Abstract
Currently in China, porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), porcine rotavirus (PoRV), and porcine deltacoronavirus (PDCoV) are the major causes of porcine viral diarrhea, and mixed infections in clinics are common, resulting in significant economic losses in pig industry. Here, a dual priming oligonucleotide (DPO)-based multiplex real-time SYBR Green RT-PCR assay were developed for accurately differentiating PEDV, TGEV, PoRV, and PDCoV in clinical specimens targeting the N gene of TGEV, PEDV, and PDCoV, and the VP7 gene of PoRV. Results showed that the DPO primer allowed a wider annealing temperature range (40–65 °C) and had a higher priming specificity compared to conventional primer, in which more than 3 nucleotides in the 3′- or 5′-segment of DPO primer mismatched with DNA template, PCR amplification efficiency would decrease substantially or extension would not proceed. DPO-based multiplex real-time RT-PCR method had analytical detection limit of 8.63 × 102 copies/μL, 1.92 × 102 copies/μL, 1.74 × 102 copies/μL, and 1.76 × 102 copies/μL for PEDV, TGEV, PoRV, and PDCoV in clinical specimens, respectively. A total of 672 clinical specimens of piglets with diarrheal symptoms were collected in Northeastern China from 2017 to 2018 followed by analysis using the assay, and epidemiological investigation results showed that PEDV, TGEV, PoRV, and PDCoV prevalence was 19.05%, 5.21%, 4.32%, and 3.87%, respectively. The assay developed in this study showed higher detection accuracy than conventional RT-PCR method, suggesting a useful tool for the accurate differentiation of the four major viruses causing porcine viral diarrhea in practice.
Keywords: Porcine viral diarrhea, Dual priming oligonucleotide (DPO), Real-time RT-PCR assay, Accurate differentiation
Highlights
-
•
DPO-based real-time RT-PCR assay for differentiating PEDV, TGEV, PoRV, and PDCoV was developed.
-
•
The assay has strong specificity and high sensitivity.
-
•
The test of clinical specimens showed that accuracy of the assay was higher than traditional RT-PCR.
-
•
The assay is useful tool for epidemiological investigation of the four viruses.
1. Introduction
Currently, porcine viral diarrhea is a major cause of high morbidity and high mortality in piglets and an important threat to the development of pig industry worldwide, resulting in significant economic losses [1]. Porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), and porcine rotavirus (PoRV) are the most common viral agents causing piglet diarrhea [2]. Moreover, porcine deltacoronavirus (PDCoV), since first reported in 2009, has become an important cause of newborn piglet diarrhea [[3], [4], [5], [6], [7], [8], [9]]. Sometimes, mixed infections in clinics are frequent. TGEV is a common cause of epidemic and endemic viral enteritis in neonates and senile pigs [10,11]. Like TGEV, PEDV can cause severe enteritis in newborn piglets, but has higher mortality rate that may reach 100% during epidemics [12,13]. PDCoV, as a newly emerging porcine intestinal coronavirus, can also cause enteritis and severe diarrhea in piglets [9], leading to numerous economic losses for farmers in China. Its infection appears to be clinically milder with lower neonatal mortality rate of 30–40% compared to typical PEDV infection. PoRV is also a common cause of viral diarrhea diseases among newborn piglets [14,15]. Infection with any of these viruses develops into similar clinical symptoms, including severe diarrhea and dehydration, and in some cases, mixed infections of these pathogens are common; therefore, it is difficult to distinguish them clinically [4,[16], [17], [18]]. Thus, it is of great significance to develop a useful method for rapid and accurate differentiation of these viruses causing viral diarrhea in piglets.
Although virus isolation, immunofluorescence assay, and enzyme-linked immunosorbent assays are standard diagnostic methods for viruses [19], these techniques are time-consuming, relatively low in specificity and sensitivity, and therefore are not suitable for early diagnosis or epidemiological investigation of enterovirus-associated diseases. Currently, polymerase chain reaction (PCR)-based methods have been proven convenient and highly sensitive for detecting porcine diarrhea-associated viruses [20,21]. Of them real-time SYBR Green PCR method that combines conventional PCR and fluorescent signal detection with advantages of high sensitivity, good repeatability, and intuitive results is widely used for epidemiological investigation and pathogen identification [[22], [23], [24]]. Moreover, the closed-tube detection of SYBR Green-based real-time PCR assay can effectively prevent aerosol pollution and reduce the incidence of false positives. However, the specificity of SYBR Green fluorescent dye is not so good that the melting curve of PCR product has to be used to confirm the amplification specificity. Therefore, primer design is the key to determining the detection specificity of SYBR Green-based real-time PCR method. However, the fundamental solution for eliminating false positive amplification still remains a challenge, which requires repeated optimization of primer parameters to achieve a high specificity, including primer specificity, primer length, melting temperature, GC content, less secondary structure, and PCR annealing temperature, in particular the development of a multiplex PCR assay.
Dual priming oligonucleotide (DPO) system, with a long 5′-segment, a short 3′-segment, and polydeoxyinosine (poly I) linker bridging 5′-and 3′-segments, is an approach to designing PCR primer first reported by Chun et al. [25], which can effectively eliminate non-specific priming without disrupting amplification of target sequences, showing higher specificity than conventional primers [[25], [26], [27], [28]]. Moreover, compared with conventional primer, the design process of DPO system is simpler and does not require repeated optimization of primer parameters. The position of 3′-segment is firstly determined at a site containing 6–12 bases with 40–80% GC content, then five deoxyinosines are designated for the poly I linker, and then the sequence upstream of 3′-segment is automatically extended to 18–25 bases until Tm > 65 °C to generate the 5′-segment [25]. Notably, DPO system allows a wide annealing temperature to effectively amplify target genes, indicating a promising tool for developing multiplex PCR assay. Several publications have reported the successful development of DPO-based multiplex PCR assay for simultaneous detection of multiple viral pathogens [26,27,29,30].
In this study, combining the strong specificity of DPO primers and high sensitivity of SYBR Green fluorescent dye, a DPO system-based multiplex real-time RT-PCR assay for the accurate differentiation of PEDV, TGEV, PoRV, and PDCoV was developed, which would provide technical support for identifying these viral pathogens causing diarrhea in piglet, and for improvement in epidemiological investigation.
2. Materials and methods
2.1. Viruses, primers, and clinical specimens
Viral strains including PEDV, TGEV, PoRV, PDCoV, bovine viral diarrhea virus (BVDV), bovine parvovirus (BPV), infectious bursal disease virus (IBDV), infectious hematopoietic necrosis virus (IHNV), bovine rotavirus (BRV), feline infectious peritonitis virus (FIPV), and bovine respiratory syncytial virus (BRSV) were used for specificity analysis in this study. A total of 672 clinical specimens of newborn piglets with diarrhea symptoms were collected from pig farms located in Northeastern China (Heilongjiang, Jilin, and Liaoning provinces) from 2017 to 2018, and were subjected to detection by the assay developed in this study. N gene of PEDV, TGEV, and PDCoV, and the VP7 gene of PoRV were used as target genes to design the primers using Oligo 6.0 software, and the details of primers are shown in Table 1 .
