Abstract
Innate immunity serves as the frontline defence against invading pathogens. Despite decades of research, new insights are constantly challenging our understanding of host-elicited immunity during microbial infections. Recently, two families of humoral innate immune proteins, pentraxins and collectins, have become a major focus of research in the field of innate immunity. Pentraxins and collectins are key players in activating the humoral arm of innate immunity, taking centre stage in immunoregulation and disease modulation. However, increasing evidence suggests that pentraxins and collectins can also mediate pathogenic effects during some infections. Herein, we discuss the protective and pathogenic effects of pentraxins and collectins, as well as their therapeutic significance.
Keywords: pentraxins, collectins, humoral innate immunity, pathogens, pentraxin 3, mannose-binding lectin
Trends
The humoral arm of innate immunity is emerging as an important determinant of host-elicited defence during pathogen invasion. Pentraxins and collectins are two families of acute-phase proteins that have demonstrated immunomodulatory effector function.
Pentraxin 3 (PTX3) is a ‘double-edged’ sword that has demonstrated host protective roles during several fungal, bacterial, and viral infections. However, emerging evidence of pathogenic properties of PTX3 was observed during arthritogenic alphavirus infections.
Collectins and ficolins can interact with PTX3 to form heterocomplexes that may possibly affect alphavirus disease progression.
PTX3 and collectins represent promising therapeutic targets for the treatment of several pathogen infections. However, such treatment should be avoided in subjects with pre-exisiting alphavirus infection.
Pentraxins and Collectins: The Humoral Modulators of Innate Immunity
The innate immune system represents the front line of host defence against invading pathogens. Regulation of innate responses is sustained by the bidirectional interaction between cellular and humoral effectors of innate immunity (Figure 1 ). The humoral arm of innate immunity includes the complement system, as well as pattern-recognition molecules (PRMs) and pattern-recognition receptors (PRRs). Among the PRMs, members of the pentraxin and the collectin superfamilies have been studied intensively in recent years.
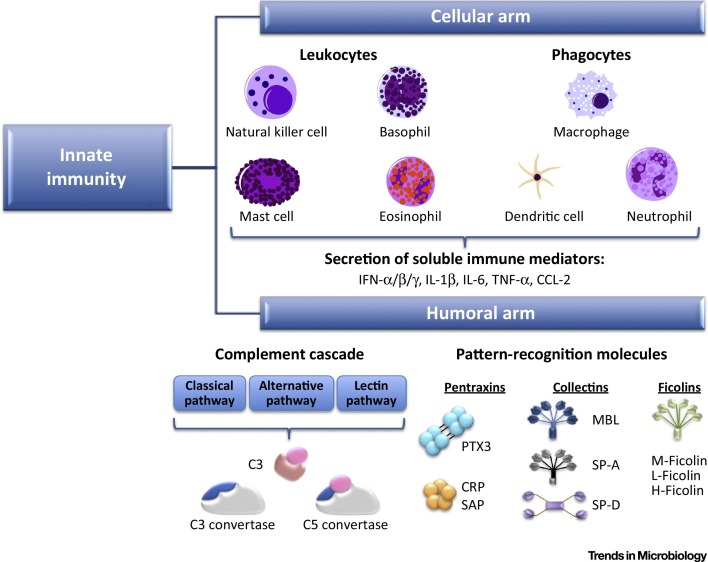
Figure 1.
The Two Arms of Innate Immunity: Cellular and Humoral Responses. The cellular arm of innate immunity is composed of immune cells, such as leukocytes and phagocytes, as well as immune mediators secreted by these cells. The humoral arm is composed of the complement cascade and pattern-recognition molecules (PRMs). Crosstalk between the two arms of innate immunity is crucial to respond promptly to stimuli and facilitate adaptive immune response.
Pentraxins belong to an evolutionarily conserved superfamily of proteins, distinguished by the presence of a C-terminal ‘pentraxin domain’ of 200 amino acids and a conserved ‘pentraxin signature’ of an eight amino acid-long sequence (HxCxS/TWxS, where x is any amino acid) [1]. This superfamily of proteins can be further classified into short and long pentraxins. Short pentraxins have an architectural structure of five or ten identical protomers arranged into a pentameric radial symmetry 2, 3. Members of the short pentraxins include C-reactive protein (CRP) and serum amyloid P component (SAP), which are acute-phase proteins secreted mainly by hepatocytes in response to proinflammatory cytokine interleukin (IL)-6 and other stimuli [4]. During the acute phase of infection, elevated levels of CRP and SAP lead to consequential activation of the classical complement cascade via interaction with C1q [5], resulting in removal of cell debris [6].
Pentraxin 3 (PTX3) was the first long pentraxin to be described in the early 1990s and is induced by tumour necrosis factor (TNF) and IL-1 7, 8. PTX3 has a structurally sophisticated octameric architecture, which is composed of two disulphide-linked tetramers giving rise to the asymmetry of the molecule [9]. Inflammation has been reported to induce PTX3 secretion from a broad range of cell types, but predominantly by monocytes, macrophages, and myeloid dendritic cells (DCs) [10]. Compared to the short pentraxins, our current understanding of PTX3 and its role in the humoral arm is limited, and has therefore been the focus of intensive research to clarify its role in a number of inflammatory and infection diseases.
Collectins are a family of collagenous Ca2+-dependent (C-type) lectins that are highly conserved in evolution and also function as soluble PRMs. C-type lectins contain a collagen-like region linked to a carbohydrate recognition domain (CRD), known as the carbohydrate-binding C-type lectin domain (CTLD), which enables binding to oligosaccharide (or lipid) structures expressed on the surface of an array of microorganisms [11]. Members of this family include the well-characterized ‘classical collectins’ mannose-binding lectin (MBL), surfactant protein (SP)-A and SP-D. Serum MBL is produced by the liver and is constitutively expressed in the blood at a concentration of ∼200 ng/ml during normal circumstances, which can be elevated to as high as ∼800 ng/ml during virus infections 12, 13. MBL plays a crucial role in the activation of the lectin complement pathway via interactions with MBL-associated serine protease (MASP). In contrast, SP-A and SP-D are predominantly found within the airways where they play a number of roles in modulating inflammation and phospholipid homeostasis [14]. Recently, a growing number of ‘novel collectins’ have been identified, which include collectin (CL) liver 1 (CL-L1) [15], CL kidney 1 (CL-K1) [16], and CL placenta 1 (CL-P1) [17], as well as the bovine-specific collectins conglutinin [18], CL-43 [19], and CL-46 [20]. As discussed below, recognition by collectins can lead to elimination of microorganisms by a range of mechanisms, including aggregation, opsonization, activation of phagocytosis, inhibition of microbial growth, or complement activation. In addition to microbial recognition, collectins have also been implicated in modulating inflammatory and allergic responses, aspects of adaptive immunity, and clearance of apoptotic cells [21]. In this review we focus on innate immune proteins – pentraxins, particularly PTX3, and collectins, discussing their role in modulating host immune responses during pathogen invasion.
