Graphical abstract
Keywords: Infection control, Self-disinfecting surfaces, Healthcare facilities, Public spaces, Microbiological risk assessment
Abstract
According to World Health Organization, every year in the European Union, 4 million patients acquire a healthcare associated infection.
Even though some microorganisms represent no threat to healthy people, hospitals harbor different levels of immunocompetent individuals, namely patients receiving immunosuppressors, with previous infections, or those with extremes of age (young children and elderly), requiring the implementation of effective control measures.
Public spaces have also been found an important source of infectious disease outbreaks due to poor or none infection control measures applied.
In both places, surfaces play a major role on microorganisms’ propagation, yet they are very often neglected, with very few guidelines about efficient cleaning measures and microbiological assessment available.
To overcome surface contamination problems, new strategies are being designed to limit the microorganisms’ ability to survive over surfaces and materials.
Surface modification and/or functionalization to prevent contamination is a hot-topic of research and several different approaches have been developed lately. Surfaces with anti-adhesive properties, with incorporated antimicrobial substances or modified with biological active metals are some of the strategies recently proposed.
This review intends to summarize the problems associated with contaminated surfaces and their importance on infection spreading, and to present some of the strategies developed to prevent this public health problem, namely some already being commercialized.
1. Introduction
Microorganisms’ spreading, and consequent infection propagation is a serious concern worldwide, yet there are still very few guidelines or legislation for infection propagation control in public spaces. The exception is made for healthcare facilities with the existence of few guidelines and orientations for infection prevention and control, nevertheless hospital acquired infection (HAI) remains a tremendous problem. One of the main causes for the high number of HAI reported by the World Health Organization (WHO) is related to the ability of the microorganisms to survive for long periods in hostile environments such as dry surfaces [1], [2].
Rooms occupied with infected patients often have their surfaces contaminated with pathogens leading to the contamination of hands and gloves of medical staff, and consequent transfer of these microorganisms to patients or onto other surfaces. This cross-contamination mechanism has already been recognized and lead to the implementation of hygiene procedures for hands when contacting with patients. Still, it is more likely for a patient to get an infection when staying in a room previously occupied by an infected patient. Thus, infected patients are the primary source of surface and material contamination. Visitors and/or asymptomatic carriers also contribute to the spread of microorganisms, especially in situations where there is an apparent sense of safety [3]. As said before, there are guidelines for surface cleaning and disinfection in hospitals, however, those recommendations are not sufficient to solve such a problem and the incorrect following of the disinfection instructions can even cause greater contamination problems [4].
This review considered English-language articles retrieved from PubMed database literature searches, bibliographies from published articles, and infection-control books and chapters, in a total of 205 references published between 2000 and 2018, considering the following criteria: the most recent studies performed on microbiological analysis on different surfaces reporting samplings performed on food contact surfaces, public spaces and hospital surfaces, where microorganisms occur naturally. For the antimicrobial and self-cleaning surfaces studies, the most recent and relevant works developed on surface modification or functionalization were selected and two main types of surfaces were considered for this review - anti-adhesive surfaces and antimicrobial surfaces, along with their subtypes. Finally, products already commercially available with enough reliable manufacturers’ information about the product and its adequacy to the topics discussed in this review were also included.
2. Role of surfaces in infection propagation: a public health problem
Contamination spreading by contact can occur either by direct contact with an infected patient, or indirectly through a contaminated object or surface [5].
Surfaces represent an important way of transmission of diseases since they can act as a reservoir of microorganisms that may spread to whoever contacts with the surface. Especially in crowded spots as public transports or public spaces and places susceptible of the presence of microorganisms like healthcare facilities, surfaces may represent a serious source of infection spreading [6]. Table 1 describes the microorganisms detected in hospital, food handling, and other environmental surfaces.
Table 1.
Examples of microorganisms isolated from different surfaces and some of their associated pathologies.
| Microorganism | Surfaces where it was found | Associated pathologies |
|---|---|---|
| Acinetobacter spp. | Hospital surfaces (mattresses, medical charts …) [33], [42], [143], [144] Environmental surfaces (telephones) [17] Food-contact surfaces [145], [146] |
Respiratory tract infection, pneumonia, wound infection, bacteremia [147], [148] |
| Aspergillus spp. | Hospital surfaces (cleaning towels, nursing stations, sink waste pipes, medical overalls…) [39], [149], [150] | Pulmonary infection, skin infection, central nervous system infection, endocarditis [151], [152], [153], [154] |
| Campylobacter spp. | Food-contact surfaces [155], [156], [157], [158] | Gastroenteritis (diarrhea) [7], [159] |
| Candida spp. | Hospital surfaces (sink, chairs…) [144], [160] | Oral and vaginal Candidiasis [161], [162] |
| Clostridium difficile | Hospital surfaces (bed rails, tables, call buttons, toilets, chairs…) [28], [163], [164] Environmental surfaces (toilets, sinks, vacuum cleaners, hotel room surfaces…) [165], [166] |
Gastroenteritis (diarrhea), Pseudomembranous colitis [167] |
| Coronavirus | Environmental surfaces (daycare center surfaces, university classroom surfaces) [16], [168] Hospital surfaces (bedrails, doorknobs, ventilator control panels, TV remote controls…) [169], [170] |
SARS (severe acute respiratory syndrome) and MERS (middle east respiratory syndrome) [171] |
| Enterococcus spp. (including vancomycin-resistant enterococci) | Hospital surfaces (mattresses, medical charts…) [33], [42], [143] Environmental surfaces (fitness centers…) [18] Food-contact surfaces [172] |
Endocarditis, meningitis, catheter-related infection [173], [174], [175] |
| Escherichia coli | Hospital surfaces (medical charts…) [143], [144], [176] Food-contact surfaces [11], [155], [172] |
Gastroenteritis (diarrhea), peritonitis, urinary tract infection [177], [178], [179] |
| Influenza virus | Environmental surfaces (toilets, toys, faucets, floors, door knobs, TV remote controls) [180], [181], [182] Hospital Surfaces [181] |
Influenza (flu) [183] |
| Klebsiella spp. | Hospital surfaces (mattresses, medical charts …) [32], [33], [143], [144] Environmental surfaces (fitness centers…) [18] Food-contact surfaces [146] |
Urinary tract infection, pneumonia, respiratory tract infection [148], [177] |
| Norovirus | Hospital surfaces (door handles, taps, light switch, telephones…) [184], [185] Environmental surfaces (carpets, door handles, soft furnishing, toilet seats, tables…) [186], [187] |
Gastroenteritis (diarrhea)[188] |
| Pseudomonas spp. | Hospital surfaces (mattresses, showers, taps, sinks, bed rails…) [32], [33], [144] | Wound infection, urinary tract infection, respiratory tract infection, pancreatitis, otitis, necrotizing pneumonia, bacteremia, sepsis [189], [190], [191], [192] |
| Rotavirus | Hospital surfaces (bed rails, chairs, cardiac monitor keyboard, door handles, TV remote controls, telephones…) [193], [194] | Rotavirosis (acute diarrhea) [159] |
| Salmonella spp. | Food-contact surfaces [146], [155], [157], [158] Environmental surfaces (fitness centers, toilets…) [18], [195] Hospital Surfaces (curtains, stethoscope…) [42], [196] |
Enteric fever, Gastroenteritis (diarrhea) [7], [159], [197] |
| Staphylococcus spp. (including MRSA) | Hospital surfaces (mattresses, curtains, ECG wires, medical charts …) [32], [33], [36], [38], [143], [144] Environmental surfaces (public telephones, fitness centers, elevator buttons, hotel room surfaces…) [17], [18], [19], [166] Food contact surfaces [155], [172], [198] |
Gastroenteritis (diarrhea), skin infection, pneumonia, catheter infection [7], [199], [200], [201] |
2.1. Food contact surfaces
It is estimated that each year, in the United States alone, 9.4 million episodes of foodborne illness occur resulting nearly 56,000 hospitalizations and over 1300 deaths. The microorganisms most commonly associated with this phenomenon are norovirus, Salmonella spp., Clostridium perfringens and Campylobacter [7].
Food contamination can result from several factors and occur at different points of the preparation process. From farming to the moment of consuming, food goes through several steps where contamination by microorganisms can occur. Temperature and storage conditions are important factors to prevent food spoilage however, there are other factors that can cause microbiological contamination [8].
Food-contact surfaces’ contamination can cause food contamination with pathogens during processing or packaging. Bacteria are naturally present in plants and animals and it is easy for them to attach to a food contact surface during handling. Due to adhesion mechanisms bacteria can remain on the surfaces and contaminate other foods [9]. Chopping boards, knifes and preparation tables are just some of the food preparation instruments found contaminated with bacteria [10].
Saad et al. [11] evaluated hygiene conditions on food preparation facilities by analysing food-contact surfaces and the results were quite adverse, suggesting fecal contamination. Coliform bacteria were found in dining tables (58%), food trays (33%), cooking pots (33%) and kitchen faucets (8%). In addition, E. coli was identified in some cooking pots.
In this study, surfaces with a count of total mesophilic aerobes of >10 CFU/cm2 or >1 CFU/cm2 for E. coli and coliforms were considered to fail hygiene criteria, according to the Commission of the European Communities guidelines [12] for hygiene conditions in fresh meat processing facilities.
Although most countries have guidelines about hygiene measures and good practice on food processing facilities not always the cleaning procedures are sufficient to prevent microorganisms propagation [4]. Most of the times the cleaning cloths used in cleaning can become contaminated if soaked with disinfectants in the wrong dilution (common fault in food handling facilities), contributing to microorganisms’ spread to previously uncontaminated surfaces [13].
In the food industry, the occurrence of biofilm formation is also a common event due to inefficient cleaning and disinfection. The remaining microorganisms can survive on the surfaces, especially if food residues remain. This will promote the development and multiplication of bacteria with consequent biofilm formation that is extremely hard to eliminate, becoming even more difficult to disinfect the surface [14].
2.2. Schools and public spaces
Schools and day-care centers are hot spots of infection spreading. Lack of hygiene habits, common among very young children, is one of the main causes of contamination between kids, leading to the contamination of other children, staff, parents or people in the community, either by direct contact or by contaminating surfaces.[15].
Ibfelt et al. [16] evaluated the presence of bacteria and viruses in day-care centers in Denmark and different surfaces from toilets, kitchens and playrooms were analyzed. The results proved contamination by several bacteria and virus. Despite the main bacteria present were non-pathogenic, several surfaces revealed the presence of coliform and nasopharyngeal bacteria. Respiratory viruses were mainly present on toys but also on pillows and playroom tables. Gastrointestinal viruses although less prevalent were found in some surfaces, mainly on playroom pillows and toilet surfaces.
A study by Jerković-Mujkić et al. [17] analyzed potential contamination in 60 public telephones and the results showed high presence of microorganisms on the surfaces. Staphylococcus epidermidis (73.3%) and Bacillus subtilis (40%) were the most common bacteria identified, however more species were isolated.
Mukherjee et al. [18] evaluated the presence of microorganisms on different surfaces of a fitness center. The samples were collected from skin-contact surfaces as floor mats and exercise instruments, regularly shared by many people. In total, 63 species were identified with the presence of some pathogenic or potential pathogenic bacteria like Salmonella, Staphylococcus or Klebsiella. Staphylococci were present in the majority of the surfaces being one the most prevalent species, namely S. aureus, S. epidermidis and Staphyloccocus saprophyticus.
Kandel et al. [19] developed a study with stupefying results. They compared microbiological contamination of toilet surfaces with buttons on hospital elevators, and the buttons showed higher colonization (61%) than the toilets (43%). The most common bacteria on both surfaces were Staphylococcus and Streptococcus, however other bacterial species were founded, as wells as fungi.
Otter and French [6] studied the presence of microorganisms in hand-touch surface in public transport system. The samples were collected from several surfaces in trains, stations and buses of London. The presence of bacteria was confirmed, and most sites presented a median bacterial concentration of 12 CFU cm−2. Even though there are no guidelines for acceptable values for bacteria's presence on surfaces of public spaces this value is higher than the recommended for food-processing surfaces [12] and hospital hand-contact surfaces [20], [21].
2.3. Hospital surfaces
It has been recognized that hospitals’ contaminated surfaces and medical equipment contribute to the contamination of patients and medical staff [3], [5]. Several factors contribute to this occurrence but the mains reason is associated with the high prevalence of microorganisms in healthcare facilities.
Pathogens with the ability to cause high rates of infection come from different sources such as patients’ endogenous flora (40–60%), hands of medical staff and assistants (20–40%), antibiotic-driven changes in patients’ flora (20–25%) and other, including contamination of the environment (20%) [22].
