Abstract
Livestock industries strive to improve the health of their animals and, in the future, they are going to be required to do this with a continued reduction in antimicrobial use. Nutraceuticals represent a group of compounds that may help fill that void because they exert some health benefits when supplemented to livestock. This review is focused on the mechanisms of action, specifically related to the immune responses and health of ruminants. The nutraceutical classes discussed include probiotics, prebiotics, phytonutrients (essential oils and spices), and polyunsaturated fatty acids.
Keywords: Health, Immune, Nutraceutical, Probiotic, Prebiotic, Phytonutrient, Polyunsaturated fatty acid
Key points
-
•
Nutraceutical is a term derived from nutrition and pharmaceutical. There are many compounds that improve immune responses and reduce the risk of disease through different mechanisms of action.
-
•
Probiotics are the supplementation of viable microorganisms that offer potential health benefits to the animal. Probiotics are predominately supplemented to improve gastrointestinal health, rumen fermentation, or nutrient utilization.
-
•
Prebiotics are a group of indigestible carbohydrates that function to improve the growth of commensal bacteria. Some fractions (eg, β-glucans and mannanoligosaccharides) have direct immunomodulatory, gram-negative pathogen binding, or hydrophobic mycotoxin absorption capacities.
-
•
Phytonutrients are a group of compounds isolated from plants with potential therapeutic applications given their intrinsic antioxidative, anti-inflammatory, and antimicrobial properties.
-
•
Polyunsaturated fatty acids influence the degree of inflammation. Generally, increasing the supplementation of omega-3 fatty acid sources decreases inflammation, whereas supplementation of omega-6 fatty acids increases inflammation. How much inflammation is needed at any specific physiologic stage is less understood in ruminant species.
Introduction
Public concerns regarding the potential association between zoonotic multidrug-resistant bacterial strains and antibiotic use in food animals has prompted significant regulatory changes to on-farm antimicrobial availability and use.1 Producers and veterinarians alike face increasingly limited options to therapeutically treat diseased animals. As a result, microbial and plant-based compounds and their derivatives received increased research and commercial attention in the past few decades. Nutraceuticals, as many of them are known, is a hybrid of nutrition and pharmaceuticals. They consist of natural compounds and/or microbes that offer potentially advantageous effects related to ruminant health and productivity, including improved feed efficiency, milk production, and disease resistance through immune modulation or decreased disease pressure.1, 2, 3
Nutraceuticals are a broad group of compounds that can be classified in several ways. Some of the more common classifications are based on the mechanism of action, chemical nature, or the feed source of the compound. In this review, we discuss the general mechanisms of action of common nutraceutical classes that are currently available to be used in ruminants. We classify and discuss each nutraceutical as either a probiotic, prebiotic, phytonutrient (eg, polyphenol, spices/essential oils), or polyunsaturated fatty acid. Finally, nutraceuticals are a rapidly developing field and it is important to note that these compounds lack governmental regulatory oversight by the Food and Drug Administration given their classification as “dietary supplements.” Therefore, product label statements regarding composition, dosage, effectiveness, and quality are not necessarily independently validated or standardized.
Probiotics
Probiotics, also known as direct-fed microbials, can improve health in many animal species. Common probiotic microorganisms currently used commercially include Lactobacillus sp. and other lactic acid-producing bacteria, Bifidobacterium sp., Bacillus sp., and Saccharomyces cerevisiae. Important considerations when supplementing probiotics include the dose (colony-forming units [CFU]), the duration of supplementation, and the age of the animal. A significant portion of the research on probiotic supplementation in ruminants focused on the health benefits to the gastrointestinal tract (GIT), which makes teleologic sense because these microorganisms may directly influence microbial communities and cellular function within the GIT. Most of the health benefits described in Table 1 report reduced incidence or duration of scours. Ancillary benefits of probiotics, both direct and indirect, in other organ systems including the respiratory tract, urogenital tract, and mammary gland have also been investigated, but are only briefly discussed in this review.
Table 1.
Effects of probiotics and prebiotics on the immune function, health, and performance of calves, dairy cows, and feedlot steers
| Animal | Strain/Type | Dose | Duration/Frequency | Health/Production Status | Outcome | Reference |
|---|---|---|---|---|---|---|
| Calf | Probiotic | 109 CFU/d multistrain | 8 wk | High risk | Decreased scours and therapy | Timmerman et al,4 2005 |
| Calf | Probiotic | 109 CFU/d LAB 6 strains | 8 wk | High risk | Decreased scours and therapy | Timmerman et al,4 2005 |
| Calf | Probiotic | 108 CFU/d LAB, Enterococcus faecium, B bifidum, S thermophilus | 90 d | Low risk | Decreased scours | Mokhber-Dezfouli et al,5 2007 |
| Calf | Probiotic | 107 CFU/d Lactobacillus acidophilus and L plantarum | 15 wk | Low risk | Increased white blood cell counts, increased IgG concentration | Al-Saiady,6 2010 |
| Calf | Probiotic | Meta-analysis on LAB | Range 14−187 d | Low risk | Decreased scours | Signorini et al,7 2012 |
| Calf | Probiotic | 2.0 × 109 CFU/d L acidophilus and Enterococcus faecium | 21 d | Moderate Salmonella enteria challenge | Reduced systemic inflammation, decreased mucosal damage, increased villi height:crypt depth in the duodenum and ileum | Liang et al,8 |
| Calf | Probiotic | 1010 CFU challenge EHEC E coli then 1010 CFU probiotic E coli | 35 d | 8–10-wk-old calves | Decreased E. coli O157:H7 GIT growth and fecal shedding | Tkalcic et al,9 2003 |
| Calf | Probiotic + yeast | 109 CFU/d L acidophilus, E faecium, Saccharomyces cerevisiae | 56 d | High risk | Decreased neutrophil oxidative burst, increased lymphocyte counts, decreased haptoglobin, decreased scours | Davis,10 2018 |
| Calf | Probiotic + yeast | 108 CFU/d LAB, 106 CFU/d S cerevisiae, 108 CFU/d LAB | 24 wk | Low risk | Decreased scours | Agarwal et al,11 2002 |
| Calf | Yeast | 109 CFU/d S cerevisiae | 2×/d for 84 d | High risk | Decreased scours | Galvao et al,12 2005 |
| Calf | BG | 5 mL/d 50% BG extract | 56 d | High risk | Increased neutrophil counts, decreased neutrophil functionality, decreased haptoglobin | Davis,10 2018 |
| Calf | BG | 113 g/d of a 1.8% BG + vitamin C | 28 d | Transport stress | Increased neutrophil counts at d 28 | Eicher et al,13 2010 |
| Calf | BG | 150 g/d of a 70% BG + vitamin C | 28 d | Transport stress | Decreased white blood cells and neutrophil phagocytosis | Eicher et al,13 2010 |
| Calf | MOS + Bs | 3 g/hd/d MOS + 109 CFU Bacillus subtilis | 56 d | High risk | Decreased lymphocyte counts and haptoglobin | Davis,10 2018 |
| Calf | MOS | 7 g/hd/d MOS | From 5 to 56 d | Low risk | Reduced antibiotic treatments and cost of scours | Kara et al,14 2015 |
| Calf | MOS | 4 g/hd/d MOS | 30 d | Low risk | Decreased fecal scores and fecal coliform counts | Ghosh & Mehla,15 2012 |
| Calf | MOS | 4 g/hd/d MOS | 5 wk | Low risk | Decreased probability of scours | Heinrichs et al,16 2003 |
| Calf | MOS | 4 or 6 g/hd/d MOS | 56 d | Low risk | No changes | Hill et al,17 2008 |
| Calf | FOS | 4 or 8 g/hd/d FOS | 56 d | Low risk | No changes | Hill et al,17 2008 |
| Calf | FOS | 3 or 6 g/hd/d FOS | 168 d | Low risk | Increased carcass weight | Grand et al,18 2013 |
| Calf | GOS | 3.4% GOS DM in milk replacer | 84 d | Low risk | Increased days with high fecal scores, increased epithelium growth in the SI, increased LAB and Bifidobacterium abundance in the SI | Castro et al,19 2016 |
| Dairy cow | Probiotic | 50 g/d Lactobacillus casei and L plantarum of 1.3 × 109 CFU/g | 30 d | Lactating | Decreased SCC, no change in milk components but increased overall milk production at 15 and 30 d of the study (75 and 90 DIM) | Xu et al,20 2017 |
| Dairy cow | Probiotic | 10, 15, or 20 g/d Saccharomyces cerevisiae and LAB, no CFU listed | 60 d | Early to mid lactation | Increased milk yield, FCM, solids, and profit | Shreedhar et al,21 2016 |
| Dairy cow | Probiotic | 10 g/hd/d probiotics, no CFU listed | 21 d | Early lactation | Increased total milk yield and fat | Musa et al,22 2017 |
| Dairy cow | Probiotic + yeast | 10, 15, or 20 g/hd/d Lactobacillus, S cerevisiae, Propionibacterium, no CFU listed | 6 wk | Lactating | Increased milk production with 20 g/d (most cost-effective), tendency to increase milk fat and protein | Vibhute et al,23 2011 |
| Dairy cow | Yeast | 9 mL/hd/d commercial yeast product in the water, no CFU listed | 10 wk | 30 DIM | Increased rumen pH, decreased BHBA, increased milk production, decreased milk protein yield | Rossow et al,24 2014 |
| Dairy cow | Yeast | 3 g/hd/d of 6.0 × 109 CFU live yeast | 5 periods of 45 d | Lactating | Increased nutrient utilization in the rumen, increased milk yield, protein, and fat | Rossow et al,25 2018 |
| Dairy cow | Yeast | 2.5 g/d of 2.5 × 1010 CFU S cerevisiae | 105 d | Lactating | Increased milk production | Maamouri et al,26 2014 |
| Dairy cow | Yeast | 40 g/d of 5.0 × 1011 CFU S cerevisiae | 90 d | 3rd lactation, early in lactation | Increased milk yield and fat, decreased SCC | Dailidaviciene et al,27 2018 |
| Dairy cow | Synbiotic | 1.0 × 107 CFU/kg diet L casei + 10 g/hd/d Dextran | 1 y | Lactating | Decreased SCC, decreased mastitis, decreased adverse impacts of heat stress, increased milk production and components | Yasuda et al,28 2007 |
| Dairy cow | Synbiotic | 10 g/d of 5.0 × 109 CFU/g Saccharomyces cerevisiae and a mix of 107 CFU L casei, Streptococcus faecium, L acidophilus | 75 d | Lactating | Increased milk fat, decreased SCC | Sretenovic et al,29 2008 |
| Feedlot steer | Probiotic | 109 CFU/d LAB | 2 y | Feedlot | Decreased fecal shedding of E coli O157:H7 | Peterson et al,30 2007 |
| Feedlot steer | Probiotic | 108 CFU/d E coli | 90 d | Feedlot | Decreased fecal shedding of E coli O157:H7 | Schamberger et al,31 2004 |
| Feedlot steer | Probiotic | 109 LAB+ 109Propionibacterium freudenreichii | 9 mo | Feedlot | Decreased fecal shedding of E coli and Salmonella | Tabe et al,32 2008 |
| Feedlot steer | Probiotic | 109 CFU/d LAB | 141 d | Feedlot | Decreased fecal shedding of E coli, decreased shedding by 80% at slaughter, only 3.4% of hides positive at harvest | Younts-Dahl et al,33 2005 |
| Feedlot steer | Probiotic | 108 CFU/d, 107 CFU/d LAB | 141 d | Feedlot | Decreased fecal shedding of E coli | Younts-Dahl et al,33 2005 |
| Feedlot steer | Probiotic | 109 CFU/d LAB NPC 747 | 45–108 d | Feedlot | Decreased fecal prevalence of E coli, 1.6% prevalence on hides | Brashears et al,34 2003 |
| Feedlot steer | Probiotic | 109 CFU/d LAB NPC 750 | 45–108 d | Feedlot | Decreased fecal prevalence of E coli, 0% prevalence on hides | Brashears et al,34 2003 |
| Feedlot steer | MOS | 0.2% MOS in diet, DM | 24 d | Feedlot | Decreased haptoglobin and endotoxin translocation from the GIT to circulation | Jin et al,35 2014 |
Concentrations of prebiotics are not standardized, so comparisons among studies are not recommended. Some prebiotics included a carrier that may or may not influence the outcome.
