Abstract
Ethnopharmacological relevance
.
Scutellaria baicalensis
Georgi (Labiatae) is a well-known traditional Chinese medicine to treat inflammation, cardiovascular diseases, respiratory and gastrointestinal infections, etc. The present study was to understand the metabolism of the root of Scutellaria baicalensis (a.k.a. Huangqin in Chinese) in the gastrointestinal tract and the correlation between the metabolites and their respective pharmacological activities.
Materials and methods
The water extract of the root of Scutellaria baicalensis (WESB) was incubated with simulated gastric and intestinal juices, and human fecal microflora for 24 h at 37 °C. The HPLC–DAD analysis was used to monitor the in vitro metabolic process and identify its metabolites by comparing their absorption spectrum and retention time with those of chemical references. The in vitro anticomplementary and antimicrobial activity was evaluated with hemolysis assay, agar disc-diffusion method and MIC value, respectively.
Results
Main constituents of WESB remain unchanged during the incubation with simulated gastric juice (pH=1.5) and intestinal juice (pH=6.8), whereas four flavones, baicalin, wogonoside, oroxyloside and norwogonoside were metabolized into their respective aglycons by human intestinal bacteria. All four metabolites were demonstrated to have higher anticomplementary and antimicrobial activity than those of WESB. The anticomplementary active metabolites were identified to be baicalein, oroxylin A and norwogonin, among them, norwogonin is the most active compound.
Conclusion
The presence of intestinal bacteria is demonstrated to play an important role in the gastrointestinal metabolism of WESB, and the pharmacological effects of Scutellaria baicalensis may be dependent on the intestinal bacteria metabolism.
Chemical compounds studied in this article: Baicalin (PubChem CID: 64982), Wogonoside (PubChem CID: 29927693), Baicalein (PubChem CID: 5281605), Wogonin (PubChem CID: 5281703), Oroxylin A (PubChem CID: 5320315), Oroxyloside (PubChem CID: 38348319), Norwogonin (PubChem CID: 5281674), Norwogonoside (PubChem CID: 44258552)
Keywords: Gastrointestinal tract, Metabolism, Scutellaria baicalensis, Anticomplementary, Antibacterial
Graphical abstract
1. Introduction
The root of Scutellaria baicalensis Georgi (Labiatae), also known as Huangqin, is a widely used herb in traditional Chinese medicine (TCM) with anticancer, antiviral, antibacterial and anti-inflammatory properties (Yoon et al., 2009). Traditionally, Huangqin has been prescribed as a diuretic, laxative, febrifuge, an antipyretic, and for hemoptysis, bloody stool, and nasal haemorrhage when prescribed in a compound recipe (The Pharmacopoeia Commission of PRC, 2010). Remarkably, Huangqin was recommended for the treatment and prevention of severe acute respiratory syndrome (SARS) by the State TCM Administration of the People's Republic of China (Miller et al., 2005, Zhang and Chen, 2008).
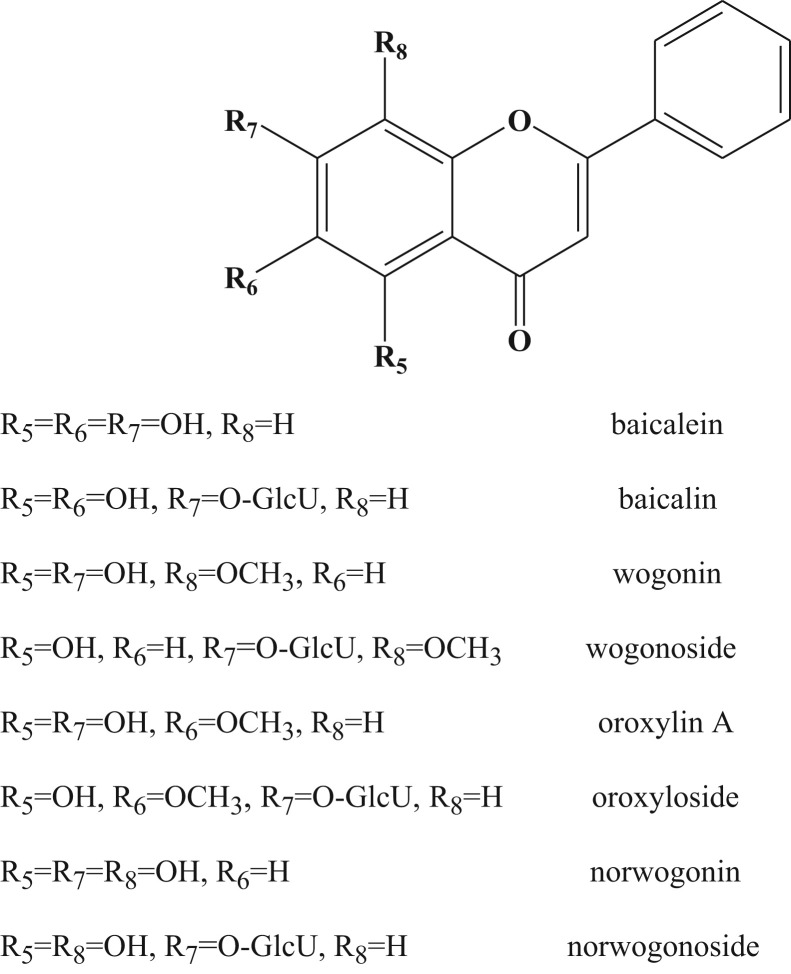
Huangqin was found to exert anti-inflammatory, antioxidant, anti-hepatitis B virus, anti-tumor, anti-allergic and anxiolytic properties (Li et al., 2009, Tong et al., 2012). The main active constituents of Huangqin are flavonoids, such as baicalin (baicalein-7-glucuronide), wogonoside (wogonin-7-glucuronide), baicalein, wogonin, oroxylin A and oroxyloside (oroxylin A-7-glucuronide) ( Fig. 1). Among these flavonoids, baicalin is regarded as the most important determinants of the quality of Huangqin (Yuan et al., 2011). Due to its orally administered as decoctions in TCM, the metabolism of the constituents often occurs in the gastrointestinal tract caused by the low gastric pH conditions, as well as the presence of digestive enzymes or intestinal bacteria (Akao et al., 1994). It was found that baicalin was initially hydrolyzed to baicalein prior to the absorption, while baicalein could be readily absorbed with a fast and extensive first pass metabolism (Akao et al., 2000, Lu et al., 2007). Wogonoside was found to be metabolized to wogonin by human fecal microflora (Trinh et al., 2009). In addition, pharmacokinetic study of Huangqin-Tang Decoction indicated that the constituents of wogonoside and oroxyloside had double-site absorption kinetics in rats which may due to the enteric circulation and enterohepatic circulation after oral dosing (Zuo et al., 2003). So far, there is no report on the metabolism of Huangqin in gastrointestinal tract. In addition, most studies on bioactivity of Huangqin were focused on antibacterial and anti-endotoxin activities but not anticomplementary activity.