Carling et al. [23], [24] have shown that in some cases less than 50% of hospital surfaces can be considered clean after terminal disinfection procedures. However, for specific surfaces the rate can be even more alarming with less than 30% of bedpan cleaners, bathroom hand-holds, light switches, and doorknobs being cleaned [25]. Contact between hands and gloves of medical staff with contaminated surfaces leads to pathogens’ spreading to other surfaces and people [5]. Particularly if they are immunocompromised patients, the risk of developing an infection is very high [26].
Stiefel et al. [27] found out that the level of contamination of gloves on medical staff after contact with patients’ skin sites was actually quite similar (40% vs 45%) to the level of contamination after contacting with surfaces on methicillin- resistant Staphylocococcus aureus (MRSA) carriers’ rooms, suggesting that environmental surfaces may pose serious risks.
An identical study from Guerrero et al. [28] found similar results for C. difficile contamination, proving that the risk of hand contamination after contact with patients’ skin was the same than when contacting with rooms’ highly-touched surfaces (50% vs 50%).
This issue is also related to the compliance of hospital workers on performing disinfection protocols. A recent study from Stahmeyer et al. [29] showed that only 42% of healthcare workers washed their hands before contact with a patient, and 50% washed their hands after contact with the patient. Also, this study found out that 94.3% of healthcare workers’ hand hygiene procedure lasted less than 15 s (average 7.6 s), when the recommendations is that it should last around 30 s.
Another study evaluating disinfection compliance by healthcare workers showed that only 26% of the enquired radiologists affirmed to disinfect their workstation daily, 24% admitted never to have disinfected their workstation, and 100% said to have never received any recommendation on how to perform the disinfection [30].
Several studies have proved that surfaces in rooms of patients infected with important pathogens are frequently contaminated, and that a person admitted to a room previously occupied by an infected patient has an increased likelihood of developing colonization or infection with that pathogen [25], [31].
A study performed in Portugal by Geadas Farias et al. [32] revealed hospital surfaces’ contamination with different microorganisms, mostly Pseudomonas and Staphylococcus. Several surfaces were contaminated but some of those with higher microbiological presence were surfaces associated with water distribution system as sinks, taps, showers and drains.
Viana et al. [33] performed an analysis on hospital mattresses, and resistant bacteria were found on about 50% of the evaluated mattresses. A. baumannii was the main species found, being present in 73.1% of the mattresses, but also other pathogens were collected as K. pneumoniae and P. aeruginosa. This study also identified the microorganisms found on mattresses and showed that in 54% of the cases the microorganisms were not related to the current patient admitted in the room but to the previous one, concluding that even after cleaning microorganisms still remain on the mattresses being a possible source of contamination to other patients.
Despite most studies evaluating the effectiveness of room disinfection have focused on some specific surfaces considered dirty (sink, toilets, etc.), all spots must be considered since contamination can be present on unsuspected surfaces [34]. Actually, White et al. [35] found out in an hospital that microbiologically the sinks and floors were cleaner than hand touch sites as chairs, beds and cardiac monitors buttons.
Shek et al. [36] developed a study where hospital curtains of burn and plastic surgery units were microbiologically tested. The results proved a considerable presence of bacteria on the curtains, even multi resistant bacteria as MRSA.
Levin et al. [37] found that in situations of poor infection control practices it is possible for patients to become a source for unresolved contamination of portable radiographic equipment with pathogenic bacteria, namely resistant bacteria.
Lestari et al. [38] performed a similar study but evaluating ECG lead wires, and among the 451 lead wires analyzed only 2 were non-contaminated with some type of microorganism. Coagulase negative staphylococci and aerobic spore forming bacteria, two important types of pathogens, were present in 96% and 71% of the wires respectively. The ineffective cleaning associated with the ability of microorganisms to survive on surfaces under hostile conditions, for long periods of time, is a serious cause of pathogens spreading to people and to other surfaces [31].
The presence of biofilms, the substances used in the cleaning process, and the method and frequency of cleaning, all interfere in the grade to which microorganisms resist to the cleaning and disinfection procedures. Badly executed cleaning procedures may bring bigger problems than not cleaning at all. The transmission of microorganisms from a contaminated surface to a clean one can occur if the cleaning method is not correct. Also, most detergents do not have antimicrobial activity they only act in the microorganisms’ removal, leading to the contamination of the cleaning cloths with viable microorganisms, and its consequent spread to non-contaminated surfaces [39].
The frequency of cleaning is also an important factor, since surfaces such as toilets, sinks, and other known as dirty surfaces tend be cleaned frequently [40]. However, other surfaces thought safe but still highly touched are often neglected, being poorly cleaned. For example, according to the Practical Guidelines for Infection Control in Health Care Facilities [41], mattresses without plastic covers must be steam cleaned if contaminated with body fluids. However, if body fluid contamination does not occur the mattresses are not so often cleaned, allowing microorganisms to accumulate with time and use, as proved by Viana et al. [33]. Also Geadas Farias et al. [32] have found that surfaces usually not considered dirty but highly touched as light switches or nursing trays were contaminated with several microorganisms.
A very recent study by Johani et al. [42] revealed the presence of biofilms in many surfaces of intensive care units despite regular disinfection procedures. Several highly touched surfaces were analyzed and in 70%, the presence of biofilm was proved by scanning electron microscopy.
In many situations the lack of adequate training of the environmental cleaning services or of the medical staff ultimately promotes the use of inappropriate detergents or germicides promoting the survival of microorganisms on the surfaces, even after cleaning [43].
Among the most used disinfectants are substances based on hypochlorite, alcohols, aldehydes, phenols and quaternary ammonium compounds [44]. However, not all of these substances are efficient against some specific types of microorganisms, namely spore forming pathogens like C. difficile [45]. Some disinfectants may cause resistance on the microorganisms if not used within specific parameters [46].
There are several guidelines [41], [47], [48] for infection control that suggest cleaning and disinfection methodologies to be applied in healthcare facilities. These guidelines not only suggest how to disinfect medical equipment but also how to clean environmental surfaces as floors, furniture or toilet seats. However, different guidelines present different opinions on the frequency of cleaning or advisable disinfectants/detergents for each situation since there is no standardization for the methods and cleaning agents to be used.
In addition, there is a lack of guidelines suggesting effective standardized methods to assess environmental cleaning. Visual evaluation is still the most used method to determine a surface cleanness, however even if a surface is apparently clean it can contain a high microbial load [49].
3. Guidelines for microbiologic limit values on surfaces
According to the Commission of the European Communities [12] guidelines for hygiene conditions in fresh meat processing facilities, food-contact surfaces with >10 CFU/cm2 for total viable count and >1 CFU/cm2 for Enterobacteriaceae are considered unacceptable.
There are no established limit values for microorganisms on surfaces for Public spaces, and there is poor control on cleaning performance. As depicted in Section 2.2 surfaces in these places are often contaminated showing values higher than those allowed on healthcare facilities (2.5–5 CFUs/cm2). More regulation should be made to assess the cleaning and disinfection efficacy and compliance on such places, namely on spaces with more susceptible people as schools, day-care centers or elderly homes. More guidelines about how to clean and how often would be a good option, as well as a higher microbiological assessment on surfaces, that is rarely or never done in public spaces.
For healthcare facilities, there is some controversy about the acceptable number of microorganisms on surfaces since there are no established thresholds, only tentative suggestions from researchers.
For some authors, clean hand-contact surfaces in hospitals must have less than 5 CFUs/cm2, with an increased risk of infection for values above that [20]. Other researchers have proposed the maximum value of 2.5 CFUs/cm2 as the limit level of contamination in clean hospital surfaces [21], [50].
For some specific microorganisms, considered indicator organisms, the limit value must be even lower. The presence of such microorganisms (for example S. aureus, C. difficile, vancomycin-resistant Enterococci or Acinetobacter spp.) is considered indicative of the need for cleaning even at levels of 1 CFUs/cm2 [20]. Some reference limit-values either from governmental entities or from research studies are listed in Table 2 .
Table 2.
Limit values suggested for aerobic colony count of microorganisms in different types of surfaces.
| Local | Aerobic colony count (limit value) | Indicator microorganisms | Entity/Researcher |
|---|---|---|---|
| Food-contact surfaces | 10 CFU/cm2 | 1 CFU/cm2 for Enterobacteriaceae | [12] |
| Public spaces’ surfaces | – | – | – |
| Hospital surfaces | 5 CFU/cm2 | 1 CFU/cm2 for S. aureus (including methicillin resistant), C. difficile, multiply resistant Gram-negative bacilli, vancomycin-resistant Enterococci, Salmonella spp, Acinetobacter spp | [20] |
| 2.5 CFU/cm2 | – | [50] | |
| 2.5 CFU/cm2 | 1 CFU/cm2 for Enterobacteriaceae and S. aureus | [21] |
The limit of <1 CFUs/cm2 for indicator microorganisms makes sense since it has been shown that an infectious dose of 1 CFUs/cm2 was sufficient to cause C. difficile infection on mice [45]. Also, it was observed that very low doses of norovirus have the capacity to cause infection [51]. These results show that microbiological contamination on surfaces poses a real risk of infection, since the environmental infectious dose can be very low for some microorganisms.
There is still the need for reaching a consensus and creating general guidelines for healthcare facilities. Establishing clear limit values and implementing frequent microbiological assessment protocols are some of the steps that should be in the horizon.
4. Antimicrobial and self-cleaning surfaces
To avoid contamination and consequent infection propagation on surfaces, several alternative strategies have been developed recently. Application of aerosols or UV light on contaminated surfaces are good examples of no-touch strategies, and they seem to be a good option to be applied in hospital environment [52]. However, these approaches still present some limitations.
UV light, in hospital facilities, can only be applied in empty rooms, therefore it cannot be used for a daily cleaning routine. Also, it only disinfects areas directly reached by the UV light, so many objects and surfaces remain contaminated after this disinfection approach. Aerosols are efficient but take a long time to decontaminate the surfaces compared to conventional cleaning or UV light. Besides that, these strategies are also exclusively used in empty rooms. This factor increases the necessary time to do the final disinfection of the room and perform the beds turnover in hospitals [53].
Cold plasma technology is another disinfection strategy that has recently been applied on surfaces. Plasma is the fourth state of mater and it can be generated from gases that became ionized with lots of ions, electrons and free radicals that will provide good electric conductivity. Plasma is known to have high levels of energy and it can change molecules composition making them highly ionized and with many free radicals moving randomly in all directions. For example, when a molecule of hydrogen peroxide is exposed to this technology the double bond on the molecule is broken, creating reactive oxygen species like hydroxyl radicals that in need to seek equilibrium will bind to surrounding microorganisms destroying them by oxidation [54], [55]
This technology has already been tested in food industry to decontaminate vegetables [56] and meat [57] with good results obtained and also to decontaminate surfaces as packaging materials. Puligundla et al. [58] have tested the elimination of different bacteria such as E. coli, Salmonella typhimurium and S. aureus, from several materials often used on food packages such as glass, polypropylene, polyethylene, nylon and paper foil. The results were quite promising with several log reductions after 5 to 10 minutes of treatment. Even though the results of this emerging technology are quite encouraging as a possible strategy to disinfect surfaces, more studies are needed to assure its safety and profitability to disinfect larger surfaces.
Surface modification/functionalization is another common procedure to obtain anti-adhesive or antimicrobial properties on different materials. This approach has several advantages over conventional disinfection techniques. For example, antimicrobial surfaces are in constant process of activity oppositely to no-touch technologies or conventional cleaning. This way the antimicrobial charge on the surfaces is reduced immediately after contact preventing its propagation and consequent contamination of surrounding surfaces or people [59], [60].
Other advantage is the possibility of these surfaces to be present in populated environments since they cause no harm to people with no need to remove people from the rooms to perform the disinfection. In addition, modified surfaces are not restricted to external surfaces. Medical devices such as urinary catheters, central venous catheters, prosthesis or contact lenses are some of the surfaces that have been functionalized to obtain better compatibility properties and lower infection risk when inserted on the human body [61], [62], [63], [64].
Self-disinfecting surfaces are an emerging topic with more and more products coming up. Some of the most recent strategies developed to obtain surfaces with anti-adhesive and antimicrobial properties are discussed below.
4.1. Anti-adhesive surfaces
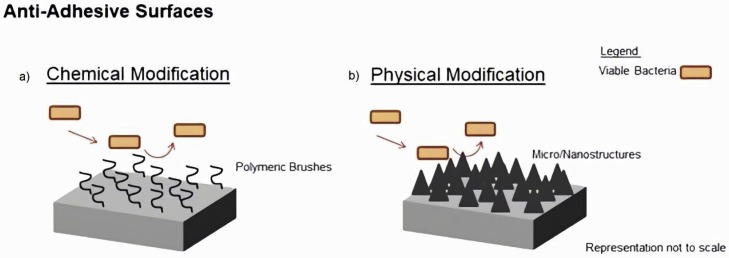
Several natural surfaces have suffered diverse evolutionary processes that turned them resistant to microorganisms’ colonization. Natural and bio-mimicked surfaces of insects’ wings, sharks’ skin, and lotus’ leaves exhibit antibiofouling properties by preventing different cells, particles or microorganisms from attaching to their surface [65]. Considering this, some of these natural anti-adhesive or self-cleaning surfaces have been investigated for their potential application on micro/nanostructured surfaces development. However it is also possible to give anti-adhesive properties to a surface by modifying the material's chemistry, namely using self-assembled monolayers or polymer brushes immobilized on the surface (Fig. 1 ) ([66], [67], [68]).
