Abstract
Cryptosporidium species are coccidian parasites with a large capacity to reproduce and to disseminate. Several species are known to infect farm animals, although the economic importance of cryptosporidiosis is highly host species dependent. This paper reviews the impact of cryptosporidial infections in livestock and poultry. For different farm animals, the Cryptosporidium spp. that occur, as well as their clinical and pathological features, and their interactions with other pathogens, are described. In addition, data concerning the prevalence, the transmission and the epidemiology of the disease are mentioned and a description of the economic losses associated with cryptosporidiosis in each of the hosts is given. Cryptosporidiosis seems to be mainly a problem in neonatal ruminants. Cryptosporidium parvum is considered to be an important agent in the aetiology of the neonatal diarrhoea syndrome of calves, lambs and goat kids, causing considerable direct and indirect economic losses. Avian cryptosporidiosis is an emerging health problem in poultry, associated with respiratory disease in chickens and other Galliformes, and with intestinal disease in turkeys and quails. Because of limited availability of effective drugs, the control of cryptosporidiosis relies mainly on hygienic measures and good management.
Keywords: Concurrent infections, Cryptosporidiosis, Cryptosporidium, Economic losses, Epidemiology, Livestock, Pathology, Prevalence, Transmission
1. Introduction
The genus Cryptosporidium was named at the beginning of this century, but was only recognised as a potential cause of disease in 1955, when it was found to be associated with diarrhoeic turkeys[1]. Although Cryptosporidium was subsequently found in a broad range of farm animals, its impact was neglected until the early 1980s when it was found to be a common, serious primary cause of outbreaks of diarrhoea in certain farm mammals2, 3. The fact that Cryptosporidium was found to infect humans[4], and could cause a life-threatening disease in immunodeficient people, especially AIDS patients[5], as well as the association of Cryptosporidium with waterborne-related human outbreaks of diarrhoea[6], has certainly given the parasite a more widespread recognition. It has encouraged the scientific work on Cryptosporidium in many domains, such as in the veterinary field, to some extent because animal husbandry is seen as a threatening source of infection for humans by the release of tremendous numbers of resistant oocysts in surface waters. Recent studies have revealed the heterogeneity of the Cryptosporidium sp. infecting both humans and animals, making the causal connection between animal and human cryptosporidiosis far more complex[7]. Nevertheless, the interest for cryptosporidiosis in the veterinary field arises even more from the fact that it concerns a harmful, difficult to control disease of many farm animals, that results in significant economic losses. This point will be addressed in this review.
2. Life cycle
The life cycle of Cryptosporidium is direct and monoxenous and it follows the patterns described for other enteric coccidia, which include a merogonic cycle with two generations of meronts, a gametogonic cycle with macrogametes, microgametes and zygotes and a sporogony (outlined in[8]). Compared with Eimeria spp., the life cycle of Cryptosporidium is characterised by a number of peculiarities, some of them of major importance for the establishment and spread of the infection and for the treatment of the disease: (i) exposure of Cryptosporidium oocysts to reducing conditions, pancreatic enzymes and bile salts, results in a high percentage of excystation. However, in contrast to most other coccidia, Cryptosporidium oocysts can liberate their sporozoites in warm aqueous solutions without any of the aforementioned special stimuli[9]. This spontaneous excystation explains, in part, the ability of Cryptosporidium to infect tissues other than the intestine, such as the conjunctiva of the eye10, 11, 12, 13and the respiratory tract10, 14, 15, 16, 17, 18, 19; (ii) the parasite develops inside the epithelial cell of the digestive or respiratory tract, although on the edge of the host cell cytoplasm and separated from it by a feeder organelle membrane. This intracellular extracytoplasmatic location is unique for the coccidia and might play a major role in the failure of many antimicrobial agents to inhibit the growth of Cryptosporidium [20]; (iii) two stages can cause auto-infection: the recycling type I meronts and the thin-walled oocysts. Consequently, in the absence of a protective immune response Cryptosporidium may persist inside a single host, even without further exposure to exogenous oocysts; (iv) the thick-walled oocysts are already fully sporulated when they leave the body with the faeces and are therefore immediately infectious. Thus, Cryptosporidium seems to have an extraordinary reproductive ability. In addition, the oocysts can travel a considerable distance following runoff[21], can survive for a relatively long time in an aqueous environment22, 23, and are infectious to a wide range of animals, thus having many potential excretors[20]. As a result, this parasite undoubtedly has an exceptional capacity to disseminate.