Abbreviations: BG, β-glucan; CFU, colony-forming units; EHEC, enterohemorrhagic E coli; FCM, fat corrected milk; FOS, fructooligosaccharide; GOS, galactooligosaccharide; LAB, lactic acid-producing bacteria; MOS, mannanoligosaccharide; SI, small intestine
The dynamics of the GIT vary between individual animals, diets, ages, environment, and management factors. Therefore, there is a significant amount of complexity involved in evaluating and comparing the efficacy of these probiotic strategies on their ability to affect health and performance. Typically, a probiotic must contain live microorganisms (usually bacteria and/or yeast) and produce one or more of the following desirable outcomes66, 67:
-
•
Regulate GIT microbial communities
-
•
Prevent adherence or colonization of potential pathogens in the GIT
-
•
Produce antimicrobial or bactericidal products against potential pathogens
-
•
Maintain host GIT integrity including improved barrier defenses
-
•
Improve mucosal adaptive immune responses
-
•
Regulate GIT inflammation
-
•
Improve ruminal fermentation and nutrient utilization
The application of probiotics in neonates is more common, which makes sense because the GIT and immune system are both developing rapidly and the risk for GIT disease is greater in the neonate. There is a shift in the microbial ecology in the GIT in early life. The neonatal GIT is colonized by many facultative anaerobic bacteria that are common in the environment, including a lot of the family of Enterobacteriaceae that include many strains of pathogenic Escherichia coli and Salmonella sp. There is a shift to more strict anaerobic bacteria as the animal ages with reduced abundance of Enterobacteriaceae. 68 Many of the probiotic microorganisms supplemented are either strict anaerobes or facultative anaerobes that commonly increase in abundance as an animal increases in age and are more often lactic acid-producing bacteria. Some of the logic is that speeding up this microbial progression will reduce GIT disease, especially by Enterobacteriaceae, because the GIT environment is less conducive to their colonization.
Probiotics can help maintain a beneficial host relationship and colonize the lumen and outer epithelium of the lower GIT creating competition for space on the mucosal surface as well as for nutrients on the mucosa that allow for bacterial adherence and growth (Fig. 1 ). The more “beneficial” bacteria present on the mucosa taking up space and nutrients the less that is available for pathogenic bacteria and potentially other enteric disease-causing microorganisms such as rotavirus, coronavirus, Cryptosporidium, and Eimeria. This mechanism is often referred to as competitive exclusion. Furthermore, increased lactic acid concentrations in the lower GIT reduces lumen pH and may help limit establishment of pathogenic Enterobacteriaceae.69
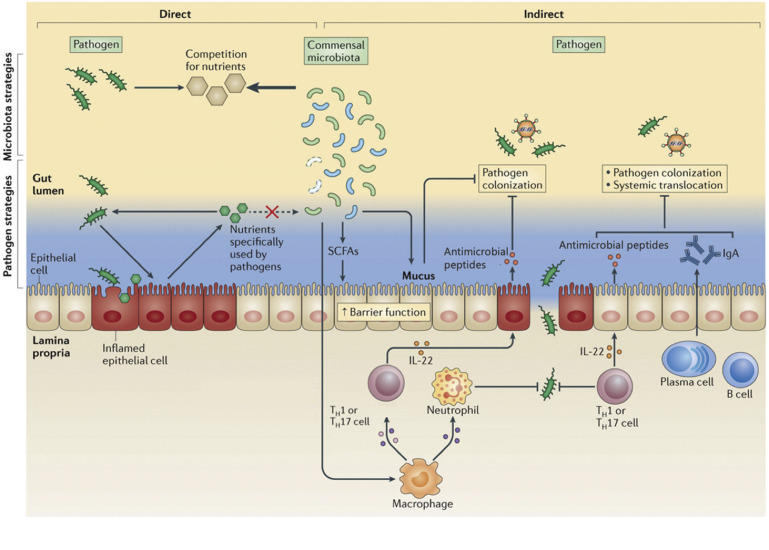
Fig. 1.
Mechanisms of action of commensal microbiota and putative probiotics on immune defenses in mucosal tissues.
(From Kamada et al. Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology 2013; 13:321-335; with permission.)
Another mechanism of action through which probiotics may influence health and performance of animals is via interaction with the GIT mucosal immune system (see Fig. 1). There are specialized cells in the GIT epithelium known as M cells that constantly sample contents from the GIT and deliver antigens to the lymphocyte-rich Peyer’s patches. Maintenance of the commensal microbial structure of the GIT helps regulate the inflammatory response in the small intestine to prevent an overreaction to the commensal microbiome and potentially other enteric pathogens.70 The presence of secretory immunoglobulin A (IgA), antimicrobial peptides, and other regulatory leukocyte responses at the GIT mucosa is an important mechanism that regulates proinflammatory responses, which are essential to maintaining GIT integrity and function. Liang et al. (in press) supplemented Jersey bull calves with 2 strains of lactic acid-producing bacteria and challenged them at 7 days of age with a moderate dose of Salmonella enterica serotype Typhimurium. Calves supplemented with probiotic bacteria had reduced systemic inflammation and less mucosal damage as demonstrated by greater villi height:crypt depth in both the duodenum and ileum compared with the unsupplemented, challenged calves.8 Localized proinflammatory responses are considered beneficial in most tissues; however, in the GIT mucosa, an excessive proinflammatory response may compromise the integrity of the GIT barrier and further exacerbate the pathogenesis of the disease.
Most of the mechanisms of action of probiotics discussed are to decrease the colonization of pathogenic microorganisms or directly improve GIT mucosal immune responses. However, supplementing probiotic species that either produce or stimulate the production of more butyrate have the potential to alter GIT mucosal defenses (see Fig. 1). Butyrate is one of the short-chain fatty acids produced by bacteria in the rumen and intestines. Butyrate is a preferred energy source for GIT epithelium that results in the proliferation and differentiation of epithelial cells in the rumen and both the small and large intestines. Butyrate can affect barrier development of the small intestine by increasing tight junction protein expression and exhibiting immunomodulatory effects on enterocytes and mucosal surfaces of the GIT by increasing neutrophil migration.71, 72 Current research suggests that butyrate has anti-inflammatory, antioxidant, and anticancer functions along with promoting epithelial cell proliferation and differentiation.73 Supplementation of butyrate to dairy heifers increased average daily gain and GIT development.74, 75 Supplementing butyrate-producing bacteria as probiotics is a relatively novel concept. Furthermore, a process known as cross-feeding has been recently described in which butyrate-producing bacteria are stimulated to proliferate in the presence of lactic acid originating from lactic acid-producing bacteria. Therefore, some of the benefits observed from supplementing common probiotic strains currently used in ruminants may partially be explained by changes in butyrate production in the GIT.
The use of probiotics in mature ruminants (eg, feedlot cattle or lactating dairy cows) can affect the rumen and lower GIT. Some probiotic microorganisms bypass the reticuloruminal and abomasal compartments and exert beneficial effects in the lower GIT, likely through similar mechanisms of action as discussed above for preweaned calves. Supplementing feedlot cattle with lactic acid-producing bacteria decreased E coli O157:H7 and Salmonella sp. fecal shedding, as well as contamination on carcass hides postharvest.9, 30, 31, 32, 33, 34 Supplementing probiotics to feedlot cattle as a preharvest food safety strategy is now a common industry practice. Most of the data currently available regarding probiotic supplementation in dairy cow rations have assessed its effect on performance and milk quality (eg, somatic cell count). Fecal pathogen shedding or manure consistency were not commonly reported variables in these studies. It is conceivable that many of the probiotic species discussed previously in neonates may have some lower GIT benefits in mature dairy cows. In fact, some of the performance benefits such as increased milk production may be partially attributable to improvements in GIT health. Furthermore, supplementing probiotics to mature dairy cows seems to have the greatest health and economic benefits during stressful events, most notably the transition period.
