Abstract
Epidemiological and clinical studies have shown that environmental factors such as infections, smoking and vitamin D are associated with the risk of developing multiple sclerosis (MS). Some of these factors also play a role in the MS disease course. We are currently beginning to understand how environmental factors may impact immune function in MS on a cellular and molecular level. Here we review epidemiological, clinical and basic immunological studies on the environmental factors, viral and parasitic infections, smoking, and vitamin D and relate epidemiological findings with their likely pathophysiology in MS.
Keywords: Multiple sclerosis, Risk factors, Epidemiology, Immunology, Autoimmunity
1. Introduction
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) in which an interplay of genetic and environmental factors leads to the chronic activation of immune cells and to neuronal injury. Epidemiological studies have identified several environmental risk factors in MS, including exposure to certain viruses and smoking; conversely, the lack of exposure to sunlight is correlated with an increased risk for MS. While most of these factors are associated with the susceptibility to developing MS, more recent studies show that some of these factors also impact on the MS disease course.
However, the identification of environmental risk factors in MS is made difficult by the fact that no single risk factor in isolation appears to be responsible for the development of the disease; most likely a multifactorial interplay of factors determines the susceptibility to MS as well as the disease course. Given the many possible factors that may influence MS susceptibility and disease course, studies need to be large enough and the data needs to be of high quality to determine the impact of individual possible risk factors. For example, diet and the exposure to organic solvents may play a role in MS, but the investigation of these risk factors is difficult, because high quality data on these factors over a long observation period is often not available. The results of studies on these factors, are therefore less reliable and sometimes contradictory [1], [2], [3], [4].
In this article we provide an overview of the immunopathogenesis of MS and discuss how environmental factors may affect aspects of immune function in MS.
2. Immunopathogenesis of MS
The etiology of MS is unresolved [5]. One hypothesis postulates that various classes of immune cells are activated in the periphery and then enter the CNS to produce pathology. An alternate hypothesis infers that the initial dysfunction is within the CNS and need not be immunological or inflammatory; potential pathologic processes include mitochondrial dysfunction in neurons or oligodendrocytes, axonal energy insufficiency, or inactivation of neural organelles such as peroxisomes [6]. Following the initial injury, the leakage of CNS antigens into draining lymph nodes contributes to the activation of T cells and other immune cell subsets that enter the CNS. Thus, whatever the origin of MS pathology, the result is the activation and recruitment of immune cell subsets into the CNS which then produce the hallmarks of MS pathology: demyelination, oligodendrocyte loss, and axonal/neuronal injury and loss [7].
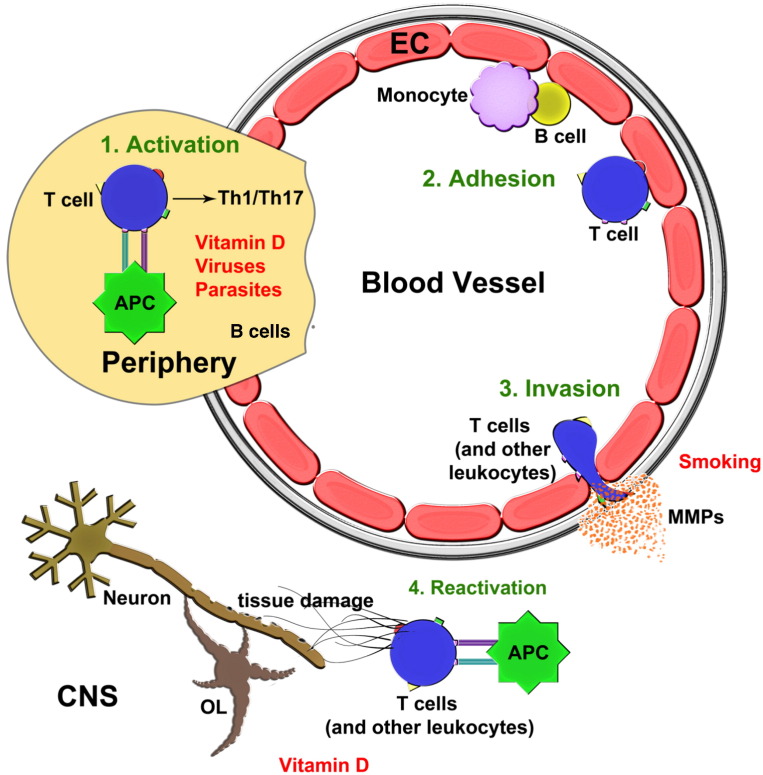
Antigen presentation (Fig. 1 ) is necessary to activate T cells. Commonly, a pathogen in exposed tissues is engulfed and broken down by antigen presenting cells (APC) such as dendritic cells. The APCs then migrate to lymph nodes, carrying a short segment of the pathogen, the antigen, bound to major histocompatibility complex (MHC) on the cell surface. In the lymph nodes, the antigen is presented to naïve T cells such that those with T-cell receptors (TCR) recognizing the antigen/MHC combination become initially engaged. The antigen/MHC and TCR interaction constitutes a first signal. A second signal, mediated by co-stimulatory molecules (e.g. B7 on antigen presenting cells and CD28 on T cells), is necessary for T-cells to undergo activation, proliferation and subsequent differentiation into effector cells.
Fig. 1.
Steps in the immunopathogenesis of MS and modulation by environmental factors.