Pentraxin 3: An Acute-Phase Immunoregulator of Pathogen-Associated Inflammation
Long pentraxin PTX3 recognizes select microorganisms, including fungi, bacteria, and viruses, and activates a number of antimicrobial effector mechanisms 4, 22. In addition, PTX3 plays an immunoregulatory role during inflammation through interactions with P-selectin, thereby modulating neutrophil recruitment as well as complement activation 4, 22, 23. The basal expression of PTX3 detected in the blood of healthy individual is approximately 2 ng/ml, which rapidly increase to a range of 200 to 800 ng/ml upon stimulation by proinflammatory cytokines during pathogen invasion 3, 24. The protective role of PTX3 during microbial infection has been long established; however, more recent studies suggest that PTX3 might also contribute to immunopathology during certain infections.
Protective Role of PTX3 in Pathogen Defence
Fungi PTX3 binds to Aspergillus fumigatus conidia, and PTX3-deficient mice show increased susceptibility to invasive aspergillosis associated with an inappropriate immune response skewed towards a Th2 response [25]. Treatment with recombinant PTX3 had therapeutic activity either alone or when combined with antifungal agents 4, 22, 25, 26, 27, 28. PTX3 is stored in neutrophil granules and is rapidly released upon cell stimulation [29]. In addition, the molecule was found in neutrophil extracellular traps (NETs), and PTX3 can opsonize A. fumigatus conidia inside these structures [29]. PTX3-deficient neutrophils were less effective in recognizing and eliminating A. fumigatus conidia, and opsonization of spores by PTX3 could reverse this phenotype 25, 29, 30. Interestingly, neutrophil-associated-PTX3 promoted the in vivo control of A. fumigatus infection [29]. Molecular mechanisms involved in this activity have been recently highlighted [30]. Briefly, the binding of PTX3-opsonized conidia to FcγRII, which has been shown to be a receptor for pentraxins [31], induces an inside-out activation of CD11b and a subsequent phagocytosis of C3b-opsonized conidia [30]. In addition, PTX3 can interact with ficolin-2 and MBL on the pathogen surface, and the formation of the heterocomplexes PTX3/ficolin-2 and PTX3/MBL can promote the deposition of complement, as observed on the surface of A. fumigatus and Candida albicans, respectively 32, 33.
The expression of PTX3 in macrophages was induced by zymosan [34]. In turn, PTX3 interacted with zymosan particles as well as with the yeast form of Paracoccidioides brasiliensis [34]. In the presence of PTX3, individual particles of zymosan were aggregated, leading to phagocytosis of a high number of particles by macrophages through a dectin-1-dependent mechanism [34].
In humans, single-nucleotide polymorphisms (SNPs) within the PTX3 gene were associated with enhanced susceptibility to infections [35]. PTX3 transcript stability might be altered by these genetic variants, and three genetic polymorphisms were associated with different PTX3 plasma levels 36, 37. Accordingly, PTX3 polymorphisms were reported to reduce the intracellular stock of PTX3 in neutrophils, leading to impaired phagocytosis and clearance of A. fumigatus [37]. Interestingly, PTX3 polymorphisms were recently associated with susceptibility to A. fumigatus infection in two cohorts of patients undergoing bone marrow transplantation [37]. The association between genetic polymorphisms and susceptibility to mold infections was recently independently confirmed in 1101 patients of the Swiss Organ Transplantation Cohort [38] and in a small cohort of lung transplantation patients [39].
Bacteria PTX3 interacts with selected bacteria, including Pseudomonas aeruginosa, Neisseria meningitidis, Klebsiella pneumoniae, and uropathogenic Escherichia coli (UPEC) 22, 25, 40, 41, 42 . PTX3 displayed opsonic activity for P. aeruginosa and UPEC, facilitating their recognition and ingestion by phagocytes 22, 42. Moreover, PTX3 had therapeutic activity in a model of chronic P. aeruginosa lung infection, reducing the bacterial load and controlling the inflammatory response [42]. In addition, PTX3, given orally to neonate mice, rapidly diffused into tissues and had therapeutic activity against P. aeruginosa lung infection [43].
Recently, PTX3 was identified as the first humoral PRM involved in defence against urinary-tract infections [35]. PTX3 was rapidly induced in uroepithelial cells in response to UPEC and amplified the phagocytosis and phagosome maturation in neutrophils [35]. Therefore, elimination of bacteria was defective in Ptx3 −/− mice and was associated with an exacerbated inflammatory response [35]. PTX3 also recognized outer membrane vesicles (OMV) from N. meningitidis and three selected meningococcal molecules (GNA0667, GNA1030, and GNA2091). Interestingly, PTX3-deficient animals displayed a defective antibody response to OMV [40]. Injection of PTX3 reversed this phenotype, and PTX3 has a protective effect in infant rats infected with N. meningitidis [40].
Genetic studies in humans support the relevance of the data obtained in animal models. Indeed, PTX3 SNPs have been associated with increased susceptibility to pulmonary tuberculosis, acute pyelonephritis, cystitis, and P. aeruginosa infections 35, 44, 45.
Viruses A protective role for PTX3 in defence against selected viruses has been proposed [35]. PTX3-deficient animals showed increased susceptibility to cytomegalovirus (CMV) and specific strains of influenza virus 46, 47. Mechanistically, PTX3 had the capacity to bind to human and murine CMV (MCMV), inhibiting the entry of virus into DCs and inducing interferon regulatory factor 3 (IRF3) activation [46]. Administration of PTX3 in BALB/c mice, known for their susceptibility to CMV infection, had therapeutic efficacy against primary infection and reactivation and protected MCMV-infected mice from invasive pulmonary aspergillosis [46].
PTX3 recognized also specific strains of H3N2 subtype influenza A viruses (IAV, H3N2) via an interaction between the glycosidic moiety of PTX3 and the haemagglutinin glycoprotein found on the surface of viruses [47]. In turn, this interaction led to a number of antiviral activities, including inhibition of viral haemagglutination and neuraminidase activities, as well as neutralization of virus infectivity [47]. As a consequence, PTX3-deficient animals had increased susceptibility to H3N2 infection, and administration of PTX3 had therapeutic activity [47]. In contrast, PTX3 did not display any protective effect during infection with both seasonal and pandemic H1N1 IAV and other H3N2 strains, likely due to a loss of interaction between the viral haemagglutinin and PTX3 48, 49.