The mechanisms of action of orally delivered probiotics on GIT health are more straightforward than how oral probiotics may improve immunity against infections in nonmucosal tissues (eg, mammary gland). The mucosal immune system is a complex network of mucosal surfaces spanning the gastrointestinal, respiratory, and urogenital tracts. Immune responses generated at one mucosal location can transfer to other mucosal surfaces through trafficking and recirculation of effector and memory leukocytes; however, this likely does not include the mammary gland. The paucity of data and the lack of a clear mechanism of action do not support the notion that oral administration of probiotics improves mammary gland health. Rainard and Foucras76 discussed the potential direct effects of either intramammary infusion or topical application of probiotics, either lactic acid-producing bacteria or Bacillus sp., that can inhibit the growth of mastitis causing pathogens in a teat dip as a more promising route of administration to improve mammary gland health. Intramammary infusion of a live lactic acid-producing bacteria culture increased the secretion of proinflammatory cytokines interleukin-8 (IL-8) and IL-1 and attracted neutrophils to the mammary gland.77 The authors suggested application of this intramammary infusion for the treatment of mastitis because of the production of a broad-spectrum bacteriocin against gram-positive bacteria and a sustained innate immune response.
Finally, a common probiotic application in ruminants is to supplement probiotics, either bacterial or fungal, to improve rumen function and health. The primary mechanisms of action are to stimulate total rumen bacterial population densities, shift or stabilize the balance of rumen bacterial populations, utilize lactic acid, and reduce the risk for ruminal acidosis, and maintain a low oxygen rumen environment.78, 79, 80, 81 Newbold and colleagues78 designed well-controlled experiments to elucidate the mechanisms by which live yeast supplements may increase rumen bacterial population densities. Oxygen utilization by live yeast supplements seems to be an important mechanism improving the growth of rumen bacteria. Silberberg and colleagues81 supplemented a live yeast during repeated rumen acidosis challenges and reported improvements in rumen pH, a more stabilized rumen bacteria population, and less systemic inflammation. Another microbial strategy to aid in the prevention of rumen acidosis is a bolus inoculation with lactic acid-utilizing bacteria such as Megasphaera elsdenii. Arik and colleagues82 administered a bolus of lactic acid-utilizing bacteria to the rumen of Holstein heifers with M elsdenii for 2 consecutive days during a subacute ruminal acidosis challenge. They observed the M elsdenii-treated heifers had a slowed decrease in rumen pH, decreased concentrations of lactic acid-producing bacteria (Streptococcus bovis), and increased protozoa counts. These studies support the notion that microbial supplementation strategies can improve rumen function and health.
Prebiotics
Prebiotics are indigestible carbohydrates, including many oligosaccharides and fructans, that can improve animal health through a variety of mechanisms. One common mode of action is that they serve as an energy source for commensal or probiotic bacteria in both the rumen and the lower GIT.83 Synbiotic is the term used when a prebiotic is fed with a probiotic and the effect together is greater than if either is fed alone (see Fig. 1). However, the focus of prebiotics in this review is more on their immunomodulatory effects, ability to bind gram-negative bacterial pathogens, and adsorbing certain mycotoxins.
Prebiotics are commonly found in plants, milk, or the cell wall of fungi (yeast and mushrooms). They are found in natural feedstuffs as well as in various extracts. Food sources for galactooligosaccharides include soybeans and milk, whereas fructooligosaccharides are found in vegetables and plants. Prebiotics isolated from yeast cell walls include mannan-oligosaccharides (MOS) and β-glucans (BGs). The composition, availability, and physical chemistry of a prebiotic in a feedstuff or in an extract influences its ability to improve the health of an animal. For example, a prebiotic extract of yeast cell wall may be very different from another extract of yeast cell wall, even if they are extracted from the same strain of yeast.84 Methods of extraction (eg, enzymatic, solvents, temperature, and time) and additional processing techniques (eg, deproteination) can significantly influence the functional properties of an extract. Making comparisons among various prebiotics are difficult in that many extracts are blended with other ingredients, so 1 g of “extract A” is likely not equivalent to 1 g of “extract B.” Therefore, caution should be taken when reviewing the dose of a prebiotic in Table 1 and when making comparisons among published studies involving different extracts.
The in vitro binding of yeast cell wall extracts with MOS showed that MOS binds to the type 1 fimbriae expressed on many gram-negative bacteria such as pathogenic Salmonella sp. and E coli. 84 These mannose -specific filaments, or type 1 fimbriae, bind to MOS and prevent pathogenic bacteria from binding to the epithelium and colonizing the GIT. Thus, supplementing yeast cell wall extracts with MOS may be a useful prevention strategy for those animals impacted by greater exposure to gram-negative pathogenic bacteria.10 Because adhesion of pathogenic bacteria to host mucosal surfaces is generally required for an infection in the GIT, the binding of MOS to these fimbriae decreases potential pathogenic adherence to the host GIT mucosa. The impacts of MOS on specific immune responses are not well characterized. Furthermore, complicating interpretation of data is that MOS extracts are predominately yeast cell wall extracts that also contain various glucan fractions including BGs. Therefore, many of the immunomodulatory effects described in the next paragraph are possible when yeast cell wall extracts are supplemented and likely attributed to the BG fraction of the extract.
β-Glucans are cell wall fractions usually isolated from fungal sources and have potential immunomodulatory effects. The β-1,3-glucans are able to bind to Dectin-1 receptors on monocytes, macrophages, and neutrophils, and, to a lesser extent on dendritic and T-cell surfaces.85 Ligation of the Dectin-1 receptor when animals are supplemented a fungal extract can cause a slight activation of various leukocyte responses and is currently considered the mechanism of action for a positive immune response. Oral supplementation of BG fractions or yeast cell wall extracts can increase phagocytosis and oxidative burst capacities of immune cells as well as activate an inflammatory response through a nuclear factor κB-mediated pathway. Oral supplementation of BG also affects systemic immune responses in addition to local GIT immune responses. The mechanism of action of BGs is thought to be mediated through specialized M cells that sample intestinal lumen contents. This increased awareness of the immune system allows for mediated inflammation and an enhanced ability of macrophages, neutrophils, and dendritic cells to identify, engulf, and destroy pathogenic bacteria before they have the opportunity to establish an infection in the host.10, 86
As discussed previously, yeast cell wall extracts contain varying levels of both MOS and BG, and are commonly supplemented to ruminants and other livestock for potential immunomodulatory effects, binding of gram-negative bacteria, or adsorbing hydrophobic mycotoxins. The quantity of MOS and BG that bypasses the rumen is not well understood. However, it does seem that an appreciable, biological amount of MOS and BG does influence lower GIT because supplementing yeast cell wall extracts reduced stress-related disease, improved mammary gland health, and increased milk production.87, 88 Supplementation strategies of yeast cell wall extracts seem to be most effective during periods of stress or increased pathogen exposure. Another application for yeast cell wall extracts is to alleviate some of the negative effects of hydrophobic mycotoxins such as zearalenone. Jouany and colleagues89 reported that BG interacted with zearalenone, in an “adsorption type” chemical interaction. Both Pereyra and colleagues90 and Fruhauf and colleagues91 reported that commercial yeast cell wall extracts with the greatest MOS and BG content adsorbed zearalenone to the greatest extent but were not effective at adsorbing aflatoxin, a hydrophilic mycotoxin. Therefore, a common mycotoxin strategy is to combine a mineral clay and a yeast cell wall extract to gain a more broad-spectrum mycotoxin adsorption capability.
Phytonutrients
Phenolic compounds represent a family of phytonutrients with potential therapeutic food animal applications given their intrinsic antioxidative and anti-inflammatory properties (Table 2 ). In nature, plants synthesize polyphenols as both a defense mechanism against exogenous pathogens and for cellular protection against ultraviolet irradiation. Rich sources of phenolic compounds fed to ruminants include a multitude of industrial fruit and vegetable coproducts, such as citrus, pomegranate, green tea, grape, and green vegetable processing residues. Following consumption, the rumen microbiome can significantly degrade polyphenols and decrease host bioavailability. However, a proportion of dietary polyphenols will bypass ruminal degradation and enter the GIT. Following intestinal absorption, phenolic compounds enter the peripheral circulation where they may exert their bioactive effects on inflammation, oxidative status, and immune function (Fig. 2 ).92, 93
Table 2.