Fig. 1.
Anti-adhesive surfaces produced by: (a) chemical and (b) physical modification.
4.1.1. Chemical modification
There are several ways to produce materials with anti-fouling properties acting at the level of surface modification with chemical structures. Functional groups exhibited by the material's surface interact with those in the microorganism’ cells determining the kinetics of microbial adhesion.
For instance, surfaces highly negatively charged, i.e. polyanionic surfaces have the ability to repulse the bacteria with polyanionic glycocalice (most of gram-positive bacteria) through electrostatic interactions. However, gram-negative bacteria have policationic glycocalices, so this surface modification mechanism is only effective against some bacterial species and sometimes only against specific strain types [69].
Zwitterionic materials, such as carboxybetaine, sulfobetaine, and phosphobetaine also have anti-fouling properties. Zwitterionic polymers have an equal number of anionic and cationic groups present in their repeating unit. This fact gives these polymers ultra-hydrophilicity and consequently great hydration ability [70], [71]. Studies concerning surface modification with zwitterionic polymers have proved to be a promising strategy to reduce microorganisms’ adhesion [72], [73]. Very recently, a study using a tyramine-conjugated sulfobetaine polymer, grafted on polyurethane showed a great reduction in S. aureus adhesion to the surface [74].
Coating with polymeric brushes can also prevent microorganisms’ adhesion. Apart from avoiding direct contact between microorganisms and the surfaces, the polymers used for brushes are usually hydrophilic, so water will be attracted into the brush forming a repellent layer in aqueous environment. Proteins and microorganisms encountering the brush surface will be repelled by steric hindrance due to the water bound in the brush and the elasticity of the polymer chains [75]. Polyethylene oxide (PEO) and polyethylene glycol (PEG) are two examples of polymers used to produce hydrophilic brush coatings on biomaterials. In a recent study by. Hadjesfandiari et al. [76] it was proved that PEO brushes covalently immobilized reduced 98% the adhesion of Staphylococci and E. coli to a surface.
4.1.2. Physical modification
Apart from the chemistry, also topography of a surface can be structured to increase anti-adhesive properties. As an attempt to find efficient alternatives over more classic antimicrobials, micro/nanostructured materials present themselves as possible solutions. Recently, have been done an attempt to elucidate if alterations on micro or nanotopography of a surface could influence colonization and consequent contamination. An example of structure modification is the application of superficial nanostructures (nanoparticles, nanofibers or nanotubes) reducing the area available for microorganisms to attach [77], [78], [79].
As said previously, mimicking the topography of anti-adhesive surfaces innately present in nature is an innovative way to obtain new self-disinfecting surfaces.
Many plants have special surface properties namely wettability. For instance, lotus leaves have micropapillae structures covered by nanostructures with fine branch-like shape. These structures along with epicuticular needle-shaped wax tubes that cover them give the lotus leaf a superhydrophobic surface and consequently antifouling properties. Water droplets roll down the lotus leaf due to its great hydrophobicity dragging out any possible particles present, and maintaining its surface clean [80].
Other example of nature that inspired anti-adhesive surfaces’ coating is sharkskin. Sharks have a special scale micropattern, which consist of a rectangular base embedded in the skin with tiny spines on the surface. The ribbed texture of these scales is responsible for the self-cleaning, anti-biofouling, hydrophobic and drag reducing properties of shark skin [81], [82]. Such properties have made of sharkskin one of the most mimicked surfaces, not only in research environment but in industrial area too.
Sharklet AF (Sharklet Technologies, Alachua, FL, USA) is a company that uses shark skin topography in different surfaces in order to avoid bacterial adhesion and biofilm formation [83], [84]. A study comparing Sharklet micropattern surfaces with regular surfaces in a clinical simulated scenario proved that shark skin microtopography reduced the number of attached bacteria by about 5 fold [85].
4.2. Antimicrobial surfaces
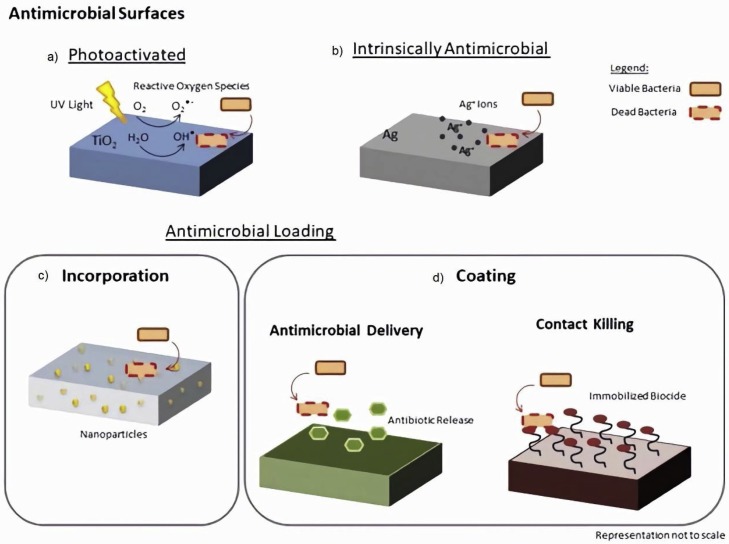
There are several materials with intrinsic antimicrobial properties, as silver, zinc, cooper, or chitosan. Nevertheless, materials can also be modified to acquire bactericidal activity [86]. Chemical or physical changes in the surface can produce antimicrobial effect. Chemical substances such as antibiotics or biocides can be incorporated in the materials to give it antimicrobial properties. However, a balance between these properties and biocompatibility must be observed since the materials will be in direct contact with users’ organism raising toxicity concerns. Photo-activated surfaces are also an example of self-disinfecting materials recently developed. Fig. 2 presents some examples of different types of antimicrobial surfaces.
Fig. 2.
Types of antimicrobial surfaces: (a) photo-activated, (b) with intrinsic antimicrobial properties, loaded with some antimicrobial, either by (c) incorporation or (d) coating.
4.2.1. Intrinsically active antimicrobial materials
Some materials have intrinsic antimicrobial properties. They do not need antimicrobial loading to exert its activity since the material by itself has the natural ability to eliminate microorganisms. Among the most known natural materials with antimicrobial properties are metals as silver, copper, or zinc and polymers like chitosan [68].
For many metals with antimicrobial properties, it is the ionic form that presents higher bactericidal activity and not the elemental metal. Silver and copper are good examples of that.
Silver has been used in medical field for many centuries due to its antimicrobial properties. Its bactericidal mechanism is based on the binding of silver atoms with thiol (SH) and disulfide (S–S) groups present in the proteins of bacterial cell membranes, leading to its disruption and eventual cell death [87]. Silver has been used for many applications on medical field especially when added in the form of silver sulfadiazine to creams or wound dressings [88]. Several wound dressing containing silver are available on market, however, not always they comply the expectations. Cavanagh et al. [89] analyzed 6 different silver dressings on market and only two of them showed bactericidal activity against S. aureus. Apart from that, silver dressing may be related with increased serum silver levels with an associated decrease on white blood cells [90]. This leads us to conclude that the delivery system of the antimicrobial is very important. A delivery mechanism that acts by contact without leaching silver ions to the surrounding areas or tissues would be good strategy to achieve antimicrobial action without compromise the safety of the product.
More recently, silver nanoparticles have been greatly studied and used to produce potential antimicrobial materials such as catheters and surgery sutures [91], [92]. However, for biomaterials implanted inside the organism there are some possible risks associated with releasing of silver ions causing local toxicity and possible accumulation in organs [93]. Silver may also have different ranges of efficacy according to the class of bacteria. Gram-positive bacteria's cell walls do not have an outer membrane as Gram-negative bacteria do, but they have several layers of peptidoglycans, making their cell wall thicker [94]; this, along with their negative charge results in the trapping of silver ions (positively charged) preventing their entrance into the bacteria [95].
AGC Glass (AGS Glass UK Ltd., Rugby, Great Britain) developed an antibacterial glass by incorporating Silver ions inside the glass. The company claims that this glass eliminates 99% of bacteria and prevents fungi proliferation which they demonstrated in the three bacterial and two fungi strains evaluated [96].
Surfacine Development Company (Tyngsborough, MA, USA) is another company that developed a silver coating (Surfacine®) that can be applied in several materials, from medical devices to food preparations and packaging industry or water distribution systems. This coating showed good antimicrobial activity against several bacteria, fungi and yeast [97].
Other company, PureThread Technologies Inc. (Cary, NC, USA) has developed PureThread®, a system that embeds silver salts into textile fibers, obtaining antimicrobial textiles. According to the company this system does not weaken or disappears during washing and kills 99.9% of microorganisms after four hours of contact with the fabric [98]. A scientific paper about a study involving hospital curtains incorporating the antimicrobial textile developed by this company proved that those curtains take more time to be contaminated for the first time when compared to regular curtains [99].
Similarly, to silver, copper has been used for many centuries as an antimicrobial agent especially for water treatment and transportation. Copper was also very used in the nautical field, to prevent adhesion and growth of organisms in hulls of ships [100]. The bactericide mechanism of copper is related to the release of copper ions that cause damage in the bacterial envelop and consequent leakage of the cell content and influx of copper ions into the bacteria. This will generate toxic radicals causing oxidative damage to cellular components and DNA degradation [101].
More recently copper came up as an alternative for preventing hospital acquired infections through its application in hospital surfaces and medical equipment [102]. A study by Souli et al. [103] performed on a hospital intensive care unit, compared two rooms, one with copper coated equipment (beds, side table, intravenous pole stands, etc.) and the other with regular equipment. The copper coated equipment room presented a reduced percentage of colonized surfaces (55.6%) compared to the regular compartment (72.5%). This study showed both reductions in colonization by gram-negative and gram-positive bacteria and in total bioburden (2.9 vs 7.6 CFU/100 cm2).
Sifri et al. [60] also developed a study where a section of an acute care unit was equipped with copper impregnated materials, hard surfaces and textiles-patients’ gowns, sinks, bed rails, tray tables, sheets and blankets. The results were quite promising with copper equipped section presenting patients’ infection reductions of 83% when caused by C. difficile and 68% when caused by multi drug resistant organisms, comparing to a non-modified section in the same hospital unit. Two companies that collaborate with each other, Cupron Medical Textiles (Cupron Inc., Richmond, VA, USA) and Cupron Enhanced EOS Surfaces (EOS Surfaces, Norfolk, VA, USA) provided the copper impregnated materials used for this study. The product developed is a hard-antimicrobial surface impregnated with copper that continuously kills 99.9% of harmful bacteria in two hours, according to its specifications. According to the company this surface is effective against S. aureus, Enterobacter aerogenes, MRSA, E. coli and P. aeruginosa [104].
4.2.2. Loading antimicrobial compounds into materials
There are several substances with antimicrobial activity that can be added to a surface to obtain an antimicrobial surface.
Antibiotics were one of the first substances to be applied to surfaces such as prosthesis or textiles for wound dressing production, to obtain antimicrobial action. Despite the promising results, it is well known that the major disadvantage of antibiotics use is the microorganisms’ ability to develop resistance. This drawback has led to an increasing search for alternatives to be used as antimicrobials. Quaternary ammonium compounds, chlorhexidine, benzalkonium chloride or nitric oxide are some examples of substances that were already tested as an alternative [105], [106], [107]. Quaternary ammonium compounds are disinfectants used in hospitals and healthcare facilities for several clinical purposes as disinfection of surfaces and medical material. However, more recently the quaternary ammonium compounds have been tested as antimicrobial loading agents to different surfaces and materials, namely polymers [108], [109].
Antimicrobial peptides (AMPs) are a class of peptides, components of innate immune system, with a broad activity against bacteria, virus, fungus and more, providing a non-specific defense against a broad spectrum of invaders [110]. Recently, numerous studies [111], [112] refer its use as antimicrobial loading agents for their excellent characteristics, presenting several advantages over classic antibiotics, namely fast and broad spectrum of action with low susceptibility to induce bacterial resistance [113]. In contrast with the mechanisms of action of antibiotics, which are based in slow processes of enzymatic inhibition and target specific cellular activities as DNA or protein, the AMPs are effective against different species of microorganisms and also reduce the risk of resistance development [113], [114], [115].