PTX3 has also been implicated in defence against coronavirus murine hepatitis virus strain 1 (MHV-1) [50]. As observed for CMV and some strains of H3N2, PTX3 bound to MHV-1 and reduced infectivity in vitro [50]. In a model of intranasal infection with MHV-1, higher disease severity was observed in PTX3-deficient animals compared to wild-type mice, and administration of PTX3 had protective activity [50].
The Emerging Concept of PTX3 Pathogenicity
PTX3 is a multifunctional humoral innate protein which has been associated with diverse immunoregulatory functions. Despite the bulk evidence demonstrating a protective role for PTX3 during microbial invasion, recent studies using murine models of inflammatory diseases have suggested its potential to promote immunopathology. The first evidence suggestive of PTX3 pathogenicity was reported in 2006, using a lethal animal model of K. pneumoniae infection [51]. Infection of PTX3 transgenic mice overexpressing PTX3 with a high inoculum of K. pneumoniae resulted in accelerated lethality and this correlated with reduced infiltration of neutrophils into the lung tissues and enhanced bacterial dissemination in blood during acute infection. Ironically, when infection was performed using low pulmonary inocula, the overt expression of PTX3 conferred a protective effect, enabling robust expression of proinflammatory cytokines, an influx of neutrophils to lungs, and enhanced phagocytosis of bacteria [51]. This study clearly demonstrated the double-edged sword characteristics of PTX3 in shaping disease outcome during an infection.
Further evidence supportive of the pathogenic role of PTX3 was recently reported in a study conducted on arthritogenic alphaviruses – chikungunya virus (CHIKV) and Ross River virus (RRV) [12]. The study conducted by Foo et al. characterized overt expression of PTX3 during the acute phase of alphavirus infection in patients and animal models. Further characterization of the alphavirus mouse models identified neutrophils and inflammatory monocytes as the cellular reservoirs of rapid PTX3 production during the acute phase of infection. The presence of PTX3 promoted early viral entry and replication events through binding interactions with alphavirus, modulating the kinetic profiles of proinflammatory cytokines, and cellular infiltration in response to alphavirus infection, which consequentially shaped the progression of alphaviral disease (Figure 2 ). This study characterized PTX3 as a pivotal immunomodulatory protein associated with the pathogenic characteristics of alphavirus infection. Additionally, this study also demonstrated how the supposedly beneficial PTX3 could be hijacked and exploited by viruses to promote viral replication in the host [12].
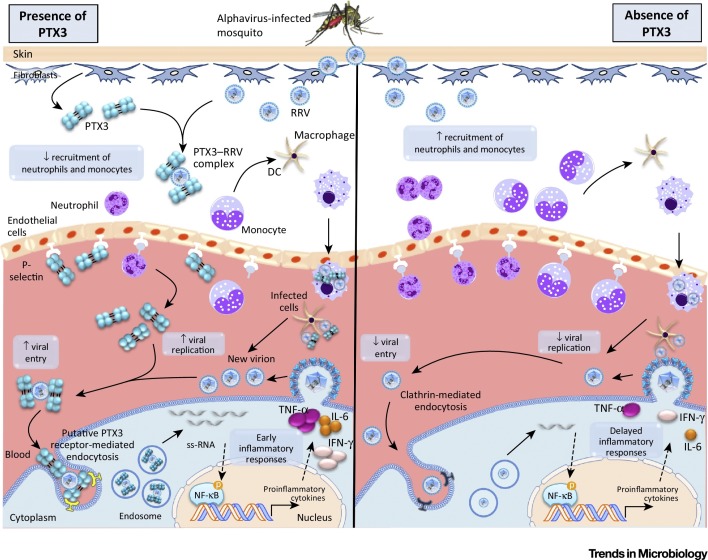
Figure 2.
Proposed Model for Pentraxin 3 (PTX3) as an Immunoregulatory Protein during Acute Alphavirus Infection. Following an infectious bite, Ross River virus (RRV) infects fibroblasts to trigger the expression of PTX3 which, in turn, binds to RRV. The presence of RRV triggers recruitment of immune cells, including neutrophils and monocytes, to the site of infection. Activated neutrophils express PTX3 which binds to P-selectin, dampening recruitment of neutrophils and monocytes. Recruited monocytes differentiate into dendritic cells (DCs) and macrophages, which phagocytose RRV and migrate back to the blood. RRV replication occurs in infected DCs and macrophages, releasing new virions that can complex with PTX3 to form PTX3–RRV complexes. The PTX3–RRV complex is recognized by a putative PTX3 receptor, enhancing viral entry through receptor-mediated endocytosis, and subsequently enhancing viral replication. Large amounts of RRV RNA released in the cytoplasm can be detected by pathogen receptors, triggering the activation of the NF-кB pathway and inducing proinflammatory immune responses. In the absence of PTX3, RRV infection triggers abundant recruitment of immune cells due to unbound P-selectin. RRV particles gain entry into the cell through clathrin-mediated endocytosis, leading to reduced viral entry and replication. The presence of fewer virions gives rise to delayed inflammatory responses. Abbreviations: IFN-γ, interferon-gamma; ss-RNA, single-stranded RNA.
In summary, the multifunctional characteristics of PTX3 can give rise to protective or pathogenic effects in response to different pathogen-induced inflammation. In a context of sterile inflammation, such as in tissue injury mediated by ischaemia and reperfusion, PTX3 can be protective (e.g., kidney) or deleterious (e.g., intestine), depending on the tissue 52, 53. PTX3 is endowed with a strong immunomodulatory role which can have a diverse impact on downstream innate immune responses, strongly suggestive of its therapeutic potential. However, taking into account the pathogenicity of PTX3 exhibited during alphavirus infections, study cohorts from future clinical trials involving PTX3 administration should be carefully assessed to avoid the inclusion of alphavirus-infected individuals as test subjects, which may result in deleterious clinical effects. To date, our understanding of PTX3 represents only the tip of an iceberg. More functional studies are warranted to further characterize the mechanism(s) underlying the immunomodulatory role of PTX3, which will enhance its utility in the development of novel therapeutics using recombinant or modified PTX3, as well as agonists and antagonists to modulate its secretion.
Collectins/Ficolins and Their Role in Pathogen Defence
Collectins have the unique ability to oligomerize into trimeric to hexameric structures that can activate the complement cascade [54]. MBL binds to a wide range of Gram-positive and Gram-negative bacteria, viruses, fungi, and protozoa, and MBL binding can activate complement via MASPs, which cleave C2 and C4 to form a C3 convertase leading to enhanced microbial clearance via opsonization and complement-mediated lysis (reviewed in [55]). However, MBL can mediate complement-independent effects, including inhibition of bacterial adhesion [56], opsonization to enhance bacterial internalization 57, 58, 59, blocking virus attachment and infection 60, 61, 62, and aggregation and opsonization to promote virus uptake via phagocytes [63].