Effects of phytonutrients on the immune function, health, and performance of calves, dairy cows, sheep, and goats
| Animal | Strain/Type | Dose | Duration/Frequency | Health/Production Status | Outcome | Reference |
|---|---|---|---|---|---|---|
| Calf | Oregano | 1%, 1.5%, or 2% oregano oil in MR | 4 d to weaning | Low risk, 4 d of age to 5 mo | Increased IgG concentrations, decreased fecal score, reduced Enterobacteriaceae shedding | Ozkaya36 2018 |
| Calf | Oregano | 100 ppm/hd/d | 120 d | Low risk, 30–150 d of age calves | No reduction in Eimeria oocyst shedding | Grandi et al,37 2016 |
| Calf | Oregano | 12.5 mg/kg | 15 d | Preweaned | Decreased incidence, severity, and duration of scours | Katsoulos et al,38 2017 |
| Calf | EO | Multiple doses 0–281 mg/calf/d | 24 wk | Low risk | Reduced health scores, scours, and antibiotic treatment | Soltan,39 2009, and Oh et al,40 2017 |
| Calf | Pomegranate | 140 mg polyphenols/g DM; about 5%–20% total DMI | 8 wk | Postweaned; apparently healthy; low risk; 11 mo old | Increased DMI, tendency for increased weight gain | Shabtay et al,41 2008 |
| Calf | Pomegranate | 5 and 10 g/d top-dressed onto starter | 70 d | Apparently healthy; low risk; 0–70 d old | Increased peripheral cytokine synthesis (IFN-γ, IL-4), improved IgG response to ovalbumin vax, no effect on fecal scores or rectal temperatures | Oliveira et al,42 2010 |
| Dairy cow | EO (garlic, oregano) | 3 mL intravaginally, 12 mL intramammary, 25 mL topical teat dip | Once daily for 3 d, 2× daily for 1 d, and 1 application | Mid to late lactation multiparous Holstein cows, Streptococcus uberus mastitis challenge | No cure of mastitis | Mullen et al,43 2108 |
| Dairy cow | EO | 0, 100, and 200 mg/d | 28 d | Lactating | Increase CD4+ T-cell response to vaccine/immune challenge, tendency to increase milk production | Oh et al,44 2016 |
| Dairy cow | Grape | 4.5 g/hd/d | 75 d | Apparently healthy, mid lactation; low risk | No effect on SCC, tendency to increase milk yield | Nielsen & Hansen,45 2004 |
| Dairy cow | Grape | 10 g/d mixed into pellets | 3 wk pre-/postcalving; 44 total day approx. | Apparently healthy; primiparous, 7 mo pregnant | Lowered leukocyte mRNA expression of SOD during initial 3 wk postpartum; no effect on glutathione peroxidase expression | Colitti & Stefanon et al,46 2006 |
| Dairy cow | Green tea | 100 μg/mL | 12 h | In vitro study; mammary epithelial cells isolated from lactating Holstein cows | Greater cell viability, protein, mRNA abundance of NFE2L2; lower intracellular ROS accumulation in response to H2O2 challenge | Ma et al,47 2018 |
| Transition dairy cow | Mixture | 150 g/d prepartum; 170 g/d postpartum | 25 d prepartum/26 d postpartum | Apparently healthy; multiparous, primiparous Holstein transition cows | Lower serum NEFA, lower NEFA:insulin postpartum, improved insulin sensitivity pre-/postpartum, higher total antioxidant capacity prepartum; lower malondialdehyde pre-/postpartum | Hashemzadeh-Cigari et al,48 2015 |
| Transition dairy cow | Pomegranate | 350 g DM/d for seeds only; 1350 g DM/d pulp (seeds + peels blend) | 25 d pre-/postpartum | Apparently healthy; multiparous, primiparous Holstein transition cows | Higher total plasma antioxidant capacity, lower TAG/FFA/BHBA at both pre-/postpartum, pulp blend increased SOD, decreased MDA postpartum, higher FCM yield | Safari et al,49 2018 |
| Transition dairy cow | Grape | 1% of DM | 3 wk prepartum until 9 wk postpartum | Multiparous, primiparous Holstein transition cows | Reduced mRNA expression of FGF21 (liver stress hormone) postpartum, no effect on hepatic inflammatory gene expression, increase daily milk yield, increase daily milk protein yield | Gessner et al,50 2015 |
| Transition dairy cow | Green tea | 0.175 g/kg feed DM | 3 wk prepartum until 9 wk postpartum | Primiparous, multiparous Holstein transition cows | Trend for reduced mRNA (haptoglobin), reduced mRNA (FGF21) postpartum; no difference (TNF), (CRP), higher ECM wk 2–9 postpartum; lower hepatic TAG, cholesterol concentrations wk 1 and 3 postpartum | Winkler et al,51 2015 |
| Sheep | Mixture (4 compounds) | Single ruminal infusion; 10% DMI | 1 d | Apparently healthy; 18-mo-old, castrated males | Grape-enhanced total plasma antioxidant capacity; reduced plasma susceptibility to liperoxidation | Gladine et al,52 2007 |
| Sheep | Green tea | 2, 4, or 6 g/kg feed DM | 8 wk | Lambs infected with Haemonchus contortus GIT parasites | Decreased serum APPs at all dosages (Hpt, LBP, a1AGP), regulate SAA in dose-dependent manner, higher ADG in infected, supplemented lambs vs. infected only lambs; reduced adult worm burden to uninfected levels at 6 g/kg group | Zhong et al,53 2014 |
| Goat | Green tea | 2, 3, or 4 g TC/kg DM feed | 60 d | Low risk | Reduced plasma glutathione most efficaciously at 2 g dosage; over 3 g dosage reduced plasma protein and globulins (bad) | Zhong et al,54 2011 |
| Goat | Green tea | 2.0% on weight:weight ratio | 90 d | Low risk, castrated male goats | Linear increase average weight gain and feed intake, increased splenic cell growth, reduced intramuscular TBARS | Ahmed et al,55 2015 |
Abbreviations: ADG, average daily gain; APP, acute-phase proteins; BHBA, beta-hydroxybutyric acid; DIM, days in milk; DM, dry matter; DMI, dry matter intake; ECM, energy-corrected milk; EO, essential oil; FCM, fat corrected milk; FFA, free fatty acids; MDA, malondialdehyde; MR, milk replacer; NEFA, nonesterified fatty acid; ROS, reactive oxygen species; SAA, serum amyloid A; SCC, somatic cell count; SOD, superoxide dismutase; TAG, triacylglycerol; TBARS, thiobarbituric acid reactive substances.
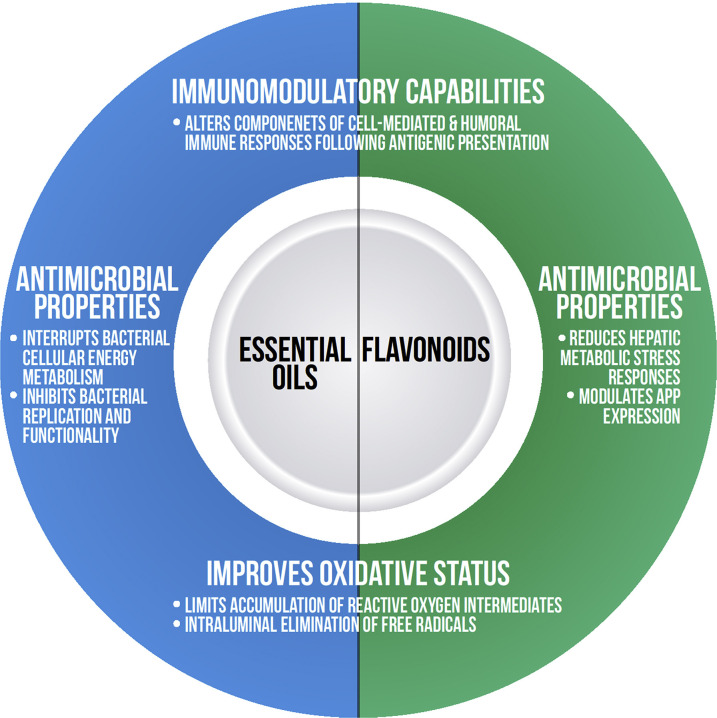
Fig. 2.
Mechanisms of action of phytonutrients on immune defenses.
Chemically, flavonoids are hydroxylated polyphenolic compounds consisting of a 15-carbon chain bound to an oxygenated heterocyclic ring structure.92, 93 Published studies on the immune effects of feeding flavonoid metabolites specifically to ruminants were limited predominantly to grape, pomegranate, and green tea derivatives.
Byproducts from wine and fruit-juicing industries include pomace, which consists of residual grape pulp, seeds, skin, and stems. Grape pomace contains high concentrations of flavonoid derivatives and tannins, thus acting as a potential reservoir of dietary antioxidant, anti-inflammatory, and antimicrobial phenolic compounds.45 Supplementing grape seed and pomace extracts can reduce mRNA abundancy of fibroblast growth factor 21 (FGF21), a stress hormone associated with hepatic fatty acid oxidation and ketogenesis, in transition dairy cows.50 Grape polyphenols lowered leukocyte mRNA expression of superoxide dismutase, an endogenous free radical scavenger, in postpartum dairy cows. This effect suggests the ability of grape polyphenols to decrease superoxide dismutase expression as a consequence of their endogenous antioxidant capacity.46
Pomegranate byproducts (eg, seeds, pulp, and peels) contain a potent source of polyphenols, predominantly flavonoids, phenolic acids, and tannins, as well as stereoisomers of vitamin E. Together, these compounds reduce reactive oxygen species and chelate metal ions to induce potentially beneficial immunomodulatory effects in ruminants.49, 94 Dairy calves supplemented with pomegranate extracts had increased in vitro synthesis of the lymphocyte-derived cytokines interferon-γ and IL-4, as well as greater total immunoglobulin G (IgG), in response to ovalbumin vaccination. By improving aspects of both humoral and cell-mediated immunity, calves supplemented with polyphenols may have an improved immune response following vaccination.42 Hydrolysable tannins may be able to reduce intraluminal oxidation thereby increasing dietary vitamin E bioavailability, suggesting a potential mechanism for antioxidants to improve health.41
Green tea leaf extracts, particularly catechins, represent another rich source of phenolic compounds with antioxidant potential. Tea polyphenols reduced accumulations of reactive oxygen intermediates in bovine mammary epithelial cells following a hydrogen peroxide challenge in vivo. 47 Green tea polyphenols also seem to modulate acute-phase protein expression upon parasitic challenge in small ruminant species.53 Reduced hepatic metabolic stress, coupled with decreased intrahepatic lipid concentrations and acute-phase protein expression, may indicate improved energy status, ultimately leading to improved ruminant health and performance.51
Essential oils represent another class of phytonutrients with potential antimicrobial and immunomodulatory capabilities. Essential oils (EOs) provide a plant with its unique color and fragrance. Chemically, EOs are composed of a lipophilic mixture of terpenoids, phenolics, and a variety of low-molecular-weight compounds.1, 95 The antibacterial mode of action of volatile oils is likely multifactorial with hypothesized mechanisms including disruption of biochemical pathways associated with cell membranes and passage of low-molecular-weight EO derivatives across inner plasma membranes of both gram-positive and gram-negative bacteria. By interrupting cellular energy metabolism, EOs can partially inhibit bacterial growth and functionality and have demonstrated microbicidal effects against common food-borne pathogens, rumen bacteria, and gastrointestinal parasites (see Fig. 2).1 Most of the immunologic effects of EOs were investigated in monogastric species. Receptor-mediated responses upon EO supplementation can result in improved mucosal blood flow, altered cytokine and neuropeptide release, and modified immune cell functionality. Volatile oils undergo significant ruminal biodegradation, likely limiting the immunomodulatory and antibacterial effects.44 Of the published studies of EOs performed specifically on ruminants, oregano, garlic, and Capsicum oleoresin oils were shown have potential health and immunomodulatory effects.
