Abstract
Background
Lactoferrin (Lf) is an 80 kDa iron-binding glycoprotein of the transferrin family. It is abundant in milk and in most biological fluids and is a cell-secreted molecule that bridges innate and adaptive immune function in mammals. Its protective effects range from anticancer, anti-inflammatory and immune modulator activities to antimicrobial activities against a large number of microorganisms. This wide range of activities is made possible by mechanisms of action involving not only the capacity of Lf to bind iron but also interactions of Lf with molecular and cellular components of both hosts and pathogens.
Scope of review
This review summarizes the activities of Lf, its regulation and potential applications.
Major conclusions
The extensive uses of Lf in the treatment of various infectious diseases in animals and humans has been the driving force in Lf research however, a lot of work is required to obtain a better understanding of its activity.
General significance
The large potential applications of Lf have led scientists to develop this nutraceutical protein for use in feed, food and pharmaceutical applications. This article is part of a Special Issue entitled Molecular Mechanisms of Iron Transport and Disorders.
Keywords: Lactoferrin, Iron-binding protein, Transferrin, Functional protein
Highlights
► It is well known that the mechanisms of action of antimicrobial activity are not fully understood. ► Important advances were made in heterologous Lf expression in prokaryotic systems. ► In the near future we will see the exploitation of the full capacity of lactoferrin expression in plants.
1. Introduction
Lactoferrin (Lf), first reported almost 50 years ago [1], [2], [3], is a non-hemic iron-binding protein. It is a member of the transferrin family, along with serum transferrin, ovotransferrin, and melanotransferrin [4], all of which function in iron transport, and inhibitors of carbonic anhydrase, Saxyphillin, and Pacifastin are also members of this superfamily. Lf is produced by mucosal epithelial cells in various mammalian species including humans, cows, goats, horses, dogs, and rodents, and it is also produced by fish [5]. This multifunctional glycoprotein is found in mucosal secretions including tears, saliva, vaginal fluids, semen [6], nasal and bronchial secretions, bile, gastrointestinal fluids, urine [7], milk and colostrum [8]. The most abundant antimicrobial proteins include lysozyme, collectin [9], [10] and lactoferrin. Lf possesses a greater iron-binding affinity, and it is the only transferrin with the ability to retain this metal over a wide range of pH values, including resistance to proteolysis. The most striking physicochemical feature of Lf is its very high affinity for iron. In both Lf and related transferrins (Tfs), two Fe+ 3 ions are bound very tightly (K ~ 1022 M) but reversibly to LF, with two synergistically bound CO3 2− ions [11], [12]. Because of its wide distribution in various tissues, Lf is a highly multifunctional protein. Indeed, it is involved in many physiological functions, including regulation of iron absorption and immune responses; it also exhibits antioxidant activity and has both anticarcinogenic and anti-inflammatory properties. However, its antimicrobial properties are its most widely studied function [8], [12], [13], [14], [15], [16]. The antimicrobial activity of Lf is driven mostly by two mechanisms. The first involves iron sequestration in sites of infection, which deprives microorganisms of this nutrient and causes a bacteriostatic effect. The second mechanism is the direct interaction of the Lf molecule with the infectious microorganism. In some cases, positively charged amino acids in Lf can interact with anionic molecules on certain bacterial, viral, fungal and parasite surfaces, causing cell lysis. The physiological capabilities of Lf in host defense combined with current pharmaceutical and nutritional needs have led to the classification of Lf as a nutraceutical protein, and for several decades, investigators have looked for the most convenient way to produce it. Three basic approaches are currently being used. First, native Lf can be commercially purified from the milk and colostrum of several mammals. Second, recombinant Lf (rLf) can be generated from bacterial, fungal and viral expression systems. Third, transgenic plants and animals have been generated with express rLF.
2. Characteristics of the Lf sequence
The nucleotide sequence of human milk Lf (hLf) was first determined by Ray et al. in 1990 [18] and compared with the amino acid sequence of human lactotransferrin determined previously [17]. Lf genes are highly conserved among species, with an almost identical organization and an mRNA of about 1900–2600 bp. A homology search in sequence databases revealed nucleotide sequences for the Lfs of 13 species: 3 primates, 7 even-toed ungulates, 1 pig, 1 cat, and 1 mouse. Pairwise sequence identities ranged from a minimum of ~ 78% to nearly 100%. The main outliers in this group were Lfs from primates, in which human and chimpanzee are quite similar (95–98%) when compared to orangutan (79%). On the other hand, horse and camel; pig, cow, yak, water buffalo, bull, and goat Lfs share 81 and 78% of sequence identity respectively when compared to human Lf as outgroup. The sequence relationships given above show that the Lfs form a highly conserved sequence family, and also sequence identity between Lfs and other Tfs is relatively high at 60–65% [19]. A characteristic feature of Lfs is their highly basic character, with a pI typically greater than 9; this property is typically predictable from their sequence. Structurally, the feature that most readily distinguishes Lfs from Tfs is the peptide linker between the two lobes, thought to have evolved from an ancient duplication event [20], which also contains several proline residues.
3. Molecular structure
Lf is an 80 kDa glycosylated protein of ~ 700 amino acids, with a high homology among species. It is comprised of a simple polypeptide chain folded into two symmetrical lobes (the N-lobe and C-lobe), which are highly homologous with one another (33–41% homology). The two lobes are connected via a hinge region containing parts of an α-helix between amino acids 333 and 343 in human Lf (hLf) [7], which confers flexibility to the molecule [6], [7], [21]. The polypeptide chain includes amino acids 1–332, comprising the N-lobe, and 344–703, comprising the C-lobe, and it is made up of α-helix and β-pleated sheet structures that create two domains within each lobe (domains I and II) [22]. Each lobe can bind a metal atom in synergy with the carbonate ion (CO3 −2). Lf is capable of binding Fe+ 2 or Fe+ 3 ions, but it has also been observed to be bound to Cu+ 2, Zn+ 2 and Mn+ 2 ions [23]. Because of its ability to reversibly bind Fe+ 3, Lf can exist free of Fe+ 3 (apo-Lf) or associated with Fe+ 3 (holo-Lf), and it has a different three-dimensional conformation depending on whether or not it is bound to Fe+ 3 [19].
Apo-Lf has an open conformation, while holo-Lf is a closed molecule with greater resistance to proteolysis [7]. Because of the common structural framework among Lfs, it is possible to model their conformations using crystallographic data from other Lf species (Fig. 1a). The amino acids directly involved at the iron-binding site in each lobe are Asp60, Tyr92, Tyr192 and His253, while Arg121 is involved in binding the CO3 −2 ion (Fig. 1b). Lf is a basic, positively charged protein with a pI of 8.0–8.5. The primary structure of Lf shows the number and position of Cys residues that allow the formation of intramolecular disulfide bridges; Asn residues in the N- and C-terminal lobes provide several potential N-glycosylation sites [25], [26].
Fig. 1.
Predicted structure of lactoferrin. a) From EU812318 (bLF) sequence using PDB ID: 1BIY (buffalo) as a template, showing two-lobe, four-domain polypeptide. b) Canonical iron-binding pocket site of lactoferrin. Fe + 3 (cream) CO3 (gray and red). Modeled using Protein Model Portal [24], and viewed using Chimera software (http://www.cgl.ucsf.edu/chimera/).
4. Antimicrobial activities of lactoferrin
Several functions have been attributed to Lf. It is considered to be a key component of the innate host defense system because it can respond to a variety of physiological and environmental changes [27]. The structural features of Lf provide additional functionalities beyond the Fe+ 3 homeostasis function common to all transferrins. Specifically, Lf exhibits strong antimicrobial activity against a broad spectrum of bacteria (Gram+ and Gram−), fungi, yeasts, viruses [14] and parasites [29], although it seems to promote the growth of beneficial bacteria like Lactobacillus and Bifidobacteria [28]. It also exhibits anti-inflammatory and anticarcinogenic activities [27] and has several enzymatic functions [30]. Lf plays a key role in maintaining cellular iron levels in the body. One of the first antimicrobial properties discovered for Lf was its role in sequestering iron from bacterial pathogens. This was believed to be the sole antimicrobial action of lactoferrin because apo-lactoferrin possessed antibacterial activity [31], [32]. It was later demonstrated that lactoferrin can also kill microorganisms through an iron-independent mechanism [33] in which lactoferrin directly interacts with the bacterial cell surface [31], [34], [35].
4.1. Antibacterial activity
The antibacterial activity of Lf has been documented in the past, both in vitro and in vivo for Gram-positive and Gram-negative bacteria and some acid-alcohol resistant bacteria. Table 1 shows the bacteria against which Lf has shown an inhibitory effect and the mechanism used by Lf to exert its effect. Particular attention should be given to bacteria listed in Table 1 because some of these are known to be resistant to antimicrobials, such as the strains of Staphylococcus aureus, Listeria monocytogenes, methicillin-resistant Klebsiella pneumoniae and Mycobacterium tuberculosis, among others. Lf has also been proven effective against strains of Haemophillus influenzae and Streptococcus mutans that were inhibited by an iron-independent interaction with the cell surface [39], [45].
Table 1.
Bacteria against which Lf has a reported effect.
| Target | Mode of action | Reference | |
|---|---|---|---|
| Gram-positive | Bacillus stearothermophilus | Iron sequestering | [36], [37] |
| Bacillus subtilis | Iron-independent interaction with bacterial cell surface | [38] | |
| Klebsiella pneumoniae | Iron-independent interaction with cell surface | [39] | |
| Listeria monocytogenes | Altering bacteria virulence | [40], [41] | |
| Staphylococcus aureus | Iron sequestering | [42], [43] | |
| Streptococcus mutans | Iron-independent interaction with cell surface | [44], [45], [46] | |
| Streptococcus parasanguinis | Altering bacterial growth | [47] | |
| Actinobacillus | Proteolytic activity | [48] | |
| S. epidemidis | Interaction with lipoteichoic acid on bacterial surface | [48] | |
| S. epidermidis | Prevents biofilm formation—through iron sequestering | [48] | |
| Gram-negative | Chlamydophila psittaci | Interferes with cell adhesion | [50] |
| Haemophillus influenzae | Altering bacteria virulence—degrading IgA1 and Hap | [39] | |
| E. coli enteropathogenic (EPEC) | Proteolytic activity of invasive mechanism | [49] | |
| E. coli enteropathogenic (EAEC) | Inhibit adherence of diffusely adherent | [51] | |
| E. coli (DAEC) | Inhibit aggregative proliferation | [51] | |
| Helicobacter felis | Prevention of matrix interaction cell-host | [52] | |
| Helicobacter pylori | Iron-independent mechanism of inhibition | [53], [54] | |
| Legionella pneumophila | Prevent intracellular proliferation | [55] | |
| Pseudomonas aeruginosa | Prevents biofilm formation | [56], [57], [58] | |
| Shigella flexneri | Disrupt bacterial type III secretion system | [59] | |
| Vibrio cholerae | Iron-independent mechanism of inhibition | [69] | |
| Mycobacterium tuberculosis | Augment cellular immunity | [61], [62] | |
| Mycoplasma bovis | Prevents biofilm formation | [57] | |
| Porphyromonas gingivalis | Disrupt biofilm formation—cell membrane permeabilization | [63] | |
| Samonella enteritidis | Interferes with polysaccharide cell content | [64] |
The sequestration of iron away from bacterial pathogens inhibits bacterial growth, limits the use of this nutrient by bacteria at the infection site and downregulates the expression of their virulence factors [60], [65]. Lf's bactericidal function has been attributed to its direct interaction with bacterial surfaces. In 1988, it was shown that Lf damages the external membrane of Gram-negative bacteria through an interaction with lipopolysaccharide (LPS) [66]. The positively charged N-terminus of Lf prevents the interaction between LPS and bacterial cations (Ca+ 2 and Mg+ 2) [67], [68] and interferes with aggregative proliferation in E. coli [52]. The interaction between Lf and LPS or other surface proteins also potentiates the action of natural antibacterials such as lysozyme, which is secreted from the mucosa at elevated concentrations along with Lf [66]. It has also been demonstrated that the N-terminal lobe of Lf possesses a serine protease-like activity [39]. In H. influenzae, Lf is able to cleave proteins in arginine-rich regions, and the protease active site is situated in the N-terminal lobe, thus attenuating virulence and preventing colonization [69]. In vitro and in vivo studies have shown that Lf has the ability to prevent the attachment of certain bacteria to the host cell. The attachment-inhibiting mechanisms are unknown, but it has been suggested that Lf's oligomannoside glycans bind bacterial adhesins, preventing their interaction with host cell receptors [48], [63]. Biofilm formation, which was proposed as a colonial organization adhesion method for Pseudomonas aeruginosa, is a well-studied phenomenon in patients suffering from cystic fibrosis. Through biofilm formation, bacteria become highly resistant to host cell defense mechanisms and antibiotic treatment [70], [71], [72]. It is well known that some bacterial strains require high levels of iron to form biofilms. Thus, Lf's function as an iron chelator has been hypothesized to effectively inhibit biofilm formation through iron sequestration [73], [74].
