Abstract
Occurrence of influenza pandemics is a worldwide phenomenon and a significant cause of mortality and morbidity throughout the globe. It is due to mutations in the influenza virus genetic material creating antigenic drift of pathogenic viral proteins resulting in emergence of new influenza virus strains. Therefore, the vaccines available for prevention of influenza offer no protection against influenza pandemics caused by new virus strains. Moreover, the existing drugs used to combat influenza may be ineffective to treat influenza pandemics due to the emergence of drug resistance in the pandemic virus strain. Therefore, a working strategy must be developed to combat influenza pandemics. In this review we have addressed this problem and reviewed the published studies on ascorbic acid in the common cold and influenza and laboratory studies on the effect of ascorbic acid on influenza virus. We have also correlated the clinical and laboratory studies and developed a hypothesis to prevent influenza pandemics.
Keywords: Pandemic, Influenza, Ascorbic acid, Vitamin C, Flu
Introduction
The common cold, or acute viral nasopharyngitis, is a mild, self-limiting infectious disease that can be caused by more than 100 different viruses. Of these, rhinovirus and coronavirus are responsible for approximately 50–70% of all common colds [1]. In contrast, influenza is an acute respiratory illness caused primarily by the influenza virus (serotypes A and B). It occurs worldwide and is responsible for considerable morbidity and mortality. Influenza, usually more severe than the common cold, typically causes fever, headache, muscle aches, and a more significant cough; however, mild cases of influenza are similar to colds. Of the two serotypes, influenza A occurs more frequently and is more dangerous [2]. The viruses causing the common cold are generally inhaled, which then attach to a protein (intercellular adhesion molecule-1) and get into the nasal epithelium and cause an inflammatory response. Inside the cell the virus proliferates, and when the host cell ruptures more viruses are available to occupy fresh intercellular adhesion molecule-1 receptors [3], [4]. Influenza A viruses are typically divided into two general subtypes that correspond to two different antigens on the surface of the virus: hemagglutinin and neuramidase. Hemagglutinin antigen (H) is a glycoprotein that allows the virus to bind to respiratory epithelial cell membrane sialic acid and fuse with the host cell membrane. After incorporation into the host cell, the viral RNA is integrated into the host cell genome, which then starts forming new virion particles that are transported to the host cell membrane, and the viral neuramidase antigen (N) breaks down sialic acid, allowing the virus to disperse from the infected cell [5]. Mutations occur in influenza viruses, resulting in the frequent emergence of new viral strains (antigenic drift) with immunologically different H and N antigens. As an example, we can observe the H5N1 avian influenza viral strain or the recent pandemic H1N1 swine flue influenza virus strain known to cause influenza pandemics [6], [7]. Consequently, the antibody raised against known influenza virus strains by vaccination programs cannot prevent the disease caused by the emergence of a new viral strain. It is needless to mention that a vaccine development to combat a new strain of influenza virus is not possible by any nation within a short time to control an influenza pandemic emergency [8]. Moreover, there are reports of drug-resistant influenza virus strains [9]. Therefore, alternative strategies to combat influenza pandemics are urgently necessary for successful control of such pandemics and in this review we address this problem to develop a hypothesis that may pave the path to combat influenza pandemics.
