Highlights
-
•
RRT-PCR detection of PEDV cannot distinguish between viable and inactivated virus.
-
•
Five classes of disinfectants were evaluated for their effects on PEDV RRT-PCR.
-
•
All evaluated disinfectants rendered PEDV non-infectious.
-
•
None of the tested disinfectants completely eliminated RRT-PCR detection of PEDV.
-
•
Sodium hypochlorite and potassium peroxymonosulfate were most successful.
Keywords: Porcine epidemic diarrhea virus, Sodium hypochlorite, Bleach, Reverse transcriptase polymerase chain reaction, Infection control, Biosecurity, Swine, Virus inactivation
Abstract
Routine detection of porcine epidemic diarrhea virus (PEDV) is currently limited to RT-PCR but this test cannot distinguish between viable and inactivated virus. We evaluated the capability of disinfectants to both inactivate PEDV and sufficiently damage viral RNA beyond RT-PCR detection. Five classes of disinfectants (phenol, quaternary ammonium compound, sodium hypochlorite, oxidizing agent, and quaternary ammonium/glutaraldehyde combination) were evaluated in vitro at varying concentrations, both in the presence and absence of swine feces, and at three different temperatures. No infectious PEDV was recovered after treatment with evaluated disinfectants. Additionally, all tested disinfectants except for 0.17% sodium hypochlorite dramatically reduced qRT-PCR values. However, no disinfectants eliminated RT-PCR detection of PEDV across all replicates; although, 0.52%, 1.03% and 2.06% solutions of sodium hypochlorite and 0.5% oxidizing agent did intermittently produce RT-PCR negatives. To simulate field conditions in a second aim, PEDV was applied to pitted aluminum coupons, which were then treated with either 2.06% sodium hypochlorite or 0.5% oxidizing agent. Post-treatment surface swabs of the coupons tested RT-PCR positive but were not infectious to cultured cells or naïve pigs. Ultimately, viable PEDV was not detected following application of each of the tested disinfectants, however in most cases RT-PCR detection of viral RNA remained. RT-PCR detection of PEDV is likely even after disinfection with many commercially available disinfectants.
1. Introduction
The recent emergence of porcine epidemic diarrhea virus (PEDV) in the United States swine herd has had severe detrimental impacts on the pork industry. Before 2013 PEDV was seen only in Asian and European swine herds but since the first reports of PEDV in Iowa in May, 2013 (Chen et al., 2013, Cima, 2013b), the highly contagious and deadly coronavirus has rapidly spread across North America. Common clinical signs include diarrhea and vomiting, which can lead to dehydration and electrolyte imbalance in infected animals. High mortality (70–100%) among neonates has led to significant economic losses (Cima, 2013a, Cima, 2013b).
Transmission of PEDV occurs mainly through the oral-fecal route with acutely infected animals shedding large quantities of virus for several days post infection. The rapid emergence of highly similar strains across the United States and the frequent detection of PEDV in livestock trailers indicates that swine transportation plays a major role in the spread of PEDV in the country (Lowe et al., 2014). Contaminated transportation equipment has been linked to the spread of several other important swine diseases (porcine reproductive and respiratory syndrome virus, Salmonella, and Escherichia coli) making trailer disinfection common among United States pork producers (Dee et al., 2004, Dee et al., 2006, Rajkowski et al., 1998). Efficient disinfection for PEDV in animal contact spaces, including trailers and trucks, is currently one of the primary methods used to control the spread of the disease.
However, PEDV is difficult to culture outside of an animal model; thus, RT-PCR assays are currently the only tests available to pork producers and swine veterinarians to directly detect PEDV. Because RT-PCR only detects the viral nucleic acid, a positive RT-PCR result only indicates detection of PEDV viral RNA, but does not mean viable and infectious virus is present. Due to the limited testing options and the implications of environmental contamination, individuals are using RT-PCR to test trailers following disinfection to ensure that the equipment is free of PEDV before contact with naïve animals. However, RT-PCR tends to underestimate disinfection efficacy compared to infectivity assays; meaning, RT-PCR positive results are obtained when in fact the trailer has been effectively disinfected. This drawback of RT-PCR has been recognized for various pathogens (Pecson et al., 2011, Poschetto et al., 2007, Suarez et al., 2003), as most disinfectants damage the protective capsid, but often, this mode of action has limited or no effect on the viral nucleic acid (Pecson et al., 2009). Although the disinfection treatments result in loss of infectivity, RT-PCR can still detect the intact viral RNA that remains within a noninfectious viral particle. While rapid progress is being made on viral culture methods, there is an immediate need for practical solutions to address the discrepancy between RT-PCR and infectivity assays. In practical terms, pork producers must consider all RT-PCR positive trailers as contaminated; the consequences of not doing so could be disastrous to their operation and the entire swine industry. However, the cost associated with extra cleaning and disinfection and additional time until a trailer tests negative is very expensive for pork producers.