For all these reasons, AMPs are being applied in the production of antimicrobial surfaces, either by simple adsorption on the surface or covalent immobilization [116]. Nevertheless, studies suggest that covalent immobilization offers many advantages toward physical adsorption, including higher local and long-term stability, and lowering toxicity [116], [117].
All those antimicrobial substances are loaded to the surface either by immobilization or by incorporation on the bulk material; recent studies on the application of each type of loading strategy are summarized next.
4.2.2.1. By incorporation
In materials’ incorporation process, the antimicrobial substances are added to the ingredients during the phase of production, to obtain a homogeneous mixture of the bulk material with the antimicrobial. This system allows antimicrobial activity throughout the bulk material, even in deeper layers and not only on the surface. This allows the material to retain its antimicrobial activity even when worn out [118], [119].
In a very recent study, Ferreira et al. [120] used Levofloxacin to load a bone cement, achieving good results against S. aureus not only on its planktonic form but also on biofilm. That was quite positive since this bacterial strain is greatly associated with biofilm formation on bone implants.
Ciprofloxacin incorporation on textile fibers by electrospinning was tested by Li et al. [79] This strategy aimed to produce a bandage to prevent wound infection and the results in vivo (rats animal model) were quite positive for E. coli and S. aureus.
In Han et al. [121] study they incorporated quaternary ammonium methacrylates in dental adhesives to prevent caries by avoiding the accumulation of bacteria and biofilm formation on teeth, testing the presence of three Staphyloccocal species (Staphylococcus mutans, Staphylococcus gordonii, and Staphylococcus sanguinis) and the results showed a decreased biofilm formation for the adhesives containing the quaternary ammonium methacrylates.
Nanoparticles containing quaternary ammonium polyethylenimine were tested in a study about endodontic sealers by Barros et al. [118]. The results were quite positive for Enterococcus faecalis, with the endodontic sealers showing good antimicrobial activity.
The AMP, LL-37, was incorporated by Cassin et al. [122] in a membrane of collagen and hyaluronic acid for the production of anti-infective films to cover injured tissues.
4.2.2.2. By coating
Antimicrobial coatings can be obtained either by adsorption of the antimicrobial substance, by covalently binding it to the material or by immobilizing it using self-assembled monolayers. This strategy can be an advantage for some substances that are too toxic to be used as bulk material but that immobilized in small amounts as surface coatings can exert their antimicrobial activity without the toxicity drawback [116], [123].
The coatings can also be divided according to their mechanism of action. They can deliver the antimicrobial substance by leaching, releasing it to the surrounding area or direct contact with the surface where the coating is immobilized.
Some coatings release substances with antibacterial properties that will interact with microorganisms acting away from the bulk. This leaching mechanism has the advantage of a larger perimeter of action around the surface where it is immobilized. It is important, though, that this release into the surrounding area is controlled to avoid toxic effects by excess of substance [124].
Coatings can also attach certain antimicrobial molecules to the materials’ surface. Several coatings can be classified as “contact biocides” since they use non-leachable substances that kill by contact with the bacteria and with no need to be released from the surface. This strategy is very interesting for both the self-sterilizing effect and the long-lasting activity, acting just through a direct contact with bacteria without consuming itself releasing from the surface without need [117], [125].
Alt et al. [126] realized a study where coating of Rifampicin and Fosfomycin was applied to prosthesis showing good efficacy against S. aureus, even the methicillin resistant strain, on a rabbit animal model. Neut et al. [127] performed a similar study using a gentamicin-releasing coating that was able to prevent Staphylococci growth on a prosthetic hip surface for at least 60 hours, showing promising results.
In a work by Iyamba et al. [106], quaternary ammonium compounds (hexadecyltrimethyl ammonium bromide and hexadecylbetainate chloride) were immobilized on medical catheters reducing S. aureus adhesion to its surface. Zanini et al. [128] also developed a work in which quaternary ammonium silanes were used to coat polyurethane catheters. The results were promising with this coating proving its antimicrobial activity against E. coli bacteria.
In a recent study, Casciaro et al. [111] successfully covalently immobilized frog skin derived AMP on contact lenses. This strategy not only reduced the adhesion of P. aeruginosa, a species highly associated with contact lenses associated keratitis, but it also proved to be non-toxic to mammalian cells and it did not compromise the lenses physical properties.
AMPs have also been immobilized on nanoparticles as loading strategy. Ma et al. [112] immobilized the AMP HHC-36 on titanium oxide (TiO2) nanotubes by physical adsorption with the nanotubes acting as nanocarriers for peptides delivery. They obtained an antimicrobial and anti-fouling surface showing a reduction in S. aureus adhesion and great antimicrobial activity (>99% activity). Braun et al. [129] immobilized the human peptide LL-37 on porous silica nanoparticles, achieving an antimicrobial delivery system.
All the studies presented above prove the good potential of modified surfaces to be applied on infection control in different scenarios; still some aspects must be taken into account and more studies are still needed. Most studies evaluating the antimicrobial/anti-adhesive activity potential of engineered surfaces do not take into account realistic physiological environments. This can affect the results, since the host's conditioning film may affect the surface in many different ways, for example, covering the surface creating a deterrent layer preventing the antimicrobial substance to leach or to contact directly with the microorganisms, reducing its antimicrobial efficacy [130].
In addition, the composition of the surface may determine which components of the conditioning film will adhere and that on its turn will influence other cells adhesion, as bacteria. Felgueiras et al. [123] showed that different concentrations of Octadecyl acrylate (C18) immobilized on polyurethane surfaces will affect the deposition of albumin and fibrinogen when the surface is in contact with human plasma, that on its turn will affect microorganisms’ adhesion to the surface since albumin avoids bacteria adhesion and fibrinogen promotes it. Besides, coatings with antimicrobial peptides can be degraded by some components of conditioning films such as proteases [131].
Despite the pointed weaknesses there are already on the market some successful cases of such as Biocote Limited (Coventry, United Kingdom) that developed an antimicrobial additive technology, Biocote®, that can be incorporated on textiles, polymers, ceramics and more. These additives are based on different antimicrobial substances such as silver, zinc or phenolic compounds, chosen according to their application and support material [132].
Sanitized AG (Burgdorf, Switzerland) is another company that developed an antimicrobial additive that can be incorporated in polymers and textiles for different areas of application such as healthcare, public transportation and food industry. This additive technology, Sanitized®, uses different active ingredients as silver, zinc pirithione, silane quat or isothiazolinone that can be added on liquid form, powder or paste according to specific production requirements of the final product [133].
4.2.3. Photo-activated surfaces (TiO2)
Photo-activated materials have been used in different technological fields namely in antimicrobial surfaces development. The most studied material used in photo-activated surfaces production is TiO2. TiO2 has high photocatalytic properties and is highly used in cosmetics and skin care products since it is not absorbed by human skin [134] becoming a very interesting material for development of antimicrobial surfaces.
In fact, its antimicrobial efficacy has already been proved against bacteria, fungi, protozoa and virus. The main advantage of this strategy is that TiO2 is not degraded so its activity can be maintained for long periods. TiO2 surfaces are only activated when irradiated by specific photon energy. When this irradiation occurs, reactions of photo-oxidation involving O2 and H2O take place on the surface with consequent formation of oxygen free radicals. These free radicals will attack the cell wall and cytoplasmic membrane of the microorganism by peroxidizing its lipids, leading to leakage of cellular components and lately cell lysis [135], [136].
Recently, Adán et al. [137] published a study where a water microfiltration system using photocatalytic membranes produced with TiO2 and porous steel showed good inactivation for the retained bacteria.
In another study by Joost et al. [138] thin films with TiO2 nanoparticles were illuminated with UV light and after 20 min of exposure no bacteria (E. coli) had survived. The analysis of bacterial cell membrane showed modifications in the chemical structure of unsaturated fatty acids and decomposition of saturated fatty acids faster than normal, confirming the peroxidation of membrane lipids.
In the market, there are already some products for microorganism elimination based on TiO2 photocatalytic action. PureHealth™ (ORION, Florence, Italy) is a system developed by ORION that coats walls and floors and is activated by special lamps of solar spectrum. This system promises to eliminate virus and bacteria [139].
5. Conclusion
This review points out the great contribution of surfaces for infection spreading, not only in healthcare facilities but also in other public spaces and food processing facilities.
There is an urgent need to pay more attention to surface contamination in different spaces since there is still a lack of guidelines for microbiologic assessment and established safe thresholds for health care facilities. In addition, it is important to establish infection control procedures for public spaces, namely schools since they often present high microbiological charges on surfaces, posing a real risk for children whose immunological system is still developing.
Both on public spaces and on healthcare facilities, it should be determined how to assess microbiologic presence on surfaces as well as how frequently this assessment should be made, and which methods should be used to obtain a realistic analysis rather than performing visual assessments. The implementation of standard acceptable limits of microorganisms on surfaces is also a key point for a better control of infection spreading. Standardized definition of which microorganisms can be considered indicator microorganisms should also be achieved.
The existing guidelines for cleaning and disinfection methodologies in healthcare facilities should come to a consensus about the disinfectants to be used in each setting, the frequency of cleaning and the methods to apply.
The implementation of self-disinfecting surfaces is a good strategy for infection control, and this review presents some successful research already developed in this area. These surfaces show clear advantages over the regular surfaces with traditional cleaning: the state of continuous disinfection and the antimicrobial activity that permanently eliminates the microorganisms.
There are still some issues to improve, like the long-term efficacy of the antimicrobial/anti-adhesive action, or the high cost of implementation of these surfaces in large areas.
Biomaterials, modified surfaces, or new scientific products in general, have to face some big challenges before they reach the market. Problems such as costs of manufacturability, scalability on production, intellectual property issues or regulatory aspects are just some of the main obstacles those products will have to overcome in order to be commercialized [140]. Some products even with great results on laboratory will fail when brought to larger scale due to their high cost of production, making them commercially unattractive. But, looking at the numbers nosocomial bacteremia alone can cost a hospital over 1 million euros per year [141], and antibiotic resistance-associated infections may cost more than 900 million euros per year in European Union.
In fact the societal cost of infections due to the selected antibiotic-resistant bacteria were estimated at about EUR 1.5 billion each year, between hospital related costs and losses in productivity due to patients’ absence from work [142]. Therefore, maybe it is worthy to invest on new technologies, betting on infection prevention and control not only to avoid higher costs but also to avoid the harm and loss of human lives.
Self-disinfecting surfaces are a step forward to the future of infection control policies. More studies involving self-disinfecting surfaces should be performed in order to realize its full potential to improve infection control and safety strategies. The reports on the application of these materials in healthcare facilities show promising results and there are already few companies supplying products with self-disinfecting properties showing that investment on prevention may be the best way to reduce the tremendous problem of infection spreading.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge the funding provided by Fundação para a Ciência e a Tecnologia (FCT - Portugal) through the scholarship SFRH/BD/130203/2017 (Micaela Machado Querido) and the project “B-SAFECOAT - Desenvolvimento de novas tintas com propriedades auto-desinfetantes” (POCI-01-0247-FEDER-017875).
