Abstract
During the last 2 decades, the continued development and the large-scale production of polyclonal immune serum globulin (ISG) preparations with improved safety and tolerability profiles have allowed treatment to focus on quality of life and long-term freedom from the complications of primary immune deficiency disease, rather than just on freedom from severe acute infections and survival. Available ISG preparations allow routine therapy by a variety of routes and regimens that can be tailored to suit individual patients. Continued vigilance is required, however, because problems with emerging diseases, and the costs and availability of ISG are likely to present continuing challenges.
Historical perspective
Today it seems quite straightforward to give IgG to patients who have immune deficiencies involving decreased antibody production. It is interesting reflect that, although serum therapy was used in the early 1900s, that treatment generally involved the use of serum from convalescent patients or from horses immunized with specific bacteria or toxins [1]. Primary immune deficiency (PID) had not yet been recognized, and penicillin had not yet been discovered; high-titered serum was the only specific therapy for common infections such as pneumococcal pneumonia. Not until World War II did concentrates of human immune globulin became available for widespread use, and it was not until 1952 that Bruton [2] published the first report of the use of immune serum globulin (ISG) in treating a patient who had PID. The studies of the Working Group on Hypogammaglobulinemia in the United Kingdom firmly established the benefit of regular ISG injections in the treatment of this family of illnesses and set the dosage at 25 mg/kg/week [3]. Not until the early 1980s were preparations of IgG that could be safely given intravenously licensed in the United States. Since that time, more purified and better-tolerated IgG preparations have become available, and there has been widespread interest in subcutaneous rather than intravenous administration. The doses of IgG used in patients who have PID have escalated steadily, with increasingly ambitious goals for prevention of infection and end-organ damage. Unfortunately, there also have been a number of reminders that treatment with blood products carries real and potential risks, including transmission of bloodborne infections. More recently, the use of high-dose intravenous immunoglobulin (IGIV) for its anti-inflammatory effects in diseases such as Kawasaki syndrome and for its immunomodulatory effects in autoimmune diseases has increased the demand for this precious commodity. To simplify the discussion of products that usually are denoted by their route of administration (ie, intravenous [IGIV], subcutaneous [IGSC], or intramuscular [IGIM]), this article uses the inclusive abbreviation “ISG” to refer to all polyclonal human immune globulin preparations.
What is immune serum globulin?
Stringent safety standards, the desire to provide antibodies against a wide range of pathogens, and the need to produce products with consistent tolerability and efficacy have led to large-scale industrial production of IgG concentrates which may contain the antibodies from 40,000 to 50,000 units of plasma per batch. These goals may seem to be at odds with the desire to assure safety by using a limited number of well-characterized, usually related, plasma donors for individual patients. The decades since IGIV was introduced have witnessed the recognition of HIV as a bloodborne virus, an outbreak of hepatitis C transmission by IGIV and other blood products in the early 1990s, and increasing concerns about the transmission of prions, which cause spongiform encephalopathies. Therefore, reducing the risk of transmission of known as well as possibly emerging bloodborne diseases has become one of the most important considerations for government regulators (the Food and Drug Administration [FDA] in the United States) and for the plasma fractionation and protein therapeutics industry. Multiple safety steps are used during the purification of therapeutic IgG concentrates from blood. These steps can be summarized as falling into four major categories, summarized in Box 1.
Box 1. Steps used to minimize risk of transmission of bloodborne diseases by immune serum globulin.
Plasma collection
Food and Drug Administration supervision of donor centers
Donor screening/deferral
Donor testing (liver function tests)
Inventory hold
Manufacturing process
Good manufacturing practices and quality assurance
Process validation
“Minipool” testing
Food and Drug Administration approval of lot release
Specific steps for viral inactivation/removal
Cold ethanol precipitation and depth filtration
Heat (pasteurization at 60°C)
Low pH
Treatment with pepsin or other proteases
Fatty alcohol/fatty acid treatment
Solvent/detergent treatment
Nanofiltration
Record keeping/recall notification
Most of the plasma used for production of therapeutic proteins such as ISG is obtained specifically for this purpose by plasmapheresis and is termed “source” plasma, although some plasma from donations of whole blood (recovered plasma) still is used as well. All products marketed in the United States must be made from plasma obtained in the United States. FDA regulations governing donor selection and plasma collection are available on the Internet [4], [5]. Donors must complete a questionnaire, undergo a physical examination, and have normal blood counts and liver function tests before use of their plasma is considered. Units of plasma are tested for serologic markers of known bloodborne diseases and are discarded if found positive. Plasma services use a “quarantine” or “inventory hold” procedure in which an individual unit, even if it tests negative for all know pathogens, is stored separately until the donor returns and provides another unit of plasma. Only when the second (and subsequent donations) also tests negative can a previously obtained unit be used. Computerized databases enable the tracking of all products derived even in part from any given plasma donation. Patients receiving blood-derived proteins are encouraged to keep careful records of the lot numbers and names of all products they receive. If a donor ever is determined to have been incubating an undetected, potentially bloodborne disease (eg, a slow-virus encephalopathy or emerging viruses such as hepatitis C in the early 1990s, or West Nile virus more recently), “lookback” programs can be activated so that any patient who received a product containing plasma from that donor can be identified, examined and tested, and treated if necessary.
The manufacturing processes of all ISG products currently marketed in the United States include at least one step of the cold-alcohol fractionation process developed by Cohn and his colleagues in the early days of World War II [6]. Different manufacturers then use different purification steps, including differing column chromatography protocols, to produce final products that are highly enriched (> 95%) in IgG. Most products are essentially free from IgM, but all contain at least small amounts of IgA. The properties of ISG products currently marketed in the United States are summarized in Table 1. All products currently available in the United States contain more than 95% IgG, and all contain all subclasses of IgG in approximately the same ratio of concentrations as in normal plasma. Testing for the spectrum and titer of multiple different specific antibodies in any given lot of a product is not required; federal standards mandate only that a minimal titer of antibody against measles virus be present. The current FDA guidelines for licensing IGIV preparations are available on the Internet [7]. Similar standards have been applied for the licensure of ISG intended for administration by the intramuscular and subcutaneous routes. Most of the products currently marketed in the United States have been licensed while these guidelines have been in effect.
Table 1.
Properties of polyclonal immune serum globulin products currently marketed in the United States
| Product | Manufacturer | IgG concentration | IgA concentration | Excipients | Viral safety |
|---|---|---|---|---|---|
| Products intended for intravenous use | |||||
| Carimmune NF | CSL-Behring | powdera | 0.72 mg/mL | sucrose | low pH, pepsin, 35 nm NF |
| Flebogamma DIF | Grifols | 5% | < 0.05 mg/mL | 50 mg/mL sorbitol; < 3 mg/mL PEG | pasteurization, S/D, 20 nm NF |
| Gammagard S/D | Baxter | powderb | < 2.2 μg/mL |
|
S/D |
| Gammagard Liq. | Baxter | 10% | 37 μg/mL | 250 mM glycine | pH 4, S/D, NF |
| Gamunex | Talecris | 10% | 46 μg/mL | 200 mM glycine | pH4, caprylate |
| Octagam | Octapharma | 5% | < 0.2 mg/mL | 10% maltose | S/D, pH 4 |
| Privigen | CSL-Behring | 10% | < 25 μg/mL | 250 mM L-proline | pH4, NF |
| Products intended for subcutaneous use | |||||
| Vivaglobin | CSL-Behring | 16% | < 1.7 mg/mL | 0.3 g/L NaCl | pasteurization |
| 250 mM glycine | fatty alcohol/low pH | ||||
| Products Intended for intramuscular use | |||||
| Gamastan | Talecris | 16% | NL | 300 mM glycine | S/D |
Abbreviations: NF, nanofiltered; NL, not listed; PEG, polyethylene glycol; S/D, solvent/detergent.