4.2. Antiviral activity
Lf possesses antiviral activity against a broad range of RNA and DNA viruses that infect humans and animals [6]. Initial work suggested that only enveloped viruses were affected by Lf, and that this activity was due to several factors, including inhibition of virus–host interaction in the herpes simplex virus (HSV) [75], [76]; inhibition of intracellular virus trafficking in the hepatitis B virus (HBV) [77], [78], [79] and human cytomegalovirus (HCMV) [80], [81]; or direct binding of lactoferrin to the viral particle in the hepatitis C virus (HCV) [82], [83], feline herpes virus (FHV-1) [16], and hepatitis G virus (HGV) [84]. The human immunodeficiency virus (HIV) remains a major medical challenge because current treatments of the syndrome that it causes are not completely effective. In vitro studies show that, among human plasma and milk proteins, Lf exerts a strong activity against HIV. This effect is due to inhibition of viral replication in the host cell [39], [85], [86]. Lf also binds to three of the many co-receptors of HIV [87] and the DC-SIGN receptor [88]. The interaction of Lf with surface nucleolin was shown to block the attachment and entry of HIV particles into HeLa P4 cells [87].
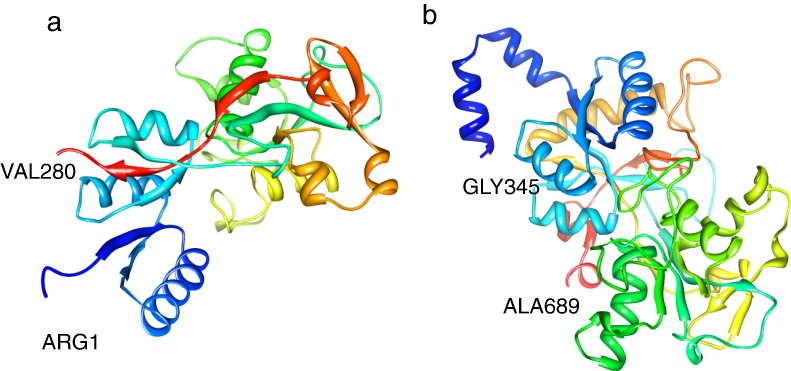
Fig. 2 illustrates structural in situ models of the C- and N-lobes. Interestingly, two large fragments of bLf, the C-lobe (aa 345–689) and the N-lobe (aa 1–280), have been shown to inhibit HSV infection [89]. The N-lobe the peptides 222–230 (ADRDQYELL) and 264–269 (EDLIWK) were also able to inhibit HSV-1 infection when chemically synthesized, albeit only when they were assayed together [89]. Additionally, smaller antibacterial peptides such as bLficin interfere with HSV-1 cell-to-cell movement [77].
Fig. 2.
Predicted structure of antiviral-active peptides using PDB ID: 1FCK as template. a) From N-lobe 1–280 aa. b) From C-lobe 345–689 aa. Modeled using DeepView software [24], and viewed using Chimera software (http://www.cgl.ucsf.edu/chimera/).
Human respiratory syncytial virus (RSV) is inhibited by Lf at concentrations ten times lower than those found in human milk. Lf also acts against non-enveloped viruses such as adenoviruses and enteroviruses [90]. Several mechanisms of action have been proposed for the antiviral activity of Lf. One of the most widely accepted hypotheses is that Lf binds to and blocks glycosaminoglycan viral receptors, especially heparan sulfate (HS). The binding of Lf and HS prevents the first contact between virus and host cell, thus preventing infection [6]. The antiviral effect of Lf has also been observed in viruses that infect animals, such as the Friend virus complex, which causes erythroleukemia in rodents [92], the feline calcivirus [93], and the feline immunodeficiency virus [16]. It appears that Lf exhibits its antiviral activity at an early phase of the infection process [16], [75], [78], [83], [84], [91], [94], [95], [96], [97]. Several viral pathogens have been shown to use the host cell surface as herpes simplex virus, as an attachment receptor during the infection process [98], [99]. Results, also suggest that the GRRRRS sequence at the amino terminus of human lactoferrin acts synergistically with an RKVR sequence at positions 28–31 to form the predominate functional GAG-binding site of human lactoferrin [100].
4.3. Antifungal activity
Candida can colonize mucosal surfaces in individuals, and it is considered to be analogous to a commensal organism that can also become an opportunistic pathogen. Kirkpatrick et al. conducted the first studies with Candida spp. in 1971 and attributed the antifungal effect of Lf to its ability to sequester Fe+ 3 [101], [102]. Both hLf and bLf, as well as the bLf-derived peptide lactoferricin, have well-documented in vitro activity toward human pathogenic fungi, especially Candida albicans and several other Candida species. Lf also has antifungal activity [103], [104], and it was observed that Lf could kill both C. albicans and C. krusei by altering the permeability of the cell surface, as it does in bacteria [105], [106].
Bovine Lf has been shown to be highly fungicidal for C. tropicalis and C. krusei and somewhat fungicidal for C. albicans and C. guilliermondii, while C. glabrata is almost resistant to Lf [107]. Lf exhibited activity against Cryptococcus neoformans and C. albicans via cytoplasmic and mitochondrial membrane permeabilization [108]. Although Lf's antifungal mechanism of action is through cell surface interaction rather than iron deprivation [109], several reports demonstrated its ability to cause cell wall damage [107], [110], [111]. In addition to direct interaction with the pathogen, Fe+ 3 sequestration is another important mechanism for fungicidal activity. In 2007, Zarember et al. showed that Fe+ 3 sequestration by neutrophil apo-Lf is important for host defense against Aspergillus fumigatus [112]. Additionally, the in vitro antifungal activity of two peptides (hLF(1–11 aa), bLf N1-domain)) derived from human lactoferrin (hLF) were compared, and dose-dependent antifungal activity was observed [113], [114]. Lf shows an interesting antifungal effect on body tineas caused by Trichophyton mentagrophytes, against which it acts indirectly, facilitating clinical improvement of skin lesions after the peak of the symptoms. Treatment of guinea pigs with bLf reduces fungal infection on the skin of the back and limbs in tinea corpus and tinea pedis, respectively [115]. It has also been demonstrated that Lf can mediate its antifungal activity through the stimulation of host cell immune mechanisms both in vitro and in vivo [102].
4.4. Antiparasitic activity
A clear understanding of the antimicrobial activities of Lf has been difficult to achieve because the mechanisms of action of Lf and the ecological niches of microbes often differ from one organism to another. The molecular mechanisms of Lf antiparasitic activity are even more complex.
Antiparasitic activities of Lf usually involve interference with iron acquisition. This activity has also been shown using peptides derived from the full molecule [116], [117]. There is also evidence supporting the occurrence of a similar mechanism during amoebiasis, which is one of the leading causes of diarrhea in children under 5 years of age and is caused by Entamoeba histolytica [118].
Apo-Lf is the milk protein with the greatest amoebicidal effect against E. hystolitica in vitro because it can bind to lipids on the trophozoite's membrane, causing membrane disruption and damage to the parasite [119], [120]. Lf appears to act as a specific iron donor and could be expected to enhance infection by other parasites such as Tritrichomonas foetus [121]. It was reported that bLF bound to components of T. brucei, and that bLF hydrolysate disrupted the sites responsible for binding to parasite proteins, causing Fe+ 3 deprivation [122]. Other in vitro studies show that serum transferrin as well as human and bovine Lf can bind the intracellular parasite Toxoplasma gondii, which causes toxoplasmosis and affects both humans and animals. However, Lf cannot prevent the parasite from entering the host. The mechanism of action in this case is inhibition of the intracellular growth of T. gondii in the host cells [123]. In animal models, a lactoferricin reduced infectivity of T. gondii and Eimeria stiedai sporozoites [124]. The effect of Lf on the hemoparasites Babesis caballi and Babesia equi depends on whether or not Lf is bound to Fe+ 3 [125]. B. caballi was found to be significantly suppressed by apo-Lf but was not inhibited by the other types of Lf, whereas none of the Lf types had an inhibitory effect against B. equi [126]. Lf also demonstrates additive or synergistic activity with clinically used antiparasitic compounds [116], [118], [119].
5. Immunomodulatory and anti-inflammatory activity
Lf displays immunological properties that influence both innate and acquired immunities [127]. Its relationship with the immune system is evident from the fact that people with congenital or acquired Lf deficiency have recurring infections [128]. Oral administration of bLf seems to influence mucosal and systemic immune responses in mice [129]. Lf can modulate both specific and non-specific expression of antimicrobial proteins, pattern recognition receptors and lymphocyte movement related proteins [130]. The role that Lf plays in regulating innate immune responses confirms its importance as a first line host defense mechanism against invading pathogens, modulating both acute and chronic inflammation [131], [132], [133], [134]. Most intriguing is the ability of Lf to induce mediators from innate immune cells that subsequently impact adaptive immune cell function. Lf's positive charge allows it to bind to negatively charged molecules on the surface of various cells of the immune system [135], and it has been suggested that this association can trigger signaling pathways that lead to cellular responses such as activation, differentiation and proliferation. Lf can be transported into the nucleus, where it can bind DNA [86], [136] and activate different signaling pathways [7].
In addition to inducing systemic immunity, Lf can promote skin immunity and inhibit allergic responses. It activates the immune system against skin allergens, causing dose-dependent inhibition of Langerhans cell migration and the accumulation of dendritic cells in lymph nodes [6]. Leukocytes exposed to Lf modulate their cytokine production; proinflammatory cytokines, TNF-α, IL-6, and IL-1β can be modulated by Lf to increase [137], [138], [139] or decrease [131], [139], [140], [141]. Production of these factors is dependent upon the type of signal recognized by the immune system. At the cellular level, Lf increases the number of natural killer (NK) and adaptative (T strain CD4+ and CD8+) cells [142], boosts the recruitment of polymorphonuclear cells (PMNs) in the blood [143], induces phagocytosis [144], and can modulate the myelopoietic process [145]. It is well documented that IL-12 plays an important role in driving development of helper T-cell type 1 immunity [146], [147]. Therefore, the role of Lf in the regulation of proinflamatory cytokines and IL-12 clearly demonstrates communication between innate and adaptative immune responses.
6. Anticarcinogenic activity
The anti-tumor properties of Lf were discovered about a decade ago and have been confirmed by numerous laboratory studies which have shown that bovine lactoferrin (bLf) significantly reduces chemically induced tumorigenesis when administered orally to rodents [148]. Since then, human clinical studies are showing that ingestion of LF can have a beneficial effect against progression of cancer [150]. bLf possesses antimetastatic effects and inhibits the growth of transplanted tumors [149], [151]. Similar to its role in inflammation, Lf has the ability to modulate the production of cytokines in cancer. Lf can induce apoptosis and arrest tumor growth in vitro; it can also block the transition from G1 to S in the cell cycle of malignant cells [7], [152]. Additionally, treatment of tumors in mice with recombinant human Lf (rhLf) inhibits their growth, increases the levels of anticarcinogenic cytokines such as IL-18, and activates NK cells and CD8+ T lymphocytes [153], [154]. Interestingly, bLf and hLf exert opposite effects on angiogenesis. Whereas orally administrated bLf inhibits angiogenesis in rats [155], [156] and tumor-induced angiogenesis in mice [154], hLf exerts a specific pro-angiogenic effect [157]. Recently, colorectal cancer was inhibited by bLf in animal models, and human Lf reduced the risk of colon carcinogenesis as demonstrated by a clinical trial [158]. Increasing evidence suggests that inhibition of the Akt signaling pathway might be a promising strategy for cancer treatment [159]. In breast cancer, Lf is able to limit the growth of tumor cells, and addition of exogenous Lf to the culture media of breast cancer cell lines (MDA-MB-231) induced cell cycle arrest at the G1/S transition [160]. Additionally, Lf induced growth arrest and nuclear accumulation of Smad-2 in HeLa cells [161]. The ability of bovine Lfcin to induce apoptosis in THP-1 human monocytic leukemic cells has also been demonstrated [162]. Although the results achieved by several researchers point to a clear anti-tumor role for Lf, the mechanisms by which it exerts these effects are not fully understood. Thus, further work on this subject is required.
7. Enzymatic activity
Lf has the ability to function as an enzyme in some catalytic reactions. A remarkable similarity of certain motifs between Lf and ribonuclease A has been observed [163]. Lf has DNA binding properties [164] and can act in transcriptional activation of specific DNA sequences [165] or as a mediator of signal transduction [166]. Lf has the highest levels of amylase and ATPase activities of all the milk proteins [167]; however, these are not its only enzymatic activities. Indeed, Lf exhibits a wide variety of activities, which can be attributed to variations in the nature of its protein characteristics; Lf has multiple isoforms, degrees of glycosylation, variations in tertiary structure (holo- or apo-Lf) and degrees of oligomerization [168], [169]. The discovery of Lf's enzymatic properties has helped to elucidate its many physiological functions.