Table 1.
Primers used in this study.
| Viruses | Primer ID | Sequences (5′–3′) | Reference | Product size |
|---|---|---|---|---|
| PEDV | PEDV-N-Fa | ATGGCTTCTGTCAGCTTTCA | GenBank DQ355221.1 |
1326 bp |
| PEDV-N-Ra | TTAATTTCCTGTATCGAAGATCTCG | |||
| PEDV-DPO-Fb | GGTATTGGAGAAAATCCTGACAGGIIIIIdGCAACAGCA | 251 bp | ||
| PEDV-DPO-Rb | GACGCATCAACACCTTTTTCGIIIIITTCCGCATC | |||
| PEDV-C-Fc | GGTATTGGAGAAAATCCTGACAGGCATAAGCAACAGCA | 251 bp | ||
| PEDV-C-Rc | GACGCATCAACACCTTTTTCGACAAATTCCGCATC | |||
| TGEV | TGEV-N-Fa | ATGGCCAACCAGGGACAA | GenBank KU729220.1 |
1149 bp |
| TGEV-N-Ra | TTAGTTCGTTACCTCATCAATTATC | |||
| TGEV-DPO-Fb | CTGTTCTTGCCGCACTTAAAAIIIIIGGTGTTGAC | 180 bp | ||
| TGEV-DPO-Rb | TAGCTCCATAAAATCTTGTCACATCIIIIITACCTGCAG | |||
| TGEV-C-Fc | CTGTTCTTGCCGCACTTAAAAAGTTAGGTGTTGAC | 180 bp | ||
| TGEV-C-Rc | TAGCTCCATAAAATCTTGTCACATCACCTTTACCTGCA | |||
| PoRV | PoRV-VP7-Fa | ATGTATGGTATTGAATATACCACAG | GenBank JN388691.1 |
981 bp |
| PoRV-VP7-Ra | CTAGACTCGGTAATAAAAGGCAG | |||
| PoRV-DPO-Fb | TGTTTTTAACAAAAGGATIIIIIACAGGGTCA | 318 bp | ||
| PoRV-DPO-Rb | CTGTAGTCGAACATCCTAIIIIIAGAGTCTGC | |||
| PoRV-C-Fc | TGTTTTTAACAAAAGGATGGCCAACAGGGTCA | 318 bp | ||
| PoRV-C-Rc | CTGTAGTCGAACATCCTATCCCGAGAGTCTGC | |||
| PDCoV | PDCoV-N-Fa | ATGGCCGCACCAGTAGTCCCTA | GenBank MH715491.1 |
1029 bp |
| PDCoV-N-Ra | CTACGCTGCTGATTCCTGCTTT | |||
| PDCoV-DPO-Fb | AGGCTACTCATCCTCAGTTTCGTGGIIIIIGAGTTCCGC | 400 bp | ||
| PDCoV-DPO-Rb | GGGCCACTTACACGCTCCTGAGGTCIIIIICTAGCGTTG | |||
| PDCoV-C-Fc | AGGCTACTCATCCTCAGTTTCGTGGCAATGGAGTTCCGC | 400 bp | ||
| PDCoV-C-Rc | GGGCCACTTACACGCTCCTGAGGTCTTCCTCTAGCGTTG |
Primers were used to amplify the full-length of the target gene for positive standard control.
DPO primers were used to establish detection method.
Conventional primers were used as control for comparison with DPO primers.
“I” means deoxyinosine.
2.2. Preparation of plasmid standards
Total RNA of PEDV, TGEV, PoRV, and PDCoV was extracted respectively from cell cultures using the TRIzol® Plus RNA Purification Kit (Invitrogen, USA) [31], followed by reverse transcription using the Superscript Reverse Transcriptase Reagent Kit (Takara, Japan) according to the manufacturer's instructions, generating complementary DNA (cDNA). Next, the full-length of target genes were amplified with primers (as shown in Table 1) using the corresponding cDNA as template. PCR system in a total volume of 25 μL was as follows: 10 × exTaq Buffer 2.5 μL, exTaq DNA polymerase 0.5 μL, dNTP mixture (10 mM each) 3 μL, cDNA 5 μL, primers (TGEV-N-F/R, PEDV-N-F/R, PoRV-VP7-F/R, or PDCoV-N-F/R) (10 μM) 1 μL for each, and ddH2O 12 μL. PCR amplification was carried out using a SensoQuest LabCycler (SensoQuest, Germany) with the conditions that 95 °C for 5 min, followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. Then, PCR product was cloned into plasmid pMD-19T [32], giving rise to recombinant plasmids pMD-TGEV-N, pMD-PEDV-N, pMD-PoRV-VP7, and pMD-PDCoV-N, respectively.
2.3. Specificity and annealing temperature sensitivity analysis of the DPO primer
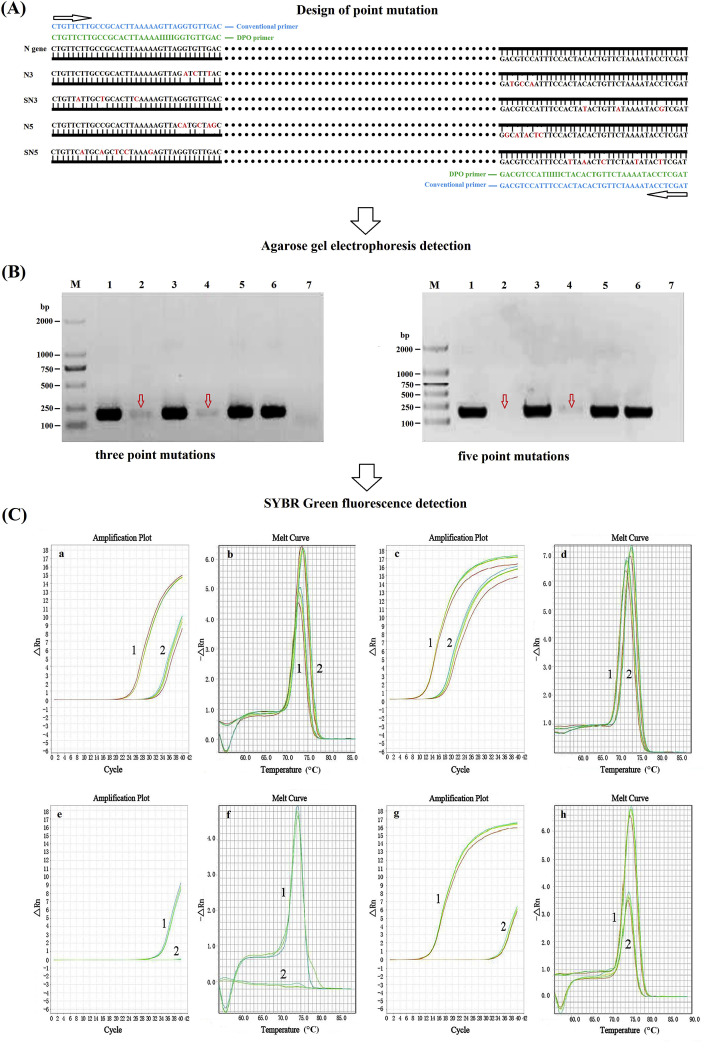
We used the N gene of TGEV as a model to evaluate the specificity of DPO primer. As shown in Fig. 1 -A, the gene sequences targeting the 5′- or 3′-segments of DPO primer were subjected to point mutation at three sites (3′-segment with three site mutations, namely N3; 5′-segment with three site mutations, namely SN3) or five sites (3′-segment with five site mutations, namely N5; 5′-segment with five site mutations namely SN5). Also, the gene sequences (PCR amplification region) with point mutations were synthesized, followed by detection using conventional PCR and SYBR Green-based real-time PCR with the conventional primer TGEV-C-F/R and DPO primer TGEV-DPO-F/R, respectively. For annealing temperature sensitivity analysis of DPO primer, a multiplex PCR assay was carried out using the mix of plasmids pMD-TGEV-N, pMD-PEDV-N, pMD-PoRV-VP7, and pMD-PDCoV-N as template with the primers TGEV-DPO-F/R, PEDV-DPO-F/R, PoRV-DPO-F/R, and PDCoV-DPO-F/R at annealing temperature from 40 °C to 65 °C to observe the simultaneous amplification effect of each target gene. In parallel, conventional PCR primers were used as control.
