Abstract
The main objective of the current study was to investigate the chemical composition of the essential oil of Artemisia asiatica together with investigating the antibacterial effects it exerts on several common respiratory infection causing bacteria including Haemophilus influenzae. Its mechanism of action was studied using various state-of-the-art assays like scanning electron microscopy, DNA, RNA and protein leakage assays, growth curve assays etc. The essential oil was extracted from the leaves of A. asiatica by supercritical CO2 fluid extraction technology. Chemical composition of essential oils was analyzed by gas chromatography-mass-spectrometry (GC-MS). The antibacterial activity was evaluated against 6 bacteria by the paper disc diffusion method. The minimum inhibitory concentration (MIC) and minimum bactericide concentration (MBC) values of the essential oil were estimated by agar dilution method. The antibacterial mechanism was evaluated by growth curve, the integrity of cell membrane and scanning electronmicroscope (SEM). Gas chromatographic analysis of the A. asiatica essential oil led to the identification of 16 chemical constituents accounting for 97.2% of the total oil composition. The major components were found to be Piperitone, (z)-davanone, p-cymene and 1, 8-cineole. The essential oil showed maximum growth inhibition against Haemophilus influenzae with a zone of inhibition of 24.5 mm and MIC/MBC values of 1.9/4.5 mg/mL respectively. Bacteria treated with the essential oil led to a rapid decrease in the number of viable cells. On adding the essential oil of A. asiatica to the bacterial culture, the constituents of the bacterial cell got released into the medium and this cell constituent release increased with increasing doses of the essential oil. SEM showed that the bacterial cells treated with the essential oil showed damaged cell wall, deformed cell morphology and shrunken cells.
Keywords: Artemisia asiatica, Essential oil, Gas chromatography, Antibacterial activity, Haemophilus influenzae
Highlights
-
•
Antibacterial activity of Artemisia asiatica essential oil evaluated.
-
•
The antibacterial mechanism evaluated by scanning electron microscope.
-
•
16 chemical constituents were identified in the essential oil.
-
•
Essential oil caused damage to the bacterial cell membrane.
-
•
The essential oil was found to be most potent against Haemophilus influenzae.
1. Introduction
Plant essential oils are known for their multiple uses in perfumery, as preservatives and antimicrobials, which are commonly extracted from leaves, branches, roots, stems, fruits and seeds of aromatic plants [1]. The plant essential oil is a mixture of various components from plants. According to the different chemical structures of the essential oil, the composition can be divided into monoterpenes, sesquiterpenes, alcohols, esters, aldehydes and ketones. The constituents are very complex which are involved in the defense of the plant against pests, herbivores, fungi, and bacteria [2], [3]. Essential oils have many applications in indigenous medicines, food flavouring and preservation as well as in drug and cosmetic industries [4]. It also has antibacterial, anti-inflammatory, anti-oxidation, anti-tumor and other functions [5], [6]. Due to thetoxicological effect of the synthetic products, renewed efforts were provided in respect of the use of essential oils as natural antioxidants and preservatives in the food processing, food supplement production andpharmaceutical industry [7].
The genus Artemisia L. (Asteraceae) comprises a variable number of species (from 200 to over 400, depending on the authors) found throughout the northern half of the world. Artemisia popularly known as sage brush or wormwood is a source of valuable drugs and essential oils. Because of medicinal importance and intricate chemical composition of several species and chemotypes, Artemisia continues to be a subject of wide interest for chemists and taxonomists [8], [9], [10]. It has been reported that most of the phytocompounds of A. asiatica comprise of terpenes and terpene alcohols, 1, 8-cineole, camphor, borneol, bornyl acetate, artemisolide, alkaloids, flavone eupatilin etc [11], [12], [13], [14].
It has been reported that a standardized extract of A. asiatica exhibited hepatoprotective activity on liver damage induced by acetaminophen and carbon tetrachloride [15]. In another study, it was reported that this standardized extract of A. asiatica (which was called as DA-9601) showed chemopreventive effects against azoxymethane-initiated and dextran sulphate sodium-promoted mouse colon cancer. DA-9601 also led to the suppression of COX-2 expression and also inhibited nuclear factor (NF)-kappa-B DNA binding in the colonic tissues [16]. The essential oil composition of A. asiatica has already been reported as well as its antibacterial and antifungal activity against a range of microbial strains [12]. However, its mode of action with regard to its antibacterial action has not been studies so far. Therefore, the aim of the study was to investigate the chemical composition and antibacterial activity of the essentialoil on several respiratory infection causing bacteria and tofurther evaluate the antibacterial mechanism against Haemophilus influenzaeby growth curve, the integrity of cellmembrane assays, the SDS-PAGE and SEM examination. Using the above assays, we expect to provide some scientific rationale for developing and utilizing this medical plant.
