Abstract
The kobuviruses represent an emerging genus in the Picornaviridae. Here we have used next generation sequencing and conventional approaches to identify the first canine kobuvirus (CaKoV) from outside the USA. Phylogenetic analysis suggests that a single lineage genotype of CaKoV now exists in Europe and the USA with 94% nucleotide similarity in the coding region. CaKoV was only identified in a single case from a case–control study of canine diarrhoea, suggesting this virus was not a frequent cause of disease in this population. Attempts to grow CaKoV in cell culture failed. Sequence analysis suggested CaKoV was distinct from human Aichi virus (AiV), and unlikely to pose a significant zoonotic risk. Serosurveys by ELISA, immunofluorescence and neutralisation tests, using AiV as antigen, suggested kobuvirus infection is prevalent in dogs. In addition, IgG antibody to AiV was also detected in cat sera, indicating for the first time that cats may also be susceptible to kobuvirus infection.
Keywords: Picornavirus, Aichivirus, Kobuvirus
1. Introduction
Kobuvirus is a relatively recently described genus of the Picornaviridae family. Human Aichi virus (AiV), its first member, was isolated from faecal samples of human patients with oyster-associated nonbacterial gastroenteritis in Japan in 1989 (Yamashita et al., 1991). Since then, AiV has been detected in many countries around the world, especially in outbreaks associated with consumption of oysters and other shell-fish, and in sporadic disease in children (Ambert-Balay et al., 2008, Kaikkonen et al., 2010, Oh et al., 2006, Pham et al., 2007, Reuter et al., 2009, Sdiri-Loulizi et al., 2008, Yang et al., 2009). Other members of the Kobuvirus genus have now been described causing enteric infections in cattle (Barry et al., 2011, Khamrin et al., 2008, Park et al., 2011), pigs (Barry et al., 2011, Khamrin et al., 2009, Reuter et al., 2008), wild boar (Reuter et al., 2012), bats (Li et al., 2010), sheep (Reuter et al., 2010) and rodents (Phan et al., 2011). Their role as a primary pathogen remains uncertain (Kaikkonen et al., 2010, Lorrot et al., 2011), and mixed infections are common (Ambert-Balay et al., 2008). Although detection of AiV itself is rare, seroprevalence of antibody to AiV is high in humans, approaching 100% in adults, suggesting infection is common (Ribes et al., 2010, Yamashita et al., 1993).
Human AiV has a linear, positive sense ssRNA genome of ∼8270 nucleotides, and exists as non-enveloped, 22–30 nm icosahedral particles (Yamashita et al., 2003). The RNA molecule contains one large open reading frame encoding a single polyprotein which like other members of the Picornaviridae family is cleaved into structural and non-structural proteins. Initially two genotypes of AiV were identified based on differences of nucleotide sequence in the 3C–3D junction (Yamashita et al., 2000). More recently a third genotype type has been identified (Ambert-Balay et al., 2008). Some unique molecular features have been described compared to other picornaviruses. Yamashita et al. (1998) showed that the VP0 capsid protein is expressed in the mature particle in Aichi virus and does not undergo cleavage (Yamashita et al., 1998) in contrast to other picornaviruses (Hellen and Wimmer, 1992). Also the 2A and L protein of Aichi virus have no protease or autocatalytic motifs as documented for other picornaviruses and their function remains unknown (Yamashita et al., 1998).
In dogs, the first kobuviruses were described in 2011. In a study of acute gastroenteritis in canine shelters in the United States, 5 of 18 dogs from two of three outbreaks tested positive for kobuviruses. Sequence analysis and distances from AiV genotypes A and B led the authors to propose these viruses be called canine kobuvirus (CaKoV) (Kapoor et al., 2011). In a separate study published around the same time, a similar virus was detected in the stools of three dogs with diarrhoea (Li et al., 2011). Based on sequence analysis, the canine virus was independently named CaKoV and tentatively classified as a novel species, closely related to AiV, in the genus Kobuvirus. Follow up PCRs of 200 sick and healthy dogs found CaKoV in 14 healthy and six diarrhoeic dogs. Both of these studies failed to identify a clear role for CaKoV in disease, suggesting that any pathogenicity was likely to be low, at least in the mostly adult canine population they tested.
Here, we describe canine kobuvirus in the UK, and provide serological evidence for widespread kobuvirus infection in cat and dog populations. The relationship of these carnivore viruses with AiV is discussed.