Fig. 1.
Comparison of priming specificity between DPO primer and conventional primer. A: Point mutations targeting gene sequences of the 5′- or 3′-segments of DPO primer (3′-segment with three/five sites mutation namely N3/N5; 5′-segment with three/five sites mutation namely SN3/SN5), followed by PCR amplification using conventional PCR assay and SYBR Green-based real-time PCR assay with conventional primer (CP) and DPO primer, respectively. B: PCR products were analyzed by agarose gel electrophoresis. (Left) M: DNA Marker DL2000; 1: N3+CP; 2: N3+DPO; 3: SN3+CP; 4: SN3+DPO; 5: N gene + CP; 6: N gene + DPO; 7: Negative control. (Right) M: DNA Marker DL2000; 1: N5+CP; 2: N5+DPO; 3: SN5+CP; 4: SN5+DPO; 5: N gene + CP; 6: N gene + DPO; 7: Negative control. C: SYBR Green fluorescence detection results. Detection result of three nucleotide mismatches (a/c: amplification plot; b/d: melt curve). 1: N3/SN3+ conventional primer; 2: N3/SN3+DPO. Detection result of five nucleotide mismatches (e/g: amplification plot; f/h: melt curve). 1: N5/SN5+CP; 2: N5/SN5+DPO.
2.4. Development of DPO-based real-time RT-PCR assay
First, DPO-based singleplex real-time RT-PCR assay for detecting PEDV, TGEV, PoRV, and PDCoV was developed, respectively. PCR system in a total volume of 20 μL was as follows: 10 μL of SYBR Green I Universal Master Mix, 0.5 μL of DPO primer pair (10 μM), 5 μL/1 μL of cDNA/plasmid DNA template, and DEPC H2O added to make up the 20 μL volume. PCR conditions were 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and 60 °C for 1 min performed in an Applied Biosystems 7500 (ABI, USA). Second, the standard curve for viral quantification was constructed using 10-fold serially diluted plasmids pMD-TGEV-N, pMD-PEDV-N, pMD-PoRV-VP7, and pMD-PDCoV-N with primary concentration of 1.52 × 108 copies/μL, 1.64 × 108 copies/μL, 2.27 × 108 copies/μL, and 1.58 × 108 copies/μL, respectively. Subsequently, DPO-based multiplex (duplex, triplex, and quadruplex) RT-PCR assays were developed for detecting different combinations of PEDV, TGEV, PoRV, and PDCoV, following optimization of the reaction system based on single real-time RT-PCR assay. And, the empty plasmid pMD-19T without target genes was used as an inhibition control for monitoring PCR inhibition in this real-time PCR assay.
2.5. Sensitivity, specificity and repeatability analysis of DPO-based real-time RT-PCR assay
To evaluate the limit of detection of the DPO-based real-time RT-PCR assay for PEDV, TGEV, PoRV, and PDCoV in clinical specimens, known PEDV-, TGEV-, PoRV-, or PDCoV-positive feces sample was subjected to 10-fold serial dilution with PBS buffer followed by the extraction of total RNA from the diluted sample. The DPO-based real-time RT-PCR assay was performed to detect the presence of viral nucleic acids, and the standard curve equation for each virus was used to calculate copy number of viral nucleic acids in each diluted feces sample. For the evaluation of detection specificity, PEDV, TGEV, PoRV, PDCoV, BVDV, BRV, BPV, IBDV, BRSV, IHNV, and FIPV were subjected to detection using DPO-based real-time RT-PCR assay developed in this study, and the plasmid standard pMD-TGEV-N, pMD-PEDV-N, pMD-PoRV-VP7, and pMD-PDCoV-N as the positive control, respectively. To assess the repeatability of detection results, plasmids pMD-TGEV-N (1.52 × 108 copies/μL), pMD-PEDV-N (1.64 × 108 copies/μL), pMD-PoRV-VP7 (2.27 × 108 copies/μL), and pMD-PDCoV-N (1.58 × 108 copies/μL) were 10-fold serially diluted, and 101-, 103-, and 105- fold diluted plasmids were used to evaluate the intra-assay repeatability and the inter-assay repeatability.
2.6. Application of DPO-based real-time RT-PCR assay
To evaluate the practicability of DPO-based real-time RT-PCR assay developed in this study, 672 piglet diarrhea samples were collected in Heilongjiang, Jilin, and Liaoning provinces located in Northeastern China from 2017 to 2018, followed by the extraction of total RNA using the TRIzol method as described above, and the assay was carried out. The results of analysis were determined in accordance with the following rules: (i) the cycle threshold (Ct) value of samples detected by the assay less than or equal to 35 was considered virus positive; (ii) the Ct value more than 40 was considered virus negative; (iii) the Ct value between 35 and 40 should be evaluated repeatedly. If Ct value was more than 40, the result was considered virus negative; otherwise, it was positive. In parallel, the detection results of RT-PCR assay with the conventional primers were used as method control.
3. Results
3.1. Priming specificity analysis of the DPO system
As shown in Fig. 1, we used the N gene of TGEV with three or five point mutations targeting the gene sequences designed for 5′- or 3′-segments of DPO primer as model (Fig. 1A) to evaluate the specificity of the DPO primer. In parallel, conventional PCR primer, designed from the same gene sequences as for DPO primer, was used as control. Following amplification, PCR products were respectively analyzed by agarose gel electrophoresis assay (Fig. 1B) and SYBR Green fluorescence assay (Fig. 1C). Our results showed that DPO primers had a higher priming specificity than that of conventional primer; moreover, when there were more than three nucleotides mismatched between the 3′- or 5′-segment of DPO primer and template, PCR amplification would be inhibited substantially and even the extension would be terminated, while the amplification efficiency of conventional PCR primers would not be affected.