Fig. 1.
Chemical structures of eight flavonoids.
In this study, the water extract of Huangqin, the root of Scutellaria baicalensis (WESB) was incubated with simulated gastric and intestinal juices, and human fecal microflora, their metabolites were identified and the in vitro anticomplementary activity was evaluated. The inhibitory activity of main compounds in Huangqin as well as their metabolites against the standard strains of methicillin-resistant Staphylococcus aureus (MRSA), methicillin sensitive Staphylococcus aureus (MSSA), Enterococcus faecalis (gram-positive bacteria), Escherichia coli, Pseudomonas aeuroginosa (gram-negative bacteria) was determined to understand the role of gastrointestinal tract conditions on pharmacological effects of Huangqin.
2. Materials and methods
2.1. Material and reagents
The roots of Scutellaria baicalensis (Huangqin) were purchased from Leiyunshang drugstore (Shanghai) and authenticated by one of the authors Mengyue Wang. Voucher specimen (HN001) has been deposited at herbarium of School of Pharmacy, Shanghai Jiao Tong University, Shanghai, China. Pepsin (1: 250) and pancreatin were purchased from Sangon Biotech Co., Ltd (Shanghai, China). General anaerobic medium broth (GAM broth) was purchased from Shanghai Kayon Biological Technology Co., Ltd (Shanghai, China). Sheep erythrocytes, rabbit erythrocytes and anti-sheep erythrocyte antibody were purchased from Shanghai Fortune Biological Technological Co., Ltd (Shanghai, China). Heparin (sodium salt, 160 IU/mg) and macroreticular resin (HZ-801) were purchased from China National Medicines Co., Ltd (Shanghai, China). Normal human serum was obtained from healthy adult donors. Guinea pigs serum was obtained from healthy guinea pigs from Laboratory Animal Research Center of Fudan University. MRSA (ATCC33591), MSSA (ATCC25923), Enterococcus faecalis (ATCC29212), Escherichia coli (ATCC8739), and Pseudomonas aeuroginosa (ATCC27853) provided by Dr. Xiuping Qian's lab.
Reference substances: Baicalein, baicalin, wogonin, wogonoside, oroxylin A, oroxyloside were purchased from Shanghai Winherb Medical Technology Co., Ltd.; Norwogonin was purchased from J&K Chemical Ltd.; Norwogonoside was isolated from Huangqin in our laboratory. All lab-made compounds were characterized by their spectra data, which were in accordance with references. All other chemicals and reagents were of analytical grade.
2.2. Preparation of WESB
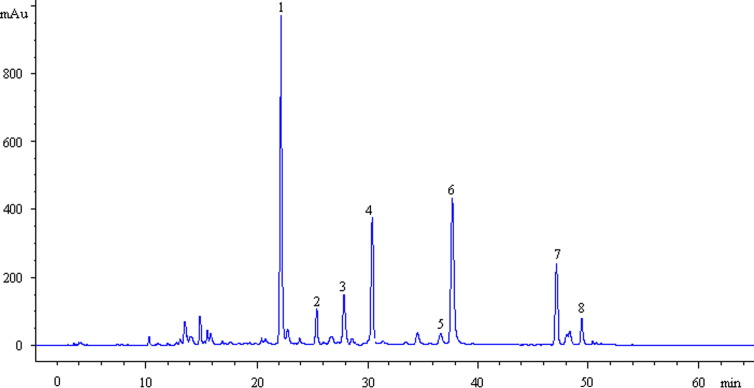
Dried Huangqin (30 g) was decocted twice with a ten-fold mass of water to boil 1 h. After filtrated, the two filtrates were combined and then concentrated to 30 ml (equivalent to 1 g (crude drug)/ml). The HPLC–DAD analysis of WESB was conducted ( Fig. 2). Peaks 1–8 were confirmed by comparing the retention times and UV spectra with reference standards, and attributed to baicalin, norwogonoside, oroxyloside, wogonoside, norwogonin, baicalein, wogonin and oroxylin A, respectively. Noted that norwogonoside was isolated from Huangqin for the first time. The contents of main constituents in Huangqin, baicalin, norwogonoside, oroxyloside, wogonoside, baicalein, wogonin and oroxylin A, were determined to be 110.72±4.25, 7.52±0.04, 16.34±0.51, 35.49±2.78, 39.27±2.93, 12.10±0.15, 4.63±0.08 mg/1 g (w/w), respectively, and those of main flavonoid glycosides in WESB, baicalin, norwogonoside, oroxyloside and wogonoside were 79.62±2.85, 3.33±0.01, 9.65±0.32 and 22.37±1.67 mg/1 ml (w/v), respectively.
Fig. 2.
HPLC chromatograms of WESB. (1) baicalin; (2) norwogonoside; (3) oroxyloside; (4) wogonoside; (5) norwogonin; (6) baicalein; (7) wogonin; (8) oroxylin A.
2.3. Stability of WESB in simulated gastric juice and intestinal juice
A 0.4 ml WESB was placed in 5 ml simulated gastric juice (0.08 M HCl containing 50 mg pepsin, pH 1.5) and incubated at 37 °C for 0, 1, 2, 4 h, and the reactions were stopped by water saturated n-butanol immediately. The mixture was centrifuged at 4800 rpm for 20 min, and then the supernatant was evaporated. The residue was dissolved in 0.5 ml methanol, filtered through a 0.22 μm membrane and analyzed by HPLC. Repeat above all steps of experiment once more.