Clinical experience: Vitamin C in management of influenza/common cold
There has been considerable interest regarding the association of vitamin C (ascorbic acid) with the common cold since the claim of the famous scientist, Linus Pauling, in 1970 that ascorbic acid has a physiologic effect on the common cold [10]. Megadoses of vitamin C (3 g/d) have been shown to prevent cold and flu symptoms in students 18–30 y of age [11]. Lower doses of vitamin C have not been shown to prevent cold symptoms in a number of trials [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. Doses of vitamin C in excess of 1 g/d taken shortly after onset of a cold symptom did not decrease the duration or severity of cold symptoms in healthy adult volunteers when compared with a vitamin C dose lower than the minimum recommended daily intake [29]. In cases of confirmed influenza, relatively few clinical trials have examined the efficacy of vitamin C [30]. In a controlled trial of 226 patients with influenza A, 114 patients received vitamin C 300 mg/d, and 112 patients served as controls; outcomes measured were development of pneumonia and duration of hospital stay. Pneumonia was reported in two subjects in the treatment group and 10 in the control group, and hospital stays for influenza or related complications averaged 9 d in the vitamin C group and 12 d in the control group [31]. There are many reviews providing meta-analyses about the matter and concluding that there is no correlation between vitamin C supplementation and the incidence of cold symptoms in the general population, but there are also persistent views that challenge these conclusions [32], [33], [34], [35], [36], [37], [38]. In fact, there is a widespread perception that the conclusion of no association of vitamin C supplementation with cold symptoms is mostly due to inaccurate review methodologies [39].
Story of vitamin C and influenza virus in the laboratory
In in vitro cell culture systems, ascorbic acid has been shown to exhibit specific antiviral effects against influenza virus [40]. In the Vero cell line, it shows 50% inhibition of influenza viral proliferation at 100 and 250 μM at 24 h. In the MDCK cell line, the specific antiviral effect of ascorbic acid is observed at higher doses (>200 μM) in a dose-dependent manner [40]. Ascorbic acid 3000 μM has been shown to inhibit the cell cycle of fibroblasts in an in vitro cell culture system at S phase with 100% inhibition of entry of those cells into G2 M phase, probably by down-modulating the genes essential for S-phase progression [41]. This ascorbic acid–induced inhibition of cells entering the G2 M phase of the cell cycle may inhibit the integration of viral genetic material into the host genome in the case of RNA viruses; for incorporation of viral genetic material into the host cell genome, the host cell cycle must be arrested at G2 M phase [42], [43]. In this regard, the influenza virus, corona virus, or picorna virus should be no exception because these are RNA viruses. Moreover, ascorbic acid has been shown to stimulate LXR-α gene expression [44], which is known to cause downregulation of the c-myc gene [45], [46]. Downregulation of the c-myc gene arrests the cell cycle at G0G1 phase [47], resulting in restricted entry of cells in S phase and reducing the number of cells at G2 M phase for viral genome integration. Also, it is a proven fact that ascorbic acid inhibits viral replication of other RNA viruses such as the human immunodeficiency virus and avian tumor virus [48], [49].
Ascorbic acid also has been observed to exhibit a sustained antiviral effect against influenza virus in the presence of iron, which may be due to the pro-oxidant effect of vitamin C. Dehydroascorbate has shown this effect more significantly compared with ascorbate [50]. In vivo ascorbic acid has been observed to play a pro-oxidant role but those observations have been criticized [51], [52], [53]. To exhibit a pro-oxidant effect on bronchial epithelium, the availability of iron and oxidants such as hydrogen peroxide should not be a limiting factor for locally available ascorbic acid because iron and hydrogen peroxide are present at the vicinity of bronchial epithelium [54], [55].