8. Bioactive peptides derived from lactoferrin
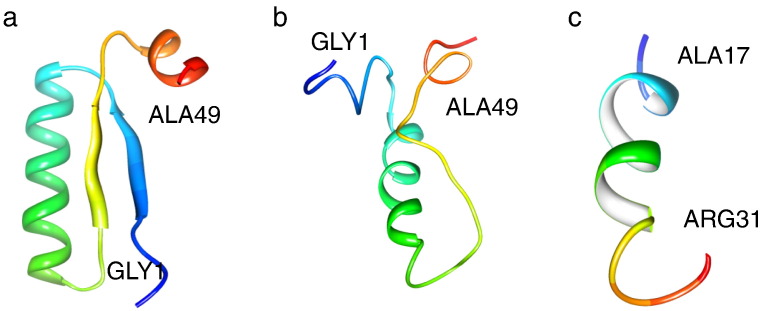
Lactoferrin was first isolated by Groves in 1960 and was recognized as a “red protein from milk” [2]. Moderate proteolysis led to a release of two Lf fragments, namely the N- and C-terminal lobes. Enzymatic treatment of bLf with pepsin produced a low molecular weight peptide with antibacterial properties against a large number of Gram-positive and Gram-negative bacteria, in addition to fungi. Bellamy et al.[170] identified a region of amino acids at the N-terminus that retains its biological activity when separated from the full molecule; this was termed lactoferricin (Lfc-B) and was shown to exhibit greater antimicrobial activity than Lf. The activity that is exerted by this region corresponds to residues 17–41 of bLf [171]. The region also includes two Cys residues linked by a disulfide bridge that contains many hydrophobic and positively charged residues.
The tertiary structure of Lfcin is markedly different when compared to its homologous region of intact Lf (Fig. 3a). An in situ model of hLfcin (Fig. 3a) and a solvated version (Fig. 3b) show structural differences at their N- and C-termini (Fig. 3a, b). A single β-sheet strand replaces the long α-helix observed in the Lf structure; such a structure may be better suited to recognize bacterial membrane topology [172]. Additionally, the bLfcin peptide contains an alpha-helix (Fig. 3a–c). This region retains high homology among other species of mammals and corresponds to amino acids 12–48 [17].
Fig. 3.
Location of field and aqueous solvated structure comparison of the human and bovine lactoferricin. a) Domain of in situ hLfcin (residues 17–41; PDB ID: 1BLF) and, aqueous solvated b) hLfcin and c) bLfcin. Viewed using Chimera software (http://www.cgl.ucsf.edu/chimera/).
It was found that minimal variations in the amino acid sequence change the antimicrobial activity of the peptide. For example, Lfampin 268–284 and Lfampin 265–284, chemically synthesized fragments from the N-terminal sequence of bLf, differ in only three amino acids (265Asp-Leu267-Ile268) but exhibit different strengths of antimicrobial activity [171]. Proteolysis of iron-free Lf could release Lf-derived active peptides in biological fluid [172].
9. Lactoferrin gene regulation
Lf has been identified in several tissues in both humans and animals. The Lf gene has been found at the chromosome level in a set of different species; in humans it was found on chromosome 3, while in mice it was found on chromosome 9 [173], and its size ranged from 23 to 35 kb. Lf genes have extensive homology among species, with an identical organization in cows, pigs and mice (17 exons, 15 encoding Lf) [174], [175]. The amount of Lf synthesized in the mammary gland is controlled by prolactin [176]. Its mRNA levels vary by tissue, suggesting tissue- or cell-specific regulation [177], [178]. The Lf full coding regions of 60 different species were analyzed, and it was found that the length of the gene varies from species to species (from 2055 to 2190 residues) due to deletions, insertions and mutations in the stop codon [179]. Lf is expressed both constitutively and inducibly. It is constitutively expressed on mucosal surfaces, while in some tissues it is induced by external agents.
The Lf promoter contains an estrogen responsive element [REF], and is consequently positively regulated by estrogen. The chicken ovalbumin upstream promoter transcription factor (COUP-TF) binding element overlaps the ERE of the lactoferrin gene [180]. COUP-TF binds to this element and competitively inhibits binding of the estrogen receptor to the lactoferrin ERE, thereby inhibiting transcription of the lactoferrin gene. Another negative regulator of LF gene transcription is repressor of estrogen receptor activity (REA). The absence of REA increases the expression of estrogen-induced Lf by up to 100-fold [181].
Lf can also be induced by compounds other than estrogens, such as retinoic acid, which stimulates gene expression in embryonic cells [182]. Lf expression is upregulated by estrogen with a magnitude of response that is cell-type-specific, and it is also upregulated by retinoic acids. Transcription factors such as Ets, PU.1, C/EBP, CDP, and KLF5 also modulate Lf gene expression, mainly in myeloid cells [183]. Lf expression is also modulated by oxidative stress, in response to infection, or during the early steps of embryogenesis [184].
10. Clinical applications of lactoferrin
Lf can be isolated from cow's milk by various purification methods, or it can be expressed by recombinant methods [51], [71], [185]. Because of the multiple functions of Lf, it has been used for clinical trials and industrial applications. One of the first applications of Lf was in infant formula. Currently it is added to immune system-enhancing nutraceuticals, cosmetics, pet care supplements, drinks, fermented milks, chewing gums, and toothpaste. The ability of Lf to prevent nososcomial infection in infants was tested [186] and the results showed lower infection levels. Several studies showed that infants fed with infant formulas had less intestinal iron absorption than breastfed infants [187], [188]. It was proposed that Lf also promotes the proliferation of lactic acid bacteria in the bowel, such as Bifidobacterium and Lactobacillus, which protect the host from harmful bacteria [186], [189].
The activity of Lf and its bioactive peptides has been documented both in vitro and in vivo against a wide variety of pathogens. Clinical trials demonstrated the efficiency of Lf for use in treating infections and inflammatory diseases. For example, Lf was tested as a second treatment against H. pylori in patients with recurring infections. The patients supplemented with bLf showed a greater recovery from infection [190]. Lf also exhibits synergistic activity with antifungal agents, thus reducing the minimum inhibitory concentrations of these agents against C. albicans and C. glabrata [101], [191]. As with antibacterials, Lf exhibits synergy with antiviral drugs in the treatment of hepatitis C, cytomegalovirus and HIV [6], [79], [192]. Lf delays the hypersensitivity response and limits the pathology caused by M. tuberculosis by increasing IL-12 and IL10 expression [193]. A clinical trial was conducted which demonstrated that ingestion of bLF could reduce the risk of colon cancer in humans [157]. Lf also offers applications in food preservation and safety because it can decrease bacterial counts in pork meat [194], [195], retard lipid oxidation [196] and limit the growth of microbes.
Lf can also be used as a molecular marker; detection of urinary Lf via electrochemical immunosensors aids in the diagnosis of urinary tract infections [197]. Increased levels of Lf serve as a clinical marker of inflammatory Severe Acute Respiratory Syndrome or septicemia [198].
11. Production of native and recombinant lactoferrin
The development of commercial production strategies for the production of recombinant Lf as a safe, effective drug and nutraceutical protein is one of the major goals in both research and industry. Purification of Lf depends on the intrinsic properties and features of the molecule, such as its net positive charge, its ability to bind Fe+ 3 and its glycosylation state [6], [199], [200]. However, the need for larger amounts of Lf has led to the development of strategies to obtain a recombinant form of the protein (rLf).
In global biotechnology, there are now three major competing approaches for the production of rLf: production in transgenic animal milk, production in microscopic fungi, and production in plants. To date, several rLf expression systems have been developed (Table 2 ) that utilize both prokaryotic and eukaryotic organisms. The first expression systems utilized Bay Hamster Kidney Cells to express human lactoferrin [221].
Table 2.
Expression of recombinant Lf by various transgenic organisms.
| Expression system | Lf origen | Expression level | Year/reference |
|---|---|---|---|
| Bacteria | |||
| E. coli | bLfc | 10 mg/L | 2007 [201] |
| Lfc | 60 mg/L | 2006 [202] | |
| bLfc | 2 mg/L | 2006 [203] | |
| Kumin Lf | 17 mg/L | 2010 [204] | |
| Lactobacillus casei | hLf | 10.6 mg/mL | 2010 [205] |
| Rhodococcus erythropolis | bLf C-lobe | 3.6 mg/mL | 2006 [206] |
| Yeast | |||
| Pichia pastoris | hLf | 115 mg/L | 2004 [207] |
| cLf | 2 mg/L | 2007 [208] | |
| pLf | 12 mg/L | 2002 [209] | |
| Yak Lf | 40 mg/L | 2006 [210] | |
| eLf | 40 mg/L | 2002 [211] | |
| hLf | 1200 mg/L | 2008 [212] | |
| Pichia methanolica | pLf | NR | 2007 [213] |
| bLfc | 90 mg/L | 2007 [214] | |
| Fungi | |||
| Aspergillus awamori | hLf | 2 g/L | 1995 [215] |
| Aspergillus oryzae | hLf | 25 mg/L | 1992 [216] |
| Insects | |||
| Spodoptera frugiperda | hLf | 9.5 mg/L | 1998 [217] |
| Bombyx mori | hLf | 65 mg/L | 2005 [218] |
| hLf | 13.5 μg/1–2 × 105 cells | 2006 [219] | |
| pLf | 205 μg/pupa | 2005 [220] | |
| Cell lines | |||
| Baby hamster kidney (BHK) | hLf | 20 mg/L | 1991 [221] |
| Cell culture (human embryo kidney) | hLf | 16 mg/L | 2009 [222] |
| Mammals | |||
| Goat | hLf | 0.756 mg/L | 2008 [223] |
| hLf | 2 g/L | 2007 [224] | |
| Mice | hLf | 2.5 mg/mL | 1997 [225] |
| hLf | 2.5 mg–200 μg/mL milk | 1997 [226] | |
| Rabbit | hLf | 2.3 mg/mL milk | 2008 [227] |
| Bovine | hLf | 1 g/L milk | 2002 [228] |
| hLf | 2.9 mg/mL | 2006 [229] | |
| Plants | |||
| Nicotiana benthamiana | hLf N-lobe | 0.6% soluble protein | 2004 [230] |
| Nicotiana tabacum | hLf | NR | 1994 [231] |
| hLf | 0.1–0.8% soluble protein | 1998 [232] | |
| hLf | 4.3% soluble protein | 2003 [233] | |
| bLf | NR | 2011 [234] | |
| Rice | hLf | 1.6 mg/g seed | 2004 [235] |
| hLf | 0.5% total biomass | 2005 [236] | |
| hLf | 2–4% soluble protein | 2003 [237] | |
| hLf | 0.1% soluble protein | 2010 [238] | |
| Potato | hLf | 0.1% soluble protein | 2000 [239] |
| Sweet potato (Ipomoea batata) | hLf | 3.2 μg/mg total protein | 2006 [240] |
| Panax ginseng | hLf | 3.0% soluble protein | 2003 [241] |
| Tomato (Lycopersicon esculentum) | hLf | NR | 2002 [242] |
| Maize | hLf | NR | 2001 [243] |
| Alfalfa (Medicago sativa) | hLf | NR | 2005 [244] |
| Barley | hLf | NR | 2011 [245] |
(h = human, b = bovine, c = caprine, p = porcine, e = equine, Lfc = lactoferricin, NR = not reported).
The transformation of the filamentous fungi Aspergillus awamory allowed for the expression of both hLf and murine Lf (mLf) [215], [216]. As shown in Table 2, expression systems were developed in yeasts, bacteria, insects and plants, which have produced human recombinant Lf (rhLf) [205], [207], [212], [217], [218], [219], [230], [231], [232], [233], [234], [235], [236], [237], [238], [239], [240], [241], [242], caprine Lf (cLF) [208], bovine Lf (bLf) [201], [203], [206], [214], equine Lf (eLf) [211], porcine Lf (pLF) [209], [220], yak Lf [210], and Kumin Lf [204], as well as Lf peptides, including Lfc [202], [203], [206], [214], which reached expression levels of 0.1 mg/L in a plant model [231] and 1200 mg/L in P. pastoris [212]. Use of viral vectors has allowed for the expression of Lf by insect infection, either in cell culture or directly in the organism, where the expression of both hLf and pLf has been successful. This practice has resulted in the production of transgenic Spodoptera frugiperda and Bombyx mori, which express 205 μg of pLf per infected pupa [220] and up to 65 mg/L in larvae [218]. Lf has also been expressed in higher eukaryotic organisms, including both plants and animals. Using microinjection and direct infection with viral vectors in the mammary gland, transgenic animals have been created that produce milk containing recombinant Lf. These animals include goats [223], [224], mice [225], [226], rabbits [227] and cows [228], [229]. Levels of up to 2 g/L have been achieved in transgenic goat milk.