Oregano (Origanum onite L) oil and its 2 main phenolic derivatives, carvacrol and thymol, offer a potential potent source of antimicrobial and antioxidant capabilities. Oregano oil increased hematologic variables in dairy calves including hemoglobin, packed cell volume, and mean corpuscular volume.96 Initial experiments have demonstrated potential benefits of using oregano to minimize scours and improve health scores in neonatal ruminants.38 Dairy calves supplemented with oregano water had increased fecal firmness, reduced Enterobacteriaceae shedding, and increased immunoglobulin concentrations.36 However, adult dairy cattle supplemented with EO products containing either garlic, thyme, or oregano failed to cure experimentally induced Streptococcus uberis mastitis following either topical or intramammary administration.43 Oregano supplementation also failed to reduce Eimeria oocyst shedding in dairy heifers.37
Garlic (Allium sativum) and garlic oils have the capacity to eliminate free radical species and reduce lipid peroxidation in vivo. The effects of garlic oil on immunologic variables in mature ruminants are inconclusive. However, Oh and colleagues97 recently described the broad immuno-stimulatory effects of garlic oil following intra-abomasal infusion via an increased neutrophil to lymphocyte ratio as well as increased helper (CD4+) T-cell proliferation.
Capsaicinoids, bioactive compounds sourced from flowering Capsicum plants, demonstrated an ability to modulate the acute-phase response in dairy cows, including in response to an intravenous lipopolysaccharide (LPS) challenge.3, 98 Furthermore, postruminal supplementation of capsaicinoids increased CD4+ T-cell proliferation.97 By potentially affecting both the innate and adaptive branches of the immune system, capsaicinoid supplementation may be particularly beneficial during vaccination events.99 However, more research is needed to validate observations using these EO-based compounds in ruminant species.
Polyunsaturated fatty acids
Dietary fat is more than just calories. The composition of fatty acids in fat sources can influence many cellular functions (Table 3 ). The fatty acid composition of cellular membranes reflects the fatty acid composition of the diet. The physical chemistry of the various fatty acids differs and increasing the incorporation of polyunsaturated fatty acids (PUFAs) can increase the membrane fluidity. Increasing the fluidity of leukocytes can disrupt the organization of cell surface receptors in the plasma membrane, and ultimately alter cellular function.100, 101, 102, 103, 104
Table 3.
Effects of polyunsaturated fatty acids on the immune function, health, and performance of calves, dairy cows, and feedlot steers
| Animal | Strain/Type | Dose | Duration/Frequency | Health/Production Status | Outcome | Reference |
|---|---|---|---|---|---|---|
| Calf | Fish oil | 1% and 2% DM of the milk replacer | 42 d | High risk | Reduced inflammatory response to LPS challenge, quadratic effect on secondary antibody response, no effect on fecal scores | Ballou et al,56 2008; Ballou & DePeters,57 2008 |
| Calf | Fish oil | 2% of the DM | 14–55 d of life | Low risk | No effect on serum haptoglobin or mitogen-induced IFN-γ secretion, no difference in fecal scores, fish oil decreased starter intake and ADG | McDonnell et al,58 2019 |
| Calf | Coconut oil | Replaced 20 or 40% of the lard in milk replacer | 56 d | Low risk | Quadratic decrease in fecal scores, no difference in starter intake or ADG | Bowen Yoho et al,59 2013 |
| Calf | Linoleic acid | 0.48% or 9.0% of the fatty acids in milk replacer as linoleic acid | 30 d | Low risk | Caused differential expression of many immune-related hepatic genes | Garcia et al,60 2016 |
| Calf | Blend of short- and medium-chain fatty acids and α-linolenic acid | 0.46%, 0.51%, 0.19%, and 0.18% of DM as butyric, lauric, myristic, and linolenic acids, respectively, in milk replacer | 56 d | Low risk | No difference in fecal score or medication, improved DM digestion and ADG | Hill et al,61 2016 |
| Dairy cow | Ca salts of palm oil, safflower oil, or fish oil | n-6 to n-3 ratios of 4, 5, or 6 | 14 d postpartum to 105 d postpartum | Early lactation | Lower n-6 to n-3 decreased plasma IL-6 and haptoglobin concentrations after a moderate intramammary LPS challenge, no effect on neutrophil phagocytosis or oxidative burst, greater DMI and 3.5% FCM in the n-6 to n-3 ratio of 4 | Greco et al,62 2015 |
| Dairy cow | Whole flaxseed | 10.4% of DM | Calving to 105 DIM | Early lactation | Transient reduction in mitogen proliferation of PBMC, no effect on humoral response, decreased prostaglandin E2 | Lessard et al,63 2003 |
| Transition dairy cow | Fish oil | 250 g prepartum and 0.92% DM postpartum | 3 wk prepartum until 10 d postpartum | Apparently healthy, multiparous Holstein cows | No effect on inflammatory response to a high dose of LPS intramammary, no difference in DMI or milk production | Ballou et al,64 2009 |
| Transition dairy cow | Ca salts of palm oil, safflower oil, or fish oil | 1.5% of DM | Safflower oil from 30 d prepartum to 35 d postpartum; fish oil 35–160 d postpartum | Apparently healthy, multiparous Holstein cows | Safflower oil increased plasma acute-phase proteins, neutrophil proinflammatory cytokines, neutrophil L-selectin, and neutrophil phagocytic and oxidative burst activities, fish oil decreased neutrophil proinflammatory cytokine | Silvestre et al,65 2011 |
Abbreviations: DIM, days in milk; DM, dry matter; n-3, omega-3 polyunsaturated fatty acids; n-6, omega-6 polyunsaturated fatty acid; PBMC, peripheral blood mononuclear cell.
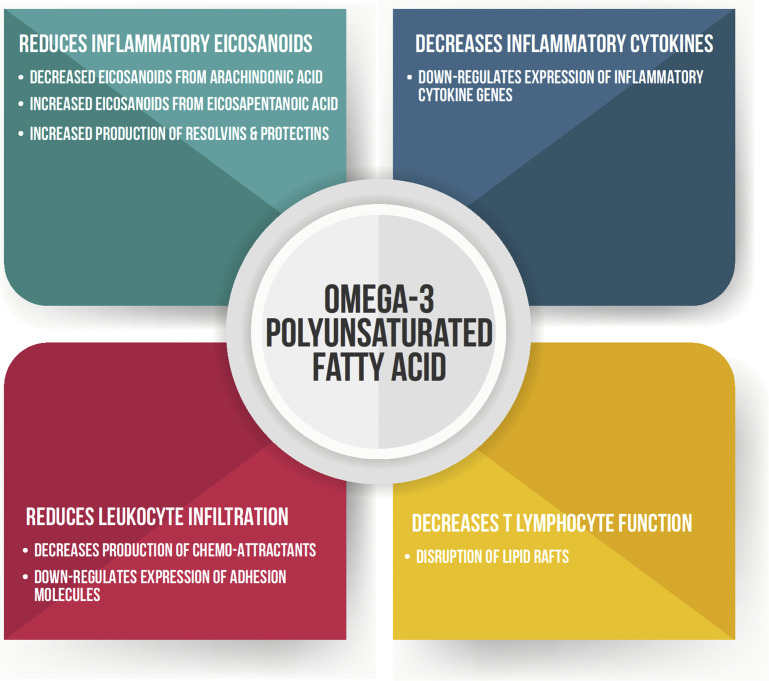
In addition to influencing membrane fluidity, the fatty acid composition of cellular membranes can alter the biosynthesis of various lipid mediators (Fig. 3 ). The eicosanoid family are some of the most well-known bioactive lipid mediators and include prostaglandins, leukotrienes, and thromboxanes. The balance of omega-6 to omega-3 PUFA in cellular membranes influences the quantity and biological potency of the eicosanoids produced. Generally, eicosanoids from omega-3 PUFAs have a reduced affinity for cyclooxygenase enzymes. They also produce many eicosanoids that have a lower biological activity than those produced from omega-6 PUFAs. This led to the concept that omega-3 PUFAs have a greater anti-inflammatory capacity than omega-6 PUFAs.
Fig. 3.
Mechanisms of action of omega-3 long-chain polyunsaturated fatty acids.