3.2. Annealing temperature sensitivity analysis of the DPO system
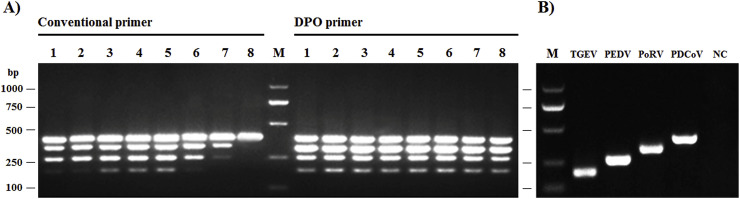
To analyze the annealing temperature sensitivity of the DPO primer, the mixture of plasmids pMD-TGEV-N, pMD-PEDV-N, pMD-PoRV-VP7, and pMD-PDCoV-N was used as the template, and multiplex PCR was performed with DPO primers at the annealing temperature range from 40 °C to 65 °C, with conventional primers as control. As shown in Fig. 2 , DPO primers allowed a wide range of annealing temperatures to efficiently amplify target genes with a nearly identical pattern, while conventional primers required a relatively rigid annealing temperature.
Fig. 2.
Comparison of effective annealing temperature between conventional primer and DPO primer (A) and specific PCR product of target gene of each virus (B). M: DNA Marker; numbers 1–8: Annealing temperature at 40 °C, 43.2 °C, 48.2 °C, 51 °C, 54 °C, 56.8 °C, 61.8 °C, and 65 °C, respectively; NC: negative control.
3.3. Standard curves
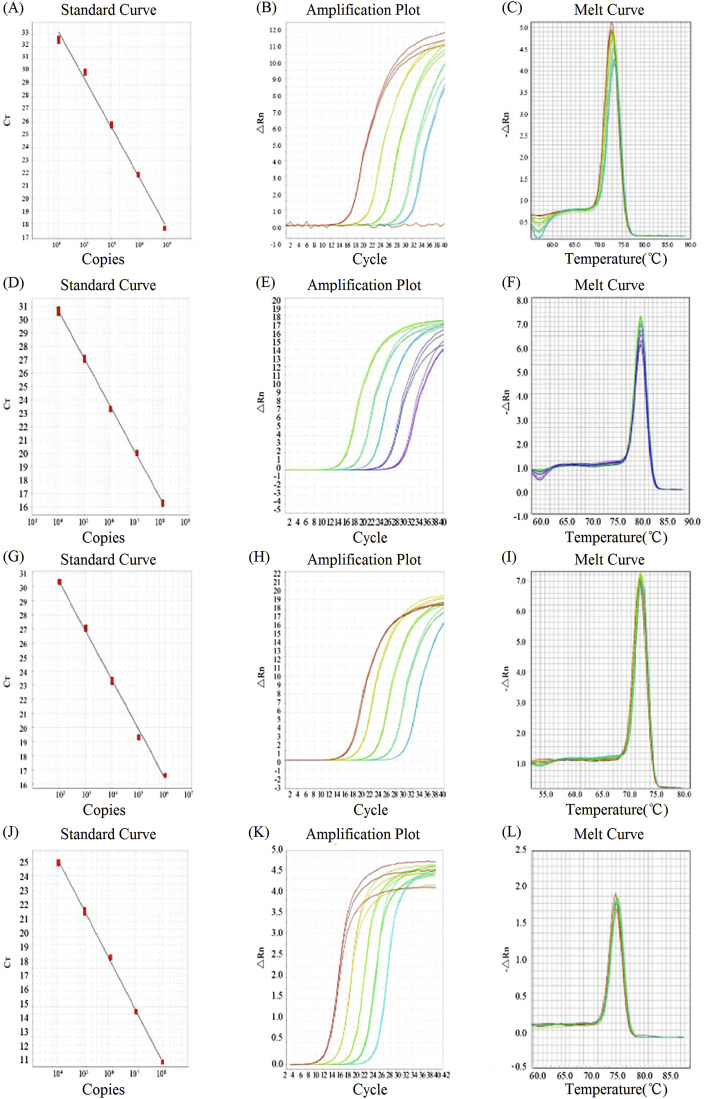
As shown in Fig. 3 , using pMD-TGEV-N, pMD-PEDV-N, pMD-PoRV-VP7, and pMD-PDCoV-N, corresponding standard curves for viral quantification of TGEV, PEDV, PoRV, and PDCoV were established, respectively. Tm value (melting point) of the melting curve for TGEV, PEDV, PoRV, and PDCoV was 73.59 °C, 79.55 °C, 71.36 °C, and 75.28 °C, respectively.
Fig. 3.
Establishment of standard curves of DPO-based real-time PCR assay for TGEV, PEDV, PoRV, and PDCoV. A, B, and C for TGEV, and corresponding standard curve equation is YTGEV = −3.7x+36.14, R2 = 0.992; D, E, and F for PEDV, and corresponding standard curve equation is YPEDV = −3.593x+45.034, R2 = 0.999; G, H and I for PoRV, and corresponding standard curve equation is YPoRV = −3.42x+37.348, R2 = 0.963; J, K and L for PDCoV, and corresponding standard curve equation is YPDCoV = −3.248x+36.825, R2 = 0.999.
3.4. Sensitivity, specificity and repeatability analysis of DPO-based real-time PCR assay
We used 10-fold serially diluted PEDV-, TGEV-, PoRV-, or PDCoV-positive feces sample to evaluate the limit of detection (LOD) of DPO-based real-time RT-PCR assay for PEDV, TGEV, PoRV, and PDCoV in clinical specimens. This was followed by calculating the copy number of viral nucleic acids with corresponding standard curve equation established in this study. Our results showed that the LOD of DPO-based real-time RT-PCR assay for detecting PEDV, TGEV, PoRV, and PDCoV in clinical specimens was 8.63 × 102 copies/μL, 1.92 × 102 copies/μL, 1.74 × 102 copies/μL, and 1.76 × 102 copies/μL, respectively (Fig. 4 A). In this work, the detection specificity of the assay for PEDV, TGEV, PoRV, and PDCoV was evaluated using TGEV, PEDV, PoRV, PDCoV, BVDV, BRV, BPV, IBDV, BRSV, IHNV and FIPV, and our results showed that only target viruses were positive for the assay without non-specific priming, while other viruses were negative, indicating a strong specificity (Fig. 4B). Moreover, the intra-assay and inter-assay detection reproducibility was estimated using the plasmid standards pMD-TGEV-N, pMD-PEDV-N, pMD-PoRV-VP7, and pMD-PDCoV-N as templates, and results showed that the coefficients of variation were all less than 1.0% (Table 2 ), indicating good repeatability.
Fig. 4.
Limit of detection and specificity of DPO-based real-time PCR assay for TGEV, PEDV, PoRV, and PDCoV. (A) Limit of detection. 10-fold serially diluted PEDV-, TGEV-, PoRV-, or PDCoV-positive feces sample was used to evaluate the LOD of DPO-based real-time RT-PCR assay in clinical specimens, followed by calculation of the copy number of viral nucleic acids with standard curve equation established in this study. a: Amplification plot with 1–8: 101, 102, 103, 104, 105, 106, 107, and 108 dilution of feces sample; b: Melt curve. (B) Specificity result. a: Amplification plot; b: Melt curve. 1: target viruses; 2–12: other viruses and negative control.