May be reconstituted to 3%, 6%, 9%, or 12% solution.
May be reconstituted to 5% or 10% solution. Data given for 5% solution.
IgG molecules in concentrated solutions tend to aggregate, which brings their crystallizable fragments (Fc) into close proximity. These Fc portions can activate complement and cross-link Fcγ-receptors, leading to the production of mediators that cause adverse reactions during IgG infusions. All the currently available ISG products contain excipients such as amino acids or sugars that are included to minimize the formation of aggregates and preserve the IgG in its monomeric state. These excipients differ in different products, as listed in Table 1. The use of products with certain excipients may be inadvisable in specific patients (eg, sucrose in patients at risk for renal damage [8], products containing proline in patients who have disorders of metabolism of that amino acid, products containing maltose in diabetic patients whose glucose monitors might give false readings because of that sugar) [9]. Many products are treated at low pH, and some are bottled at low pH as well. The buffering capacity of the low-pH solutions is limited, however, and low pH has not been problematic. None of the products currently marketed in the United States, even those intended for intramuscular or subcutaneous use, contain thimerosal or any other preservative. Some products require refrigerated storage, but others have been shown to have satisfactory stability at room temperature. All should be brought to room temperature before administration. The content of salt and the concentration of IgG itself vary as well, and not all products are licensed for administration by all routes. Thus, the selection of the most appropriate product must be individualized for each patient. Despite the use of large donor pools, the spectrum of antibodies, concentrations of certain specific antibodies, and the presence of trace amounts of other plasma proteins in different preparations also differ. These differences may lead to unpredictable and idiosyncratic differences in tolerance of different products by individual patients. Therefore, IgG products should not be considered as interchangeable generics. Physicians should be notified and may want to slow the rate of infusion, administer premedications, and/or give the IgG under close observation if a patient must be given a product he or she has not received previously.
Minimizing the risk of bloodborne pathogen transmission
A dilemma arises in assuring the absence of bloodborne pathogens in IgG preparations, because the IgG itself may complex with and impair detection of viruses and other pathogens without actually inactivating them. Complexes of virus particles with bound IgG usually have chemical properties that differ from the viruses themselves. Thus, the presence of antibodies against a virus may cause partitioning of the virus-antibody complex away from the product during purification steps that would not remove the uncomplexed virus itself. This phenomenon probably contributed to the transmission of hepatitis C when plasma containing antibodies to that virus was excluded from pools used to manufacture ISG at a time when sensitive tests for the virus itself were not available. To avoid this problem, animal viruses and prions that have properties similar to important human pathogens but against which humans are not likely to have antibodies are “spiked” into units of donor plasma that then are run through scaled-down production steps to assess the ability of each step to remove and/or inactivate the virus [10]. These viruses are listed in Table 2 together with the human viruses they are intended to simulate. Note that significant removal of many intact viruses can be accomplished by the Cohn cold-alcohol precipitation steps used for the initial purification of the IgG-containing fraction of plasma. The two major targets for viral inactivation are the protein coat of nonenveloped viruses and the lipid envelope of enveloped viruses. In general, surface proteins of nonenveloped viruses are more sensitive to inactivation by low pH, proteolytic enzymes, and heat (pasteurization) than are the lipid and protein structures of enveloped viruses. Inactivation of enveloped viruses generally requires dissolution of the lipid envelope, which is accomplished in some products by treatment with fatty acids, fatty alcohols, or solvent/detergent combinations such as tri-(N-butyl) phosphate/Triton X-100 [11]. Many products also are processed by passage through filters with nanometer pore sizes that can remove many virus particles by size, regardless of their chemical characteristics.
Table 2.
Viruses used to test efficacy of viral removal/inactivation steps during IgG preparation
| Virus | Nucleic acid | Size (nm) | Human virus modeled |
|---|---|---|---|
| Enveloped viruses | |||
| HIV-1 | RNA | 80–100 | HIV 1 & 2 |
| Pseudorabies | DNA | 120–200 | Herpes |
| Bovine viral diarrhea | RNA | 50–70 | Hepatitis C |
| West Nile virus | RNA | 50–70 | West Nile |
| Nonenveloped viruses | |||
| Encephalomyocarditis | RNA | 25–30 | Hepatitis A |
| Minute virus of mice | DNA | 18–24 | Parvovirus B19 |
| Reovirus | RNA | 70 | Rotavirus |
| Porcine or bovine parvovirus | DNA | 20–30 | Parvovirus B19 |
| Prion (agent of Creutzfeldt-Jacob disease) | |||
| Rodent-adapted hamster scrapie | |||
Who needs it?
Assessing the need for IgG replacement or augmentation in any given patient usually requires an in-depth understanding of the patient's condition and underlying diagnosis. In patients who have confirmed diagnoses known to result in severe antibody deficiency, such as severe combined immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and hyper-IgM syndromes, the diagnosis alone may provide sufficient grounds to initiate IgG replacement therapy as soon as it is established. The pattern of infections, family history, physical examination, and flow cytometry usually will lead readily to a diagnosis that can be confirmed by molecular analysis of the patient's mutation [12], [13]. For patients who present with recurrent infections or with symptom complexes that may or may not be caused by infection, the decision to start IgG therapy should be based on laboratory data demonstrating the antibody deficiency and a deficient response to appropriate vaccines, as well as on evidence for increased morbidity caused by infection. The use of measurements of specific antibody titers and vaccine responses to help determine whether IgG supplementation may be indicated for a given patient has been discussed extensively elsewhere [14], [15], [16] and is beyond the scope of this article. Guidelines for the diagnosis and management of PID and the use of IgG replacement in antibody-deficiency diseases have been promulgated by the Immune Deficiency Foundation and the Joint Council on Allergy, Asthma and Allergy in the United States (JCAAI) [12], [13] and presented at a meeting of the European Society for Immune Deficiency [17]. A very helpful scheme abbreviated from the latter is reproduced in Box 2.
Box 2. Guidelines for IgG replacement/augmentation presented at the 2006 meeting of the European Society for Immune Deficiency.
When is IgG replacement/supplementation indicated?
IgG < 200 mg/dL: All patients
IgG 200–500 mg/dL: If a specific antibody deficiency is identified and frequent infections are documented.
IgG > 500: If a specific antibody deficiency identified and severe/recurrent infections are documented
In many patients the antibody deficiency may be only transient. These patients include very low birth weight babies, infants with delayed development of the full spectrum of necessary humoral responses, older children and adults who have been given cytotoxic chemotherapy for cancer, and patients who have been given cytotoxic or immunosuppressive therapy for autoimmune disease and/or to prevent transplant rejection. Nearly all patients who receive hematopoietic stem cell transplants for severe combined immunodeficiency require IgG supplementation for at least a year. Many recipients of stem cell transplants have poor B-cell engraftment or function and require replacement therapy for life. Patients, particularly children under 5 years of age, who do not have confirmed genetic diagnoses of PID disease should be re-evaluated periodically to determine if IgG replacement/augmentation is still necessary. Re-evaluation typically is performed after the patient has been off IgG therapy for at least several months and usually involves measuring the specific antibody response after administration of protein and polysaccharide vaccines. There are no commercially available preparations of enriched IgA or IgM, so patients who have isolated IgA deficiency generally are not considered candidates for IgG supplementation. Patients who have significant morbidity associated with low or absent IgA levels should be checked for specific IgG antibodies and vaccine responses, however, because these conditions may coexist as part of a broader immune deficiency. Because most laboratories use very broad ranges for “normal” IgG levels, patients who do not meet rigorous criteria for common variable hypogammaglobulinemia (also termed “common variable immune deficiency”) still may benefit from IgG supplementation. Conversely, patients who have IgG levels below a laboratory's normal ranges for the age of the patient do not necessarily require IgG replacement therapy if they have satisfactory specific antibody concentrations and vaccine responses.