Preweaned calves fed milk replacer with either 1% or 2% of the total dry matter (DM) replaced as fish oil, which is rich in omega-3 PUFAs, reduced the proinflammatory response when challenged with a large dose of LPS to mimic sepsis.56 In a recent study replacing 2% of the total DM with fish oil fed to preweaned calves did not reduce the serum haptoglobin concentrations or mitogen-induced interferon-γ secretion.58 In contrast, Garcia and colleagues60 supplemented preweaned calves with either a low or high omega-6 linoleic acid milk replacer (0.46% or 9.0% of the total fatty acids, respectively) and reported that the high linoleic acid treatment had many differentially expressed genes in the liver that should predict a reduced risk of infection. The linoleic fatty acid compositions used in the study of Garcia and colleagues likely reflect milk diets based on milk fat (low linoleic acid) and those in which the primary fat source is based on lard (high linoleic acid). The exact PUFA composition or balance of omega-6 to omega-3 PUFAs in milk solids that improves immune responses and overall health remains to be determined. Finally, although short-chain fatty acids are not PUFAs it is important to emphasize their importance in GIT development as we discussed previously in the section on probiotics. Supplementing short-chain fatty acids such as butyrate can improve GIT development and calf performance.61 Furthermore, the effect may be more pronounced in milk replacer diets in which the primary fat source is not milk fat. Milk fat already contains a significant quantity of butyrate; however, the exact concentrations of these short-chain fatty acids to supplement in milk replacer are not known.59, 61
Supplementing omega-3 PUFAs and altering the omega-6 to omega-3 PUFA ratio to transition and early lactating cows was investigated as a strategy to improve health and production performance. Feeding whole flaxseed as a source of the omega-3 PUFA, specifically α-linolenic acid, from calving until 105 days in milk (DIM) decreased the serum concentrations of the proinflammatory eicosanoid, prostaglandin E2.63 Furthermore, decreasing the omega-6 to omega-3 PUFA ratio using calcium salts of fish oil from 14 to 105 DIM decreased the proinflammatory response following a mild intramammary LPS challenge.62 In contrast, there was no difference in the proinflammatory response in cows when challenged with 10 times the dose of LPS in the mammary gland at 7 DIM whether they were supplemented with fish oil or not.64 The authors attributed the lack of an effect on the severity of the LPS challenge. Silvestre and colleagues65 supplemented 1.5% of the total DM as safflower oil as a source of omega-6 PUFA from 30 d prepartum to 35 DIM. Observations included increased plasma acute-phase proteins, neutrophil proinflammatory cytokines, neutrophil L-selectin, and neutrophil phagocytic and oxidative burst capacities during the periparturient period. Furthermore, cows were switched to a breeding diet at 35 DIM that contained 1.5% of the total DM as calcium salts of fish oil and fed through 160 DIM. Supplementing cows with the calcium salts of fish oil decreased neutrophil proinflammatory cytokine secretion. These data further support that the balance of omega-6 to omega-3 PUFAs in the diets of both calves and cows can influence various immune responses. However, it remains to be determined at what age, physiologic stage, and conditions more inflammation is desirable and vice versa when less inflammation is preferred.105, 106
Summary
Nutraceuticals represent a group of compounds that may help fill that void because they exert some health benefits when supplemented to livestock. There remains significant ambiguity regarding nutraceuticals because this field is evolving at a rapid pace without regulatory oversight. In this review, we broadly classify and discuss nutraceuticals as either probiotics, prebiotics, phytonutrients, or PUFAs. These compounds work through various mechanisms of action including stabilizing commensal microbial communities, improving mucosal immune responses and barrier function, binding or adsorbing potential pathogens or toxins, improving antioxidant capacity, direct antimicrobial activity, and increasing or decreasing systemic immune responses.
Footnotes
Disclosure: M.A. Ballou has equity ownership in MB Nutritional Sciences, LLC. The rest of the authors have nothing to disclose.
References
- 1.Benchaar C., Calsamiglia S., Chaves A., et al. A review of plant-derived essential oils in ruminant nutrition and production. Anim Feed Sci Technol. 2008;145(1–4):209–228. [Google Scholar]
- 2.Braun H., Schrapers K., Mahlkow-Nerge K., et al. Dietary supplementation of essential oils in dairy cows: evidence for stimulatory effects on nutrient absorption. Animal. 2019;13(3):518–523. doi: 10.1017/S1751731118001696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh J., Wall E., Bravo D., et al. Host-mediated effects of phytonutrients in ruminants: a review. J Dairy Sci. 2017;100(7):5974–5983. doi: 10.3168/jds.2016-12341. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman H.M., Mulder L., Everts H., et al. Health and growth of veal calves fed milk replacers with or without probiotics. J Dairy Sci. 2005;88(6):2154–2165. doi: 10.3168/jds.S0022-0302(05)72891-5. [DOI] [PubMed] [Google Scholar]
- 5.Mokhber-Dezfouli M.R., Tajik P., Bolourchi M., et al. Effects of probiotics supplementation in daily milk intake of newborn calves on body weight gain, body height, diarrhea occurrence and health condition. Pak J Biol Sci. 2007;10(18):3136–3140. doi: 10.3923/pjbs.2007.3136.3140. [DOI] [PubMed] [Google Scholar]
- 6.Al-Saiady M.Y. Effect of probiotic bacteria on immunoglobulin G concentration and other blood components of newborn calves. J Anim Vet Adv. 2010;9(3):604–609. [Google Scholar]
- 7.Signorini M.L., Soto L.P., Zbrun, et al. Impact of probiotic administration on the health and fecal microbiota of young calves: a meta-analysis of randomized controlled trials of lactic acid bacteria. Res Vet Sci. 2012;93(1):250–258. doi: 10.1016/j.rvsc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Liang Y., Hudson R.E., Ballou M.A. Supplementing neonatal Jersey calves with a blend of probiotic bacteria improves the pathophysiological response to an oral Salmonella enterica challenge. Jacobs Journal of Veterinary Science and Research. 2019;6(3):050. doi: 10.3168/jds.2019-17480. [DOI] [PubMed] [Google Scholar]
- 9.Tkalcic S., Zhao T., Harmon B., et al. Fecal shedding of enterohemorrhagic Escherichia coli in weaned calves following treatment with probiotic Escherichia coli. J Food Prot. 2003;66(7):1184–1189. doi: 10.4315/0362-028x-66.7.1184. [DOI] [PubMed] [Google Scholar]
- 10.Davis E.M. Texas Tech University; Lubbuck (TX): 2018. Impacts of various milk replacer supplements on the health and performance of high-risk dairy calves. Master’s thesis. [Google Scholar]
- 11.Agarwal N., Kamra D., Chaudhary L., et al. Microbial status and rumen enzyme profile of crossbred calves fed on different microbial feed additives. Lett Appl Microbiol. 2002;34:329–336. doi: 10.1046/j.1472-765x.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- 12.Galvao K.N., Santos J.E.P., Coscioni A., et al. Effect of feeding live yeast products to calves with failure of passive transfer on performance and patterns of antibiotic resistance in fecal Escherichia coli. Reprod Nutr Dev. 2005;44(6):427–440. doi: 10.1051/rnd:2005040. [DOI] [PubMed] [Google Scholar]
- 13.Eicher S.D., Wesley I.V., Sharma V.K., et al. Yeast cell-wall products containing β-glucan plus ascorbic acid affect neonatal Bos taurus calf leukocytes and growth after a transport stressor. J Anim Sci. 2010;88(3):1195–1203. doi: 10.2527/jas.2008-1669. [DOI] [PubMed] [Google Scholar]
- 14.Kara C., Cihan H., Temizel M., et al. Effect of supplemental mannanoligosaccharides on growth performance, faecal characteristics, and health in dairy calves. Asian-Australas J Anim Sci. 2015;28(11):1599–1605. doi: 10.5713/ajas.15.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh S., Mehla R.K. Influence of dietary supplementation of prebiotics (mannanoligosaccharide) on the performance of crossbred calves. Trop Anim Health Prod. 2012;44(3):617–622. doi: 10.1007/s11250-011-9944-8. [DOI] [PubMed] [Google Scholar]
- 16.Heinrichs A.J., Jones C.M., Heinrichs B.S. Effects of mannan oligosaccharides or antibiotics in neonatal diets on health and growth of dairy calves. J Dairy Sci. 2003;86(12):4064–4069. doi: 10.3168/jds.S0022-0302(03)74018-1. [DOI] [PubMed] [Google Scholar]
- 17.Hill T.M., Bateman H.G., Aldrich J.M., et al. Oligosaccharides for dairy calves. Professional Animal Scientist. 2008;24(5):460–464. [Google Scholar]
- 18.Grand E., Respondek F., Martineau C., et al. Effects of short-chain fructooligosaccharides on growth and performance of preruminant veal calves. J Dairy Sci. 2013;96(2):1094–1101. doi: 10.3168/jds.2011-4949. [DOI] [PubMed] [Google Scholar]
- 19.Castro J., Gomez A., White B., et al. Changes in the intestinal bacterial community, short-chain fatty acid profile, and intestinal development of preweaned Holstein calves. 1. Effects of prebiotic supplementation depend on site and age. J Dairy Sci. 2016;99(12):9682–9702. doi: 10.3168/jds.2016-11006. [DOI] [PubMed] [Google Scholar]
- 20.Xu H., Huang W., Hou Q., et al. The effect of probiotics administration on the milk production, milk components, and fecal bacteria microbiota of dairy cows. Sci Bull (Beijing) 2017;62(11):767–774. doi: 10.1016/j.scib.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Shreedhar J.N., Patil M., Kumar P. Effect of probiotics supplementation on milk yield and its composition in lactating Holstein Fresien and Deoni cross bred cows. J Med Bioeng. 2016;5(1):19–23. [Google Scholar]
- 22.Musa A., Pandey N., Pandy R. Effects of feeding sodium bicarbonate and multi-strain probiotics on milk yield and milk composition of lactating Holstein Frisian crossbred cows. J Pharmacogn Phytochem. 2017;6(6):1912–1916. [Google Scholar]