As an enveloped virus, a wide variety of disinfectants effectively inactivate PEDV (Pospischil et al., 2002) but we cannot detect this biological inactivation with RT-PCR. Presently there is a paucity of data examining disinfectant usage on PEDV RT-PCR results. Data from other pathogens indicate that some disinfectants (e.g., accelerated peroxide-based compounds and/or sodium hypochlorite) would better disrupt the viral RNA and produce more meaningful RT-PCR results (Charrel et al., 2001, Ma et al., 1994, Ojeh et al., 1995, Suarez et al., 2003). Therefore, we examined the effect of disinfectants on RT-PCR results for PEDV and explored practical solutions to produce RT-PCR negative trailers after they have been contaminated with PEDV.
2. Methods
2.1. In vitro evaluation of disinfectants
Five commonly used disinfectants were evaluated for efficacy in inactivating PEDV and for their capability to disrupt PEDV RNA beyond the detection limits of RT-PCR. The disinfectants included were a phenolic disinfectant; a quaternary ammonia compound; sodium hypochlorite; an oxidizing agent; and a quaternary ammonium/glutaraldehyde combination product (Table 1 ). Since oxidizing agents and sodium hypochlorite are known to disrupt the RNA of other viruses, three different dilutions of the oxidizing agent and four different dilutions of sodium hypochlorite were tested. All disinfectants were tested at three different temperatures (37 °C, 4 °C, or −20 °C).
Table 1.
Disinfectants and concentrations tested against a tissue culture adapted porcine epidemic diarrhea virus strain in both cell culture media and 10% (v/v) swine feces slurry. Testing was performed in triplicate resulting in 72 samples for each temperature tested. This procedure was replicated for each temperature (37 °C, 4 °C and −20 °C) for a grand total of 216 samples.
| Study group | PEDV status to contaminate petri dishes | Treatment with disinfectant (dilution) | Temperatures | Contact time (min) |
|---|---|---|---|---|
| Negative control | Neg; in culture medium or fecal slurry | Water | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Positive control | Pos; in culture medium or fecal slurry | Water | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Quaternary ammoniuma | Pos; in culture medium or fecal slurry | 1.5:128 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Phenolb | Pos; in culture medium or fecal slurry | 1:256 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Quaternary ammonium/glutaraldehyde combinationc | Pos; in culture medium or fecal slurry | 1:256 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Oxidizing agentd (0.5%) | Pos; in culture medium or fecal slurry | 1:200 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Oxidizing agentd (1%) | Pos; in culture medium or fecal slurry | 1:100 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Oxidizing agentd (2%) | Pos; in culture medium or fecal slurry | 1:50 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Sodium hypochloritee (0.17%) | Pos; in culture medium or fecal slurry | 1:50 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Sodium hypochloritee (0.52%) | Pos; in culture medium or fecal slurry | 1:16 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Sodium hypochloritee (1.03%) | Pos; in culture medium or fecal slurry | 1:8 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
| Sodium hypochloritee (2.06%) | Pos; in culture medium or fecal slurry | 1:4 | 37 °C, 4 °C, or −20 °C | 60 or 90 |
Roccal-D Plus; Zoetis, Florham Park, New Jersey.
1-Stroke Environ; STERIS Corporation, Mentor, Ohio.
Synergize; Preserve International, Reno, Nevada.
Virkon S; DuPont, Wilmington, Delaware.
Clorox Regular-Bleach (8.25% sodium hypochlorite); The Clorox Company, Oakland, California.
