Abstract
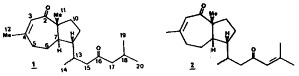
The structures of reiswigins A(1) and B(2), new reduced azulene diterpene enones have been determined by combined one and two dimensional NMR and mass spectral techniques.
The structures of reiswigins A(1) and B(2) have been determined by combined one and two dimensional NMR and mass spectral techniques.
References
- 1.Taxonomic identification of the sponge was made by S. Pomponi and C. Diaz. M.C. Diaz, B. Alvarez, and R.W.M. VanSoest, New Species of Demospongiae(Porifera) from the National Park “Archipelago de los Roques,” Venezuela. Bijdr. Dierk., In Press.
- 2.Antiviral assays for herpes simplex virus type 1 (HSV-1) and vesicular stomatitis virus (VSV) were plaque reduction tests in infected CV-1 monkey kidney cells. Coronavirus strain A59 inhibitions were measured by reduction in cell fusion and cytopathic effects in NCTC 1469 mouse liver cells. Readings in these assays range from “-” for no observable viral inhibition, to “3+”, denoting complete inhibition of virus by the test compound. Reiswigin A(1) was nontoxic at 2μg and completely inhibited HSV-1 and VSV. A59 was partially inhibited at 20μg with a 2+ reading. Reiswigin B(2) showed the same activity as 1 against HSV-1 and A-59.
- 3.Faulkner D.J. Nat. Prod. Rep. 1986:1–33. doi: 10.1039/np9860300001. [DOI] [PubMed] [Google Scholar]
- 4.Reiswigin A(1): MS(HREI) m/z 304,C20H32O2, 1.8%, 0.3ppm deviation, 1H NMR(360 MHz,CDCl3): δ 5.85s(1H,H-5), 2.5dt(1H,H-5,J=18.3,4.9), 2.44brd(1H,H-15,J=12.9), 2.38m(2H,H-17), 2.35d(1H,H-13,J=6.6), 2.33m(1H,H-5), 2.27brd(1H,H-15,J=14.5), 2.21sept(1H,H-18,J=6.6,0.6), 1.98s(3H,Me-12), 1.96m(1H,H-10), 1.90m(1H,H-6, 1.85m(1H,H-8), 1.80m(2H,H-6,H-9), 1.74dd(1H,H-10,J=9.6,1.6), 1.68ddd (H7,J=10.2,6.5,2.7), 1.39m(1H,H-9), 1.20s(3H,Me-11), 1.0d(6H,Me-19,Me-14,J=6.6), 0.98d(3H,me-20, J=6.6), IR(CHCl3): 2940br, 1700, 1630 cm−1, UV(heptane): 223nm(ε=2300) 205m(ε=5700), optical rotation [α]D20 =−10° (c= 0.1,CHCl3)
- 5.Reiswigin B(2): MS(HREI) m/z 302,0.5%) C20H30O2, 1H NMR(360 MHz,CDCl3)δ 6.04sht(1H,H-17,J=1.3), 5.82s(1H,H-3), 2.41m(2H,H-15), 2.27m(2H,H-5), 2.20m(1H,H-13), 2.13s(3H,Me-20), 1.88s(6H,Me-19,Me-12). 1.82m(1H,H-10) 1.74m(1H,H-8), 1.68m(3H,H-6,H-10), 1.64dt(1H,H-7,J=9.3,1.5), 1.32m(2H,H-9), 1.11s(3H,Me-11), 0.93d(3H,Me-14,J=6.5), 13C NMR(90 MHz, CDCl3): 207.9s, 201.3s, 155.6s, 153.2s, 127.8d, 124.8d, 57.1s, 48.6d, 47.0t, 45.8d, 35.7t, 35.3t, 30.8t, 29.1q, 28.2q, 25.2t, 22.8t, 21.2q, 20.6q, 20.4q, IR(CHCl3): 2900br, 1650, 1605 cm−1, UV(heptane): 234nm(ε=6700) optical rotation [α]D20 =−20° (c= 0.1,CHCl3)
- 6.Nagayama K. J. Mag. Res. 1980;40:321. [Google Scholar]
- 7.Bax A., Morris G. J. Mag. Res. 1981;42:501. [Google Scholar]
- 8.Standard HETCOR pulse sequence with suitable delays to emphasize JCH = 10Hz and 7Hz
- 9.Mareci T.H., Freeman R. J. Mag. Res. 1982;48:158. [Google Scholar]
- 10.Experimental conditions for INADEQUATE experiment. 300mg sample in 0.4ml CDCl3, Sweep width in F1 and F2 18.5KHz, 256 experiments (t1 increments) at 256 scans per experiment were recorded. Zero filling in F1 to 1K, no zero filling in F2 to give a total 2D matrix 2K X 2K, Effective resolution in both F1 and F2 9 Hz/point. Transformed 2D matrix was symmetrized about the diagonal.
- 11.Bodenhausen G., Kogler H., Ernst R.R. J. Mag. Res. 1984;58:370. doi: 10.1016/j.jmr.2011.08.033. [DOI] [PubMed] [Google Scholar]