Molecular farming, which involves the utilization of plants as bioreactors, is well depicted as a tool for the production of valuable therapeutic and industrial proteins. This process is advantageous because of the lack of contamination from human or animal pathogens and endotoxins in the final product. Furthermore, higher plants are able to synthesize proteins with the proper folding, glycosylation and functional activity, and plant cells can direct the protein of interest to environments that reduce its degradation. hLf was expressed in plant expression models (Table 2) and bLf was only expressed in tobacco plants [235]. However, none of these nuclear transformation methods are able to promote the accumulation of a large amount of rLf. Therefore, new promoters or regulatory elements as well as other transformation methods should be tested to increase accumulation and stability of recombinant proteins. The expression of Lf in plant models could significantly improve crop quality by increasing its resistance to some diseases; additionally, it provides a source for high quality Lf protein.
12. Concluding remarks
Lf is a multifunctional protein that takes part in a large number of important physiological processes. The beneficial effect of Lf in the treatment of various infectious diseases caused by bacteria, fungi, protozoa, and viruses in animals and humans is described above; nevertheless, much research and many experiments still need to be performed to obtain a better understanding of its activity. The usefulness of Lf has led scientists to develop this health-enhancing nutraceutical protein for use in food and pharmaceutical applications.
Acknowledgements
This work was supported in part by an internal grant from Facultad de Ciencias Químicas, Universidad Autónoma de Chihuahua, and Muuu-Technologies de México, and García-Montoya thanks CONACYT for the MC studies grant.
Footnotes
This article is part of a Special Issue entitled Molecular Mechanisms of Iron Transport and Disorders.
References
- 1.Montreuil J., Tonnelat J., Mullet S. Preparation and properties of lactosiderophilin (lactotransferrin) of human milk. Biochim. Biophys. Acta. 1960;45:413–421. doi: 10.1016/0006-3002(60)91478-5. [DOI] [PubMed] [Google Scholar]
- 2.Groves M.L. The isolation of the red protein from milk. J. Am. Chem. Soc. 1960;82:3345–3350. [Google Scholar]
- 3.Johanson B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960;14:510–512. [Google Scholar]
- 4.Shanbacher F.L., Goodman R.E., Talhouk R.S. Bovine mammary lactoferrin: Implications from messenger ribonucleic acid (mRNA) sequence and regulation contrary to other milk proteins. J. Dairy Sci. 1992;76:3812–3831. doi: 10.3168/jds.S0022-0302(93)77725-5. [DOI] [PubMed] [Google Scholar]
- 5.Torres J.M., Concepción J.L., Vielma J.R. Detección de lysozima y lactoferrina por western blot en ovas de trucha arcoíris (Oncorhynchus mykiss) Mundo Pecuario. 2006;2:57–59. [Google Scholar]
- 6.Van Der Strate B.W.A., Belijaars L., Molema G., Harmsen M.C., Meijer D.K.F. Antiviral activities of lactoferrin. Antiviral Res. 2001;52:225–239. doi: 10.1016/s0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- 7.Öztaş Yeşim E.R., Özgüneş N. Lactoferrin: a multifunctional protein. Adv. Mol. Med. 2005;1:149–154. [Google Scholar]
- 8.Rodríguez D.A., Vázquez L., Ramos G. Actividad Antimicrobiana de la lactoferrina: Mecanismos and aplicaciones clínicas potenciales. Rev. Latinoam. Microbiol. 2005;47:102–111. [PubMed] [Google Scholar]
- 9.Brogan T.D., Ryley H.C., Neale L., Yassa J. Soluble proteins of bronchopulmonary secretions from patients with cystic fibrosis, asthma, and bronchitis. Thorax. 1975;30:72–79. doi: 10.1136/thx.30.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim D.J., Chun Y.M., Lee H.Y., Moon S.K., Chang K.H., Li J.-D., Andalibi A. Cell biology of tubotympanum in relation to pathogenesis of otitis media e a review. Vaccine. 2000;19:S17–S25. doi: 10.1016/s0264-410x(00)00273-5. [DOI] [PubMed] [Google Scholar]
- 11.Aisen P., Leibman A. Lactoferrin and transferrin: a comparative study. Biochim. Biophys. Acta. 1972;257:314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- 12.Baker E.N. Structure and reactivity of transferrins. Adv. Inorg. Chem. 1994;41:389–463. [Google Scholar]
- 13.Duarte D.C., Nicolau A., Teixeira J.A., Rodrigues L.R. The effect of bovine milk lactoferrin on human breast cancer cell lines. J. Dairy Sci. 2011;94:66–76. doi: 10.3168/jds.2010-3629. [DOI] [PubMed] [Google Scholar]
- 14.Drago S.M.E. Actividades antibacterianas de la lactoferrina. Enferm. Infecc. Microbiol. 2006;26:58–63. [Google Scholar]
- 15.Marchetti M., Superti F., Ammendolia M.G., Rossi P., Valenti P., Seganti L. Inhibition of poliovirus type 1 infection by iron-, manganese-, and zinc-saturated lactoferrin. Med. Microbiol. Immunol. (Berl) 1999;187:199–204. doi: 10.1007/s004300050093. [DOI] [PubMed] [Google Scholar]
- 16.Sato R., Inanami O., Tanaka Y., Takase S.E., Naito Y. Oral administration of bovine lactoferrin for treatment of intractable stomatitis in feline immunodeficiency virus (FIV)-positive and (FIV)-negative cats. Am. J. Vet. Res. 1996;57:1443–1446. [PubMed] [Google Scholar]
- 17.Metz-Boutigue M.H., Jollès J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur. J. Biochem. 1984;145:659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 18.Rey M.W., Woloshuk S.L., deBoer H.A., Pieper F.R. Complete nucleotide sequence of human mammary gland lactoferrin. Nucleic Acids Res. 1990;18:5288. doi: 10.1093/nar/18.17.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wally J., Buchanan S.K. A structural comparison of human serum transferrin and human lactoferrin. BioMetals. 2007;20:249–262. doi: 10.1007/s10534-006-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert L.A., Perri H., Meehan T.J. Evolution of duplications in the transferrin family of proteins. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2005;140:11–25. doi: 10.1016/j.cbpc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Haridas M., Anderson B.F., Baker E.N. Structure of human diferric lactoferrin refined at 2.2 Å resolution. Acta Crystallogr. 1995;51:629–646. doi: 10.1107/S0907444994013521. [DOI] [PubMed] [Google Scholar]
- 22.Mazurier J., Spik G. Comparative study of the iron-binding properties of human transferrins: I. complete and sequential iron saturation and desaturation of the Lactotransferrin. Biochim. Biophys. Acta. 1980;629:399–408. doi: 10.1016/0304-4165(80)90112-9. [DOI] [PubMed] [Google Scholar]
- 23.Baker H.M., Anderson B.F., Baker E.N. Dealing with iron: common structural principles in proteins that transport iron and heme. Proc. Natl. Acad. Sci. U.S.A. 2004;100:3579–3583. doi: 10.1073/pnas.0637295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold K., Kiefer F., Kopp J., Battey J.N., Podvinec M., Westbrook J.D., Berman H.M., Bordoli L., Schwede T. The protein model portal. J. Struct. Funct. Genomics. 2009;10:1–8. doi: 10.1007/s10969-008-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson B.F., Baker H.M., Dodson E.J., Norris G.E., Rumball S.V., Waters J.M., Baker E.N. Structure of human lactoferrin at 3.2-A° resolution. Proc. Natl. Acad. Sci. U.S.A. 1987;84:1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan J.A., Kumar P., Paramasivam M., Yadav R.S., Sahani M.S., Sharma S., Srinivasana A., Singh T.P. Camel lactoferrin, a transferrin-cum-lactoferrin: crystal structure of camel apolactoferrin at 2.6 Å resolution and structural basis of its dual role. J. Mol. Biol. 2001;309:751–761. doi: 10.1006/jmbi.2001.4692. [DOI] [PubMed] [Google Scholar]
- 27.Connely O.M. Antiinflammatory activities of lactoferrin. J. Am. Coll. Nutr. 2001;438:389S–395S. doi: 10.1080/07315724.2001.10719173. [DOI] [PubMed] [Google Scholar]
- 28.Sherman M.P., Bennett S.H., Hwang F.F., Yu C. Neonatal small bowel epithelia: enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. BioMetals. 2004;17:285–289. doi: 10.1023/b:biom.0000027706.51112.62. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi K., Wakabayashi H., Shin K., Takase M. Bovine lactoferrin: benefits and mechanism of action against infections. Biochem. Cell Biol. 2006;84:291–296. doi: 10.1139/o06-054. [DOI] [PubMed] [Google Scholar]
- 30.Leffell S., Spitznagel J.K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect. Immun. 1972;6:761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalmar J.R., Arnold R.R. Killing of Actinobacillus actinomycetemcomitans by human lactoferrin. Infect. Immun. 1988;56:2552–2557. doi: 10.1128/iai.56.10.2552-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamauchi K., Tomita M., Giehl T.J., Ellison R.T., III Antibacterial activity of lactoferrin and a pepsin derived lactoferrin peptide fragment. Infect. Immun. 1993;61:719–728. doi: 10.1128/iai.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenti P., Antonini G. Lactoferrin: an important host defense against microbial and viral attack. Cell. Mol. Life Sci. 2005;62:2576–2587. doi: 10.1007/s00018-005-5372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bortner C.A., Arnold R.R., Miller R.D. Bactericidal effect of lactoferrin on Legionella pneumophila: effect of the physiological state of the organism. Can. J. Microbiol. 1989;35:1048–1051. doi: 10.1139/m89-174. [DOI] [PubMed] [Google Scholar]
- 35.Farnaud S., Evans R.W. Lactoferrin: a multifunctional protein with antimicrobial properties. Mol. Immunol. 2005;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 36.O´leay J., Busta F.F. Effect of food components on growth of Basillius stearothermophilus. J. Food Sci. 1974;39:1157–1160. [Google Scholar]
- 37.Oliver S.P., Duby R.T., Prange R.W., Tritschler J.P., II Residues in colostrum following antibiotic dry cow therapy. J. Dairy Sci. 1984;67:3081–3084. doi: 10.3168/jds.S0022-0302(84)81676-8. [DOI] [PubMed] [Google Scholar]
- 38.Oram J.D., Reitera B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim. Biophys. Acta. 1968;170:351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- 39.Qiu J., Hendrixson D.R., Baker E.N., Murphy T.F., St Geme J.W., III, Plaut A.G. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12641–12646. doi: 10.1073/pnas.95.21.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellamy W., Takasea M., Yamauchia K., Wakabayashia H., Kawasea K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-F. [DOI] [PubMed] [Google Scholar]
- 41.Lee H.Y., Park J.H., Seok S.H., Baek M.W., Kim D.J., Lee B.H., Kang P.D., Kim Y.S., Park J.H. Potential antimicrobial effects of human lactoferrin against oral infection with Listeria monocytogenes in mice. J. Med. Microbiol. 2005;54:1049–1054. doi: 10.1099/jmm.0.45918-0. [DOI] [PubMed] [Google Scholar]
- 42.Hammerschmidt S., Bethe G., Remane P.H., Chatwal G.S. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 1999;67:1683–1687. doi: 10.1128/iai.67.4.1683-1687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhimani R.S., Vendrov Y., Furmanski P. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J. Appl. Microbiol. 1999;86:135–144. doi: 10.1046/j.1365-2672.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 44.Berlutti F., Ajello M., Bosso P., Morea C., Andrea P., Giovanni A., Piera V. Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. BioMetals. 2004;17:271–278. doi: 10.1023/b:biom.0000027704.53859.d3. [DOI] [PubMed] [Google Scholar]
- 45.Arnold R.R., Russell J.E., Champion W.J., Gauthier J.J. Bactericidal activity of human lactoferrin: influence of physical conditions and metabolic state of the target microorganism. Infect. Immun. 1981;32:655–660. doi: 10.1128/iai.32.2.655-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalmastri C., Valenti P., Visca P., Vittorioso P., Orsi N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica. 1988;11:225–230. [PubMed] [Google Scholar]
