Abstract
Influenza is a viral zoonosis of birds and mammals that has probably existed since antiquity. Attack rates of influenza are relatively high but mortality is relatively low. Influenza mortality is highest in the very young, the very old, and the immunosuppressed. Influenza has the potential for rapid spread and may involve large populations. This article examines the swine influenza (H1N1) strain of recent origin, and compares the microbiology, epidemiology, clinical presentation, differential, clinical, and laboratory diagnosis, therapy, complications, and prognosis with previous recorded outbreaks of avian and human seasonal influenza pneumonias.
Keywords: Pandemic influenza, CPK, Relative lymphopenia, Thrombocytopenia, CA-MRSA CAP
History
Influenza is a viral zoonosis of birds and mammals that has probably existed since antiquity. In antiquity, populations were small and less concentrated, limiting influenza potential. As animals became domesticated and lived in close proximity to human populations, populations concentrated in urban centers, setting the stage for the introduction of zoonotic influenza into the human population. Influenza can maintain itself in rural populations because of ongoing changes in immunity mediated by influenza viral antigenic drift and shift. Because influenza immunity is strain-specific, influenza may be maintained and may result in epidemics in large, closed urban populations. Attack rates of influenza are relatively high but mortality is relatively eg ∼ 1 % low. Influenza mortality is highest in the very young, the very old, and the immunosuppressed. Influenza (human seasonal) has the potential for rapid spread and may involve large populations. Even though the mortality associated with epidemics is relatively low, the total number of fatalities involved may be huge. In the 1918–1919 influenza A (H1N1) pandemic, the mortality rate was 1%, but because massive numbers of people were infected, there were 50 million fatalities.1, 2, 3
Clinical descriptions of influenza were first clearly described by Caus in 1551. The English physician described a “sweating disease” characterized by fever, headache, and myalgias that killed some patients rapidly but lasted only a few days in those that survived. Although the English sweating sickness might have been due to influenza, the findings were nonspecific and did not resemble the first recognized influenza epidemic, which occurred in Germany, England, and Italy in 1173. Subsequent influenza epidemics occurred in France and Italy in 1323 and 1387. Villaini and Segui were the first to use the term “una influenza,” referring to some “celestial influence” that was thought to be responsible for the infection. The French described influenza as the grip, because patients appeared to be gripped or seized by influenza. Thousands of people were affected by these early epidemics but the first pandemic spread through Europe in 1510. In 1679, Sydenem provided the first accurate clinical description of influenza. In 1933, Smith isolated influenza A virus. Three years later, Burnette was the first to culture the influenza virus in embryonated eggs. In 1941, Hearst was the first to describe the hemagglutination reactions of influenza. Frances, in 1939, was the first to isolate influenza B and later in 1950, Taylor was the first to isolate influenza C.2, 3, 4
The great influenza pandemic of 1918 to 1919 was due to an H1N1 strain. In 1997, and again recently, outbreaks of avian influenza (H5N1) virus have occurred, affecting humans as well as poultry. Since 1997, avian influenza (H5N1) emerged in terms of lethality with a high mortality rate ie, 60% among young healthy adults, and has as yet unrealized pandemic potential. Avian influenza (H5N1) strain resembles the 1918 to 1919 pandemic strain. Fortunately, avian influenza (H5N1) outbreaks over the past decade have been limited with minimal person-to-person spread. However, avian influenza (H5N1), like the influenza A (H1N1) strain of 1918 to 1919, has pandemic potential if the virus mutates, permitting efficient person-to-person transmission. The influenza A (H1N1) pandemic of 1918 to 1919 was remarkable in many respects. The pandemic was unprecedented in its mortality and virulence, and was accompanied by 2 unusual sequelae, namely, encephalitis and postinfluenza Parkinson disease.5, 6, 7 The 1918 to 1919 influenza A (H1N1) pandemic was noteworthy in that the death rate was highest in young healthy adults.6, 8, 9, 10 The majority of the early deaths in young healthy individuals were due to influenza pneumonia alone and not simultaneous/subsequent bacterial pneumonia.4, 11, 12, 13, 14, 15, 16
The swine influenza A (H1N1) pandemic began in 2009.17 Swine influenza (H1N1) is a novel influenza A virus comprising a reassortment of 4 distinct genetic elements, namely, swine, human, avian, and Eurasian swine genetic components, which combine into a single influenza virus, swine influenza (H1N1). The swine influenza (H1N1) pandemic rapidly spread from Mexico to the United States and the world in the spring of 2009. The initial spread from Mexico was via tourists/travelers returning home from Mexico. Foci of swine influenza (H1N1) strains became established across wide geographic areas.17, 18, 19, 20 As with other influenza A pandemics, the initial “herald wave” (in the late spring/summer) involved large numbers of individuals with relatively few deaths. Lethal swine influenza (H1N1) pneumonia affected primarily young and healthy adults. The epidemiologic hallmark of pandemic influenza is its, “pandemic signature,” ie, most early mortalities are among young healthy adults. Human seasonal influenza affects primarily the very young, elderly, and those with comorbidities. Repeated passages through hosts, usually results in increased viral virulence. Excluding the effect of the (H1N1) vaccine, there is concern that subsequent waves of swine influenza (H1N1) may be more lethal than the first.8, 11, 21
Microbiology
Members of the family Orthomyxoviridae have a segmented negative strand RNA genome. In the Orthomyxoviridae family are the influenza A, B, and C viruses. The single-stranded RNA influenza viruses differ in the length of their RNA segments, that is, influenza A and B have 8 RNA segments whereas influenza C has 7 segments. Influenza A, B, and C viruses are spherical and covered by a lipid envelope from host cell membrane. Influenza B and C have no subtypes. Subtypes are characteristic of the influenza A virus. Influenza A viruses are classified on the basis of their surface glycoproteins of the hemagglutinin (HA) and neuraminidase agglutinating (NA) proteins. Influenza A viruses have 16 different HA and 9 different NA subtypes. However, only 3 HA subtypes and 2 NA subtypes have been implicated in human influenza epidemics. The 3 HA subtypes in human outbreaks are H1, H2, and H3, and the 2 NA subtypes are N1 and N2. The surface HA proteins are HA glycoproteins in a rod-shaped spike with a hydrophobic carboxy terminal based in the viral envelope. The hydrophobic end of the rod-shaped spike projects from the virus and is the binding site for sialic acid residues in host receptor cells. The HA surface glycoprotein is responsible for viral adherence to the host cell, whereas the HA glycoprotein is responsible for attachment of the virus and penetration of the influenza A virus into the host cell. The NA surface glycoprotein is mushroom-shaped and is also implanted in the lipid envelope at the amino acid end of the glycoprotein. NA glycoprotein cleaves terminal sialic acid residues, which are responsible for release of virus from infected host cells and spread of the influenza virus to other cells in the respiratory tract. Influenza viruses also have M2 proteins, which are important in uncoding the virus. M2 proteins, which are the matrix proteins, are present only in influenza A viruses and appear on the surface of infected host cells. M1 proteins are surface structural proteins important in viral budding.22, 23, 24
Influenza A and B viruses bind to sialic acid receptors on the host cell surface and begin the infectious process. Human influenza viruses attach preferentially to the α2, 6 linkage to galactose containing oligosaccharides. In contrast, avian influenza viruses preferentially bind to α23 linkages. Influenza C viruses bind to 9-O-acetyl-N-acetyl-neuraminic acid receptors. Influenza A viruses are widely distributed in nature. Avian species are the primary reservoir for all 16 HA and 9 NA influenza A subtypes. Other natural hosts of influenza A viruses are humans, horses, and swine. Human influenza viruses replicate in respiratory epithelial cells, whereas avian influenza A virus is replicated in respiratory and gastrointestinal epithelial cells.22, 23, 24, 25
Swine influenza is an influenza A virus that consists of distinctive genetic influenza A elements from swine, human, avian, and Eurasian swine strains of influenza (H1N1). The swine influenza (H1N1) pandemic was totally unpredicted. As with the “herald waves” of previous influenza A pandemics, large numbers of people were infected but relatively few died. The virulence of avian influenza (H5N1) remains worrisome.21 To date, avian influenza (H5N1) has been highly lethal in relatively small numbers of patients. It is thought that avian influenza (H5N1) is relatively inefficient in bird-to-human and human-to-human transmission, thus far limiting its human pandemic potential. With the swine influenza (H1N1) pandemic, although mortality rates are low, the potential for pandemic spread has been demonstrated during the spring/summer initial “herald wave”. Lethal, swine influenza (H1N1) affects primarily young healthy adults.5, 6, 22, 23, 24, 25
Epidemiology
Influenza viruses are spread by airborne aerosols/droplets from infected individuals while speaking, coughing, or sneezing. Fomites are also implicated in the transmission of influenza. Nonhuman influenza infections are spread from respiratory/gastrointestinal infections of the host animal.2, 3, 26 Influenza viruses remain viable at cool temperatures under conditions of low humidity on nonporous surfaces.