A 0.4 ml WESB was placed in 5 ml simulated intestinal juice (0.05 M KH2PO4 containing 50 mg pancreatin, pH 6.8) and incubated at 37 °C for 0, 2, 4, 6 h. The treatment of samples was same to the simulated gastric juice.
2.4. Metabolism of WESB by human intestinal bacteria in vitro
Fresh fecal samples, obtained from five healthy subjects, were immediately homogenized with 25 volumes of GAM broth. The sediments were removed by filtration through three pieces of gauze. The suspension was incubated at 37 °C in an anaerobic incubator in which the air had been replaced with a gas mixture (H2 5%, CO2 10%, N2 85%). A 1 ml WESB was added to human fecal suspension (100 ml) and the mixture was incubated at 37 °C in an anaerobic incubator for 24 h. The cultured mixture was respectively taken out and extracted with water saturated n-butanol. The extract was evaporated, and then the residue was dissolved in methanol (0.5 ml) and analyzed with HPLC–DAD.
2.5. HPLC analysis
The HPLC system consisted of a multi-solvent delivery pump (Agilent 1200, USA), equipped with a quaternary solvent delivery system, an auto sampler and a DAD detector. Waters XTerra reversed-phase column (particle size 5 μm, 4.6 mm×250 mm i.d., Agilent Ltd., USA) were used. The purity of compounds and metabolic samples were analyzed under chromatographic condition as follows: acetonitrile (A)–water–phosphoric acid (100: 0.1, v/v, B) with gradient elution 0–10 min (A: 10–20%), 10–15 min (A: 20–22%), 15–22 min (A: 22–25%), 22–32 min (A: 25–30%), 32–45 min (A: 30–40%), 45–50 min (A: 40–85%), 50–65 min (A: 85–100%) appropriately. The addition of acid into the mobile phase was carried out in order to improve the peak shapes of analysis. UV absorption was monitored at 280 nm. The column temperature was set at 35 °C. The flow rate was 1.0 ml/min and sample injection volume was 10 μl.
2.6. Isolation and identification of norwogonoside from Huangqin
The dried Huangqin (2.0 kg) were extracted for three times with 75% ethanol by heating reflux for 1.5 h each time, with the solvent removed under reduced pressure. The 75% ethanolic extract was suspended in water, and then purified with a column of macroreticular resin (column, 7.0 cm×65 cm) washed with distilled water, 30% ethanol, 60% ethanol and 90% ethanol. The 60% ethanol soluble fraction (50 g) were firstly separated by silica gel column chromatography (12.0 cm×70 cm, eluted with a gradient system of methanol and chloroform), and then by Sephadex LH-20 column (2.5 cm×70 cm, eluted with methanol) to obtain norwogonoside. 1H and 13C spectra data agree with those reported in the literature (Shibata, 1988).
Norwogonin-7-O-glucuronide Yellow powder, the absorbance maximum (λ max) of UV absorption is at 246, 285 and 357 nm in methanol, suggesting the compound to be a flavonoid. 1H NMR (500 MHz, DMSO-d6) δ: 12.23 (1H, s, 5-OH), 8.10–8.15 (2H, m, H2', 6'), 7.58–7.64 (3H, m, H3', 5'), 7.02 (1H, s, H-3), 6.65 (1H, s, H-6); 13C NMR (125 MHz, DMSO-d6) δ: 182.64 (C-4), 163.58 (C-2), 158.06 (C-7), 152.21 (C-9), 151.47 (C-5), 132.32 (C-4'), 130.58 (C-1'), 127.76 (C-8), 129.12 (C3', 5'), 126.56 (C-2', 6'), 105.13 (C-3), 105.56 (C-10), 99.36 (C-6).
2.7. Anticomplementary activity through the classical pathway
Based on Mayer’s (1961) modified method, sensitized erythrocytes (EA) were prepared by incubation of sheep erythrocytes with rabbit anti-sheep erythrocyte antibody in GVB2+ (Veronal buffer saline containing 0.1% gelatin, 0.5 mM Mg2+ and 0.15 mM Ca2+). The compounds and heparin sodium, used as reference, were dissolved in GVB2+. Guinea pigs serum was used as the complement source. The 1:40 diluted guinea pigs serum was chosen to give sub maximal lysis in the absence of complement inhibitors. First, various dilutions of tested samples (100 μl) were pre-incubated with 100 μl guinea pigs serum and 200 μl GVB2+ at 37 °C for 10 min. Then, 200 μl EA was added and the mixture was incubated at 37 °C for 30 min. The different assay controls were incubated in the same conditions: (1) vehicle control, 200 μl EA in 400 μl GVB2+; (2) 100% lysis control, 200 μl EA in 400 μl water; (3) sample control, 100 μl dilution of each sample in 500 μl GVB2+. The reaction mixture was centrifuged immediately. Optical density of the supernatant was measured at 405 nm with a spectrophotometer (Thermo, Varioskan Flash). The absorbance of sample (A1), sample control (A0) and 100% lysis control (A) were recorded.
2.8. Anticomplementary activity through the alternative pathway
According to the method of Klerx et al. (1983), each sample was dissolved in GVB-Mg-EGTA (Veronal buffer saline containing 0.1% gelatin, 2.5 mM Mg2+ and 8 mM ethylene glycol tetraacetic acid), and various dilutions were made. After pre-incubation of dilutions of each sample (150 μl) with 1:10 diluted normal human serum (150 μl) in enzyme label plate at 37 °C for 10 min, 200 μl rabbit erythrocytes (ER) were added, followed a second incubation step at 37 °C for 30 min. After incubation, the resulting mixture was centrifuged immediately, and the optical density of the supernatant was measured at 405 nm.