- 23.Vibhute V., Shelke R., Chavan S., et al. Effect of probiotics supplementation on the performance of lactating crossbred cows. Vet World. 2011;4(12):557–561. [Google Scholar]
- 24.Rossow H., DeGroff D., Parsons M. Performance of dairy cows administered probiotic in water troughs. Professional Animal Scientist. 2014;30(5):527–533. [Google Scholar]
- 25.Rossow H., Riordan T., Riordan A. Effects of addition of a live yeast product on dairy cattle performance. J Appl Anim Res. 2018;46(1):159–163. [Google Scholar]
- 26.Maamouri O., Selmi H., M’hamdi N. Effects of yeast (Saccharomyces cerevisiae) feed supplement on milk production and its composition in Tunisian Holstein Fresian cows. Scientia Agriculturae Bohemica. 2014;45(3):170–174. [Google Scholar]
- 27.Dailidaviciene J., Budreckiene R., Gruzauskas R., et al. The influence of probiotic additives or multienzyme composition on blood biochemical parameters and milk quality of Lithuanian Black-and-White cattle. Arq Bras Med Vet Zootec. 2018;70(3):939–945. [Google Scholar]
- 28.Yasuda K., Hashikawa S., Sakamoto H., et al. New synbiotic consisting of Lactobacillus casei subsp. casei and dextran improves milk production in Holstein dairy cows. J Vet Med Sci. 2007;69(2):205–208. doi: 10.1292/jvms.69.205. [DOI] [PubMed] [Google Scholar]
- 29.Sretenovic Lj., Petrovic M., Aleksic S., et al. Influence of yeast, probiotics and enzymes in rations on dairy cow performances during transition. Biotechnology in Animal Husbandry. 2008;24(5-6):33–43. [Google Scholar]
- 30.Peterson R., Klopfenstein T., Erickson G., et al. Effect of Lactobacillus acidophilus strain NP51 on Escherichia coli O157:H7 fecal shedding and finishing performance in beef feedlot cattle. Journal of Food Protection. 2007;70(2):287–291. doi: 10.4315/0362-028x-70.2.287. [DOI] [PubMed] [Google Scholar]
- 31.Schamberger G., Phillips R., Jacobs J., et al. Reduction of Escherichia coli and O157:H7 populations in cattle by addition of Colicin E7-Producing E. coli to feed. Appl Environ Microbiol. 2004;70(10):6053–6060. doi: 10.1128/AEM.70.10.6053-6060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabe E., Oloya J., Doetkott D., et al. Comparative effect of direct-fed microbials on fecal shedding of Escherichia coli O157:H7 and Salmonella in naturally infected feedlot cattle. J Food Prot. 2008;71(3):539–544. doi: 10.4315/0362-028x-71.3.539. [DOI] [PubMed] [Google Scholar]
- 33.Younts-Dahl S., Osborn G., Galyean M., et al. Reduction of Escherichia coli O157 in finishing beef cattle by various doses of Lactobacillus acidophilus in direct-fed microbials. J Food Prot. 2005;68(1):6–10. doi: 10.4315/0362-028x-68.1.6. [DOI] [PubMed] [Google Scholar]
- 34.Brashears M., Galyean M., Loneragan G., et al. Prevalence of Escherichia coli O157:H7 and performance by beef feedlot cattle given Lactobacillus direct-fed microbials. J Food Prot. 2003;66(5):748–754. doi: 10.4315/0362-028x-66.5.748. [DOI] [PubMed] [Google Scholar]
- 35.Jin L., Dong G., Lei G., et al. Effects of dietary supplementation of glutamine and mannan oligosaccharides on plasma endotoxin and acute phase protein concentrations and nutrient digestibility in finishing steers. J Appl Anim Res. 2014;42(2):160–165. [Google Scholar]
- 36.Ozkaya S., Erbas S., Ozkan O., et al. Effect of supplementing milk replacer with aromatic oregano (Oreganum onites L.) water on performance, immunity and general health profiles of Holstein calves. Anim Prod Sci. 2018;58(10):1892–1900. [Google Scholar]
- 37.Grandi G., Kramer L., Quarantelli A., et al. Influence of oregano essential oil (OEO) on prevalence and oocyst shedding dynamics of naturally acquired Eimeria spp. infection in replacement dairy heifers. Ann Anim Sci. 2016;16(1):171–179. [Google Scholar]
- 38.Katsoulos P.D., Karatzia M., Dovas C., et al. Evaluation of the in-field efficacy of oregano essential oil administration on the control of neonatal diarrhea syndrome in calves. Res Vet Sci. 2017;115:478–483. doi: 10.1016/j.rvsc.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soltan M. Effect of essential oils supplementation on growth performance, nutrient digestibility, health condition of Holstein male calves during pre- and post-weaning periods. Pak J Nutr. 2009;8(5):642–652. [Google Scholar]
- 40.Oh J., Harper M., Bravo D., et al. Effects of rumen-protected Capsicum oleoresin on productivity and responses to a glucose tolerance test in lactating dairy cows. J Dairy Sci. 2017;100(3):1888–1901. doi: 10.3168/jds.2016-11665. [DOI] [PubMed] [Google Scholar]
- 41.Shabtay A., Eitam H., Tadmor Y., et al. Nutritive and antioxidative potential of fresh and stored pomegranate industrial byproduct as a novel beef cattle feed. J Agric Food Chem. 2008;56(21):10063–10070. doi: 10.1021/jf8016095. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira R., Narciso C., Bisinotto R., et al. Effects of feeding polyphenols from pomegranate extract on health, growth, nutrient digestion, and immunocompetence of calves. J Dairy Sci. 2010;93(9):4280–4291. doi: 10.3168/jds.2010-3314. [DOI] [PubMed] [Google Scholar]
- 43.Mullen K., Lyman R., Washburn S., et al. Effect of 3 phytoceutical products on elimination of bacteria in experimentally induced Streptococcus uberis clinical mastitis. J Dairy Sci. 2018;101(11):10409–10413. doi: 10.3168/jds.2017-14279. [DOI] [PubMed] [Google Scholar]
- 44.Oh J., Bravo D., Wall E., et al. Rumen disappearance of capsaicin and dihydrocapsaicin in lactating dairy cows. J Anim Sci. 2016;94(5):801. [Google Scholar]
- 45.Nielsen B., Hansen H. Effect of grape pomace rich in flavonoids and antioxidants on production parameters in dairy production. J Anim Feed Sci. 2004;13(1):535–538. [Google Scholar]
- 46.Colitti M., Stefanon B. Effect of natural antioxidants on superoxide dismutase and glutathione peroxidase mRNA expression in leukocytes from periparturient dairy cows. Vet Res Commun. 2006;30(1):19–27. doi: 10.1007/s11259-005-3208-x. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y., Zhao L., Gao M., et al. Tea polyphenols protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative damage in vitro by activating NFE2L2/HMOX1 pathways. J Dairy Sci. 2018;102(2):1658–1670. doi: 10.3168/jds.2018-15047. [DOI] [PubMed] [Google Scholar]
- 48.Hashemzadeh-Cigari F., Ghorbani G., Khorvash M., et al. Supplementation of herbal plants differently modulated metabolic profile, insulin sensitivity, and oxidative stress in transition dairy cows fed various extruded oil seeds. Prev Vet Med. 2015;118(1):45–55. doi: 10.1016/j.prevetmed.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Safari M., Ghasemi E., Alikhani M., et al. Supplementation effects of pomegranate by-products on oxidative status, metabolic profile, and performance in transition dairy cows. J Dairy Sci. 2018;101(12):11297–11309. doi: 10.3168/jds.2018-14506. [DOI] [PubMed] [Google Scholar]
- 50.Gessner D., Koch C., Romberg F., et al. The effect of grape seed and grape marc meal extract on milk performance and the expression of genes of endoplasmic reticulum stress and inflammation in the liver of dairy cows in early lactation. J Dairy Sci. 2015;98(12):8856–8868. doi: 10.3168/jds.2015-9478. [DOI] [PubMed] [Google Scholar]
- 51.Winkler A., Gessner D., Koch C., et al. Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Arch Anim Nutr. 2015;69(6):425–441. doi: 10.1080/1745039X.2015.1093873. [DOI] [PubMed] [Google Scholar]
- 52.Gladine C., Rock E., Morand C., et al. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br J Nutr. 2007;98(4):691–701. doi: 10.1017/S0007114507742666. [DOI] [PubMed] [Google Scholar]
- 53.Zhong R.Z., Li H., Sun H., et al. Effects of supplementation with dietary green tea polyphenols on parasite resistance and acute phase protein response to Haemonchus contortus infection in lambs. Vet Parasitol. 2014;205(1–2):199–207. doi: 10.1016/j.vetpar.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Zhong R., Xiao W., Ren G., et al. Dietary tea catechin inclusion changes plasma biochemical parameters, hormone concentrations and glutathione redox status in goats. Asian-Australas J Anim Sci. 2011;24(12):1681–1689. [Google Scholar]
- 55.Ahmed S., Lee J., Mun H., et al. Effects of supplementation with green tea by-products on growth performance, meat quality, blood metabolites and immune cell proliferation in goats. J Anim Physiol Anim Nutr. 2015;99(6):1127–1137. doi: 10.1111/jpn.12279. [DOI] [PubMed] [Google Scholar]
- 56.Ballou M., Cruz G., Pittroff W., et al. Modifying the acute phase response of Jersey calves by supplementing milk replacer with omega-3 fatty acids from fish oil. J Dairy Sci. 2008;91(9):3478–3487. doi: 10.3168/jds.2008-1016. [DOI] [PubMed] [Google Scholar]
- 57.Ballou M.A., DePeters E.J. Supplementing milk replacer with omega-3 fatty acids from fish oil on immunocompetence and health of Jersey calves. J Dairy Sci. 2008;91(9):3488–3500. doi: 10.3168/jds.2008-1017. [DOI] [PubMed] [Google Scholar]
- 58.McDonnell R., O’Doherty J., Earley B., et al. Effect of supplementation with n-3 polyunsaturated fatty acids and/or β-glucans on performance, feeding behavior and immune status of Holstein Friesian bull calves during the pre- and post-weaning periods. J Anim Sci Biotechnol. 2019;10(7):1–17. doi: 10.1186/s40104-019-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowen Yoho W., Swank V., Eastridge M., et al. Jersey calf performance in response to high-protein, high-fat liquid feeds with varied fatty acid profiles: intake and performance. J Dairy Sci. 2013;96(4):2494–2506. doi: 10.3168/jds.2012-6099. [DOI] [PubMed] [Google Scholar]
- 60.Garcia M., Greco L., Lock A., et al. Supplementation of essential fatty acids to Holstein calves during late uterine life and first month of life alters hepatic fatty acid profile and gene expression. J Dairy Sci. 2016;99(9):7085–7101. doi: 10.3168/jds.2015-10472. [DOI] [PubMed] [Google Scholar]
- 61.Hill T., Quigley J., Suarez-Mena F., et al. Effect of milk replacer feeding rate and functional fatty acids on dairy calf performance and digestion of nutrients. J Dairy Sci. 2016;99(8):6352–6361. doi: 10.3168/jds.2015-10812. [DOI] [PubMed] [Google Scholar]