The samples were generated using 147.8 cm2 plastic petri dishes marked on the exterior with 5 dots in a 7 cm square (1 dot per corner with the 5th dot in the center of the square); three petri dishes were used for each substrate to be tested. A cell-culture-adapted PEDV strain (PC22A) was used for all experiments in the present study (Oka et al., 2014). One mililiter (1 × 106 TCID50/ml) of PEDV suspension was added to each petri dish and spread evenly to cover the surface of each dish using a sterile cell spreader. Dulbecco’s modified eagle medium (DMEM) with 7 ug/ml trypsin, 1% penicillin-streptomycin, and 0.3% tryptose phosphate broth was used as the negative control. The inoculum was allowed to dry completely in each petri dish in biosafety cabinets. Once dry, the dishes were incubated for 15 min at the selected temperature. After the incubation, 1 ml of each disinfectant was added to its respectively labeled petri dishes and spread evenly with a spreader. All disinfectants were allowed to dry in open petri dishes in biosafety cabinets (60 min). Once the disinfectants were dry, the lids were replaced on the petri dishes and the dishes were incubated for 15 min at the selected temperature. Double distilled water was used as the sham disinfectant for the positive and negative controls. A polyester-tipped swab (Catalog number: 23-400-111, Fisher Scientific, Pittsburg, PA) was pre-moistened in collection medium (DMEM with 7 ug/ml trypsin, 1% penicillin-streptomycin, and 0.3% tryptose phosphate broth mixed l:1 with Dey–Engley neutralizing broth) prior to swabbing the respective petri dish. Each petri dish was swabbed in a “W” pattern following the 5 marks on each petri dish, rotated 90° clockwise, and swabbed in a “W” pattern again with the same swab. The swabs were then placed into 1 ml of collection medium, immediately vortexed, and incubated at room temperature (21 °C) for 30 min to allow the neutralizing broth time to deactivate any residual disinfectant.
One hundred microliters of each sample was used for RNA extraction using the MagMAX™-96 Viral RNA Isolation kit and the MagMax™ Express Magnetic Particle Processor (Applied Biosystems, Foster, CA) according to the manufacturer’s instructions for a 50 μl elution volume. Following extraction, the RNA was subjected to real-time RT-PCR using a one-step multiplex QRT-PCR kit (Life Technologies Grand Island, NY. Path ID kit [cat#4442135]) in a 25 μl reaction mixture containing 12.5 μl 2× Multiplex buffer, 1.25 μl of the enzyme mix, 4.312 μl water, 1 μl of 5 μM probe, 0.469 μl of 40 μM forward primer, 0.469 μl of 40 μM reverse primer, and 5 μl extracted RNA. The primers and probes targeting the partial N gene were previously described (Jung et al., 2014). The reactions were performed on a QPCR system (Life Technologies 7500 Fast Real-Time PCR System) under the following thermocycling conditions: Stage 1–48 °C for 10 min, Stage 2–95 °C for 10 min, and Stage 3–45 cycles of 95 °C for 15 s and 60 °C for 40 s. Cycle threshold (Ct) values were calculated for each sample by setting the threshold at 5% of the positive control at cycle 40; samples with a Ct of ≤40 were considered positive. A standard curve of the estimated TCID50 was generated for each RT-PCR run by making 10 fold dilutions of the positive control standard to estimate the TCID50 value of each positive sample. Briefly, 100 μl of stock PEDV was included with each batch of samples and 5 μl of the extracted RNA was used undiluted as the starting point in the standard curve. Five additional 10-fold dilutions were generated to give a standard curve range of 1 × 104 to 0.1 TCID50. Estimated TCID50 values were calculated based off the standard curves generated with each RT-PCR run from the stock virus extracted with samples each time. Estimated TCID50 values were used for ease of comparison to actual TCID50 values obtained from the positive controls. Estimated TCID50 values from qRT-PCR were log (1 + x) transformed prior to comparison.
All samples were inoculated onto 96 well cell culture plates containing monolayers of Vero cells (CCL-81; American Type Culture Collection, Manassas, VA) to determine if each disinfectant biologically inactivated the virus. Ten-fold dilutions of each sample were inoculated in octuplicate and the wells were observed daily for cytopathic effects (CPE), with 72 h post inoculation serving as the final endpoint. Cytopathic effects were defined as the visualization of fusion cell (giant cell) formation, syncytia, and/or vacuolation in the monolayer of small cuboidal cells (Hofmann and Wyler, 1988). Since PEDV infection causes the Vero cells to join together and become multinucleated giant round cells, disruption of the monolayer (i.e., cytotoxicity) was not consider evidence of CPE. Observation of CPE was used to determine the TCID50 value of each sample using the Reed and Muench (1938) method.
The above procedures were repeated with a 10% (v/v) fecal slurry made from PEDV negative swine feces and double distilled water. For the fecal samples, 1 ml of the 10% fecal slurry was added with the 1 ml of PEDV virus to the petri dishes. This increased volume resulted in a 30 min increase in drying time.