Influenza viruses may be detected in the general population throughout the year, but peak in the winter months. In the northern hemisphere, peak human seasonal influenza season is in February whereas in the southern hemisphere, where winter months occur between May and August, flu usually peaks in June or July. Epidemics of influenza A are characterized by a sudden increase in acute respiratory illnesses. In epidemics, usually influenza A or influenza B predominates, although both influenza A and B may occur together in epidemics. Avian influenza (H5N1) may be transmitted from direct contact with infected birds or humans.2, 5, 22, 23
Influenza has a short incubation period of approximately 2 days (range, 1–5 days). During the initial phase of the illness, there are high concentrations of influenza virus in respiratory secretions responsible for its spread via airborne transmission. One of the characteristics of influenza viruses is their changing antigenicity. Minor changes in surface glycoproteins resulting from stepwise point mutations in HA/NA glycoproteins are called “antigenic drift.” Sequential amino acid changes occurring over a period of years resulting in HA/NA glycoprotein changes occur every 2 to 3 years and are termed “antigenic shift.” Influenza epidemics occur every 6 to 10 years and are due to antigenic shifts exposing the population to strains to which it has not been exposed previously. For this reason, pandemic influenza A has a high attack rate in individuals of all age groups who are not susceptible, particularly children. Unlike epidemics of influenza that occur during the winter months, pandemic influenza may occur at any time and spread rapidly from country to country, facilitated by travel. The twentieth century has experienced 3 influenza pandemics, 2 of which have emerged from China. The highest late excess fatalities occur are among the elderly, who succumb to influenza because of comorbid conditions/bacterial complications, whereas early deaths among young healthy patients are most often due to severe influenza pneumonia alone.5, 22, 23, 24, 25, 26
Influenza has also been responsible for nosocomial outbreaks. Nosocomial influenza is usually due to influenza A and, less commonly, influenza B strains. In contrast to community-acquired infections, nosocomial outbreaks are brief, lasting 1 to 3 weeks versus months in community-acquired outbreaks. Health care workers may be important in initiating or spreading influenza in nursing homes, chronic care facilities, or hospitals. Nosocomial influenza has occurred in immunized hosts, suggesting that there is suboptimal protection in elderly nursing home residents.23, 24, 25, 26
Swine influenza (H1N1) is spread primarily via aerosols/droplets and to a lesser extent via hand-to-face transmission. Avian (H5N1) and swine influenza (H1N1) may be transmitted in aircraft (unrecirculated cabin air) via aerosol/droplet transmission. Because swine influenza (H1N1), like avian influenza (H5N1), is often accompanied by gastrointestinal symptoms (ie, diarrhea), there may be potential for viral spread from feces. As with seasonal human influenza A, swine influenza (H1N1) may be spread nosocomially.27
Clinical presentation
Mild to moderate influenza is clinically indistinguishable from illnesses caused by other respiratory viruses that present as influenzalike illnesses (ILIs). Peak incidence of influenza historically is in February in the northern hemisphere, but the influenza season may start earlier and last into early spring. Avian influenza (H5N1) has a nonseasonal distribution, and swine influenza (H1N1) has occurred in summer and winter. Excluding pandemics and epidemics, influenza is a relatively mild 3-day illness, that is, a mild respiratory infection with a high attack rate and low mortality. Pandemic influenza also has a high attack rate, but due to highly virulent strains has a high mortality.22, 23, 24, 25, 28
In adults, severe influenza A has a distinctive clinical presentation, which is not easily confused with other causes of viral pneumonia.23, 29, 30 Like severe acute respiratory syndrome (SARS), hantavirus pulmonary syndrome (HPS), and adenovirus, severe influenza A pneumonia begins abruptly, but influenza A patients often can recall the exact time of onset of the illness. Severe human seasonal influenza A is accompanied by fever higher than 39°C/102°F and chills, accompanied by severe myalgias and dry cough with or without hemoptysis. Purulent sputum suggests superimposed bacterial community-acquired pneumonia (CAP) due to MSSA/CA-MRSA. Another noteworthy feature of human seasonal influenza is severe debilitating fatigue rapidly making victims bedridden. Prostration is profound and is accompanied by headache and prominent myalgias. Some infections present with myalgias, but the myalgias of influenza are peculiarly severe and are localized to the neck and back. Retro-orbital pain is common, and conjunctival suffusion may be present in severe seasonal human influenza A. Conjunctival suffusion may be present in avian influenza (H5N1) but is uncommon with swine influenza (H1N1). The clinical constellation of an ILI with fever higher than 39°C/102°F and severe myalgias especially of the neck/back, with profound prostration, clinically differentiates severe influenza A in adults from ILIs (Table 1 ).22, 23, 24, 25, 28, 30
Table 1.
Winthrop-University Hospital Infectious Disease Division's point system for diagnosing severe influenza A in adults (modified)
| Symptomsa | Point Score | Signsa | Point Score | Laboratory Testsa | Point Score |
|---|---|---|---|---|---|
| Hyperacute onset | +3 | Fever (>39°C/102°F) | +2 | Leukocytosisc | −5 |
| Severe prostration | +5 | Dry cough | +1 | Leukopenia | +3 |
| Generalized muscle aches | +3 | Conjunctival suffusion | +5 | Relative lymphopenia | +3 |
| Retro-orbital pain | +5 | Hemoptysis | +3 | Thrombocytopenia | +3 |
| Severe back of neck/lumbar aches | +5 | Localized ralesb | −3 | Chest radiograph | |
| Cyanosis | +5 | No/minimal infiltrates (<48 hours) | +3 | ||
| Bilateral patchy infiltratesb (>48 hours) | +5 | ||||
| Focal/segmental infiltratesb | −5 | ||||
| Likelihood of severe influenza A | |||||
| Total points | >20 = Severe influenza A highly probable 10–20 = Mild/moderate influenza A likely <10 = Influenza A unlikely |
||||
Adapted from Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008;36:92–3; Cunha BA. Pneumonia essentials. 3rd edition. Sudbury (MA): Jones & Bartlett; 2010.
Otherwise unexplained, acute, and related to influenza.
Unless with bacterial CAP.
Leukocytosis without relative lymphopenia and thrombocytopenia.
The clinical presentation of avian influenza (H5N1) resembles severe human influenza A in adults and affects primarily young healthy adults, whereas nonepidemic pandemic/epidemic human influenza A primarily affects the very young, elderly, and debilitated. Pandemic influenza A affects primarily young healthy adults (ie, “pandemic signature”) rather than the very young, elderly, and immunocompromised. Swine influenza (H1N1) has demonstrated its “pandemic signature,” that is, most fatal cases have occurred in young healthy adults.28, 31
Several nonspecific laboratory test abnormalities are associated with human influenza A in adults. The severity of human influenza A is related to the degree/duration of leukopenia and relative lymphopenia.31, 32 The nonspecific laboratory test hallmark of influenza A (human, avian, and swine) is otherwise unexplained relative lymphopenia. Relative lymphopenia is an inconsistent feature of swine influenza (H1N1) in children; lymphocytosis is more common. In adults, relative lymphopenia is usually accompanied by thrombocytopenia. In adults with human and avian (H5N1) influenza, leukopenia if present may be an indicator of severity. Leukopenia in swine influenza (H1N1) when present usually occurs with relative lymphopenia and thrombocytopenia.33 Atypical lymphocytes are not a usual feature of swine influenza (H1N1) in adults but may be present in children.
From a radiographic perspective, influenza (human, avian, swine) has no distinctive features. Initially, the chest radiograph (CXR) shows no or minimal infiltrates. Later, (>48 hours) bilateral patchy interstitial infiltrates appear as influenza pneumonia progresses. The presence of focal segmental/lobar infiltrates on the admission CXR of adults with influenza indicates simultaneous bacterial CAP, due to Staphylococcus aureus (MSSA/CA-MRSA) CAP.30 Patients with influenza/ILI and simultaneous S aureus CAP present with purulent sputum, high spiking fevers, often with cyanosis/hypotension. S aureus CAP, whether due to methicillin-sensitive S aureus (MSSA) or methicillin-resistant S aureus (MRSA), presents in the same way clinically and on CXR. The CXR of S aureus (MSSA/MRSA) CAP in patients with influenza pneumonia are characterized by rapid cavitation in 72 hours or less. In adults, S aureus (MSSA/MRSA) CAP occurs only in patients with influenza/ILI (Table 2 ).30, 31, 34, 35, 36, 37, 38
Table 2.
Clinical presentations and diagnostic features of severe influenza A pneumonia
| Influenza Pneumonia | Influenza withSimultaneous Bacterial CAP | Influenza Followed by Sequential Bacterial CAP | |
|---|---|---|---|
| Usual pathogen | Influenza Aa | Influenza A withStaphylococcus aureus (MSSA/CA-MRSA) CAP | Influenza A followed byStreptococcus pneumoniae or Haemophilus influenzae CAP |
| Presentation of CAP | Subacute/acute | Acute | Influenza then an interval of clinical improvement (5–7 days) followed by CAP |
| Symptoms | Severe myalgias (neck/back)Debilatating fatigueRetro-orbital painDry cough (± mild hemoptysis)Shortness of breath ± pleuritic chest pain | Same as influenza A plus hemoptysis, productive cough/purulent sputum ± pleuritic chest pain | After 5–7 days following influenza, new fevers and productive cough/purulent sputum ± pleuritic chest pain |
| Signs | FeverConjunctival suffusion Dyspnea (± cyanosis) No rales | Same as influenza plus localized rales ± consolidation | Localized rales ± consolidation |
| Laboratory tests | Hypoxemia (A-a gradient >35)Relative lymphopenia Thrombocytopenia± Leukopenia Sputum: WBC with normal/or no flora | Same as influenza plus LeukocytosisSputum: WBCs with Gram + cocci (in clusters) | Minimal/no hypoxemia (A-a gradient <35)LeukocytosisSputum: WBCs with Gram + cocci (in pairs) or GNBs |
| Chest radiograph | No infiltrates (early)Bilateral patchy interstitial infiltrates (later) No/small pleural effusion(s) | Focal segmental/lobar infiltrates with rapid cavitation <72 h | Focal segmental/lobar infiltrates without cavitation ± consolidation± pleural effusion |
| Mortality | +++ | ++++ | + |
Abbreviations: A-a, alveolar arterial gradient; CAP, community-acquired pneumonia; GNBs, gram-negative bacilli; MRSA, methicillin-resistant S aureus; MSSA, methicillin-sensitive S aureus; WBC, white blood cell count.
Data from Cunha BA. Pneumonia essentials. 3rd edition. Sudbury (MA): Jones & Bartlett; 2010.
Uncomplicated influenza is a 3-day illness.
