosinophils in dogs of groups A and B (Fig. 4 ) were both significantly higher than those of the control group at 120, 135 and 165 p.i. (p ≤ 0.05).
Fig. 4.

Average eosinophils values in control dogs, inoculated dogs with trophozoites and inoculated dogs with cysts of Giardia duodenalis. The symbol (*) represents significant difference between groups (p ≤ 0.05).
Biochemical analyses of total protein, albumin, alanine aminotransferase, gamma glutamyltransferase, urea and creatinine revealed no statistically significant differences between the groups.
4. Discussion
Although investigations on interspecies transmission, principally between humans and other mammals, have been carried out for more than 80 years (Hegner, 1926, Padchenko and Stolyarchuk, 1969, Hewlett et al., 1982), most have obtained inconclusive results and have not clarified points related to infectivity, pathogenic pattern and the real epidemiological importance of small animals in human infections.
The results of experimental transmission studies have been questioned due to the uncertainty of the animals being free of Giardia, the common use of cysts in quantities that do not represent natural infections, and the use of isolates from axenic cultures that could reduce virulence (Thompson et al., 2000, Monis and Thompson, 2003). However, it should be emphasized that the lack of success in some experiments could be related not only to the above problems, but also to genetic diversity, differences in the viability of the cysts, variation in the immune response of the experimental host or even in the sensitivity in the technique for cyst detection (Faubert, 2000, Monis and Thompson, 2003, Thompson, 2004). Despite these controversies, these studies remain as sources of information on the epidemiological and zoonotic perspectives of G. duodenalis transmission.
The results of the present study confirm that dogs can be infected with genotypically uncharacterized cysts from human and by axenic cultures of molecularly characterized G. duodenalis trophozoites. The trophozoite is not usually considered to be involved in transmission although infection by this form is possible. Hewlett et al. (1982) suggested that the trophozoite could be infective and that cysts from different people or animals, or even those produced on different days, varied in infectivity.
The prepatent period of 5–6 days encountered in the present study was concordant with those observed by Barlough (1979), Hewlett et al. (1982), Kirkpatrick (1987), Zajac (1992) and Bordeau (1993), where dogs infected both naturally and experimentally demonstrated prepatent periods of 5–16 days. Differences between the forms and quantities of the protozoan used for each group did not influence the prepatent period.
The intermittent elimination of cysts over approximately 3 months demonstrated that dogs may be susceptible to human isolates of G. duodenalis and possibly act as carriers. Intermittent cyst elimination was described by Bemrick (1963), Barr and Bowman (1994) and Zajac et al. (2002). This pattern of elimination, together with the low sensitivity of detection by conventional methods, underestimates the real prevalence of G. duodenalis in animals held in groups (Thompson, 2004).
Little information is available on the behavior of G. duodenalis and the duration of infection in dogs. In this study dogs inoculated with trophozoites (Fig. 1) presented negative results from 91 to 105 p.i. becoming positive again soon afterwards. Bemrick (1963) noted that the cysts apparently disappeared spontaneously and this may be characteristic natural behavior of the parasite. This behavior may explain false-negative diagnoses or under-estimations. Bemrick (1963) noted that although some animals may develop persistent infections and the possibility of re-infection cannot be rejected. In this study it should be emphasized that hygiene, water quality, food and environmental conditions were controlled throughout the experimental period, to avoid contamination and re-infection. Zajac et al. (1992) observed experimentally infected dogs to be positive after treatment and suggested that they suppressed parasite levels, with latent potential for recrudescence of infection. However, the possibility of re-infection cannot be rejected. In this case, it is possible that treatment eliminates infection but does not confer immunity.
The absence of significant clinical signs in experimentally inoculated dogs agrees with the observations of several authors who commented on the asymptomatic nature of infection in most dogs with giardiasis (Leib and Zajac, 1999, Anderson et al., 2004, Thompson, 2004). However clinical giardiasis is reported and is associated with dogs (Leib and Zajac, 1995). The factors that determine the pathogenicity of infection are poorly understood, but clinical disease may be associated with the number or altered distribution of trophozoites in the duodenal mucosa (Zajac, 1992). It is also possible that human isolates used in these studies did not produce significant pathogenicity in dogs.
Based on the results of the red blood cell data, it was observed that dogs inoculated with trophozoites presented discrete anemia at 15 p.i., suggesting an association between G. duodenalis and nutrient deficiency. Studies such as those of Olivares et al. (2002), Olivares et al. (2004) and Heazlewood (1987), reported deficiencies of iron, Vitamin B12 and folic acid in people infected with G. duodenalis. However, the mechanism that the parasite uses to promote these alterations in humans is unclear. There is no information in the literature on deficiencies in iron, Vitamin B12 and folic acid in dogs with giardiasis. The profile of anemia and the lower mean values of group A in relation to the other groups may be related to the type of inoculum administered and/or the numbers of the form inoculated, which could result in the development of pathogenicity. Another possibility is that a physical barrier was created between the intestinal epithelium and lumen, resulting from rapid multiplication of the trophozoites by binary fission and interfering with nutrient absorption (Faubert, 2000).