Many patients who do not have antibody-deficiency diseases receive IGIV for its immunomodulatory and/or anti-inflammatory properties. The most common such condition for which IGIV is used in pediatrics is Kawasaki syndrome [18]. IGIV also is used in toxigenic bacterial infections and in idiopathic thrombocytopenic purpura (ITP), Guillain-Barre syndrome, chronic idiopathic demyelinating polyneuropathy (multifocal motor neuropathy), and other autoimmune diseases. A recent evaluation of the evidence for the use of IGIV in different conditions is available elsewhere [19] and is beyond the scope of this article.
Alternatives to immune serum globulin therapy
Patients who have confirmed antibody deficiency may be maintained without IgG replacement for periods of time, using relative isolation and/or prophylactic antibiotics. If exposed or potentially exposed to viral pathogens (eg, in a local outbreak of chickenpox or mumps or when traveling to a developing nation), such patients may be given hyperimmune (eg, Varicella-zoster immune globulin) or standard intramuscular ISG preparations. This approach may be satisfactory for patients who reasonably can be expected to overcome a developmental delay in antibody production or to recover from the effects of a treatment regimen that has been completed. This approach should not be considered a long-term strategy for patients who have clearly diagnosed PID disease. IGIV has been found to be helpful in certain HIV-infected infants, but with prenatal treatment reducing the maternal transmission of HIV and improved antiviral chemotherapy, this setting is not a major use of ISG. In a previous era, plasma, often from a parent or relative, was used for antibody replacement in children who had PID disease. This approach is rarely used in the United States, mainly because of the time and effort required to be sure that the donor plasma is free from any bloodborne pathogens (which maybe clinically unapparent) and the desire to provide a broad range of protective antibodies.
How to administer immune serum globulin: intravenously or subcutaneously?
Although individual doses of ISG may be given intramuscularly, the pain and risks associated with deep intramuscular injections limit the amount that can be administered this way. Routine therapy by the intramuscular route is rarely used now, although it was the mainstay of treatment for PID disease for more than 30 years. Most IgG replacement/augmentation regimens now employ intravenous or subcutaneous administration. In general, doses are in the range of 300 to 800 mg/kg per month (see next section). Intravenous infusions of IgG generally are well tolerated. The introduction of ISG preparations that could be given safely by the intravenous route in the early 1980s was a major advance in the care of patients who have PID disease. Obstacles that had to be overcome included stabilizing the IgG molecules so they would not aggregate in solution and purification to remove traces of proinflammatory molecules such as activators of the kallikrein-kinin and clotting cascades. As noted earlier, a major breakthrough was the recognition of the importance of including excipients such as amino acids and/or sugars in the final preparations. Most adverse reactions to IGIV are related to the rate of infusion. Patients who are naive to IgG replacement, who have had interruptions in their therapy, and/or who are actively or chronically infected have an increased risk of infusion-related adverse effects. These effects may be related, in part, to the formation of antigen-antibody complexes while the IgG is being given and/or the rapid release of lipopolysaccharide or other components of pathogens already present in the recipient. The risk of these reactions may be reduced by making sure patients are afebrile and that active infections are being treated with antibiotics before beginning IGIV therapy or giving any scheduled infusion. Some studies have shown that the incidence of reactions is increased when patients already receiving therapy are given a different brand of IGIV [20]. To minimize rate-related adverse effects, infusions should be started slowly, at rates not above 0.01 mL/kg/minute (equaling 0.5 mg/kg/minute of 5% solution or 1 mg/kg/minute of 10% solution). Vital signs should be checked frequently during IGIV infusions, particularly in naive patients. If the patient remains comfortable and stable, the rate of the infusion may be increased in a stepwise manner, usually at 15- to 30-minute intervals, up to the maximum tolerated by the patient. Most preparations are labeled for administration at a maximum rate of 0.08 mL/kg/minute (4 or 8 mg/kg/minute of 5% or 10% solution, respectively). Infusion-related adverse reactions frequently mimic the signs of infection, including chills and even rigors, arthralgias and/or myalgias, and headache. Because most of these symptoms are related to the rate of infusion, slowing or temporarily stopping the infusion may allow the symptoms to subside; then the infusion can be resumed at the previously tolerated rate. If these precautions fail to prevent these symptoms, premedication with antipyretics, antihistamines, and/or corticosteroids may help ameliorate the symptoms. During prolonged infusions, such medications may be repeated if necessary. In some cases, patients report systemic reactions including back pain, chest tightness, and a feeling of anxiety or a sense of impending doom, frequently in association with flushing and tachycardia. This type of reaction may resemble anaphylaxis but usually does not involve IgE. Therefore, this type of reaction has been termed “anaphylactoid.” A key difference between the anaphylactoid reactions that accompany IGIV infusions and true IgE-mediated anaphylaxis is that the former usually are associated with hypertension, rather than hypotension, as would be expected in true anaphylaxis. True anaphylaxis may occur in patients receiving IGIV, particularly in those who are deficient in IgA but still have the capacity to produce IgE [21]. True anaphylaxis occurs very rarely but may be life threatening. Therefore, any practitioner or facility that administers IGIV should be equipped to treat anaphylaxis if it occurs. The risk of true anaphylaxis can be minimized by screening patients for complete IgA deficiency, by starting initial infusions extremely slowly, by using products with the lowest concentration of IgA, and by testing the patient for IgE-antibodies against IgA if this is a concern. Many IgA-deficient patients also are deficient in other immunoglobulin isotypes and/or in specific IgG antibodies against important pathogens. Such patients should not be denied ISG therapy because of the IgA deficiency, but caution should be used in its administration.
Headaches may occur during or after intravenous infusions and sometimes repeatedly follow the infusions by as much as 48 to 72 hours. These headaches may have the character of migraines and are more common in patients who suffer from migraines independently of their IGIV infusions. In rare cases, headaches following IGIV infusions may be accompanied by meningismus, and aseptic meningitis has been well documented. Headaches sometimes may be prevented by the use of corticosteroids as premedication or for a day or two following the infusion, or by the use of triptans or other migraine treatments. Additional, rare complications of IGIV therapy include transfusion-related acute lung injury, renal failure, and thromboses. A comprehensive review of the adverse effects and complications of IGIV infusions is available and should be read by everyone who administers this form of therapy [22]. Quite often, patients who experience headaches or other adverse effects with one brand of IGIV may tolerate another with no problem. Therefore, switching products may be indicated to obviate the adverse effects. Conversely, because not all products are tolerated equally by a given patient, substitutions should be made carefully, and under supervision. Generally when switching products, slow infusion rates should be used, at least initially.
The pharmacokinetics of IGIV have been well described [23]. By the end of an intravenous bolus of IgG, the IgG is mostly intravascular, and its concentration can be expected to rise by 100 to 200 mg/dL for every 100 mg/kg given. It thus is common for peak serum IgG concentrations to rise by as much as 1000 mg/dL following doses in the conventional replacement range (300–800 mg/kg). Over the next 48 to 72 hours, the IgG becomes distributed into the total extracellular fluid volume, and the serum concentration may drop by 25% to 40%. After this re-equilibration, IgG is catabolized with first-order kinetics and has a half-life of around 22 days. Currently marketed ISGs contain intact IgG molecules that have not undergone any chemical modification. Thus, the distribution and half-life of intravenously administered IgG is essentially identical to that of endogenously produced native IgG [24], [25], [26], [27]. For this reason, most intravenous regimens repeat doses at 21- to 28-day intervals. Depending on the dose and whether there is any endogenous IgG production at all, dosing intervals of 28 days or longer frequently leave patients with serum IgG concentrations in the range of 400 to 500 mg/dL, or even less, at the end of the dosing interval. Many patients report flulike symptoms, increased fatigue, and/or malaise when the trough serum IgG level falls so low, and they may have increased susceptibility to infection at that time. In that situation, the dose may be increased or the interval shortened. Higher doses and/or shorter intervals also will be necessary in patients who have gastrointestinal or renal protein loss and/or increased catabolism of IgG, as discussed later.