Gastrointestinal symptoms (ie, nausea, vomiting, or diarrhea) are more common with avian and swine influenza (H1N1) than with human influenza. Serum aspartate and alanine transaminases (AST/ALT) are often mildly/transiently elevated in avian (H5N1) and swine influenza (H1N1). Another key laboratory finding in swine influenza (H1N1) patients is an elevated creatine phosphokinase (CPK). Some patients with highly elevated CPKs may also have rhabdomyolysis. Seasonal human influenza A and avian influenza A (H5N1) may have mild to moderate elevations of the serum lactate dehydrogenase (LDH). Whereas certain nonspecific laboratory abnormalities are typical of swine influenza (H1N1), for example, otherwise unexplained relative lymphopenia, thrombocytopenia, mildly elevated AST/ALT, and elevated CPKs, other findings argue against the diagnosis, for example, elevated serum ferritin levels, hypophosphatemia, elevated cold agglutinin titers, and so forth.30, 31, 32, 34 In influenza (human, avian, swine) pneumonia, hypoxemia is a reflection of the severity of influenza in the interstitium of the lungs. Viral involvement of the lung interstitium may result in an oxygen diffusion defect manifested as hypoxemia and an increased A-a gradient. The greater the degree/duration of hypoxemia (A-a gradient >35), the more severe and potentially fatal is influenza (human, avian, swine) pneumonia.30, 31
As with avian influenza (H5N1), hospitalized adults with swine influenza (H1N1) pneumonia rarely present with or subsequently develop bacterial pneumonia CAP.33, 36 During the 1957 to 1958 Asian influenza A pandemic, some patients presented with influenza A pneumonia and simultaneous S aureus CAP. Still others, especially the elderly, later developed Streptococcus pneumoniae or Haemophilus influenzae CAP 1 to 2 weeks into their recovery. As with avian influenza (H5N1), young healthy adults with swine influenza (H1N1) pneumonia, have only rarely presented with superimposed S aureus (MSSA/CA-MRSA) CAP. Adult swine influenza (H1N1), like avian influenza (H5N1) pneumonia simultaneous or subsequent bacterial CAP remains relatively rare.36, 37, 38, 39, 40
Differential diagnosis
The differential diagnosis of severe viral CAPs in normal hosts includes cytomegalovirus (CMV), and adenovirus, and in compromised hosts RSV and CMV. Viral pneumonias presenting as severe CAPs have a similar radiological appearance, that is, no/minimal infiltrates early (<48 hours), followed later (>48 hours) by bilateral diffuse patchy interstitial infiltrates. Adult influenza A pneumonia (human, avian, swine) as well as other severe viral CAPs are accompanied by variable degrees of hypoxemia and a high A-a gradient >35. Bacterial CAP rarely, if ever, complicates severe CMV or adenoviral CAP.30
In general, viral pneumonias are not accompanied by pleuritic chest pain because pathophysiologically they are interstitial and not pleurally based but influenza A may be accompanied by bilateral “pleuritic” chest pain. The “pleuritic chest pain” of influenza A pneumonia is due to direct viral involvement of the intercostal muscles mimicking pleuritic chest pain. The pleuritic chest pain of bacterial CAPs is unilateral and related to the location of the underlying infiltrate.30 Influenza/ILI complicated by simultaneous CA-MRSA/MSSA CAP is characterized by a necrotizing pneumonia with rapidly cavitating infiltrates on CXR with or without pleuritic chest pain. MSSA/CA-MRSA (PVL+ strains) with influenza/ILI is often accompanied by cyanosis/hypotension (see Table 2).36, 37, 38, 39, 40
Swine influenza (H1N1) resembles avian influenza (H5N1) in its predilection for young healthy adults.39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 The epidemiologic clue to avian influenza (H5N1) is recent close contact with infected poultry or people in Europe or Asia. The diagnosis of avian influenza (H5/N1) may be missed if the hemagglutinin inhibition (HI) test is used to diagnose influenza A because this test is insensitive to avian hemagglutinins.30, 39 The diagnosis of avian influenza (H5N1) is by hemagglutinin-specific reverse transcription-polymerase chain reaction (RT-PCR) for avian influenza (H5N1).
The other illness that does not clinically resemble influenza A is SARS. Unlike adult human seasonal influenza A, loose stools/diarrhea occurs in some and the illness lasts longer than 3 days. In contrast to influenza/avian influenza, the white blood cell (WBC) count and platelet counts in SARS are usually normal but may be slightly decreased. Relative lymphopenia is present, as are mild increases in the serum transaminases (AST/ALT). Like seasonal human influenza A, elevations of the CPK and LDH are not uncommon. Diagnosis of SARS is by specific serology or viral isolation.30, 51
The clinical clue to SARS is the recent contact history with infected poultry or someone with SARS from Europe or Asia. Unlike seasonal human influenza A, SARS is a biphasic infection. At onset, fever decreases after a few days and the patient improves, but unlike seasonal human influenza A, fever recurs in a few days when the patient re-presents with signs and symptoms of viral pneumonia ie, CXR shows bilateral patchy interstitial infiltrates. Like avian influenza (H5N1), SARS has not been complicated by bacterial CAP.30, 50, 51
In the differential diagnosis of influenza/ILIs, HPS should be considered if there has been close contact usually after about 2 weeks postexposure to a rodent.52 Like SARS, HPS is a biphasic illness, that is, the initial phase is the febrile phase, followed by the cardiopulmonary phase. Later, HPS patients progress through an oliguric/diuretic phase and finally to a convalescent phase. The distinguishing clinical feature of HPS is noncardiogenic pulmonary edema. HPS is not a 3-day illness like influenza. In HPS leukocytosis (up to 90,000/mm3) rather than leukopenia is the rule. The nonspecific laboratory hallmark of HPS is the presence of immunoblasts.30, 52 Diagnosis of HPS is by IgM enzyme-linked immunosorbent assay or RT-PCR (Table 3 ).
Table 3.
Differential diagnosis of severe influenza/influenza like illnesses
| Clinical Features | Influenza (Human Seasonal/Swine) | Avian Influenza (H5N1) | SARS | HPS |
|---|---|---|---|---|
| Epidemiology | 2 d | <7 d | 5 d | 4 d |
| Incubation period (mean) | (1–4 d) | (2–5 d) | (2–10 d) | (2–15 d) |
| Recent exposure | ||||
| Influenza | + | − | + | − |
| Birds | − | + | + | − |
| Rodents | − | − | − | + |
| Asian travel | − | + | + | − |
| Symptoms | ||||
| Biphasic illness | − | + | ± | − |
| Fever/chills | + | + | + | + |
| Profound weakness | + | − | − | + |
| Headache/muscle aches | + | + | + | + |
| Dry cough | + | + | − | + |
| Sore throat | + | + | − | − |
| Runny nose | + | ± | − | − |
| Hemoptysis | ± | ± | − | − |
| SOB → early | + | + | − | − |
| → late | ± | ± | + | + |
| Substernal discomfort/burning | ± | − | − | ± |
| Pleuritic chest pain | ± | − | − | + |
| Loose stools/diarrhea | ± | + | ± | + |
| Abdominal pain | − | − | − | + |
| Signs | ||||
| Fever >39°C/102°F | + | + | ± | ± |
| Conjunctival suffusion | + | + | + | − |
| Injected oropharynx | + | + | − | +a |
| Laboratory testsf | ||||
| Leukopenia | ±∗ | + | − | +d |
| Relative lymphopenia | + | + | + | − |
| Atypical lymphocytes | − | − | − | − |
| Immunoblasts | − | − | − | + |
| Thrombocytopenia | ± | ± | ± | + |
| Mildly elevated serum transaminases (AST/ALT) | ± | + | + | + |
| Elevated LDH | − | + | + | + |
| Elevated CPK | + | + | + | + |
| CXR | ||||
| Minimal/no infiltrates (early) | + | + | + | + |
| Bilateral patchy infiltrates (late) | + | + | + | +e |
| Focal segmental/lobar infiltrates | −b | −b | +c | − |
Abbreviations: CPK, creatinine phosphokinase; CXR, Chest radiograph; HPS, hantavirus pulmonary syndrome; LDH, lactate dehydrogenase; SARS, severe acute respiratory syndrome; SOB, shortness of breath.
*Usually normal WBC count.
Data from Cunha BA. Pneumonia essentials. 3rd edition. Sudbury (MA): Jones & Bartlett; 2010.
With exudates.
Unless bacterial CAP.
Infiltrates often ovoid or round.
Leukocytosis later with hemoconcentration and increase in severity.
Noncardiogenic pulmonary edema.
HI test for influenza A negative in avian influenza (H5N1); use PCR to diagnose avian influenza (H5N1).