References
- 1.Cardoso T., Almeida M., Carratalà J., Aragão I., Costa-Pereira A., Sarmento A.E., Azevedo L. Microbiology of healthcare-associated infections and the definition accuracy to predict infection by potentially drug resistant pathogens: a systematic review. BMC Infect. Dis. 2015;15:565. doi: 10.1186/s12879-015-1304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber D.J., Anderson D., Rutala W.A. The role of the surface environment in healthcareassociated infections. Curr. Opin. Infect. Dis. 2013;26:345–351. doi: 10.1097/QCO.0b013e3283630adf. [DOI] [PubMed] [Google Scholar]
- 3.Otter J.A., Yezli S., French G.L. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect. Control Hosp. Epidemiol. 2011;32:687–699. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- 4.Rutala W.A., Weber D.J. Guideline for disinfection and sterilization in health care facilities: what clinicians need to know. Clin. Infect. Dis. 2008;39:702–709. doi: 10.1086/423182. [DOI] [PubMed] [Google Scholar]
- 5.Morgan D.J., Rogawski E., Thom K.A., Johnson J.K., Perencevich E.N., Shardell M., Leekha S., Harris A.D. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit. Care Med. 2012;40:1045–1051. doi: 10.1097/CCM.0b013e31823bc7c8.Transfer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otter J.A., French G.L. Bacterial contamination on touch surfaces in the public transport system and in public areas of a hospital in London. Lett. Appl. Microbiol. 2009;49:803–805. doi: 10.1111/j.1472-765X.2009.02728.x. [DOI] [PubMed] [Google Scholar]
- 7.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadamori Y., Gooneratne R., Hussain M.A. Outbreaks and factors influencing microbiological contamination of fresh produce. J. Sci. Food Agric. 2016 doi: 10.1002/jsfa.8125. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen V.T., Fegan N., Turner M.S., Dykes G.A. Role of attachment to surfaces on the prevalence and survival of Campylobacter through food systems. J. Food Prot. 2012;75:195–206. doi: 10.4315/0362-028X.JFP-11-012. [DOI] [PubMed] [Google Scholar]
- 10.Osimani A., Garofalo C., Clementi F., Tavoletti S., Aquilanti L. Bioluminescence ATP monitoring for the routine assessment of food contact surface cleanliness in a university canteen. Int. J. Environ. Res. Public Health. 2014;11:10824–10837. doi: 10.3390/ijerph111010824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad M., See T.P., Abdullah M.F.F., Nor N.M. Use of rapid microbial kits for regular monitoring of food-contact surfaces towards hygiene practices. Procedia – Soc. Behav. Sci. 2013;105:273–283. doi: 10.1016/j.sbspro.2013.11.029. [DOI] [Google Scholar]
- 12.T.C. of the E. Communities Commission Decision of 8 June 2001. Off. J. Eur. Comm. 2001;165:48–53. https://doi.org/http://eur-lex.europa.eu/pri/en/oj/dat/2003/l_285/l_28520031101en00330037.pdf [Google Scholar]
- 13.Masuku S.M., Babu D., Martin E.M., Koo O.K., O’Bryan C.A., Crandall P.G., Ricke S.C. Cleaning and decontamination efficacy of wiping cloths and silver dihydrogen citrate on food contact surfaces. J. Appl. Microbiol. 2012;113:89–95. doi: 10.1111/j.1365-2672.2012.05318.x. [DOI] [PubMed] [Google Scholar]
- 14.Srey S., Jahid I.K., Ha S.D. Biofilm formation in food industries: a food safety concern. Food Control. 2013;31:572–585. doi: 10.1016/j.foodcont.2012.12.001. [DOI] [Google Scholar]
- 15.Lee M.B.E.A. 2008. Review of Enteric Outbreaks in Child Care Centers: Effective Infection Control Recommendations. [PubMed] [Google Scholar]
- 16.Ibfelt T., Engelund E.H., Permin A., Schultz A.C., Andersen L.P. Presence of pathogenic bacteria and viruses in the daycare environment. J. Environ. Health. 2005;78:24–30. [PubMed] [Google Scholar]
- 17.Jerković-Mujkić A., Besta R., Memisevic S. Bacterial contamination of public telephones in the downtown area of Sarajevo. Afr. J. Microbiol. Res. 2013;7:1664–1667. doi: 10.5897/AJMR12.313. [DOI] [Google Scholar]
- 18.Mukherjee N., Dowd S.E., Wise A., Kedia S., Vohra V., Banerjee P. Diversity of bacterial communities of fitness center surfaces in a U.S. metropolitan area. Int. J. Environ. Res. Public Health. 2014;11:12544–12561. doi: 10.3390/ijerph111212544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandel C.E., Simor A.E., Redelmeier D.A. Elevator buttons as unrecognized sources of bacterial colonization in hospitals. Open Med. 2014;8:81–86. [PMC free article] [PubMed] [Google Scholar]
- 20.Dancer S.J. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J. Hosp. Infect. 2004;56:10–15. doi: 10.1016/j.jhin.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis T., Griffith C., Gallo M., Weinbren M. A modified ATP benchmark for evaluating the cleaning of some hospital environmental surfaces. J. Hosp. Infect. 2008;69:156–163. doi: 10.1016/j.jhin.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein R.A. Epidemiology and control of nosocomial infections in adult intensive care units. Am. J. Med. 1991;91:179S–184S. doi: 10.1016/0002-9343(91)90366-6. [DOI] [PubMed] [Google Scholar]
- 23.Carling P.C., Parry M.M., Rupp M.E., Po J.L., Dick B., Von Beheren S. Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect. Control Hosp. Epidemiol. 2008;29:1035–1041. doi: 10.1086/591940. [DOI] [PubMed] [Google Scholar]
- 24.Carling P.C., Parry M.F., Bruno-Murtha L.A., Dick B. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission. Crit. Care Med. 2010;38:1054–1059. doi: 10.1097/CCM.0b013e3181cdf705. [DOI] [PubMed] [Google Scholar]
- 25.Weber D.J., Rutala W.A., Miller M.B., Huslage K., Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control. 2010;38:S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Li Y., Zhang X., Li H. Characteristics of nosocomial infection and its effects on the survival of chemotherapy patients with advanced non-small cell lung cancer. Oncol. Lett. 2017;14:7379–7383. doi: 10.3892/ol.2017.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiefel U., Cadnum J.L., Eckstein B.C., Guerrero D.M., Tima M.A., Donskey C.J. Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infect. Control Hosp. Epidemiol. 2011;32:185–187. doi: 10.1086/657944. [DOI] [PubMed] [Google Scholar]
- 28.Guerrero D.M., Nerandzic M.M., Jury L.A., Jinno S., Chang S., Donskey C.J. Acquisition of spores on gloved hands after contact with the skin of patients with Clostridium difficile infection and with environmental surfaces in their rooms. Am. J. Infect. Control. 2012;40:556–558. doi: 10.1016/j.ajic.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Stahmeyer J.T., Lutze B., von Lengerke T., Chaberny I.F., Krauth C. Hand hygiene in intensive care units: a matter of time? J. Hosp. Infect. 2017;95:338–343. doi: 10.1016/j.jhin.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Quon J.S., Dilauro M., Ryan J.G. Disinfection of the radiologist workstation and radiologist hand hygiene: a single institution practice quality improvement project. Can. Assoc. Radiol. J. 2017;68:270–275. doi: 10.1016/j.carj.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Otter J.A., Yezli S., Salkeld J.A.G., French G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control. 2013;41:S6–S11. doi: 10.1016/j.ajic.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Geadas Farias P., Gama F., Reis D., Alarico S., Empadinhas N., Martins J.C., de Almeida A.F., Morais P.V. Hospital microbial surface colonization revealed during monitoring of Klebsiella spp., Pseudomonas aeruginosa, and non-tuberculous mycobacteria. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2017;110:863–876. doi: 10.1007/s10482-017-0857-z. [DOI] [PubMed] [Google Scholar]
- 33.Viana R.E.H., dos Santos S.G., Oliveira A.C. Recovery of resistant bacteria from mattresses of patients under contact precautions. Am. J. Infect. Control. 2016;44:465–469. doi: 10.1016/j.ajic.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Huslage K., Rutala W.A., Bennett E.S., Weber D.J. A quantitative approach to defining “High-Touch” surfaces in hospitals. Source Infect. Control Hosp. Epidemiol. 2010;31:850–853. doi: 10.1086/655016. [DOI] [PubMed] [Google Scholar]
- 35.White L.F., Dancer S.J., Robertson C., McDonald J. Are hygiene standards useful in assessing infection risk? Am. J. Infect. Control. 2008;36:381–384. doi: 10.1016/j.ajic.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Shek K., Patidar R., Kohja Z., Liu S., Gawaziuk J.P., Gawthrop M., Kumar A., Logsetty S. Rate of contamination of hospital privacy curtains on a burns and plastic surgery ward: a cross-sectional study. J. Hosp. Infect. 2017;96:54–58. doi: 10.1016/j.jhin.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Levin P.D., Shatz O., Sviri S., Moriah D., Or-Barbash A., Sprung C.L., Moses A.E., Block C. Contamination of portable radiograph equipment with resistant bacteria in the ICU. Am. Coll. Chest Physicians. 2009;136:426–432. doi: 10.1378/chest.09-0049. [DOI] [PubMed] [Google Scholar]
- 38.Lestari T., Ryll S., Kramer A. Microbial contamination of manually reprocessed, ready to use ECG lead wire in intensive care units. GMS Hyg. Infect. Control. 2013;8 doi: 10.3205/dgkh000207. Doc07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sifuentes L.Y., Gerba C.P., Weart I., Engelbrecht K., Koenig D.W. Microbial contamination of hospital reusable cleaning towels. Am. J. Infect. Control. 2013;41:912–915. doi: 10.1016/j.ajic.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Barker A.K., Alagoz O., Safdar N. Interventions to reduce the incidence of hospital-onset Clostridium difficile infection: an agent-based modeling approach to evaluate clinical effectiveness in adult acute care hospitals. Clin. Infect. Dis. 2017:1–12. doi: 10.1093/cid/cix962/4589130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization . World Health Organization; 2004. Practical Guidelines for Infection Control in Health Care Facilities Practical Guidelines for Infection Control in Health Care Facilities. [Google Scholar]
- 42.Johani K., Abualsaud D., Costa D.M., Hu H., Whiteley G., Deva A., Vickery K. Characterization of microbial community composition, antimicrobial resistance and biofilm on intensive care surfaces. J. Infect. Public Health. 2017 doi: 10.1016/j.jiph.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Pappas D.E., Hendley J.O., Schwartz R.H. Respiratory viral RNA on toys in pediatric office waiting rooms. Pediatr. Infect. Dis. J. 2010;29:102–104. doi: 10.1097/INF.0b013e3181b6e482. [DOI] [PubMed] [Google Scholar]
- 44.Sattar S. Promises and pitfalls of recent advances in chemical means of preventing the spread of nosocomial infections by environmental surfaces. Am. J. Infect. Control. 2010;38:S34–S40. doi: 10.1016/j.ajic.2010.04.207. [DOI] [PubMed] [Google Scholar]
- 45.Lawley T.D., Clare S., Deakin L.J., Yen J.L., Raisen C., Brandt C., Lovell J., Cooke F., Clark T.G., Lawley T.D., Clare S., Deakin L.J., Goulding D., Yen J.L., Raisen C., Brandt C., Lovell J., Cooke F., Clark T.G., Dougan G. Use of purified Clostridium difficile spores to facilitate evaluation of health care disinfection regimens. Appl. Environ. Microbiol. 2010;76:6895–6900. doi: 10.1128/AEM.00718-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirose R., Nakaya T., Naito Y., Daidoji T., Watanabe Y., Yasuda H., Konishi H., Itoh Y. Viscosity is an important factor of resistance to alcohol-based disinfectants by pathogens present in mucus. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-13732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention . 2011. Basic Infection Control and Prevention Plan for Outpatient Oncology Settings. [Google Scholar]
- 48.Health Protection Scotland . HPS; Glasgow: 2017. Standard Infection Control Precautions Literature Review: Routine Cleaning of the Environment in the Hospital Setting. [Google Scholar]
- 49.Watanabe R., Shimoda T., Yano R., Hayashi Y., Nakamura S., Matsuo J., Yamaguchi H. Visualization of hospital cleanliness in three Japanese hospitals with a tendency toward long-term care. BMC Res. Notes. 2014;7:121. doi: 10.1186/1756-0500-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffith C.J., Cooper R.A., Gilmore J., Davies C., Lewis M. An evaluation of hospital cleaning regimes and standards. J. Hosp. Infect. 2000;45:19–28. doi: 10.1053/jhin.1999.0717. [DOI] [PubMed] [Google Scholar]