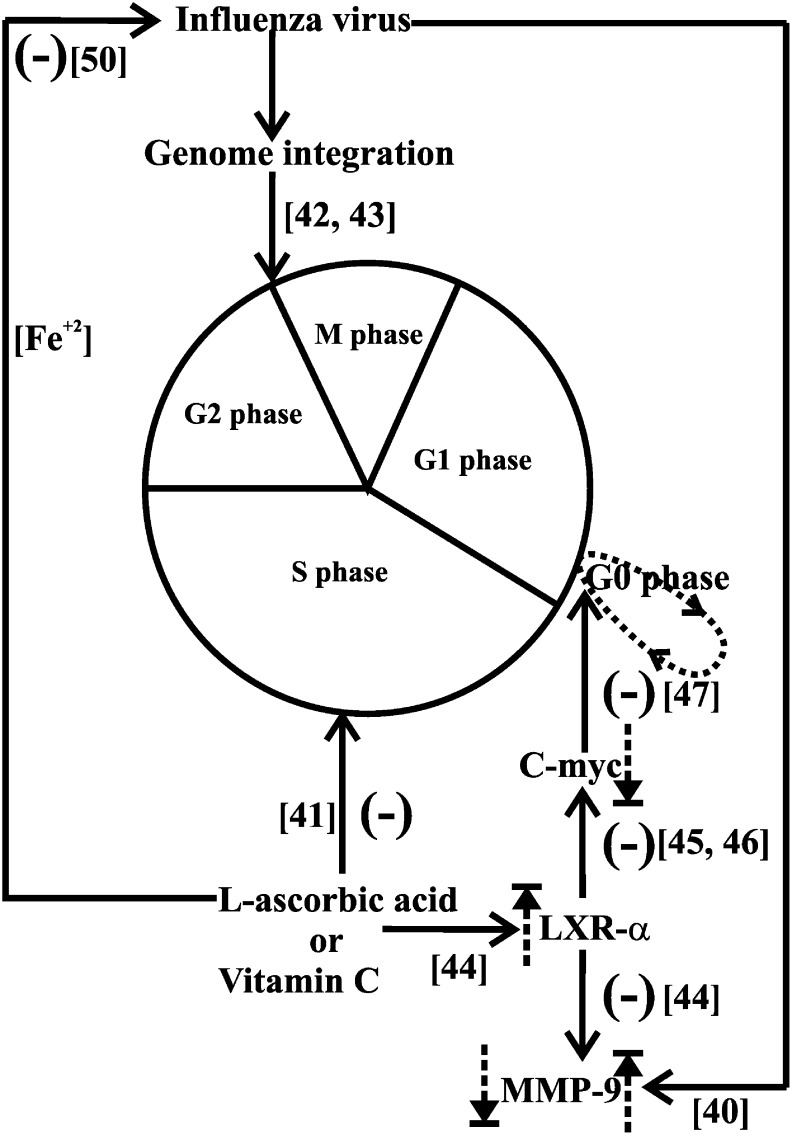
Influenza virus infection is known to produce matrix metalloprotease (MMP) in epithelial cells, which is thought to be a mechanism of spread of the virus [40]. Vero cells infected with influenza virus have been shown to produce substantial MMP-9 [56]. Clinical cases with influenza encephalitis also have been proved to have significantly more serum MMP-9 concentration [57]. Ascorbic acid has been shown to reduce MMP-9 gene expression in peripheral blood-derived mononuclear cells [44]. It also has been shown to decrease MMP-9 synthesis induced by hydrogen peroxide in an in vitro chorioamniotic membrane model [58]. It is well known that MMPs are important for cancer metastasis and vitamin C has been proved to inhibit the migration of cancer cells independent of its antioxidant activity [59]. Therefore, it is likely that availability of vitamin C in the respiratory epithelium will inhibit the possible lethal mediator of inflammation, MMP-9, and reduce the extent of harm due to influenza virus infection. Supporting this view, a study in an animal model system has demonstrated enhanced lung pathology in an influenza virus–infected vitamin C–deficient mouse model [60]. All these experimental evidences indicating vitamin C–induced inhibition of influenza virus proliferation are represented in Figure 1 .
Fig. 1.
Diagram showing possible antiviral mechanisms of high concentrations of vitamin C by arresting the cell cycle and modulating host gene expression .The possible pro-oxidant action of vitamin C in the presence of iron to render antiviral effects is also represented. The cell cycle inhibition effect of vitamin C is shown at S phase directly and at G0G1 phase through LXR-α overexpression and subsequent c-myc underexpression, which reduces the availability of cells at G2 M phase, required for viral genome integration, a mandatory event for successful causation of influenza. Vitamin C–induced LXR-α overexpression and subsequent downregulation of MMP-9 gene are also shown, which are important to inhibit the spread of influenza infection. Relevant references are indicated within square brackets. Upregulation and downregulation of gene expression are indicated by upward and downward dashed arrows. A hyphen within parentheses represents inhibition of that particular process. MMP, matrix metalloprotease.