Collectins: MBL
In humans, MBL is encoded by a single gene whereas in rodents two homologous proteins exist, MBL-A and MBL-C, and MBL-null mice lacking both proteins show increased susceptibility to a range of microbes, including Staphylococcus aureus [64], P. aeruginosa [65], herpes simplex virus (HSV)-2, [66], IAV [67], and the intracellular protozoan Trypanasoma cruzi [68].
While MBL is generally associated with protective host defence, emerging literature suggests a fine balance between the beneficial and detrimental outcomes associated with MBL-mediated recognition, particularly in the context of viral infections. For example, the MBL pathway of complement activation was also shown to play a critical role in the pathogenesis of alphaviral-induced inflammatory disease in mice infected with RRV, and MBL levels in serum and synovial fluids correlated with severity of disease in humans diagnosed with RRV [13]. MBL-deficient mice were more susceptible to infection with highly glycosylated MBL-sensitive strains of IAV [67], whereas infection with MBL-resistant strains was associated with reduced disease and airway inflammation [50], arguing that MBL may represent a risk factor during certain IAV infections. Of interest, MBL-mediated recognition resulted in enhanced infection of human cells by Ebola, Hendra, Nipah, and West Nile viruses by macropinocytosis in low-complement conditions [69]. MBL-mediated enhancement of HIV-1 infection of the brain occurs via alternative mechanisms, whereby gp120 shed by HIV-1 can be internalized via CXCR4 on neuronal cells, then bound and trafficked by intracellular MBL where it has been proposed to induce gp120-mediated neuronal apoptosis 70, 71.
Due to its promising therapeutic potential, several preclinical studies have evaluated the antimicrobial effect of MBL therapy. To evaluate the potential of MBL therapy in the context of Ebola virus infection, a lethal murine model of Ebola infection was utilized. High doses of MBL given to Ebola-challenged mice increased the survival rate by 40%, and mice exhibited protective immunity when rechallenged with Ebola virus [72]. The concentration of MBL in human serum varies greatly and is affected by mutations in the promoter and coding regions of the human MBL gene [73]. MBL deficiency is associated with susceptibility to various infections, although MBL-deficient individuals are generally healthy [74]. The concentration of plasma MBL in humans ranges widely between 5 to 10 000 ng/ml, resulting from polymorphisms in the MBL gene [75]. MBL-deficiency has been commonly observed in humans, with approximately 25% of the Caucasians having low levels (<500 ng/ml) of MBL, which are likely to be inadequate for protection against invading pathogens [76]. Indeed, MBL-null mice are susceptible to S. aureus infection, which resulted in 100% mortality 48 h postinfection [64].
Collectins: SP-A and SP-D
In contrast to MBL, SP-A and SP-D are synthesized by alveolar type II and Clara cells and constitutively expressed in the airways 77, 78, 79, and levels increase further during infection and/or inflammation of the airways 80, 81. Moreover, both SP-A and SP-D have been detected at extrapulmonary sites, including the gastrointestinal tract and kidney [82]. In addition to their role in innate host defence, SP-A and SP-D play a number of important physiological roles related to airway homeostasis [83]. In vitro studies indicate that SP-A and SP-D bind to a range of Gram-positive and Gram-negative bacteria and contribute to bacterial clearance by a number of mechanisms, including opsonization to increase phagocytosis by alveolar macrophages 84, 85 and neutrophils [63] as well as direct antimicrobial effects against Gram-negative bacteria 86, 87. Mice deficient in SP-A or SP-D, or with combined deficiency in both, have been used to demonstrate important protective roles for both pulmonary collectins against a range of different bacteria [88] and to show that the functions of SP-A and SP-D are not completely redundant during bacterial infections [89].
Both SP-A and SP-D have been reported to bind viruses, including IAV, respiratory syncytial virus (RSV), and HSV, and recognition is generally associated with virus aggregation and/or neutralization of virus infectivity. Of interest, SP-D and SP-A (and other collectins) potentiate virus uptake and virus-induced respiratory burst responses by neutrophils [90], and SP-A was reported to enhance phagocytosis of HSV-1 by alveolar macrophages [91]. Studies in SP-A−/− and SP-D−/− mice indicate that both can play protective roles during IAV infection; however, the relative importance of each is determined by strain-specific factors, such as the degree of virus glycosylation 80, 81, 92.
While a number of reports indicate that interactions between MBL and particular pathogens may be deleterious for the host, to date there is little evidence to implicate pulmonary collectins in disease exacerbation. While the ability of SP-A and SP-D to promote phagocytosis of extracellular bacteria by macrophages contributes to effective host defence, one could speculate that uptake of intracellular pathogens into their intracellular niche has the potential to exacerbate disease severity. Both SP-A and SP-D bind and agglutinate Mycobacterium tuberculosis, and SP-A enhances phagocytosis via upregulation of functional mannose receptor at the cell surface [93], whereas SP-D inhibits phagocytosis by macrophages [94]. However, SP-A−/−, SP-D−/−, or SP-A/D−/− mice displayed no major defects in uptake or control of M. tuberculosis in a low-dose, aerosol challenge model of tuberculosis, indicating that either or both pulmonary collectins are dispensable in the mouse model [95]. Both SP-A and SP-D also bind to Legionella pneumophila and suppress, rather than promote, intracellular growth in macrophages [96]. Of interest, HIV replication does occur in the lung, particularly during advanced disease [97], and SP-A has been reported to promote transfer of HIV-1 from dendritic cells to T cells [98].
Ficolins
Ficolins are structurally similar to MBL in that they possess a collagen-like domain, while a fibrinogen-like domain replaces the CRD of the collectins. Ficolins bind to acetylated compounds, including acetylated sugars found on the surface of some microbes [99]. Protective roles of MBL and ficolins have been reported during several microbial infections (Figure 3 ). In humans, there are three different forms of ficolin (H-, L-, and M-ficolin), which resemble MBL in overall structure, Ca2+-dependent binding to pathogens, and ability to activate complement independently of antibody (reviewed in [100]). In general terms, it is well established that ficolins bind a range of Gram-negative bacteria (e.g., Salmonella enterica serovar Typhimurium and P. aeruginosa) and Gram-positive bacteria (e.g., S. aureus and Aerococcus viridans) where they can serve as opsonins to increase phagocytosis and/or activate the lectin pathway of complement (reviewed in [101]). Recently, L-ficolin was shown to promote conidial uptake and killing of A. fumigatus by macrophages and neutrophils [102]. In the context of viral infections, L- and H-ficolins bind to IAV glycoproteins to inhibit virus infection in vitro and in vivo 103, 104, 105. Binding of L-ficolin to viral N-glycans expressed by hepatitis C virus (HCV) and HIV was reported to trigger activation of the lectin pathway complement 101, 106, and L-ficolin can neutralize HCV infectivity 107, 108.