2. Methods
2.1. Samples
The faecal samples in this study were from an existing case–control study to determine the role of canine enteric coronavirus (CECoV) and other potential pathogens in enteric disease (Stavisky et al., 2011). As well as using PCR to detect known pathogens, electron microscopy was also conducted on all cases (N = 86) and on 60 controls. In two cases and one control, myxovirus-like particles were described. These samples tested negative using conventional PCR for canine distemper and parainfluenza (data not presented). One of these samples (UK003) was from a two month-old Staffordshire-bull terrier-cross that presented collapsed with diarrhoea and vomiting. This puppy was also shedding type II CECoV and Campylobacter upsaliensis, as well as Toxacara canis and Toxacara leonina. Sample UK003 was used for next generation sequencing.
AiV strain A846/88 (genotype A), was isolated by Yamashita et al. (1991), and kindly provided by Pierre Pothier (University of Dijon, France). This strain was propagated in Vero cells, recovered from cell lysates, and clarified by centrifugation, and the supernatant was divided into aliquots, which were stored at −80 °C. The stock virus was titrated by immunofluorescence on Vero cells.
Anonymized canine (N = 198) and feline (N = 97) serum samples were obtained as part of routine diagnostic work being carried out on animals attending the University of Liverpool Small Animal Teaching Hospital. For confidentiality reasons, clinical data could not be obtained with the samples.
2.2. Sample preparation, sequencing and informatics
Faecal samples were purified and nucleic acid extracted essentially according to published protocols with minor modification (Finkbeiner et al., 2008, Wang et al., 2003). Briefly, chips of frozen archived faeces (∼30–150 mg) were resuspended in 6 volumes of PBS, clarified by centrifugation (9700 × g, 10 min), and the supernatants passed through 0.45 μm filters. RNA was extracted from 100 μl of the filtrate (Qiagen RNA extraction kit) without the DNase step, reverse transcribed (Superscript) using primer A (5′-GTTTCCCAGTCACGATCNNNNNNNNN-3′), and amplified using primer B (5′-GTTTCCCAGTCACGATC-3′). Smears of DNA were gel purified and submitted for library preparation according to standard protocols.
Whole-genome shotgun pyrosequencing was performed by generating a standard DNA fragment library (Roche Applied Sciences, Indianapolis, IN) and sequenced with a GS-FLX using Titanium chemistry (454 Life Sciences, Roche Applied Sciences). The 454 reads were assembled with Newbler (v2.3 Roche Applied Sciences), and results compared to a viral protein database downloaded from NCBI Jan 2009 using BlastX.
The full genome of UK003 was determined by sequencing overlapping amplicons generated by multiple PCRs using primers based on the draft next generation genome, and 5′/3′ RACE. Maximum likelihood trees with 1000 bootstrap replicates were calculated using PHYML 3.0 (Guindon and Gascuel, 2003) using the most appropriate model of evolution, which was identified using the model selection approach implemented in Topali v2.5 (Milne et al., 2009).
2.3. Viral prevalence by PCR detection
Based on the results of next generation sequencing, a diagnostic PCR assay was designed to screen the case–control study (80 cases and 147 controls) for the presence of a putative Aichi/kobu viruses. RNA was extracted from 140 μl of virus transport medium containing the faecal sample using the QIAamp viral RNA extraction kit (Qiagen), and first strand cDNA synthesis was carried out using the RNA SuperscriptIII kit (Invitrogen), following the manufacturer's instructions. Reactions contained 1 μl oligodT or random hexamers, 3 μl RNA, 2 μl 5 mM dNTP mix, 8 μl H2O, 4 μl first-strand buffer, 1 μl 0.1 M DTT and 1 μl SuperscriptIII. Reactions were incubated at 25 °C for 5 min, 50 °C for 60 min and 70 °C for 15 min. The resulting cDNA was used as the template for the PCR assay.
Five separate oligonucleotide primers were designed (three forward and two reverse), based on the known sequences of three kobuviruses: AiV (AB010145), porcine kobuvirus (EU787450) and our putative CaKoV. The primers sequences were chosen to match our CaKoV sequence in regions of high similarity to the other kobuviruses. The five primers were tested in all combinations with the following conditions: initial denaturation 94 °C for 5 min: 30 cycles of 30 s at 94 °C, 30 s at 55 °C, 30 s at 72 °C and a final elongation step of 7 min at 72 °C, using UK003 as positive control. The most successful combination (DogF3; 5′-CACCTACGACTGGGCCACGCTCC-3′ and DogR2; 5′-GTGGGGAAGGTAGAGAAGTAGAGGTC-3′), having the clearest, brightest product was selected for use in future diagnostic PCR assays. These primers amplified ∼650 bases of kobuvirus RNA, equivalent to bases 6205–6861 of AiV AB010145 3CD region.
As well as this single stage PCR, a nested PCR was developed using DogF3 and DogR2, followed by primers DogF4 (5′-GTCCACACCCCTACCTCCCGCC-3′) and DogR1 (5′-CGTGTTTGAGGAAGAGTTGGGTGTC-3′), using the cycling conditions described above.