Clinical diagnosis
Sporadic seasonal human influenza A cases may occur throughout the year, but influenza activity in the northern hemisphere peaks in February (November–March), and in the southern hemisphere in July (May–September). Influenza occurring during the peak influenza season is often more severe than that which occurs sporadically throughout the year.22, 23, 24, 25
In adults with swine influenza (H1N1), pneumonia presents in adults as an ILI with a temperature higher than 39°C (102°F) accompanied by prominent myalgias; these are the key clinical findings. Many patients also have headache, sore throat, or dry cough, with or without loose stools/diarrhea. Conjunctival suffusion is rare. The onset of swine influenza (H1N1) pneumonia is often abrupt but a preceeding ILI prodrome is common. Mild cases of swine influenza (H1N1) are indistinguishable from ILIs. Adult patients who require hospitalization have characteristic clinical nonspecific laboratory abnormalities.17, 18, 19, 20
In adults hospitalized with swine influenza (H1N1) pneumonia, otherwise unexplained relative lymphopenia is uniformly present.34 However, clinicians must be careful to not ascribe relative lymphopenia in patients to swine influenza (H1N1) pneumonia until the patient's medical history is reviewed for other disorders associated with relative lymphopenia. Besides human seasonal and avian influenza other infectious causes of relative lymphopenia include CMV, human herpesvirus (HHV)-6, HHV-8, human immunodeficiency virus (HIV), miliary tuberculosis (TB), Legionnaire's disease, typhoid fever, Q fever, brucellosis, SARS, malaria, babesiosis, Rocky Mountain spotted fever (RMSF), histoplasmosis, dengue fever, Chickungunya fever, ehrlichiosis, parvovirus B19, HPS, West Nile encephalitis (WNE), and viral hepatitis (early). Noninfectious causes of relative lymphopenia include cytotoxic drugs, steroids, sarcoidosis, systemic lupus erythematosus (SLE), lymphoma, rheumatoid arthritis (RA), radiation therapy, Wiskott-Aldrich syndrome, Whipple's disease, severe combined immunodeficiency disease (SCID), common variable immune deficiency (CVID), Di George's syndrome, Nezelof's syndrome, intestinal lymphangiectasia, constrictive pericarditis, tricuspid regurgitation, Kawasaki's disease, idiopathic CD4 cytopenia, Wegener's granulomatosis, acute/chronic renal failure, hemodialysis; myasthenia gravis, celiac disease, alcoholic cirrhosis, coronary bypass, congestive heart failure (CHF), acute pancreatitis, and carcinomas.30
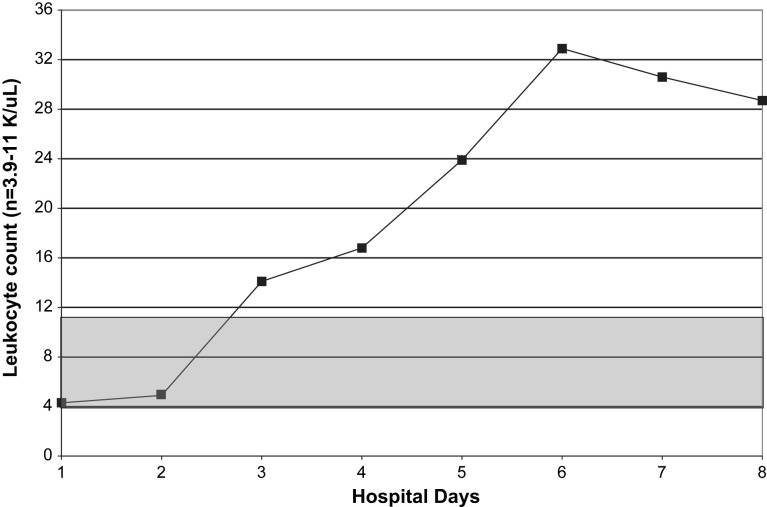
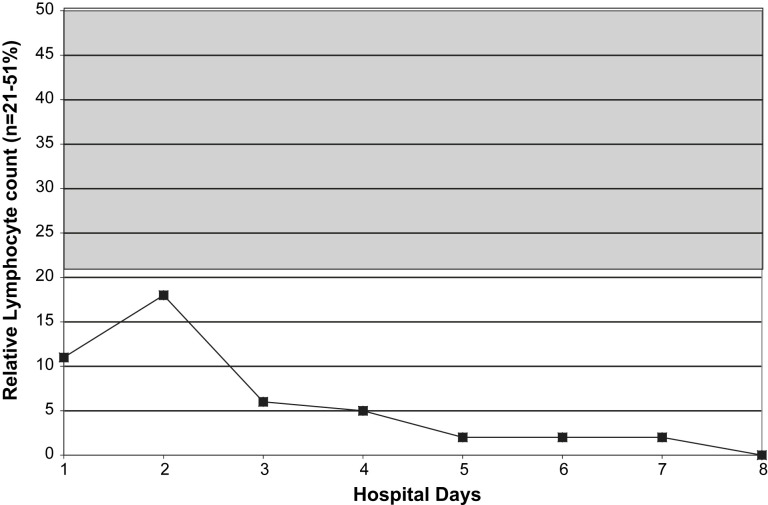
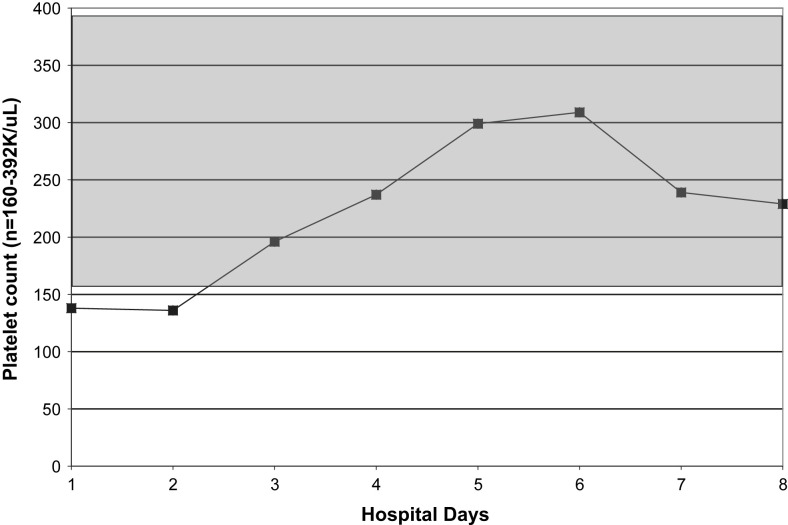
Relative lymphopenia with swine influenza (H1N1) pneumonia may be profound and prolonged. The degree/duration of relative lymphopenia also has prognostic implications. Typically, an increase in the percentage of lymphocytes precedes clinical improvement.30 Atypical lymphocytes are present in a variety of viral illnesses but are not usually present in adults with influenza A or avian influenza (H5N1). Also, atypical lymphocytes are not a feature of adult swine influenza (H1N1) but may be present in children with swine influenza (H1N1).30 With swine influenza (H1N1) pneumonia, thrombocytopenia does not occur as an isolated entity but accompanies/follows relative lymphopenia. Leukocytosis, rather than leukopenia, is the rule in hospitalized adults with swine influenza (H1N1) pneumonia. When leukopenia is present, it occurs together with the relative lymphopenia or thrombocytopenia. Also, as with severe seasonal human influenza A, leukopenia may rarely be an isolated finding in fatal cases of swine influenza (H1N1) pneumonia in adults (Fig. 1, Fig. 2, Fig. 3, Fig. 4 ).30, 53
Fig. 1.
Serial WBC counts in a case of fatal swine influenza (H1N1) pneumonia. (From Cunha BA, Syed U, Mikail N. Rapid clinical diagnosis in fatal swine influenza (H1N1) pneumonia in adult with negative rapid influenza diagnostic tests (RIDTs): diagnostic swine influenza triad. Heart & Lung 2010;39:78–86; with permission.)
Fig. 2.
Relative lymphopenia in a case of fatal swine influenza (H1N1) pneumonia. (From Cunha BA, Syed U, Mickail N, et al. Rapid clinical diagnosis in fatal swine influenza (H1N1) pneumonia in an adult with negative rapid influenza diagnostic tests (RIDTs): Diagnostic swine influenza triad. Heart Lung 2010;39:78–86: with permission.)
Fig. 3.
Serial platelet counts in a case of fatal swine influenza (H1N1) pneumonia. (From Cunha BA, Syed U, Mickail N, et al. Rapid clinical diagnosis in fatal swine influenza (H1N1) pneumonia in an adult with negative rapid influenza diagnostic tests (RIDTs): Diagnostic swine influenza triad. Heart Lung 2010;39:78–86: with permission.)
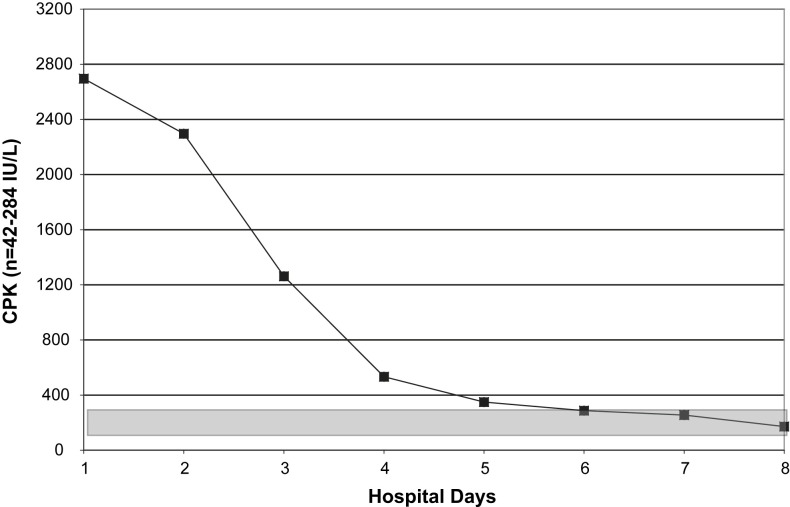
Fig. 4.
Serial CPK in a case of fatal swine influenza (H1N1) pneumonia. (From Cunha BA, Syed U, Mickail N, et al. Rapid clinical diagnosis in fatal swine influenza (H1N1) pneumonia in an adult with negative rapid influenza diagnostic tests (RIDTs): Diagnostic swine influenza triad. Heart Lung 2010;39:78–86; with permission.)