Table 2.
Repeatability of DPO-based real-time RT-PCR assay.
| Standards | Concentration (copies/μL) | n | Intra-assay variability |
Inter-assay variability |
||
|---|---|---|---|---|---|---|
| X±SD (Ct) | CV (%) | X±SD (Ct) | CV (%) | |||
| pMD-TGEV-N | 1.52 × 107 | 5 | 13.28 ± 0.08 | 0.60 | 12.83 ± 0.12 | 0.83 |
| 1.52 × 105 | 5 | 21.43 ± 0.13 | 0.22 | 21.19 ± 0.13 | 0.52 | |
| 1.52 × 103 | 5 | 26.84 ± 0.09 | 0.36 | 28.19 ± 0.19 | 0.67 | |
| pMD-PEDV-N | 1.64 × 107 | 5 | 14.13 ± 0.09 | 0.63 | 13.73 ± 0.15 | 0.83 |
| 1.64 × 105 | 5 | 19.51 ± 0.11 | 0.56 | 20.26 ± 0.14 | 0.69 | |
| 1.64 × 103 | 5 | 26.16 ± 0.08 | 0.31 | 27.54 ± 0.16 | 0.58 | |
| pMD-PoRV-VP7 | 2.27 × 107 | 5 | 14.97 ± 0.07 | 0.46 | 13.51 ± 0.22 | 0.72 |
| 2.27 × 105 | 5 | 19.23 ± 0.12 | 0.62 | 20.79 ± 0.29 | 0.89 | |
| 2.27 × 103 | 5 | 25.81 ± 0.10 | 0.38 | 28.11 ± 0.17 | 0.60 | |
| pMD-PDCoV-N | 1.58 × 107 | 5 | 15.38 ± 0.19 | 0.93 | 14.48 ± 0.12 | 0.83 |
| 1.58 × 105 | 5 | 19.96 ± 0.23 | 0.85 | 20.76 ± 0.25 | 0.97 | |
| 1.58 × 103 | 5 | 26.01 ± 0.16 | 0.62 | 26.71 ± 0.28 | 0.95 | |
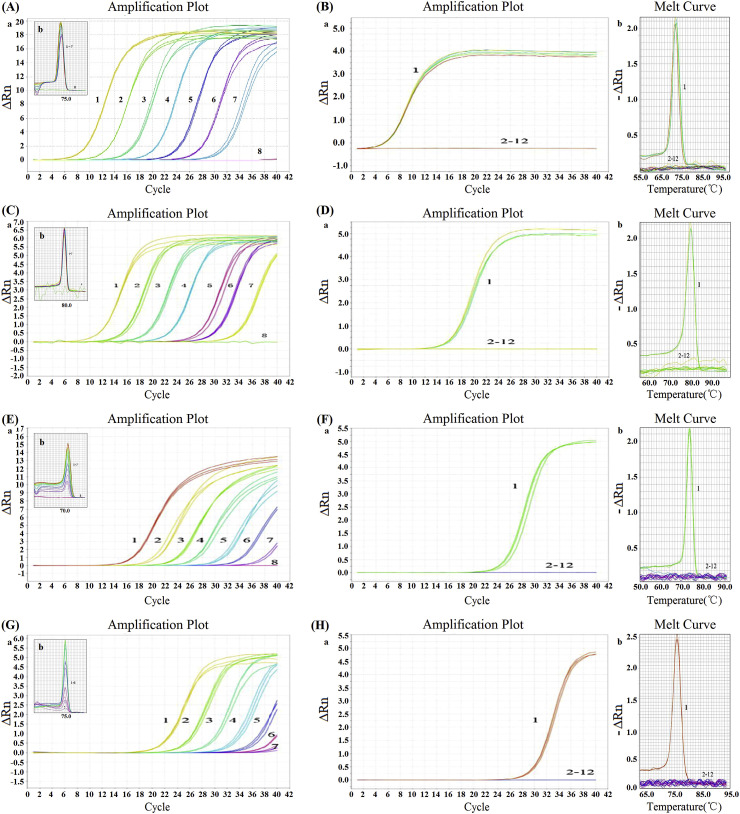
3.5. Development of DPO-based multiplex real-time RT-PCR assay
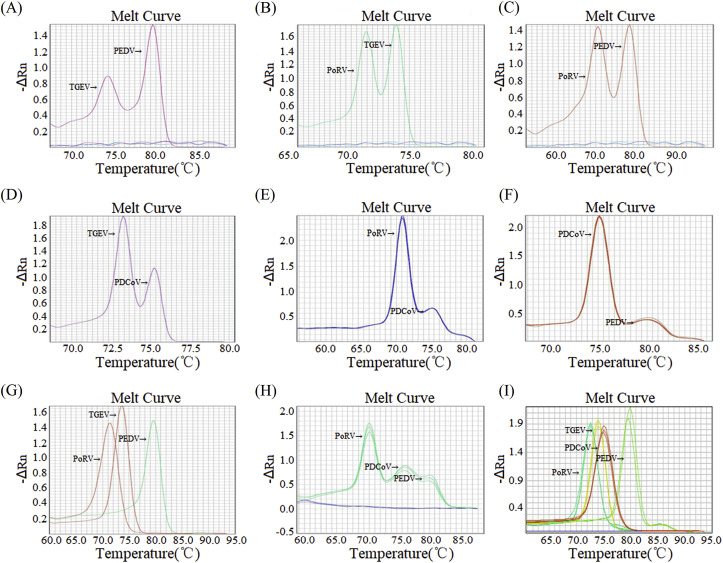
In this work, DPO-based multiplex (duplex, triplex, and quadruplex) real-time RT-PCR assays were developed for the detection of different combinations of PEDV, TGEV, PoRV, and PDCoV, following the optimization of reaction system based on the single real-time RT-PCR assay. As shown in Fig. 5 , using DPO-based multiplex real-time RT-PCR assays developed in this study, different target viral pathogens were accurately differentiated in a single reaction.
Fig. 5.
DPO-based duplex, triplex, and quadruplex real-time RT-PCR assays.