Correlation of clinical findings and laboratory results to develop the hypothesis
The laboratory reports stating an antiviral effect of ascorbic acid against influenza virus has not been challenged by any report thus far. The antiviral activity of ascorbic acid against influenza virus is not due to its interaction with the H and N antigens of the virus but may be due to its pro-oxidant effect, its genomic effect of cell cycle arrest at S phase, or perhaps both. Therefore, the antiviral activity of ascorbic acid should occur irrespective of the antigenic drift of the virus, which changes the N or H antigen, causing an influenza pandemic emergency, and renders the existing influenza vaccines ineffective for prevention of a pandemic influenza virus strain infection or causes drug resistance of the neuraminidase inhibitor (N).
In the community, although many trials have assessed the relation of cold and flu symptoms and vitamin C supplementation, no trial has assessed the specific viral load in an influenza epidemic/pandemic in a population with ascorbic acid supplementation. The supplementation trials have been reviewed several times, but the individual studies considered in those trials mostly have not estimated the viral load and ascorbic acid concentration available in the respiratory secretion before and after vitamin C supplementation. Therefore, the available reviews and meta-analyses speaking for and against vitamin C supplementation may not exist without substantial errors.
Availability of ascorbic acid in bronchial secretion is a proven fact [61]. It has been shown that after 1-g/d supplementation of vitamin C, ascorbic acid concentration in the bronchial secretion is around 90 μM, a concentration ineffective to exhibit an antiviral effect on the MDCK cell line or to arrest the cell cycle at S phase in fibroblasts [62]. Therefore, we are of the opinion that, if a sustained high dose of vitamin C is available in the respiratory secretion, it can exhibit substantial anti influenza virus activity.
How to test the hypothesis
Oral megadoses of ascorbic acid in a normal population are not advisable because these have been reported to cause diarrhea in normal individuals and there is no conclusive study reporting that oral high doses of ascorbic acid deliver sufficient amounts of ascorbic acid to bronchial secretion [63]. An exploration of the antitumor activity of ascorbic acid through intravenous route has been proposed [64]. Analogously, to achieve a high concentration of ascorbic acid in the bronchial epithelium, we propose ascorbic acid supplementation by an inhalational route. In recent times, when steroid inhalers are routinely used in asthmatic patients, the technologic development for an ascorbic acid delivery system by an inhalational route is expected to be developed quickly without much difficulty. In addition to ascorbic acid supplementation by an inhalational route, a 1-g/d oral supply of ascorbic acid should be combined because oral ascorbic acid supplementation is known to enhance iron absorption from the gastrointestinal tract, which may be responsible for killing the influenza virus in the presence of ascorbic acid in the bronchial epithelium. There is every reason to believe that this combined mode of ascorbic acid supplementation may render the host resistant to influenza virus infection, even to a newly emerged pandemic strain. Before starting this trial directly in humans, preclinical studies in animal models must be conducted to determine the possible toxicities of this approach. If preclinical studies show encouraging results, then appropriate clinical trials should be designed and available ascorbic acid concentrations in bronchial secretions and viral load must be correlated to interpret the outcome and adequate monitoring of such trials for any harm. Only this combination approach of ascorbic acid supplementation and correlation of available ascorbic acid at the bronchial epithelium and viral load can conclusively decide that the widespread anticipation about ascorbic acid as a preventive measure of influenza is right or wrong.
Summary and conclusion
Influenza virus infects the respiratory tract epithelial cells and frequently causes flu pandemic emergencies due to emergence of a new strain of the virus by possible antigenic shift/drift of viral proteins that renders the disease unpreventable by vaccination of the host against known influenza viral strains. Vitamin C has long been expected to offer protection against influenza, but the clinical studies conducted thus far have failed to provide an unbiased conclusion about the matter, and contradictory views on the topic are reported. Conversely, the laboratory studies show promising antiviral effects of vitamin C by multiple ways at higher concentrations that are unlikely to be attained at the respiratory tract epithelium by oral supplementation of vitamin C. Therefore, combined oral and inhalational routes of vitamin C delivery will provide higher concentrations of vitamin C at the respiratory epithelium, the very site of influenza virus infection, and possibly impart anti–influenza virus immunity irrespective of infection caused by new or old virus strains. If this approach works, we propose to name it the Pauling vaccine. That would be our tribute to Linus Pauling who strongly believed that vitamin C supplementation could protect mankind from cold and flu.