The inhibition rate of haemolysis was calculated by the following formula: ×100%, where A, A1 and A0 represented the absorbance of 100% lysis control, the sample and the sample control, respectively. The CH50 (concentrations resulted in 50% inhibition of sheep erythrocytes) or AP50 (concentrations that resulted in 50% hemolysis inhibition of rabbit erythrocytes) value was obtained by plotting the hemolysis inhibition percentage against the logarithm of sample concentrations (Lg C). Heparin sodium salt was used as the positive control.
2.9. Antibacterial activity by the agar disc-diffusion method and determination of minimal inhibitory concentration (MIC)
The antibacterial activity was carried out on WESB, metabolic sample incubated 24 h, the blank of intestinal bacterial liquid and eight flavonoids from Huangqin with five test bacterial strains, and all of samples were three replicates. The positive controls were ampicillin for gram-positive bacteria, gentamycin for gram-negative bacteria and negative control was sterile distilled water. The antimicrobial effect of all samples was in petri dishes with 20 ml of nutrient agar plus 0.2 ml of microorganism suspension (108 CFU/ml, O.D 0.1, λ=590 nm). Once the agar had solidified, 200 µl of each sample and sterile distilled water (negative control) was added to wells of 3 mm diameter. The plates were incubated at 37 °C for 24 h, and the inhibited halos were evaluated (mm). The antibacterial effect was determined by measure of inhibited halos formed around the wells (Murray et al., 1995, Boussaada et al., 2008).
The MIC value was determined by the dilution method according to National Committee for Clinical Laboratory Standards (1985). The active samples and positive controls were dissolved in dimethylsulphoxide at a concentration of 2000 µg/ml. Two fold serial dilutions of the solution were then prepared (2000, 1000, 500, …, 3.9 µg/ml). Inoculated nutrient broth with 1% test bacteria strains (108 CFU/ml, O.D 0.1, λ=590 nm) was prepared. Ten concentrations of each sample×three test bacterial strains×three wells as repetitions were carried out in the sterile round-bottom 96-well plates by comparison of the sample with the non-inoculated nutrient broth. In each well, 0.9 ml inoculated nutrient broth plus 0.1 ml sample. Plates were incubated at 37 °C for 24 h. The MIC values were determined as the lowest concentration that inhibited visible growth of the bacterial as detected by unaided eye.
3. Results
3.1. Stability of WESB in simulated gastric juice and simulated intestinal juice
WESB was found to be stable in simulated gastric juice (pH=1.5) and intestinal juice (pH=6.8). After incubated with artificial juices, main constituents of WESB remained unchanged based on the comparison of peak areas of individual constituents during the course of incubation (RSD<3.0%).
3.2. Metabolism of WESB by human intestinal bacteria in vitro
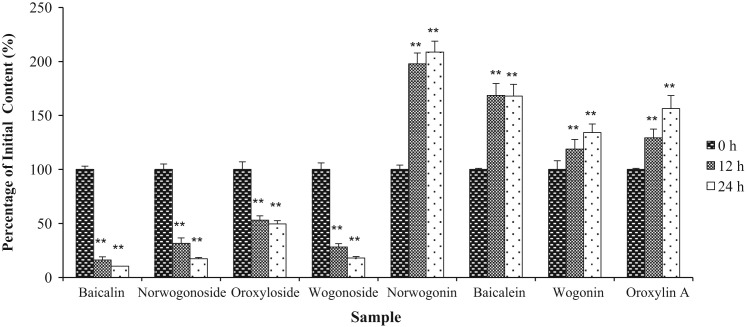
WESB was anaerobically incubated for 24 h with a bacterial mixture from human feces. The content changes of eight ingredients in WESB were analyzed at incubation time points of 0, 12 and 24 h. ( Fig. 3). According to the content changes as well as the chemical structure of the eight ingredients in WESB, we concluded that four flavone glycosides, baicalin, wogonoside, oroxyloside and norwogonoside were converted to their respective aglycons, i.e. baicalein, wogonin, oroxylin A and norwogonin by intestinal bacteria.
Fig. 3.
The content changes of the main constituents in WESB when incubated in human intestinal bacteria. Data are presented as mean±SD, n=3. ⁎⁎p<0.01 as compared with the initial content of the compound.
3.3. Anticomplementary activity through the classical pathway and the alternative pathway
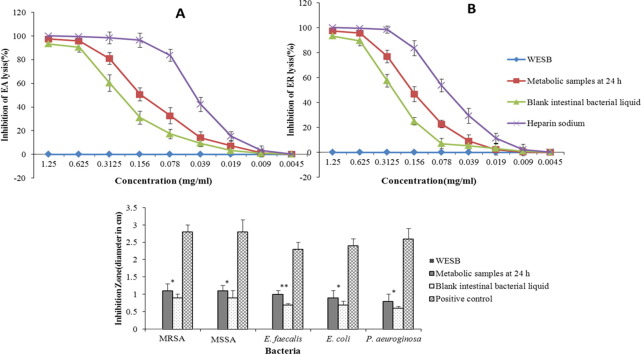
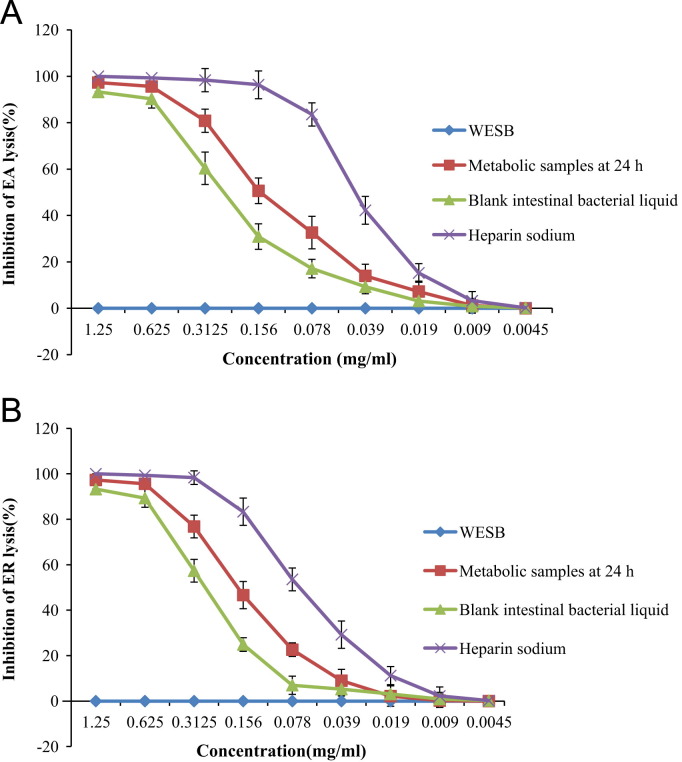
The effect on activation of guinea pig complement inhibition through the classical pathway was examined in 1:40 diluted guinea pig serum and heparin sodium was used as the positive control. The percent of activation that 1:40 diluted guinea serum occurred in the classical pathway was 98.34±5.23% in the complement control group. The percentage of activation that 1:10 diluted normal human serum occurred in the alternative pathway was 97.26±7.85% in the complement control group. WESB showed no anticomplementary effect through the classical pathway or alternative pathway. The CH50 and AP50 values of the metabolic samples at 24 h were both smaller than those of the blank of intestinal bacterial liquid as shown in Fig. 4. It indicated that the metabolic sample is more effective than the blank of intestinal bacterial liquid in complement inhibition. These results suggested that anticomplementary metabolites may be generated during the metabolic process.