3.6. Detection of clinical samples
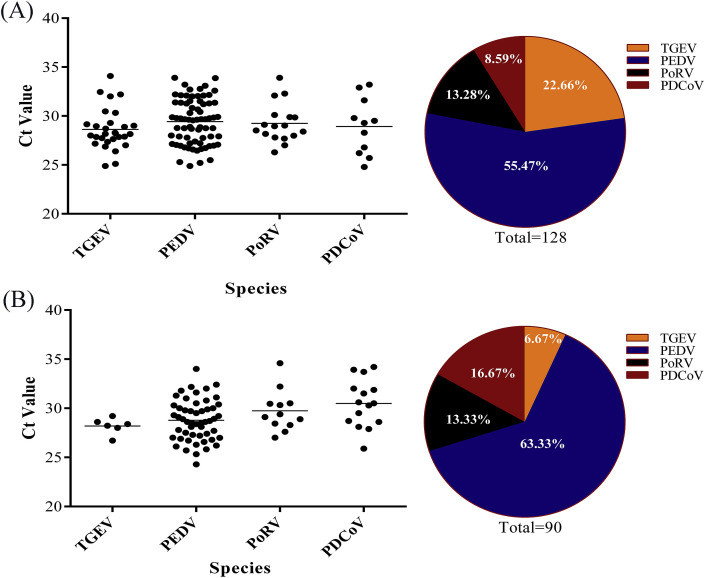
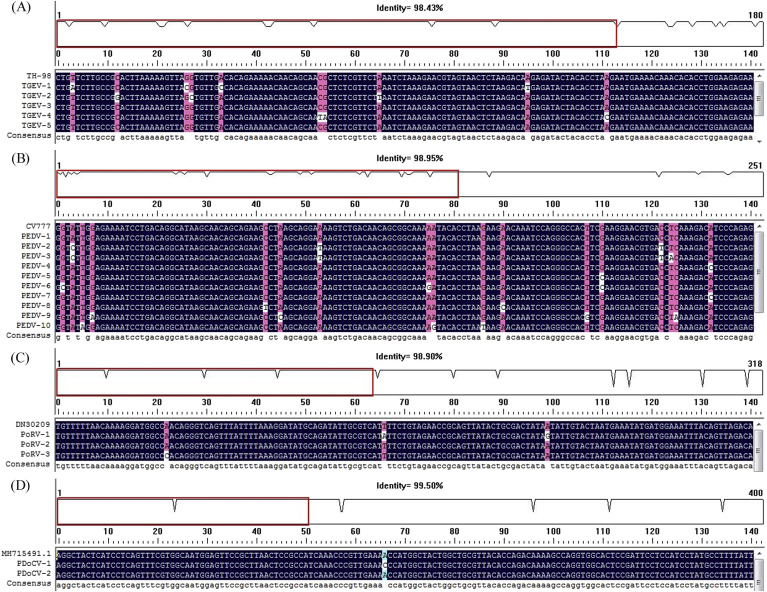
A total of 672 piglet diarrhea samples collected from Heilongjiang, Jilin, and Liaoning provinces in Northeastern China from 2017 to 2018 were used to evaluate the feasibility of the assay developed in this study, using conventional RT-PCR assay as control. As shown in Table 3 , the prevalence rate of PEDV, TGEV, PoRV, and PDCoV detected by the DPO-based real-time RT-PCR assay was 19.05% (128/672), 5.21% (35/672), 4.32% (29/672), and 3.87% (26/672), respectively, while that detected by conventional RT-PCR assay was 17.56% (118/672), 4.46% (30/672), 3.87% (26/672), and 3.57% (24/672), respectively. The positive coincidence rate of these two detection methods for PEDV, TGEV, PoRV, and PDCoV was 92.18%, 85.71%, 89.66%, and 92.31%. We also detected mixed virus infections present in some samples. With the results of DPO-based real-time RT-PCR in terms of Ct value as shown in Fig. 6 , it was clear that the quantities of the viruses detected in different virus-positive samples varied greatly with Ct values of 20–25, 25–30, and 30–35, respectively. Moreover, from the virus-positive results of the clinical samples, PEDV is the major cause of piglet viral diarrhea disease. Subsequently, to confirm the detection accuracy of the assay developed in this study, the samples with different detection results by the two methods were subjected to sequence analysis of target genes. As shown in Fig. 7 , the sequencing results verified that the detection results of DPO-based real-time RT-PCR assay were accurate.
Table 3.
Detection results of clinical samples.
| Year | n | Conventional RT-PCR assay |
Prevalence rate TGEV/PEDV/PoRV/PDCoV | DPO-based real-time RT-PCR assay |
Prevalence rate TGEV/PEDV/PoRV/PDCoV |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TGEV +/− |
PEDV +/− |
PoRV +/− |
PDCoV +/− |
TGEV +/− |
PEDV +/− |
PoRV +/− |
PDCoV +/− |
||||
| 2017 | 376 | 25/351 | 64/312 | 15/361 | 10/366 | 6.65%/17.02%/3.99%/2.66% | 29/347 | 71/305 | 17/359 | 11/365 | 7.71%/18.88%/4.52%/2.93% |
| 2018 | 296 | 5/291 | 54/242 | 11/285 | 14/282 | 1.69%/18.24%/3.72%/4.73% | 6/290 | 57/239 | 12/284 | 15/281 | 2.03%/19.26%/4.05%/5.07% |
| Total | 672 | 30/642 | 118/554 | 26/646 | 24/648 | 4.46%/17.56%/3.87%/3.57% | 35/637 | 128/544 | 29/643 | 26/646 | 5.21%/19.05%/4.32%/3.87% |
| Coincidence ratesa between conventional RT-PCR assay and DPO-based real-time RT-PCR assay | TGEV 85.71% | PEDV 92.19% | PoRV 89.66% | PDCoV 92.31% | |||||||
Coincidence rate was the positive results detected by conventional RT-PCR/the positive results detected by DPO-based real-time RT-PCR.
Fig. 6.
Virus-positive results of the clinical samples and the rate of PEDV, TGEV, PoRV, and PDCoV in the positive samples.
Fig. 7.
Confirmation of detection results of DPO-based real-time RT-PCR assay by sequencing.
4. Discussion
Porcine viral diarrhea disease still seriously endangers the development of the pig industry, and leads to significant economic losses for pig farmers worldwide. Clinically, PEDV, TGEV, PoRV, and PDCoV are the major causative agents of viral diarrhea in piglets. In some cases, mixed infections with two or more these viruses are common, which seriously interfere with clinical diagnosis [8,16,18,33,34]. Moreover, with the increasing growth of international trades and animal transports, the risk of transboundary spread of porcine viral diarrhea disease has also increased significantly. Therefore, to differentiate accurately infections of TGEV, PEDV, PoRV, and PDCoV in clinical specimens, and to prevent transboundary spread of porcine viral diarrhea disease, it is necessary to develop a rapid, accurate, and multi-target diagnostic method for the differentiation of TGEV, PEDV, PoRV, and PDCoV.
Currently, PCR-based methods are commonly recognized as useful tools for the molecular diagnosis of various viral or bacterial pathogens. The most common PCR method used is the real-time PCR method, which includes probe assay such as Taqman-probe and fluorescence dye assay such as SYBR Green dye. Both assays have their advantages and disadvantages. Of them both Taqman-probe and SYBR Green fluorescence dye have high sensitivity, and TaqMan-probe has high specificity but poor versatility, which is only suitable for specific gene detection, while SYBR Green fluorescence dye has strong versatility. Moreover, the cost of TaqMan-probe is higher than that of SYBR Green fluorescence dye. Therefore, in order to reduce the cost of detection, we utilized SYBR Green fluorescence dye to develop the real-time PCR assay. Primer design is the key to determine the specificity of both probe- and fluorescence dye-based real-time PCR method, which requires very rigid primer parameters. Generally, to obtain reliable primers, there should be repeated optimization of primer parameters, such as specificity, length, melting temperature, GC content, less secondary structure, and PCR annealing temperature, which is time-consuming, in particular the design of primers for developing a multiplex PCR assay. In this study, we introduced DPO primer that was different from the conventional primer to develop real-time RT-PCR assay with SYBR Green fluorescence dye for the detection of TGEV, PEDV, PoRV, and PDCoV. Compared with the conventional primer, DPO primer is easier because it does not require repeated optimization of primer parameters.