An initial activation of T cells by antigen presenting cells in the periphery [1], particularly in lymph nodes, leads to the increased generation of Th1 and/or Th17 pro-inflammatory subsets in MS. These cells, and other leukocyte subsets including monocytes and B cells, transit in blood where they can adhere [2] onto inflamed endothelial cells (EC). Predominantly at post-capillary venules in the CNS, the leukocyte populations produce MMPs to invade [3] across the glia limitans into the CNS parenchyma. T cells may undergo reactivation within the CNS and they, together with other leukocyte populations that have entered the CNS, contribute to tissue injury [4]. This figure also displays where the environmental factors that influence MS may act in the cascade of immunopathogenesis.
CD8 + T cells are activated by the engagement of TCR on CD8 + T cells with antigen/MHC class I complex, while CD4 + cells are activated by the engagement of TCR on CD4 + T cells with antigen/MHC class II complex. CD4 + T cells are particularly interesting in MS as they can differentiate into pro-inflammatory T helper (Th) 1 or 17 subsets, anti-inflammatory Th2 cells, or into cells with regulatory/anti-inflammatory properties (Tregs), depending on the microenvironment and cytokine milieu [8]. CD4 + T cells in MS patients tend to differentiate into Th1 and/or Th17 subsets, which are not only pro-inflammatory [9], but potentially neurotoxic as well [10]. In contrast to the propensity to generate Th1/17, there is an apparent deficiency of the regulatory/anti-inflammatory Th2 and Tregs subsets in MS, on the whole favoring a pro-inflammatory milieu [11]. Besides the CD4 + subsets, CD8 + T cells also have several tissue damaging roles in MS [12].
B cells are also important contributors to disease activity in MS. The best supporting evidence for this is that monoclonal antibodies that target the B cell antigen, CD20, are effective therapies in MS [13], [14]. Oligoclonal bands in the cerebrospinal fluid, representing immunoglobulins of many specificities, are common in MS patients, and B cell follicular-like structures can be found in the meninges of many patients with MS [15]. Besides the production of antibodies targeting CNS structures such as proteins at the node of Ranvier [16], the pathogenic roles of B cells are thought to be related to their cellular functions such as antigen presentation and providing help for T cells [17].
When activated, immune cells upregulate various adhesion molecules and adhere to endothelial cells of post-capillary venules in the CNS. After crossing the endothelial cell barrier, immune cells readily cross the endothelial basement membrane. Then, in the presence of proteolytic activity largely furnished by the family of matrix metalloproteinases (MMPs), they migrate across a second basement membrane barrier, the glia limitans or parenchymal basement membrane, into the CNS parenchyma (Fig. 1). Without proteolytic activity, immune cells are trapped in the perivascular space that separates both basement membrane layers [18]. However, MMPs are upregulated in MS which facilitates the transmigration of activated immune cells into the CNS parenchyma [19]. MMPs themselves are induced by an upstream switch, Extracellular Matrix Metalloproteinase Inducer, EMMPRIN (CD147) [20].
Upon entering the CNS parenchyma, T cells can become reactivated through repeated antigen presentation by APCs such as microglia, macrophages, B cells and dendritic cells. The products of activated immune cell subsets include free radicals, glutamate and other excitotoxins, proteases, and cytokines, all of which can cause or promote neuronal injury and loss [10], [21].
3. Viral infections and MS
Findings from migration studies have led to the idea that a viral infection may trigger the development of MS. Several studies have shown that people migrating from a country of high MS prevalence to one of lower prevalence are at lower risk of developing MS than they would be in their country of origin. Those migrating from a low-risk country to a high-risk one retain the low risk of their country of origin, while their children have a risk comparable to the country they emigrate to [for review see [22]]. Further studies suggested that this effect of migration on the risk of developing MS was stronger in those migrating before the age of 15 [23], suggesting that an infection at a young age may predispose to the later development of MS. In addition to these migration studies, some classical studies on the incidence and prevalence of MS have suggested that there may have been ‘MS epidemics’ in several locations, with the rise in MS incidence after the second world war in the Faroe islands as the best known example [24], [25]. Similar increases in the Shetland islands [26], Ferrara [27] and Sardinia [28] have been taken to suggest that an infectious agent may be involved in the pathogenesis of MS.
3.1. Epidemiological and clinical studies
Several viruses have been implicated as factors that may influence MS risk. These include the Epstein–Barr-Virus (EBV), Herpes simplex virus (HSV) types 1 and 2, human herpesvirus 6, measles, mumps and rubella. The evidence for and against a role of most of these viruses in MS is inconsistent, except for that supporting EBV.
Studies on the influence of EBV in MS are generally difficult due to the very high prevalence of EBV seropositivity in the general population (around 95% seropositivity by adulthood) which translates into very large sample sizes to achieve adequate power in epidemiological studies. Despite this methodological challenge, seronegativity for EBV is associated with a very low risk of MS. A meta-analysis including 1779 MS patients and 2526 non-MS controls showed an OR of 0.06 (95% CI: 0.03–0.13) for developing MS in seronegative persons compared to seropositives [29]. A history of symptomatic EBV infection (infectious mononucleosis), on the other hand, roughly doubled the risk of developing MS, as shown in a recent meta analysis including 19,390 patients and 16,007 controls (relative risk: 2.17, 95% CI: 1.97–2.39) [30].