Although many patients tolerate long-term intravenous IgG replacement regimens with no problems, surveys show that a high proportion of patients describe the conditions under which they receive their infusions as inconvenient, because they must travel to a hospital or infusion center, miss school or work, and/or experience difficulty with intravenous access. Subcutaneous regimens offer freedom from those concerns. Bruton treated the first agammaglobulinemic patient to be reported with subcutaneous injections of ISG, but other investigators used intramuscular injections, which became the standard of care for nearly 3 decades. In the early 1980s, the use of small, battery-powered syringe driver pumps to give subcutaneous infusions of 16% ISG intended for intramuscular use over several hours was described [28], [29], [30]. This method of administration is free from the pain associated with the deep intramuscular injections and was much better tolerated by the patients, so higher doses could be administered routinely [31]. In general, 25- to 27-gauge needles extending 6 to 11 mm under the skin are inserted perpendicularly. A wide variety of special needles and infusion sets are now available specifically for IGSC therapy [32]. Depending on the size of the patient, 5 to 20 mL are delivered per site, usually over 2 hours or so. Several sites may be used for each infusion of ISG, so weekly dosing is a common regimen. Frequently used sites include the abdomen, inner or anterior thighs, and the backs of the arms. In general any site at which one can “pinch an inch” is acceptable for subcutaneous infusions of 10 to 20 mL of ISG. A very nice illustration of potential subcutaneous infusion sites and good technique for inserting subcutaneous needles is available on-line from the nursing staff of the National Institutes of Health Clinical Center [33]. The author and colleagues have found that the size of the patient and the time over which the infusion is given are important factors in determining the maximum volume that can be infused at any given site. Thus, some patients may give themselves 40 to 60 mL of ISG in a single site, over a period of time as long as 8 hours or more. Some patients prefer this type of regimen and take their IGSC while they sleep. In contrast, other patients may prefer to take small doses of IGSC on a daily basis (eg, by pushing 10 mL into a single site over several minutes). In a series of 20 patients using 15% or 16% ISG preparations reported from UH/Rainbow Babies & Children's Hospital, most of the regimens were within the parameters of 0.12 to 0.24 mL/kg/site/hour [34] Basically, once the monthly dose of IGSC has been calculated, a wide variety of schedules may be used to tailor the infusion regimen to the patient's preferences.
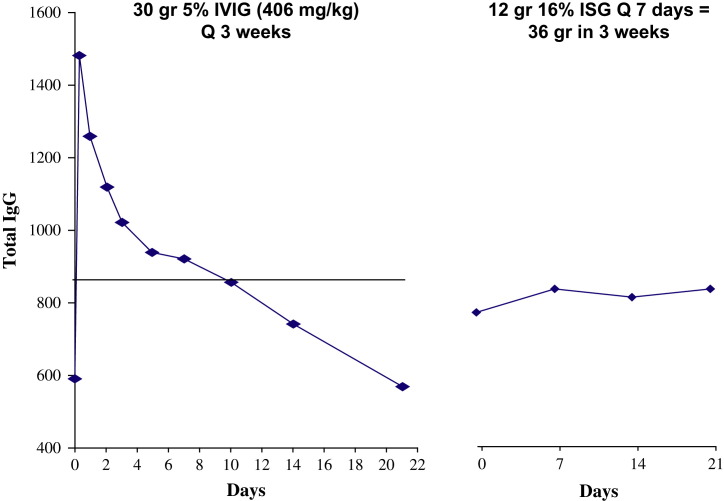
Although most physicians and patients in the United States adopted the intravenous route when preparations that could be administered intravenously became available, patients in other countries and those who experience severe adverse effects from intravenous preparations continue to use the subcutaneous route [35], [36]. Serious systemic adverse effects are rarely a problem with subcutaneous infusions; most studies have reported that less than 1% of infusions are associated with systemic adverse events [37]. Because the expertise and experience necessary to establish and maintain intravenous access are not required for subcutaneous administration, and because of the low risk of serious reactions, subcutaneous infusions usually are administered at home by the patients themselves or their parents or partners. The major disadvantage of subcutaneous infusions is the limitation on the volume of ISG that can be given at one time. Because the infusions can be given at home and/or while the patient is performing other activities, most patients readily adapt to weekly or more frequent infusions. The division of the monthly dose of ISG into weekly or even more frequent infusions and the slower absorption of IgG into the circulation from the subcutaneous site than from intravenous boluses tends to flatten out the curve of serum IgG concentration over time, as seen in Fig. 1. Elimination of the high peaks and low troughs associated with intermittent intravenous boluses ameliorates most infusion-related adverse effects and also the feelings of malaise and fatigue associated with the low troughs. Subcutaneous infusions frequently are associated with local swelling, redness, and an itching or burning sensation, but these effects are rarely serious and usually subside over several hours. If the patient continues with subcutaneous infusions, these local reactions tend to lessen with time, but the reasons for this change are not known. Although many patients have used the subcutaneous route for more than 25 years, chronic changes, fibrosis, or lipodystrophy at the infusion sites have not been problematic. It is rare to be able to identify the site at which a subcutaneous IgG infusion was given more than 24 hours after the infusion has been completed. Because it is easier for the patients to adjust their infusion schedules to their school or work schedule, rather than vice versa, and because patients no longer have to travel to infusion centers or the hospital for their treatments, many patients find that home IGSC regimens increase their sense of independence and autonomy. In turn, this response results in improved quality of life experienced by many, but not all, patients [37], [38], [39], [40].
Fig. 1.
Serum IgG concentrations in a 34-year-old man who has X-linked agammaglobulinemia. (Left panel) The diamonds and blue line indicate serum IgG concentrations at various times after a single intravenous infusion of 30 g (406 mg/kg). The black line indicates the average daily IgG level calculated by interpolation from points indicated by diamonds. Mean ± SEM = 850.6 ± 43 mg/dL. (Right panel) Serum IgG concentrations in the same patient receiving 12 g 16% IgG every 7 days. Mean ± SEM = 816 ± 16 mg/dL. (From Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol 2004;112:1–7; with permission.)
At present, only one ISG preparation marketed in the United States is licensed for subcutaneous administration. Small series and anecdotal reports, however, suggest that most preparations licensed for administration by other routes are well tolerated when administered by the subcutaneous route. Several preparations are marketed for subcutaneous use in Europe, and it is likely that additional products with concentrations as high as 16% or even 20% will be available in the United States in the next few years. A comparison of the volumes required to give comparable amounts of IgG by the subcutaneous and intravenous routes using currently available preparations is given in Box 3, along with some sample subcutaneous regimens.
Box 3. Comparison of intravenous and subcutaneous dosing.