References
- 1.Gwaltney J.M., Jr. In: Principles and practice of infectious diseases. 4th ed. Mandell G.L., Bennett J.E., Dolin R., editors. Volume 1. Churchill Livingstone; London, UK: 1995. pp. 561–566. (The common cold). [Google Scholar]
- 2.Centers for Disease Control and Prevention. Seasonal influenza (flu). Available at: http://www.cdc.gov/flu/. Accessed June 30, 2009.
- 3.Douglas R.G.J. Pathogenesis of rhinovirus common colds in human volunteers. Ann Otol Rhinol Laryngol. 1970;79:563–571. doi: 10.1177/000348947007900320. [DOI] [PubMed] [Google Scholar]
- 4.Abraham G., Colonno R.J. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984;51:340–345. doi: 10.1128/jvi.51.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zambon M.C. The pathogenesis of influenza in humans. Rev Med Virol. 2001;11:227–241. doi: 10.1002/rmv.319. [DOI] [PubMed] [Google Scholar]
- 6.Mossad S.B. The resurgence of swine-origin influenza A (H1N1) Cleveland Clinic Journal of Medicine. 2009;76:337–343. doi: 10.3949/ccjm.76a.09047. [DOI] [PubMed] [Google Scholar]
- 7.Thomas J.K., Noppenberger J. Avian influenza: a review. Am J Health Syst Pharm. 2007;64:149–165. doi: 10.2146/ajhp060181. [DOI] [PubMed] [Google Scholar]
- 8.García-García J., Ramos C. Influenza, an existing public health problem. Salud Publica Mex. 2006;48:244–267. doi: 10.1590/s0036-36342006000300009. [DOI] [PubMed] [Google Scholar]
- 9.Fleming D.M., Elliot A.J., Meijer A., Paget W.J. Influenza virus resistance to oseltamivir: what are the implications? Eur J Public Health. 2009;19:238–239. doi: 10.1093/eurpub/ckp012. [DOI] [PubMed] [Google Scholar]
- 10.Hemilä H. Vitamin C supplementation and the common cold—was Linus Pauling right or wrong? Int J Vitam Nutr Res. 1997;67:329–335. [PubMed] [Google Scholar]
- 11.Gorton H.C., Jarvis K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. J Manipulative Physiol Ther. 1999;22:530–533. doi: 10.1016/s0161-4754(99)70005-9. [DOI] [PubMed] [Google Scholar]
- 12.Cowan D.W., Diehl S.H., Baker A.B. Vitamins for the prevention of colds. JAMA. 1942;120:1268–1271. [Google Scholar]
- 13.Dahlberg G., Engel A., Rydin H. The value of ascorbic acid as a prophylactic against common colds. Acta Med Scand. 1944;119:540–561. [Google Scholar]
- 14.Franz W.L., Heyl H.L., Sands G.W. Blood ascorbic acid level in bioflavonoid and ascorbic acid therapy of common cold. JAMA. 1956;162:1224–1226. doi: 10.1001/jama.1956.02970300024009. [DOI] [PubMed] [Google Scholar]
- 15.Ritzel G. Critical evaluation of vitamin C as a prophylactic and therapeutic agent in colds. Helv Med Acta. 1961;28:63–68. [PubMed] [Google Scholar]
- 16.Anderson T.W., Reid D.B., Beaton G.H. Vitamin C and the common cold: a double-blind trial. Can Med Assoc J. 1972;107:503–508. [PMC free article] [PubMed] [Google Scholar]
- 17.Charleston S.S., Clegg K.M. Ascorbic acid and the common cold. Lancet. 1972;1:1401–1402. doi: 10.1016/s0140-6736(72)91143-9. [DOI] [PubMed] [Google Scholar]
- 18.Wilson C.W., Loh H.S., Foster F.G. Common cold symptomatology and vitamin C. Eur J Clin Pharmacol. 1973;6:196–202. doi: 10.1007/BF00558286. [DOI] [PubMed] [Google Scholar]