2.4. Cell culture
Briefly, confluent cell lines A72 [ATCC CRL-1542; p179–182], MDCK [ATCC CCL-34; p156–166] and Vero cells [ATCC CCL-81; p64–68] were grown in EMEM (Sigma) with 1% FBS. Cells were inoculated with 100 μl of filtered faecal sample (from a pre wetted 0.2 μl filter). These samples had been stored in virus transport medium or pooled brain heart infusion media and incubated for up to 7 days in maintenance media. Samples were checked daily for cytopathic effect (cpe) and compared to negative controls. Each passage was frozen at −80°C, thawed and repeated once.
Detection of specific antibodies against Aichi virus by ELISA: viral antigen was partially purified from AiV genotype A A846/88-infected cells by ultracentrifugation. When the cpe was 80–90%, the cell cultures were frozen and thawed three times, and clarified by low-speed centrifugation (15,450 × g for 25 min). The supernatants were concentrated by ultracentrifugation at 50,000 rpm for 2 h at 4 °C using a Beckman 70 Ti rotor. Pellets were resuspended in 300 μl of TNC (0.05 M Tris–HCl, 0.15 M NaCl, 0.01 M CaCl2). The protein concentration was determined by the Bradford method (Bio-Rad) and the viral antigen preparations stored at −80 °C.
Ninety-six-well polystyrene microtiter plates (Costar) were coated with 10 μg/well with partially purified antigen diluted in 0.05 M carbonate/bicarbonate buffer (pH 9.6), incubated for 2 h at 37 °C, and washed three times with 0.5% Tween 20 in phosphate buffered saline (PBS-T). Plates were incubated for 1.5 h at 37 °C with 100 μl dilutions of serum samples in PBS containing 1% bovine serum albumin (PBS–BSA). Dilutions were 1:100 for antibody screening or 100 μl of serially diluted serum samples for antibody titration. After three washes with PBS-T, 100 μl of horseradish peroxidase (HRP)-conjugated anti-dog (or anti-cat) IgG antibody (Novus Biologicals) diluted 1:2000 in PBS–BSA were added to each well and incubated at 37 °C for 1 h. The plates were washed four times with PBS-T and bound antibody detected with 50 μl of o-phenylenediamine (OPD), followed by incubation at room temperature for 10 min. The reaction was stopped with 3 M H2SO4 and the absorbance was read at 492 nm (Multiskan FC spectrophotometer, Thermo Scientific). Since the semi-purified AiV preparation could contain cellular debris, serum samples were tested simultaneously against antigens of uninfected Vero cells extracted by the same procedure. The absorbance value of each serum sample against the cellular antigens was subtracted from the absorbance value obtained against the viral antigen. A pool of AiV-positive dog sera were used in follow up experiments as positive controls, and three dog sera known to have no antibodies to this virus were included as the negative controls in every assay. The cut-off value was calculated as the mean of the negative controls plus 3 times the standard deviations of all negative controls.
2.5. Immunofluorescence assay (IFA)
A random selection of serum samples testing positive and negative by ELISA were also analysed by IFA. Vero cells were grown on round coverslips (Marienfeld GmbH & Co., KG) in 24-well plates (Costar) at a concentration suitable to form a confluent monolayer within 24 h. The cells were infected with AiV genotype A A846/88 in MEM medium and adsorption was performed at 37 °C for 1 h. The inocula were removed, maintenance medium (MEM with 2% SBF) was added and the infected cell cultures were incubated 24 h at 37 °C. The cells were washed with PBS, fixed with methanol:acetone for 15 min on ice, washed two further times with PBS and incubated with dog/cat serum samples diluted 1:100 in PBS containing 1% bovine serum albumin (BSA) (Sigma) for 1 h at 37 °C. The coverslips were washed three times with PBS and incubated with a fluoresceine isothiocyanate (FITC)-labelled rabbit serum anti-dog or anti-cat IgG (Santa Cruz Biotechnology) for 1 h at 37 °C. The monolayers were washed five times in PBS and stained with Evans blue. Finally, the coverslips were mounted onto glass slides with buffered glycerol (Light Diagnostics), examined under a fluorescence microscope and images were captured using a Nikon Digital Sight DS-Fi1 camera.