-
1.For a 20-kg 6-year-old receiving 500 mg/kg per month = 10 g IgG
- = 200 mL of 5% intravenous solution
- = 100 mL of 10% intravenous solution
- = 62.5 mL of 16% subcutaneous solution
-
2.In subcutaneous treatment regimens using the unit dose approach to deliver approximately 10 g IgG per month to a 20-kg 6-year-old child, the volume of 16% solution required = approximately 62.5 mL
- = 16 mL/week (one 10-mL and two 3-mL bottles of 16% solution) administered as one infusion into two or three sites = 2.56 g/week = 10.24 g/month
- = 10 mL (one 10-mL bottle of 16% solution) administered every fifth day (six times/month) into one or two sites = 9.6 g/month
- = 6 mL (two 3-mL bottles of 16% solution) administered every third day as one infusion into one site = 0.96 g/dose = 9.6 g/month
-
3.In subcutaneous regimens for a 30-kg-child receiving approximately 15 g/month, the volume of 16% solution required = approximately 94 mL
- = 10 mL/week (one 10-mL bottle) administered every third day into one or two sites = 16 g/month
- = 20 mL administered once a week into two or three sites, with one extra infusion per month = 16 g/month
- = 3 mL administered daily into one site over 15 minutes (“push”) = 14.4 g/month
-
4.In subcutaneous regimens for a 40-kg-child receiving approximately 20 g/month, the volume of 16% solution required = 125 mL
- = 30 mL/week administered into one or two sites once a week = 4.8 g/week = 19.2 g/month
- = 20 mL administered every fifth day (six times per month) into one site = 19.2 g/month
- = 16 mL administered every Saturday and Sunday into one or two sites = 20.5 g/month
- = 10 mL delivered every Monday, Wednesday, and Friday (weekends off) by push or into one site = 19.2 g/month
How much to give
The efficacy of IgG replacement in patients who have PID disease has been clearly demonstrated in studies going back to the 1950s and 1960s. A recent survey documented the decrease in the incidence of pneumonia after patients who had PID disease were put on IGIV treatment [41]. The doses of IgG given in standard replacement regimens has increased over time, as more convenient routes of administration have become practical. In classic studies in the early 1960s, the British Medical Research Council working group on hypogammaglobulinemia reported that 50 mg/kg/week was more effective than 25 mg/kg/week in preventing febrile episodes, otitis media, and pneumonia, but the differences did not seem to be of sufficient clinical importance to warrant widespread use of the higher dosage [3]. Licensing studies of the first generation of intravenous products in the United States used doses in the range of 100 to 200 mg/kg/month [42], [43], [44]. Studies by Roifman and his colleagues [45], [46] beginning in the mid-1980s showed that patients receiving doses of 200 mg/kg/month did not achieve trough serum IgG levels higher than 500 mg/dL. In contrast, when the patients were crossed over to the arm that received 600 mg/kg/month, they did sustain trough levels above 500 mg/kg. In turn, the incidence of both major and minor infections was reduced greatly when the patients received the higher dose and maintained higher levels. Similar findings were presented by Roifman and colleagues [47] from a more recent study in which patients were maintained on doses of IGIV selected by their physicians: patients receiving higher doses and maintaining higher serum trough IgG levels had fewer infections. A crossover study comparing higher IGIV doses (600 mg/kg/month in adults and 800 mg/kg/month in children) with “standard” doses (300 mg/kg/month in adults and 400 mg/kg/month in children) showed statistically significant reductions in the incidence of infection and cumulative days of illness in the higher-dose groups [48]. Licensing studies of the current generation of IGIV products marketed in the United States have used doses in the range of 400 to 500 mg/kg/month, reflecting the dose the patient had been receiving before entering the study [20], [24], [25], [26], [27]. These regimens all resulted in an annual incidence of serious bacterial infections (meeting FDA definitions) of 0.1 infection/patient/year or less and an overall incidence of infection of two to four infections per patient per year. The Practice Parameters promulgated by the JCAAI call for maintaining trough serum IgG levels of 500 mg/dL as a useful guideline [13]. This level typically requires doses in the range of 300 to 600 mg/kg/month. In patients who have gastrointestinal and/or renal protein loss, higher doses and/or shorter dosing intervals may be necessary to maintain trough levels above 500 mg/dL and keep the patient free from infection. Higher doses—up to 800 mg/kg/month or even higher—are recommended for patients who have chronic lung and/or sinus disease [13], [45], [46], [47], [48]. There have been several reports of progressive lung disease and dysfunction in patients who seem clinically free from pneumonia and who report few or no symptoms of chronic bronchitis or bronchiectasis [49], [50]. These reports are consistent with the anecdotal experience of most immunologists who have large numbers of antibody-deficient patients in their practices. Close monitoring, which may include annual high-resolution CT scans of the chest, formal pulmonary function testing, and even bronchoscopy in some cases, is recommended for all patients who have these diseases. In the current era, most experts agree that prevention of pneumonia and serious infection is no longer sufficient indication that the patient's management is optimal. Efforts aimed at normalizing pulmonary function tests, maximizing the patients' ability to participate in a full range of activities, and preventing progressive loss of lung function seem warranted [51]. Preventing and/or arresting chronic lung and sinus disease in patients who have PID disease frequently requires IgG replacement doses higher than 750 mg/kg/month, particularly in patients who already have chronic infection and structural damage before therapy is started. Such patients usually benefit by comprehensive approaches, including intense antibiotic therapy, bronchodilators and/or inhaled corticosteroids, mucolytics, and physical therapy, to improve pulmonary toilet and/or sinus surgery. Patients who have selective or lacunar antibody deficiencies and who do not have severe hypogammaglobulinemia per se still require full doses of ISG replacement to maintain adequate titers of the specific antibodies they cannot produce on their own. Similarly, antibody-deficient patients who have high IgG levels caused by polyclonal B-cell activation, as occurs in systemic lupus erythematosus or HIV infection, and patients who have monoclonal gammopathies actually may have elevated serum IgG levels but still need full doses of ISG replacement to provide the spectrum of antibodies necessary for protection against the pathogens to which they may be exposed.
Some evidence suggests that the bioavailability of IgG is decreased is when it is given subcutaneously instead of intravenously [52]. The tendency toward higher trough levels with weekly subcutaneous treatment may counterbalance any decreased efficacy caused by tissue degradation of the IgG administered by the subcutaneous route, however. Data that would allow determination of the effects of maintaining higher trough levels by fractionating the cumulative dose that otherwise would be given by a single monthly intravenous bolus are sparse. In particular, the relative importance of the high peaks achieved with the intermittent boluses is unclear. Two major efficacy studies of the single ISG product currently licensed for subcutaneous administration in the United States have been performed. In the United States pivotal trial, a dose increase of 37% above the previous intravenous dose was used to meet the FDA requirement that the area under the curve of serum IgG concentration versus time be the same for both routes of therapy [52]. In contrast, a study performed contemporaneously in Europe and Brazil used one fourth of the previous monthly intravenous dose as the weekly subcutaneous dose. In both studies, equal rates of infection were obtained: 0.04 serious bacterial infections per patient per year, and 4.4 infections per patient per year overall. The range of subcutaneous doses spanned 34 to 352 mg/kg/week in the United States study and 51 to 147 mg/kg/week in the European/Brazil study [53]. These dose ranges and results are quite consistent with those used in licensing trials of intravenous products in the United States in the past few years.
Monitoring therapy
Most patients who have PID disease require IgG replacement therapy for life. It therefore is extremely important to monitor them closely to be sure that (1) the treatment itself is associated with as few adverse events and as little interference with normal activity as possible; (2) the treatment regimen is adequate and maintains control of acute infections as well as chronic complications of the underlying disease; (3) patients do not acquire any bloodborne infections or other long-term complications of their therapy.