All of the above procedures were performed at three separate temperatures (37 °C, 4 °C, or −20 °C). Ultimately, each disinfectant was tested in triplicate at all three temperatures both with and without the 10% fecal slurry resulting in a total of 216 samples.
2.1.1. Simulation of field conditions
Based on the in vitro testing, two disinfectants (2.06% sodium hypochlorite and 0.5% oxidizing agent) were selected for further evaluation. To mimic the surface of a washed but not yet disinfected livestock trailer, 10.4 × 10.4 × 0.6 cm aluminum coupons (bare plate aluminum 6061-T651) were used. Aluminum coupons were cleaned with nucleic acid removing wipes (DNA AWAY, Molecular Bioproducts San Diego, CA), dried, and rinsed with distilled water. The surface of the coupons were then pitted (Davis, 1999) by submersion in a 5% acetic acid (distilled white vinegar) bath for 16 h, after which they were rinsed with distilled water, and air dried to simulate field conditions. The aluminum coupons were then autoclaved at 121 °C for 15 min and the sterilized coupons were aseptically placed into sterile petri dishes. Twenty coupons were used for each disinfectant. Coupons were pre-heated to 37 °C before one ml of stock PEDV (1 × 106 TCID50/ml) was added to each aluminum coupon, spread over the entire coupon using a spreader, and allowed to dry completely (60 min) in a biological safety cabinet at room temperature. Once dry the lids were replaced on the petri dishes and the dishes with aluminum coupons were incubated at 37 °C for 15 min. After incubation the dishes were moved back to biosafety cabinets, the lids were removed, and 1 ml of respective disinfectant (2.06% sodium hypochlorite or 0.5% oxidizing agent) was added to each aluminum coupon. The disinfectant was spread over the entire surface of the coupon using a spreader and allowed to dry completely. Double distilled water was used as the sham disinfectant for the positive and negative controls. Once dry, the lids were replaced on the petri dishes and the dishes with aluminum coupons were again incubated at 37 °C for 15 min. A single swab was premoistened in viral storage medium (viral collection medium without Dey–Engley neutralizing broth) was used to swab the entire surface of the aluminum coupon. The swab was put into 2 ml of viral storage medium and mixed by vortexing. The 20 samples of each treatment group were pooled together. The viral load in the samples was quantified with qRT-PCR and TCID50 assays as described above; infectivity was assessed with cell culture and a bioassay.
2.1.2. Bioassay
All procedures involving pigs were performed with the approval of The Ohio State University Institutional Animal Care and Use Committee (protocol no. 2014A00000016). Twelve 3-week-old PEDV naïve pigs were obtained from the university’s teaching herd and were randomly divided into four challenge groups (n = 3 per group): negative control, sodium hypochlorite, oxidizing agent, and positive control. Upon arrival at the animal facility, the pigs were given a 72 h acclimation period. RT-PCR confirmed the pigs to be negative for PEDV upon arrival and throughout the acclimation period. The bedding and feed tested PEDV negative prior to the start of the bioassay. Each pig was inoculated orally with 8.5 ml of the pooled aluminum coupon sample of the respective treatment group. Rectal swabs were collected daily for RT-PCR testing and the pigs were monitored for clinical signs of disease. Pigs were euthanized on day 10 post inoculation and tissues were collected and fixed in 10% neutral buffered formaldehyde. The tissues were paraffin embedded then sectioned utilizing routine methods for histopathologic evaluation. The primary tissues evaluated in this study included the stomach, duodenum, jejunum, ileum and colon.
3. Results
3.1. In vitro evaluation of disinfectants without a fecal slurry
Viable PEDV was not detected in any of the treated samples when tested by virus isolation. Irrespective of temperature, all the disinfectants reduced the estimated amount of PEDV on qRT-PCR as compared to the positive control (Fig. 1 ). None of the disinfectants were able to produce qRT-PCR results that were negative across all replicates (Table 2 ); however, strong solutions of sodium hypochlorite (0.52%, 1.03% and 2.06%) and 0.5% oxidizing agent did produce several negative or nearly negative qRT-PCR test results (Fig. 1 and Table 2). Numerically, fewer qRT-PCR positive samples were detected at 37 °C (n = 18) than 4 °C (n = 27), or −20 °C (n = 27).
Fig. 1.