2.6. Seroneutralisation assay
Four canine and four feline sera that showed high antibody titres to AiV genotype A by ELISA were inactivated for 30 min at 56 °C and serially diluted 4-fold in minimal essential medium (MEM) (Gibco-BRL). All dilutions were mixed with an AiV suspension containing 50–100 fluorescent focus-forming units (ffu) for 1 h at 37 °C. These mixtures were then added in duplicate to Vero cell monolayers growing in 96-well plates, and incubated for 1 h at 37 °C. Viral inocula were removed and the cells were further incubated for 8 h, before fixing in methanol–acetone. Viral antigens were detected by indirect immunofluorescence. Briefly, fixed cell monolayers were rinsed with PBS and incubated for 1 h at 37 °C with a hyperimmune human serum against Aichi virus diluted 1:500 in PBS–BSA. The cells were washed twice with PBS and incubated for 1 h at 37 °C with a FITC-conjugated antibody against human IgG (Sigma) diluted 1:2000 in PBS–BSA. The cells were then washed three times with PBS and observed under a Nikon Eclipse fluorescence microscope E600. Aichi virus antibody-negative and positive human sera were included as negative and positive controls, respectively. Neutralising antibody titres were considered the inverse of the sample serum dilution that showed a >60% reduction in AiV ffu compared to the control.
3. Results
3.1. Sequencing
The only novel viral sequences identified in these faecal samples were related to Kobo virus, and were found in a single sample UK003. The 454 assembly had a total length of 7594 bp with a GC% of 58.4, and greater than 25-fold coverage.
The final sequence of CaKoV UK003 was 8213 characters in length, including a 612base 5′NTR, 7332base polyprotein excluding the STOP codon, and a 2443′NTR. The coding nucleotide sequence was 94% similar to both published CaKoV sequences, and 78% similar to the published sequence for AiV strain A846/88 (genotype A; Genbank AB010145). The GenBank accession number for the genome sequence of CaKoV UK003 is KC161964.
Maximum likelihood phylogeny based on a 459 bp alignment of the 3C–3D region showed CaKoV UK003 to be closely related to the two canine strains from the USA, distinct from AiV genotypes A–C, and the mouse kobuvirus.
3.2. Attempts to grow virus in cell culture
Attempts to grow virus from sample UK003, as well as from a representative panel of other samples failed. No cpe was observed in any of the samples, and RT-PCR on media and cells failed to identify viral genome.
3.3. Viral prevalence by PCR
The only sample testing consistently positive for kobuvirus from the case control study was UK003. This suggested the prevalence of CaKoV-like viruses was 1.25% (95%, 1 of 80: CI 0.1%–7.7%) for cases and 0% (95% CI 0.1–3.2%) for the 147 controls. Fifty-one samples screened by nested PCR also tested negative (Fig. 1 ).
Fig. 1.
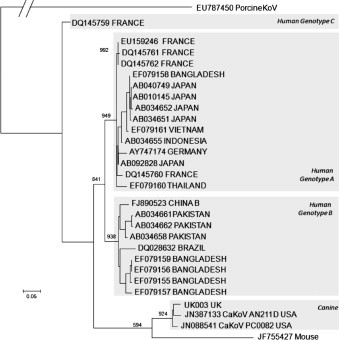
Maximum likelihood phylogeny of sequence UK003, with representative sequences of canine and murine kobuvirus, and the three genotypes of human AiV, rooted to porcine kobuvirus. Branch lengths are to scale except in the outgroup. Numbers at major nodes are bootstraps out of 1000 replicates.
3.4. Serology
Aichi virus-specific IgG antibodies were detected in 74 (37.4%) out of 198 dog serum samples and in 67 (69.9%) of 97 cat serum samples. Titres of Aichi virus-specific antibody in dog and cat serum samples are shown in Fig. 2 . The highest antibody titre detected was 1:2000, which was found in 4 dog sera and in 4 cat sera.
Fig. 2.
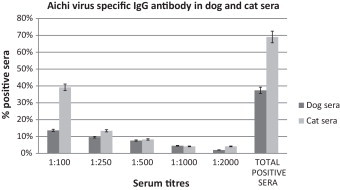
Titration by enzyme immunoassay of anti-AiV IgG in dog and cat serum samples collected in England. Sera were diluted two-fold starting from 1:100 and tested using human AiV strain A846/88 as antigen. Error bars indicate 95% confidence intervals.
To confirm that dog and cat sera contained IgG antibodies specifically reacting to AiV antigens, IFA was performed on AiV-infected Vero cells with canine and feline serum samples that previously tested positive and negative by ELISA. A characteristic granular perinuclear cytoplasmic fluorescence was observed with positive dog (Fig. 3 B) and cat sera (Fig. 3D), consistent with the pattern observed with control positive human sera (Fig. 3A). There was a good correlation between both ELISA and IFA, discriminating positive and negative samples (results not shown).
Fig. 3.