2. Materials and methods
2.1. Plant material
Artemisia asiatica leaves were collected from a local region of Zhengzhou, Henan, China in September of 2015 and the voucher specimen was deposited in Zhengzhou University herbarium under accession number ZUH5A786/2015. The leaves of the plant were chopped, shade-dried at 40 °C, then finely powdered before storing in the dark for future use.
2.2. Essential oil extraction
The essential oil of A. Asiatica was extracted by supercritical CO2 fluid extraction technology. Many factors affect the total oil yield. The optimum condition is at a pressure of 18 MPa and a temperature of 40 °C and an extraction time of 100 min. The essential oil was stored in tightly closed dark vials and covered with aluminum foil at 4 °C prior to analysis. The essential oil was obtained as a light yellow liquid and had characteristic aroma.
2.3. Microbial strains and culture conditions
The antibacterial activity of the essential oil was tested against 6 bacteria includingStaphylococcus aureus (ATCC6538), Streptococcus pyogenes (ATCC-12344), Listeria monocytogenes (ATCC19115), Pseudomonas aeruginosa (ATCC27853), Klebsiella pneumoniae (ATCC46117), Haemophilus influenzae (ATCC-33391). These bacteria were stored with liquid paraffin wax at 4 °C. All strains were cultured at 37 °C on Mueller-Hintonmedium.
2.4. Gas chromatography-mass spectrometry analysis
The analysis of the essential oil was carried out using a GC/MS-QP 2010 Ultra System (Shimadzu, Kyoto, Japan) and a Shimadzu GC-2010 Plus gas chromatograph. A Rxi-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness, Restek, Bellefonte, USA) was used. Helium was the carrier gas at 1 mL/min with following temperature program: at 60 °C for 1 min, increased to 280 °C at the rate of 3 °C/min held for 5 min. Samples were injected at a temperature of 250 °C with a split ratio of 1/40 during 1 min. An electron impact ionization system with ionization energy of 70 eV and electron ionization spectra with a mass scan range of 30–500 m/z were used.
2.5. Antibacterial activity evaluation
2.5.1. Disc-diffusion method
The antibacterial activity of the essential oil was evaluated by the paper-disk agar diffusion method described by Özer et al. [17] with some modification. Suspension in sterile water of the tested bacteria (0.1 mL of 105 cells per mL) was spread on the solid MH media plates. Paper disc (8 mm in diameter) were impregnated with the essential oil (25 mg/mL) and then placed on the surface of inoculated plates. These plates were incubated at 37 °C for 24 h. The diameter of the inhibition zones (DIZ) was measured by vernier calipers. Penicillin, chloramphenicol and streptomycin (2.5 μg/mL) were used as positive controls and the DMSO solution as negative control.
2.5.2. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC and MBC values of the essential oil were assessed by agar dilution method. The essential oil of A. asiaticawas dissolved in DMSO (2%), and added in the 20 mL sterile nutrient broth (NB) medium to achieve a concentration of 25 mg/mL. The essential oil was tested in the concentrations ranging from 25 to 1.2 mg/mL. Then each plate added 100 μL bacterial suspensions (adjusted to 1 × 105 CFU/mL). Theplates allowed to incubate for 24 h at 37 °C. All experiments were performed in triplicate.
2.5.3. Growth curve experiment
The growth curve protocol was used to examine the bactericidal effects of the essential oil. Haemophilus influenzae bacteria were treated with the essential oil at 1×MIC, 2×MIC and the control containing only DMSO (1%). Then the bacteriawere cultivated at 37 °C with shaking 200 (rpm) for 0, 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36 h respectively. At selected time intervals, the OD600 of supernatants were determined by UV-Vis spectrophotometer. All measurements were carried out in triplicate. Through the assay above, the growth curve of Haemophilus influenzae was measured, the time as the horizontal axis (0 h–36 h), the OD600 of supernatant as the vertical axis.
2.6. Experiments involving DNA and RNA leakage via the bacterial cell membrane
The integrity of cell membrane could be monitored by the release of cytoplasmic constituents of the cell [18]. The experiments were carried out as follows: Haemophilus influenzaebacteria were incubated in NB medium at 37 °C for 12 h. Logarithmic growth phase cells of bacteria was treated with the essential oil at 1× MIC, 2×MIC value except the control. Then the samples were incubated at 37 °C for 1, 4, 8 and 24 h respectively. The samples were then immediately filtered with 0.2 μm organic membrane. To determine the amounts of the DNA and RNA released from the cytoplasm, the supernatant was used to measure the optical density at 260 nm.