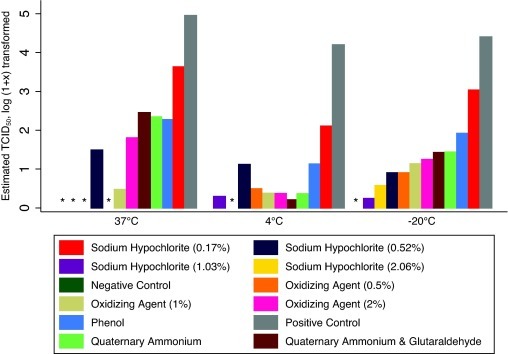
Mean qRT-PCR results (estimated TCID50/ml that have been log (1 + x) transformed) for the disinfectants tested against porcine epidemic diarrhea virus in cell culture medium. An (*) denotes treatments that tested negative.
Table 2.
Number of samples for each treatment group that tested positive for porcine epidemic diarrhea virus (PEDV) with RT-PCR for the disinfectants tested in the presence and absence of a 10% (v/v) fecal slurry at three different temperatures. Each treatment was tested in triplicate.
| 37 °C |
4 °C |
−20 °C |
||||
|---|---|---|---|---|---|---|
| Disinfectant | Cell culture medium | Fecal slurry | Cell culture medium | Fecal slurry | Cell culture medium | Fecal slurry |
| Quaternary ammonium | 3 | 3 | 3 | 3 | 3 | 3 |
| Phenol | 3 | 3 | 3 | 3 | 3 | 3 |
| Quaternary ammonium/glutaraldehyde combination | 3 | 3 | 3 | 3 | 3 | 3 |
| Oxidizing agent (0.5%) | 0 | 3 | 3 | 3 | 3 | 3 |
| Oxidizing agent (1%) | 1 | 3 | 3 | 3 | 3 | 3 |
| Oxidizing agent (2%) | 3 | 3 | 3 | 3 | 3 | 3 |
| Sodium hypochlorite (0.17%) | 3 | 3 | 3 | 3 | 3 | 3 |
| Sodium hypochlorite (0.52%) | 2 | 2 | 3 | 3 | 3 | 3 |
| Sodium hypochlorite (1.03%) | 0 | 0 | 3 | 0 | 1 | 2 |
| Sodium hypochlorite (2.06%) | 0 | 1 | 0 | 0 | 2 | 1 |
| Negative controls | 0 | 0 | 0 | 0 | 0 | 0 |
| Positive controlsa | 3 | 3 | 3 | 3 | 3 | 3 |
Viable PEDV was recovered from the positive control samples but PEDV could not be recovered from any of the disinfectant treated or negative control samples when tested with virus isolation.
3.2. In vitro evaluation of disinfectants with fecal slurry
In the presence of a fecal slurry, infectious PEDV was not detected from any of the disinfectant treated samples when tested with virus isolation. Additionally, all the disinfectants except for 0.17% sodium hypochlorite produced reductions in the number of PEDV copies estimated by qRT-PCR and this was only apparent at 4 °C (Fig. 2 ). Strong solutions of sodium hypochlorite (1.03% and 2.06%) were able to intermittingly produce negative RT-PCR results (Table 2).
Fig. 2.
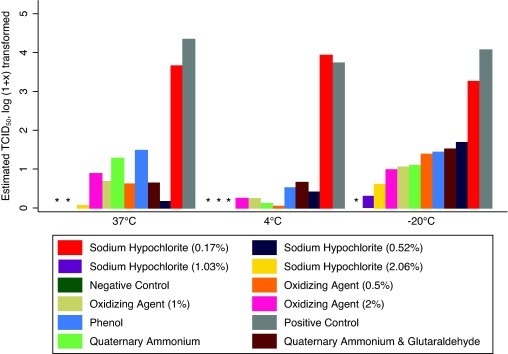
Mean QRT-PCR results (estimated TCID50/ml that have been log (1 + x) transformed) for the disinfectants tested against porcine epidemic diarrhea virus in a 10% (v/v) fecal slurry. An (*) denotes treatments that tested negative.