- 19.Anderson T.W., Suranyi G., Beaton G.H. The effect on winter illness of large doses of vitamin C. Can Med Assoc J. 1974;111:31–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Coulehan J.L., Reisinger K.S., Rogers K.D., Bradley D.W. Vitamin C prophylaxis in a boarding school. N Engl J Med. 1974;290:6–10. doi: 10.1056/NEJM197401032900102. [DOI] [PubMed] [Google Scholar]
- 21.Carson M., Cox H., Corbett M., Pollitt N. Vitamin C and the common cold. J Soc Occup Med. 1975;25:99–102. doi: 10.1093/occmed/25.3.99. [DOI] [PubMed] [Google Scholar]
- 22.Karlowski T.R., Chalmers T.C., Grenkel L.D., Kapikian A.Z., Lewis T.L., Lynch J.M. Ascorbic acid for the common cold. A prophylactic and therapeutic trial. JAMA. 1975;231:1038–1042. [PubMed] [Google Scholar]
- 23.Coulehan J.L., Eberhard S., Kapner L., Taylor F., Rogers K., Garry P. Vitamin C and acute illness in Navajo school children. N Engl J Med. 1976;295:973–977. doi: 10.1056/NEJM197610282951802. [DOI] [PubMed] [Google Scholar]
- 24.Elwood P.C., Lee H.P., St Leger A.S., Baird M., Howard A.N. A randomized controlled trial of vitamin C in the prevention and amelioration of the common cold. Br J Prev Soc Med. 1976;30:193–196. doi: 10.1136/jech.30.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludvigsson J., Hansson L.O., Tibbling G. Vitamin C as a preventive medicine against common colds in children. Scand J Infect Dis. 1977;9:91–98. doi: 10.3109/inf.1977.9.issue-2.07. [DOI] [PubMed] [Google Scholar]
- 26.Miller J.Z., Nance W.E., Norton J.A., Wolen R.L., Griffith R.S., Rose R.J. Therapeutic effect of vitamin C. A co-twin control study. JAMA. 1977;237:248–251. [PubMed] [Google Scholar]
- 27.Carr A.B., Einstein R., Lai L.Y.C., Martin N.G., Starmer G.A. Vitamin C and the common cold: a second MZ Cotwin control study. Acta Genet Med Gemellol (Roma) 1981;30:249–255. doi: 10.1017/s0001566000006450. [DOI] [PubMed] [Google Scholar]
- 28.Cowan D.W., Diehl H.S. Antihistaminic agents and ascorbic acid in the early treatment of the common cold. JAMA. 1950;143:421–424. doi: 10.1001/jama.1950.02910400013004. [DOI] [PubMed] [Google Scholar]
- 29.Audera C., Patulny R.V., Sander B.H., Douglas R.M. Mega-dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Aust. 2001;175:359–362. doi: 10.5694/j.1326-5377.2001.tb143618.x. [DOI] [PubMed] [Google Scholar]
- 30.Roxas M., Jurenka J. Colds and influenza: a review of diagnosis and conventional, botanical, and nutritional considerations. Altern Med Rev. 2007;12:25–48. [PubMed] [Google Scholar]
- 31.Kimbarowski J.A., Mokrow N.J. Colored precipitation reaction of the urine according to Kimbarowski (FARK) as an index of the effect of ascorbic acid during treatment of viral influenza. Dtsch Gesundheitsw. 1967;22:2413–2418. [PubMed] [Google Scholar]
- 32.Hemilä H. Does vitamin C alleviate the symptoms of the common cold?—a review of current evidence. Scand J Infect Dis. 1994;26:1–6. doi: 10.3109/00365549409008582. [DOI] [PubMed] [Google Scholar]
- 33.Douglas R.M., Chalker E.B., Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2000;2 doi: 10.1002/14651858.CD000980. CD000980. [DOI] [PubMed] [Google Scholar]
- 34.Douglas R.M., Hemilä H., D'Souza R., Chalker E., Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2004;4 doi: 10.1002/14651858.CD000980.pub2. CD000980. [DOI] [PubMed] [Google Scholar]