Another important nonspecific laboratory clue in adult influenza (human, swine, and avian) are elevated CPKs. Severe swine influenza (H1N1) pneumonia, like seasonal human influenza A and avian influenza (H5N1) pneumonia, may be accompanied by high elevations of CPK, with or without rhabdomyolysis. Highly elevated cold agglutinin titers are not a feature of swine influenza (H1N1) pneumonia in adults, and their presence should suggest another diagnosis, for example, Mycoplasma pneumoniae CAP. In adults with swine influenza (H1N1) pneumonia, serum transaminases (AST/ALT) are often transiently mildly/moderately elevated. Highly elevated serum ferritin levels or hypophosphatemia are not features of swine influenza (H1N1) pneumonia and should suggest an alternate diagnosis, for example, Legionnaire's disease. In adults, a quick way to differentiate ILIs from swine influenza (H1N1) pneumonia is a fever higher than 39°C/102°F accompanied by severe myalgias with otherwise unexplained relative lymphopenia thrombocytopenia, elevated serum transaminases (AST/ALT) or an elevated CPK.30, 53
A diagnostic weighted point score system was developed at Winthrop-University Hospital by the Infectious Disease Division during the “herald wave” of swine influenza (H1N1) pandemic to clinically differentiate admitted adults with swine influenza (H1N1) pneumonia from ILIs and other patients with cardiac/pulmonary symptoms. While the diagnostic point score system was accurate and useful early in the pandemic, a more simplified/rapid approach was developed; the swine influenza diagnostic triad. The Winthrop-University Hospital Infectious Disease Division's diagnostic triad was then used as the basis for making a probable clinical diagnosis of swine influenza (H1N1) in rapid influenza diagnostic tests (RIDT)-negative hospitalized adults, to determine which patients should be on influenza precautions and treated with oseltamivir (Table 4 , Box 1 ).54
Table 4.
Swine influenza (H1N1) pneumonia: Winthrop-University Hospital Infectious Disease Division's clinical weighted diagnostic point score system for adults and negative rapid influenza diagnostic tests (RIDTs)
| Adults with an ILI with dry cough, fever >39°C/102°F and a CXR with no focal/segmental lobar infiltrates and negative RIDTsa: | |
| Key clinical finding: | |
|
+5 |
| +5 | |
|
+3 |
|
+2 |
|
+5 |
| Argues against the diagnosis of swine influenza (H1N1) pneumonia: | |
|
−5 |
|
−2 |
|
−1 |
|
−5 |
|
−3 |
| Swine influenza Diagnostic Point Score totals: | Maximum score: 20 |
| Probable swine influenza (H1N1) pneumonia | >15 |
| Possible swine influenza (H1N1) pneumonia | 10–15 |
| Unlikely swine influenza (H1N1) pneumonia | <10 |
Data from Cunha BA, Syed U, Stroll S, et al. Winthrop-University Hospital Infectious Disease Division's swine influenza (H1N1) pneumonia diagnostic weighted point score system for hospitalized adults with influenza-like illnesses (ILIs) and negative rapid influenza diagnostic tests (RIDTs). Heart Lung 2009;38:534–8.
Diagnostic tests negative for all other viral CAP pathogens (CMV, SARS, HPS, RSV metapneumoviruses, parainfluenza viruses, adnoviruses).
Other causes of relative lymphopenia: Infectious causes: CMV, HHV-6, HHV-8, HIV, military TB, Legionella, typhoid fever, Q fever, brucellosis, SARS, malaria, babesiosis, influenza, avian influenza, RMSF, histoplasmosis, dengue fever, Chickungunya fever, ehrlichiosis, parvovirus B19, HPS, WNE, viral hepatitis (early); Noninfectious causes: cytotoxic drugs, steroids, sarcoidosis, SLE, lymphoma, RA, radiation therapy, Wiskott-Aldrich syndrome, Whipple disease, severe combine immunodeficiency disease (SCID), common variable immune deficiency (CVID), Di George's syndrome, Nezelof's syndrome, intestinal lymphangiectasia, constrictive pericarditis, tricuspid regurgitation, Kawasaki's disease, idiopathic CD4 cytopenia, Wegener's granulomatosis, acute/chronic renal failure, hemodialysis; myasthenia gravis, celiac disease, alcoholic cirrhosis, coronary bypass, CHF, acute pancreatitis, carcinomas (terminal).
Otherwise unexplained.
Box 1. Swine influenza (H1N1) pneumonia: case definitions in hospitalized adults.
Definite swine influenza (H1N1) pneumonia (laboratory criteria)
- ILI with dry cough, temperature higher than 39°C/102°F and a CXR with no focal/segmental lobar infiltrates plus one or more of these positive tests:
-
•Rapid influenza A test
-
•Respiratory fluorescent antibody (FA) viral panel
-
•RT-PCR for swine influenza (H1N1)
-
•
Probable swine influenza (H1N1) pneumonia (clinical criteria)
- ILI with temperature higher than 39°C/102°F with severe myalgias and a CXR with no focal/segmental lobar infiltrates with negative influenza tests (see above)a plus this diagnostic triad: (any 3)
-
•Relative lymphopeniab
-
•Thrombocytopeniab
-
•Elevated serum transaminasesb
-
•Elevated CPKsb
-
•
aDiagnostic tests negative for other viral CAP pathogens (CMV, SARS, HPS, RSV metapneumoviruses, parainfluenza viruses, adenoviruses).
bOtherwise unexplained.
Data from Cunha BA, Syed U, Stroll S, et al. Winthrop-University Hospital Infectious Disease Division's swine influenza (H1N1) pneumonia diagnostic weighted point score system for hospitalized adults with influenza-like illnesses (ILIs) and negative rapid influenza diagnostic tests (RIDTs). Heart Lung 2009;38:534–8.
Also, a key component in the Winthrop-University Hospital Infectious Disease Division's diagnostic swine influenza triad for the diagnosis of hospitalized adults with swine influenza (H1N1) pneumonia was dry cough, fever, and a CXR without focal segmental/lobar infiltrates.30, 53 Adult patients presenting with an ILI, fever, and shortness of breath with an admission CXR with focal segmental/lobar infiltrates invariably had an alternate diagnosis, namely, CHF, asthma, acute exacerbation of chronic bronchitis (AECB), preexisting lung disease, or bacterial CAP. In an adult hospitalized with an ILI without the diagnostic swine influenza triad, focal segmental/lobar infiltrate on the admission CXR effectively rules out the diagnosis of swine influenza (H1N1) pneumonia.33
As the result of the experience in the 1957 to 1958 Asian influenza pandemic, there has been concern about the potential for simultaneous or sequential bacterial CAPs with pandemic influenza A. Patients presenting with human influenza A and CXR infiltrates that rapidly cavitate (within <72 hours) should suggest MSSA/CA-MRSA CAP superimposed underlying influenza/ILI. This presentation has been reported in the literature sporadically with seasonal human influenza A during the past few years.55, 56, 57, 58 In adults, seasonal human influenza A pneumonia may also be complicated by subsequent bacterial CAP after a period of improvement (usually 5–7 days). Adults with human seasonal influenza A improve, then may re-present with new onset of fever and new focal segmental/lobar infiltrates on CXR. The pathogen in this setting is either S pneumoniae or H influenzae, not S aureus (MSSA/CA-MRSA). Adults with influenza A pneumonia presenting simultaneous MSSA/CA-MRSA CAP are critically ill, with high spiking fevers, cyanosis, and hypotension and a fatal outcome is not uncommon. However, most admitted adult patients with severe influenza A pneumonia have not been complicated by simultaneous or subsequent bacterial CAP.
In the recent avian influenza (H5N1) pneumonia experience in Europe and Asia, this has also been the case, that is, severe or fatal cases have not been complicated by simultaneous or subsequent bacterial CAP.41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Deaths from severe avian influenza (H5N1) pneumonia have been due to severe hypoxemia, which is also the case with most fatal cases of seasonal human influenza A. In the “herald wave” of recent pandemic, the majority of swine influenza (H1N1) pneumonia deaths occurred primarily in young healthy adults without comorbidities.56 The cause of death in most fatal swine influenza (H1N1) pneumonia cases has been due to severe hypoxemia and not due to simultaneous/subsequent bacterial CAP.35, 36, 37, 38, 39, 40
The admission CXR is of critical importance in evaluating swine influenza (H1N1) pneumonia. Because CAP coinfections are rare, the presence of a focal segmental/lobar infiltrates should suggest another diagnosis, for example, bacterial CAP. In hospitalized adults with swine influenza (H1N1) pneumonia, the admission CXR typically is clear without infiltrates or shows an accentuation of basilar markings/atelectasis which may be unilateral or bilateral. On CXR in severe cases, within 48 hours there is rapid progression to bilateral patchy interstitial infiltrates. Unilateral or bilateral small pleural effusions are not uncommon. Adult hospitalized patients with swine influenza (H1N1) pneumonia who present with, or rapidly develop bilateral patchy interstitial infiltrates, may also have small pleural effusions. Consolidation is unusual and if present it occurs, later in patients on ventilatory support. Cavitation is not a feature of swine influenza (H1N1) pneumonia in adults.33
The Winthrop-University Hospital Infectious Disease Division's diagnostic swine influenza triad has been useful for the rapid clinical presumptive diagnosis of swine influenza (H1N1) pneumonia in hospitalized adults with negative RIDTs and a negative CXR, that is, accentuated basilar markings/atelectasis, but not focal segmental/lobar infiltrates, and differentiates swine influenza (H1N1) pneumonia from ILIs as well as bacterial pneumonias.33, 54
Mimics of Swine Influenza (H1N1) Pneumonia
During “herald wave” of the swine influenza (H1N1) pandemic, an unexplained increase in Legionnaire's disease occurred before the usual late summer/early fall peak incidence of legionella CAP. This may be analogous to the increased incidence/severity of acute bacterial appendicitis during human influenza A/B outbreaks. Regardless of the mechanism, clinicians should be alert for Legionnaire's disease mimicing swine influenza (H1N1) pneumonia.