3.3. PEDV detection and infectivity following simulation of field conditions
When the samples generated on the aluminum coupons were inoculated onto Vero cells, only the positive control samples yielded recovery of PEDV. PEDV was detected with RT-PCR from the pooled samples collected from the aluminum coupons treated with 2.06% sodium hypochlorite and 0.5% oxidizing agent (Table 3 ). Back titration of the inoculum used for the positive control pigs was calculated to be 4.14 × 104 TCID50. In the bioassay, positive control pigs tested PEDV positive from rectal swabs on days 4 (n = 1) and 5 (n = 3) post inoculation; all three positive control pigs tested PEDV positive for the remainder of the study. PEDV was not detected in the rectal swabs from the negative control pigs nor the pigs challenged with the material collected from the swabbing of the aluminum coupons treated with 2.06% sodium hypochlorite and 0.5% oxidizing agent. Histologic evaluation of the positive control group demonstrated moderate to marked villus atrophy with collapse and fusion of the lamina propria. Epithelial cell vacuolization interpreted as hydropic degeneration was observed sporadically in this group. The changes observed in the experiment groups and the negative control group consisted of mild inflammatory changes which predominately eosinophilic in nature. This inflammation was considered an incidental background lesion.
Table 3.
Mean qRT-PCR results (estimated TCID50/ml) for the disinfectants tested against porcine epidemic diarrhea virus (PEDV) in cell culture medium applied to pitted aluminum coupons.
| Mean Ct value | Mean QRT-PCR results (estimated TCID50/ml) | |
|---|---|---|
| Oxidizing agent (0.5%) | 26.27 | 28 |
| Hypochlorite (2.06%) | 24.29 | 1.10 × 102 |
| Negative control | Negative | 0 |
| Positive controla | 14.46 | 1.27 × 104 |
Infectious PEDV was detected in the positive control samples but viable PEDV was not found in any of the disinfectant treated or negative control samples when tested with virus isolation and swine bioassay.
4. Discussion
Under the conditions described above, no infectious PEDV was detected in any of the disinfected treated samples. In contrast, only a few of the disinfectants were able to disrupt the viral RNA to the point that PEDV could not be detected by RT-PCR. While the qualitative results imply that the phenol, the quaternary ammonium, and the quaternary ammonium/glutaraldehyde combination had little impact on RT-PCR results (Table 2), the quantitative data show that these three classes of disinfectants did decrease the number of estimated viral copies per ml (Fig. 1, Fig. 2). Despite a number of medically important human and animal coronaviruses, the mechanisms of action for disinfectants against these viruses are poorly understood. Perhaps the best supported mechanism is for glutaraldehyde where the chemical is believed to react with amino or sulfhydryl groups in capsid proteins, thereby damaging the capsid and inactivating the virus (McDonnell and Russell, 1999). Clearly this is an area in great need of future research.
Due to the fact that a strong solution of sodium hypochlorite (2.06%) and 0.5% oxidizing agent did produce several negative and very low estimated qRT-PCR test results (Table 2, Fig. 1, Fig. 2), those two disinfectants were used in our simulation of field conditions. The samples collected from pitted aluminum coupons to simulate a livestock trailer were not infectious in cell culture or in naïve pigs. Yet, neither of these two disinfectants were able to produce completely negative PCR results. The PEDV qRT-PCR results recorded after treatment with 2.06% sodium hypochlorite and 0.5% oxidizing agent were near the limit of detection and much less than the positive control; nonetheless, PEDV was still detectable with qRT-PCR post disinfection. The results from the in vitro experiments in the plastic petri dishes did not translate perfectly to the aluminum coupons. One possible explanation for these discrepancies is the pitted surface of the aluminum coupons. Following treatment with 5% acetic acid, the surface of the aluminum was rough and pitted. We hypothesize that these surface irregularities may have harbored inactive virus particles and that this situation is likely common in used livestock trailers. It is also important to note that the entire 108.16 cm2 surface of the aluminum coupons were swabbed, whereas only 47.8 cm2 of the petri dishes were swabbed. This increased sampling increased the likelihood of detecting residual PEDV RNA.
In the present study, we were unable to detect viable PEDV following treatment with any of the tested disinfectants. While this is a significant finding, it is important to note that the failure to detect PEDV may not mean absolute inactivation of PEDV but rather that the quantity of viable PEDV was decreased below the detection threshold of our TCID50 assay. Under the described conditions, we were confident that we would detect a minimum of 99.9% (3-log) inactivation of PEDV. Aim 2 confirmed the inactivation of the PEDV with 2.06% sodium hypochlorite and 0.5% oxidizing as no pigs became infected after challenge with material collected after either of the two treatments.