2.7. Experiment involving leakage of proteins through the bacterial membrane
The cell integrity was further examined by determining the release of proteins into the supernatant. Theconcentrations of proteins in supernatants were determined by the Bradford'smethod [19]. Logarithmic growth phase cells of Haemophilus influenzae bacteria were treated with the essential oil at 1×MIC, 2×MIC except thecontrol. Then all samples were incubated at 37 °C for 1, 3, 9 and 24 h respectively. Then bacteria were separatedby centrifugation (12,000 g) for 10 min at 4 °C. To determine the concentrations of proteins released from the cytoplasm, the supernatant was used to measure the optical density at 595 nm.
2.8. Scanning electron microscope (SEM) study of bacterial ultrastructure
Scanning electron microscope was used to observe the morphological changes of bacteria as per themethod already described in literature [20]. Haemophilus influenzae bacteria were incubated in NBmedium at 37 °C for 12 h. Logarithmic growth phase cells of K. pneumoniae was treated with the essential oil at2×MIC value except the control. The samples were incubated at 37 °C for 3, 9 and 24 h respectively. Then thebacteria were centrifuged (12,000 g, 10 min, 4 °C), fixed with 2.0% (v/v) glutaraldehyde (4 °C, 3 h) and washed with 0.1 M PBS (pH 7.2, 2 times). After centrifugation, the cells were fixed with 1% osmic acid (4 °C, 1.5 h), then washed 0.1 M PBS (pH 7.2). Then the cells were dehydrated in a graded series of ethanol (50%, 70%, 80%, 90% and 100%). Finally, the samples were sputter-coated with gold 157 under vacuum, followed by microscopicexaminations using a SEM (Hitachi S-3000H; Hitachi, Ltd., Tokyo, Japan).
2.9. Statitical analysis
The experiments were carried out in triplicates and expressed as mean ± SD. Student's test was used for statistical analysis and p values were considered significant at p < .01.
3. Results
3.1. Chemical compositions of the essential oil of Artemisia asiatica
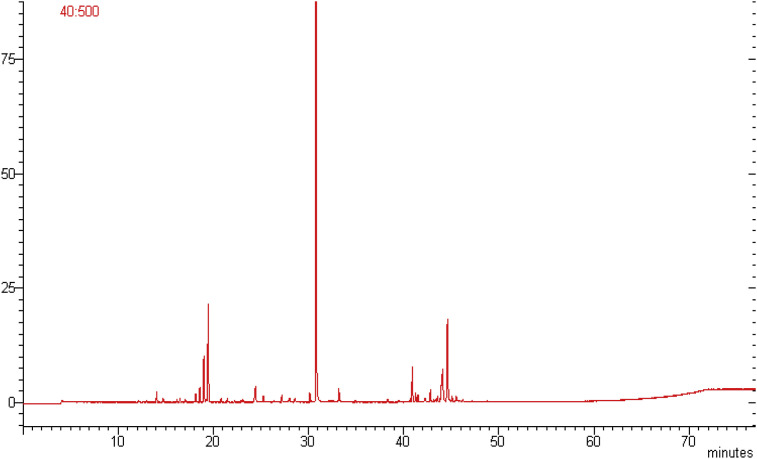
The essential oil was obtained by supercritical CO2 fluid extraction technology. The chemical compositions of the essential oil were analyzed by GC-MS. The yield of essential oil from the leaves of the A. asiatica was 0.4%Gas chromatographic analysis of the A. asiatica essential oil led to the identification of 16 chemical constituents accounting for 97.2% of the total oil composition. The major components were found to be Piperitone, (z)-davanone, p-cymene and 1, 8-cineole. Oxygenated monoterpenes were found to be the dominant class of terpenes, followed by the monoterpene hydrocarbons. Fig. 1 shows the GC-MS Total Ion Chromatogram (TIC) of the essential oil of A. asiatica while as Table 1 lists the identified chemical components.
Fig. 1.
The GC-MS Total Ion Chromatogram (TIC) of the essential oil of A. asiatica.
Table 1.