Studies on the association of EBV with the disease course of MS have shown diverging results. One small prospective cohort study (n = 73) found that patients with a higher serum antibody titer to EBV early antigen (EA) had significantly more gadolinium enhancing lesions on MRI, while their disability and other clinical characteristics did not differ [31]. Another prospective cohort study followed 100 patients over a period of five years and found significant but relatively weak correlations (Spearman's rank correlation r = 0.27 to 0.33) between serum anti-Epstein Barr nuclear antigen-1 (EBNA1) IgG titers and the number of gadolinium enhancing lesions, change in T2 lesion volume, and change in EDSS scores [32].
The possible influence of EBV on the early stages of the disease process in MS was investigated in another cohort study which compared 147 patients with CIS with 50 matched controls. Increased levels of serum EBNA1 IgG titers were found among the CIS patients, as well as significant, although weak correlations of serum EBNA1 IgG titers with gadolinium enhancing lesions, the number of T2 lesions, and EDSS scores (Spearman's rank correlation r = 0.21 to 0.3) [33]. This apparent relationship of EBV antibody titers and measures of disease activity has not consistently been found however, as Ingram and co-workers found no correlation between serum EBNA1 IgG titers and EDSS and MSSS scores in their investigation of 75 MS patients [34].
Most observational studies compared healthy controls to MS patients and limited their analyses to EBV serology. A clearer picture has recently emerged through studies with different controls. Pohl and colleagues found elevated antibody indices to EBV, but also to other neurotropic viruses in the CSF and serum from 43 children and 50 adults with MS. Interestingly, the antibody indices for measles, rubella, varicella zoster virus and herpes simplex virus were all higher than those for EBV. This suggests that the humoral immune response that elevates EBV titers in MS is not exclusively directed against EBV antigens but is part of a general polyspecific immune response [35]. These findings were confirmed by a similar study in patients with CIS or MS [36].
3.2. Immunology of viral infections and MS
There are several hypotheses to explain how viral infections are associated with MS [37]. One hypothesis is that of “bystander activation” which postulates that autoreactive T cells are activated by nonspecific inflammatory molecules occurring during infections, such as cytokines, superantigens and toll-like receptor ligands [38].
Yet another hypothesis is that viruses activate immune cells through the process of “molecular mimicry” [39], [40]. The hypothesis postulates that upon exposure to a pathogen, the pathogen/MHC conformation on an APC bears molecular similarity to that of a endogenous peptide, such as a fragment from myelin basic protein (MBP), presented within an MHC. If co-stimulatory molecules are properly engaged, the result could be the expansion and differentiation of not only the pathogen-reactive T cells, a properly directed immune response, but also the expansion of MBP-reactive T cells, an improper outcome. If both pools differentiate into Th1 or Th17 pro-inflammatory subsets (Fig. 1), these can become reactivated within the CNS to promote pathology. In support of this idea, T cell lines isolated from MS patients demonstrate cross-reactivity between MBP and coronavirus [41] or EBV [42] antigens. Moreover, crystal structure analyses revealed a significant degree of structural similarity between the DRB5*0101-EBV peptide complex to the DRB1*1501-MBP peptide complex at the cell surface for TCR recognition [43].
Overall, given that there are several pathogens with molecular similarity to a number of myelin peptides and other molecules within the CNS, the probability is high that several viruses can lead to the improper expansion of CNS-reactive T cells that can promote pathology within the CNS. For this reason it will be difficult to pinpoint a single infectious pathogen uniquely associated with MS.
There are other immunological bases to the association of EBV with MS. The follicular-like structures under the meninges harbor B cells that are infected with EBV in many patients and this is thought to be a Trojan horse-like mechanism by which EBV infects the brain [44], [45]. MS subjects have antibodies that cross-react between MBP and EBV [46], providing a mechanism by which EBV-reactive antibodies may disrupt myelin. Moreover, cellular immune responses such as EBV-reactive CD8 + T cells that are restricted by HLA-B7, a common allele in MS, are dysregulated in MS [47]. Pender has postulated an initial CD8 + T cell deficiency in MS that impairs the capacity to control EBV infection with the result that EBV-infected B cells accumulate in the CNS where they produce pathogenic autoantibodies and provide survival signals to autoreactive T cells [48].
4. Parasitic infections and MS
4.1. Clinical studies
Although there is no evidence that parasitic infections impact MS risk they may impact disease activity. Report from a single MS center in Argentina noted that 12 patients with relapsing-remitting MS patients had eosinophilia, caused by mild, asymptomatic intestinal parasitosis. These patients were compared with an age and gender-matched relapsing-remitting MS group with no evidence of intestinal parasites and prospectively followed for more than seven years [49], [50]. Interestingly, the number of new relapses, the accumulation of disability and the number of new enhancing lesions on MRI were all much lower in the parasite infected group than in the uninfected control group. When four of the 12 patients became symptomatic with regards to their infection they received anti-parasite medication. This resulted in clearing of the parasitic infection but they experienced an increase in relapses, MRI lesions and disability [50].
These findings encouraged others to undertake a pilot trial of five relapsing-remitting MS patients where treatment consisted of parasites being introduced by ingestion of helminth eggs. Preliminary results showed that the number of new enhancing MRI lesions was reduced during treatment [51].
4.2. Immunology
Exposure to parasites results in a characteristic immunological response aimed at eradicating the parasites: a CD4 + T helper cell response that is predominantly Th2 biased, increases in the frequency of regulatory T cells and regulatory B cells, amongst others. These are, on balance, anti-inflammatory or regulatory and can return an active immune response back to homeostasis. In the context of MS, the elevation of these anti-inflammatory and regulatory immune responses would help overcome the pro-inflammatory conditions seen in MS. Indeed, in the infected MS patients described in the Argentinean study, they had higher levels of Th2 anti-inflammatory cells, and regulatory T and B cells compared to the uninfected MS control group [49], [50], [52].