While all disinfectants tested in the current study prevented the virological detection of PEDV, RT-PCR detection of residual inactivated virus was common after disinfection with most of these disinfectants. Several classes of disinfectants are known to cause little or no damage to nucleic acid. In fact, investigators have used phenolic disinfectants to biologically inactivate influenza A virus in order to safely ship non-infectious, but still PCR detectable material, between laboratories for quality assurance testing (Spackman and Suarez, 2005). Proper cleaning and surface preparation is critical to the success of any disinfection protocol. Organic material is known to inactivate many classes of disinfectants. Interestingly, presence of the fecal slurry did not impact the total number of RT-PCR positives in the present study. It is important to note that the cell culture medium used in the present study contained many organic compounds necessary for Vero cell growth; thus, even the samples generated in the absence of the fecal slurry may have contained significant disinfectant inhibitors. The present study is also limited by a very small sample size.
Other important factors in disinfection effectiveness are concentration, contact time and temperature. In both objectives of the current study, we allowed the applied solutions to fully dry before proceeding to the next step of the protocol because most field disinfection protocols allow for complete drying following application of disinfectants. Complete drying took as long as 90 min in some situations and this impacted the temperature of the substrates (i.e., temperature changed during drying in the biosafety cabinets). Thus, the results of the 4 °C and −20 °C are likely overestimating the effectiveness of the disinfectants at those temperatures because colder temperatures will increase drying time and are known to have a negative effect on disinfectant activity (Shaker et al., 1986, Vaneseltine and Rahn, 1949). This limitation was unavoidable because the procedures had to be performed in a biosafety cabinet to maintain biocontainment of PEDV. Despite this limitation, we still observed temperature dependent differences in the data (Table 2).
Because most PEDV strains do not grow in cell culture, the pork industry must rely upon RT-PCR for testing. Results of the present study indicate that certain oxidizing agents and sodium hypochlorite are most likely to produce negative RT-PCR results. Caution must be exercised when considering field application of these results. The very strong concentration of sodium hypochlorite (2.06%), the most effective in our study, is a serious hazard to human health (Racioppi et al., 1994). Contact with bare skin can result in chemical burns and inhalation of sodium hypochlorite irritates the respiratory tract and can cause pulmonary edema in some situations (Hostynek et al., 1990, Luttrell, 2001). Additionally, sodium hypochlorite will lead to corrosion of metal equipment and deterioration of rubber objects (Babb et al., 1980).
Ultimately, all disinfectants tested herein inactivated PEDV but few prevented RT-PCR detection of viral RNA. RT-PCR detection of PEDV is likely even after disinfection with many commercially available disinfectants. Pork producers attempting to disinfect transportation equipment should take steps whenever possible to properly clean the equipment prior to disinfectant application, increase the ambient temperature of the equipment, and use an appropriate disinfectant according to labeled directions.
Acknowledgements
The authors wish to thank Josh Filson and Jennifer Staten for their assistance with the animal housing and care. This work was supported by the National Pork Checkoff and the Ohio Pork Producers Council under project number 14-151.
References
- Babb J.R., Bradley C.R., Ayliffe G.A.J. Sporicidal activity of glutaraldehydes and hypochlorites and other factors influencing their selection for the treatment of medical equipment. J. Hosp. Infect. 1980;1:63–75. doi: 10.1016/0195-6701(80)90033-x. [DOI] [PubMed] [Google Scholar]
- Charrel R.N., de Chesse R., Decaudin A., De Micco P., de Lamballerie X. Evaluation of disinfectant efficacy against hepatitis C virus using a RT-PCR-based method. J. Hosp. Infect. 2001;49:129–134. doi: 10.1053/jhin.2001.1048. [DOI] [PubMed] [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak in US swine. J. Clin. Microbiol. 2013 doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima G. Fighting a deadly pig disease. J. Am. Vet. Med. Assoc. 2013;243:467–470. [PubMed] [Google Scholar]
- Cima G. Viral disease affects U.S. pigs: porcine epidemic diarrhea found in at least 11 states. J. Am. Vet. Med. Assoc. 2013;243:30–31. [PubMed] [Google Scholar]