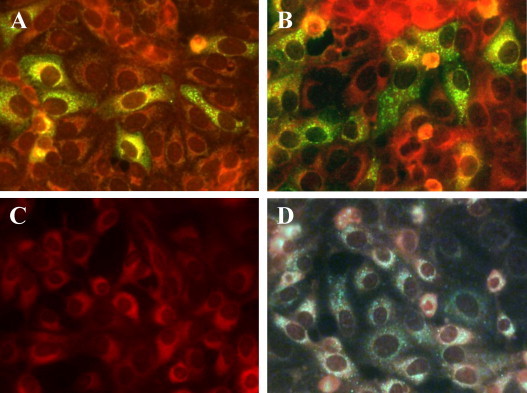
Immunofluorescence assay of serum IgG antibodies against AiV on infected Vero cells. (A) Human serum at 1:400 dilution as the positive control; (B) dog serum sample diluted 1:100; (C) negative control with PBS; (D) cat serum at 1:100 dilution. Vero cells were infected with AiV and further incubated for 8 h. After washing and fixing the cells with methanol:acetone, they were incubated with each serum dilution, washed and IgG antibodies were detected using FITC-labelled appropriate secondary antibodies.
A neutralisation assay was performed on a subset of 4 dog and 4 cat sera with high antibody titres to AiV detected by ELISA, to verify whether these sera also inhibited AiV infection in Vero cells. All samples showed significant viral neutralising activity, but with no clear correlation to their ELISA titres (Table 1 ). The results also showed that dog sera had stronger neutralising activity than cat sera despite having similar titres by ELISA.
Table 1.
ELISA and neutralisation titres of antibodies to human Aichi virus in four dog sera and in four cat sera.
| Dog serum samples | ELISA titres | Neutralisation titres |
|---|---|---|
| S-112 | 1:1000 | 1:4096 |
| S-114 | 1:2000 | 1:512 |
| S-159 | 1:1000 | 1:4096 |
| S-194 | 1:1000 | 1:512 |
| Cat serum samples | ||
| S-46 | 1:2000 | 1:64 |
| S-71 | 1:2000 | 1:32 |
| S-64 | 1:2000 | 1:256 |
| S-77 | 1:2000 | 1:32 |
4. Discussion
Kobuviruses represent a relatively new and emerging genus of the Picornaviridae with members infecting both humans and other animal species including cattle, pigs, bats, sheep, rodents and dogs (Kapoor et al., 2011, Li et al., 2011, Phan et al., 2011, Reuter et al., 2010, Reuter et al., 2011). These viruses have been implicated in diarrhoeal disease but the evidence for a primary pathogenic role remains controversial. We report here the first observation of kobuvirus in the UK. The virus was discovered by next generation sequencing, a powerful tool for the detection of novel viruses (Radford et al., 2012). Although actual infection was rare in dogs with diarrhoea, the seroprevalence study for AiV cross-reactive antibodies in dogs and cats showed infection with kobuviruses to be common in both these species.
Our analysis is the first to compare sequences of CaKoV obtained in more than one country. The phylogenetic analysis reported here suggests one clade of canine kobuvirus is now present both in the USA (Kapoor et al., 2011, Li et al., 2011) and Europe. The CaKoV lineage is phylogenetically distinct from the three known AiV genotypes, and also other published kobuviruses, probably representing a single recent transfer into this species. Our failure to grow the CaKoV in various cell culture systems, is in contrast to AiV (Yamashita et al., 1991), and suggests these two viruses are currently adapted to independent niches, although clearly we cannot rule out the virus being inactivated during storage. These results suggest CaKoV is unlikely to pose a significant zoonotic risk. That said, it is clear that geography also plays a strong part in the human kobuvirus phylogeny, and it will therefore be important to sequence AiV strains from the UK, and/or canine strains from other countries.
The low prevalence of virus we report by RT-PCR is in broad agreement with that previously described for CaKoV. Kapoor et al. (2011) found 5 of 18 dogs from two of three outbreaks in the USA tested positive for CaKoV. In a second study, PCRs of 200 sick and healthy dogs found CaKoV in 14 healthy and six diarrhoeic dogs (Li et al., 2011). Both studies concluded there was no clear link between CaKoV infection and disease. These levels of infection are similar for those reported for other kobuviruses in sporadic disease (Ambert-Balay et al., 2008), and suggest these viruses either cause in apparent infection or are an uncommon cause of sporadic clinical disease in the general population.
In humans, AiV infection is most frequently reported in children (Kaikkonen et al., 2010, Reuter et al., 2009, Sdiri-Loulizi et al., 2009). In our case control study, the mean age of cases and controls was 68 and 65 months respectively, with few juvenile (<6 months) animals included. The sample in our study that did test positive for CaKoV was from a two month-old puppy that presented with severe disease, collapsed with diarrhoea and vomiting. This puppy was also shedding type II CECoV and C. upsaliensis, as well as T. canis and T. leonina. Kobuviruses are frequently found with other known pathogens such as canine parvovirus in dogs (Li et al., 2011), and rota-, astro and noroviruses in humans (Ambert-Balay et al., 2008, Sdiri-Loulizi et al., 2008). In further studies, it will be important to assess CaKoV prevalence in a cohort of younger animals, and in association with mixed infections, to see whether co-infection is associated with disease severity.