- 51.Teunis P.F.M., Moe C.L., Liu P., Miller S.E., Lindesmith L., Baric R.S., Pendu J.L., Calderon R.L. Norwalk virus: how infectious is it? Anticancer Res. 2010;30:4799–4804. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 52.Boyce J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control. 2016;5:10. doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber D.J., Kanamori H., Rutala W.A. ‘No touch’ technologies for environmental decontamination. Curr. Opin. Infect. Dis. 2016;29:1–8. doi: 10.1097/QCO.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 54.Chizoba Ekezie F.G., Sun D.W., Cheng J.H. A review on recent advances in cold plasma technology for the food industry: current applications and future trends. Trends Food Sci. Technol. 2017;69:46–58. doi: 10.1016/j.tifs.2017.08.007. [DOI] [Google Scholar]
- 55.Izadjoo M., Zack S., Kim H., Skiba J. Medical applications of cold atmospheric plasma: state of the science. J. Wound Care. 2018;27(Sup9) doi: 10.12968/jowc.2018.27.sup9.s4. [DOI] [PubMed] [Google Scholar]
- 56.Oh Y.J., Song A.Y., Min S.C. Inhibition of Salmonella typhimurium on radish sprouts using nitrogen-cold plasma. Int. J. Food Microbiol. 2017;249:66–71. doi: 10.1016/j.ijfoodmicro.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Han L., Ziuzina D., Heslin C., Boehm D., Patange A., Sango D.M., Valdramidis V.P., Cullen P.J., Bourke P. Controlling microbial safety challenges of meat using high voltage atmospheric cold plasma. Front. Microbiol. 2016;7:1–12. doi: 10.3389/fmicb.2016.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puligundla P., Lee T., Mok C. Inactivation effect of dielectric barrier discharge plasma against foodborne pathogens on the surfaces of different packaging materials. Innov. Food Sci. Emerg. Technol. 2016;36:221–227. doi: 10.1016/j.ifset.2016.06.027. [DOI] [Google Scholar]
- 59.Hasan J., Crawford R.J., Ivanova E.P. Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol. 2013;31:295–304. doi: 10.1016/j.tibtech.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Sifri C.D., Burke G.H., Enfield K.B. Reduced health care-associated infections in an acute care community hospital using a combination of self-disinfecting copper-impregnated composite hard surfaces and linens. Am. J. Infect. Control. 2016;44:1565–1571. doi: 10.1016/j.ajic.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Ferraris S., Spriano S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C. 2016;61:965–978. doi: 10.1016/j.msec.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 62.Lim K., Chua R.R.Y., Ho B., Tambyah P.A., Hadinoto K., Leong S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015;15:127–138. doi: 10.1016/j.actbio.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 63.Querido M.M., Felgueiras H.P., Rai A., Costa F., Monteiro C., Borges I., Oliveira D., Ferreira L., Cristina M., Martins L. 2018. Cecropin – Melittin Functionalized Polyurethane Surfaces Prevent Staphylococcus epidermidis Adhesion without Inducing Platelet Adhesion and Activation 1801390; pp. 1–10. [DOI] [Google Scholar]
- 64.Willcox M.D.P., Hume E.B.H., Aliwarga Y., Kumar N., Cole N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J. Appl. Microbiol. 2008;105:1817–1825. doi: 10.1111/j.1365-2672.2008.03942.x. [DOI] [PubMed] [Google Scholar]
- 65.Xu Q., Zhang W., Dong C. Biomimetic self-cleaning surfaces: synthesis, mechanism and applications. J. R. Soc. Interface. 2016;13 doi: 10.1098/rsif.2016.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao G., Yu K., Kindrachuk J., Brooks D.E., Hancock R.E.W., Kizhakkedathu J.N. Antibacterial surfaces based on polymer brushes: investigation on the influence of brush properties on antimicrobial peptide immobilization and antimicrobial activity antibacterial surfaces based on polymer brushes: investigation on the influence of Bru. Biomacromolecules. 2011 doi: 10.1021/bm2009697. [DOI] [PubMed] [Google Scholar]
- 67.Junter G.A., Thébault P., Lebrun L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016;30:13–25. doi: 10.1016/j.actbio.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Tripathy A., Sen P., Su B., Briscoe W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017;248:85–104. doi: 10.1016/j.cis.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campoccia D., Montanaro L., Arciola C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 70.Mi L., Jiang S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. 2014;53:1746–1754. doi: 10.1002/anie.201304060. [DOI] [PubMed] [Google Scholar]
- 71.Schlenoff J.B. Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir. 2014;30:9625–9636. doi: 10.1021/la500057j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Q., Li B., Zhou X., Ge Y., Wang S., Li M., Ren B., Wang H., Zhang K., Xu H.H.K., Peng X., Feng M., Weir M.D., Chen Y., Cheng L. Anti-caries effects of dental adhesives containing quaternary ammonium methacrylates with different chain lengths. Materials (Basel) 2017;10 doi: 10.3390/ma10060643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venault A., Subarja A., Chang Y. Zwitterionic polyhydroxybutyrate electrospun fibrous membranes with a compromise of bioinert control and tissue-cell growth. Langmuir. 2017;33:2460–2471. doi: 10.1021/acs.langmuir.6b04683. [DOI] [PubMed] [Google Scholar]
- 74.Kwon H.J., Lee Y., Phuong L.T., Seon G.M., Kim E., Park J.C., Yoon H., Park K.D. Zwitterionic sulfobetaine polymer-immobilized surface by simple tyrosinase-mediated grafting for enhanced antifouling property. Acta Biomater. 2017;61:169–179. doi: 10.1016/j.actbio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Gao G., Lange D., Hilpert K., Kindrachuk J., Zou Y., Cheng J.T.J., Kazemzadeh-Narbat M., Yu K., Wang R., Straus S.K., Brooks D.E., Chew B.H., Hancock R.E.W., Kizhakkedathu J.N. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials. 2011;32:3899–3909. doi: 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 76.Hadjesfandiari N., Yu K., Mei Y., Kizhakkedathu J.N. Polymer brush-based approaches for the development of infection-resistant surfaces. J. Mater. Chem. B. 2014;2:4968. doi: 10.1039/C4TB00550C. [DOI] [PubMed] [Google Scholar]
- 77.Aslan S., Deneufchatel M., Hashmi S., Li N., Pfefferle L.D., Elimelech M., Pauthe E., Van Tassel P.R. Carbon nanotube-based antimicrobial biomaterials formed via layer-by-layer assembly with polypeptides. J. Colloid Interface Sci. 2012;388:268–273. doi: 10.1016/j.jcis.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 78.Knetsch M.L.W., Koole L.H. New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers (Basel) 2011;3:340–366. doi: 10.3390/polym3010340. [DOI] [Google Scholar]
- 79.Li H., Williams G.R., Wu J., Lv Y., Sun X., Wu H., Zhu L.M. Thermosensitive nanofibers loaded with ciprofloxacin as antibacterial wound dressing materials. Int. J. Pharm. 2017;517:135–147. doi: 10.1016/j.ijpharm.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto M., Nishikawa N., Mayama H., Nonomura Y., Yokojima S., Nakamura S., Uchida K. Theoretical explanation of the lotus effect: superhydrophobic property changes by removal of nanostructures from the surface of a lotus leaf. Langmuir. 2015;31:7355–7363. doi: 10.1021/acs.langmuir.5b00670. [DOI] [PubMed] [Google Scholar]
- 81.Jaggessar A., Shahali H., Mathew A., Yarlagadda P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017;15:64. doi: 10.1186/s12951-017-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sullivan T., Regan F. The characterization, replication and testing of dermal denticles of Scyliorhinus canicula for physical mechanisms of biofouling prevention. Bioinspir. Biomim. 2011;6 doi: 10.1088/1748-3182/6/4/046001. [DOI] [PubMed] [Google Scholar]
- 83.Mann E.E., Magin C.M., Mettetal M.R., May R.M., Henry M.K.M., DeLoid H., Prater J., Sullivan L., Thomas J.G., Twite M.D., Parker A.E., Brennan A.B., Reddy S.T. Micropatterned endotracheal tubes reduce secretion-related lumen occlusion. Ann. Biomed. Eng. 2016;44:3645–3654. doi: 10.1007/s10439-016-1698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharklet Technologies, Inc. (WWW Document), n.d. https://www.sharklet.com/contact-us/ (accessed 10.12.17).
- 85.Reddy S.T. Surface micropattern resists bacterial contamination transferred by healthcare practitioners. J. Microbiol. Exp. 2014;1:1–7. doi: 10.15406/jmen.2014.01.00032. [DOI] [Google Scholar]
- 86.Wnek G., Bowlin G. Inf. Healthc.; 2008. Encyclopedia of Biomaterials and Biomedical Engineering. [Google Scholar]
- 87.Gordon O., Slenters T.V., Brunetto P.S., Villaruz A.E., Sturdevant D.E., Otto M., Landmann R., Fromm K.M. Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2010;54:4208–4218. doi: 10.1128/AAC.01830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao W., Liu H., Liu X., Wang S., Wu J., Zhang R., Min H., Huang M. Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbohydr. Polym. 2015;132:351–358. doi: 10.1016/j.carbpol.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 89.Cavanagh M.H., Burrell R.E., Nadworny P.L. Evaluating antimicrobial efficacy of new commercially available silver dressings. Int. Wound J. 2010;7:394–405. doi: 10.1111/j.1742-481X.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi H., Castillo B., Seminario-Vidal L. Silver absorption in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis treated with silver-impregnated dressings. A case series. Int. Wound J. 2018:4–6. doi: 10.1111/iwj.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhas S.P., Anbarasan S., Mukherjee A., Chandrasekaran N. Biobased silver nanocolloid coating on silk fibers for prevention of post-surgical wound infections. Int. J. Nanomed. 2015;10:159–170. doi: 10.2147/IJN.S82211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu K., Yang Y., Zhang Y., Deng J., Lin C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015;10:7241–7252. doi: 10.2147/IJN.S92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hadrup N., Lam H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver – a review. Regul. Toxicol. Pharmacol. 2014;68:1–7. doi: 10.1016/j.yrtph.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2:1–16. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawahara K., Tsuruda K., Morishita M., Uchida M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 2000;16:452–455. doi: 10.1016/S0109-5641(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 96.AGS Glass (WWW Document), n.d. https://www.agc-yourglass.com/gb/en/brands/antibacterial-glass (accessed 10.12.17).
- 97.Surfacine (WWW Document), n.d. http://www.surfacine.com/ (accessed 10.12.17).
- 98.PureThread Technologies Inc. (WWW Document), n.d. http://www.purthread.com/ (accessed 02.09.18).
- 99.Schweizer M., Graham M., Ohl M., Heilmann K., Boyken L., Diekema D. Novel hospital curtains with antimicrobial properties: a randomized, controlled trial. Infect. Control Hosp. Epidemiol. 2012;33:1081–1085. doi: 10.1086/668022. [DOI] [PubMed] [Google Scholar]
- 100.Vincent M., Hartemann P., Engels-Deutsch M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health. 2016;219:585–591. doi: 10.1016/j.ijheh.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 101.Luo J., Hein C., Mücklich F., Solioz M. Killing of bacteria by copper, cadmium, and silver surfaces reveals relevant physicochemical parameters. Biointerphases. 2017;12:020301. doi: 10.1116/1.4980127. [DOI] [PubMed] [Google Scholar]
- 102.Casey A.L., Adams D., Karpanen T.J., Lambert P.A., Cookson B.D., Nightingale P., Miruszenko L., Shillam R., Christian P., Elliott T.S.J. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010;74:72–77. doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 103.Souli M., Antoniadou A., Katsarolis I., Mavrou I., Paramythiotou E., Papadomichelakis E., Drogari-Apiranthitou M., Panagea T., Giamarellou H., Petrikkos G., Armaganidis A. Reduction of environmental contamination with multidrug-resistant bacteria by copper-alloy coating of surfaces in a highly endemic setting. Infect. Control Hosp. Epidemiol. 2017;38:765–771. doi: 10.1017/ice.2017.52. [DOI] [PubMed] [Google Scholar]
- 104.Cupron Enhanced EOS Surfaces (WWW Document), n.d. http://eoscu.com/the-product/ (accessed 10.18.17).