- Davis J.R. ASM International; Materials Park, OH: 1999. Corrosion of aluminum and aluminum alloys. vii, 313 p. [Google Scholar]
- Dee S.A., Deen J., Otake S., Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of transmission of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can. J. Vet. Res. 2004;68:128–133. [PMC free article] [PubMed] [Google Scholar]
- Dee S.A., Deen J., Pijoan C. Evaluation of an industry-based sanitation protocol for full-size transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. J. Swine Health Prod. 2006;14:307–311. [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostynek J.J., Wilhelm K.P., Cua A.B., Maibach H.I. Irritation factors of sodium hypochlorite solutions in human skin. Contact Dermat. 1990;23:316–324. doi: 10.1111/j.1600-0536.1990.tb05165.x. [DOI] [PubMed] [Google Scholar]
- Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20:662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Gauger P., Harmon K., Zhang J., Connor J., Yeske P., Loula T., Levis I., Dufresne L., Main R. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerg. Infect. Dis. 2014;20:872–874. doi: 10.3201/eid2005.131628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell W. Toxic tips: sodium hypochlorite. Chem. Health Saf. 2001;8:24–26. [Google Scholar]
- Ma J.F., Straub T.M., Pepper I.L., Gerba C.P. Cell culture and PCR determination of poliovirus inactivation by disinfectants. Appl. Environ. Microbiol. 1994;60:4203–4206. doi: 10.1128/aem.60.11.4203-4206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeh C.K., Cusack T.M., Yolken R.H. Evaluation of the effects of disinfectants on rotavirus RNA and infectivity by the polymerase chain reaction and cell-culture methods. Mol. Cell. Probes. 1995;9:341–346. doi: 10.1016/s0890-8508(95)91652-0. [DOI] [PubMed] [Google Scholar]
- Oka T., Saif L.J., Marthaler D., Esseili M.A., Meulia T., Lin C.M., Vlasova A.N., Jung K., Zhang Y., Wang Q. Cell culture isolation and sequence analysis of genetically diverse US porcine epidemic diarrhea virus strains including a novel strain with a large deletion in the spike gene. Vet. Microbiol. 2014;173:258–269. doi: 10.1016/j.vetmic.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecson B.M., Ackermann M., Kohn T. Framework for using quantitative PCR as a nonculture based method to estimate virus infectivity. Environ. Sci. Technol. 2011;45:2257–2263. doi: 10.1021/es103488e. [DOI] [PubMed] [Google Scholar]
- Pecson B.M., Martin L.V., Kohn T. Quantitative PCR for determining the infectivity of bacteriophage MS2 upon inactivation by heat, UV-B radiation, and singlet oxygen: advantages and limitations of an enzymatic treatment to reduce false-positive results. Appl. Environ. Microbiol. 2009;75:5544–5554. doi: 10.1128/AEM.00425-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschetto L.F., Ike A., Papp T., Mohn U., Bohm R., Marschang R.E. Comparison of the sensitivities of noroviruses and feline calicivirus to chemical disinfection under field-like conditions. Appl. Environ. Microbiol. 2007;73:5494–5500. doi: 10.1128/AEM.00482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil A., Stuedli A., Kiupel M. Update on porcine epidemic diarrhea. J. Swine Health Prod. 2002;10:81–85. [Google Scholar]
- Racioppi F., Daskaleros P.A., Besbelli N., Borges A., Deraemaeker C., Magalini S.I., Martinez Arrieta R., Pulce C., Ruggerone M.L., Vlachos P. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem. Toxicol. 1994;32:845–861. doi: 10.1016/0278-6915(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Rajkowski K.T., Eblen S., Laubauch C. Efficacy of washing and sanitizing trailers used for swine transport in reduction of Salmonella and Escherichia coli. J. Food Protect. 1998;61:31–35. doi: 10.4315/0362-028x-61.1.31. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent end points. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Shaker L.A., Russell A.D., Furr J.R. Aspects of the action of chlorhexidine on bacterial-spores. Int. J. Pharm. 1986;34:51–56. [Google Scholar]
- Spackman E., Suarez D.L. Use of a novel virus inactivation method for a multicenter avian influenza real-time reverse transcriptase-polymerase chain reaction proficiency study. J. Vet. Diagn. Invest. 2005;17:76–80. doi: 10.1177/104063870501700117. [DOI] [PubMed] [Google Scholar]
- Suarez D.L., Spackman E., Senne D.A., Bulaga L., Welsch A.C., Froberg K. The effect of various disinfectants on detection of avian influenza virus by real time RT-PCR. Avian Dis. 2003;47:1091–1095. doi: 10.1637/0005-2086-47.s3.1091. [DOI] [PubMed] [Google Scholar]
- Vaneseltine W.P., Rahn O. The effect of temperature upon bacteriostasis. J. Bacteriol. 1949;57:547–554. doi: 10.1128/jb.57.5.547-554.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]