In adults the best non-specific laboratory test markers helpful in differentiating Legionnaire's disease from swine influenza (H1N1) pneumonia are the presence of focal segmental/lobar infiltrates on CXR, relative bradycardia, hypophosphatemia, and highly elevated ferritin levels. Serum procalcitonin levels (PCT) are not elevated in swine influenza (H1N1) pneumonia, but are elevated in Legionnaire's disease. The other atypical CAP that may be mimic Legionnaire's disease or swine influenza (H1N1) pneumonia is Q fever. Q fever CAP has many of the features of Legionnaire's disease, namely, elevated serum ferritin levels, mildly increased serum transaminases (AST/ALT), and relative bradycardia. However, Q fever CAP is often accompanied by splenomegaly, which is not a feature of Legionnaire's disease or swine influenza (H1N1) pneumonia. As with M pneumoniae, elevated cold agglutinins may occur with Q fever CAP but are not a feature of Legionnaire's disease or swine influenza (H1N1) pneumonia. Relative lymphopenia is common with Legionnaire's disease, Q fever, and swine influenza (H1N1) pneumonia, but leukopenia and thrombocytopenia are not laboratory markers of either Legionnaire's disease or Q fever CAP.30, 33, 55
Laboratory diagnosis
Influenza virus is present in respiratory secretions early in the illness. Nasopharyngeal swabs or washings may be used for viral isolation. In general, higher concentrations of virus are found in nasal secretions than in oropharyngeal secretions. During influenza A epidemics, oropharyngeal secretions contain higher concentrations of influenza virions than nasal secretions. In alveolar macrophages, influenza A viruses produce cytopathogenic effects (CPEs), but are nonspecific and difficult to detect. CPEs due to influenza A are apparent in approximately 50% of cultures within 3 days of inoculation and in approximately 90% within 5 days. Identification of cells showing CPE may be verified using immunofluorescent technique or type/subtype specific antisera. The most common rapid method of influenza laboratory diagnosis is by FA techniques. For FA testing for respiratory viruses, nasopharyngeal aspirates are preferred to swabs. FA influenza assays identify viral antigens in infected respiratory epithelial cells.22, 23, 24, 25
During the “herald wave” of the swine influenza (H1N1) pandemic in New York, it was realized that there were problems with screening tests, that is, RIDTs. It was hoped that rapid screening tests for influenza A would detect swine influenza (H1N1), an influenza A virus. Rapid influenza A test positivity was usually predictive of RT-PCR positivity for swine influenza (H1N1). It was quickly realized that negative rapid influenza A testing did not rule out swine influenza (H1N1). False negative rapid influenza A testing for swine influenza (H1N1) was 30%. Respiratory FA viral testing detects influenza A, influenza B, metapneumoviruses, RSVs, parainfluenza viruses, and adenoviruses. Unfortunately, respiratory FA viral testing did not correlate with either rapid influenza A testing or RT-PCR testing. Although the definitive test for swine influenza (H1N1) diagnosis remains RT-PCR, during the pandemic, RT-PCR testing was restricted. Restricted RT-PCR testing resulted in tremendous difficulties in making/ruling out the diagnosis of swine influenza (H1N1) and in initiating/discontinuing influenza precautions.53, 54, 55, 59
Because of restricted swine influenza (H1N1) RT-PCR testing, clinical criteria were developed for patients presenting with ILIs who had a negative rapid influenza A test. By combining key clinical and nonspecific laboratory features, a clinical diagnosis of probable swine influenza was developed at Winthrop-University Hospital by the Infectious Disease Division. This permitted an operational clinical approach to placing admitted adult patients on influenza precautions/treatment, and was also useful in differentiating swine influenza (H1N1) from its mimics.54 (see Box 1, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Table 3, Table 4, Table 5 ).
Table 5.
Winthrop-University Hospital Infectious Disease Division's swine influenza (H1N1) pneumonia diagnostic weighted point system in adults with negative rapid influenza diagnostic tests (RIDTs)
| Clinical Features | Point Scores | Swine Influenza (H1N1) Laboratory Diagnosed | Swine Influenza (H1N1) Clinically Diagnosed | ILIs not Swine Influenza (H1N1) | CMV CAP | Q Fever CAP | Legionella CAP |
|---|---|---|---|---|---|---|---|
| Adults with an ILI with dry cough, fever >39°C/102°F and a CXR with no focal/segmental lobar infiltratesa | |||||||
|
+5 | +5 | +5 | 0 | 0 | 0 | 0 |
|
+5 | +5 | +5 | 0 | +5 | +5 | +5 |
|
+3 | +3 | +3 | 0 | 0 | 0 | +5 |
|
+2 | +2 | +2 | 0 | +2 | +2 | +2 |
|
+5 | +5 | +5 | 0 | +5 | +2 | 0 |
| Argues against the diagnosis of (H1N1): | |||||||
|
−5 | 0 | 0 | 0 | 0 | 0 | −5 |
|
−2 | 0 | 0 | 0 | 0 | 0 | 0 |
|
−1 | 0 | 0 | 0 | 0 | 0 | 0 |
|
−5 | 0 | 0 | 0 | 0 | 0 | −5 |
|
−3 | 0 | 0 | 0 | 0 | 0 | −3 |
| Swine influenza Diagnostic Point Score totals: | Total score: | 20 | 20 | 0 | 12 | 9 | −1 |
| Probable swine influenza (H1N1) pneumonia = >15 Possible swine influenza (H1N1) pneumonia = 10–15 Unlikely swine influenza (H1N1) pneumonia = <10 | |||||||
Abbreviation: ILIs, Influenzalike illnesses.
Data from Cunha BA, Syed U, Stroll S, et al. Winthrop-University Hospital infectious disease division's swine influenza (H1N1) pneumonia diagnostic weighted point score system for adults with Influenza Like Illnesses (ILIs) and negative Rapid Influenza Diagnostic Tests (RIDTs). Heart Lung 2009;38:534–8.
Q fever and legionnaire's disease CAPs usually have focal segmental/labor infiltrates.
Other causes of relative lymphopenia: Infectious causes: CMV, HHV-6, HHV-8, HIV, military TB, Legionella, typhoid fever, Q fever, brucellosis, SARS, malaria, babesiosis, influenza, avian influenza, RMSF, histoplasmosis, dengue fever, Chickungunya fever, ehrlichiosis, parvovirus B19, HPS, WNE, viral hepatitis (early); Noninfectious causes: cytotoxic drugs, steroids, sarcoidosis, SLE, lymphoma, RA, radiation therapy, Wiskott-Aldrich syndrome, Whipple disease, severe combine immunodeficiency disease (SCID), common variable immune deficiency (CVID), Di George syndrome, Nezelof syndrome, intestinal lymphangiectasia, constrictive pericarditis, tricuspid regurgitation, Kawasaki disease, idiopathic CD4 cytopenia, Wegener granulomatosis, acute/chronic renal failure, hemodialysis; myasthenia gravis, celiac disease, alcoholic cirrhosis, coronary bypass, CHF, acute pancreatitis, carcinomas (terminal).
Therapy
Until relatively recently, there were no effective anti-influenza antivirals. Amantadine and rimantadine has been used for influenza prophylaxis/therapy and act by interfering with the attachment of the virus to uninfected hosts' respiratory epithelial cells. The current anti-influenza antivirals currently available are zanamivir and oseltamivir. Both of these antivirals decrease the duration/severity of illness. Oseltamivir is administered orally but zanamivir is administered via an inhaler, which is problematic for some patients.30 Unfortunately, there is now widespread resistance to oseltamivir, greatly diminishing the therapeutic options in influenza and avian influenza (H5N1). At present, oseltamivir remains effective against most strains of swine influenza (H1N1). Oseltamivir is usually given for 5 days, but in severe cases, therapy has been given for 10 days. For severely ill hospitalized patients with swine influenza (H1N1) pneumonia unable to take oral medications, eg, oseltamivir, peramivir is available from the CDC for IV administration. In severe cases of influenza A, amantadine may have some role because of its effect on dilatation of the distal bronchioles. By increasing distal bronchiolar aeration, oxygenation may be improved, which may be critical in severe influenza A.60, 61, 62, 63, 64, 65, 66, 67
Complications and prognosis
The prognosis of influenza A is related primarily to strain virulence. Most patients with mild to moderate influenza A recover without specific anti-influenza therapy. However, influenza A may be severe, and may be fatal in the very young and the elderly with impaired cardiopulmonary function. With pandemic influenza A, the prognosis depends on the virulence of the strain and may be fatal in young, healthy adults. Such has clearly been the case in the recent avian influenza (H5N1) epidemic in Asia whose fatalities, as in the 1918 to 1919 pandemic, were due in the main to influenza pneumonia and was frequently fatal without bacterial superinfection. Severe influenza A pneumonia with superimposed S aureus CAP have the worst prognosis. Others who initially present with influenza may later be complicated by subsequent bacterial pneumonia due to S pneumoniae or H influenzae, and have the same prognosis as noninfluenza patients with S pneumoniae or H influenzae CAP.
There is a late excess mortality with influenza A, that occurs after the acute episode eg, elderly patients with borderline cardiopulmonary function succumb to heart failure or acute myocardial infarction from the hypoxemia/stress of swine influenza (H1N1) pneumonia. In severe cases of respiratory failure/ARDS, extracorporeal membrane oxygenation (ECMO) has been lifesaving in some.68 Although influenza is a 3-day illness, postinfluenza fatigue may last for weeks or months. In influenza, prognosis is worse in those patients who develop encephalitis. Following the 1918 to 1919 influenza pandemic, some patients developed Parkinson disease decades later. The prognosis in influenza depends on several factors including the virulence of the strain, the age/immune status of the host, cardiopulmonary function, and complications, for example, encephalitis or bacterial CAP.1, 2, 16 The most important intervention in adults with severe swine influenza (H1N1) pneumonia is prolonged ventilatory support. Because these patients are not complicated by simultaneous or subsequent bacterial CAP, empiric antimicrobial therapy seems to be unnecessary in swine influenza (H1N1) pneumonia. The degree and duration of relative lymphopenia/leukopenia as well as the severity and duration of hypoxemia are key prognostic indicators. An improvement in relative lymphopenia and a decrease in oxygen (FIO2) are predictive of recovery.