- 47.Van der Kraan M.I., Van Marle J., Nazmi K., Groenink J., Van't Hof W., Veerman E.C., Bolscher J.G., Amerongen A.V. Ultrastructural effects of antimicrobial peptides from bovine lactoferrin on the membranes of Candida albicans and Escherichia coli. Peptides. 2005;26:1537–1542. doi: 10.1016/j.peptides.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Roseanu A., Florian P., Condei M., Cristea D., Damian M. Antibacterial activity of lactoferrin and lactoferricin against oral Streptococci. Rom. Biotechnol. Lett. 2010;15:5788–5792. [Google Scholar]
- 49.Ochoa T.J., Noguera-Obenza M., Ebel F., Guzman C.A., Gomez H.F., Cleary T.G. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 2003;71:5149–5155. doi: 10.1128/IAI.71.9.5149-5155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beeckman D.S.A., Van Droogenbroeck C.M., De Cock J.A., Oostveldt P.V., Vanrompay D.C.G. Effect of ovotransferrin and lactoferrins on Chlamydophila psittaci adhesion and invasion in HD11 chicken macrophages. Vet. Res. 2007;38:729–739. doi: 10.1051/vetres:2007028. [DOI] [PubMed] [Google Scholar]
- 51.Nacimiento A., Giugliano L.O. Human milk fractions inhibit the adherence of diffusely adherent Escherichia coli (DAEC) and enteroaggregative E. coli (EAEC) to HeLa cells. FEMS Microbiol. Lett. 2000;184:91–94. doi: 10.1111/j.1574-6968.2000.tb08996.x. [DOI] [PubMed] [Google Scholar]
- 52.Dial E.J., Romero J.J., Headon D.R., Lichtenberger L.M. Recombinant human lactoferrin is effective in the treatment of Helicobacter felis-infected mice. J. Pharm. Pharmacol. 2000;52:1541–1546. doi: 10.1211/0022357001777595. [DOI] [PubMed] [Google Scholar]
- 53.Wang X., Hirmo S., Willén R., Wadström T. Inhibition of Helicobacter pylori infection by bovine milk glycoconjugates in a BAlb/cA mouse model. J. Med. Microbiol. 2001;50:430–435. doi: 10.1099/0022-1317-50-5-430. [DOI] [PubMed] [Google Scholar]
- 54.Husson M.O., Legrand D., Spik G., Leclerc H. Iron acquisition by Helicobacter pylori: importance of human lactoferrin. Infect. Immun. 1993;61:2694–2697. doi: 10.1128/iai.61.6.2694-2697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldoni P., Sinibaldi L., Valentiu P., Orsi N. Metal complexes of lactoferrin and their effect on the intracellular multiplication of Legionella pneumophila. BioMetals. 2000;13:15–22. doi: 10.1023/a:1009221616623. [DOI] [PubMed] [Google Scholar]
- 56.Berlutti F., Morea C., Battistoni A., Sarli S., Cipriani P., Superti F., Ammendolia M.G., Valenti P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2005;18:661–670. doi: 10.1177/039463200501800407. [DOI] [PubMed] [Google Scholar]
- 57.Asfour H.A.E., Yassin M.H., Goma A.M. Anti-bacterial activity of bovine milk lactoferrin against some mastitis causative pathogens with special regard to mycoplasma. Int. J. Microbiol. Res. 2010;97:97–105. [Google Scholar]
- 58.Rogan M.P., Taggart C.C., Greem C.M., Murphy P.G., O'Neill S.J., McElvaney N.G. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with fibrosis cystic. J. Infect. Dis. 2004;190:1245–1253. doi: 10.1086/423821. [DOI] [PubMed] [Google Scholar]
- 59.Willer E.M., Lima R.L., Giuigliano L.G. In vitro adhesion and invasion inhibition of Shigella dysentariae, Shigella flexneri and Shigella sonnei clinical strains by human milk proteins. BMC Microbiol. 2004;4:18–24. doi: 10.1186/1471-2180-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnold R.R., Cole M.F., McGhee J.R. A bactericidal effect for human lactoferrin. Science. 1977;197:263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- 61.Schaible U.E., Collins H.L., Priem F., Kaufmann S.H. Correction of the iron overload defect in 2-microglobulin knockout mice by lactoferrin abolishes their susceptibility to tuberculosis. J. Exp. Med. 2002;196:1507–1513. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hwang S.A., Kruzel M.L., Actor J.K. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int. Immunopharmacol. 2005;5:591–599. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Wakabayashi H., Kondo I. Periodontitis, periodontopathic bacteria and lactoferrin. BioMetals. 2010;23:419–424. doi: 10.1007/s10534-010-9304-6. [DOI] [PubMed] [Google Scholar]
- 64.Del Olmo A., Calzada J., Nuñez M. Antimicrobial effect of lactoferrin and its amidated and pepsin-digested derivatives against Salmonella enteriditids and Pseudomona fluorescence. J. Dairy Sci. 2010;93:3965–3969. doi: 10.3168/jds.2010-3152. [DOI] [PubMed] [Google Scholar]
- 65.Reyes R.E., Manjarrez H.A., Drago M.E. El hierro y la virulencia bacteriana. Enferm. Infecc. Microbiol. 2005;25:104–107. [Google Scholar]
- 66.Ellison R.T., Giehl T.J., Laforce F.M. Damage of the membrane of enteric Gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988;56:2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coughlin R.T., Tonsager S., McGroaty E.J. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry. 1983;22:2002–2007. doi: 10.1021/bi00277a041. [DOI] [PubMed] [Google Scholar]
- 68.Leitch C.A., Willcox M.D. Elucidation of the antistaphylococcal action of lactoferrin and lysozyme. J. Med. Microbiol. 1999;48:867–871. doi: 10.1099/00222615-48-9-867. [DOI] [PubMed] [Google Scholar]
- 69.Hendrixson D.H., Qiu J., Shewry S.C., Fink D.L., Petty S., Baker E.N., Plaut A.G., St. Geme J.W., III Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 2003;47:607–617. doi: 10.1046/j.1365-2958.2003.03327.x. [DOI] [PubMed] [Google Scholar]
- 70.Odeh R., Quinn J.P. Problem pulmonary pathogens: Pseudomonas aeruinosa. Semin. Respir. Crit. Care Med. 2000;21:331–339. doi: 10.1055/s-2000-9861. [DOI] [PubMed] [Google Scholar]
- 71.Singh P.K., Parsek M.R., Greenberg E.P., Welsh M.J. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 72.Caraher E.M., Gumulapurapu K., Taggart C.C., Murphy P., McClean S., Callaghan M. The effect of recombinant human lactoferrin on growth and the antibiotic susceptibility of the cystic fibrosis pathogen Burkholderia cepacia complex when cultured planktonically or as biofilms. J. Antimicrob. Chemother. 2007;60:546–554. doi: 10.1093/jac/dkm222. [DOI] [PubMed] [Google Scholar]
- 73.Leitch E.C., Willcox M.D. Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Curr. Eye Res. 1999;19:12–19. doi: 10.1076/ceyr.19.1.12.5342. [DOI] [PubMed] [Google Scholar]
- 74.Weinberg E.D. Suppression of bacterial biofilm formation by iron limitation. Med. Hypotheses. 2004;63:863–865. doi: 10.1016/j.mehy.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Andersen J.H., Jenssen H., Sandvik K., Gutteberg T.J. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 2004;74:262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- 76.Hasegawa K., Motsuchi W., Tanaka S., Dosako S. Inhibition with lactoferrin of in vitro infection with human herpes virus. J. Med. Sci. Biol. (Jpn) 1994;47:73–85. doi: 10.7883/yoken1952.47.73. [DOI] [PubMed] [Google Scholar]
- 77.Marr A.K., Jenssen H., Roshan Moniri M., Hancock R.E.W., Panté N. Bovine lactoferrin and lactoferricin interfere with intracellular trafficking of Herpes simplex virus-1. Biochimie. 2009;91:160–164. doi: 10.1016/j.biochi.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 78.Hara K., Ikeda M., Saito S., Matsumoto S., Numata K., Kato N., Tanaka K., Sekihara H. Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol. Res. 2002;24:228. doi: 10.1016/s1386-6346(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 79.Viani R.M., Gutteberg T.J., Lathey J.L., Spector S.A. Lactoferrin inhibits HIV-1 replication in vitro and exhibits synergy when combined with zidovudine. AIDS. 1999;13:1273–1274. doi: 10.1097/00002030-199907090-00018. [DOI] [PubMed] [Google Scholar]
- 80.Beljaars L., Van Der Strate B.W., Bakker H.I. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antiviral Res. 2004;63:197–208. doi: 10.1016/j.antiviral.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Andersen J.H., Osbakk S.A., Vorland L.H., Traavik T., Gutteberg T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 2001;51:141–149. doi: 10.1016/S0166-3542(01)00146-2. [DOI] [PubMed] [Google Scholar]
- 82.Ikeda M., Nozakia A., Sugiyama K., Tanaka T., Naganuma A., Tanaka K., Sekihara H., Shimotohno K., Saito M., Kato N. Characterization of antiviral activity of lactoferrinagainst hepatitis C virus infection in human cultured cells. Virus Res. 2000;66:51–63. doi: 10.1016/s0168-1702(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda M., Sugiyama K., Tanaka T., Tanaka K., Sekiharab H., Shimotohno K., Kato N. Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes. Biochem. Biophys. Res. Commun. 1998;245:549–553. doi: 10.1006/bbrc.1998.8481. [DOI] [PubMed] [Google Scholar]
- 84.Beaumont S.L., Maggs D.J., Clarke H.E. Effects of bovine lactoferrin on in vitro replication of feline herpesvirus. Vet. Ophthalmol. 2003;6:245–250. doi: 10.1046/j.1463-5224.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 85.Marchetti M., Longhi C., Conte M.P., Pisani S., Valenti P., Seganti L. Lactoferrin inhibits herpes simplex virus type 1 adsorption to Vero cells. Antiviral Res. 1996;29:221–231. doi: 10.1016/0166-3542(95)00840-3. [DOI] [PubMed] [Google Scholar]
- 86.Swart P.J., Kuipers M.E., Smit C., Pauwels R., De Béthune M.P., De Clercq E., Meijer D.K.F., Huisman J.G. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vitro. AIDS Res. Hum. Retroviruses. 1996;12:769–775. doi: 10.1089/aid.1996.12.769. [DOI] [PubMed] [Google Scholar]
- 87.Legrand D., Vigie´ K., Said E.A., Elass E., Masson M., Slomianny M.C., Carpentier M., Briand J.P., Mazurier J., Hovanessian A.G. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur. J. Biochem. 2004;271:303–317. doi: 10.1046/j.1432-1033.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 88.Groot F., Geijtenbeek T.B., Sanders R.W., Baldwin C.E., Sanchez-Hernandez M., Floris R., van Kooyk Y., de Jong E.C., Berkhout B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN–gp120 interaction. J. Virol. 2005;79:3009–3015. doi: 10.1128/JVI.79.5.3009-3015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siciliano R., Bega R., Marchetti M., Seganti L., Antonini G., Valenti P. Bovine lactoferrin peptidic fragments involved in inhibition of herpes simplex virus type 1 infection. Biochem. Biophys. Res. Commun. 1999;264:19–23. doi: 10.1006/bbrc.1999.1318. [DOI] [PubMed] [Google Scholar]
- 90.Seganti L., Di Biase A.M., Marchetti M., Pietrantoni A., Tinari A., Superti F. Antiviral activity of lactoferrin towards naked viruses. BioMetals. 2004;17:295–299. doi: 10.1023/b:biom.0000027708.27142.bc. [DOI] [PubMed] [Google Scholar]
- 91.Lin T.Y., Chu C., Chiu C.H. Lactoferrin inhibits enterovirus infection of human embryonal rhabdomyosarcoma cells in vitro. J. Infect. Dis. 2002;186:161–1164. doi: 10.1086/343809. [DOI] [PubMed] [Google Scholar]
- 92.Lu L., Hangoc G., Oliff A., Chen L.T., Shen R.N., Broxmeyer H.E. Protective influence of lactoferrin on mice infected with the polycythemia-inducing strain of Friend virus complex. Cancer Res. 1987;47:4184–4188. [PubMed] [Google Scholar]
- 93.Addie D.D., Radford A., Yam P.S., Taylor D.J. Cessation of feline calicivirus shedding coincident with resolution of chronic gingivostomatitis in a cat. J. Small Anim. Pract. 2003;44:172–176. doi: 10.1111/j.1748-5827.2003.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 94.Andersen H.J., Jenssen H., Sandvik K., Gutteberg T.J. The anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulfate at the cell surface. J. Med. Virol. 2004;74:262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- 95.Berkhout B., Van Wamel J.L., Beljaars L., Meijer D.K., Visser S., Floris R. Characterization of the anti-HIV effects of native lactoferrin and other milk proteins and protein-derived peptides. Antiviral Res. 2002;55:341–355. doi: 10.1016/s0166-3542(02)00069-4. [DOI] [PubMed] [Google Scholar]