The results of our seroprevalence study in dogs and cats suggest that high percentages of these animals contain IgG antibodies against AiV genotype A. In the initial screening assay at 1:100 dilution, 37.4% dog serum samples and 69.1% cat serum samples were positive, suggesting that infection with AiV-cross-reactive kobuviruses is quite common among these animals. In humans, studies in Japan (Yamashita et al., 1993), Germany (Oh et al., 2006), France (Goyer et al., 2008), and Spain (Ribes et al., 2010) show that up to 80–95% of the population have antibodies to AiV when they are 30–40 years old. It has also been reported that AiV infection can affect all age groups (Drexler et al., 2011). Whether the high seroprevalence we report results from symptomatic or asymptomatic infection is unknown.
Our inability to replicate CaKoV in cell culture limited our choices for serological assays. Future serological assays could be based on cloned capsid antigens, but were beyond the scope of this project. We therefore chose to screen canine (and feline samples) for the prevalence of antibodies cross-reacting with human AiV genotype A. To our knowledge, there is no published data on the serological cross-reactivity between different members of the kobuvirus genus, nor between members of distinct AiV genotypes. Therefore the virological origin of the high seroprevalence detected in this study cannot be clear. For example, it is not known whether these seropositive animals represent kobuvirus infections that serologically cross-react with genotype A AiV, or whether they indeed represent previous AiV infections. Although published data generally suggests kobuviruses are host specific, some cross-species infections have been detected among farm animals (Khamrin et al., 2010, Park et al., 2011, Reuter et al., 2011), and clearly the lifestyle of some pet dogs and cats would provide opportunity for viral transmission between species (Chomel and Sun, 2011).
This is the first paper to report possible kobuvirus infections in cats, although clearly we cannot definitely say our assay is not cross-reacting with other feline picornaviruses (Lau et al., 2012). Clearly the identity of the virus or viruses responsible needs to be determined, along with their relationship to AiV and CaKoV. There are now many examples of possible transmission of various microbiological agents between these species (Clegg et al., 2012, Dawson et al., 2000, Radford et al., 2007), perhaps suggesting CaKoV might also be common to both the cat and the dog. It will clearly be important to assess the role of kobuviruses in feline disease, and also as a source of infection to dogs.
5. Conclusion
In conclusion, we have demonstrated the presence of a single clade of CaKoV in dogs in Europe and the USA. Evidence for a role for the virus in disease in this species is lacking, although high seroprevalence in both dogs and cats suggests infection is common. In future studies it will be important to clarify the serological cross-reactivity between the members of this emerging picornavirus genus, and the genetic relatedness of animal and human kobuviruses from similar geographic regions, to rule out any potential for zoonosis.
Acknowledgements
The sequencing and epidemiological work in this project was supported by MSD Animal Health. N.C.-V. is a Ph.D. academic fellow of the “V Segles” programme of the University of Valencia. The serology was partly supported by a grant from the Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Spain (Project No. PS 09/02065). The sequencing was conducted at the Centre for Genomics Research, University of Liverpool. The authors are grateful to Ruth Ryvar and Kathy Freeman for their diligent attempts to culture these viruses.