5. Smoking
5.1. Epidemiological and clinical studies
Smoking has recently been recognized as an environmental risk factor in MS. Convincing evidence that smoking increases the susceptibility to MS comes from an analysis of the The Nurses' Health Study, which showed that the relative incidence rate of MS in current smokers compared to never smokers was 1.6 (95% confidence interval (CI) 1.2–2.1), with a dose–response dependent on pack years smoked [53]. Two studies have also shown that second hand smoke exposure increased the risk of developing MS among children (adjusted rate ratio of 2.12, 95% CI 1.43–3.15) [54] and adults (OR 1.3, 95% CI 1.1–1.6) [55].
Smoking also seems to impact inflammatory outcomes in MS. A study in patients with a clinically isolated syndrome (CIS) showed an increased risk of conversion to clinically definite MS in smokers compared to non-smokers (HR: 1.8 (95% confidence interval: 1.2–2.8)) [56]. One imaging cross-sectional study showed that smokers had more gadolinium enhancing lesions, a greater T2-lesion load and more brain atrophy than non-smokers [57]. Another study followed patients for an average of 2.8 years and reported a quicker increase in T2 lesion volume and brain atrophy in smokers [58].
The association between smoking and disease progression, however, is less clear. A study from a hospital based MS cohort suggested that smoking was neither associated with the risk of secondary progression (HR: 0.89, 95% CI: 0.60–1.32) nor with that of reaching EDSS 4.0 (HR: 0.93, 95% CI: 0.66–1.33) or EDSS 6.0 (HR: 0.88, 95% CI: 0.61–1.28) [59]. An analysis of data collected by general practitioners however suggested that smoking is associated with a greater risk of evolving to a secondary progressive course (hazard ratio (HR): 3.6, 95% CI: 1.3–9.9) [60]. The most recent large hospital based cohort-study also found smoking to be associated with the risk of secondary progression (HR: 2.50, 95% CI: 1.42–4.41), but not with an increase in EDSS scores [58]; and in a prospective Australian study, smoking was associated with an increase in EDSS scores during two years of follow-up [61]. Thus, while it appears that smoking influences the early disease course of MS, more research is needed to ascertain its relative impact on early versus late disease stages of MS.
5.2. Immunology of smoking in MS
It is not known how smoking increases the risk of MS and although cigarette smoke contains many mutagens which potentially may affect long lasting immunity, a recent review indicates that smoking generally leads to an immunosuppressant state [62]. Nonetheless, aspects of immune functions are promoted by cigarette smoking and genetic studies indicate an interaction between smoking and genes that regulate immune function [63]. It may be instructive to determine whether constituents of tobacco alter signaling through the aryl hydrocarbon receptor, a transcription factor affected by polycyclic aromatic hydrocarbons and polychlorinated dioxins; the latter can regulate T cell polarization and alter the course of experimental autoimmune encephalomyelitis [64], an animal model of MS.
One potential mechanism by which smoking affects MS is by upregulating MMPs since immune cells and biological fluids of smokers tend to have higher levels of several MMPs [65]. The higher expression of MMPs in smokers could be advantageous for immune cells to cross the blood brain barrier into the CNS parenchyma. It is noteworthy that a comparison of MRI scans from smokers and non-smokers with MS showed more contrast enhancing lesions among the smokers, which suggests an increased breakdown of the blood brain barrier [57].
6. Sunlight exposure and vitamin D
6.1. Epidemiological and clinical studies
That MS is more prevalent in regions of higher latitude has been shown in many populations [66] and more recently this phenomenon has been associated with sunlight (UV) exposure and the subsequent production of vitamin D [67]. In a retrospective study Van der Mei and colleagues showed that the risk of MS is decreased in people with greater exposure to sunlight [68]. More recently it was demonstrated that the risk of developing MS decreased with increasing serum 25-hydroxy-vitamin D levels (OR: 0.59; 95% CI: 0.36–0.97 per 50 nmol/L increase) in a prospective nested case–control study; 25-hydroxy-vitamin D levels from routine serum samples were related to the subsequent risk of developing MS in US military personnel [69]. In a study that calculated the risks of MS posed by various suspected environmental factors in MS, the lack of UV exposure was found to be the most significant risk factor [70].
There is also mounting evidence that vitamin D influences the disease course of MS. Cross-sectional studies showed that low levels of vitamin D and one consequence of low vitamin D levels, lower bone mineral density, are common in MS [71], and that lower vitamin D levels are associated with higher levels of disability [72]. Evidence for an association between vitamin D and relapses comes from a prospective cohort study which showed a decreased risk of relapse during the subsequent six months in patients with higher serum vitamin D levels (HR per 10 nmol/L increase 0.91, 95% CI: 0.85–0.97) [73]. A retrospective cohort study showed similar results in children with MS [74].
There is also some evidence to suggest that vitamin D supplementation may be an effective treatment for MS. Burton and co-workers performed a randomized controlled trial investigating high-dose vitamin D treatment in MS and found a trend towards decreased relapses during treatment [75].