- 96.Pietrantoni A., Di Biase A.M., Tinari A., Marchetti M., Valenti P., Seganti L. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob. Agents Chemother. 2003;47:2688–2691. doi: 10.1128/AAC.47.8.2688-2691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Waarts B.L., Aneke O.J., Smit J.M., Kimata K., Bittman R., Meijerb D., Wilschut J. Antiviral activity of human lactoferrin: Inhibition of alphavirus interaction with heparan sulfate. Virology. 2005;333:284–292. doi: 10.1016/j.virol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 98.Mettenleiter T.C. Brief overview on cellular virus receptors. Virus Res. 2002;82:3–8. doi: 10.1016/s0168-1702(01)00380-x. [DOI] [PubMed] [Google Scholar]
- 99.Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie. 2001;83:811–817. doi: 10.1016/s0300-9084(01)01290-1. [DOI] [PubMed] [Google Scholar]
- 100.Mann D.M., Romm E., Migliorini M. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J. Biol. Chem. 1994;269:23661–23667. [PubMed] [Google Scholar]
- 101.Kirkpatrick C.H., Green I., Rich R.R., Schadeet L.A. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 1971;124:539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- 102.Viejo-Díaz M., Andres M.T., Fierro J.F. Modulation of in vitro fungicidal activity of human lactoferrin against Candida albicans by extra cellular cation concentration and target cell metabolic activity. Antimicrob. Agents Chemother. 2004;48:1242–1248. doi: 10.1128/AAC.48.4.1242-1248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arnold R.R., Brewer M., Gauthier J.J. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 1980;28:893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bellamy W., Wakabayashi H., Takase M., Kawase S., Shimamura S., Tomita M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993;182:97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- 105.Wakabayashi H., Abe S., Okutomi T., Tansho S., Kawase K., Yamaguchi H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 1996;40:821–825. doi: 10.1111/j.1348-0421.1996.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 106.Kuipers M.E., de Vries H.G., Eikenboom M.C., Meijer D.K., Swart P.J. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 1999;43:2635–2641. doi: 10.1128/aac.43.11.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu Y.Y., Samaranayake Y.H., Samaranayake L.P., Nikawa H. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 1999;37:35–41. doi: 10.1046/j.1365-280x.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 108.Kondori N., Baltzer L., Dolphin G.T., Mattsby-Baltzer I. Fungicidal activity of human lactoferrin-derived peptides based on the antimicrobial αβ region. Int. J. Antimicrob. Agents. 2011;37:51–57. doi: 10.1016/j.ijantimicag.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 109.Valenti P., Visca P., Antonini G., Orsi N. Interaction between lactoferrin and ovotransferrin and Candida cells. FEMS Microbiol. Lett. 1986;33:271–275. [Google Scholar]
- 110.Nikawa H., Samarayanake L.P., Tenovuo J., Pang K.M., Hamada T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral Biol. 1993;38:1057–1063. doi: 10.1016/0003-9969(93)90167-k. [DOI] [PubMed] [Google Scholar]
- 111.Nikawa H., Samarayanake L.P., Hamada T. Modulation of the anti-Candida activity of apo-lactoferrin by dietary sucrose and tunicamycin in vitro. Arch. Oral Biol. 1995;40:581–584. doi: 10.1016/0003-9969(94)00195-h. [DOI] [PubMed] [Google Scholar]
- 112.Zarember K.A., Sugui J.A., Chang Y.C., Kwon-Chung K.J., Gallin J.I. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J. Immunol. 2007;178:6367–6373. doi: 10.4049/jimmunol.178.10.6367. [DOI] [PubMed] [Google Scholar]
- 113.Lupetti A., Van Dissel J.T., Brouwer C.P.J.M., Nibbering P.H. Human antimicrobial peptides antifungal activity against Aspergillus fumigatus. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:1125–1129. doi: 10.1007/s10096-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 114.van der Kraan M.I.A., Groenink J., Nazmi K., Veerman E.C.I., Bolscher J.G.M., Amerongen A.V.N. Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides. 2004;25:177–183. doi: 10.1016/j.peptides.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Wakabayashi H., Uchida K., Yamauchi K., Teraguchi S., Hayasawa H., Yamaguchi H. Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J. Antimicrob. Chemother. 2000;46:595–601. doi: 10.1093/jac/46.4.595. [DOI] [PubMed] [Google Scholar]
- 116.Weinberg G.A. Iron chelators as therapeutic agents against Pneumocystis carinii. Antimicrob. Agents Chemother. 1994;38:997–1003. doi: 10.1128/aac.38.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cirioni O., Giacometti A., Barchiesi F., Scalise G. Inhibition of growth of Pneumocystis carinii by lactoferrins alone and in combination with pyrimethamine, clarithromycin and minocycline. J. Antimicrob. Chemother. 2000;46:577–582. doi: 10.1093/jac/46.4.577. [DOI] [PubMed] [Google Scholar]
- 118.Leon-Sicairos N., Reyes-López M., Ordaz-Pichardo C., de la Garza M. Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in Entamoeba histolytica. Biochem. Cell Biol. 2006;84:327–336. doi: 10.1139/o06-060. [DOI] [PubMed] [Google Scholar]
- 119.León-Sicartios N., López-Soto S.F., Reyes-López M., Godínez-Vargas D., Ordaz-Pichardo C., de la Garza M. Amoebicidal activity of milk, apo-lactoferrin, sIgA and lysozyme. Clin. Med. Res. 2006;4:106–113. doi: 10.3121/cmr.4.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.F. López-Soto, N. León-Sicairos, K. Nazmi, J.G. Bolscher, M. de la Garza, Microbicidal effect of the lactoferrin peptides lactoferricin 17–30, actoferrampin 265–284, and lactoferrin chimera on the parasite Entamoeba histolytica, Biometals 23 (2010) 563–568. doi:10.1007/s10534-010-9295-3. [DOI] [PubMed]
- 121.Tachezy J., Kulda J., Bahnikova I., Suchan P., Razga J., Schrevel J. Tritrichomonas foetus: iron acquisition from lactoferrin and transferrin. Exp. Parasitol. 1996;83:216–228. doi: 10.1006/expr.1996.0068. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka T., Abe Y., Inoue N., Kim W.S., Kumura H., Nagasawa H. The detection of bovine lactoferrin binding protein on Trypanosoma brucei. J. Vet. Med. Sci. 2004;66:619–625. doi: 10.1292/jvms.66.619. [DOI] [PubMed] [Google Scholar]
- 123.Katarzyna D., Bożena D., Jarosław D., Henryka D. Toxoplasma gondii: inhibition of the intracellular growth by human lactoferrin. Pol. J. Microbiol. 2007;56:25–32. [PubMed] [Google Scholar]
- 124.Omata Y., Satake M., Maeda R., Saito A., Shimazaki K., Yamauchi K., Uzuka Y., Tanabe S., Sarashina T., Mikami T. Reduction of the infectivity of Toxoplasma gondii and Eimeria stiedai sporozoites by treatment with bovine lactoferricin. J. Vet. Med. Sci. 2001;63:187–190. doi: 10.1292/jvms.63.187. [DOI] [PubMed] [Google Scholar]
- 125.Botteon P., Massard C., Botteon R. Seroprevalence of Babesia equi in three breeding systems of equines. Parasitol. Latinoam. (Bras) 2002;57:141–145. [Google Scholar]
- 126.Ikada H., Tanaka T., Shibahara N. Short report: inhibitory effect of lactoferrin on in vitro growth of Babesia caballi. Am. J. Trop. Med. Hyg. 2005;73:710–712. [PubMed] [Google Scholar]
- 127.Legrand D., Elass E., Carpentier M., Mazurier J. Interaction of lactoferrin with cells involved in immune function. Biochem. Cell Biol. 2006;84:282–290. doi: 10.1139/o06-045. [DOI] [PubMed] [Google Scholar]
- 128.Breton-Gorius J., Mason D., Buriot D., Vilde J.-L., Griscelli C. Lactoferrin deficiency as a consequence of a lack of specific granules in neutrophils from a patient with recurrent infections. Detection by immunoperoxidase staining for lactoferrin and cytochemical electron microscopy. Am. J. Pathol. 1980;99:413–428. [PMC free article] [PubMed] [Google Scholar]
- 129.Sfeir R.M., Dubarry M., Boyaka P.N., Rautureau M., Tome D. The mode of oral bovine lactoferrin administration influences mucosal and systemic immune responses in mice. J. Nutr. 2004;134:403–409. doi: 10.1093/jn/134.2.403. [DOI] [PubMed] [Google Scholar]
- 130.Wakabayashi H., Takakura N., Yamauchi K., Yoshitaka T. Modulation of immune-related gene expression in small intestine of mice by oral administration of lactoferrin. Clin. Vaccine Immunol. 2006;13:239–245. doi: 10.1128/CVI.13.2.239-245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kruzel M.L., Harari Y., Mailman D., Actor J.K., Zimecki M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002;130:25–31. doi: 10.1046/j.1365-2249.2002.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kruzel M.L., Bacsi A., Choudhury B., Sur S., Boldogh I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology. 2006;119:159–166. doi: 10.1111/j.1365-2567.2006.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Legrand D., Elass E., Carpentier M., Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol. Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kane S.V., Sandborn W.J., Rufo P.A., Zholudev A., Boone J., Lyerly D., Camilleri M., Hanauer S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 135.Baker E.N., Baker H.M. Lactoferrin molecular structure, binding properties and dynamics of lactoferrin. Cell Mol. Life Sci. 2005;62:2531–2539. doi: 10.1007/s00018-005-5368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bennett R.M., Davis J. Lactoferrin interacts with deoxyribonucleic acid: a preferential reactivity with double-stranded DNA and dissociation of DNA–anti-DNA complex. J. Lab. Clin. Med. 1982;99:127–138. [PubMed] [Google Scholar]
- 137.Sorimachi K., Akimoto K., Hattori Y., Ieiri T., Niwa A. Activation of macrophages by lactoferrin: secretion of TNF-alpha, IL-8 and NO. Biochem. Mol. Biol. Int. 1997;43:79–87. doi: 10.1080/15216549700203841. [DOI] [PubMed] [Google Scholar]
- 138.Zimecki M., Wlaszczyk A., Wojciechowski R., Dawiskiba J., Kruzel M. Lactoferrin regulates the immune responses in post-surgical patients. Arch. Immunol. Ther. Exp. 2001;49:325–333. [PubMed] [Google Scholar]
- 139.Machnicki M., Zimecki M., Zagulski T. Lactoferrin regulates the release of tumor necrosis factor alpha and interleukin in vivo. Int. J. Exp. Pathol. 1993;74:433–439. [PMC free article] [PubMed] [Google Scholar]
- 140.Zimecki M., Spiegel K., Wlaszczyk A., Kubler A., Kruzel M.L. Lactoferrin increases the output of neutrophil precursors and attenuates the spontaneous production of TNF-alpha and IL-6 by peripheral blood cells. Arch. Immunol. Ther. Exp. (Warsz) 1999;47:113–118. [PubMed] [Google Scholar]
- 141.Zimecki M., Dawiskiba J., Zawirska B., Krawczyk Z., Kruzel M. Bovine lactoferrin decreases histopathological changes in the liver and regulates cytokine production by splenocytes of obstructive jaundiced rats. Inflamm. Res. 2003;52:305–310. doi: 10.1007/s00011-003-1178-4. [DOI] [PubMed] [Google Scholar]
- 142.Haversen L., Ohlsson B.G., Hahn-Zoric M., Hanson L.A., Mattsby-Baltzer I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell. Immunol. 2002;220:83–95. doi: 10.1016/s0008-8749(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 143.Shimizu K., Matsuzawa H., Okada K., Tazume S., Dosako S., Kawasaki Y., Hashimoto K., Koga Y. Lactoferrin-mediated protection of the host from murine cytomegalovirus infection by T-cell-dependent augmentation of natural killer cell activity. Arch. Virol. 1996;141:1875–1889. doi: 10.1007/BF01718201. [DOI] [PubMed] [Google Scholar]
- 144.Kurose I., Yamada T., Wolf R., Granger D.N. P-selectin-dependent leukocyte recruitment and intestinal mucosal injury induced by lactoferrin. J. Leuckoc. Biol. 1994;55:771–777. doi: 10.1002/jlb.55.6.771. [DOI] [PubMed] [Google Scholar]
- 145.Szuter C.A., Kaminska T., Kandefer S.M. Phagocytosis-enhancing effect of lactoferrin on bovine peripheral blood monocytes in vitro and in vivo. Arch. Vet. 1995;35:63–71. [PubMed] [Google Scholar]