3. Cryptosporidium infection in cattle
3.1. Historical background
Infection by Cryptosporidium spp. in cattle were first reported in the early 1970s24, 25, 26. However, because of the association with other viral or bacterial enteropathogens, the role of Cryptosporidium spp. as primary enteropathogens was uncertain until 1980, when Tzipori et al.[2]attributed an outbreak of neonatal diarrhoea to cryptosporidial infection alone. In the following years methods to free the infective oocysts from other contaminating pathogens became available, which permitted the experimental demonstration that Cryptosporidium was capable of causing clinical diarrhoea in calves27, 28.
In cattle two species of the genus Cryptosporidium can be distinguished: Cryptosporidium parvum infecting the distal small intestine, and Cryptosporidium muris infecting the abomasum. Substantial differences in the size and shape of C. parvum (5.0 μm× 4.5 μm and sperical29, 30) and C. muris (7.4 μm×5.6 μm and ovoid[30]) oocysts enables the two species to be distinguished readily on microscopical examination. Only C. parvum has been associated with neonatal diarrhoea. Cryptosporidium muris is much less prevalent and was only found in weaned calves or adult cattle31, 32, 33and C. muris infection is considered to be clinically mild, affecting weight gain[34]and milk production[35].
3.2. Clinical and pathological features
The kinetics of oocyst shedding of experimentally C. parvum infected neonatal calves, revealed a prepatent and a patent period ranging from 3–6 and 4–13 days, respectively[36]. In practice, oocyst excretion has been described as early as 3 days of age, which means that calves are already susceptible for infection during or shortly after birth37, 38, 39. Calves raised in isolation from Cryptosporidium remain susceptible to infection at older age, but the clinical signs become less severe[40]. In neonates, a great variability was observed in the severity and duration of diarrhoea due to cryptosporidiosis, even when the animals were exposed to similar conditions. In most calves diarrhoea has already began 3–5 days p.i., and lasted from 4 to 17 days[36]. In fattening units, where the prevalence of C. parvum is known to be high (93%), up to 38% of the calves developed a liquid diarrhoea, that could be attributed to cryptosporidiosis[41]. Cryptosporidial diarrhoea is associated with the excretion of tremendous numbers of oocysts[42]. High mortality due to cryptosporidiosis has been reported, even in the absence of other enteropathogens[43], and this would occur more often in the Belgian Blue–White, and the French Limousin and Charolais meat breeds[44].
Cryptosporidium infections are mainly concentrated in the distal small intestine but lesions were also found in the caecum and colon43, 45, and occasionally in the duodenum[43]. The pathological findings associated with Cryptosporidium are a mild to moderate villous atrophy, villous fusion, and changes in the surface epithelium (reviewed in[46]). Further, infiltration of mononuclear cells and neutrophils was seen in the lamina propria[43]. After primary exposure, α/β T-cells, both CD4+ and CD8+, and γ/δ T-cells accumulated in the intestinal villi, while in challenged immune animals only an increase in the number of CD8+ T-cells was found[47]. Respiratory cryptosporidiosis has been reported[19], but can be considered to be of less economic importance than the enteritic form.
3.3. Interactions with other enteropathogens and prevalence
The neonatal diarrhoea syndrome, both in large and small (see Section 4.3) ruminants, is a clear example of a multifactorial disease governed by a wide range of factors related to the animal, conditions of the environment and husbandry, and a variety of viruses, bacteria and protozoan parasites.
In calves, enterotoxigenic Escherichia coli (ETEC), with thermolabile and/or thermostable enterotoxins and with colonisation factors[48], are recognised as a common cause of diarrhoea in calves under 3 days old. This age-dependence is caused by a diminished adherence of ETEC to enterocytes after the first few days of life[49].
Between 4 days and 6 weeks of age digestive problems can mostly be attributed to C. parvum and/or a variety of viruses, with rotavirus, coronavirus and bovine viral diarrhoea (BVD) virus being the most important. Data from the Veterinary and Agrochemical Research Centre in Brussels, on the proportional occurence of viral enteropathogens and C. parvum in samples from diarrhoeic calves (Fig. 1 ), highlighted the increasing importance of Cryptosporidium. During the period July 1982–June 1983 Cryptosporidium infections made up only 6.7% (3.9% as a single pathogen, 2.8% in association) of the digestive problems, whereas the impact of cryptosporidiosis was considerably greater in 1992–1993. Indeed, almost 40% of the diarrhoeic calves shed C. parvum; in 30.7% of the cases Cryptosporidium was the only pathogen present, and in 8.9% it was found in association with a viral infection. For comparison: rotavirus, coronavirus and BVD-virus were found as a single pathogen in, respectively, 11.2, 23.7 and 8.9% of the diagnosed samples. The decreased number of cases of rotavirus, shown in Fig. 1, is probably due to the use of commercialised rotavirus vaccines that became available halfway during the studied period (Vanopdenbosch, personal communication). The proportional occurrence of these pathogens may differ according to geographic parameters and the studied period, but in the last decade several studies described C. parvum as the most commonly detected enteropathogenic agent in calves50, 51, 52.