Chemical composition of essential oil from the leaves of A. asiatica.
| S. No | Compound | % Peak Area | Methods of identification |
|---|---|---|---|
| 1 | α-Pinene | 3.5 | MS, RI |
| 2 | β-Pinene | 0.2 | MS, RI |
| 3 | δ-3-Carene | 0.5 | MS, RI |
| 4 | α-Terpinene | 1.9 | MS, RI |
| 5 | P-Cymene | 14.5 | MS, RI, Std |
| 6 | 1,8-Cineole | 23.4 | MS, RI, Std |
| 7 | γ-Terpinene | 0.9 | MS, RI |
| 8 | (Z)-β-Ocimene | 4.2 | MS, RI |
| 9 | 4-Terpineol | 1.2 | MS, RI |
| 10 | (E)-Piperitol | 4.2 | MS, RI |
| 11 | Piperitone | 21.2 | MS, RI, Std |
| 12 | Ascaridole | 1.3 | MS, RI |
| 13 | α-Curcumene | 3.3 | MS, RI |
| 14 | Germacrene D | 5.2 | MS, RI |
| 15 | α-Bergamotene | 2.2 | MS, RI |
| 16 | (Z)-Davanone | 9.7 | MS, RI, Std |
| Total | 97.9% |
3.2. Antibacterial activity of the essential oil
The essential oil was tested for its antibacterial activity against 6 kinds of bacteria. The zone of inhibition values of the essential oil from A. Asiatica are presented in Table 2 . The results showed that the essential oil showed some antibacterial effect against all the tested bacteria including both Gram-positive and Gram-negative bacteria. In order to determine the antibacterial spectrum of the essential oil, 3 well-known standard drugs viz., Penicillin, chloramphenicol and streptomycin were used as positive controls. The results revealed that essential oil of A. asiatica exhibited a broad spectrum of antibacterial activity against the various microbial strains. The essential oil showed maximum growth inhibition against Haemophilus influenzae with a zone of inhibition of 24.5 mm and MIC/MBC values of 1.9/4.5 mg/mL respectively. However, the essential oil showed lowest growth inhibition against Pseudomonas aeruginosawith a zone of inhibition of 9.2 mm and MIC/MBC values of 5.8/9.4 mg/mL respectively. This is not unexpected because Pseudomonas aeruginosais a Gram negativebacteria which usually are resistant to most of the drugs used. The MIC/MBC values of the essential oil indicated that the antibacterial activity of the oil is somehow comparable to the standard positive controls. MIC was the minimum essential oil concentration that can prevent the bacteria from obvious growth. MBC was the lowest concentration of the essential oil that could kill the inoculum bacteria [21]. Thus from zone of inhibition values and MIC/MBC values, it was shown that Haemophilus influenza was the most affected bacteria, therefore further mechanistic studies were done on this bacterial strain.
Table 2.
Antibacterial activity of A. asiatica essential oil against some common respiratory infection causing bacteria.
| Bacterial strain (cat. no.) | Zone of inhibition (mm) |
|||||
|---|---|---|---|---|---|---|
| Essential oil (μg/ml) | Penicillin | Chloramphenicol | Streptomycin | MIC (mg/ml) | MBC (mg/ml) | |
| Staphylococcus aureus (ATCC6538) | 12.7 | 34.2 | 33.6 | 32.3 | 2.5 | 5.2 |
| Streptococcus pyogenes (ATCC-12344) | 14.3 | 34.7 | 32.7 | 33.7 | 3.1 | 6.7 |
| Listeria monocytogenes (ATCC19115) | 18.1 | 36.2 | 28.5 | 26.5 | 4.2 | 8.3 |
| Pseudomonas aeruginosa (ATCC27853) | 9.2 | 24.7 | 23.9 | 28.4 | 5.8 | 9.4 |
| Klebsiella pneumoniae ATCC46117 | 19.2 | 34.9 | 31.1 | 34.5 | 2.3 | 5.8 |
| Haemophilus influenzae ATCC-33391 | 24.5 | 26.4 | 29.4 | 25.6 | 1.9 | 4.5 |
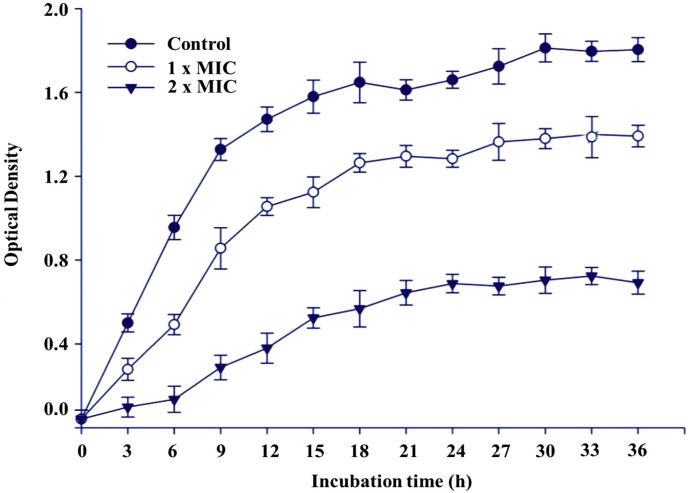
3.3. Growth curve studies
According to the sensitivity of the tested bacteria, Haemophilus influenzae was selected as the model bacteria for further study to confirm the antibacterial mechanism of the essential oil from A. asiatica. The results are shown in Fig. 2 . Compared to the bacteria treated with the essential oil, the control showed a fast increase in bacterial number. On the contrary the bacteria treated with the essential oil led to a rapid decrease in the number of viable cells. The growth of bacteria was suppressed by the essential oil. In the treatments (1×MIC) showed a slow decrease in bacterial number over the first 12 h period of the test. Unlike the changing trend of the bacterial number at 1×MIC, the number of viable cells with the treatment of 2×MIC reduced significantly in the first three hours after cultivation. The results indicated that different incubation time and concentration of the essential oil of A. Asiatica had great effects on antibacterial activities.