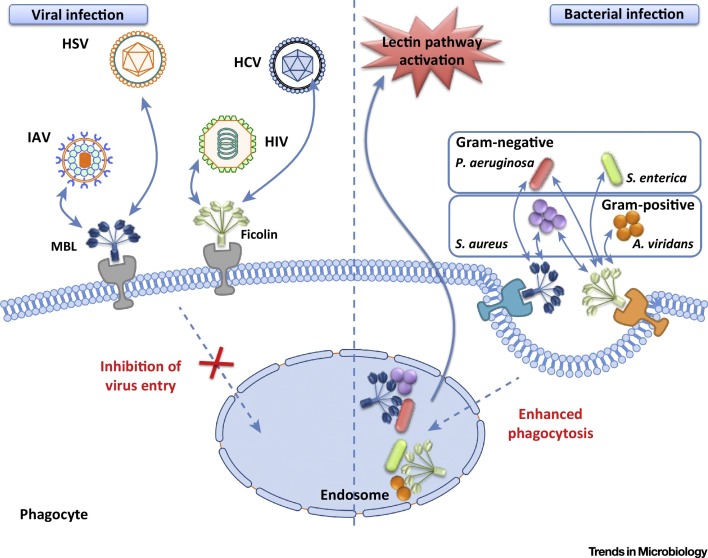
Figure 3.
Protective Roles of Collectins and Ficolins during Pathogen Attack. Collectins and ficolins have been reported to bind several microbes, including viruses and bacteria. In the context of viral infections, MBL can bind to viruses such as herpes simplex virus (HSV) and influenza A virus (IAV), while ficolin binds to hepatitis C virus (HCV) and human immunodeficiency virus (HIV), resulting in virus neutralization and inhibition of receptor-mediated endocytosis. During bacterial infections, MBL binds to Pseudomonas aeruginosa and Staphylococcus aureus, while ficolins bind both of these bacteria as well as Salmonella enterica and Aerococcus viridans, forming immunocomplexes and gaining entry into host cells through phagocytosis.
Impact of the Crosstalk between PTX3 and Collectins/Ficolins on Infectious Disease
Apart from interacting with microbial moieties, PTX3 has also demonstrated binding potential to several components of the complement cascade, including C1q of the classical pathway [109], Factor H of alternative pathway [110], as well as ficolins and MBL of the lectin pathway 32, 33, 111, 112.
Ficolin–PTX3 Complex Formation Promotes Complement Activation
Ficolins have been reported to recognize and bind several microbial moieties. Previously, L-ficolin has been shown to bind both A. fumigatus and PTX3. Interestingly, the binding affinity between L-ficolin and A. fumigatus was enhanced in the presence of PTX3, which promoted complement C4 deposition on the surface of A. fumigatus. Further characterization identified that a T236 M amino acid substitution on the fibrinogen-like domain of L-ficolin can lead to reduced binding capacity to PTX3 and A. fumigatus [32].
Other members of the ficolin family, such as M-ficolin, can also complex with PTX3 on apoptotic or necrotic cells, but not with A. fumigatus. The binding sites that enable the heterocomplex formation between M-ficolin and PTX3 were located on the structurally unique N-terminal domain of PTX3 and fibrinogen-like domain of M-ficolin, which is dependent on its sialic acid-binding ability 111, 112. The complex formation subsequently promoted phagocytosis of apoptotic cells and suppressed the production of IL-8 in human monocyte-derived macrophages, preventing excessive inflammatory responses and neutrophil recruitment [111]. These studies demonstrated the importance of crosstalk between ficolins and PTX3 in amplifying the innate immune responses through activation of the lectin complement pathway.
What Is the Effect(s) of MBL–PTX3 Complex Formation during Microbial Infections?
A recent structural study using MBL demonstrated binding to, and formation of, heterocomplexes with PTX3, giving rise to the cross-activation of complement pathways [33]. The presence of the MBL–PTX3 heterocomplex facilitated C1q recruitment and complement C3 and C4 deposition on C. albicans, promoting opsonophagocytosis by polymorphonuclear leukocytes [33].
The formation of MBL–PTX3 heterocomplexes and subsequent activation of lectin pathways have been largely associated with beneficial effects for the host. However, emerging evidence regarding the pathogenicity of MBL and PTX3 suggests a potentially pathogenic role for MBL–PTX3 heterocomplexes during alphavirus infections. Recent studies identified the N-terminus as a functional domain of PTX3 which modulates pathogenicity during alphavirus infection [12]. In a separate study, MBL was shown to induce deposition of complement component C3 on inflamed tissues, resulting in alphavirus-induced arthritis during acute RRV infection [13]. Therefore, based on the functional roles identified for PTX3, MBL, and MBL–PTX3 heterocomplexes, one can speculate that PTX3 and MBL are likely to complex during alphavirus infection and act in synergy to modulate viral replication and innate immunity. The presence of MBL–PTX3 complexes may trigger excessive activation of the lectin pathway, which, in turn, could give rise to extensive tissue destruction and exacerbated disease outcome during alphavirus infections (Figure 4 ). Future investigations are essential to dissect the immunological roles of MBL–PTX3 complexes, and these humoral innate immune complexes may be effective therapeutic targets in the defence against alphavirus infection (see Outstanding Questions).
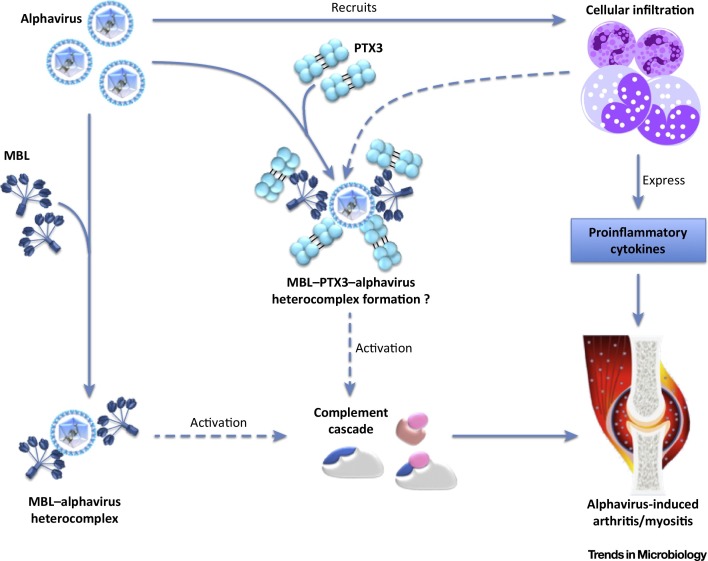
Figure 4.