Fig. 1.
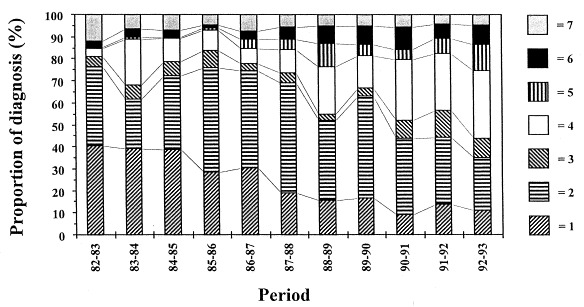
Proportional occurrence of different enteropathogens in samples from diarrhoeic calves. Over a 10-year period, the number of cases with Cryptosporidium as single pathogen present, increased significantly. 1=rotavirus, 2=coronavirus, 3=BVD-virus, 4=Cryptosporidium as single pathogen, 5=other single pathogens, 6=Cryptosporidium in association with other pathogens, 7=other associations.
Attaching and effacing E. coli have been implicated in diarrhoea and dysentery in 2- to 8-week-old calves53, 54, with a calculated mean age of 5 weeks[55]. Verotoxigenic E. coli, a group of bacteria that produces potent cytotoxins known as verocytotoxins, were found to be occasionally associated with calf diarrhoea[56]. They were detected in 6.0 and 9% of diarrhoeic calves in Germany[57]and Spain[58], respectively. There are reports of viruses other than the aforementioned ones, populating the intestine of young calves (picobirnavirus[59], calici- and astrovirus[60], adenovirus[61], enterovirus[62]and parvovirus[63]), but their pathogenicity is still uncertain. Giardia infections have also been found to be exceptionally frequent in suckling and weanling calves, although the role of this protozoan parasite in the aetiology of diarrhoea in calves remains unclear[39]. Finally, the problems related to clinical salmonellosis are only minor in dairy, beef[64]and veal units[65].
Cryptosporidium parvum oocysts were detected in bovines ranging from 3 days old to adults, although the prevalence was significantly higher in suckling calves39, 66, 67, 68. In a Spanish epidemiological study on the prevalence and age distribution of C. parvum infections, as many as 44.4% of calves aged 3–4 days were infected, but infection rates peaked (76.7%) at 6–15 days of age. Prevalence was also high in weanling calves aged 1.5–4 months (14%), fattening calves and heifers 4–24 months old (7.7%) and adults (17.8%). However, Cryptosporidium infection was only statistically associated with diarrhoea in suckling calves[39].
3.4. Transmission and epidemiological factors
The transmission occurs by the faecal-oral route. The majority of adult cattle can be described as excretors of C. parvum oocysts when sensitive detection methods are used. Nevertheless, their importance in the transmission of the disease remains questionable, since oocyst excretion by adult cattle was similar in herds with serious problems of cryptosporidial neonatal diarrhoea and in those without[68]. So far, in cattle, no increased output of C. parvum oocysts around parturition has been observed67, 68, 69. However, these findings should be considered with great caution, as it was shown that more frequent samplings and more sensitive detection methods in sheep revealed a periparturient rise in oocyst score (Requejo-Fernandez JA, Pereira-Bueno J, Pilar-Izquierdo M, Rojo-Vazquez FA, Ortego-Mora LM. Periparturient rise in ovine cryptosporidiosis. In: Proc COST 820 WG3 Meeting. Brussels, 1997, p. 5).