Fig. 4.
Anticomplementary activity of WESB, metabolic samples at 24 h and the blank of intestinal bacterial liquid. (A) The classical pathway; (B) the alternative pathway. Data are presented as mean±SD, n=3.
None of the flavonoid glycosides, baicalin, wogonoside, oroxyloside and norwogonoside, showed anticomplementary activities through the classical or alternative pathway, however, their respective metabolites, baicalein (CH50=0.182±0.008 mg/ml; AP50=0.243±0.013 mg/ml), oroxylin A (CH50=0.536±0.032 mg/ml; AP50=0.835±0.043 mg/ml) and norwogonin (CH50=0.138±0.004 mg/ml; AP50=0.147±0.008 mg/ml) did display an interesting anticomplementary activity through both classical and alternative pathways, while wogonin had no anticomplementary activity. Remarkably, norwogonin demonstrated significant activity comparable to the positive inhibitor (heparin sodium, CH50=0.056±0.005 mg/ml; AP50=0.075±0.011 mg/ml).
3.4. Determination of the antibacterial activity
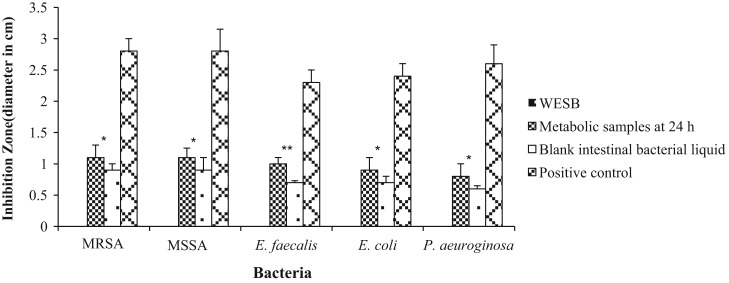
The primary antimicrobial screening was carried out using the agar disc-diffusion. As showed in Fig. 5, the WESB (1.25 mg/ml) had no inhibitory effect against any bacteria tested. The metabolic samples incubated for 24 h exhibited better antibacterial activity than the blank of intestinal bacterial liquid. Specifically, baicalin, baicalein, wogonin and norwogonin revealed inhibitory effects against all bacteria studied, whereas oroxylin A was limited to MRSA, MSSA and Pseudomonas aeuroginosa, while wogonoside, oroxyloside and norwogonoside had no antimicrobial effect. The MIC values of baicalin, baicalein, wogonin, oroxylin A and norwogonin against the bacteria were summarized in Table 2. Norwogonin displayed an overall highest antimicrobial activity, followed by baicalein, oroxylin A and wogonin, and then baicalin.
Fig. 5.
Antimicrobial activity of WESB, metabolic samples at 24 h and the blank of intestinal bacterial liquid. Data are presented as mean±SD, n=3. ⁎p<0.05, ⁎⁎p<0.01, metabolic samples compared with the blank of intestinal bacterial liquid.
Table 2.
MIC of main constituents in WESB and their metabolites against five bacterias.
| Sample | MIC (mg/ml) |
||||
|---|---|---|---|---|---|
| MRSA | MSSA | Enterococcus faecalis | Escherichia coli | Pseudomonas aeuroginosa | |
| WESB | NEa | NE | NE | NE | NE |
| Baicalin | 1 | 1 | 2 | 2 | 2 |
| Baicalein | 0.125 | 0.125 | 0.125 | 0.5 | 0.25 |
| Wogonoside | NE | NE | NE | NE | NE |
| Wogonin | 0.5 | 0.5 | 1 | 1 | 2 |
| Oroxyloside | NE | NE | NE | NE | NE |
| Oroxylin A | 0.125 | 0.125 | NE | 0.5 | NE |
| Norwogonoside | NE | NE | NE | NE | NE |
| Norwogonin | 0.0625 | 0.0625 | 0.125 | 0.25 | 0.25 |
| Positive controlb | 0.0039 | 0.0039 | 0.0078 | 0.0156 | 0.03125 |
NE denotes that this compound has no inhibitory effect at the maximal concentration tested.
Positive control: Ampicillin for gram-positive bacteria and gentamycin for gram-negative bacteria.
4. Discussion
In order to identify the effective substances of Huangqin by oral administration in human body, the metabolism in the gastrointestinal tract was investigated in vitro. Although no change was observed in artificial gastric or intestinal juice, the main constituents, baicalin, wogonoside and oroxyloside, were found to be metabolized into their respective aglycons by intestinal bacteria. These findings are consistent with the reports that monomeric compounds baicalin, wogonoside and oroxyloside could be converted to metabolites baicalein, wogonin and oroxylin A by intestinal bacteria, respectively (Akao et al., 2000, Trinh et al., 2009, Zuo et al., 2002). The biotransformation of individual compound was the same as that in whole herb decoctions. Moreover, a low content norwogonoside was isolated and identified from Huangqin for the first time, and confirmed to be metabolized to norwogonin. Baicalein, wogonin and oroxylin A were detected in rat plasma and urine samples after oral administration of Gegen-Qinlian-Wan (GQW, including Huangqin) by UHPLC/DAD/qTOF-MS, but not norwogonin which was present in our metabolic study in vitro (Miao et al., 2013). It is probably due to the difference between human and rat or the low content in specimens. Nevertheless, both results suggest that flavonoid O-glycosides from Huangqin could be easily transformed into their aglycones.