Proposed Heterocomplex Formation between Pentraxin 3 (PTX3) and Mannose-Binding Lectin (MBL) during Alphavirus Infection. During an alphavirus infection, immune cells such as inflammatory monocytes and neutrophils are rapidly recruited to the site of infection. These activated immune cells express high levels of proinflammatory cytokines during alphavirus infection which promote tissue damage, as well as overt expression of humoral MBL and PTX3. MBL can, in turn, bind to alphavirus to form the MBL–alphavirus heterocomplexes, or it can bind to PTX3 to form MBL–PTX3-alphavirus heterocomplexes. These heterocomplexes can then activate the complement cascade, resulting in arthritis and myositis.
Concluding Remarks
Components from the cellular and humoral arms of the innate immune system must remain in a delicate balance to ensure effective detection and response to invading pathogens. The multifunctional roles of humoral pentraxins and collectins add to the complexity of eliciting appropriate innate immune responses. Despite intensive research efforts, our understanding of how the innate immune system detects and responds to different pathogens to shape, limit, or exacerbate disease severity is still limited. This review has discussed two families of humoral innate immune proteins which can mediate potential antimicrobial and immunoregulatory activities but, if dysregulated or activated inappropriately, can also act as potent inducers of immunopathology. Currently, further studies are required to clarify the functional and physiological roles of heterocomplexes formed between pentraxins and collectins or ficolins. Heterocomplex formation is dependent on sialylated moeities which are expressed on the N-terminal domain of PTX3 and CRD of collectins. Hence, computational and structural studies investigating those particular glycosylation sites (as well as the nature of the glycans expressed) on pentraxins and collectins will provide new insights into our current understanding regarding heterocomplex formation. Identification of key glycosylation sites that affect the functional role of these proteins may serve as the first step towards the development of new therapeutic strategies for intervention with a broad spectrum of microbial infections in the near future.
Outstanding Questions.
What are the putative receptors for pentraxin 3 (PTX3) expression on the cell surface? Do these receptors interfere with pathogen entry processes?
What are the exact structural features of PTX3, and how do these features interact with a pathogen to render a neutralized or enhanced pathogen infection?
Does PTX3–MBL complex formation exacerbate alphavirus infections?
Acknowledgment
S.M. is the recipient of an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellowship (APP1059167). The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
References
- 1.Garlanda C. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 2.Emsley J. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 3.Deban L. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 2011;343:237–249. doi: 10.1007/s00441-010-1018-0. [DOI] [PubMed] [Google Scholar]
- 4.Bottazzi B. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 5.Roumenina L.T. Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry. 2006;45:4093–4104. doi: 10.1021/bi052646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauta A.J. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24:148–154. doi: 10.1016/s1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 7.Lee G.W. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J. Immunol. 1993;150:1804–1812. [PubMed] [Google Scholar]
- 8.Breviario F. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J. Biol. Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 9.Inforzato A. The angiogenic inhibitor long pentraxin PTX3 forms an asymmetric octamer with two binding sites for FGF2. J. Biol. Chem. 2010;285:17681–17692. doi: 10.1074/jbc.M109.085639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alles V.V. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- 11.Drickamer K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999;9:585–590. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 12.Foo S.S. Role of pentraxin 3 in shaping arthritogenic alphaviral disease: from enhanced viral replication to immunomodulation. PLoS Pathog. 2015;11:e1004649. doi: 10.1371/journal.ppat.1004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunn B.M. Mannose binding lectin is required for alphavirus-induced arthritis/myositis. PLoS Pathog. 2012;8:e1002586. doi: 10.1371/journal.ppat.1002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crouch E. Collectins and pulmonary innate immunity. Immunol. Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohtani K. Molecular cloning of a novel human collectin from liver (CL-L1) J. Biol. Chem. 1999;274:13681–13689. doi: 10.1074/jbc.274.19.13681. [DOI] [PubMed] [Google Scholar]
- 16.Keshi H. Identification and characterization of a novel human collectin CL-K1. Microbiol. Immunol. 2006;50:1001–1013. doi: 10.1111/j.1348-0421.2006.tb03868.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohtani K. The membrane-type collectin CL-P1 is a scavenger receptor on vascular endothelial cells. J. Biol. Chem. 2001;276:44222–44228. doi: 10.1074/jbc.M103942200. [DOI] [PubMed] [Google Scholar]
- 18.Haurum J.S. Studies on the carbohydrate-binding characteristics of human pulmonary surfactant-associated protein A and comparison with two other collectins: mannan-binding protein and conglutinin. Biochem. J. 1993;293:873–878. doi: 10.1042/bj2930873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen S. Genomic and molecular characterization of CL-43 and its proximal promoter. Biochim. Biophys. Acta. 2003;1625:1–10. doi: 10.1016/s0167-4781(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 20.Hansen S. CL-46, a novel collectin highly expressed in bovine thymus and liver. J. Immunol. 2002;169:5726–5734. doi: 10.4049/jimmunol.169.10.5726. [DOI] [PubMed] [Google Scholar]
- 21.Winkler C., Hohlfeld J.M. Surfactant and allergic airway inflammation. Swiss Med. Wkly. 2013;143:w13818. doi: 10.4414/smw.2013.13818. [DOI] [PubMed] [Google Scholar]
- 22.Jaillon S. The long pentraxin PTX3 as a key component of humoral innate immunity and a candidate diagnostic for inflammatory diseases. Int. Arch. Allergy Immunol. 2014;165:165–178. doi: 10.1159/000368778. [DOI] [PubMed] [Google Scholar]
- 23.Bonavita E. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J. Clin. Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 25.Garlanda C. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 26.Lo Giudice P. Efficacy of PTX3 in a rat model of invasive aspergillosis. Antimicrob. Agents Chemother. 2010;54:4513–4515. doi: 10.1128/AAC.00674-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biagi E. PTX3 as a potential novel tool for the diagnosis and monitoring of pulmonary fungal infections in immuno-compromised pediatric patients. J. Pediatr. Hematol. Oncol. 2008;30:881–885. doi: 10.1097/MPH.0b013e318180bc1d. [DOI] [PubMed] [Google Scholar]