- 105.Gjorgievska E.S., Nicholson J.W., Coleman N.J., Booth S., Dimkov A., Hurt A. Component release and mechanical properties of endodontic sealers following incorporation of antimicrobial agents. Biomed. Res. Int. 2017;2017:1–6. doi: 10.1155/2017/2129807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iyamba J.-M.L., Okombe D.T., Zakanda F.N., Malongo T.K., Unya J.W., Lukukula C.M., Balega N.Z. Adherence of Staphylococcus aureus to catheter tubing inhibition by quaternary ammonium compounds. Pan Afr. Med. J. 2016;26:25–50. doi: 10.11604/pamj.2016.25.50.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pérez-Köhler B., García-Moreno F., Bayon Y., Pascual G., Bellón J.M. Inhibition of Staphylococcus aureus adhesion to the surface of a reticular heavyweight polypropylene mesh soaked in a combination of chlorhexidine and allicin: an in vitro study. PLoS One. 2015;10:e0126711. doi: 10.1371/journal.pone.0126711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ji W., Koepsel R.R., Murata H., Zadan S., Campbell A.S., Russell A.J. Bactericidal specificity and resistance profile of poly(quaternary ammonium) polymers and protein-poly(quaternary ammonium) conjugates. Biomacromolecules. 2017;18:2583–2593. doi: 10.1021/acs.biomac.7b00705. [DOI] [PubMed] [Google Scholar]
- 109.Rego G.F., Vidal M.L., Viana G.M., Cabral L.M., Schneider L.F.J., Portela M.B., Cavalcante L.M. Antibiofilm properties of model composites containing quaternary ammonium methacrylates after surface texture modification. Dent. Mater. 2017;33:1149–1156. doi: 10.1016/j.dental.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 110.Brogden N.K., Brogden K.A. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int. J. Antimicrob. Agents. 2011;38:217–225. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Casciaro B., Dutta D., Loffredo M.R., Marcheggiani S., Mcdermott A.M., Willcox M.D., Mangoni M.L. Esculentin-1a derived peptides kill Pseudomonas aeruginosa biofilm on soft contact lenses and retain antibacterial activity upon immobilization to the lens surface. Biopolymers. 2017:1–9. doi: 10.1002/bip.23074. [DOI] [PubMed] [Google Scholar]
- 112.Ma M., Kazemzadeh-Narbat M., Hui Y., Lu S., Ding C., Chen D.D.Y., Hancock R.E.W., Wang R. Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections. J. Biomed. Mater. Res. A. 2012;100A:278–285. doi: 10.1002/jbm.a.33251. [DOI] [PubMed] [Google Scholar]
- 113.Sibel Akalın A. Dairy-derived antimicrobial peptides: action mechanisms, pharmaceutical uses and production proposals. Trends Food Sci. Technol. 2014;36:79–95. doi: 10.1016/j.tifs.2014.01.002. [DOI] [Google Scholar]
- 114.Lee T., Hall K.N., Aguilar M. 2016. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure; pp. 25–39. [DOI] [PubMed] [Google Scholar]
- 115.Monteiro C., Fernandes M., Pinheiro M., Maia S., Seabra C.L., Ferreira-Da-Silva F., Costa F., Reis S., Gomes P., Martins M.C.L. Antimicrobial properties of membrane-active dodecapeptides derived from MSI-78. Biochim. Biophys. Acta – Biomembr. 2015;1848:1139–1146. doi: 10.1016/j.bbamem.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 116.Costa F., Carvalho I.F., Montelaro R.C., Gomes P., Martins M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011;7:1431–1440. doi: 10.1016/j.actbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Costa F., Maia S.R., Gomes P.A.C., Martins M.C.L. Dhvar5 antimicrobial peptide (AMP) chemoselective covalent immobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials. 2015;52:531–538. doi: 10.1016/j.biomaterials.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 118.Barros J., Silva M.G., Rodrigues M.A., Alves F.R.F., Lopes M.A., Pina-Vaz I., Siqueira J.F. Antibacterial, physicochemical and mechanical properties of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. Int. Endod. J. 2014;47:725–734. doi: 10.1111/iej.12207. [DOI] [PubMed] [Google Scholar]
- 119.Cleophas T.C., Sjollema J., Busscher H.J., Kruijtzer J.A.W., Liskamp R.M.J. Characterization and activities of a bactericidal PEG-hydrogel with immobilized antimicrobial peptide. Biomac. 2014;15:3390–3395. doi: 10.1021/bm500899r. [DOI] [PubMed] [Google Scholar]
- 120.Ferreira M., Rzhepishevska O., Grenho L., Malheiros D., Gonçalves L., Almeida A.J., Jordão L., Ribeiro I.A., Ramstedt M., Gomes P., Bettencourt A. Levofloxacin-loaded bone cement delivery system: highly effective against intracellular bacteria and Staphylococcus aureus biofilms. Int. J. Pharm. 2017;532:241–248. doi: 10.1016/j.ijpharm.2017.08.089. [DOI] [PubMed] [Google Scholar]
- 121.Han Y., Xu X., Tang J., Shen C., Lin Q., Chen H. Bottom-up fabrication of zwitterionic polymer brushes on intraocular lens for improved biocompatibility. Int. J. Nanomed. 2017;12:127–135. doi: 10.2147/IJN.S107491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cassin M.E., Ford A.J., Orbach S.M., Saverot S.E., Rajagopalan P. The design of antimicrobial LL37-modified collagen-hyaluronic acid detachable multilayers. Acta Biomater. 2016;40:119–129. doi: 10.1016/j.actbio.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 123.Felgueiras H.P., Wang L.M., Ren K.F., Querido M.M., Jin Q., Barbosa M.A., Ji J., Martins M.C.L. Octadecyl chains immobilized onto hyaluronic acid coatings by thiol–ene “Click Chemistry” increase the surface antimicrobial properties and prevent platelet adhesion and activation to polyurethane. ACS Appl. Mater. Interfaces. 2017;9:7979–7989. doi: 10.1021/acsami.6b16415. [DOI] [PubMed] [Google Scholar]
- 124.Seabra A.B., Da Rocha L.L., Eberlin M.N., De Oliveira M.G. Solid films of blended poly(vinyl alcohol)/poly(vinyl pyrrolidone) for topical S-nitrosoglutathione and nitric oxide release. J. Pharm. Sci. 2005;94:994–1003. doi: 10.1002/jps.20314. [DOI] [PubMed] [Google Scholar]
- 125.Rai A., Pinto S., Evangelista M.B., Gil H., Kallip S., Ferreira M.G.S., Ferreira L. High-density antimicrobial peptide coating with broad activity and low cytotoxicity against human cells. Acta Biomater. 2016;33:64–77. doi: 10.1016/j.actbio.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 126.Alt V., Kirchhof K., Seim F., Hrubesch I., Lips K.S., Mannel H., Domann E., Schnettler R. Rifampicin-fosfomycin coating for cementless endoprostheses: antimicrobial effects against methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) Acta Biomater. 2014;10:4518–4524. doi: 10.1016/j.actbio.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 127.Neut D., Dijkstra R.J.B., Thompson J.I., Van Der Mei H.C., Busscher H.J. A gentamicin-releasing coating for cementless hip prostheses-longitudinal evaluation of efficacy using in vitro bio-optical imaging and its wide-spectrum antibacterial efficacy. J. Biomed. Mater. Res. A. 2012;100 A:3220–3226. doi: 10.1002/jbm.a.34258. [DOI] [PubMed] [Google Scholar]
- 128.Zanini S., Polissi A., Maccagni E.A., Dell’Orto E.C., Liberatore C., Riccardi C. Development of antibacterial quaternary ammonium silane coatings on polyurethane catheters. J. Colloid Interface Sci. 2015;451:78–84. doi: 10.1016/j.jcis.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 129.Braun K., Pochert A., Lindén M., Davoudi M., Schmidtchen A., Nordström R., Malmsten M. Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J. Colloid Interface Sci. 2016;475:161–170. doi: 10.1016/j.jcis.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 130.Slate A.J., Wickens D., Wilson-Nieuwenhuis J., Dempsey-Hibbert N., West G., Kelly P., Verran J., Banks C.E., Whitehead K.A. The effects of blood conditioning films on the antimicrobial and retention properties of zirconium-nitride silver surfaces. Colloids Surf. B Biointerfaces. 2019;173:303–311. doi: 10.1016/j.colsurfb.2018.09.060. [DOI] [PubMed] [Google Scholar]
- 131.Ji S., Li W., Zhang L., Zhang Y., Cao B. Cecropin A-melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem. Biophys. Res. Commun. 2014;451:650–655. doi: 10.1016/j.bbrc.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 132.Biocote Limited (WWW Document), n.d. https://www.biocote.com/ (accessed 01.3.18).
- 133.Sanitized AG (WWW Document), n.d. https://www.sanitized.com/ (accessed 01.02.18).
- 134.Sadrieh N., Wokovich A.M., Gopee N.V., Zheng J., Haines D., Parmiter D., Siitonen P.H., Cozart C.R., Patri A.K., McNeil S.E., Howard P.C., Doub W.H., Buhse L.F. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol. Sci. 2010;115:156–166. doi: 10.1093/toxsci/kfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Foster H.A., Ditta I.B., Varghese S., Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kubacka A., Diez M.S., Rojo D., Bargiela R., Ciordia S., Zapico I., Albar J.P., Barbas C., Martins Dos Santos V.A.P., Fernández-García M., Ferrer M. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014;4:1–9. doi: 10.1038/srep04134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adán C., Marugán J., Mesones S., Casado C., van Grieken R. Bacterial inactivation and degradation of organic molecules by titanium dioxide supported on porous stainless steel photocatalytic membranes. Chem. Eng. J. 2017;318:29–38. doi: 10.1016/j.cej.2016.04.091. [DOI] [Google Scholar]
- 138.Joost U., Juganson K., Visnapuu M., Mortimer M., Kahru A., N??mmiste E., Joost U., Kisand V., Ivask A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015;142:178–185. doi: 10.1016/j.jphotobiol.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 139.PureHealth (WWW Document), n.d. http://www.purehealth.it (accessed 10.12.17).
- 140.Serban M.A. Translational biomaterials – the journey from the bench to the market – think “product”. Curr. Opin. Biotechnol. 2016;40:31–34. doi: 10.1016/j.copbio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 141.Riu M., Chiarello P., Terradas R., Sala M., Garcia-Alzorriz E., Castells X., Grau S., Cots F. Cost attributable to nosocomial bacteremia. Analysis according to microorganism and antimicrobial sensitivity in a university hospital in Barcelona. PLoS One. 2016;11:1–12. doi: 10.1371/journal.pone.0153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.European Center for Disease Prevention and Control Agency, E.M . 2009. The Bacterial Challenge: Time to React. Stockholm. [Google Scholar]
- 143.Chen K.H., Chen L.R., Wang Y.K. Contamination of medical charts: an important source of potential infection in hospitals. PLoS One. 2014;9:1–7. doi: 10.1371/journal.pone.0078512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yue D., Song C., Zhang B., Liu Z., Chai J., Luo Y., Wu H. Hospital-wide comparison of health care-associated infection among 8 intensive care units: a retrospective analysis for 2010–2015. Am. J. Infect. Control. 2017;45:e7–e13. doi: 10.1016/j.ajic.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 145.Fagerlund A., Møretrø T., Heir E., Briandet R., Langsrud S. Cleaning and disinfection of biofilms composed of listeria monocytogenes and background microbiota from meat processing surfaces. Appl. Environ. Microbiol. 2017;83:1–21. doi: 10.1128/AEM.01046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Staskel D.M., Briley M.E., Field L.H., Barth S.S. Microbial evaluation of foodservice surfaces in texas child-care centers. J. Am. Diet. Assoc. 2007;107:854–859. doi: 10.1016/j.jada.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 147.Güldoğan C.E. Clinical infection in burn patients and the consequences. Turk. J. Trauma Emerg. Surg. 2017;23:2–7. doi: 10.5505/tjtes.2017.16064. [DOI] [PubMed] [Google Scholar]
- 148.Werarak P., Kiratisin P., Thamlikitkul V. Hospital-acquired pneumonia and ventilator-associated pneumonia in adults at Siriraj Hospital: etiology, clinical outcomes, and impact of antimicrobial resistance. J. Med. Assoc. Thail. 2010;93:126–138. [PubMed] [Google Scholar]
- 149.Alberti C., Bouakline A., Ribaud P., Lacroix C., Rousselot P., Leblanc T., Derouin F. Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J. Hosp. Infect. 2001;48:198–206. doi: 10.1053/jhin.2001.0998. [DOI] [PubMed] [Google Scholar]
- 150.McLellan E., Fenton P. Fungal contamination of hospital healthcare workers’ overalls. J. Hosp. Infect. 2007;66:87–88. doi: 10.1016/j.jhin.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 151.Fukuda Y., Homma T., Suzuki S., Takuma T., Tanaka A., Yokoe T., Ohnishi T., Niki Y., Sagara H. High burden of Aspergillus fumigatus infection among chronic respiratory diseases. Chron. Respir. Dis. 2018 doi: 10.1177/1479972318761654. 147997231876165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ortega-loubon C., Fernández-molina M., Tobar-ruiz J., Arce N., Fulquet-Carreras E. Rapid fulminant case of aspergillus prosthetic valve endocarditis. Cureus. 2017;9:1–5. doi: 10.7759/cureus.1652. https://www.ncbi.nlm.nih.gov/pubmed/29142800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schwartz S., Kontoyiannis D.P., Harrison T., Ruhnke M. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Glob. Health. 2018;4422:1–11. doi: 10.1016/S1474-4422(18)30030-9. [DOI] [PubMed] [Google Scholar]
- 154.Sharma S., Yenigalla B.M., Naidu S.K., Pidakala P. Primary cutaneous aspergillosis due to Aspergillus tamarii in an immunocompetent host. BMJ Case Rep. 2013:2–4. doi: 10.1136/bcr-2013-010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Borrusso P.A., Quinlan J.J. Prevalence of pathogens and indicator organisms in home kitchens and correlation with unsafe food handling practices and conditions. J. Food Prot. 2017;80:590–597. doi: 10.4315/0362-028X.JFP-16-354. [DOI] [PubMed] [Google Scholar]