Fig. 2.
The growth curves of the Haemophilus influenzae affected by the essential oil from the leaves of A. asiatica. The results were considered significant at p < .01.
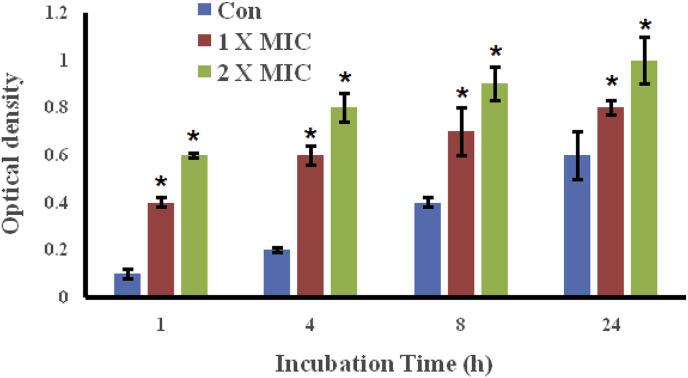
3.4. The essential oil of A. asiatica led to the leakage of DNA and RNA through bacterial cell membrane
Further antibacterial mechanism of the essential oil of Artemisia asiatica against the Haemophilus influenzae was tested using the assay of Leakage of DNA and RNA through the membrane of these bacteria. Fig. 3 shows the results when Haemophilus influenzae were treated with different concentrations of the essential oil from A. Asiatica for 1, 4, 8 and 24 h, respectively. Results showed that on adding the essential oil of A. asiatica to the bacterial culture, the constituents of the bacterial cell got released into the medium and this cell constituent release increased with increasing doses of the essential oil. The optical density (OD260) value of supernatant from Haemophilus influenzaetreated with essential oil (1xMIC) was much higher as that of the untreated control for 1, 4, 8 and 24 h respectively. The optical density values were even much higher when the concentration of the essential oil was 2 x MIC. It is also important to mention here that the optical density values also increased with the incubation intervals of 1, 4, 8 and 24 h. This assay confirms that the essential oil of A. asiatica led to the damage of the cell membrane of Haemophilus influenzae which results in the leakage of the macromolecules including DNA and RNA.
Fig. 3.
The essential oil of A. asiatica led to the DNA and RNA leakage from the Haemophilus influenzae bacteria. The results were considered significant at *p < .01.