Monitoring during infusions
Before beginning any infusion, particularly an intravenous infusion, the patient should be reassessed. It is important to note any changes in medications and signs/symptoms of chronic or acute infection. Adverse effects occurring for up to 72 hours after the previous infusion should be noted. Changes in risk factors for adverse effects also should be noted. For example, starting oral contraceptives or increased cigarette smoking may increase the risk of thrombotic complications of IGIV infusions. Many patients who have PID disease and chronic bronchitis/bronchiectasis have some degree of reversible airway obstruction. If increased secretions and/or bronchospasm are present, bronchodilators may be helpful before or during the infusion. The author and colleagues find it useful to measure and record expiratory flow rates with an office spirometer or even a simple peak flow meter to help with this assessment and to document a patient's status over time. Having the airways as open as possible at the beginning of the infusion may help prevent serious problems if the patient experiences bronchospasm/chest tightness during the infusion. If patients are or recently have been febrile, it usually is helpful to pretreat with antipyretics. If there are signs/symptoms of acute bacterial infection, it may be desirable to defer the infusion for a few days while initiating antibiotic therapy, to avoid shaking chills and severe myalgias/headache during the infusion. The IgG may be needed to help resolve the infection, however, so this delay must be done with caution. Patients who have intercurrent acute gastroenteritis may benefit from antispasmodic or antiemetic treatment before or during the infusion. Some patients may require antipyretics, antiemetics, and/or antimigraine premedication on a routine basis, and some also may require corticosteroids. The author and colleagues often have the latter type of patient take a dose of steroids orally several hours before the infusion is initiated. The patient's hydration status should be assessed carefully, and it usually is a good idea to record the patient's weight at the time of each infusion to allow comparisons. It is possible that dehydration may increase the risk of renal complications, hyperviscosity, and thromboses. It may be prudent to give patients intravenous fluids before actually starting the IGIV to minimize these risks. Conversely, patients who have or are at risk for congestive heart failure may require deferring the fluid/salt/protein load of an infusion if they have recently gained weight, have increased dyspnea and/or rales on chest examination, and/or have increased peripheral edema. Patients who have congestive heart failure and/or fluid retention from other causes may benefit from the administration of diuretics before, during, or following their infusions. Obviously, monitoring such patients closely during the infusion for signs of dyspnea/fluid retention is important.
Before the infusion begins, vital signs should be recorded. It may be important to be sure that the patient has time to relax and adjusted to the ambient conditions before recording the “baseline” vital signs to avoid the phenomenon of “white coat hypertension” that then might lead to a misinterpretation of decreased blood pressure as the patient relaxes once the intravenous has been placed and the infusion is running. If the patient is comfortable or sleeping, and particularly if the pulse has decreased rather than increased, blood pressure drops of 10% or 20% or even more can be expected and do not necessarily mean that shock is imminent, especially if the patient is not flushed or having dyspnea. Intravenous infusions usually are started at 0.01 mL/kg/minute, and the rates are increased or doubled at 15- to 30-minute intervals to a maximum of 0.08 or 1.0 mL/kg/minute in most cases. Stepwise increases in rates should be made only if the patient is tolerating the infusion well. Therefore vital signs and the patient's condition should be recorded before and 5 to 10 minutes after each rate change. The maximum rate tolerated by the patient at previous infusions should be exceeded only with caution, and it is important to realize that stepwise increases in rates and the maximum infusion rate may vary when the same patient is given different products. Therefore, when any patient must switch products, close monitoring of the patient's condition and vital signs is necessary, because the infusion rates and/or intervals between rate increases may require adjustment.
Subcutaneous infusions of ISG very rarely are associated with systemic adverse effects or significant changes in vital signs. Inadvertent intravascular administration should be avoided by checking to be sure there is no blood return from the needle before actually starting the ISG infusion. Because with most subcutaneous regimens the total monthly dose is fractionated into four or more individual doses, because the IgG is given more slowly, and because adsorption from the subcutaneous site into the circulation is slower than with intravenous infusions, this route may be preferred in patients at risk from cardiovascular, thrombotic and/or renal complications. Many patients who complain of severe headaches during or following intravenous ISG infusions have less severe problems after subcutaneous infusions, but migraines still may occur, and medication may be required for up to 48 hours after the infusion has been completed. Vital signs usually are not monitored repeatedly during subcutaneous infusions, but the infusion site should be observed by the patient a few times during the infusion. Most subcutaneous infusions are accompanied by swelling with or without redness, and many patients report local pruritus or a burning sensation. These symptoms may be obviated by treatment with antihistamines before or during the infusion. Pretreatment with corticosteroids or antiemetics is rarely needed. One area of special concern with subcutaneous infusions is the risk of cellulitis or other local infection at the infusion sites. Because most patients have swelling and redness, and the injected fluid may lead to a feeling of fluctuance, distinguishing this manifestation from infection sometimes can be difficult. Application of warm compresses or gentle massage may increase local circulation and help dissipate the infused product. In general, most local reactions to IGSC infusions subside within hours after the infusion is completed. Any site at which redness, swelling, or warmth is increasing with time after the infusion should be considered potentially infected and observed very closely or examined by a professional. Patients who self-infuse at home always should be able to contact a physician or nurse on-call. In some cases it may be helpful to ask the patient to mark the size of any local reaction with a pen, so that potential enlargement can be tracked objectively. Although most home-care services and physicians in the United States prescribe preloaded epinephrine injectors to be kept at home, that practice is no longer followed in the United Kingdom [53].
Monitoring adequacy of dose and control of disease
Selection of the appropriate dose has been discussed in a previous section. Selection of the initial dose and monitoring its adequacy over time should be individualized. Certainly freedom from acute bacterial infections would be a readily agreed-upon goal, but many patients who have chronic bronchitis or bronchiectasis and some who seem to have reactive airways actually may be experiencing progressive subclinical lung disease. Similarly, patients may have progressive erosive sinus disease but may have learned to “live with” the symptoms. Monthly measurements of the white blood cell count, sedimentation rate, and C-reactive protein often are used to assess the presence of subclinical infection. In turn, these data are used to adjust ISG and antibiotic therapy. Frequent sputum cultures and physical examinations also may be useful. Although the practice parameters of the JCAAI suggest that 500 mg/dL is a suitable target serum IgG level, patients should be treated more according to their clinical condition than to achieve any designated level. Nevertheless, monitoring serum IgG levels at routine intervals is important for several reasons. They may serve as markers for adequacy of therapy and enable comparison of one regimen with another. Thus, in patients who experience exacerbations of underlying infection and/or chronic nonspecific symptoms when the IgG trough level falls below a certain value during treatment with intermittent IGIV infusions, that value may serve a target for subcutaneous therapy. Monitoring the serum IgG level also helps assess whether a patient is having increased gastrointestinal or renal protein loss and requires a higher dose or shorter dosing interval to maintain protection against infections. In patients who do not have severe hypogammaglobulinemia per se and/or who actually have elevated serum IgG levels because of monoclonal gammopathy or nonspecific polyclonal B-cell activation, monitoring the trough levels of specific antibody titers (eg, against pneumococcal polysaccharides) may be preferable to using the total IgG level for monitoring the adequacy of therapy.
Besides control of infection, it is important to remember that patients who have common variable immunodeficiency and some other PID diseases are at greater risk for developing malignancies and autoimmunity. Thus, part of the routine monitoring of all such patients, even if they are free from infection, should include careful review of the interval history and physical examination. In addition, selected patients may require radiographic and/or radionuclide studies at regular intervals. In many cases, the immunologist sees the patient much more frequently than any other physician. Sometimes the immunologist, by default, becomes the principal caregiver for the patient and must be diligent to be sure that routine health screening/maintenance is not neglected by the patient's primary care provider, if there is one. Clear communication is required to make sure that developmental and lead screenings are being performed on young children and that monitoring of serum lipids and other risk factors for cardiovascular disease, common malignancies (ie, mammograms, stool guaiac testing, prostate examinations, among others), and bone density is being performed as appropriate in adult patients. Bone density monitoring may be particularly important in postmenopausal women and even in some men who chronically require oral or high-dose inhaled corticosteroids.