References
- Ambert-Balay K., Lorrot M., Bon F., Giraudon H., Kaplon J., Wolfer M., Lebon P., Gendrel D., Pothier P. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J. Clin. Microbiol. 2008;46:1252–1258. doi: 10.1128/JCM.02140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A.F., Ribeiro J., Alfieri A.F., van der Poel W.H., Alfieri A.A. First detection of kobuvirus in farm animals in Brazil and the Netherlands. Infect. Genet. Evol. 2011;11:1811–1814. doi: 10.1016/j.meegid.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Chomel B.B., Sun B. Zoonoses in the bedroom. Emerg. Infect. Dis. 2011;17:167–172. doi: 10.3201/eid1702.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S.R., Coyne K.P., Dawson S., Spibey N., Gaskell R.M., Radford A.D. Canine parvovirus in asymptomatic feline carriers. Vet. Microbiol. 2012;157:78–85. doi: 10.1016/j.vetmic.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Dawson S., Jones D., McCracken C.M., Gaskell R.M., Hart C.A., Gaskell C.J. Bordetella bronchiseptica infection in cats following contact with infected dogs. Vet. Rec. 2000;146:46–48. doi: 10.1136/vr.146.2.46. [DOI] [PubMed] [Google Scholar]
- Drexler J.F., Baumgarte S., de Souza Luna L.K., Eschbach-Bludau M., Lukashev A.N., Drosten C. Aichi virus shedding in high concentrations in patients with acute diarrhea. Emerg. Infect. Dis. 2011;17:1544–1548. doi: 10.3201/eid1708.101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Allred A.F., Tarr P.I., Klein E.J., Kirkwood C.D., Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 2008;4:e1000011. doi: 10.1371/journal.ppat.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer M., Aho L.S., Bour J.B., Ambert-Balay K., Pothier P. Seroprevalence distribution of Aichi virus among a French population in 2006-2007. Arch. Virol. 2008;153:1171–1174. doi: 10.1007/s00705-008-0091-0. [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hellen C.U., Wimmer E. Maturation of poliovirus capsid proteins. Virology. 1992;187:391–397. doi: 10.1016/0042-6822(92)90440-z. [DOI] [PubMed] [Google Scholar]
- Kaikkonen S., Rasanen S., Ramet M., Vesikari T. Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol. Infect. 2010;138:1166–1171. doi: 10.1017/S0950268809991300. [DOI] [PubMed] [Google Scholar]
- Kapoor A., Simmonds P., Dubovi E.J., Qaisar N., Henriquez J.A., Medina J., Shields S., Lipkin W.I. Characterization of a canine homolog of human Aichi virus. J. Virol. 2011;85:11520–11525. doi: 10.1128/JVI.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamrin P., Maneekarn N., Hidaka S., Kishikawa S., Ushijima K., Okitsu S., Ushijima H. Molecular detection of kobuvirus sequences in stool samples collected from healthy pigs in Japan. Infect. Genet. Evol. 2010;10:950–954. doi: 10.1016/j.meegid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Khamrin P., Maneekarn N., Kongkaew A., Kongkaew S., Okitsu S., Ushijima H. Porcine kobuvirus in piglets. Thai. Emerg. Infect. Dis. 2009;15:2075–2076. doi: 10.3201/eid1512.090724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamrin P., Maneekarn N., Peerakome S., Okitsu S., Mizuguchi M., Ushijima H. Bovine kobuviruses from cattle with diarrhea. Emerg. Infect. Dis. 2008;14:985–986. doi: 10.3201/eid1406.070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Yip C.C., Choi G.K., Wu Y., Bai R., Fan R.Y., Lai K.K., Chan K.H., Yuen K.Y. Identification of a novel feline picornavirus from the domestic cat. J. Virol. 2012;86:395–405. doi: 10.1128/JVI.06253-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Pesavento P.A., Shan T., Leutenegger C.M., Wang C., Delwart E. Viruses in diarrhoeic dogs include novel kobuviruses and sapoviruses. J. Gen. Virol. 2011;92:2534–2541. doi: 10.1099/vir.0.034611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Victoria J.G., Wang C., Jones M., Fellers G.M., Kunz T.H., Delwart E. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 2010;84:6955–6965. doi: 10.1128/JVI.00501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrot M., Bon F., El Hajje M.J., Aho S., Wolfer M., Giraudon H., Kaplon J., Marc E., Raymond J., Lebon P., Pothier P., Gendrel D. Epidemiology and clinical features of gastroenteritis in hospitalised children: prospective survey during a 2-year period in a Parisian hospital, France. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:361–368. doi: 10.1007/s10096-010-1094-9. [DOI] [PubMed] [Google Scholar]
- Milne I., Lindner D., Bayer M., Husmeier D., McGuire G., Marshall D.F., Wright F. TOPALi v2: a rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics. 2009;25:126–127. doi: 10.1093/bioinformatics/btn575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.Y., Silva P.A., Hauroeder B., Diedrich S., Cardoso D.D., Schreier E. Molecular characterization of the first Aichi viruses isolated in Europe and in South America. Arch. Virol. 2006;151:1199–1206. doi: 10.1007/s00705-005-0706-7. [DOI] [PubMed] [Google Scholar]