- 35.Douglas R.M., Hemilä H., Chalker E., Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD000980.pub3. CD000980. [DOI] [PubMed] [Google Scholar]
- 36.Hickey S., Roberts H. Misleading information on the properties of vitamin C. PLoS Med. 2005;2:e307. doi: 10.1371/journal.pmed.0020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemilä H., Herman Z.S. Vitamin C and the common cold: a retrospective analysis of Chalmers' review. J Am Coll Nutr. 1995;142:116–123. doi: 10.1080/07315724.1995.10718483. [DOI] [PubMed] [Google Scholar]
- 38.Hemilä H. Vitamin C, the placebo effect, and the common cold: a case study of how preconceptions influence the analysis of results. J Clin Epidemiol. 1996;49:1079–1084. doi: 10.1016/0895-4356(96)00189-8. [DOI] [PubMed] [Google Scholar]
- 39.Hemilä H. Vitamin C supplementation and common cold symptoms: problems with inaccurate reviews. Nutrition. 1996;12:804–809. doi: 10.1016/s0899-9007(96)00223-7. [DOI] [PubMed] [Google Scholar]
- 40.Jariwalla R., Roomi M.W., Gangapurkar B., Kalinovsky T., Niedzwiecki A., Rath M. Suppression of influenza A virus nuclear antigen production and neuraminidase activity by a nutrient mixture containing ascorbic acid, green tea extract and amino acids. Biofactors. 2007;31:1–15. doi: 10.1002/biof.5520310101. [DOI] [PubMed] [Google Scholar]
- 41.Belin S., Kaya F., Duisit G., Giacometti S., Ciccolini J., Fontés M. Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PLoS ONE. 2009;4:e4409. doi: 10.1371/journal.pone.0004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y., Yongky A., Yin J. Growth of an RNA virus in single cells reveals a broad fitness distribution. Virology. 2009;385:39–46. doi: 10.1016/j.virol.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davy C., Doorbar J. G2/M cell cycle arrest in the life cycle of viruses. Virology. 2007;368:219–226. doi: 10.1016/j.virol.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaul D., Baba M.I. Genomic effect of vitamin ‘C’ and statins within human mononuclear cells involved in atherogenic process. Eur J Clin Nutr. 2005;59:978–981. doi: 10.1038/sj.ejcn.1602203. [DOI] [PubMed] [Google Scholar]
- 45.Kaul D., Gautam A., Sikand K. Importance of LXR-α transcriptome in the modulation of innate immunity. Mol Cell Biochem. 2006;292:53–57. doi: 10.1007/s11010-006-9216-5. [DOI] [PubMed] [Google Scholar]
- 46.Anand P.K., Kaul D. Oxysterol receptor LXRa regulates SREBP gene expression in HL-60 cells exposed to differentiating agents. Curr Sci. 2002;82:136–137. [Google Scholar]
- 47.Wang H., Mannava S., Grachtchouk V., Zhuang D., Soengas M.S., Gudkov A.V. c-Myc depletion inhibits proliferation of human tumor cells at various stages of the cell cycle. Oncogene. 2008;27:1905–1915. doi: 10.1038/sj.onc.1210823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bissell M.J., Hatie C., Farson D.A., Schwarz R.I., Soo W.J. Ascorbic acid inhibits replication and infectivity of avian RNA tumor virus. Proc Natl Acad Sci U S A. 1980;77:2711–2715. doi: 10.1073/pnas.77.5.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harakeh S., Jariwalla R.J., Pauling L. Suppression of human immunodeficiency virus replication by ascorbate in chronically and acutely infected cells. Proc Natl Acad Sci U S A. 1990;87:7245–7249. doi: 10.1073/pnas.87.18.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuya A., Uozaki M., Yamasaki H., Arakawa T., Arita M., Koyama A.H. Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int J Mol Med. 2008;22:541–545. [PubMed] [Google Scholar]