6.2. Immunology
Although other immune modulatory mechanisms potentially exist for ultraviolet radiation [76], the association of sunlight with MS is most likely due to the ultraviolet B radiation of sunlight (290–320 nm) converting cutaneous 7-dehydrocholesterol to previtamin D3, which then spontaneously forms vitamin D3. The latter then undergoes 2 hydroxylations, most notably in the liver and kidney by d-25-hydroxylase (CYP2R1) and 25-hydroxyvitamin d-1α-hydroxylase (CYP27B1), to produce the biologically active form of vitamin D, 1,25-dihydroxyvitamin D3. Notably, variants of the CYP27B1 gene have been found to be associated with increased risk of MS [77]. Another study found association of MS with CYP27B1 and a second region containing another vitamin D associated gene, CYP24A1 [78], a vitamin D response element lies close to the promoter region of HLA-DRB1, the main risk allele for MS [79].
The utility of vitamin D in MS is almost certainly due to its multiple immune-regulating properties that impact on the majority of the immunopathogenic steps outlined in Fig. 1. Vitamin D suppresses the maturation and activity of APCs, including dendritic cells, or increases their “tolerogenic” phenotype [80], thereby reducing the probability of presenting self-antigens to generate autoreactive cells, or decreasing the likelihood of expanding autoreactive cells through molecular mimicry.
The differentiation of CD4 + T helper cells is also affected by vitamin D, with a reduced production of pro-inflammatory Th1 and Th17 cells [81]; concurrently, Th2 cells are increased [82], thereby helping to reduce the pro-inflammatory state that typifies MS. Other major immunologic consequences of vitamin D treatment are the increased activity of regulatory T cells [81] and the reduction of pro-inflammatory molecules produced by stimulated monocytes [83]. These immunologic changes caused by vitamin D can be detected in culture, in samples obtained from MS patients, and in EAE. In EAE, treatment with vitamin D either prevents the development of symptoms when given early in the disease course [84], or reduces the severity of EAE when treatment is initiated at onset of clinical signs or at peak disease [85].
Vitamin D is known to penetrate into the CNS, and it may potentially have several activities within the CNS including the reduction of antigen presentation to reduce neuroinflammation. More intriguing is the possibility that vitamin D may be neuro-protective. The enzymes necessary to synthesize the bioactive 1,25-dihydroxyvitamn D3 are present in the brain [86]. In rats that are deficient of vitamin D during gestation, abnormal brain development such as alteration of brain shape and enlargement of lateral ventricle volume has been observed. Mice with gestational vitamin D deficiency have impaired learning in adulthood [87]. In tissue culture, glutamate excitotoxicity to cortical, cerebellar or hippocampal neurons is reduced by vitamin D [88]. To what extent these neuroprotective activities of vitamin D account for its effect in MS remains to be clarified. Overall, there is substantial evidence that vitamin D corrects many of the immune abnormalities seen in MS. It is not clear which of its multiple mechanisms are most critical to its therapeutic efficacy. Whether some of the benefits of vitamin D may relate to its actions within the CNS remains to be determined.
7. Conclusion
We are now at a point where the separate fields of MS epidemiology and basic laboratory research come together to improve our overall understanding of the disease process of MS. Recent epidemiological studies have identified new risk factors, not only associated with the risk of developing MS, but also, and perhaps more immediately important, with the MS disease course. While previous factors associated with MS disease course, such as gender or the age at disease onset, are not modifiable, the newly identified factors such as smoking and vitamin D levels are modifiable through individual and public health measures.
Future studies on risk factors in MS will need to build upon the classical epidemiological studies and integrate the new factors into the “big picture”. Such studies will need to unravel how the whole set of classic and newly identified factors interact with each other, how they are modulated by immunomodulatory drugs, genetic factors, gender and hormones, and at which stages of disease they have their greatest impact.
Basic laboratory research can help us understand the mechanisms that underlie these epidemiological associations, and hopefully lead to useful experimental models and eventually new treatments.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Ascherio A., Munger K.L. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. Jun 2007;61(6):504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 2.Grønning M., Albrektsen G., Kvåle G., Moen B., Aarli J.A., Nyland H. Organic solvents and multiple sclerosis: a case–control study. Acta Neurol Scand. Oct 1993;88(4):247–250. doi: 10.1111/j.1600-0404.1993.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen J.T., Brønnum-Hansen H., Rasmussen K. Multiple sclerosis and organic solvents. Epidemiology. Mar 1998;9(2):168–171. [PubMed] [Google Scholar]