With respect to the differential leukocyte count, the kinetics of immature and segmented neutrophils presented inverse profiles at 15 p.i., suggesting migration of segmented neutrophils to the tissues and the rapid liberation of band neutrophils into the peripheral blood (Fig. 3). Neutrophils are attracted to the tissues in response to stimuli from the inflamed area, influencing the number of these cells in the circulating blood, as well as the liberation of immature neutrophils. These neutrophils that migrate to the tissues do not return to the circulating blood but are directed to organs such as the lungs, intestinal tract, liver and spleen (Osgood, 1954). The presence of G. duodenalis in the intestinal tract is associated with the development of the inflammatory process in the epithelium (Jimenez et al., 2004), which could have influenced in the dynamics of the neutrophils observed in the present study.
According to Jain (1986), eosinophils are present in the blood and tissues, predominantly the skin, lungs, intestines and urogenital tract, their numbers generally being proportional to the degree of antigenic stimulation and resulting principally from the presence of parasites. Bordeau (1993) described dogs with giardiasis as presenting a tendency to eosinophilia. Although our results cannot be characterized as demonstrating absolute eosinophilia, the oscillations observed in groups A and B relative to the control may be associated with the presence of G. duodenalis, principally at 15 p.i., and thus concurrent with observed alterations in the erythrogram. Studies with mice inoculated with G. duodenalis demonstrate that eosinophils participate actively in the host immune response (Jimenez et al., 2004).
The biochemical parameters analyzed for total proteins, albumin, alanine aminotransferase, gamma glutamyltransferase, urea and creatinine did not present alterations or statistically significant differences that could be associated with G. duodenalis in the inoculated dogs. There are no reports in the literature on the blood biochemical profile in dogs inoculated with G. duodenalis.
The results of the present study suggest that trophozoites of G. duodenalis genetically defined as Portland 1 (ATCC 30888) from human isolates, as well as those uncharacterized isolates from children with clinical giardiasis and chronic cyst elimination, were able to infect dogs without producing disease symptoms, with minimal systemic manifestations. Elimination of cysts during the experimental period proves that infection by human isolates is not restricted to humans and dogs can be infected with G. duodenalis. More detailed studies are needed on the role of dogs and other animals in the transmission of the protozoan.
Acknowledgements
Thanks to Conselho Nacional de Pesquisa (CNPq) for financial support; Centroeste Nutrição Animal Ltda. for supplying dog's vaccines; Dr. Ednaldo Carvalho Guimarães for help with statistical analyses, and Dr. Bruce Alexander for English review.
References
- Anderson K.A., Brooks A.S., Morrison A.L., Reid-Smith R.J., Martin S.W., Benn D.M., Peregrine A.S. Impact of Giardia vaccination on asymptomatic Giardia infections in dogs at a research facility. Can. Vet. J. 2004;45:924–930. [PMC free article] [PubMed] [Google Scholar]
- Barlough J.E. Canine giardiases: a review. J. Small Anim. Pract. 1979;20:613–623. doi: 10.1111/j.1748-5827.1979.tb06670.x. [DOI] [PubMed] [Google Scholar]
- Barr S.C., Bowman D.D. Giardiasis in dogs and cats. Comp. Contin. Educ. Pract. Vet. 1994;16(5):603–614. [Google Scholar]
- Bemrick W.J. Observations on dogs infected with Giardia. J. Parasitol. 1963;49:1031–1032. [PubMed] [Google Scholar]
- Bordeau P. Les giardioses des carnivores. Rec. Méd. Vét. 1993;169(5–6):393–400. [Google Scholar]
- Dace J.V., Lewis S.M. J&A Churchill Ltd., Livingstone; London: 1984. Practical Haematology. pp. 536. [Google Scholar]
- Diamond L.P., Harlow D.R., Cunnick C.C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Faubert G. Imune response to Giardia duodenalis. Clin. Microbiol. Rev. 2000;13(1):35–54. doi: 10.1128/cmr.13.1.35-54.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E.C., D’Antoni I.C., Odon V. A critical study of clinical laboratory technics for the diagnosis of protozoan cysts and helminths eggs in feces. Preliminary communication. Am. J. Trop. Med. 1938;18:169–183. [Google Scholar]
- Ferreira Neto J.M., Viana E.S., Magalhães L.M. Minas Gerais; Brasil: 1981. Patologia Clínica Veterinária. Rabelo Belo Horizonte. pp. 293. [Google Scholar]
- Heazlewood V.J. Giardiasis and Vitamin B12 deficiency. Aust. NZJ. Med. 1987;17:261. doi: 10.1111/j.1445-5994.1987.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Hegner R. The biology of host-parasite relationships among protozoa living in man. Q. Rev. Biol. 1926;1:393–418. [Google Scholar]
- Hewlett E.L., Andrews J.S., Ruffier J., Schaefer F.W. Experimental infection of mongrel dogs with Giardia lamblia cysts and cultured trophozoites. J. Infect. Dis. 1982;145:89. doi: 10.1093/infdis/145.1.89. [DOI] [PubMed] [Google Scholar]
- Jacobs, S.R., Forrester, C.P.R., Yang, J., 2001. A survey of the prevalence of Giardia in dogs presented to Canadian veterinary practices. Can. Vet. J. 42 (1), 45–46. In: Anderson, K.A., Brooks, A.S., Morrison,A.L., Reid-Smith, R.J., Martin, S.W., Benn, D.M., Peregrine, 2004. Impact of Giardia vaccination on asymptomatic Giardia infections in dogs at a research facility. A.S. (Eds.), Can. Vet. J. 45, 924–930.