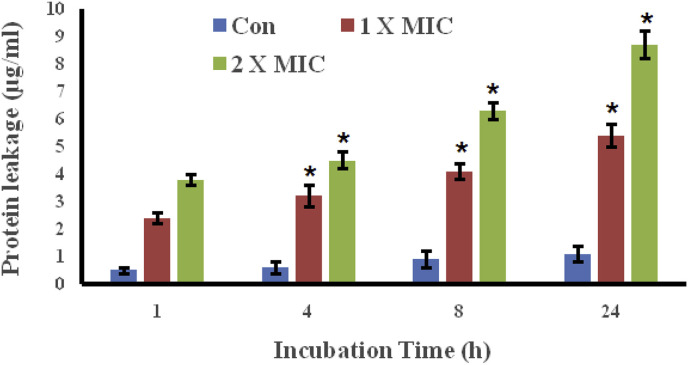
3.5. The essential oil of A. asiatica led to the leakage of proteins through bacterial cell membrane
Proteins play vital roles within bacterial cell. In this assay, the effect of A. asiatica essential oil on the protein leakage from the Haemophilus influenzae bacteria was studied. The results which are shown in Fig. 4 indicate that the essential oil of A. asiatica resulted in the leakage of essential proteins from the Haemophilus influenza bacterial cells by damaging the bacterial cell membrane. The leakage of proteins at the start in the untreated control was much lower as compared to the leakage of proteins treated with A. asiatica essential oil. The leakage of the proteins at 1 h incubation was 0.5 μg/mL in the untreated control, while as the leakage of proteins increased to 2.4 μg/mL (1 x MIC) and 3.8 μg/mL (2 x MIC). The leakage of proteins not only increased with essential oil dose but also increased with the treatment time. The results indicated that the essential oil of A. asiaticadecreased the content of cellular proteins by permeating and disrupting cell membrane.
Fig. 4.
The essential oil of A. asiatica induces leakage of essential proteins from Haemophilus influenzae. The results were considered significant at *p < .01.
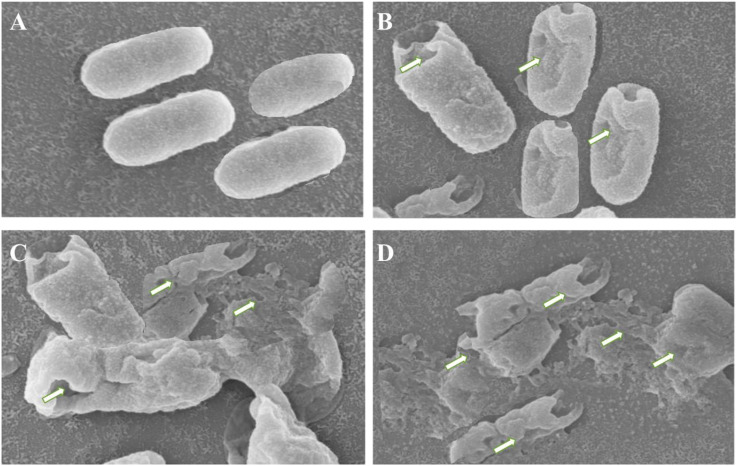
3.6. Morphological evaluation of the bacteria using scanning electron microscope (SEM)
In this assay, we evaluated the effects of the essential oil on the ultrastructural features of Haemophilus influenzaeusing SEM. The results of this assay are shown in Fig. 5 and reveal that as compared to the untreated control bacteria, the essential oil treated bacterial cells revealed serious damage to the cell. The bacterial cells treated with the essential oil showed damaged cell wall, deformed cell morphology and shrunken cells. The damage to the cells seemed to enhance with increasing doses of the essential oil. In contrast, untreated cells showed rod shaped, regular, and intact morphologywhich were uniform in size and shape. This supported the results of the growth curve study and integrity of cell membrane assays, and showed that the non-reversible damage to the cell wall and membrane occurred.
Fig. 5.
SEM images of Haemophilus influenzae after treating with 1 x MIC dose of the A. asiatica essential oil for 4 h (B), 8 h (C) and 24 h (D) respectively. The arrows indicate deformed cell morphology, withering of cells and finally cell membrane rupture leading to leakage of the DNA, RNA and proteins through the membrane.
4. Discussion
Respiratory tract infections are characterized by acute infection usually involving nose, larynx, sinuses and pharynx. The different kinds of upper respiratory tract infections include the cold, laryngitis, pharyngitis, sinusitis etc [22]. The main contributing factors towards these nasty infections are the infections caused by certain bacteria like Haemophilus influenzae, Streptococcus pneumoniae, Bacillus anthracis, Streptococcus pyogenes etc. Some viruses like coronavirus, para influenza virus etc can also cause certain types of upper respiratory tract infections [23], [24]. Many of the bacterial strains have acquired resistance against the commonly used antibiotics resulting in limited efficacy of these drugs. As such there is an urgent need for novel, promising and cheap antimicrobial drugs which do not develop resistance easily against these microbes. Essential oils have wide range of applications in medicine and aromatherapy owing to their multiple chemical constituents in their mixtures giving rise to multifunctionality. Essential oils have been reported to exhibit antibacterial activity against a range of Gram-positive as well as Gram-negative bacterial strains [25]. It has been reported that traditionally essential oils and their constituents have been used for the treatment of various upper respiratory tract infections including colds. Inhalation therapy using essential oils has been successfully used to treat acute disorders like sinusitis, acute and chronic bronchitis and even for reducing the tracheal inflammation [26], [27], [28], [29].