Summary and historical perspective
There are differences of opinion on the cause of early deaths in influenza pandemics. Most early deaths in young healthy individuals in the 1918–1919 pandemic seemed to be caused by influenza A pneumonia alone. Based on autopsy specimen cultures, which are notoriously unreliable, some believe the high mortality rate of the 1918–1919 influenza pandemic was due to superimposed bacterial pneumonia, for example, S pneumoniae. Most lung specimens from autopsies of young healthy military recruits clearly show the pathologic changes of influenza alone. Based on the pathologic comparisons of viral and bacterial pneumonia, it seems that the majority of early deaths in young healthy military recruits was due to severe hypoxemia. Late excess mortality seems to have been due to influenza precipitated decompensation in those with antecedent borderline cardiopulmonary function/reserve.
Most recently, S aureus was recognized as an important pathogen in the 1957– 1958 Asian influenza A pandemic. In 1957–1958 Asian influenza A pandemic, influenza presented as pneumonia alone or simultaneously with S aureus or subsequently with S pneumoniae or H influenzae CAP. Because the 1957–1958 influenza A pandemic clinicians had modern bacteriologic and virologic diagnostic methods, clinicians attributed the excess mortality to late bacterial CAP. The unusual severity of the 1918–1919 influenza A pandemic seems to have been related to a particularly virulent strain of influenza A (H1N1), not unlike the virulence of avian influenza (H5N1). In the recent experience with avian influenza (H5N1), it is interesting to note that despite high mortality of ∼ 60% in young adults, avian influenza deaths have been due to avian influenza (H5N1) pneumonia alone and not simultaneous/subsequent bacterial CAP.69, 70
A summary of lessons learned during the “herald wave” of the swine influenza (H1N1) pandemic is presented here in tabular form (Table 6, Table 7 ). The important take-home lessons for clinicians in the summary relate to laboratory and clinical diagnosis, infection control concerns, and empiric antibiotic use.71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85
Table 6.
Lessons learned during the “herald wave” of the swine influenza (H1N1) pandemic in spring/summer of 2009 at Winthrop-University Hospital
| Laboratory Diagnosis: Rapid Influenza and RT-PCR Testing | Clinical Diagnosis: Winthrop-University Hospital Infectious Disease Division's Diagnostic Swine Influenza (H1N1) Triad | Infection Control Considerations |
|---|---|---|
|
|
|
Abbreviation: ILI, influenzalike illness.
Table 7.
Clinical summary of lessons learned during the “herald wave” of the swine influenza (H1N1) pandemic
| Diagnostic Difficulties | Infection Control Problems | Severity Indicators | |
|---|---|---|---|
| Laboratory Diagnostic Difficulties | Clinical Diagnostic Difficulties | Influenza Precautions (Droplet and Contact) | Laboratory Test Indicators |
|
|
|
|
| Swine Influenza (H1N1) Prophylaxis | Swine Influenza (H1N1) Therapy | Mimics of Swine Influenza (H1N1) Pneumonia | Complications of Swine Influenza (H1N1) |
|---|---|---|---|
| Prophylaxis | Therapy | Adults | Pulmonary |
|
|
|
|
Otherwise unexplained.
References
- 1.Ewald P.W. Evolution of infectious disease. Oxford University Press; New York: 1994. Influenza; pp. 10–116. [Google Scholar]
- 2.Crosby A.W. Influenza. In: Kiple K.F., editor. The Cambridge world history of human disease. Cambridge University Press; New York: 1993. pp. 807–811. [Google Scholar]
- 3.Aufderheide A.C., Rodriguez-Martin C. Influenza. In: Aufderheide A.C., Rodriguez-Martin C., editors. The Cambridge encyclopedia of human paleopathology. Cambridge University Press; New York: 1998. pp. 210–212. [Google Scholar]
- 4.Sydenham T. Influenza. In: Major R.H., editor. Classic descriptions of disease. Charles C. Thomas, Publisher; Springfield (MA): 1978. pp. 201–202. [Google Scholar]
- 5.Douglas R.G., Jr. Influenza in man. In: Kilbourne E.D., editor. The influenza viruses and influenza. Academic Press; Orlando (FL): 1975. p. 395. [Google Scholar]
- 6.Cunha B.A. Influenza: historical aspects of epidemics and pandemics. Infect Dis Clin North Am. 2004;18:141–156. doi: 10.1016/S0891-5520(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 7.Ravenholt R.T., Foege W.H. Before our time: 1918 influenza, encephalitis lethargica, parkinsonism. Lancet. 1982;2:860–864. doi: 10.1016/s0140-6736(82)90820-0. [DOI] [PubMed] [Google Scholar]
- 8.Conner L.A. The symptomatology and complications of influenza. JAMA. 1919;73:321–325. [Google Scholar]
- 9.Kolte I.V., Skinhoj P., Keiding N. The Spanish flu in Denmark. Scand J Infect Dis. 2008;40:538–546. doi: 10.1080/00365540701870903. [DOI] [PubMed] [Google Scholar]
- 10.Sheretz R.J., Sheretz H.J. Influenza in the preantibiotic era. Infect Dis Clin Pract. 2006;14:127. [Google Scholar]
- 11.Winternitz M.C., Wason I.M., McNamara F.P. Yale University Press; New Haven (CT): 1920. The pathology of influenza. [Google Scholar]
- 12.Wolbach S.B. Comments on the pathology and bacteriology of fatal influenza cases as observed at Camp Devens, Mass. Bull Johns Hopkins Hosp. 1919;30:104–105. [Google Scholar]
- 13.Stevens K.M. The pathophysiology of influenzal pneumonia in 1918. Perspect Biol Med. 1918;25:115–125. doi: 10.1353/pbm.1981.0059. [DOI] [PubMed] [Google Scholar]
- 14.Mulder J., Hers J.F. Wolters-Noordhoff; Groningen (The Netherlands): 1979. Influenza. [Google Scholar]
- 15.Klotz O. Studies on epidemic influenza, comprising clinical and laboratory investigations by members of the faculty of the school of medicine. University of Pittsburgh Press; Pittsburgh (PA): 1919. The pathology of epidemic influenza; pp. 255–261. [Google Scholar]
- 16.Andrewes F.W. Great Britain Ministry of Health. Reports on public health and medical subjects, no. 4: report on the pandemic of influenza, 1918–1919. His Majesty's Stationery Office; London: 1920. The bacteriology of influenza; pp. 110–126. [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–470. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:470–472. [PubMed] [Google Scholar]
- 19.Gallaher W.R. Towards a sane and rational approach to management of Influenza H1N1 2009. Virol J. 2009;6:51. doi: 10.1186/1743-422X-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezza G. Swine-origin influenza virus A (H1N1)v: lessons learnt from the early phase of the epidemic. Eur J Public Health. 2009;19:572–573. doi: 10.1093/eurpub/ckp156. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J., Enserink M. Infectious disease. As swine flu circles globe, scientists grapple with basic questions. Science. 2009;324:572–573. doi: 10.1126/science.324_572. [DOI] [PubMed] [Google Scholar]
- 22.Kilbourne E.D., editor. The influenza viruses and influenza. Academic Press; Orlando (FL): 1975. [Google Scholar]
- 23.Debré R., Couvreur J. Influenza: clinical features. In: Debré R., Celers J., editors. Clinical virology: the evaluation and management of human viral infections. WB Saunders; Philadelphia: 1970. pp. 507–515. [Google Scholar]
- 24.Nicholson K.G., Webster R.G., Hay A.J., editors. Textbook of influenza. Blackwell Science; Oxford (UK): 1998. [Google Scholar]
- 25.Van Voris L.P., Young J.F., Bernstein J.M. Influenza viruses. In: Belshe R.B., editor. Textbook of human virology. PSG Publishing Company; Littleton (MA): 1984. pp. 267–281. [Google Scholar]
- 26.Atmar R.L. Influenza viruses. In: Murray P.R., Baron E.J., Jorgensen J.H., editors. Manual of clinical microbiology. 9th edition. ASM Press; Washington, DC: 2007. pp. 1340–1351. [Google Scholar]
- 27.Hayden F.G., Palese P. Influenza virus. In: Richman D.D., Whitley R.J., Hayden F.G., editors. Clinical virology. 3rd edition. ASM Press; Washington, DC: 2009. pp. 943–976. [Google Scholar]
- 28.Sym D., Patel P.M., El-Chaar G.M. Seasonal, avain and novel H1N1 influenza: prevention and treatment modalities. Ann Pharmacother. 2009;43:2001–2011. doi: 10.1345/aph.1M557. [DOI] [PubMed] [Google Scholar]
- 29.Harper S.A., Bradley J.S., Englund J.A. Seasonal influenza in adults and children: diagnosis, treatment, chemoprophylaxis and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–1032. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunha B.A. 3rd edition. Jones & Bartlett; Sudbury (MA): 2010. Pneumonia essentials. [Google Scholar]
- 31.Cunha B.A. The clinical diagnosis of severe viral influenza A. Infection. 2008;36:92–93. doi: 10.1007/s15010-007-7255-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim H.M., Lee Y.W., Lee K.J. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol. 2008;82:4265–4274. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mollura D.J., Asnis D.S., Crupi R.S. Imaging findings in a fatal case of pandemic swine-origin influenza A (H1N1) AJR Am J Roentgenol. 2009;193:1500–1503. doi: 10.2214/AJR.09.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal P.P., Cinti S., Kazerooni E.A. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009;193:1488–1493. doi: 10.2214/AJR.09.3599. [DOI] [PubMed] [Google Scholar]
- 35.Cunha B.A. A useful clinical approach to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) infections. J Hosp Infect. 2008;68 doi: 10.1016/j.jhin.2007.12.001. 271–73. [DOI] [PubMed] [Google Scholar]
- 36.Cunha B.A. Methicillin-resistant Staphylococcus aureus: clinical manifestations and antimicrobial therapy. Clin Microbiol Infect. 2005;11:33–42. doi: 10.1111/j.1469-0691.2005.01162.x. [DOI] [PubMed] [Google Scholar]