- 156.Guyard-Nicodème M., Tresse O., Houard E., Jugiau F., Courtillon C., El Manaa K., Laisney M.J., Chemaly M. Characterization of Campylobacter spp. Transferred from naturally contaminated chicken legs to cooked chicken slices via a cutting board. Int. J. Food Microbiol. 2013;164:7–14. doi: 10.1016/j.ijfoodmicro.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 157.Mattick K., Durham K., Hendrix M., Slader J., Griffith C., Sen M., Humphrey T. The microbiological quality of washing-up water and the environment in domestic and commercial kitchens. J. Appl. Microbiol. 2003;94:842–848. doi: 10.1046/j.1365-2672.2003.01904.x. [DOI] [PubMed] [Google Scholar]
- 158.Sarjit A., Dykes G.A. Transfer of Campylobacter and Salmonella from poultry meat onto poultry preparation surfaces. J. Food Prot. 2017;80:750–757. doi: 10.4315/0362-028X.JFP-16-414. [DOI] [PubMed] [Google Scholar]
- 159.Yu J., Jing H., Lai S., Xu W., Li M., Wu J., Liu W., Yuan Z. Etiology of diarrhea among children under the age five in China: results from a five-year surveillance. J. Infect. 2015;71:19–27. doi: 10.1080/10937404.2015.1051611.INHALATION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lesho E.P., Bronstein M.Z., Mcgann P., Stam J., Kwak Y., Maybank R., Mcnamara J., Callahan M., Campbell J., Hinkle M.K., Walsh E.E. Importation, mitigation, and genomic epidemiology of Candida auris at a large teaching hospital. Infect. Control Hosp. Epidemiol. 2018;39:53–57. doi: 10.1017/ice.2017.231. [DOI] [PubMed] [Google Scholar]
- 161.Achkar J.M., Fries B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010;23:253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gaetti-Jardim E., Jardim E.C.G., Schweitzer C.M., da Silva J.C.L., Oliveira M.M., Masocatto D.C., dos Santos C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018 doi: 10.1016/J.ARCHORALBIO.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 163.Ali S., Muzslay M., Wilson P. A novel quantitative sampling technique for detection and monitoring of Clostridium difficile contamination in the clinical environment. J. Clin. Microbiol. 2015;53:2570–2574. doi: 10.1128/JCM.00376-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yui S., Ali S., Muzslay M., Jeanes A., Wilson A.P.R. Identification of Clostridium difficile reservoirs in the patient environment and efficacy of aerial hydrogen peroxide decontamination. Infect. Control Hosp. Epidemiol. 2017;38:1487–1492. doi: 10.1017/ice.2017.227. [DOI] [PubMed] [Google Scholar]
- 165.Shaughnessy M.K., Bobr A., Kuskowski M.A., Johnston B.D., Sadowsky M.J., Khoruts A., Johnson J.R. Environmental contamination in households of patients with recurrent clostridium difficile infection. Appl. Environ. Microbiol. 2016;82 doi: 10.1128/AEM.03888-15. AEM.03888-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu C., Weese S.J., Namvar A., Warriner K. Sanitary status and incidence of methicillin-resistant Staphylococcus aureus and Clostridium difficile within Canadian hotel rooms. J. Environ. Health. 2015;77:8–15. [PubMed] [Google Scholar]
- 167.Burke K.E., Lamont J.T. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014;8:1–6. doi: 10.5009/gnl.2014.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bonny T.S., Yezli S., Lednicky J.A. Isolation and identification of human coronavirus 229E from frequently touched environmental surfaces of a university classroom that is cleaned daily. Am. J. Infect. Control. 2018;46:105–107. doi: 10.1016/j.ajic.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B., Mederski B., White D., Low D.E., McGeer A., Simor A., Vearncombe M., Downey J., Jamieson F.B., Tang P., Plummer F. Detection of airborne Severe Acute Respiratory Syndrome (SARS) Coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dowell S.F., Simmerman J.M., Erdman D.D., Wu J.S.J., Chaovavanich A., Javadi M., JyhYuan Y., Anderson L.J., Tong S.X., MeiShang H. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin. Infect. Dis. 2004;39:652–657. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hui D.S. Epidemic and emerging coronaviruses (Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome) Clin. Chest Med. 2017;38:71–86. doi: 10.1016/j.ccm.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sampers I., Jacxsens L., Luning P.A., Marcelis W.J., Dumoulin A., Uyttendaele M. Performance of food safety management systems in poultry meat preparation processing plants in relation to Campylobacter spp. contamination. J. Food Prot. 2010;73:1447–1457. doi: 10.4315/0362-028x-73.8.1447. [DOI] [PubMed] [Google Scholar]
- 173.Olmos C., Vilacosta I., Fernández-Pérez C., Bernal J.L., Ferrera C., García-Arribas D., Pérez-García C.N., San Román J.A., Maroto L., Macaya C., Elola F.J. The evolving nature of infective endocarditis in spain: a population-based study (2003 to 2014) J. Am. Coll. Cardiol. 2017;70:2795–2804. doi: 10.1016/j.jacc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 174.Pintado V., Cabellos C., Moreno S., Meseguer M.A., Ayats J., Viladrich P.F. Enterococcal meningitis: a clinical study of 39 cases and review of the literature. Medicine (Baltimore) 2003;82:346–364. doi: 10.1097/01.md.0000090402.56130.82. [DOI] [PubMed] [Google Scholar]
- 175.Wagner M., Bonhoeffer J., Erb T.O., Glanzmann R., Häcker F.M., Paulussen M., Weibel D., Heininger U. Prospective study on central venous line associated bloodstream infections. Arch. Dis. Child. 2011;96:827–831. doi: 10.1136/adc.2010.208595. [DOI] [PubMed] [Google Scholar]
- 176.Dziri R., Klibi N., Alonso C.A., Jouini A., Ben Said L., Chairat S., Bellaaj R., Boudabous A., Ben Slama K., Torres C. Detection of CTX-M-15-producing Escherichia coli isolates of lineages ST131-B2 and ST167-A in environmental samples of a Tunisian hospital. Microb. Drug Resist. 2016;22:399–403. doi: 10.1089/mdr.2015.0354. [DOI] [PubMed] [Google Scholar]
- 177.Foxman B. Urinary tract infection syndromes. Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014;28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 178.García-Laorden M.I., Stroo I., Terpstra S., Florquin S., Medema J.P., Vańt Veer C., De Vos A.F., Van Der Poll T. Expression and function of granzymes A and B in Escherichia coli peritonitis and sepsis. Mediators Inflamm. 2017 doi: 10.1155/2017/4137563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim Y., Kim H.-J., Lim S., Bae K.-S., Han S.B., Jeong D.C., Kang J.H., Shin G.J., Lee G.D., Yeon-Joon Park Community-acquired Escherichia coli Enteritis in Korean children: the clinical application of a stool polymerase chain reaction assay. Infect. Chemother. 2017;49:275–281. doi: 10.3947/ic.2017.49.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boone S.A., Gerba C.P. The occurrence of influenza A virus on household and day care center fomites. J. Infect. 2005;51:103–109. doi: 10.1016/j.jinf.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 181.Killingley B., Greatorex J., Digard P., Wise H., Garcia F., Varsani H., Cauchemez S., Enstone J.E., Hayward A., Curran M.D., Read R.C., Lim W.S., Nicholson K.G., Nguyen-Van-Tam J.S. The environmental deposition of influenza virus from patients infected with influenza A(H1N1)pdm09: implications for infection prevention and control. J. Infect. Public Health. 2016;9:278–288. doi: 10.1016/j.jiph.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 182.Simmerman J.M., Suntarattiwong P., Levy J., Gibbons R.V., Cruz C., Shaman J., Jarman R.G., Chotpitayasunondh T. Influenza virus contamination of common household surfaces during the 2009 Influenza A (H1N1) Pandemic in Bangkok, Thailand: implications for contact transmission. Clin. Infect. Dis. 2010;51:1053–1061. doi: 10.1086/656581. [DOI] [PubMed] [Google Scholar]
- 183.Xue K.S., Moncla L.H., Bedford T., Bloom J.D. Within-host evolution of human Influenza virus. Trends Microbiol. 2018;0:1–13. doi: 10.1016/j.tim.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gallimore C.I., Taylor C., Gennery A.R., Cant A.J., Galloway A., Xerry J., Adigwe J., Gray J.J. Contamination of the hospital environment with gastroenteric viruses: comparison of two pediatric wards over a winter season. J. Clin. Microbiol. 2008;46:3112–3115. doi: 10.1128/JCM.00400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gallimore C.I., Taylor C., Gennery A.R., Cant A.J., Galloway A., Iturriza-gomara M., Gray J.J. Environmental monitoring for gastroenteric viruses in a pediatric primary immunode ciency unit. Society. 2006;44:395–399. doi: 10.1128/JCM.44.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cheesbrough J.S., Green J., Gallimore C.I., Wright P.A., Brown D.W.G. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol. Infect. 2000;125:93–98. doi: 10.1017/S095026889900432X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park G.W., Lee D., Treffiletti A., Hrsak M., Shugart J., Vinjé J. Evaluation of a new environmental sampling protocol for detection of human norovirus on inanimate surfaces. Appl. Environ. Microbiol. 2015;81:5987–5992. doi: 10.1128/AEM.01657-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Biscaro V., Piccinelli G., Gargiulo F., Ianiro G., Caruso A., Caccuri F., De Francesco M.A. Detection and molecular characterization of enteric viruses in children with acute gastroenteritis in Northern Italy. Infect. Genet. Evol. 2018 doi: 10.1016/j.meegid.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 189.De Oliveira F.P., Pires B.M.F.B., De Cássia Ferreira De Almeida Silva K., De Carvalho B.T.F., Teixeira L.A., De Paula G.R., De Oliveira B.G.R.B. Prevalence, antimicrobial susceptibility, and clonal diversity of pseudomonas aeruginosa in chronic wounds. J. Wound Ostomy Cont. Nurs. 2017;44:528–535. doi: 10.1097/WON.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 190.Heward E., Cullen M., Hobson J. Microbiology and antimicrobial susceptibility of otitis externa: a changing pattern of antimicrobial resistance. J. Laryngol. Otol. 2018:1–4. doi: 10.1017/S0022215118000191. [DOI] [PubMed] [Google Scholar]
- 191.Nazareth R., Gonçalves-Pereira J., Tavares A., Miragaia M., de Lencastre H., Silvestre J., Freitas P., Gonçalves E., Martins F., Mendes V., Tapadinhas C., Póvoa P. Community-associated methicillin-resistant Staphylococcus aureus infection in Portugal. Rev. Port. Pneumol. 2012;18:34–38. doi: 10.1016/j.rppneu.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 192.Yetkin G., Otlu B., Cicek A., Kuzucu C., Durmaz R. Clinical, microbiologic, and epidemiologic characteristics of Pseudomonas aeruginosa infections in a University Hospital, Malatya, Turkey. Am. J. Infect. Control. 2006;34:188–192. doi: 10.1016/j.ajic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 193.Ganime A.C., Leite J.P.G., Figueiredo C.E.D.S., Carvalho-Costa F.A., Melgaço F.G., Malta F.C., Fumian T.M., Miagostovich M.P. Dissemination of human adenoviruses and rotavirus species A on fomites of hospital pediatric units. Am. J. Infect. Control. 2016;44:1411–1413. doi: 10.1016/j.ajic.2016.04.207. [DOI] [PubMed] [Google Scholar]
- 194.Ganime A.C., Carvalho-Costa F.A., Mendonça M.C.L., Vieira C.B., Santos M., Costa Filho R., Miagostovich M.P., Leite J.P.G. Group A rotavirus detection on environmental surfaces in a hospital intensive care unit. Am. J. Infect. Control. 2012;40:544–547. doi: 10.1016/j.ajic.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 195.Barker J., Bloomfield S.F. Survival of Salmonella in bathrooms and toilets in domestic homes following salmonellosis. J. Appl. Microbiol. 2000;89:137–144. doi: 10.1046/j.1365-2672.2000.01091.x. [DOI] [PubMed] [Google Scholar]
- 196.Da Fonseca T.A.P., Pessôa R., Felix A.C., Sanabani S.S. Diversity of bacterial communities on four frequently used surfaces in a large Brazilian teaching hospital. Int. J. Environ. Res. Public Health. 2016;13:1–11. doi: 10.3390/ijerph13020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bhetwal A., Maharjan A., Khanal P.R., Parajuli N.P. Enteric fever caused by Salmonella enterica serovars with reduced susceptibility of fluoroquinolones at a community based teaching hospital of Nepal. Int. J. Microbiol. 2017;2017:1–6. doi: 10.1155/2017/2869458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rodríguez M., Valero A., Posada-Izquierdo G.D., Carrasco E., Zurera G. Evaluation of food handler practices and microbiological status of ready-to-eat foods in long-term care facilities in the andalusia region of Spain. J. Food Prot. 2011;74:1504–1512. doi: 10.4315/0362-028X.JFP-10-468. [DOI] [PubMed] [Google Scholar]
- 199.Lawrence K.R., Golik M.V., Davidson L. The role of primary care prescribers in the diagnosis and management of community-associated methicillin-resistant staphylococcus aureus skin and soft tissue infections. Am. J. Ther. 2009;16:333–338. doi: 10.1097/MJT.0b013e31817fdea8. [DOI] [PubMed] [Google Scholar]
- 200.Pham J., Asif T., Hamarshi M.S. Community-acquired Pneumonia with methicillin-resistant Staphylococcus aureus in a patient admitted to the intensive care unit: a therapeutic challenge. Cureus. 2018;10:1–7. doi: 10.7759/cureus.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zakhour R., Chaftari A.-M., Raad I.I. Catheter-related infections in patients with haematological malignancies: novel preventive and therapeutic strategies. Lancet Infect. Dis. 2016;16:e241–e250. doi: 10.1016/S1473-3099(16)30213-4. [DOI] [PubMed] [Google Scholar]