Nevertheless, infected newborn calves excrete oocyst numbers of the order of 106 to 107 g−1 of faeces[42]and were considered to be a more dangerous source of infection. Infection can rapidly spread from calf to calf when animals are communally housed and overcrowded, or from cow to calf via the udders when they are contaminated with infected calf faeces in the lying area of the dams[46]. This might explain the association between cryptosporidial diarrhoea and the farm type and/or its specific hygienic conditions. Cryptosporidium infections were more common in single or multiple suckler beef herds[70]and dairy farms with multiple-cow maternity facilities[71]. In the so-called fattening units, where calves are purchased through markets, almost 100% of the animals become infected during transit from the market to the rearing unit or soon after their arrival[41].
Other potential risk factors are herd size[71]and season. In a Canadian study of beef calves, higher prevalence was found in winter and spring, the period related to calving season and consequently the period with the greatest number of calves in the high risk group (1- to 3-weeks-old)[72]. However, in American dairy farms, where calvings tend to be year-round, and environmental contamination level is less subjected to fluctuations, cryptosporidiosis was more prevalent in the summer[71].
Little information is available on differences in infectivity, excretion patterns, virulence or immunogenicity among different isolates of C. parvum from cattle or other sources[36]. This is at least partially due to the lack of single oocyst cloned isolates since all reported isolates are heterogenic mixtures.
3.5. Economic losses
The economic losses due to cryptosporidial infections of neonatal calves are related to diarrhoea: dehydratation, growth retardation and to a lesser extent mortality[43]. Diarrhoeic problems of calves demand special care: feeding of electrolyte solutions, i.v. fluid therapy, drug administration, hygienic measures, etc. which are costly as well as labour and time-consuming. In Belgium, mortality due to the neonatal diarrhoea syndrome is estimated between 5–10% (Vanopdenbosch, personal communication). Cryptosporidium parvum is considered as the most commonly found enteropathogen in calves during their first weeks of life50, 51, 52. The parasite frequently acts alone, but the losses are more severe when concurrent infections occur (Vanopdenbosch, personal communication), as has been demonstrated for viral and bacterial enteropathogens[73].
4. Cryptosporidium infection in sheep and goat
4.1. Historical background
In sheep, infection by Cryptosporidium was first described in Australia in 1- to 3-week-old lambs with diarrhoea[25]. Its role as a primary aetiological agent was confirmed in the early 1980s in studies on experimental infections in the absence of other enteropathogenic agents3, 74. Since then, Cryptosporidium has been attributed an increasingly important role in neonatal diarrhoea syndrome in this domestic species and is currently associated with high morbidity rates and, depending on environmental conditions and the presence of other intestinal pathogens, mortality46, 75, 76.
In goats, infection by this agent was also first described in Australia, in a 2-week-old kid with diarrhoea[77]. Since then, the infection has been diagnosed in outbreaks of diarrhoea in goat kids in several European countries78, 79, 80and is now considered to be one of the principal enteropathogens in these animals[76].
Only one of the two species of the genus Cryptosporidium described in domestic ruminants, C. parvum, has been associated with diarrhoea in small ruminants. In a recent work, a bovine isolate of C. muris did not produce infection in goats[81].
4.2. Clinical and pathological features
In lambs, the prepatent period oscillated between 2 and 7 days[82]and around 4 days in goat kids[83]. This period increases with a reduction in the dose of the infectious agent[84]or with an increase in the age of the animal[85]. The main clinical manifestations of cryptosporidiosis in neonate small ruminants are: apathy and depression, anorexia, abdominal pain, and mainly diarrhoea accompanied by the shedding of a large number of oocysts74, 83, 85, 86, 87, 88. The faeces are usually yellow, have a soft or liquid consistency and give off a strong unpleasant odour. In experimental infections in lambs[85]the dry weight of the faeces can drop from 24 to 10%. In milder cases of the disease the animals have diarrhoea for 3 to 5 days and in more severe cases for 1 to 2 weeks. The diarrhoea usually coincides with the period of oocyst shedding. The duration of the oocyst shedding depends on factors such as the age or immune status of the animals. The kinetics of the shedding is usually similar in experimentally and naturally infected animals. After the first 2 or 3 days of the patent period there is a progressive increase in the number of oocysts shed which reaches a maximum 5–6 days p.i. and drops sharply between days 10 and 15 p.i.83, 85, 89. In addition to the diarrhoea and oocyst shedding the animals characteristically manifest anorexia74, 83, 85which results in weight loss and retarded growth during the first few weeks of life[90]. Studies on the development of the lesions over the incubation period and the clinical course of the disease74, 83have shown that, over this period, the parasite mainly proliferates in the jejunum and the ileum. After the start of oocyst shedding (3 days after the start of infection) the lesions can spread to other parts of the small and large intestine.