In the current study, we evaluated the antibacterial effects of Artemisia asiatica essential oil along with demonstrating its mechanism of action by using different state-of-the-art assays including DNA, RNA and protein leakage assays, dynamic curve assay and scanning electron microscopy assay. The mechanistic studies were performed on Haemophilus influenzae which was shown to be most susceptible to the essential oil treatment. The essential oil showed significant and broad spectrum antibacterial activity against the various microbial strains. The essential oil showed maximum growth inhibition against Haemophilus influenzae with a zone of inhibition of 24.5 mm and MIC/MBC values of 1.9/4.5 mg/mL respectively. However, the essential oil showed lowest growth inhibition against Pseudomonas aeruginosa with a zone of inhibition of 9.2 mm and MIC/MBC values of 5.8/9.4 mg/mL respectively. Growth curve studies revealed that as compared to the bacteria treated with the essential oil, the control showed a fast increase in bacterial number. On the contrary the bacteria treated with the essential oil led to a rapid decrease in the number of viable cells. Our results are well in agreement with previous which have reported the essential oil of other Artemisia species to exert significant antimicrobial activities [30]. The essential oil components observed in case of other Artemisia were more or less similar as observed in the present study [30]. Furthermore the results showed that on adding the essential oil of A. asiatica to the bacterial culture, the constituents of the bacterial cell got released into the medium and this cell constituent release increased with increasing doses of the essential oil. SEM investigations revealed that the essential oil treatment led to damaged cell wall, deformed cell morphology, shrunken cells and also caused DNA damage. The damage to the bacterial cells seemed to enhance with increasing doses of the essential oil. Our results are information with previous studies wherein essential oils have been reported to inhibit cell wall synthesis via interaction with a number of cellular enzymes [31], [32]. Besides, essential oils have also been reported to induce DNA damage in bacterial cells and hence further confirming our results [33]. Given the promising results of the present study, we believe that further in vivo investigation should be carried out on the essential of A. asiatica to establish it as an important antimicrobial agent.
5. Conclusion
In brief, the current study reports the chemical composition of the essential oil of Artemisia asiatica along with its antibacterial activity against some common respiratory infection causing microbes. The essential oil exhibited significant antibacterial activity against Haemophilus influenzae. Firstly, the essential oil was seen to damage the cell membrane which further led to the leakage of DNA, RNA, and proteins. Further, the SEM experiment was performed to examine the effects of the essential oil of A. asiatica on cell morphology of Haemophilus influenzae. The SEM experiment showed that severe morphological changes appeared in the cell wall and membrane, and the microscopy of treated cells showed many irregularly deformed and shrunken bacteria.
Conflicts of interest
The authors declare that there is no conflict of interest to reveal.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.micpath.2017.12.032.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.AlgeriaLila H.M., Boudria A. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crop. Prod. 2015;78:148–153. [Google Scholar]
- 2.Tajkarimi M.M., Ibrahima S.A., Cliver D.O. Antimicrobial herb and spice compounds in food. Food Contr. 2010;21:1199–1218. [Google Scholar]
- 3.Batish D.R., Singh H.P. Eucalyptus essential 314 oil as a natural pesticide. Forest EcolManag. 2008;256:2166–2174. [Google Scholar]
- 4.Abdel N.E.G., Michele L. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai, Egypt. Ind. Crop. Prod. 2015;67:201–207. [Google Scholar]
- 5.Li Y.Q., Han Q. Antibacterial characteristics and mechanisms of e-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Contr. 2014;43:22–27. [Google Scholar]
- 6.Tian J., Ban X.Q. Chemical composition and antifungal activity of essential oil from Cicutavirosa L. var. latisectaCelak. Int. J. Food Microbiol. 2011;145:464–470. doi: 10.1016/j.ijfoodmicro.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Wei A., Shibamoto T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007;55:1737–1742. doi: 10.1021/jf062959x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L., Zhang H.Y. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015;187:370–377. doi: 10.1016/j.foodchem.2015.04.108. [DOI] [PubMed] [Google Scholar]