- 146.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 147.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 148.Bezault J., Bhimani R., Wiprovnick J., Furmanski P. Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res. 1994;54:2310–2312. [PubMed] [Google Scholar]
- 149.Varadhachary A., Wolf J.S., Petrak K., O'Malley B.W., Jr., Spadaro M., Curcio C., Forni G., Pericle F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer. 2004;111:398–403. doi: 10.1002/ijc.20271. [DOI] [PubMed] [Google Scholar]
- 150.Hayes T.G., Falchook G.F., Varadhachary G.R., Smith D.P., Davis L.D., Dhingra H.M., Hayes B.P., Varadhachary A. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest. New Drugs. 2006;24:233–240. doi: 10.1007/s10637-005-3690-6. [DOI] [PubMed] [Google Scholar]
- 151.Iigo M., Shimamura M., Matsuda E., Fujita K., Nomoto H., Satoh J., Kojima S., Moore M.A., Tsuda H. Orally administered bovine lactoferrin induces caspase-1 and interleukin-18 in the mouse intestinal mucosa: a possible explanation for inhibition of carcinogenesis and metastasis. Cytokine. 2004;25:36–44. doi: 10.1016/j.cyto.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 152.Crouch S.P.M., Slate K.J., Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992;80:235–240. [PubMed] [Google Scholar]
- 153.Wang W.P., Ligo M., Sato J., Sekine K., Adachi I., Tsuda H. Activation of mucosal intestinal immunity in tumor-bearing mice by lactoferrin. J. Cancer Res. (Jpn) 2000;91:1022–1027. doi: 10.1111/j.1349-7006.2000.tb00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shimamura M., Yamamoto Y., Ashino H., Oikawa T., Hazato T., Tsuda H., Ligo M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer. 2004;111:111–116. doi: 10.1002/ijc.20187. [DOI] [PubMed] [Google Scholar]
- 155.Norrby K., Mattsby-Baltzer I., Innocenti M., Tuneberg S. Orally administered bovine lactoferrin systemically inhibits VEGF-mediated angiogenesis in the rat. Int. J. Cancer. 2001;91:236–240. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1024>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 156.Shimamura M., Yamamoto Y., Ashino H., Oikawa T., Hazato T., Tsuda H., Iigo M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer. 2004;111:111–116. doi: 10.1002/ijc.20187. [DOI] [PubMed] [Google Scholar]
- 157.Kozu T., Iinuma G., Ohashi Y., Saito Y., Akasu T., Saito D., Alexander D.B., Iigo M., Kakizoe T., Tsuda H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial Takahiro Kozu. Cancer Prev Res. 2009;2:975–983. doi: 10.1158/1940-6207.CAPR-08-0208. [DOI] [PubMed] [Google Scholar]
- 158.Nakajima K., Kanno Y., Nakamura M., Gao X.D., Kawamura A., Itoh F., Ishisaki A. Bovine milk lactoferrin induces synthesis of the angiogenic factors VEGF and FGF2 in osteoblasts via the p44/p42 MAP kinase pathway. BioMetals. 2011;23:1–10. doi: 10.1007/s10534-011-9439-0. [DOI] [PubMed] [Google Scholar]
- 159.Xu X.X., Jiang H.R., Li H.B., Zhang T.N., Zhou Q., Liu N. Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin. J. Dairy Sci. 2010;93:2344–2350. doi: 10.3168/jds.2009-2926. [DOI] [PubMed] [Google Scholar]
- 160.Damiens E., Yazidi I., Mazurier J., Duthile I., Spik G., Boilly-Marer Y. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J. Cell. Biochem. 1999;74:486–498. [PubMed] [Google Scholar]
- 161.Zemann N., Klein P., Wetzel E., Huettinger F., Huettinger M. Lactoferrin induces growth arrest and nuclear accumulation of Smad-2 in HeLa cells. Biochimie. 2010;92:880–884. doi: 10.1016/j.biochi.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 162.Yoo Y.C., Watanabe R., Koike Y., Mitobe M., Shimazaki K., Watanabe S., Azuma I. Apoptosis in human leukemic cells induced by lactoferricin, a bovine milk protein-derived peptide: involvement of reactive oxygen species. Biochem. Biophys. Res. Commun. 1997;237:624–628. doi: 10.1006/bbrc.1997.7199. [DOI] [PubMed] [Google Scholar]
- 163.Ramaswamy H., Swamy C.V., Das M.R. Purification and characterization of a high molecular weight ribonuclease from human milk. J. Biol. Chem. 1993;268:4181–4187. [PubMed] [Google Scholar]
- 164.Bennett R.M., Merrit M.M., Gabor G. Lactoferrin binds to neutrophilic membrane DNA. Br. J. Haematol. 1986;63:105–117. doi: 10.1111/j.1365-2141.1986.tb07500.x. [DOI] [PubMed] [Google Scholar]
- 165.He J., Furmanski P. Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA. Nature. 1995;373:721–724. doi: 10.1038/373721a0. [DOI] [PubMed] [Google Scholar]
- 166.Brandl N., Zemann A., Kaupe I., Marlovits S., Huettinger P., Goldenberg H., Huettinger M. Signal transduction and metabolism in chondrocytes is modulated by lactoferrin. Osteoarthr. Cartil. 2010;18:117–125. doi: 10.1016/j.joca.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 167.Kanyshkova T.G., Babina S.E., Semenov D.V., Isaeva N., Vlassov A.V., Neustroev K.N., Kulminskaya A.A., Buneva V.N., Nevinsky G.A. Multiple enzymatic activities of human milk lactoferrin. Eur. J. Biochem. 2003;270:3353–3361. doi: 10.1046/j.1432-1033.2003.03715.x. [DOI] [PubMed] [Google Scholar]
- 168.Devy A.S., Das M.R., Pandir M.W. Lactoferrin contains structural motifs of ribonuclease. Biochem. Biophys. Acta. 1994;114:299–306. doi: 10.1016/0167-4838(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 169.Furmanski P., Li Z.P., Fortuna M.B. Multiple molecular forms of human lactoferrin. J. Exp. Med. 1989;170:415–429. doi: 10.1084/jem.170.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bellamy W., Takase M., Wakabayashi H. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992;73:472–479. doi: 10.1111/j.1365-2672.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- 171.Van Der Kraan M.I., Nazmi K., Vant Hof W. Distinct bactericidal activities of bovine lactoferrin peptides LFampin 268–284 and Lfampin 265–284; Asp-Leu-Ile makes a difference. Biochem. Cell Biol. 2006;84:358–362. doi: 10.1139/o06-042. [DOI] [PubMed] [Google Scholar]
- 172.Gifford J.L., Hunter H.N., Vogel H.J. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Teng C.T., Pentecost B.T., Marshall A., Solomon A., Bowman B.H., Lalley P.A., Naylor S.L. Assignment of the lactotransferrin gene to human chromosome 3 and to mouse chromosome 9. Somat. Cell Mol. Genet. 1997;13:689–693. doi: 10.1007/BF01534490. [DOI] [PubMed] [Google Scholar]
- 174.Teng C.T., Beard C., Gladwell W. Differential expression and estrogen response of lactoferrin gene in the female reproductive tract of mouse, rat, and hamster. Biol. Reprod. 2002;67:1439–1449. doi: 10.1095/biolreprod.101.002089. [DOI] [PubMed] [Google Scholar]
- 175.Teng C.T. Lactoferrin gene expression and regulation: an overview. Biochem. Cell Biol. 2002;80:7–16. doi: 10.1139/o01-215. [DOI] [PubMed] [Google Scholar]
- 176.Green M.R., Pastewka J.V. Lactoferrin is a marker for prolactin response in mouse mammary explants. Endocrinology. 1978;103:1510–1513. doi: 10.1210/endo-103-4-1510. [DOI] [PubMed] [Google Scholar]
- 177.Masson P.L., Heremans J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. Biol. 1971;39:119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- 178.Masson P.L., Heremans J.F., Dive C. An iron-binding protein common to many external secretions. Clin. Chim. Acta. 1966;14:735–739. [Google Scholar]
- 179.Kang J.F., Li X.L., Zhou R.Y. Bioinformatics analysis of lactoferrin gene for several species. Biochem. Genet. 2008;46:312–322. doi: 10.1007/s10528-008-9147-9. [DOI] [PubMed] [Google Scholar]
- 180.Liu D., Yang N., Teng C.T. COUP-TF acts as a competitive repressor for estrogen receptor-mediated activation of the mouse lactoferrine gene. Mol. Cell. Biol. 1993;13:1836–1846. doi: 10.1128/mcb.13.3.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park S.E., Xu J., Frolova A., Liao L., O'Malley B.W., Katzenellenboge B.S. Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo. Mol. Cell. Biol. 2005;25:1989–1999. doi: 10.1128/MCB.25.5.1989-1999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Geng K., Li Y., Bezault J., Furmanskit P. Induction of lactoferrin expression in marine cells by retinoic and estrogen. Exp. Cell Res. 1998;254:214–220. doi: 10.1006/excr.1998.4266. [DOI] [PubMed] [Google Scholar]
- 183.Teng C.T. Factors regulating lactoferrin gene expression. Biochem. Cell Biol. 2006;84:263–267. doi: 10.1139/o06-034. [DOI] [PubMed] [Google Scholar]
- 184.Ward P.P., Paz E., Conneely O.M. Multifunctional roles of lactoferrin: a critical overview. Cell. Mol. Life Sci. 2005;62:2540–2548. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wakabayashi H., Yamauchi K., Takase M. Lactoferrin research, technology and applications. Int. Dairy J. 2006;16:1241–1251. [Google Scholar]
- 186.Manzoni P., Rinaldi M., Cattani S. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. J. Am. Med. Assoc. 2009;302:1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 187.Saarinen U.M., Siimes M.A. Iron absorption from infant milk formula and the optimal level of iron supplementation. Acta Paediatr. Scand. 1977;66:719–722. doi: 10.1111/j.1651-2227.1977.tb07978.x. [DOI] [PubMed] [Google Scholar]
- 188.Siimes M.A., Salmenperä L., Perheentupa J. Exclusive breast-feeding for 9 months: risk of iron deficiency. J. Pediatr. 1984;104:196–199. doi: 10.1016/s0022-3476(84)80991-9. [DOI] [PubMed] [Google Scholar]
- 189.Wang Y.Z., Shan T.Z., Xu Z.R., Liu J., Feng J. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed. Sci. Technol. 2006;135:263–272. [Google Scholar]
- 190.Tursi A., Elisei W., Brandimarte G., Glorgetti G., Modeo E., Aiello F. Effect of lactoferrin supplementation on the effectiveness and tolerability of a 7-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med. Sci. Monit. 2007;13:187–190. [PubMed] [Google Scholar]
- 191.Naidu A.S., Chen J., Martinez C., Tulpinski J., Pal B., Fowler R.S. Activated lactoferrin's ability to inhibit Candida growth and block yeast adhesion to the vaginal epithelial monolayer. J. Reprod. Med. 2004;49:859–866. [PubMed] [Google Scholar]
- 192.Kaito M., Iwasa M., Fujita N., Kobayashi Y., Kojima Y., Ikoma J., Imoto I., Adachi Y., Hamano H., Yamauchi K. Effect of lactoferrin in patients with chronic hepatitis C: combination therapy with interferon and ribavirin. J. Gastroenterol. Hepatol. 2007;22:1894–1897. doi: 10.1111/j.1440-1746.2007.04858.x. [DOI] [PubMed] [Google Scholar]
- 193.Wilk K.M., Hwang S.A., Actor J.K. Lactoferrin modulation of antigen-presenting-cell response to BCG infection. Postepy. Hig. Med. Dosw. 2007;61:277–282. [PMC free article] [PubMed] [Google Scholar]
- 194.Umuhumuza L.C., Wei-min N., Sun X. Effect of bovine lactoferrin and casein peptide powder on microbial growth and glucose utilization by microorganisms in pork meat during storage at 4 °C. Pak. J. Nutr. 2011;10:208–213. [Google Scholar]
- 195.Naidu A.S. Activated lactoferrin—a new approach to meat safety. Food Technol. 2002;56:40–45. [Google Scholar]
- 196.Medina I., Tombo I., Satue-Gracia M.T., German J.B., Frankel E.N. Effects of natural phenolic compounds on the antioxidant activity of lactoferrin in liposomes and oil-in-water emulsions. J. Agric. Food Chem. 2002;50:2392–2399. doi: 10.1021/jf011126y. [DOI] [PubMed] [Google Scholar]
- 197.Pann Y., Sonn G.A., Sin M.L.Y., Mach K.E., Shih M., Gau V., Wong P.K., Liao J.C. Electrochemical immunosensor detection of urinary lactoferrin in clinical samples for urinary tract infection diagnosis. Biosens. Bioelectron. 2010;26:649–654. doi: 10.1016/j.bios.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reghunathan R., Jayapal M., Hsu Y., Chng H.H., Tai D., Leung B.P., Melendez A.J. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. 2005;6:2. doi: 10.1186/1471-2172-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tomita M., Wakabayashi H., Yamauchi K., Teraguchi S., Hayasawa H. Bovine lactoferrin and lactoferricina derived from milk: production and applications. Biochem. Cell Biol. 2002;80:109–112. doi: 10.1139/o01-230. [DOI] [PubMed] [Google Scholar]