4.3. Interactions with other enteropathogens and prevalence
A recent study showed that the most frequent aetiologic agent involved in outbreaks of diarrhoea in lambs was C. parvum (65% of the outbreaks and 45% of the individuals) followed by E. coli potentially pathogenic (61% of the outbreaks and 30% of the individuals). Other agents such as rotavirus were less important (7% of the outbreaks and 2% of the individuals). In goat kids, C. parvum was present in 40% of the outbreaks and 42% of the individuals, followed by E. coli (36 and 22%), rotavirus (14 and 22%), Clostridium perfringens (20 and 11%) and Salmonella spp. (7 and 3%)[76]. In a parallel study, it has been shown that E. coli strains isolated from diarrhoeic lambs and kids older than 4 days (mainly between 7 and 15 days old) are not generally toxigenic and belong to a large number of serogroups91, 92. In small ruminants older than 4 weeks, infections by Eimeria spp. are increasingly important accompanied by dietary changes and situations of stress[93]. The role of other intestinal protozoa such as Giardia sp. is controversial and although infection by this parasite is often diagnosed during the first month of life[94]it is not clearly associated with the production of diarrhoea39, 95.
The prevalence of infection by C. parvum in lambs and goat kids has been studied in both outbreaks of diarrhoea and in randomly selected farms (Table 1 ), although more research has been done on cattle. In outbreaks of diarrhoea, morbidity can be very high in lambs98, 100and goat kids86, 102, 103, 104. Mortality increases when the disease is associated with concurrent infections or deficiencies in nutrition or husbandry82, 87, 100. In goat herds in France and Hungary, C. parvum was considered to be the predominant aetiological agent in neonate goat kids with diarrhoea79, 102. In Spain, in a study on 97 sheep farms and 31 goat farms, all randomly selected, corresponding to a total of 2204 lambs and 367 goat kids under 5 weeks old, flock prevalence was 47 and 36% and individual prevalence was 15 and 11% for lambs and goat kids, respectively[80].
Table 1.
Prevalence of C. parvum infections in lambs and kid goats
| Prevalence | |||||
| % (number infected/number sampled) | |||||
| Host species | Country | Type of study∗ | Flock | Individual | Reference |
| Sheep | Canada | 3 | 24 (21/89) | [95] | |
| Sheep | Iran | 3 | 3 (9/280) | 4 (18/433) | [96] |
| Sheep | Italy | 1 | 8 (5/59) | 12 (18/156) | [97] |
| Sheep | Spain | 2 | 73 (16/22) | 40 (53/132) | [98] |
| Sheep | Spain | 1 | 47 (43/92) | 15 (331/2,204) | [80] |
| Sheep | Spain | 2 | 65 (30/46) | 45 (82/183) | [76] |
| Sheep | Trinidad and Tobago | 3 | 20 (18/90) | [99] | |
| Sheep | USA | 2 | 85 (27/32) | [100] | |
| Goat | Hungary | 2 | 25 (10/40) | [79] | |
| Goat | Italy | 1 | 13 (2/15) | 19 (8/42) | [97] |
| Goat | Spain | 1 | 35 (11/31) | 11 (40/367) | [80] |
| Goat | Spain | 2 | 40 (6/15) | 42 (15/36) | [76] |
| Goat | Trinidad and Tobago | 3 | 20 (4/20) | [99] | |
| Goat | USA | 3 | 26 (5/19) | [101] | |
∗1=Cross-sectional study, 2=diarrhoea outbreak, 3=other.
4.4. Transmission and epidemiological factors
Infection occurs mainly by the ingestion of oocysts previously eliminated with the faeces of infected neonates or asymptomatic adult carriers[100]. Daily excretion of oocysts by infected lambs can exceed 2×109 and more than 1010 oocysts are shed during the patent period85, 89. Moreover, adult sheep can act as asymptomatic carriers shedding small numbers of oocysts to the environment which was shown to increase in number in the perinatal period and contribute to maintaining the infection between lambing periods. Between 75 and 100% of lambs born in this environment become infected in the first few weeks of life[105]. In a recent study, the viability of oocysts shed was shown to be greater between days 5 and 11 p.i. than between days 11 and 15 p.i.[106]. Other possible sources of infection, such as farm rodents, should also be considered[107].