Moreover, DPO primer has 2 obvious advantages, compared with conventional primer. Firstly, the priming specificity of DPO primer is stronger than that of conventional primer. We used the TGEV N gene with point mutations targeting the gene sequences designed for the 3′- or 5′-segment of DPO primer as model to evaluate the priming specificity of the DPO primer. It was found that when five nucleotides mismatched with the template in the 5′-segment of the DPO primer, the PCR amplification efficiency decreased substantially, while the PCR products were still observed via agarose gel electrophoresis assay and SYBR Green fluorescence assay. Additionally, when five nucleotides mismatched with the template in the 3′-segment of DPO primer, the PCR amplification terminated and could not be analyzed by agarose gel electrophoresis assay, whereas a very weak fluorescence signal with Ct value of approximately 33 was observed via SYBR Green fluorescence assay. Our results were somewhat inconsistent with previous report [25], in which the researchers claimed that if there were 3 bp mismatched in the 3′-segment or 5′-segment of the DPO primer, the extension would not proceed. In any case, the priming specificity of DPO primer is stronger than that of conventional primers, which have a single priming region, and extension might proceed even in the presence of mismatch between primer and template. Moreover, the high specificity of DPO primers could effectively reduce the competitive effect between different primers, promoting the amplification efficiency indirectly, and although the optimal PCR product size generally recommended for SYBR Green-based protocols was below 200 nucleotides, there was little effect on the amplification efficiency for TGEV (180 bp), PEDV (251 bp), PoRV (318 bp), and PDCoV (400 bp) designed in this work, showing 94.551%, 101.283%, 95.347%, and 102.305%, respectively. Secondly, due to the specific structure of DPO primer, there is wide range of annealing temperature to effectively amplify the target gene with a nearly identical pattern. However, the conventional primers required optimal annealing temperature to amplify effectively, suggesting that the DPO primer was a powerful tool to develop multiplex PCR assay.
In this study, we combined the DPO primer and SYBR Green fluorescence dye to develop the DPO-based real-time RT-PCR assay for clinically differentiating the infection of TGEV, PEDV, PoRV, and PDCoV, which showed high specificity, sensitivity, and detection repeatability. Generally, to demonstrate the new detection method, it is necessary to test the clinical samples and compare the results with that of control method. For this purpose, we collected 672 diarrhea samples of piglet from pig farms located in Northeastern China from 2017 to 2018 to evaluate the diagnostic capability of the assay developed in this study, using conventional RT-PCR assay as control. By contrast, the detection accuracy of DPO-based real-time RT-PCR assay is higher than that of conventional RT-PCR assay, indicating a better diagnostic capability in clinical samples. Moreover, from the epidemiological investigation, we know that PEDV still is the major cause of piglet viral diarrhea disease in China, and PDCoV as a novel animal coronaviruses that emerged in China [35], has its infection on the rise.
In conclusion, DPO-based real-time RT-PCR assay for differentiating TGEV, PEDV, PoRV, and PDCoV developed in this study has both high specificity of the DPO primer and high sensitivity of SYBR Green fluorescence dye, and shows good diagnostic capability in practice, providing a useful tool for the rapid and accurate diagnosis and epidemiological investigation of these viral pathogens causing piglet diarrhea. Moreover, since SYBR Green-based PCR protocols rely on complex melting curves, they are no more sufficient for human diagnostics laboratories. Therefore, there protocols need further development to an internal probe-based detection system using different colors in order to comply with current diagnostic standards, even if this test is not being applied for humans.
Funding
This work was funded by the National Key Research and Development (R&D) Program of China (grant number 2016YFD0501003, 2016YFD0500704).
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mcp.2019.101435.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhao J., Shi B.J., Huang X.G., Peng M.Y., Zhang X.M., He D.N., Pang R., Zhou B., Chen P.Y. A multiplex RT-PCR assay for rapid and differential diagnosis of four porcine diarrhea associated viruses in field samples from pig farms in East China from 2010 to 2012. J. Virol. Methods. 2013;194:107–112. doi: 10.1016/j.jviromet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Rodak L., Valicek L., Smid B., Nevorankova Z. An ELISA optimized for porcine epidemic diarrhoea virus detection in faeces. Vet. Microbiol. 2005;105:9–17. doi: 10.1016/j.vetmic.2004.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Byrum B., Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 2014;20:1227–1230. doi: 10.3201/eid2007.140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y., Zhang Y., Liang X., Lou F., Oglesbee M., Krakowka S., Li J. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio. 2015;6 doi: 10.1128/mBio.00064-15. e00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J. Porcine deltacoronavirus: overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016;226:71–84. doi: 10.1016/j.virusres.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang G., Lee K.K., Kim S.H., Lee C. Prevalence, complete genome sequencing and phylogenetic analysis of porcine deltacoronavirus in South Korea, 2014-2016. Transbound. Emerg. Dis. 2017;64:1364–1370. doi: 10.1111/tbed.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajayi T., Dara R., Misener M., Pasma T., Moser L., Poljak Z. Herd-level prevalence and incidence of porcine epidemic diarrhoea virus (PEDV) and porcine deltacoronavirus (PDCoV) in swine herds in Ontario, Canada, Transbound. Emerg. Dis. 2018;65:1197–1207. doi: 10.1111/tbed.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederwerder M.C., Hesse R.A. Swine enteric coronavirus disease: a review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada, Transbound. Emerg. Dis. 2018;65:660–675. doi: 10.1111/tbed.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H., Liang Q., Li B., Cui X., Wei X., Ding Q., Wang Y., Hu H. Prevalence, phylogenetic and evolutionary analysis of porcine deltacoronavirus in Henan province, China. Prev. Vet. Med. 2019;166:8–15. doi: 10.1016/j.prevetmed.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saif L.J., van Cott J.L., Brim T.A. Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet. Immunol. Immunopathol. 1994;43:89–97. doi: 10.1016/0165-2427(94)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu M., Wang L., Ma S., Wang X., Wang Y., Xiao Y., Jiang Y., Qiao X., Tang L., Xu Y., Li Y. Immunogenicity of eGFP-marked recombinant Lactobacillus casei against transmissible gastroenteritis virus andporcine epidemic diarrhea virus. Viruses. 