- 9.Watson L.E., Bates P.L. Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol. Biol. 2002;2:17. doi: 10.1186/1471-2148-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kordali S., Cakir A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J. Agric. Food Chem. 2005;53:1408–1416. doi: 10.1021/jf048429n. [DOI] [PubMed] [Google Scholar]
- 11.Kaku T., Yosimura K. Artemisia asiatica Nakai. I. Chemical studies. Pharmacology. 1938;11:115. [Google Scholar]
- 12.Kalemba D., Kusewicz D., Swiader K. Antimicrobial properties of the essential oil of Artemisia asiatica Nakai. Phytother Res. 2002;16:288–291. doi: 10.1002/ptr.856. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Alonso M.J., Velasco-Negueruela A. Variations in the essential oil composition of Artemisia pedemontana gathered in Spain: chemotype camphor-1,8-cineole and chemotype davanone. Biochemist. 2003;31:77–84. [Google Scholar]
- 14.Kim D.H., Na-Hye K. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochem. Pharmacol. 2004;68:1081–1087. doi: 10.1016/j.bcp.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Ryu B.K., Ahn B.O. Studies on protectiveeffect of DA-9601, Artemisia asiatica extract, on acetaminophen- andCCl4-induced liver damage in rats. Arch Pharm. Res. (Seoul) 1998;21:508–513. doi: 10.1007/BF02975366. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.S., Kundu J.K. Chemopreventiveeffects of the standardized extract (DA-9601) of Artemisia asiatica onazoxymethane-initiated and dextran sulfate sodium-promoted mouse coloncarcinogenesis. Nutr. Canc. 2008;60:90–97. doi: 10.1080/01635580802404170. [DOI] [PubMed] [Google Scholar]
- 17.Özer H., Sökmen M., Güllüce M. Chemical composition and antimicrobial and antioxidant activities of the essential oil and methanol extract of Hippomarathrummicrocarpum (Bieb.) from Turkey. J. Agric. Food Chem. 2007;55:937–942. doi: 10.1021/jf0624244. [DOI] [PubMed] [Google Scholar]
- 18.Chen C.Z., Stuart L.C. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/s0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 19.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of proteinutilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Bajpai V.K., Sharma A., Baek K.H. Antibacterial mode of action of Cudraniatricuspidatafruitessential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Contr. 2013;32:582–590. [Google Scholar]
- 21.Zhang Y.B., Liu X.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Contr. 2016;59:282–289. [Google Scholar]
- 22.Eccles M.P., Grimshaw J.M., Johnston M. Applying psychological theories to evidence-based clinical practice: identifying factors predictive of managing upper respiratory tract infections without antibiotics. Implement. Sci. 2007;2:26. doi: 10.1186/1748-5908-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mäkelä M.J., Puhakka T., Ruuskanen O. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H., Yang T., Li F.Y., Yao Y., Sun Z.M. Antibacterial activity and mechanism of action of Monardapunctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int. J. Clin. Exp. Pathol. 2014;7:7389–7398. [PMC free article] [PubMed] [Google Scholar]
- 25.Edris A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 26.Rantzsch U., Vacca G., Dück R., Gillissen A. Anti-inflammatory effects of Myrtol standardized and other essential oils on alveolar macrophages from patients with chronic obstructive pulmonary disease. Eur. J. Med. Res. 2009;14:205–209. doi: 10.1186/2047-783X-14-S4-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Federspil P., Wulkow R., Zimmermann T. Effect of standardized Myrtol in therapy of acute sinusitis - results of a double-blind, randomized multicenter study compared with placebos. Laryngo-Rhino-Otol. 1997;76:23–27. doi: 10.1055/s-2007-997381. [DOI] [PubMed] [Google Scholar]
- 28.Schindl R. Inhalational effect of volatile oils. Wien Med. Wochenschr. 1972;122:591–593. [PubMed] [Google Scholar]
- 29.Shubina L.P., Siurin S.A., Savchenko V.M. Inhalation of essential oils in the combined treatment of patients with chronic bronchitis. Vrach. 1990;5:66–67. [PubMed] [Google Scholar]
- 30.Rashid S., Rather M.A., Shah W.A., Bhat B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013;138(1):693–700. doi: 10.1016/j.foodchem.2012.10.102. [DOI] [PubMed] [Google Scholar]
- 31.Oussalah M., Caillet S., Lacroix M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J. Food Protect. 2006;69(5):1046–1055. doi: 10.4315/0362-028x-69.5.1046. [DOI] [PubMed] [Google Scholar]
- 32.Rather M.A., Dar B.A., Dar M.Y., Wani B.A., Shah W.A., Bhat B.A., Ganai B.A., Bhat K.A., Anand R., Qurishi M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine. 2012;19(13):1185–1190. doi: 10.1016/j.phymed.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.