- 200.Nuijens J.H., Van Berkel P.H.C., Schanbacher F.L. Structure and iologicalactions of lactoferrin. J. Mammary Gland Biol. Neoplasia. 1996;1:285–295. doi: 10.1007/BF02018081. [DOI] [PubMed] [Google Scholar]
- 201.Tian Z.G., Teng D., Yang Y.L., Luo J., Feng X.J., Ying F., Zhang F., Wang J.H. Multimerization and fusion expression of bovine lactoferrin derivative Lfcin B15-W4, 10 in Escherichia coli. Appl. Microbiol. Biotechnol. 2007;75:117–124. doi: 10.1007/s00253-006-0806-7. [DOI] [PubMed] [Google Scholar]
- 202.Kim H.K., Chun D.S., Kim J.S., Yun C.H., Lee J.H., Hong S.K., Kang D.K. Expression of the cationic antimicrobial peptide lactoferricin fussed with the anionic peptide in Escherichia coli. Appl. Microbiol. Biotechnol. 2006;72:330–338. doi: 10.1007/s00253-005-0266-5. [DOI] [PubMed] [Google Scholar]
- 203.Feng X.J., Wang J.H., Shan A.S., Teng D., Yang Y.L., Yao Y., Yang G.P., Shao Y.C., Liu S., Zhang F. Fusion expression of bovine lactoferricin in Escherichia coli. Protein Expr. Purif. 2006;47:110–117. doi: 10.1016/j.pep.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 204.Wang J., Tian Z., Teng D., Yang Y., Hu J., Wang J. Cloning, expression and characterization of Kunming mice lactoferrin and its N-lobe. BioMetals. 2010;23:523–530. doi: 10.1007/s10534-010-9294-4. [DOI] [PubMed] [Google Scholar]
- 205.Chen H.L., Lai Y.W., Chen C.S., Chu T.W., Lin W., Yen C.C., Lin M.F., Tu M.Y., Chen C.M. Probiotic Lactobacillus casei expressing human lactoferrin elevates antibacterial activity in the gastrointestinal tract. BioMetals. 2010;23:543–554. doi: 10.1007/s10534-010-9298-0. [DOI] [PubMed] [Google Scholar]
- 206.Kim W.S., Shimazaki K.I., Tamura T. Expression of bovine lactoferrin C-lobe in Rhodococcus erythropolis and its purification and characterization. Biosci. Biotechnol. Biochem. 2006;70:2641–2645. doi: 10.1271/bbb.60245. [DOI] [PubMed] [Google Scholar]
- 207.Ying G., Wu S.H., Wang J., Zhao X.D., Chen J.M., Zhang X.G. Producing human lactoferrin by high density fermentation recombinant Pichia pastoris. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2004;18:181–185. [PubMed] [Google Scholar]
- 208.Chen G.H., Yin L.J., Chiang I.H., Jiang S.T. Expression and purification of goat lactoferrin from Pichia pastoris expression system. J. Food Sci. 2007;72:M67–71. doi: 10.1111/j.1750-3841.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 209.Wang S.H., Yang T.S., Lin S.M., Tsai M.S., Wu S.C., Mao S. Expression, characterization and purification of recombinant porcine lactoferrin in Pichia pastoris. Protein Expr. Purif. 2002;25:41–49. doi: 10.1006/prep.2001.1607. [DOI] [PubMed] [Google Scholar]
- 210.Dong Z.Y., Zhang Y.Z. Molecular cloning and expression of yak (Bos grunniens) lactoferrin cDNA in Pichia pastoris. Biotechnol. Lett. 2006;28:1285–1292. doi: 10.1007/s10529-006-9092-9. [DOI] [PubMed] [Google Scholar]
- 211.Paramasivam M., Saravanan K., Uma K., Sharma S., Singh T.P., Srinivasan A. Expression, purification and characterization of equine lactoferrin in Pichia pastoris. Protein Expr. Purif. 2002;26:28–34. doi: 10.1016/s1046-5928(02)00528-4. [DOI] [PubMed] [Google Scholar]
- 212.Jiang T., Chen L., Jia S., Chen L., Ma Y. High-level expression and production of human lactoferrin in Pichia pastoris. Dairy Sci. Technol. 2008;88:173–181. [Google Scholar]
- 213.Shan T.Z., Wang Y.Z., Liu G.F., Huang H.Q. Expression of recombinant porcine lactoferrin N lobe in Pichia methanolica and its antibacterial activity. Biochemistry. 2008;72:173–181. [Google Scholar]
- 214.Wang H.K., Zhao X.H., Lu F.P. Heterologous expression of bovine lactoferricin in Pichia mehtanolica. Biochemistry. 2007;72:640–643. doi: 10.1134/s0006297907060065. [DOI] [PubMed] [Google Scholar]
- 215.Ward P.P., Piddington C.S., Cunningham G., Zhou X., Wyat R., Conneely O. A system for production of commercial quantities of human lactoferrin: a broad spectrum natural antibiotic. Nat. Biotechnol. 1995;13:498–503. doi: 10.1038/nbt0595-498. [DOI] [PubMed] [Google Scholar]
- 216.Wad P.P., Lo J.Y., Duke M., May G., Headon D., Conneely O. Production of biologically active recombinant human lactoferrin in Aspergillus oryzae. Nat. Biotechnol. 1992;10:784–789. doi: 10.1038/nbt0792-784. [DOI] [PubMed] [Google Scholar]
- 217.Zhang D.B., Jiang Y.L., Wu X.F., Hong M.M. Expression of human lactoferrin cDNA in insect cells. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shangai) 1998;30:575–578. [PubMed] [Google Scholar]
- 218.Liu T., Zhang Y.Z., Wu X.F. High level expression of functionally active human lactoferrin in silkworm larvae. J. Biotechnol. 2005;118:245–256. doi: 10.1016/j.jbiotec.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 219.Liu T., Zhang Y.Z., Wu X.F. Recombinant functional human lactoferrin expressed in baculovirus system. Acta Biochim. Biophys. Sin. 2006;38:201–206. doi: 10.1111/j.1745-7270.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 220.Wang Y., Wu X., Liu G., Cao C., Huang H., Xu Z., Liu J. Expression of porcine lactoferrin by using recombinant baculovirus in silkworm, Bombyx mori L. and its purification and characterization. Appl. Microbiol. Biotechnol. 2005;69:385–389. doi: 10.1007/s00253-005-1998-y. [DOI] [PubMed] [Google Scholar]
- 221.Stowell K.M., Rado T.A., Funk W.D., Tweedie J.W. Expression of cloned human lactoferrin in baby-hamster kidney cells. Biochem. J. 1991;276:349–355. doi: 10.1042/bj2760349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tutykhina I.L., Bezborodova O.A., Verkhovskaya L.V., Shmarov M.M., Logunov D.Y., Nemtsova E.R., Naroditskii B.S., Yakubovskaya R.I., Ginzburg A.I. Recombinant pseudoadenovirus nanostructure with the human lactoferrin gene: production and study of lactoferrin expression and properties in vivo. Mol. Gen. Microbiol. Virol. 2009;24:32–36. [PubMed] [Google Scholar]
- 223.Zhang J., Li L., Cai Y., Xu X., Chen J., Wu Y., Yu H., Yu G., Zhang A., Chen J., Cheng G. Expression of active recombinant human lactoferrin in the milk of transgenic goats. Protein Expr. Purif. 2008;57:127–135. doi: 10.1016/j.pep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 224.Han Z.S., Li Q.W., Zhang Z.Y., Xiao B., Gao D.W., Wu S.Y., Zhao H.W., Jiang Z.L., Hu J.H. High-level expression of human lactoferrin in the milk of goats by using replication-defective adenoviral vectors. Protein Expr. Purif. 2007;53:225–231. doi: 10.1016/j.pep.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 225.Nuijens J.H., Van Berkel P.H., Geerts M.E.J., Hartevelt P.P., Boer H.A., van Veen H., Pieper F.R. Characterization of recombinant human lactoferrin secrete in milk of transgenic mice. J. Biol. Chem. 1997;272:8802–8807. doi: 10.1074/jbc.272.13.8802. [DOI] [PubMed] [Google Scholar]
- 226.Kim S.J., Lee K.W., Yu D.Y., Han Y.M., Lee C.S., Nam M.S., Moon H.B., Lee K.K. Expression analysis of a bovine B-casein/human lactoferrin hybrid gene in transgenic mice. J. Reprod. Dev. 1997;43:143–149. doi: 10.1262/jrd.43.143. [DOI] [Google Scholar]
- 227.Han Z.S., Li Q.W., Zhang Z.Y., Yu Y.S., Xiao B., Wu S.Y., Jiang Z.L., Zhao H.W., Zhao R., Li J. Adenoviral vector mediates high expression levels of human lactoferrin in the milk of rabbits. J. Microbiol. Biotechnol. 2008;18:153–159. [PubMed] [Google Scholar]
- 228.Van Berkel P., Welling M.M., Geerts M., Van Veen H., Ravensbergen B., Salaheddine M., Pauwels E., Pieper F., Nuijens J.H., Nibbering P.H. Large scale production of recombinant human lactoferrin in the milk of transgenic cows. Nat. Biotechnol. 2002;20:484–487. doi: 10.1038/nbt0502-484. [DOI] [PubMed] [Google Scholar]
- 229.Hyvӧnen P., Suojala L., Orro T., Haaranen J., Simola O., RØntved C., Pyӧrӓlӓ S. Transgenic cows that produce recombinant human lactoferrin in milk are not protected from experimental Escherichia coli intramammary infection. Infect. Immun. 2006;74:6206–6212. doi: 10.1128/IAI.00238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li Y., Geng Y., Song H., Zheng G., Huan L., Qiu B. Expression of human lactoferrin N-lobe in Nicotiana benthmiana with potato virus x-based agroinfection. Biotechnol. Lett. 2004;26:953–957. doi: 10.1023/b:bile.0000030038.27358.20. [DOI] [PubMed] [Google Scholar]
- 231.Mitra A., Zhang Z. Expression of a human lactoferrin cDNA in tobacco cells produces antibacterial proteins. Plant Physiol. 1994;106:977–981. doi: 10.1104/pp.106.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang Z., Coyne D., Vidaver A., Mitra A. Expression of human lactoferrin cDNA confers resistance to Ralstonia sdanacearum in transgenic tobacco plants. Phytopathology. 1998;88:730–734. doi: 10.1094/PHYTO.1998.88.7.730. [DOI] [PubMed] [Google Scholar]
- 233.Choi S.M., Lee O.S., Kwon S.Y., Kwak S.S., Yu D.Y., Lee H.S. High expression of a human lactoferrin in transgenic tobacco cell cultures. Biotechnol. Lett. 2003;25:213–218. doi: 10.1023/a:1022341917735. [DOI] [PubMed] [Google Scholar]
- 234.Nguyen T.C., Lakshaan D.K., Han J., Galvez L., Mitra A. Transgenic plants expressing antimicrobial lactoferrin protein are resistant to a fungal pathogen. J. Plant Mol. Biol. Biotechnol. 2011;2:1–8. [Google Scholar]
- 235.Rachmawati D., Mori T., Hosaka T., Takaiwa F., Inove E., Anzai H. Expression and localization of recombinant human lactoferrin in transgenic Javanica rice cv rojolele. Milk Sci. 2004;53:247–249. [Google Scholar]
- 236.Nandi S., Yalda D., Lu S., Nikolv Z., Misaki R., Fujiyama K., Huang N. Process development and economic evaluation of recombinant human lactoferrin expressed in rice grain. Transgenic Res. 2005;14:237–249. doi: 10.1007/s11248-004-8120-6. [DOI] [PubMed] [Google Scholar]
- 237.Suzuki Y., Kelleher S., Yalda D., Wu L., Huang J., Huang N., Lӧnnerdal B. Expression, characterization, and biologic activity of recombinant human lactoferrin in rice. J. Pediatr. Gastroenerol. Nutr. 2003;36:190–199. doi: 10.1097/00005176-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 238.Chong D., Lanagridge W. Expression of full-length bioactive antimicrobial human lactoferrin in potato plants. Transgenic Res. 2000;9:71–78. doi: 10.1023/a:1008977630179. [DOI] [PubMed] [Google Scholar]
- 239.Conesa C., Calvo M., Sánchez L. Recombinant human lactoferrin: a valuable protein for pharmaceutical products and functional foods. Biotechnol. Advan. 2010;28:831–838. doi: 10.1016/j.biotechadv.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 240.Min S.R., Woo J.W., Jeong W.J., Han S.K., Lee Y.B., Liu J.R. Production of human lactoferrin in transgenic cell suspension cultures of sweet potato. Biol. Plant. 2006;50:131–134. [Google Scholar]
- 241.Kwon S.Y., Jo S.H., Lee O.S., Choi S.M., Kwak S.S., Lee H.S. Transgenic ginseng cell lines that produce high levels of a human lactoferrin. Planta Med. 2003;69:1005–1008. doi: 10.1055/s-2003-45146. [DOI] [PubMed] [Google Scholar]
- 242.Lee T.J., Coyne P.P., Clement T.E., Mitra A. Partial resistance to bacterial wilt in transgenic tomato plants expressing antibacterial lactoferrin gene. J. Am. Soc. Hortic. Sci. 2002;127:158–168. [Google Scholar]
- 243.Samyn-Petit B., Gruber V., Flahaut C., Wajder-Dubos J.P., Ferrer S., Pons A., Desmazieres G., Slomianny M.C., Theisen M., Delannoy P. N-Glycosylation potential of maize: the human lactoferrin used as a model. Glycoconj. J. 2001;18:519–527. doi: 10.1023/a:1019640312730. [DOI] [PubMed] [Google Scholar]
- 244.Vlahova M., Stefannova G., Petkov P., Barbulova A., Petkova D., Kalushkov P., Atanassov A. Genetic modification of alfalfa (Medicago sativa L.) for quality improvement and production of novel compounds. Biotechnol. Biotechnol. Equip. 2005;19:56–62. [Google Scholar]
- 245.Tanasienko I.V., Yemets A.I., Pirko Y.V., Korhkovyy V.I., Abumhadi N., Blume Y.B. Generation of transgenic barley lines producing human lactoferrin using mutant alpha-tubulin gene as the selective marker. Cytol. Genet. 2005;45:1–6. doi: 10.3103/S0095452711010026. [DOI] [Google Scholar]