- 4.Riise T., Moen B.E., Kyvik K.R. Organic solvents and the risk of multiple sclerosis. Epidemiology. Nov 2002;13(6):718–720. doi: 10.1097/00001648-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Trapp B.D., Nave K.-A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 6.Kassmann C.M., Lappe-Siefke C., Baes M., Brügger B., Mildner A., Werner H.B. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet. Aug 2007;39(8):969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- 7.Yong V.W., Marks S. The interplay between the immune and central nervous systems in neuronal injury. Neurology. Jan 5 2010;74(Suppl. 1):S9–S16. doi: 10.1212/WNL.0b013e3181c97d04. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. May 11 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 9.Becher B., Segal B.M. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol. Dec 2011;23(6):707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siffrin V., Radbruch H., Glumm R., Niesner R., Paterka M., Herz J. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. Sep 24 2010;33(3):424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Viglietta V., Baecher-Allan C., Weiner H.L., Hafler D.A. Loss of functional suppression by CD4+CD25 + regulatory T cells in patients with multiple sclerosis. J Exp Med. Apr 5 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena A., Martin-Blondel G., Mars L.T., Liblau R.S. Role of CD8 T cell subsets in the pathogenesis of multiple sclerosis. FEBS Lett. Dec 1 2011;585(23):3758–3763. doi: 10.1016/j.febslet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 13.Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. Feb 14 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 14.Kappos L., Li D., Calabresi P.A., O'Connor P., Bar-Or A., Barkhof F. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. Nov 19 2011;378(9805):1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 15.Magliozzi R., Howell O., Vora A., Serafini B., Nicholas R., Puopolo M. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. Apr 2007;130(Pt 4):1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 16.Meinl E., Derfuss T., Krumbholz M., Pröbstel A.-K., Hohlfeld R. Humoral autoimmunity in multiple sclerosis. J Neurol Sci. Jul 15 2011;306(1–2):180–182. doi: 10.1016/j.jns.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 17.von Büdingen H.-C., Bar-Or A., Zamvil S.S. B cells in multiple sclerosis: connecting the dots. Curr Opin Immunol. Dec 2011;23(6):713–720. doi: 10.1016/j.coi.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrawal S., Anderson P., Durbeej M., van Rooijen N., Ivars F., Opdenakker G. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. Apr 17 2006;203(4):1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal S.M., Lau L., Yong V.W. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. Feb 2008;19(1):42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal S.M., Silva C., Tourtellotte W.W., Yong V.W. EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci. Jan 12 2011;31(2):669–677. doi: 10.1523/JNEUROSCI.3659-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikić I., Merkler D., Sorbara C., Brinkoetter M., Kreutzfeldt M., Bareyre F.M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. Apr 2011;17(4):495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- 22.Gale C.R., Martyn C.N. Migrant studies in multiple sclerosis. Prog Neurobiol. Dec 1995;47(4–5):425–448. [PubMed] [Google Scholar]
- 23.Alter M., Leibowitz U., Speer J. Risk of multiple sclerosis related to age at immigration to Israel. Arch Neurol. Sep 1966;15(3):234–237. doi: 10.1001/archneur.1966.00470150012002. [DOI] [PubMed] [Google Scholar]
- 24.Kurtzke J.F., Hyllested K. Multiple sclerosis in the Faroe Islands: I. Clinical and epidemiological features. Ann Neurol. Jan 1979;5(1):6–21. doi: 10.1002/ana.410050104. [DOI] [PubMed] [Google Scholar]
- 25.Joensen P. Multiple sclerosis: variation of incidence of onset over time in the Faroe Islands. Mult Scler. Feb 2011;17(2):241–244. doi: 10.1177/1352458510386997. [DOI] [PubMed] [Google Scholar]
- 26.Poskanzer D.C., Sheridan J.L., Prenney L.B., Walker A.M. Multiple sclerosis in the Orkney and Shetland Islands. II: The search for an exogenous aetiology. J Epidemiol Community Health. Dec 1980;34(4):240–252. doi: 10.1136/jech.34.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casetta I., Granieri E., Malagù S., Tola M.R., Paolino E., Caniatti L.M. Environmental risk factors and multiple sclerosis: a community-based, case–control study in the province of Ferrara, Italy. Neuroepidemiology. 1994;13(3):120–128. doi: 10.1159/000110369. [DOI] [PubMed] [Google Scholar]
- 28.Rosati G., Aiello I., Granieri E., Pirastru M.I., Becciu S., Demontis G. Incidence of multiple sclerosis in Macomer, Sardinia, 1912–1981: onset of the disease after 1950. Neurology. Jan 1986;36(1):14–19. doi: 10.1212/wnl.36.1.14. [DOI] [PubMed] [Google Scholar]
- 29.Ascherio A., Munger K.L. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. Apr 2007;61(4):288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 30.Handel A.E., Williamson A.J., Disanto G., Handunnetthi L., Giovannoni G., Ramagopalan S.V. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS ONE [Internet] 2010;5(9) doi: 10.1371/journal.pone.0012496. [cited 2011 Nov 1; Available from: http://www.ncbi.nlm.nih.gov/pubmed/20824132] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buljevac D., van Doornum G.J.J., Flach H.Z., Groen J., Osterhaus A.D.M.E., Hop W. Epstein–Barr virus and disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. Oct 2005;76(10):1377–1381. doi: 10.1136/jnnp.2004.048504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell R.A., Antony D., Wall G.R., Clark D.A., Fisniku L., Swanton J. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology. Jul 7 2009;73(1):32–38. doi: 10.1212/WNL.0b013e3181aa29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lünemann J.D., Tintoré M., Messmer B., Strowig T., Rovira A., Perkal H. Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann Neurol. Feb 2010;67(2):159–169. doi: 10.1002/ana.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingram G., Bugert J.J., Loveless S., Robertson N.P. Anti-EBNA-1 IgG is not a reliable marker of multiple sclerosis clinical disease activity. Eur J Neurol. Nov 2010;17(11):1386–1389. doi: 10.1111/j.1468-1331.2010.03083.x. [DOI] [PubMed] [Google Scholar]