- 51.Podmore I.D., Griffiths H.R., Herbert K.E., Mistry N., Mistry P., Lunec J. Vitamin C exhibits pro-oxidant properties. Nature (Lond) 1998;392:559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 52.Rehman A., Collis C.S., Yang M., Kelly M., Diplock A.T., Halliwell B., Rice-Evans C. The effects of iron and vitamin C co-supplementation on oxidative damage to DNA in healthy volunteers. Biochem Biophys Res Commun. 1998;246:293–298. doi: 10.1006/bbrc.1998.8592. [DOI] [PubMed] [Google Scholar]
- 53.Carr A., Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 54.Gutteridge J.M.C., Mumby S., Quinlan G.J., Chung K.F., Evans T.W. Pro-oxidant iron is present in human pulmonary epithelial lining fluid: implications for oxidative stress in the lung. Biochem Biophys Res Commun. 1996;220:1024–1027. doi: 10.1006/bbrc.1996.0518. [DOI] [PubMed] [Google Scholar]
- 55.Jöbsis R.Q., Schellekens S.L., Fakkel-Kroesbergen A., Raatgeep R.H.C., de Jongste J.C. Hydrogen peroxide in breath condensate during a common cold. Mediat Inflamm. 2001;10:351–354. doi: 10.1080/09629350120102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeo S.J., Kim S.J., Kim J.H., Lee H.J., Kook Y.H. Influenza A virus infection modulates the expression of type IV collagenase in epithelial cells. Arch Virol. 1999;144:1361–1370. doi: 10.1007/s007050050592. [DOI] [PubMed] [Google Scholar]
- 57.Takashi I., Tsuneo M., Madoka K., Takeshi M., Tomoyo M., Susumu F. Matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 in influenza-associated encephalopathy. Pediatr Infect Dis J. 2007;26:542–544. doi: 10.1097/INF.0b013e31803994a0. [DOI] [PubMed] [Google Scholar]
- 58.Hernández Guerrero C.A., Vázquez Vela M.E., Herrerías Canedo T., Flores Herrera H., Meraz Cruz N. Vitamin C decreases MMP-9 synthesis induced by hydrogen peroxide in an in vitro chorioamniotic membrane model. Ginecol Obstet Mex. 2006;74:3–12. [PubMed] [Google Scholar]
- 59.Wybieralska E., Koza M., Sroka J., Czyż J., Madeja Z. Ascorbic acid inhibits the migration of walker 256 carcinosarcoma cells. Cell Mol Biol Lett. 2008;13:103–111. doi: 10.2478/s11658-007-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W., Maeda N., Beck M.A. Vitamin C deficiency increases the lung pathology of influenza virus–infected Gulo−/− mice. J Nutr. 2006;136:2611–2616. doi: 10.1093/jn/136.10.2611. [DOI] [PubMed] [Google Scholar]
- 61.Cross C.E., van der Vliet A., O'Neill C.A., Louie S., Halliwell B. Oxidants, antioxidants, and respiratory tract lining fluid. Environ Health Perspect. 1994;102:185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischer H., Schwarzer C., Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cathcart R.F. Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med Hypotheses. 1981;7:1359–1376. doi: 10.1016/0306-9877(81)90126-2. [DOI] [PubMed] [Google Scholar]
- 64.Chen Q., Espey M.G., Sun A.Y., Pooput C., Kirk K.L., Krishna M.C. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]