- 28.Gaziano R. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob. Agents Chemother. 2004;48:4414–4421. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaillon S. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moalli F. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 31.Lu J. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y.J. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J. Biol. Chem. 2009;284:28263–28275. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y.J. Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system. J. Biol. Chem. 2011;286:3405–3417. doi: 10.1074/jbc.M110.190637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diniz S.N. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J. Leukoc. Biol. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 35.Jaillon S. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Barbati E. Influence of pentraxin 3 (PTX3) genetic variants on myocardial infarction risk and PTX3 plasma levels. PLoS ONE. 2012;7:e53030. doi: 10.1371/journal.pone.0053030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha C. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 2014;370:421–432. doi: 10.1056/NEJMoa1211161. [DOI] [PubMed] [Google Scholar]
- 38.Wójtowicz A. PTX3 Polymorphisms and invasive mold infections after solid organ transplantation. Clin. Infect. Dis. 2015;61:619–622. doi: 10.1093/cid/civ386. [DOI] [PubMed] [Google Scholar]
- 39.Cunha C. PTX3-based genetic testing for risk of aspergillosis after lung transplantation. Clin. Infect. Dis. 2015 doi: 10.1093/cid/civ679. Published online August 10, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Bottazzi B. Recognition of Neisseria meningitidis by the long pentraxin PTX3 and its role as an endogenous adjuvant. PLoS ONE. 2015;10:e0120807. doi: 10.1371/journal.pone.0120807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeannin P. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Moalli F. Pathogen recognition by the long pentraxin PTX3. J. Biomed. Biotechnol. 2011;2011:830421. doi: 10.1155/2011/830421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaillon S. Prototypic long pentraxin PTX3 is present in breast milk, spreads in tissues, and protects neonate mice from Pseudomonas aeruginosa lung infection. J. Immunol. 2013;191:1873–1882. doi: 10.4049/jimmunol.1201642. [DOI] [PubMed] [Google Scholar]
- 44.Chiarini M. PTX3 genetic variations affect the risk of Pseudomonas aeruginosa airway colonization in cystic fibrosis patients. Genes Immun. 2010;11:665–670. doi: 10.1038/gene.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olesen R. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 2007;8:456–467. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 46.Bozza S. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 2006;108:3387–3396. doi: 10.1182/blood-2006-03-009266. [DOI] [PubMed] [Google Scholar]
- 47.Reading P.C. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 48.Job E.R. A single amino acid substitution in the hemagglutinin of H3N2 subtype influenza A viruses is associated with resistance to the long pentraxin PTX3 and enhanced virulence in mice. J. Immunol. 2014;192:271–281. doi: 10.4049/jimmunol.1301814. [DOI] [PubMed] [Google Scholar]
- 49.Job E.R. Pandemic H1N1 influenza A viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. J. Immunol. 2010;185:4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 50.Ling M.T. Mannose-binding lectin contributes to deleterious inflammatory response in pandemic H1N1 and avian H9N2 infection. J. Infect. Dis. 2012;205:44–53. doi: 10.1093/infdis/jir691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soares A.C. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006;8:1321–1329. doi: 10.1016/j.micinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Souza D.G. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am. J. Pathol. 2009;174:1309–1318. doi: 10.2353/ajpath.2009.080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao Y. Pentraxin 3 inhibits acute renal injury-induced interstitial fibrosis through suppression of IL-6/Stat3 pathway. Inflammation. 2014;37:1895–1901. doi: 10.1007/s10753-014-9921-2. [DOI] [PubMed] [Google Scholar]
- 54.Yokota Y. Oligomeric structures required for complement activation of serum mannan-binding proteins. J. Biochem. 1995;117:414–419. doi: 10.1093/jb/117.2.414. [DOI] [PubMed] [Google Scholar]
- 55.Jack D.L., Turner M.W. Anti-microbial activities of mannose-binding lectin. Biochem. Soc. Trans. 2003;31:753–757. doi: 10.1042/bst0310753. [DOI] [PubMed] [Google Scholar]
- 56.Zuo D.M. Protective role of mouse MBL-C on intestinal mucosa during Shigella flexneri invasion. Int. Immunol. 2009;21:1125–1134. doi: 10.1093/intimm/dxp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack D.L. Mannose-binding lectin enhances phagocytosis and killing of Neisseria meningitidis by human macrophages. J. Leukoc. Biol. 2005;77:328–336. doi: 10.1189/jlb.0604342. [DOI] [PubMed] [Google Scholar]
- 58.Kuhlman M. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polotsky V.Y. Interaction of human mannose-binding protein with Mycobacterium avium. J. Infect. Dis. 1997;175:1159–1168. doi: 10.1086/520354. [DOI] [PubMed] [Google Scholar]
- 60.Ezekowitz R.A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J. Exp. Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ip W.K. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kase T. Human mannan-binding lectin inhibits the infection of influenza A virus without complement. Immunology. 1999;97:385–392. doi: 10.1046/j.1365-2567.1999.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartshorn K.L. Human mannose-binding protein functions as an opsonin for influenza A viruses. J. Clin. Invest. 1993;91:1414–1420. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi L. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moller-Kristensen M. Deficiency of mannose-binding lectin greatly increases susceptibility to postburn infection with Pseudomonas aeruginosa. J. Immunol. 2006;176:1769–1775. doi: 10.4049/jimmunol.176.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gadjeva M. Mannan-binding lectin modulates the response to HSV-2 infection. Clin. Exp. Immunol. 2004;138:304–311. doi: 10.1111/j.1365-2249.2004.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang W.C. Lack of the pattern recognition molecule mannose-binding lectin increases susceptibility to influenza A virus infection. BMC Immunol. 2010;11:64. doi: 10.1186/1471-2172-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothfuchs A.G. Mannose-binding lectin regulates host resistance and pathology during experimental infection with Trypanosoma cruzi. PLoS ONE. 2012;7:e47835. doi: 10.1371/journal.pone.0047835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brudner M. Lectin-dependent enhancement of Ebola virus infection via soluble and transmembrane C-type lectin receptors. PLoS ONE. 2013;8:e60838. doi: 10.1371/journal.pone.0060838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bachis A. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J. Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teodorof C. Intracellular mannose binding lectin mediates subcellular trafficking of HIV-1 gp120 in neurons. Neurobiol. Dis. 2014;69:54–64. doi: 10.1016/j.nbd.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michelow I.C. High-dose mannose-binding lectin therapy for Ebola virus infection. J. Infect. Dis. 2011;203:175–179. doi: 10.1093/infdis/jiq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner M.W., Hamvas R.M. Mannose-binding lectin: structure, function, genetics and disease associations. Rev. Immunogenet. 2000;2:305–322. [PubMed] [Google Scholar]