- 35.Pohl D., Rostasy K., Jacobi C., Lange P., Nau R., Krone B. Intrathecal antibody production against Epstein–Barr and other neurotropic viruses in pediatric and adult onset multiple sclerosis. J Neurol. Feb 2010;257(2):212–216. doi: 10.1007/s00415-009-5296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otto C., Oltmann A., Stein A., Frenzel K., Schroeter J., Habbel P. Intrathecal EBV antibodies are part of the polyspecific immune response in multiple sclerosis. Neurology. Apr 12 2011;76(15):1316–1321. doi: 10.1212/WNL.0b013e318215286d. [DOI] [PubMed] [Google Scholar]
- 37.Kakalacheva K., Münz C., Lünemann J.D. Viral triggers of multiple sclerosis. Biochim Biophys Acta. Feb 2011;1812(2):132–140. doi: 10.1016/j.bbadis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sospedra M., Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 39.Libbey J.E., McCoy L.L., Fujinami R.S. Molecular mimicry in multiple sclerosis. Int Rev Neurobiol. 2007;79:127–147. doi: 10.1016/S0074-7742(07)79006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chastain E.M.L., Miller S.D. Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol Rev. Jan 2012;245(1):227–238. doi: 10.1111/j.1600-065X.2011.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talbot P.J., Paquette J.S., Ciurli C., Antel J.P., Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann Neurol. Feb 1996;39(2):233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng W., Ma Y., Gong F., Hu C., Qian L., Huang Q. Cross-reactivity of autoreactive T cells with MBP and viral antigens in patients with MS. Front Biosci. 2012;17:1648–1658. doi: 10.2741/4010. [DOI] [PubMed] [Google Scholar]
- 43.Lang H.L.E., Jacobsen H., Ikemizu S., Andersson C., Harlos K., Madsen L. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. Oct 2002;3(10):940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 44.Serafini B., Severa M., Columba-Cabezas S., Rosicarelli B., Veroni C., Chiappetta G. Epstein–Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: implications for viral persistence and intrathecal B-cell activation. J Neuropathol Exp Neurol. Jul 2010;69(7):677–693. doi: 10.1097/NEN.0b013e3181e332ec. [DOI] [PubMed] [Google Scholar]
- 45.Meier U.-C., Giovannoni G., Tzartos J.S., Khan G. Translational Mini-Review Series on B cell subsets in disease. B cells in multiple sclerosis: drivers of disease pathogenesis and Trojan horse for Epstein–Barr virus entry to the central nervous system? Clin Exp Immunol. Jan 2012;167(1):1–6. doi: 10.1111/j.1365-2249.2011.04446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabibov A.G., Belogurov A.A., Jr., Lomakin Y.A., Zakharova M.Y., Avakyan M.E., Dubrovskaya V.V. Combinatorial antibody library from multiple sclerosis patients reveals antibodies that cross-react with myelin basic protein and EBV antigen. FASEB J. Dec 2011;25(12):4211–4221. doi: 10.1096/fj.11-190769. [DOI] [PubMed] [Google Scholar]
- 47.Jilek S., Schluep M., Harari A., Canales M., Lysandropoulos A., Zekeridou A. HLA-B7-restricted EBV-specific CD8 + T cells are dysregulated in multiple sclerosis. J Immunol. May 1 2012;188(9):4671–4680. doi: 10.4049/jimmunol.1103100. [DOI] [PubMed] [Google Scholar]
- 48.Pender M.P. CD8 + T-cell deficiency, Epstein–Barr Virus infection, vitamin D deficiency, and steps to autoimmunity: a unifying hypothesis. Autoimmune Dis. 2012;2012:189096. doi: 10.1155/2012/189096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Correale J., Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. Feb 2007;61(2):97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 50.Correale J., Farez M.F. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. Apr 2011;233(1–2):6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Fleming J., Isaak A., Lee J., Luzzio C., Carrithers M., Cook T. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler. Jun 2011;17(6):743–754. doi: 10.1177/1352458511398054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Correale J., Farez M., Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. Aug 2008;64(2):187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 53.Hernán M.A., Olek M.J., Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol. Jul 1 2001;154(1):69–74. doi: 10.1093/aje/154.1.69. [DOI] [PubMed] [Google Scholar]
- 54.Mikaeloff Y., Caridade G., Tardieu M., Suissa S. Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain. Oct 2007;130(Pt 10):2589–2595. doi: 10.1093/brain/awm198. [DOI] [PubMed] [Google Scholar]
- 55.Hedström A., Bäärnhielm M., Olsson T., Alfredsson L. Exposure to environmental tobacco smoke is associated with increased risk for multiple sclerosis. Mult Scler. Jul 2011;17(7):788–793. doi: 10.1177/1352458511399610. [DOI] [PubMed] [Google Scholar]
- 56.Di Pauli F., Reindl M., Ehling R., Schautzer F., Gneiss C., Lutterotti A. Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. Mult Scler. Sep 2008;14(8):1026–1030. doi: 10.1177/1352458508093679. [DOI] [PubMed] [Google Scholar]
- 57.Zivadinov R., Weinstock-Guttman B., Hashmi K., Abdelrahman N., Stosic M., Dwyer M. Smoking is associated with increased lesion volumes and brain atrophy in multiple sclerosis. Neurology. Aug 18 2009;73(7):504–510. doi: 10.1212/WNL.0b013e3181b2a706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Healy B.C., Ali E.N., Guttmann C.R.G., Chitnis T., Glanz B.I., Buckle G. Smoking and disease progression in multiple sclerosis. Arch Neurol. Jul 2009;66(7):858–864. doi: 10.1001/archneurol.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch M., van Harten A., Uyttenboogaart M., De Keyser J. Cigarette smoking and progression in multiple sclerosis. Neurology. Oct 9 2007;69(15):1515–1520. doi: 10.1212/01.wnl.0000277658.78381.db. [DOI] [PubMed] [Google Scholar]