The human complement system plays an important role in the host defense foreign invasive organisms. Its effects are normally beneficial to the host (Min et al., 2003), but over-activation of the system may lead to pathological reaction in a variety of inflammatory and degenerative diseases such as rheumatoid arthritis, type I diabetes mellitus, systemic lupus erythematosus, SARS, vasculitis and many others (Valeriya et al., 2011). Numerous naturally occurring agents have been reported to effectively block the complement activation, which provide the prospect of novel anticomplementary drugs in non-toxicity based on abundant herb resources. The best described anticomplementary agents are polysaccharides, flavones and triterpenes (Xu et al., 2007). In the present study, WESB (1.25 mg/ml) showed no anticomplementary activity through the classical pathway or the alternative pathway, even though WESB was abundant in flavonoids. Our studies on main flavonoid glycosides from WESB, baicalin, norwogonoside, oroxyloside and wogonoside generated the similar results (Table 1). However, their metabolites, especially norwogonin, showed obvious anticomplementary activity in inhibiting the classical pathway and the alternative pathway, suggesting a correction between 5,7-dihydroxyflavone and the anticomplementary effect. Specifically, the inhibitory potencies against complement system were enhanced by the number of free hydroxyls on A ring of 5,7-dihydroxyflavone (Xi et al., 2012). This finding also agrees with the anticomplementary properties in some acylated flavonol glycosides obtained from Magnolia fargesii (Jung et al., 1998) and Persicaria lapathifolia (Park et al., 1999). On the other hand, glycosylation on A ring of flavones might play an important role in its anticomplementary effect that most of the effect even disappeared (Yao et al., 2013). These results were also coincident with the previous suggestion that the intestinal flora might regulate the metabolism of compounds administered orally or excreted into bile (Zuo et al., 2002), and the herbal components should be transformed to bioactive compounds by the intestinal bacteria prior to their pharmacological actions (Lee et al., 2002a, Sousa et al., 2008). Further study should be carried out to elucidate their anticomplementary activity in vivo.
Table 1.
Anticomplementary activity of main constituents in WESB and their metabolites.
| Sample | Classical pathway |
Alternative pathway |
|---|---|---|
| CH50 (mg/ml)a | AP50 (mg/ml)a | |
| WESB | NEb | NE |
| Baicalin | NE | NE |
| Baicalein | 0.182±0.008 | 0.243±0.013 |
| Wogonoside | NE | NE |
| Wogonin | NE | NE |
| Oroxyloside | NE | NE |
| Oroxylin A | 0.536±0.032 | 0.835±0.043 |
| Norwogonoside | NE | NE |
| Norwogonin | 0.138±0.004 | 0.147±0.008 |
| Heparin sodium | 0.056±0.005 | 0.075±0.011 |
Data are expressed as the mean±SD of independent experiments performed in triplicate.
NE denotes that this compound has no inhibitory effect at the maximal concentration tested.
Published literature strongly support that the antimicrobial activities of some plant extracts are likely to be due to their high flavonoid content (Cushnie and Lamb, 2005, Zhang et al., 2011). In our studies, baicalin, baicalein, oroxylin A and norwogonin, four typical flavones possessed different degree of antimicrobial activities. A small number of research groups have investigated the relationship between structure and antibacterial activity of flavonoid to identify common structural features among active compounds. Tsuchiya's study indicated that 5,7-dihydroxylation of the A ring in the flavone structure was important for anti-MRSA activity (Tsuchia et al., 1996). It is consistent that baicalein, wogonin, oroxylin A and norwogonin, all featured such structures (shown in Fig. 1), and display better effective antimicrobial properties than other compounds. Interestingly, a report by Stapleton demonstrated that substitution with C-8 chain also enhanced the antistaphylococcal activity of flavonoids (Stapleton et al., 2004). Independently, our data also revealed that norwogonin, 5,7,8-trihydroxyflavone has the highest antibacterial activity. These results suggest that the overall antimicrobial effect of Huangqin may be dependent on its metabolism by intestinal bacteria. Moreover, gram-negative bacteria Escherichia coli and Pseudomonas aeuroginosa have been inhibited to a less extent as compared to the gram-positive bacteria MRSA, MSSA, and Enterococcus faecalis as shown in Table 2. This result is in agreement with that flavonoids of the plant origin appear to have greater activity against gram-positive than gram-negative bacteria (Ikigai et al., 1993) and could selectively inhibit gram-positive bacteria (Wyman and Van-Etten, 1978). Further characterization of the interaction between these antimicrobial flavonoids and their target sites are needed, however.
In the present study, norwogonin, one of the metabolites of WESB, presented most significant anticomplementary and antimicrobial activities. Specifically, norwogonin has the most potent activity against multidrug-resistant Acinetobacter baumannii strains (MIC=0.256 mg/ml) among all identified antimicrobial compounds (Miyasaki et al., 2013), and exhibited more potent cytotoxicity than wogonin according to the MTT and LDH release assays in leukemia HL-60 cells (Chow et al., 2008). Norwogonin also exhibited potent inhibitory activity toward VHR (IC50=1.1 μM), but had no inhibitory activity against T-cell protein tyrosine phosphatase or serine/threonine protein phosphatase 1 (Lee et al., 2002b). Norwogonin possessed significant antioxidant activity in AAPH (2,2'-azobis (2-amidinopropane hydrochloride)-induced haemolysis (Liu et al., 2004). In addition, it is reported that norwogonin was determined in test strains differing in excision-repair capability and in the presence or absence of plasmid pKM101 (MacGregor and Wilson, 1988). Although some biological effects of norwogonin have been observed, the mechanism of action still remains unclear and requires further investigation.