- Park S.J., Kim H.K., Song D.S., Moon H.J., Park B.K. Molecular detection and genetic characterization of kobuviruses in fecal samples collected from diarrheic cattle in Korea. Infect. Genet. Evol. 2011;11:1178–1182. doi: 10.1016/j.meegid.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Pham N.T., Khamrin P., Nguyen T.A., Kanti D.S., Phan T.G., Okitsu S., Ushijima H. Isolation and molecular characterization of Aichi viruses from fecal specimens collected in Japan, Bangladesh, Thailand, and Vietnam. J. Clin. Microbiol. 2007;45:2287–2288. doi: 10.1128/JCM.00525-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.G., Kapusinszky B., Wang C., Rose R.K., Lipton H.L., Delwart E.L. The fecal viral flora of wild rodents. PLoS Pathog. 2011;7:e1002218. doi: 10.1371/journal.ppat.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford A.D., Chapman D., Dixon L., Chantrey J., Darby A.C., Hall N. Application of next-generation sequencing technologies in virology. J. Gen. Virol. 2012;93:1853–1868. doi: 10.1099/vir.0.043182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford A.D., Coyne K.P., Dawson S., Porter C.J., Gaskell R.M. Feline calicivirus. Vet. Res. 2007;38:319–335. doi: 10.1051/vetres:2006056. [DOI] [PubMed] [Google Scholar]
- Reuter G., Boldizsar A., Kiss I., Pankovics P. Candidate new species of Kobuvirus in porcine hosts. Emerg. Infect. Dis. 2008;14:1968–1970. doi: 10.3201/eid1412.080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Boldizsar A., Papp G., Pankovics P. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch. Virol. 2009;154:1529–1532. doi: 10.1007/s00705-009-0473-y. [DOI] [PubMed] [Google Scholar]
- Reuter G., Boros A., Pankovics P. Kobuviruses – a comprehensive review. Rev. Med. Virol. 2011;21:32–41. doi: 10.1002/rmv.677. [DOI] [PubMed] [Google Scholar]
- Reuter G., Boros A., Pankovics P., Egyed L. Kobuvirus in domestic sheep, Hungary. Emerg. Infect. Dis. 2010;16:869–870. doi: 10.3201/eid1605.091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Nemes C., Boros A., Kapusinszky B., Delwart E., Pankovics P. Porcine kobuvirus in wild boars (Sus scrofa) Arch. Virol. 2012 doi: 10.1007/s00705-012-1456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes J.M., Montava R., Tellez-Castillo C.J., Fernandez-Jimenez M., Buesa J. Seroprevalence of Aichi virus in a Spanish population from 2007 to 2008. Clin. Vaccin. Immunol. 2010;17:545–549. doi: 10.1128/CVI.00382-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdiri-Loulizi K., Gharbi-Khelifi H., de Rougemont A., Chouchane S., Sakly N., Ambert-Balay K., Hassine M., Guediche M.N., Aouni M., Pothier P. Acute infantile gastroenteritis associated with human enteric viruses in Tunisia. J. Clin. Microbiol. 2008;46:1349–1355. doi: 10.1128/JCM.02438-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdiri-Loulizi K., Hassine M., Gharbi-Khelifi H., Sakly N., Chouchane S., Guediche M.N., Pothier P., Aouni M., Ambert-Balay K. Detection and genomic characterization of Aichi viruses in stool samples from children in Monastir, Tunisia. J. Clin. Microbiol. 2009;47:2275–2278. doi: 10.1128/JCM.00913-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavisky J., Radford A.D., Gaskell R., Dawson S., German A., Parsons B., Clegg S., Newman J., Pinchbeck G. A case–control study of pathogen and lifestyle risk factors for diarrhoea in dogs. Prev. Vet. Med. 2011;99:185–192. doi: 10.1016/j.prevetmed.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Urisman A., Liu Y.T., Springer M., Ksiazek T.G., Erdman D.D., Mardis E.R., Hickenbotham M., Magrini V., Eldred J., Latreille J.P., Wilson R.K., Ganem D., DeRisi J.L. Viral discovery and sequence recovery using DNA microarrays. PLoS Biol. 2003;1:E2. doi: 10.1371/journal.pbio.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Ito M., Kabashima Y., Tsuzuki H., Fujiura A., Sakae K. Isolation and characterization of a new species of kobuvirus associated with cattle. J. Gen. Virol. 2003;84:3069–3077. doi: 10.1099/vir.0.19266-0. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Kobayashi S., Sakae K., Nakata S., Chiba S., Ishihara Y., Isomura S. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J. Infect. Dis. 1991;164:954–957. doi: 10.1093/infdis/164.5.954. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Ishihara Y., Isomura S., Utagawa E. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J. Clin. Microbiol. 1993;31:2938–2943. doi: 10.1128/jcm.31.11.2938-2943.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Tsuzuki H., Suzuki Y., Ishikawa N., Takeda N., Miyamura T., Yamazaki S. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 1998;72:8408–8412. doi: 10.1128/jvi.72.10.8408-8412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Sugiyama M., Tsuzuki H., Sakae K., Suzuki Y., Miyazaki Y. Application of a reverse transcription-PCR for identification and differentiation of Aichi virus, a new member of the Picornavirus family associated with gastroenteritis in humans. J. Clin. Microbiol. 2000;38:2955–2961. doi: 10.1128/jcm.38.8.2955-2961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhang W., Shen Q., Yang Z., Zhu J., Cui L., Hua X. Aichi virus strains in children with gastroenteritis, China. Emerg. Infect. Dis. 2009;15:1703–1705. doi: 10.3201/eid1510.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]