- 60.Hernán M.A., Jick S.S., Logroscino G., Olek M.J., Ascherio A., Jick H. Cigarette smoking and the progression of multiple sclerosis. Brain. Jun 2005;128(Pt 6):1461–1465. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- 61.Pittas F., Ponsonby A.-L., van der Mei I.A.F., Taylor B.V., Blizzard L., Groom P. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J Neurol. 2009;256(4):577–585. doi: 10.1007/s00415-009-0120-2. [DOI] [PubMed] [Google Scholar]
- 62.Gonçalves R.B., Coletta R.D., Silvério K.G., Benevides L., Casati M.Z., da Silva J.S. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. May 2011;60(5):409–424. doi: 10.1007/s00011-011-0308-7. [DOI] [PubMed] [Google Scholar]
- 63.Hedström A.K., Sundqvist E., Bäärnhielm M., Nordin N., Hillert J., Kockum I. Smoking and two human leukocyte antigen genes interact to increase the risk for multiple sclerosis. Brain. Mar 2011;134(Pt 3):653–664. doi: 10.1093/brain/awq371. [DOI] [PubMed] [Google Scholar]
- 64.Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. May 1 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 65.Ozçaka O., Biçakci N., Pussinen P., Sorsa T., Köse T., Buduneli N. Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis. Oral Dis. Jan 2011;17(1):68–76. doi: 10.1111/j.1601-0825.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- 66.Simpson S., Jr., Blizzard L., Otahal P., Van der Mei I., Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(10):1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 67.Ascherio A., Munger K.L., Simon K.C. Vitamin D and multiple sclerosis. Lancet Neurol. Jun 2010;9(6):599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 68.van der Mei I.A.F., Ponsonby A.-L., Dwyer T., Blizzard L., Simmons R., Taylor B.V. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case–control study. BMJ. 2003;327(7410):316. doi: 10.1136/bmj.327.7410.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Munger K.L., Levin L.I., Hollis B.W., Howard N.S., Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. Dec 20 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 70.Sloka S., Silva C., Pryse-Phillips W., Patten S., Metz L., Yong V.W. A quantitative analysis of suspected environmental causes of MS. Can J Neurol Sci. Jan 2011;38(1):98–105. doi: 10.1017/s0317167100011124. [DOI] [PubMed] [Google Scholar]
- 71.Nieves J., Cosman F., Herbert J., Shen V., Lindsay R. High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis. Neurology. Sep 1994;44(9):1687–1692. doi: 10.1212/wnl.44.9.1687. [DOI] [PubMed] [Google Scholar]
- 72.Smolders J., Menheere P., Kessels A., Damoiseaux J., Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. Nov 2008;14(9):1220–1224. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 73.Simpson S., Taylor B., Blizzard L., Ponsonby A.-L., Pittas F., Tremlett H. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. Aug 2010;68(2):193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 74.Mowry E.M., Krupp L.B., Milazzo M., Chabas D., Strober J.B., Belman A.L. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. May 2010;67(5):618–624. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 75.Burton J.M., Kimball S., Vieth R., Bar-Or A., Dosch H.-M., Cheung R. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. Jun 8 2010;74(23):1852–1859. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hart P.H., Gorman S., Finlay-Jones J.J. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. Sep 2011;11(9):584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 77.Ramagopalan S.V., Dyment D.A., Cader M.Z., Morrison K.M., Disanto G., Morahan J.M. Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann Neurol. Dec 2011;70(6):881–886. doi: 10.1002/ana.22678. [DOI] [PubMed] [Google Scholar]
- 78.Sawcer S., Hellenthal G., Pirinen M., Spencer C.C.A., Patsopoulos N.A., Moutsianas L. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. Aug 11 2011;476(7359):214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramagopalan S.V., Maugeri N.J., Handunnetthi L., Lincoln M.R., Orton S.-M., Dyment D.A. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. Feb 2009;5(2):e1000369. doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Széles L., Keresztes G., Töröcsik D., Balajthy Z., Krenács L., Póliska S. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. Feb 15 2009;182(4):2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 81.Correale J., Ysrraelit M.C., Gaitán M.I. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. May 2009;132(Pt 5):1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 82.Sloka S., Silva C., Wang J., Yong V.W. Predominance of Th2 polarization by Vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;8:56. doi: 10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almerighi C., Sinistro A., Cavazza A., Ciaprini C., Rocchi G., Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. Mar 2009;45(3):190–197. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 84.Lemire J.M., Archer D.C. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. Mar 1991;87(3):1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cantorna M.T., Hayes C.E., DeLuca H.F. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. Jul 23 1996;93(15):7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smolders J., Moen S.M., Damoiseaux J., Huitinga I., Holmøy T. Vitamin D in the healthy and inflamed central nervous system: access and function. J Neurol Sci. Dec 15 2011;311(1–2):37–43. doi: 10.1016/j.jns.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 87.Fernandes de Abreu D.A., Nivet E., Baril N., Khrestchatisky M., Roman F., Féron F. Developmental vitamin D deficiency alters learning in C57Bl/6J mice. Behav Brain Res. Apr 2 2010;208(2):603–608. doi: 10.1016/j.bbr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 88.Brewer L.D., Thibault V., Chen K.C., Langub M.C., Landfield P.W., Porter N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001 Jan 1;21(1):98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]