The influence of the dose of the infectious agent on the course of the disease has been studied by several authors. There do not seem to be any differences between natural or experimental infections with respect to clinical symptoms, oocyst shedding or serum antibody responses[108]. However, the prepatent period is a few days longer in experimental infections with low infectious doses[84]. In gnotobiotic lambs the minimum infectious dose can even correspond to a single oocyst and the average infectious dose is around five oocysts[84].
At present, no intraspecific differences in pathogenicity have been found between isolates obtained from domestic ruminants. However, in the laboratory of one of the authors (Ortega-Mora, personal communication), two different isolates obtained from diarrhoeic ruminants showed significant differences in the number of oocysts shed and the severity of the diarrhoea after experimental infection in lambs. However, further research is necessary to confirm this finding.
In both natural and experimental infections the clinical process that accompanies infection has been reported to be more common in lambs and goat kids under 30 days old76, 82, 85. Extension of the prepatent period and reduction of oocyst shedding occur as the age of the lambs at infection increases. In a recent study, diarrhoea was only present in lambs infected at 6 days old and in a small percentage of lambs infected at 28 days old, but not in lambs infected at 56 days old. The size of the specific serum (IgG and IgA) and faecal (IgA) humoral response was similar and apparently independent of the age, suggesting the participation of mechanisms other than the humoral response in the development of protection[85]. However, a greater intestinal mucus production and increased glucoprotein concentration in the mucus was observed in animals infected at 28 or 56 days old. The kinetics of the IgA in the intestinal mucus response varied with the age of the animal at infection, the most rapid responses and highest titres occurred in animals which were older when infected (Quintanilla-Gozalo A, Wright S, Pereira-Bueno J, Rojo-Vazquez FA, Ortega-Mora LM. Changes in the intestinal mucus during infection by Cryptosporidium parvum in lambs. In: Proc 1995 COST 820 Annual Workshop. Prague, 1995, p. 70).
In natural infections, the majority of the infected lambs and kids were also less than 2 weeks old and the proportion of infected animals underwent a pronounced decrease in animals more than 3 weeks old76, 80, 109.
4.5. Economic losses
The economic losses associated with this disease are not only due to the resulting mortality, but also to the retarded growth of the animals, the cost of drugs, veterinary assistance and the increased labour involved. In the absence of other enteropathogens, mortality is higher in lambs than in calves[88]and morbidity can reach 100%46, 75. The anorexia is very pronounced at the start of the process in both lambs74, 82, 85and goat kids83, 90. In lambs naturally infected with C. parvum, there was a mean weight difference of 2 kg at 4 weeks old compared with non-infected control lambs of this age (Ortega-Mora, personal communication).
5. Cryptosporidiosis in chickens and other birds
5.1. Historical background
First reports of a Cryptosporidium sp. in avian species were from Tyzzer[110]. He found a parasite in the caecal epithelium of chickens with structural similarities to C. parvum, found in mice. At present, only two Cryptosporidium spp. are recognised as valid in avian hosts: (i) Cryptosporidium meleagridis, first reported in turkeys[1]; and (ii) Cryptosporidium baileyi, isolated from broiler chickens[111]. Cryptosporidium meleagridis and C. baileyi can be distinguished by morphological differences between their oocysts (length and width)[112]. There is possibly a third species from bobwhite quail113, 114and fourth species from ostrich[115]. Infection by Cryptosporidium spp. has been detected in over 30 species of birds including domesticated chickens, turkeys, ducks, geese, quails, pheasants, peacocks, and a wide variety of wild and captive birds116, 117.
5.2. Clinical and pathological features
Cryptosporidium meleagridis may infect the intestinal tract, bursa of Fabricius (BF) and cloaca of turkeys[118]and chickens[112], but infection was only associated with illness, including diarrhoea and moderate mortality in turkeys[119].
Cryptosporidium baileyi may infect the respiratory tract (larynx, trachea, primary and secondary bronchi, air sacs), BF and cloaca of chickens120, 121, turkeys[122]and ducks[123]. It is the most prevalent Cryptosporidium sp. in poultry and the most commonly associated with disease in chickens. Following experimental inoculation, C. baileyi can develop infections in many anatomic sites of its avian hosts, and it seems that the route of oocyst administration strongly determines the site where the infection finally establishes (reviewed in[124]).
A Cryptosporidium sp. was responsible for intestinal disease, including diarrhoea, in quails113, 114. Sometimes, the disease can be severe and mortality can reach 90% in young quails[125].