- Jain N.C. Lea & Febiger; Philadelphia: 1986. Schalm's Veterinary Hematology. pp. 1221. [Google Scholar]
- Jimenez J.C., Fontaine J., Grzych J.M., Deicas E., Capron M. Systemic and mucosal responses to oral administration of excretory and secretory antigens from Giardia intestinalis Clinical and Diagnostic Laboratory. Imunology. 2004;11(1):152–160. doi: 10.1128/CDLI.11.1.152-160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C.E. Giardiasis. Vet. Clin. N. Am. Small Anim. Pract. 1987;17(6):1377–1387. doi: 10.1016/s0195-5616(87)50007-9. [DOI] [PubMed] [Google Scholar]
- Lane S., Lloyd D. Current Trends in Research into the Waterborne Parasite Giardia. Crit. Rev. Microbiol. 2002;28(2):123–147. doi: 10.1080/1040-840291046713. [DOI] [PubMed] [Google Scholar]
- Leib M.S., Zajac A.M. Giardia: diagnosis and treatment. In: Bonagura J.D., editor. Current Veterinary Therapy XII: Small Animal Practice. WB Saunders; Philadelphia: 1995. pp. 716–719. [Google Scholar]
- Leib M.S., Zajac A.M. Giardiasis in dogs and cats. Vet. Med. 1999;9:793–802. [Google Scholar]
- Monis P.T., Thompson R.C.A. Cryptosporidium and Giardia—zoonoses: fact or fiction. Infect. Genet. Evol. 2003;3:233–244. doi: 10.1016/j.meegid.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Olivares J.L., Fernandez R., Fleta F., Ruiz M.Y., Clavel A. Vitamin B12 and Folic acid in children with intestinal parasitic infection. J. Am. Coll. Nutr. 2002;21(2):109–113. doi: 10.1080/07315724.2002.10719202. [DOI] [PubMed] [Google Scholar]
- Olivares J.L., Fernandez R., Fleta J., Ruiz M.Y., Clavel A., Moreno L.A. Iron deficiency in children with Giardia lamblia and Enterobius vermicularis. Nutr. Res. 2004;24:1–5. [Google Scholar]
- Osgood E.E. Number and distribution of human hemic cells. Blood. 1954;9:544–549. [PubMed] [Google Scholar]
- Padchenko I.K., Stolyarchuk N.Q. On the possible circulation of Lamblia in nature. Prog. Protozool. 1969;3:311–312. [Google Scholar]
- Roberts-Thomson I.C., Stevens D.P., Mahmoud A.A., Warren K.S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976;71:57–61. [PubMed] [Google Scholar]
- Thompson R.C.A., Hopkins R.M., Homan W.L. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol. Today. 2000;16(5):210–214. doi: 10.1016/s0169-4758(99)01624-5. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet. Parasitol. 2004;126:15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Van Keulen H., Homam W.L., Erlandsen S.L., Jarroll E.L. A three nucleotide signature sequence in small subunit rRNA divides human Giardia in two different genotypes. J. Eukaryot. Microbiol. 1995;42:392–394. doi: 10.1111/j.1550-7408.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Van Keulen H., Macechko P.T., Wade S., Schaaf S., Wallis P.M., Erlandsen S.L. Presence of human Giardia in domestic, farm and wild animals, and environmental samples suggests a zoonotic potential for giardiasis. Vet. Parasitol. 2002;108:97–107. doi: 10.1016/s0304-4017(02)00181-4. [DOI] [PubMed] [Google Scholar]
- Zajac A.M. Giardiasis. Comp. Cont. Educ. Pract. Vet. 1992;14(5):604–610. [Google Scholar]
- Zajac A.M., Leib M.S., Burkholdert W.J. Giardia infection in a group of experimental dogs. J. Small Anim. Pract. 1992;33:257–260. [Google Scholar]
- Zajac A.M., Leib M., Thompson R.C.A. Conferencing Room, The Veterinary Information Network, Inc.; 2002. Giardia A GI Parasite with Many Faces. ( http://www.vspn.org/Library/Rounds/VSPN_VSPN020407.htm) [Google Scholar]