5. Conclusion
Our results demonstrated that the presence of intestinal bacteria played an important role in the gastrointestinal metabolism of WESB. The process of metabolism by intestinal microbiota is involved in hydrolytic reactions generating non-polar low molecular weight byproducts. Active metabolites were generated during the metabolic process. The results indicated that pharmacological effects of Huangqin may be dependent on its metabolism by intestinal bacteria, consistent with the fact that WESB has no anticomplementary and antimicrobial activities in vitro.
Acknowledgments
This work was financially supported by the National Project in Significant Creation of New Drugs during the Eleventh Five-Year Plan Period (2009ZX09502-013). The authors are grateful to Dr. Xiuping Qian's lab to provide all the bacteria strains and technical support of antimicrobial test, and Dr. James L. Fu for the careful English revision.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jep.2013.12.056.
Appendix A. Supplementary materials
Supplementary material
References
- Akao T., Hayashi T., Kobashi K., Kanaoka M., Kato H., Kobayashi M., Takeda S., Oyama T. Intestinal bacterial hydrolysis is indispensable to absorption of 18 beta-glycyrrhetic acid after oral administration of glycyrrhizin in rats. J. Pharm. Pharmacol. 1994;46:135–137. doi: 10.1111/j.2042-7158.1994.tb03756.x. [DOI] [PubMed] [Google Scholar]
- Akao T., Kawabata K., Yanagisawa E., Ishihara K., Mizuhara Y., Wakui Y., Sakashita Y., Kobashi K. Baicalin, the predominant flavone glucuronide of Scutellariae Radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J. Pharm. Pharmacol. 2000;52:1563–1568. doi: 10.1211/0022357001777621. [DOI] [PubMed] [Google Scholar]
- Boussaada O., Chriaa J., Nabli R., Ammar S., Saidana D., Mahjoub M.A., Chraeif I., Helal A.N., Mighri Z. Antimicrobial and antioxidant activities of methanol extracts of Evax pygmaea (Asteraceae) growing wild in Tunisia. World J. Microbiol. Biotechnol. 2008;24:1289–1296. [Google Scholar]
- Chow J.M., Huang G.C., Shen S.C., Wu C.Y., Lin C.W., Chen Y.C. Differential apoptotic effect of wogonin and nor-wogonin via stimulation of ROS production in human leukemia cells. J. Cell. Biochem. 2008;103:1394–1404. doi: 10.1002/jcb.21528. [DOI] [PubMed] [Google Scholar]
- Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikigai H., Nakae T., Hara Y., Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- Jung K.Y., Oh S.R., Park S.H., Lee I.S., Ahn K.S., Lee J.J., Lee H.K. Anticomplement activity of tiliroside from the flower buds of Magnolia fargesi. Biol. Pharm. Bull. 1998;21:1077–1078. doi: 10.1248/bpb.21.1077. [DOI] [PubMed] [Google Scholar]
- Klerx J.P., Beukelman C.J., Van D.H., Willers J.M. Microassay for colorimetric estimation of complement activity in guinea pig, human and mouse serum. J. Immunol. Methods. 1983;63:215–220. doi: 10.1016/0022-1759(83)90425-8. [DOI] [PubMed] [Google Scholar]
- Li C.R., Zhou L.M., Lin G., Zuo Z. Contents of major bioactive flavones in proprietary traditional Chinese medicine products and reference herb of Radix scutellariae. J. Pharm. Biomed. Anal. 2009;50:298–306. doi: 10.1016/j.jpba.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Liu Z.Q., Luo X.Y., Sun Y.X., Wu W., Liu C.M., Liu Z.Q., Liu S.Y. The antioxidative effect of icariin in human erythrocytes against free-radical-induced haemolysis. J. Pharm. Pharmacol. 2004;56:1557–1562. doi: 10.1211/0022357044869. [DOI] [PubMed] [Google Scholar]
- Lee D.S., Kim Y.S., Ko C.N., Cho K.H., Bae H.S., Lee K.S., Kim J.J., Park E.K., Kim D.H. Fecal metabolic activities of herbal components to bioactive compounds. Arch. Pharmacal Res. 2002;25:165–169. doi: 10.1007/BF02976558. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Oh W.K., Kim B.Y., Ahn S.C., Kang D.O., Sohn C.B., Osada H., Ahn J.S. Inhibition of VHR dual-specificity protein tyrosine phosphatase activity by flavonoids isolated from Scutellaria baicalensis: structure–activity relationships. Planta Med. 2002;68:1063–1065. doi: 10.1055/s-2002-36351. [DOI] [PubMed] [Google Scholar]
- Lu T., Song J., Huang F., Deng Y.X., Xie L., Wang G.J., Liu X. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J. Ethnopharmacol. 2007;110:412–418. doi: 10.1016/j.jep.2006.09.036. [DOI] [PubMed] [Google Scholar]
- MacGregor J.T., Wilson R.E. Flavone mutagenicity in Salmonella typhimurium: dependence on the pKM101 plasmid and excision-repair deficiency. Environ. Mol. Mutagen. 1988;11:315–322. doi: 10.1002/em.2850110304. [DOI] [PubMed] [Google Scholar]
- Mayer M.M. Complement and complement fixation. In: Kabat EA, Mayer MM, editors. Experimental Immunochemistry. (second ed.) Springfield Publications; 1961. pp. 133–240. [Google Scholar]
- Miao W.J., Wang Q., Bo T., Ye M., Qiao X., Yang W.Z., Guo D.A. Rapid characterization of chemical constituents and rats metabolites of the traditional Chinese patent medicine Gegen-Qinlian-Wan by UHPLC/DAD/qTOF-MS. J. Pharm. Biomed. Anal. 2013;72:99–108. doi: 10.1016/j.jpba.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Miller, R., Yu, H.W., Zhong, S.M., 2005. Extracts of Scutellaria for the Treatment of SARS. GB, CN200580006085.3.