Monitoring for complications of therapy with blood products
Because all ISG products are prepared from large pools of plasma, and there are always threats of emerging diseases and of lapses in standard safety procedures, patients should be monitored for any sign of development of a potential bloodborne disease, including spongiform encephalopathies. Although there have been reports of chronic, slowly progressive neurodegenerative disease in patients receiving IGIV therapy for PID disease, it is not clear whether these conditions might represent chronic viral infections of the central nervous system or other complications of the PID disease per se [54]. Transient Coombs' positivity has been reported with certain IGIV preparations, but clinically significant hemolytic anemia is rare as a complication of IGIV therapy [22]. Patients who have PID disease, especially those who have common variable immunodeficiency and hyper-IgM syndromes, also may develop hematologic and/or hepatic abnormalities resulting from their underlying disease or treatment with other medications. Obviously it is important to follow renal function in patients receiving repeated therapeutic infusions of fluid and protein. Careful initial characterization of the patient's baseline, including documentation of hematologic, hepatic, and renal function, and developmental screening thus are essential. In patients who do have some antibody production, tests for exposure to Epstein-Barr virus and cytomegalovirus, as well as HIV and viral hepatidies should be documented before therapy is begun because the presence of passively acquired antibody may make this documentation more difficult. Documentation of a patient's baseline virologic status may have important clinical and medicolegal implications in the future. To monitor for complications of therapy, the complete blood cell count, hepatic and renal function tests, and urinalysis should be repeated every 6 to 12 months. To monitor for bloodborne and other infectious diseases, nucleic acid tests such as polymerase chain reaction or reverse transcription polymerase chain reaction, when available, are preferred to serologic screening tests, because patients who have PID disease generally are antibody deficient and may not produce antibody as expected after exposure to a pathogen. These tests probably should be repeated once a year.
Hyperimmune and other specific immune globulins
Besides standard preparations of polyclonal ISG, several special hyperimmune globulins are available. These are listed and described in detail elsewhere [55]. In general, hyperimmune globulins are prepared from the plasma of individuals who have been exposed accidentally (eg, to snake or other venoms), are convalescing from specific infections (eg, chickenpox), or whose plasma has been found on testing to contain high titers of certain desirable antibodies. For a few antigens, plasma is drawn from persons specifically immunized against unusual but potentially important pathogens such as vaccinia or from healthy normal donors repeatedly immunized with common vaccines such as tetanus toxoid. Most pediatricians are familiar with Varicella zoster immune globulin and with the use of anti-Rh(D) to prevent alloimmunization of mothers. Guidelines for plasma donations from which these products are produced and the steps used to assure that they are free from bloodborne pathogens are the same as for standard polyclonal ISG products, as discussed previously.
Unanswered questions
Although great progress has been made since the painful intramuscular injections of ISG in the 1950s and 1960s, many questions about the characteristics and uses of ISG remain unanswered. Important issues about the mechanisms of action of polyclonal IgG in inflammatory and autoimmune diseases remain, and it is not clear to what extent specifically modified or monoclonal preparations might obviate the use of polyclonal IgG for those conditions. Reports describing the spectrum of antibodies and the appropriate uses of ISG for prevention and/or management of influenza are scarce, and it is even less clear what will be done if new pandemic strains emerge. As the population of plasma donors shifts from those who have recovered from natural infection with measles, mumps, and similar viruses in childhood to those who were immunized and never had the wild-type disease, antibody levels in ISG preparations are changing. Concerns have been raised about how well the protection of antibody-deficient individuals will be maintained. On the other hand, a steadily increasing number of new vaccines has been introduced in recent years, now including meningococcal conjugate vaccine and human papilloma virus vaccine. Studies of the antibody content and efficacy of standard ISG preparations in preventing those infections are not available. In addition, with the experience of rapid, worldwide transmission of severe acute respiratory syndrome and West Nile virus infection in recent years, the threat of emerging diseases is no longer an exotic science fiction scenario. Thus, production of ISG and other blood-derived products and optimal care of patients who have PID disease will continue to evolve.
Summary
Advances in the large-scale production of polyclonal ISG preparations during the last 2 decades have greatly improved the management of patients who have PID disease. The continued development of products with improved safety and tolerability profiles has allowed treatment to focus on quality of life and long-term freedom from the complications of PID disease rather than just on freedom from severe acute infections and survival. Currently available ISG preparations allow routine therapy by a variety of routes and regimens that can be tailored to suit any individual patient. Continued vigilance is required, however, because problems with emerging diseases and the costs and availability of ISG are likely to present continuing challenges.
References
- 1.von Behring E. Serum therapy in therapeutics and medical science. Nobel Lecture, December 12, 1901. Available at: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1901/behring-lecture.html. Accessed February 29, 2008.
- 2.Bruton O. Agammaglobulinemia. Pediatrics. 1952;9:722–728. [PubMed] [Google Scholar]
- 3.Her Majesty's Stationery Office; London: 1971. MRC Working Party on Hypogammaglobulinaemia, Hypogammaglobulinaemia in the United Kingdom. [Google Scholar]
- 4.US SARS Compliance program guidance manual. Chapter 42—blood and blood products inspection of source plasma establishments, brokers, testing laboratories, and contractors - 7342.002. http://www.fda.gov/cber/cpg/7342002bld.htm#p6 Available at: Accessed January 1, 2008.
- 5.US SARS Guide to inspections of source plasma establishments. http://www.fda.gov/ora/inspect_ref/igs/Source_Plasma/default.htm Available at: Accessed January 1, 2008.
- 6.Oncley J.L., Melin M., Richert D.A. The separation of the antibodies. isoagglutinins, prothrombin, plasmonogen and beta-lipoprotein into subfractions of human plasma. J Am Chem Soc. 1949;71:541–550. doi: 10.1021/ja01170a048. [DOI] [PubMed] [Google Scholar]
- 7.US FDA Guidance for Industry: safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency. http://www.fda.gov/cber/gdlns/igivimmuno.htm Available at: Accessed July 1, 2008.
- 8.Renal insufficiency and failure associated with immune globulin intravenous therapy—United States, 1985–1998. MMWR Morb Mortal Wkly Rep. 1999;48:518–521. [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration Center for Biologics Evaluation and Research. Important safety information on interference with blood glucose measurement following use of parenteral maltose/parenteral galactose/oral xylose-containing products. http://www.fda.gov/cber/safety/maltose110405.htm Available at: Accessed January 1, 2008.
- 10.Biesert L. Virus validation studies of immunoglobulin preparations. Clin Exp Rheumatol. 1996;14(Suppl 15):S47–S52. [PubMed] [Google Scholar]
- 11.Horowitz B., Wiebe M.E., Lippin A. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion. 1985;25:516–522. doi: 10.1046/j.1537-2995.1985.25686071422.x. [DOI] [PubMed] [Google Scholar]
- 12.Buckley R. Immune Deficiency Foundation; Towson (MD): 2006. Diagnostic and clinical care guidelines for primary immunodeficiency diseases.www.primaryimmune.org Available at: Accessed February 29, 2008. [Google Scholar]
- 13.Bonilla F.A., Bernstein I.L., Khan D.A. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl 1):S1–S63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal S., Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol. 2007;99:281–283. doi: 10.1016/S1081-1206(10)60665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paris K., Sorensen R.U. Assessment and clinical interpretation of polysaccharide antibody responses. Ann Allergy Asthma Immunol. 2007;99:462–464. doi: 10.1016/S1081-1206(10)60572-8. [DOI] [PubMed] [Google Scholar]
- 16.Chouksey A, Berger M. Evaluation of antibody responses to protein antigens. Ann Allergy Asthma Immunol;99:(in press).
- 17.Fasth A. European Society for Immune Deficiency. Budapest, Hungary, October 2006.