Naturally occurring cryptosporidiosis in chickens usually manifests as respiratory disease, and only occasionally as intestinal or renal disease[124]. Symptoms (depression, anorexia, emaciation, coughing, sneezing, dyspnoea), pathological consequences (excessive mucoid exudate, local inflammation, airsacculitis, deciliation, epithelial hypertrophy and hyperplasia), as well as increased mortality are most often associated with respiratory cryptosporidiosis15, 126, 127. The primary respiratory disease potential of C. baileyi was proven in the absence of other detected pathogens, and the negative effect of cryptosporidiosis on growth performance and carcass pigment was clearly shown[121]. Severe air sac disease15, 121which resulted in heavy carcass condemnation at processing, places C. baileyi among the agents to be considered in the respiratory disease complex[121].
The infection of immunocompetent birds is self-limiting. Two-week-old broiler chicks inoculated orally with C. baileyi oocysts and capable of clearing the endogenous stages from the cloaca and/or BF, were resistant to subsequent oral challenge[128]. Furthermore, there is an age-related resistance to clinical disease. Older chickens are less susceptible to C. baileyi infection, exhibiting a longer prepatent and a shorter patent period129, 130, 131. Unlike younger (7-day-old) chicks[121], food conversion and body weight gain were not influenced by C. baileyi infection in 26-day-old broilers[132]. In practice, infections with Cryptosporidium are known to occur only in chickens less than 11 weeks of age[133], and not in adult birds127, 134.
5.3. Interaction with other pathogens and prevalence
When appropriate diagnostic tools are used, Cryptosporidium spp. appear to be present wherever avian hosts are raised commercially[135]. Cryptosporidium baileyi has been reported in domestic chickens from Asia, Australia, Europe, North America[136]and North Africa[137]. The prevalence of Cryptosporidium spp. in chickens and other Galliformes has been studied both in outbreaks of respiratory and/or intestinal disease and in randomly selected flocks (Table 2 ). Histological observations demonstrated a similar incidence of Cryptosporidium infection in broilers and commercial egg-layer-type pullets[134]. However, limited epizootiological data suggest that the infection in the latter[126]is under-reported compared with that in broilers[136]. Goodwin et al.[148]found that 41% (23/56) of the northern Georgia broiler flocks had C. baileyi tracheitis. Parasitism rates among C. baileyi-infected flocks ranged from a low of 10% to a high of 60%. Cryptosporidium baileyi tracheitis was very highly correlated to severity of tracheitis, negatively correlated with average body weight, and correlated with airsacculitis and condemnations. This study indicated that C. baileyi infection rates in Georgia were much higher than previously suspected.
Table 2.
Prevalence of Cryptosporidium infections in Galliformes
| Prevalence |
||||||
| % (number infected/number sampled) |
||||||
| Host species (type) | Age | Country (period of study) | Diagnostic technique∗ | Flock | Individual | Reference |
| Chicken | Scotland | 3 | 88 (22/25) | [138] | ||
| Chicken (broiler) | 4–7 weeks | Scotland | 1 | 19 (26/139) | [139] | |
| Chicken (layer) | 31–86 days | Japan (1976–1983) | 1, 4 | 37 (25/68) | [126] | |
| Chicken (broiler) | 40–48 days | Japan (1975–1981) | 1, 4 | 33 (4/12) | [126] | |
| Chicken (broiler) | 21–42 days | USA, Deleware | 1 | 25 (2/8) | [140] | |
| Chicken (broiler) | 49 days | USA (1985) | 3 | 38 (7/18) | [141] | |
| Chicken (broiler) | 63 days | USA (1985) | 3 | 50 (9/18) | [141] | |
| Chicken (broiler) | 49 days | USA (1987) | 3 | 22 (100/454) | [141] | |
| Chicken (broiler) | 5–50 days | Greece | 1, 4 | 24 (17/70) | [142] | |
| Chicken | 14–51 days | USA, Georgia (1986) | 1 | 6 (105/1065) | [134] | |
| Chicken (broiler) | 25–48 days | USA, North Carolina | 2 | 27 (9/33) | [143] | |
| Chicken (broiler breeder) | 27–59 days | USA, North Carolina | 2 | 10 (3/30) | [143] | |
| Chicken (layer) | 42 days | USA, North Carolina | 2 | 6 (1/17) | [143] | |
| Turkey | 17–24 days | USA, Iowa | 2 | 45 (54/120) | [144] | |
| Chicken, turkey | USA, Georgia (1974–1984) | 1 | 1 (63/6050) | [145] | ||
| Chicken, turkey | USA, Georgia (1985–1988) | 1 | 6 (157/2622) | [145] | ||
| Chicken | 4 weeks | Korea | 2 | 15 (75/500) | [146] | |
| Chicken (broiler) | USA, Georgia (1987–1992) | 1 | 1 (84/7007) | [147] | ||
| Chicken (broiler) | 49–52 days | USA, Georgia | 1 | 41 (23/56) | [148] | |
| Chicken (broiler) | 26–50 days | Marocco | 1, 4 | 37 (14/38) | 24 (54/225) | [137] |
∗1=Histology, 2=faecal examination, 3=serology, 4=other.