- 62.Greco L., Neves Neto J., Pedrico, et al. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on performance and inflammatory responses to a lipopolysaccharide challenge in lactating Holstein cows. J Dairy Sci. 2015;98(1):602–617. doi: 10.3168/jds.2014-8805. [DOI] [PubMed] [Google Scholar]
- 63.Lessard M., Gagnon N., Petit H. Immune response of postpartum dairy cows fed flaxseed. J Dairy Sci. 2003;86(8):2647–2657. doi: 10.3168/jds.S0022-0302(03)73860-0. [DOI] [PubMed] [Google Scholar]
- 64.Ballou M.A., Gomes R., Juchem S., et al. Effects of dietary supplemental fish oil during the peripartum period on blood metabolites and hepatic fatty acid compositions and total triacylglycerol concentrations of multiparous Holstein cows. J Dairy Sci. 2009;92(2):657–669. doi: 10.3168/jds.2008-1196. [DOI] [PubMed] [Google Scholar]
- 65.Silvestre F., Carvalho T., Francisco N., et al. Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: I. Uterine and metabolic responses, reproduction, and lactation. J Dairy Sci. 2011;94(1):189–204. doi: 10.3168/jds.2010-3370. [DOI] [PubMed] [Google Scholar]
- 66.Isolauri E., Sutas Y., Kankaanpaa P., et al. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444–450. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 67.Gaggia F., Mattarelli P., Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 68.Meale S.J., Chaucheyras-Durand F., Berends H., et al. From pre- to postweaning: transformation of the young calf’s gastrointestinal tract. J Dairy Sci. 2017;100(7):5984–5995. doi: 10.3168/jds.2016-12474. [DOI] [PubMed] [Google Scholar]
- 69.Frizzo L.S., Soto L.P., Zbrun M.V., et al. Lactic acid bacteria to improve growth performance in young calves fed milk replacer and spray-dried whey powder. Anim Feed Sci Technol. 2010;157(3–4):159–167. [Google Scholar]
- 70.Jung C., Hugot J., Barreau F. Peyer’s patches: the immune sensors of the intestine. Int J Inflamm. 2010;2010:1–12. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bocker U., Nebe T., Herweck F., et al. Butyrate modulates intestinal epithelial cell-mediated neutrophil migration. Clin Exp Immunol. 2003;131(53):53–60. doi: 10.1046/j.1365-2249.2003.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng L., Li Z., Green R., et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knudsen K., Lerke H., Hedemann, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10:1–19. doi: 10.3390/nu10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorka P., Kowalskim Z., Zabielski R., et al. Invited Review. Use of butyrate to promote gastrointestinal tract development in calves. J Dairy Sci. 2018;101(6):4785–4800. doi: 10.3168/jds.2017-14086. [DOI] [PubMed] [Google Scholar]
- 75.Rice E., Aragona K., Moreland S., et al. Supplementation of sodium butyrate to postweaned heifer diets: effects on growth performance, nutrient digestibility, and health. J Dairy Sci. 2019;102(4):3121–3130. doi: 10.3168/jds.2018-15525. [DOI] [PubMed] [Google Scholar]
- 76.Rainard P., Foucras G. A critical appraisal of probiotics for mastitis control. Front Vet Sci. 2018;5:1–13. doi: 10.3389/fvets.2018.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beecher C., Daly M., Berry D., et al. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1 and IL-8 gene expression. J Dairy Res. 2009;76:340–348. doi: 10.1017/S0022029909004154. [DOI] [PubMed] [Google Scholar]
- 78.Newbold C.J., Wallace R.J., Chen X.B., et al. Different strains of Saccharomyces cerevisiae differ in their effects on ruminal bacterial numbers in vitro and in sheep. J Anim Sci. 1995;73(6):1811–1818. doi: 10.2527/1995.7361811x. [DOI] [PubMed] [Google Scholar]
- 79.Chaucheyras-Durand F., Fonty G. Establishment of cellulolytic bacteria and development of fermentative activities in the rumen of gnotobiotically reared lambs receiving the microbial additive Saccharomyces cerevisiae CNCM I1077. Reprod Nutr Dev. 2001;41(1):57–68. doi: 10.1051/rnd:2001112. [DOI] [PubMed] [Google Scholar]
- 80.Mosoni P., Chaucheyras-Durand F., Bera-Maillet C., et al. Quantification by real-time PCR of cellulolytic bacteria in the rumen of sheep after supplementation of a forage diet with readily fermentable carbohydrates: effect of a yeast additive. J Appl Microbiol. 2007;103(6):2676–2685. doi: 10.1111/j.1365-2672.2007.03517.x. [DOI] [PubMed] [Google Scholar]
- 81.Silberberg M., Chaucheyras-Durand F., Commun L., et al. Repeated acidosis challenges and live yeast supplementation shape rumen microbiota and fermentations and modulate inflammatory status in sheep. Animal. 2013;7(12):1910–1920. doi: 10.1017/S1751731113001705. [DOI] [PubMed] [Google Scholar]
- 82.Arik H., Gulsen N., Hayirli A., et al. Efficacy of Megasphaera elsdenii inoculation in subacute ruminal acidosis in cattle. J Anim Physiol Anim Nutr. 2018;103(2):416–426. doi: 10.1111/jpn.13034. [DOI] [PubMed] [Google Scholar]
- 83.Liu F., Li P., Chen Y., et al. Fructooligosaccharide (FOS) and galactooligosaccharides (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in heathy young population. Sci Rep. 2017;11 doi: 10.1038/s41598-017-10722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ganner A., Stoiber C., Uhlik J.T., et al. Quantitative evaluation of E. coli F4 and Salmonella typhimurium binding capacity of yeast derivatives. AMB Express. 2013;3(1):1–12. doi: 10.1186/2191-0855-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor P.R., Brown G.D., Reid D.M., et al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169(7):3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 86.Gantner B.N., Simmons R.M., Underhill D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24(6):1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nocek J., Holt M., Oppy J. Effects of supplementation with yeast culture and enzymatically hydrolyzed yeast on performance of early lactation dairy cattle. J Dairy Sci. 2011;94(8):4046–4056. doi: 10.3168/jds.2011-4277. [DOI] [PubMed] [Google Scholar]
- 88.Hall L., Rivera F., Villar F., et al. Evaluation of OmniGen-AF in lactating heat-stressed Holstein cows. 2014. http://dairy.ifas.ufl.edu/RNS/2014/collier.pdf Available at: Accessed September 12, 2019.
- 89.Jouany J., Yiannikouris A., Bertin G. The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Archiva Zootechnica. 2005;8:26–50. [Google Scholar]
- 90.Pereyra C., Cavaglieri L., Chiacchiera S., et al. The corn influence on the adsorption levels of aflatoxin B1 and zearalenone by yeast cell wall. J Appl Microbiol. 2013;114(3):655–662. doi: 10.1111/jam.12082. [DOI] [PubMed] [Google Scholar]
- 91.Fruhauf S., Schwartz H., Ottner F., et al. Yeast cell-based feed additives: studies on aflatoxin B1 and zearalenone. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29(2):217–231. doi: 10.1080/19440049.2011.630679. [DOI] [PubMed] [Google Scholar]
- 92.Gessner D., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr. 2017;101(4):605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- 93.Kalantar M. The importance of flavonoids in ruminant nutrition. Archives of Animal Husbandry & Dairy Science. 2018;1(1) AAHDS.MS.ID.000504. [Google Scholar]
- 94.Stover M.G., Watson R.R. In: Polyphenols in human health and disease. Watson R.R., Preedy V.R., Zibadi S., editors. Elsevier; New York: 2014. Polyphenols in foods and dietary supplements: role in veterinary medicine and animal health; pp. 3–7. [Google Scholar]
- 95.Yang W., Benchaar C., Ametaj B., et al. Effects of garlic and juniper berry essential oils on ruminal fermentation and on the site and extent of digestion in lactating cows. J Dairy Sci. 2007;90(12):5671–5681. doi: 10.3168/jds.2007-0369. [DOI] [PubMed] [Google Scholar]
- 96.Seirafy H., Sobhanirad S. Effects of oregano (Origanum vulgare) and thyme (Thymus vulgaris) oils on growth performance and blood parameters in Holstein suckling calves. Iran J Appl Anim Sci. 2017;7(4):585–593. [Google Scholar]
- 97.Oh J., Hristov A., Lee C., et al. Immune and production responses of dairy cows to postruminal supplementation with phytonutrients. J Dairy Sci. 2013;96(12):7830–7843. doi: 10.3168/jds.2013-7089. [DOI] [PubMed] [Google Scholar]
- 98.Oh J., Giallongo F., Frederick T., et al. Effects of dietary Capsicum oleoresin on productivity and immune responses in lactating dairy cows. J Dairy Sci. 2015;98(9):6327–6339. doi: 10.3168/jds.2014-9294. [DOI] [PubMed] [Google Scholar]
- 99.Lee S., Lillehoj H., Jang S., et al. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Vet Parasitol. 2011;181(2):97–105. doi: 10.1016/j.vetpar.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Leroy J., Sturmey R., Hoeck V., et al. Dietary lipid supplementation on cow reproductive performance and oocyte and embryo viability: a real benefit? Anim Reprod. 2013;10(3):258–267. [Google Scholar]
- 101.Sun Y., Bu D., Wang J., et al. Supplementing different ratios of short- and medium-chain fatty acids to long-chain fatty acids in dairy cows: changes in milk fat production and milk fatty acids composition. J Dairy Sci. 2013;96(4):2366–2373. doi: 10.3168/jds.2012-5356. [DOI] [PubMed] [Google Scholar]
- 102.Welter K., Martins C., Vizeu de Palma A., et al. Canola oil in lactating dairy cow diets reduces milk saturated fatty acids and improves its omega-3 and oleic fatty acid content. PLoS One. 2016;11(3):1–16. doi: 10.1371/journal.pone.0151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang X., Sheng W., Sun G., et al. Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem Int. 2010;58(3):321–329. doi: 10.1016/j.neuint.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simons K., Sampaio J. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3(10):1–17. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ballou M.A. Growth and Development Symposium: inflammation: role in the etiology and pathophysiology of clinical mastitis in dairy cows. J Anim Sci. 2012;90(5):1466–1478. doi: 10.2527/jas.2011-4663. [DOI] [PubMed] [Google Scholar]
- 106.Bradford B., Yuan K., Farney J., et al. Invited Review. Inflammation during the transition to lactation: new adventures with an old flame. J Dairy Sci. 2015;98(10):6631–6650. doi: 10.3168/jds.2015-9683. [DOI] [PubMed] [Google Scholar]