2017;9:274. doi: 10.3390/v9100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun R.Q., Cai R.J., Chen Y.Q., Liang P.S., Chen D.K., Song C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China, Emerg. Inf. Disp. 2012;18:161–163. doi: 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi W., Jia S., Zhao H., Yin J., Wang X., Yu M., Ma S., Wu Y., Chen Y., Fan W., Xu Y., Li Y. Novel approach for isolation and identification of porcine epidemic diarrhea virus (PEDV) strain NJ using porcine intestinal epithelial cells. Viruses. 2017;9:19. doi: 10.3390/v9010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlasova A.N., Amimo J.O., Saif L.J. Porcine rotaviruses: epidemiology, immune responses and control strategies. Viruses. 2017;9:48. doi: 10.3390/v9030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dou X., Li Y., Han J., Zarlenga D.S., Zhu W., Ren X., Dong N., Li X., Li G. Cholesterol of lipid rafts is a key determinant for entry and post-entry control of porcine rotavirus infection. BMC Vet. Res. 2018;14:45. doi: 10.1186/s12917-018-1366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung K., Hu H., Eyerly B., Lu Z., Chepngeno J., Saif L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015;21:650–654. doi: 10.3201/eid2104.141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesonero-Escuredo S., Strutzberg-Minder K., Casanovas C., Segales J. Viral and bacterial investigations on the aetiology of recurrent pig neonatal diarrhoea cases in Spain. Porc. Heal. Manag. 2018;4:5. doi: 10.1186/s40813-018-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuanthap S., Phupolphan C., Luengyosluechakul S., Duangin A., Theamboonlers A., Wattanaphansak S., Vongpunsawad S., Amonsin A., Poovorawan Y. Porcine rotavirus C in pigs with gastroenteritis on Thai swine farms, 2011-2016. Peerj. 2018;6 doi: 10.7717/peerj.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belak S. The molecular diagnosis of porcine viral diseases: a review. Acta Vet. Hung. 2005;53:113–124. doi: 10.1556/AVet.53.2005.1.11. [DOI] [PubMed] [Google Scholar]
- 20.Ben Salem A.N., Sergei A.C., Olga P.B., Olga G.A., Mahjoub A., Larissa B.P. Multiplex nested RT-PCR for the detection of porcine enteric viruses. J. Virol. Methods. 2010;165:283–293. doi: 10.1016/j.jviromet.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G., Jiang Y., Opriessnig T., Gu K., Zhang H., Yang Z. Detection and differentiation of five diarrhea related pig viruses utilizing a multiplex PCR assay. J. Virol. Methods. 2019;263:32–37. doi: 10.1016/j.jviromet.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Escutenaire S., Mohamed N., Isaksson M., Thoren P., Klingeborn B., Belak S., Berg M., Blomberg J. SYBR Green real-time reverse transcription-polymerase chain reaction assay for the generic detection of coronaviruses. Arch. Virol. 2007;152:41–58. doi: 10.1007/s00705-006-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun Y.H., Jeong Y.J., Park S.I., Hosmillo M., Shin D.J., Kwon H.J., Kang S.Y., Woo S.K., Kang M.I., Park S.J., Cho K.O. Development of one-step real-time reverse transcription polymerase chain reaction assays for rapid detection of porcine group C rotaviruses. J. Vet. Diagn. Investig. 2010;22:74–77. doi: 10.1177/104063871002200113. [DOI] [PubMed] [Google Scholar]
- 24.Han H.Y., Zheng H.H., Zhao Y., Tian R.B., Xu P.L., Hou H.L., Chen H.Y., Yang M.F. Development of a SYBR green I-based duplex real-time fluorescence quantitative PCR assay for the simultaneous detection of porcine epidemic diarrhea virus and porcine circovirus 3. Mol. Cell. Probes. 2019;44:44–50. doi: 10.1016/j.mcp.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun J.Y., Kim K.J., Hwang I.T., Kim Y.J., Lee D.H., Lee I.K., Kim J.K. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007;35:e40. doi: 10.1093/nar/gkm051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C.S., Kang B.K., Lee D.H., Lyou S.H., Park B.K., Ann S.K., Jung K., Song D.S. One-step multiplex RT-PCR for detection and subtyping of swine influenza H1, H3, N1, N2 viruses in clinical samples using a dual priming oligonucleotide (DPO) system. J. Virol. Methods. 2008;151:30–34. doi: 10.1016/j.jviromet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim S.R., Ki C.S., Lee N.Y. Rapid detection and identification of 12 respiratory viruses using a dual priming oligonucleotide system-based multiplex PCR assay. J. Virol. Methods. 2009;156:111–116. doi: 10.1016/j.jviromet.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma X., Xu H., Shi L., Yang P., Zhang L., Sun X., Zhen W., Hu K. A multiplex PCR assay for the detection of five influenza viruses using a dual priming oligonucleotide system. BMC Infect. Dis. 2015;15:93. doi: 10.1186/s12879-015-0818-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh J.Y., Lee J.H., Seo H.J., Park J.Y., Moon J.S., Cho I.S., Choi I.S., Park S.Y., Song C.S., Lee J.B. Simultaneous detection of rift valley fever, bluetongue, rinderpest, and peste des petits ruminants viruses by a single-tube multiplex reverse transcriptase-PCR assay using a dual-priming oligonucleotide system. J. Clin. Microbiol. 2011;49:1389–1394. doi: 10.1128/JCM.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan W.L., Wang Z.W., Qin Y., Sun C., Liu Z.M., Jiang Y.P., Qiao X.Y., Tang L.J., Li Y.J., Xu Y.G. Use of dual priming oligonucleotide system-based multiplex RT-PCR combined with high performance liquid chromatography assay for simultaneous detection of five enteric viruses associated with acute enteritis. J. Virol. Methods. 2017;243:80–82. doi: 10.1016/j.jviromet.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Feng B., Niu C., Jia S., Sun C., Wang Z., Jiang Y., Cui W., Wang L., Xu Y. Dendritic cell targeting of bovine viral diarrhea virus E2 protein expressed by Lactobacillus casei effectively induces antigen-specific immune responses via oral vaccination. Viruses. 2019;11:575. doi: 10.3390/v11060575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X.W., Ma Y.Y., Wang Z., Bai J., Jia S., Feng B.H., Jiang Y.P., Cui W., Tang L.J., Li Y.J., Wang L., Xu Y.G. Oral immunization of mice with a probiotic Lactobacillus casei constitutively expressing the α-toxoid induces protective immunity against Clostridium perfringens α-toxin. Virulence. 2019;10:166–179. doi: 10.1080/21505594.2019.1582975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amimo J.O., Vlasova A.N., Saif L.J. Detection and genetic diversity of porcine group A rotaviruses in historic (2004) and recent (2011 and 2012) swine fecal samples in Ohio: predominance of the G9P 13 genotype in nursing piglets. J. Clin. Microbiol. 2013;51:1142–1151. doi: 10.1128/JCM.03193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q., Gauger P., Stafne M., Thomas J., Arruda P., Burrough E., Madson D., Brodie J., Magstadt D., Derscheid R., Welch M., Zhang J. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology. 2015;482:51–59. doi: 10.1016/j.virol.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B., Liu Y., Ji C.M., Yang Y.L., Liang Q.Z., Zhao P., Xu L.D., Lei X.M., Luo W.T., Qin P., Zhou J., Huang Y.W. Porcine deltacoronavirus engages the transmissible gastroenteritis virus functional receptor porcine aminopeptidase N for infectious cellular entry. J. Virol. 2018;92 doi: 10.1128/JVI.00318-18. e00318-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.