- 18.Newburger J.W., Fulton D.R. Kawasaki disease. Curr Opin Pediatr. 2004;16:508–514. doi: 10.1097/01.mop.0000137796.23813.64. [DOI] [PubMed] [Google Scholar]
- 19.Orange J.S., Hossny E.M., Weiler C.R. Use of intravenous immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2006;117:S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Berger M., Pinciaro P.J. Safety, efficacy and pharmacokinetics of Flebogamma 5% for replacement therapy in primary immunodeficiency diseases. J Clin Immunol. 2004;24:389–396. doi: 10.1023/B:JOCI.0000029108.18995.61. [DOI] [PubMed] [Google Scholar]
- 21.Burks A.W., Sampson H.A., Buckley R.H. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. N Engl J Med. 1986;314:560–564. doi: 10.1056/NEJM198602273140907. [DOI] [PubMed] [Google Scholar]
- 22.Pierce L.R., Jain N. Risks associated with the use of intravenous immunoglobulin. Transfus Med Rev. 2003;17:241–254. doi: 10.1016/s0887-7963(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 23.Mankarious S., Lee M., Fischer S. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med. 1988;112:634–640. [PubMed] [Google Scholar]
- 24.Ballow M., Berger M., Bonilla F.A. Pharmacokinetics and tolerability of a new intravenous immunoglobulin preparation, IGIV-C, 10% (Gamunex, 10%) Vox Sang. 2003;84:202–210. doi: 10.1046/j.1423-0410.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 25.Church J.A., Leibl H., Stein M.R. US-PID-IGIV 10% -Study Group. Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin [IGIV 10%] in patients with primary immunodeficiency. J Clin Immunol. 2006;26:388–395. doi: 10.1007/s10875-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 26.Ochs H.D., Pinciaro P.J. Octagam 5%, an intravenous IgG product, is efficacious and well tolerated in subjects with primary immunodeficiency diseases. J Clin Immunol. 2004;24:309–314. doi: 10.1023/B:JOCI.0000025453.23817.3f. [DOI] [PubMed] [Google Scholar]
- 27.Andressen I., Kovarik J.M., Spycher M. Product equivalence study comparing the tolerability, pharmacokinetics and pharmacodynamics of various human IgG formulations. J Clin Pharmacol. 2000;40:722–730. doi: 10.1177/00912700022009477. [DOI] [PubMed] [Google Scholar]
- 28.Berger M., Cupps T.R., Fauci A.S. Immunoglobulin replacement by slow subcutaneous infusion. Ann Intern Med. 1980;98:55–56. doi: 10.7326/0003-4819-93-1-55. [DOI] [PubMed] [Google Scholar]
- 29.Ugazio A.G., Duse M., Re R. Subcutaneous Infusions of gammaglobulin in management of agammaglobulinemia. Lancet. 1982;I:226. doi: 10.1016/s0140-6736(82)90793-0. [DOI] [PubMed] [Google Scholar]
- 30.Roord J.J., Van der Meer J.W.M., Kuis M. Home treatment in patients with antibody deficiency by slow subcutaneous infusion of gammaglobulin. Lancet. 1982;I:689–690. [PubMed] [Google Scholar]
- 31.Berger M., Cupps T.R., Fauci A.S. High dose immunoglobulin replacement therapy during pregnancy. J Am Med Assoc. 1982;247:2824–2825. [PubMed] [Google Scholar]
- 32.Berger M., Duff K., Beal C. Information for patients: pumps and needles used for Subcu IgG. http://www.rainbowbabies.org/subcu Available at: Accessed January 1, 2008.
- 33.National Institutes of Health Clinical Center Nurses patient information publications. Giving a subcutaneous injection. www.cc.nih.gov/ccc/patient_education/pepubs/subq.pdf Available at: Accessed January 1, 2008.
- 34.Chouksey A., Duff K., Wasserbauer N. Subcutaneous IgG replacement therapy with preparations currently available in the US for IV or IM use: reasons and regimens. Allergy, asthma and Clinical Immunology. 2005;1:120–130. doi: 10.1186/1710-1492-1-3-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardulf A., Anderson V., Bjorkander J. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995;345:365–369. doi: 10.1016/s0140-6736(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 36.Welch M.J., Steihm E.R. Slow subcutaneous immunoglobulin therapy in a patient with reactions to IM immunoglobulin. J Clin Immunol. 1983;3:285–286. doi: 10.1007/BF00915353. [DOI] [PubMed] [Google Scholar]
- 37.Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7. doi: 10.1016/j.clim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Gardulf A., Nicolay U., Math D. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114:936–942. doi: 10.1016/j.jaci.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 39.Nicolay U., Kiessling P., Berger M. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26:65–72. doi: 10.1007/s10875-006-8905-x. [DOI] [PubMed] [Google Scholar]
- 40.Kittner J.M., Grimbacher B., Wulff W. Patients' attitude to subcutaneous immunoglobulin substitution as home therapy. J Clin Immunol. 2006;26:400–405. doi: 10.1007/s10875-006-9031-5. [DOI] [PubMed] [Google Scholar]
- 41.Busse P.J., Razvi S., Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109:1001–1004. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 42.Pirofsky B. Intravenous immune globulin therapy in hypogammaglobulinemia. Am J Med. 1984;76(3):53–60. doi: 10.1016/0002-9343(84)90320-6. [DOI] [PubMed] [Google Scholar]
- 43.Ochs H.D., Buckley R.H., Pirofsky B. Safety and patient acceptability of intravenous immune globulin in 10% maltose. Lancet. 1980;II:1158–1159. doi: 10.1016/s0140-6736(80)92594-5. [DOI] [PubMed] [Google Scholar]
- 44.Eibl M. Intravenous immunoglobulins: clinical and experimental studies. In: Alving B.M., Finlayson J.S., editors. Immunoglobulins: characteristics and uses of intravenous preparations. US Food and Drug Administration, US Department of Health and Human Services; Bethesda (MD): 1980. pp. 23–30. [Google Scholar]
- 45.Roifman C.M., Levison H., Gelfand E.W. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet. 1987;1:1075–1077. doi: 10.1016/s0140-6736(87)90494-6. [DOI] [PubMed] [Google Scholar]
- 46.Gelfand E.W., Reid B., Roifman C.M. Intravenous immune serum globulin replacement in hypogammaglobulinemia. A comparison of high- versus low-dose therapy. Monogr Allergy. 1988;23:177–186. [PubMed] [Google Scholar]
- 47.Roifman C.M., Schroeder H., Berger M. Comparison of the efficacy of IGIV- C, 10% (caprylate/chromatography) and IGIV-SD, 10% as replacement therapy in primary immune deficiency. A randomized double-blind trial. Int Immunopharmacol. 2003;3:1325–1333. doi: 10.1016/s1567-5769(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 48.Eijkhout H.W., van Der Meer J.W., Kallenberg C.G. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinaemia: a randomized, double-blinded, multicenter crossover trial. Ann Intern Med. 2001;135:165–174. doi: 10.7326/0003-4819-135-3-200108070-00008. [DOI] [PubMed] [Google Scholar]
- 49.Kainulainen L., Varpula M., Liippo Pulmonary abnormalities in patients with primary hypogammaglobulienaemia. J Allergy Clin Immunol. 1999;104:1031–1036. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- 50.Busse P.J., Farzan S., Cunningham-Rundles C. Pulmonary complications of common variable immunodeficiency. Ann Allergy Asthma Immunol. 2007;98:1–11. doi: 10.1016/S1081-1206(10)60853-8. [DOI] [PubMed] [Google Scholar]
- 51.Berger M. Goals of therapy in antibody deficiency syndromes. J Allergy Clin Immunol. 1999;104:911–913. doi: 10.1016/s0091-6749(99)70067-9. [DOI] [PubMed] [Google Scholar]
- 52.Ochs H.D., Gupta S., Kiessling P. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–273. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 53.Sewell W.A.C., editor. Sucutaneous IgG therapy medical education programme. Watermeadow PLC; Oxfordshire (UK): 2006. [Google Scholar]
- 54.Ziegner U.H., Kobayashi R.H., Cunningham-Rundles C. Progressive neurodegeneration in patients with primary immunodeficiency disease on IVIG treatment. Clin Immunol. 2002;102:19–24. doi: 10.1006/clim.2001.5140. [DOI] [PubMed] [Google Scholar]
- 55.Grabenstein John D., editor. ImunoFacts, 2008. Lippincott, Williams and Wilkins; Philadelphia: 2007. [Google Scholar]