- 37.Tacconelli E., De Angelis G. Pneumonia due to methicillin-resistant Staphylococcus aureus: clinical features, diagnosis and management. Curr Opin Pulm Med. 2009;15:218–248. doi: 10.1097/MCP.0b013e3283292666. [DOI] [PubMed] [Google Scholar]
- 38.Kallen A.J., Brunkard J., Moore Z. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. 2009;53:358–365. doi: 10.1016/j.annemergmed.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Cheng V.C., Lau Y.K., Lee K.L. Fatal co-infection with swine origin influenza virus A/H1N1 and community-acquired methicillin-resistant Staphylococcus aureus. J Infection. 2009;259:1–5. doi: 10.1016/j.jinf.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Cunha B.A., Syed U., Strollo S. Bacterial pneumonia rare with fatal swine influenza (H1N1) pneumonia: if chest films have no focal segmental/lobar infiltrates, empiric antibiotic therapy is unnecessary. J Chemotherapy. 2010;21:584–585. doi: 10.1179/joc.2009.21.5.584. [DOI] [PubMed] [Google Scholar]
- 41.Hien N.D., Ha N.H., Van N.T. Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004–2005. Emerg Infect Dis. 2009;15:19–23. doi: 10.3201/eid1501.080073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Writing Committee of the Second World Health Organization consultation on clinical aspects of human infection with avian influenza A (H5N1) virus. Update on avian influenza A (H5N1) virus infection in humans N Engl J Med. 2008;358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 43.Thomas J.K., Noppenberger J. Avian influenza: a review. Am J Health Syst Pharm. 2007;64:149–165. doi: 10.2146/ajhp060181. [DOI] [PubMed] [Google Scholar]
- 44.Ozbay B., Sertogullarindan B., Tekin M. Influenza-associated pneumonia in a Turkish area with endemic avian influenza. Respirology. 2008;13:444–446. doi: 10.1111/j.1440-1843.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 45.Sandrock C., Kelly T. Clinical review: update of avian influenza A infections in humans. Crit Care. 2007;11:209–218. doi: 10.1186/cc5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.To K.F., Chan P.K., Chan K.F. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 47.Tran T.H., Nguyen T.L., Nguyen T.D. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 48.Uyeki T.M. Human infection with highly pathogenic avian influenza A (H5N1) virus: review of clinical issues. Clin Infect Dis. 2009;49:279–290. doi: 10.1086/600035. [DOI] [PubMed] [Google Scholar]
- 49.Gambotto A., Barratt-Boyes S.M., de Jong M.D. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- 50.Yuen K.Y., Chan P.K.S., Peiris M. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 51.Rainer T.H. Severe acute respiratory syndrome: clinical features, diagnosis, and management. Curr Opin Pulm Med. 2004;10:159–165. doi: 10.1097/00063198-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Rhodes L.V., Huang C., Sanchez A.J. Hantavirus pulmonary associated with Monongahela virus, Pennsylvania. Emerg Infect Dis. 2000;6:616–621. doi: 10.3201/eid0606.000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunha B.A., Pherez F.M., Strollo S. Swine influenza H1N1: diagnostic dilemma early in the pandemic. Scand J of Infect. 2009;41 doi: 10.3109/00365540903222465. 900–2. [DOI] [PubMed] [Google Scholar]
- 54.Cunha B.A., Syed U., Strollo S. Winthrop-University Hospital infectious disease division's swine influenza (H1N1) pneumonia diagnostic weighted point score system for adults with Influenza Like Illnesses (ILIs) and negative Rapid Influenza Diagnostic Tests (RIDTs) Heart Lung. 2009;38 doi: 10.1016/j.hrtlng.2009.09.005. 534–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunha B.A., Pherez F.M., Schoch P.E. The diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis. 2009;49:1454–1456. doi: 10.1086/644496. [DOI] [PubMed] [Google Scholar]
- 56.Louria D.B., Blumenfeld H.L., Ellis J.T. Studies on influenza in the pandemic of 1957–1958. Pulmonary complications of influenza. J Clin Invest. 1959;38:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robertson L., Caley J.P., Moore J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet. 1958;2:233–236. doi: 10.1016/s0140-6736(58)90060-6. [DOI] [PubMed] [Google Scholar]
- 58.Petersdorf R.G., Fusco J.J., Harter D.H. Pulmonary infections complicating Asian influenza. Arch Intern Med. 1959;103:262–272. doi: 10.1001/archinte.1959.00270020090010. [DOI] [PubMed] [Google Scholar]
- 59.Vasoo S., Stevens J., Singh K. Rapid antigen tests for diagnosis of pandemic (Swine) influenzae A/H1N1. Clin Infect Dis. 2009;49:1090–1093. doi: 10.1086/644743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunha B.A. Amantadine may be lifesaving in severe influenza A. Clin Infect Dis. 2006;43:1574–1575. doi: 10.1086/508665. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention (CDC) Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58 433–5. [PubMed] [Google Scholar]
- 62.Influenza Project Team Oseltamivir resistance in human seasonal influenza viruses (A/H1N1) in EU and EFTA countries: an update. Euro Surveill. 2008;13:8032. [PubMed] [Google Scholar]
- 63.Kawai N., Ikematsu H., Iwaki N. A change in the effectiveness of amantadine for the treatment of influenza over the 2003–2004, 2004–2005, and 2005–2006 influenza seasons in Japan. J Infect Chemother. 2007;13:314–319. doi: 10.1007/s10156-007-0538-3. [DOI] [PubMed] [Google Scholar]
- 64.Meijer A., Lackenby A., Hungnes O. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg Infect Dis. 2009;15:552–560. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van der Vries E., van den Berg B., Schutten M. Fatal oseltamivir-resistant influenza virus infection. N Engl J Med. 2008;359:1074–1076. doi: 10.1056/NEJMc0803120. [DOI] [PubMed] [Google Scholar]
- 66.Couzin-Frankel J. Swine flu outbreak. What role for antiviral drugs? Science. 2009;324:705. doi: 10.1126/science.324_705. [DOI] [PubMed] [Google Scholar]
- 67.Hurt A.C., Selleck P., Komadina N. Susceptibility of highly pathogenic A (H5N1)Avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Res. 2007;73:228–231. doi: 10.1016/j.antiviral.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Davies A., Jones D., Bailey M. Extracorporeal Membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 69.Andreasen V., Viboud C., Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197:270–278. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steel J, Palese P. The 1918 Influenza pandemic: lessons from the past raise questions for the future. In: Klenk HD, Matrosovich MN, Stech J, editors. Avian Influenza. Monogr Virol, vol 27. Basel: Karger; 2008. p. 272–86.
- 71.Cunha B.A., Thekkel V., Cohan C. Swine influenza (H1N1): contact investigation burden because of failure to institute influenza precautions with negative rapid influenza A diagnostic test results. Infect Control Hosp Epidemiol. 2010;31:102–104. doi: 10.1086/649762. [DOI] [PubMed] [Google Scholar]
- 72.Cunha B.A., Thekkel V., Krilov L. Nosocomial swine influenza (H1N1) pneumonia: lessons learned from an illustrative case. J Hosp Infect. 2009;72 doi: 10.1016/j.jhin.2009.08.024. in press. [DOI] [PubMed] [Google Scholar]
- 73.Charlier C., Enouf V., Lanternier F. Kinetics of nasopharyngeal shedding of novel H1N1 (swine-like) influenza A virus in an immunocompetent adult under oseltamivir therapy. Clin Microbiol Infect. 2009;15:1189–1191. doi: 10.1111/j.1469-0691.2009.03007.x. [DOI] [PubMed] [Google Scholar]
- 74.Dominguez-Cherit G., Lapinsky S.E., Macias A.E. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 75.MMWR. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009, 77. [PubMed]
- 76.MMWR Hospitalized patients with novel influenza A (H1N1) infection—California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58 536–41. [PubMed] [Google Scholar]
- 77.Kumar A., Zarychanski R., Pinto R. Critically ill patients with 2009 influenza A (H1N1) in Canada. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 78.MMWR Intensive-care patients with severe novel influenza A (H1N1) virus infection—Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009;58:749–752. [PubMed] [Google Scholar]
- 79.Bin C., Xingwang L., Yeulong S. Clinical & epidemiologic characteristics of 3 early cases of influenza A pandemic (H1N1) 2009 virus infection, People's Republic of China, 2009. Emerging Infect Dis. 2009;15:1418–1422. doi: 10.3201/eid1509.090794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cunha B.A. Swine influenza (H1N1) pneumonia: bacterial airway colonization common but fatalities due to bacterial pneumonia remain relatively rare. J Clin Virol. 2009 doi: 10.1016/j.jcv.2009.11.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 81.Barlow GD, on behalf of the BSAC Council. Swine flu and antibiotics. J Antimicrob Chemother. 2009;10:1–6. doi: 10.1093/jac/dkp313. [DOI] [PubMed] [Google Scholar]
- 82.Mauad I., Hajjar L.A., Callegari C.A. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200909-1420OC. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 83.Cunha B.A., Klein N.C., Strollo S. Legionnaire's disease mimicking swine influenza (H1N1) pneumonia. Heart & Lung. 2010;39 doi: 10.1016/j.hrtlng.2009.10.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klein N., Chak A., Chengot A. Case report: a fatal case of severe H1N1 influenza A (Swine flu) pneumonia in an HIV positive patient. Emerg Infect Dis. 2010;16:149–150. doi: 10.3201/eid1601.090930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cunha B.A., Syed U., Mikail N. Rapid clinical diagnosis in fatal swine influenza (H1N1) pneumonia in adult with negative rapid influenza diagnostic tests (RIDTs): diagnostic swine influenza triad. Heart & Lung. 2010;39:78–86. doi: 10.1016/j.hrtlng.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]