- Min B.S., Lee S.Y., Kim J.H., Lee J.K., Kim T.J., Kim D.H., Kim Y.H., Joung H., Lee H.K., Nakamura N., Miyashiro H., Hattori M. Anti-complement activity of constituents from the stem-bark of Juglans mandshurica. Biol. Pharm. Bull. 2003;26:1042–1044. doi: 10.1248/bpb.26.1042. [DOI] [PubMed] [Google Scholar]
- Miyasaki Y., Rabenstein J.D., Rhea J., Crouch M.L., Mocek U.M., Kittell P.E., Morgan M.A., Nichols W.S., Van, Benschoten M.M., Hardy W.D., Liu G.Y. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS One. 2013;8:e61594. doi: 10.1371/journal.pone.0061594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H. American Society for Microbiology; Washington DC, USA: 1995. Manual of Clinical Microbiology; pp. 1327–1341. [Google Scholar]
- National Committee for Clinical Laboratory Standards . National Committee for Clinical Laboratory Standards; Villanova, PA, USA: 1985. Approved Standard Document M7A. [Google Scholar]
- Park S.H., Oh S.R., Jung K.Y., Lee I.S., Ahn K.S., Kim J.H., Kim Y.S., Lee J.J., Lee H.K. Acylated flavonol glycosides with anti-complement activity from Persicaria lapathifolia. Chem. Pharm. Bull. 1999;47:1484–1486. doi: 10.1248/cpb.47.1484. [DOI] [PubMed] [Google Scholar]
- Shibata S. Studies of the constituents of Scytellaria species. I.: The flavonoid glucuronides of “Bo Ye Huang Chin”, Scutellaria Ikonnikovii Juz. Chem. Pharm. Bull. 1988;36:3206–3209. [Google Scholar]
- Sousa T., Paterson R., Moore V., Carlsson A., Abrahamsson B., Basit A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008;363:1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Stapleton P.D., Shah S., Hamilton-Miller J.M., Hara Y., Nagaoka Y., Kumagai A., Uesato S., Taylor P.W. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents. 2004;24:374–380. doi: 10.1016/j.ijantimicag.2004.03.024. [DOI] [PubMed] [Google Scholar]
- The Pharmacopoeia Commission of PRC . vol. 1. Chinese Medical Science and Technology Press; Beijing: 2010. p. 2010. (Pharmacopoeia of the People's Republic of China, version). [Google Scholar]
- Tong L., Wan M.X., Zhang L.H., Zhu Y.H., Sun H., Bi K.S. Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, oroxylin A and chrysin of Radix scutellariae extract in rat plasma by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012;70:6–12. doi: 10.1016/j.jpba.2012.03.051. [DOI] [PubMed] [Google Scholar]
- Trinh H.T., Jang S.Y., Han M.J., Kawk H.Y., Beak N.I., Kim D.H. Metabolism of wogonoside by human fecal microflora and its anti-pruritic effect. Biomol. Ther. 2009;17:211–216. [Google Scholar]
- Tsuchia H., Sato M., Miyazaki T., Fujiwara S., Tanigaki S., Ohyama M., Tanaka T., Iinuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- Valeriya G., Kalina A., Alexandre M., Petya D., Nina I., Christiane H., Thomas B., Milen G. Anti-inflammatory activity of Devil's claw in vitro systems and their active constituents. Food Chem. 2011;125:171–178. [Google Scholar]
- Wyman J.G., Van-Etten H.D. Antibacterial activity of selected isoflavonoids. Phys. Biol. 1978;68:583–589. [Google Scholar]
- Xi Z.X., Chen W.S., Wu Z.J., Wang Y., Zeng P.Y., Zhao G.J., Li X., Sun L.N. Anti-complementary activity of flavonoids from Gnaphalium affine D. Don. Food Chem. 2012;130:165–170. [Google Scholar]
- Xu H., Zhang Y.Y., Zhang J.W., Chen D.F. Isolation and characterization of an anti-complementary polysaccharide D3-S1 from the roots of Bupleurum smithii. Int. Immunopharmacol. 2007;7:175–182. doi: 10.1016/j.intimp.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Yao S., Xu N.Y., Chu C.J., Zhang J., Chen D.F. Chemical constituents of Rabdosia japonica var. glaucocalyx and their anti-complementary activity. Chin. J. Chin. Mater. Med. 2013;38:199–203. [PubMed] [Google Scholar]
- Yoon S.B., Lee Y.J., Park S.K., Kim H.C., Bae H., Kim H.M., Ko S.G., Choi H.Y., Oh M.S., Park W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009;125:286–290. doi: 10.1016/j.jep.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Liu Y.J., Luo Y.J., Huang L.Q., Chen S.Q., Yang Z.C., Qin S.S. High temperature effects on flavones accumulation and antioxidant system in Scutellaria baicalensis Georgi cells. Afr. J. Biotechnol. 2011;10:5182–5192. [Google Scholar]
- Zhang L., Ravipati A.S., Koyyalamudi S.R., Jeong S.C., Reddy N., Smith P.T., Bartlett J., Shanmugam K., Münch G., Wu M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011;59:12361–12367. doi: 10.1021/jf203146e. [DOI] [PubMed] [Google Scholar]
- Zhang T., Chen D.F. Anticomplementary principles of a Chinese multiherb remedy for the treatment and prevention of SARS. J. Ethnopharmacol. 2008;117:351–361. doi: 10.1016/j.jep.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo F., Zhou Z.M., Yan M.Z., Liu L.M., Xiong Y.L., Zhang Q., Song H.Y., Ye W.H. Metabolism of constituents in Huangqin-Tang, a prescription in Traditional Chinese Medicine, by human intestinal flora. Biol. Pharm. Bull. 2002;25:558–563. doi: 10.1248/bpb.25.558. [DOI] [PubMed] [Google Scholar]
- Zuo F., Zhou Z.M., Zhang Q., Mao D., Xiong Y.L., Wang Y.L., Yan M.Z., Liu M.L. Pharmacokinetic study on the multi-constituents of Huangqin-Tang decoction in rats. Biol. Pharm. Bull. 2003;26:911–919. doi: 10.1248/bpb.26.911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material