- 74.Eisen D.P., Minchinton R.M. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 2003;37:1496–1505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 75.Steffensen R. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods. 2000;241:33–42. doi: 10.1016/s0022-1759(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 76.Valdimarsson H. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand. J. Immunol. 2004;59:97–102. doi: 10.1111/j.0300-9475.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 77.Crouch E. Surfactant protein D: subcellular localization in nonciliated bronchiolar epithelial cells. Am. J. Physiol. 1992;263:L60–L66. doi: 10.1152/ajplung.1992.263.1.L60. [DOI] [PubMed] [Google Scholar]
- 78.Crouch E.C. Structure, biologic properties, and expression of surfactant protein D (SP-D) Biochim. Biophys. Acta. 1998;1408:278–289. doi: 10.1016/s0925-4439(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 79.Wohlford-Lenane C.L., Snyder J.M. Localization of surfactant-associated proteins SP-A and SP-B mRNA in rabbit fetal lung tissue by in situ hybridization. Am. J. Respir. Cell Mol. Biol. 1992;7:335–343. doi: 10.1165/ajrcmb/7.3.335. [DOI] [PubMed] [Google Scholar]
- 80.Hawgood S. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J. Virol. 2004;78:8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LeVine A.M. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 2001;167:5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 82.van Rozendaal B.A. Localization and functions of SP-A and SP-D at mucosal surfaces. Pediatr. Pathol. Mol. Med. 2001;20:319–339. [PubMed] [Google Scholar]
- 83.Crouch E., Wright J.R. Surfactant proteins a and d and pulmonary host defense. Annu. Rev. Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 84.Pikaar J.C. Opsonic activities of surfactant proteins A and D in phagocytosis of Gram-negative bacteria by alveolar macrophages. J. Infect. Dis. 1995;172:481–489. doi: 10.1093/infdis/172.2.481. [DOI] [PubMed] [Google Scholar]
- 85.Restrepo C.I. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1999;21:576–585. doi: 10.1165/ajrcmb.21.5.3334. [DOI] [PubMed] [Google Scholar]
- 86.Schaeffer L.M. Bordetella pertussis lipopolysaccharide resists the bactericidal effects of pulmonary surfactant protein A. J. Immunol. 2004;173:1959–1965. doi: 10.4049/jimmunol.173.3.1959. [DOI] [PubMed] [Google Scholar]
- 87.Wu H. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuroki Y. Pulmonary collectins in innate immunity of the lung. Cell. Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 89.Giannoni E. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 2006;34:704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White M.R. Respiratory innate immune proteins differentially modulate the neutrophil respiratory burst response to influenza A virus. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L606–L616. doi: 10.1152/ajplung.00130.2005. [DOI] [PubMed] [Google Scholar]
- 91.van Iwaarden J.F. Surfactant protein A is opsonin in phagocytosis of herpes simplex virus type 1 by rat alveolar macrophages. Am. J. Physiol. 1991;261:L204–L209. doi: 10.1152/ajplung.1991.261.2.L204. [DOI] [PubMed] [Google Scholar]
- 92.LeVine A.M. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 93.Beharka A.A. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J. Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- 94.Ferguson J.S. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J. Immunol. 1999;163:312–321. [PubMed] [Google Scholar]
- 95.Lemos M.P. Dispensability of surfactant proteins A and D in immune control of Mycobacterium tuberculosis infection following aerosol challenge of mice. Infect. Immun. 2011;79:1077–1085. doi: 10.1128/IAI.00286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sawada K. Pulmonary collectins protect macrophages against pore-forming activity of Legionella pneumophila and suppress its intracellular growth. J. Biol. Chem. 2010;285:8434–8443. doi: 10.1074/jbc.M109.074765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakata K. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am. J. Respir. Crit. Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 98.Gaiha G.D. Surfactant protein A binds to HIV and inhibits direct infection of CD4+ cells, but enhances dendritic cell-mediated viral transfer. J. Immunol. 2008;181:601–609. doi: 10.4049/jimmunol.181.1.601. [DOI] [PubMed] [Google Scholar]
- 99.Endo Y. Carbohydrate-binding specificities of mouse ficolin A, a splicing variant of ficolin A and ficolin B and their complex formation with MASP-2 and sMAP. Immunogenetics. 2005;57:837–844. doi: 10.1007/s00251-005-0058-1. [DOI] [PubMed] [Google Scholar]
- 100.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol. Immunol. 2007;44:3875–3888. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Ren Y. Ficolins and infectious diseases. Virol. Sin. 2014;29:25–32. doi: 10.1007/s12250-014-3421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bidula S. The serum opsonin L-ficolin is detected in lungs of human transplant recipients following fungal infections and modulates inflammation and killing of Aspergillus fumigatus. J. Infect. Dis. 2015;212:234–246. doi: 10.1093/infdis/jiv027. [DOI] [PubMed] [Google Scholar]
- 103.Chang W.C. Recombinant chimeric lectins consisting of mannose-binding lectin and L-ficolin are potent inhibitors of influenza A virus compared with mannose-binding lectin. Biochem. Pharmacol. 2011;81:388–395. doi: 10.1016/j.bcp.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pan Q. L-ficolin binds to the glycoproteins hemagglutinin and neuraminidase and inhibits influenza A virus infection both in vitro and in vivo. J. Innate Immun. 2012;4:312–324. doi: 10.1159/000335670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verma A. Human H-ficolin inhibits replication of seasonal and pandemic influenza A viruses. J. Immunol. 2012;189:2478–2487. doi: 10.4049/jimmunol.1103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J. Specifically binding of L-ficolin to N-glycans of HCV envelope glycoproteins E1 and E2 leads to complement activation. Cell. Mol. Immunol. 2009;6:235–244. doi: 10.1038/cmi.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hamed M.R. Recombinant human L-ficolin directly neutralizes hepatitis C virus entry. J. Innate Immun. 2014;6:676–684. doi: 10.1159/000362209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y. Ficolin-2 inhibits hepatitis C virus infection, whereas apolipoprotein E3 mediates viral immune escape. J. Immunol. 2014;193:783–796. doi: 10.4049/jimmunol.1302563. [DOI] [PubMed] [Google Scholar]
- 109.Bottazzi B. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J. Biol. Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 110.Deban L. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J. Immunol. 2008;181:8433–8440. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 111.Ma Y.J. Ficolin-1-PTX3 complex formation promotes clearance of altered self-cells and modulates IL-8 production. J. Immunol. 2013;191:1324–1333. doi: 10.4049/jimmunol.1300382. [DOI] [PubMed] [Google Scholar]
- 112.Gout E. M-ficolin interacts with the long pentraxin PTX3: a novel case of cross-talk between soluble pattern-recognition molecules. J. Immunol. 2011;186:5815–5822. doi: 10.4049/jimmunol.1100180. [DOI] [PubMed] [Google Scholar]