Avian cryptosporidiosis has been reported in concurrence with immunosupressive viruses like reovirus[113], Marek's disease virus (MDV)149, 150, 151, infectious bursal disease virus139, 150, and chicken anemia virus[152]. In chickens, the synergistic effect of C. baileyi and these viruses was experimentally confirmed (reovirus[153], MDV [Abbassi H, Coudert F, Cherel Y, Naciri M. Effect of oncogenic Marek's disease virus and vaccinal virus on the pathology induced by Cryptosporidium baileyi. In: Proc VIIth International Coccidiosis Conference and European Union COST 820 Workshop. Oxford, 1997, pp. 108–109], infectious bursal disease virus[154], chicken anaemia virus[155]). Marek's disease virus may induce the establishment of the parasite in inhabitual sites ([156], Abbassi H, Coudert F, Cherel Y, Brugere-Picoux J, Naciri M. Experimental reproduction of renal cryptosporidiosis (C. baileyi) in SPF chickens after oral inoculation of parasite. In: AAAP Meeting. Baltimore, 1998, pp. 210–211). The severity of respiratory disease induced by C. baileyi can be enhanced by other respiratory pathogens, such as infectious bronchitis virus and E. coli (157, Hoerr FJ, Blagburn BL, Lindsay DS, Giambrone JJ. Interactions of Cryptosporidium with other infectious pathogens in chickens. In: Proc of the 124th Annual Meeting of the Am Vet Med Assoc, Chicago, USA, 1987, p. 134). Moreover, C. baileyi infection affects the development of humoral immunity to heterologeous antigens121, 158, 159, and can diminish cell-mediated immunity, as shown by decreased delayed hypersensitivity indices of infected chickens[121].
5.4. Transmission and epidemiological factors
Chickens can become infected by ingestion or inhalation and/or aspiration of oocysts present in the environment (litter, faeces, water, breeding materials, dust, etc.). As few as 100 oocysts can result in intestinal or respiratory infections[135]. Since C. baileyi can infect a variety of wild birds they must be considered as possible sources of infection. Although C. baileyi does not infect rodents (mice and rats) or insects, they may serve as mechanical carriers of oocysts[135].
Hygienic conditions and management strongly influences the incidence and persistance of avian cryptosporidiosis. The disease was shown to be more prevalent in flocks with defective hygiene[137]. Goodwin et al.[145], suggested that the increase of intestinal and respiratory cryptosporidiosis infection from 1% (63/6050) during 1974–1984 to 6% (157/2622) during 1984–1988, could be explained in part by the relatively widespread practice of cleaning out poultry houses less frequently. The increased use of `built-up litters' could increase exposure to and infection of chicks by Cryptosporidium [145]. In quails, rigorous clean up and disinfection with hypochlorite acid was effective in preventing continued infection, morbidity and mortality attributed to Cryptosporidium sp.[125].
Furthermore, outbreaks of cryptosporidiosis seem to be related to climatologic parameters. It was found that the percentage of cryptosporidiosis cases in winter was significantly lower than in other seasons[134]. Differences in the incidence between geographic locations were related to the number of days of frost[147].
5.5. Economic losses
For a long time, avian cryptosporidiosis was considered to be an opportunistic disease, but it is now recognised as a first order disease120, 121, 127. The economic losses associated with this disease are due to poor flock performance (growth retardation and increased consumption index) and/or mortality121, 140, 141, 148. In addition, cost of therapy, although most drugs are inefficient122, 125, 142, 160, and carcass condemnation at processing due to air sacculitis15, 121, should also be taken into account. When C. baileyi occurs concurrently with immunosuppressive or other respiratory pathogenic agents, the consequences are more serious, and the losses more important15, 127, 157. Vaccination with the attenuated serotype 1 MDV can enhance the severity of subsequent cryptosporidial respiratory disease, resulting in unexpected losses (Abbassi H, Couder F, Cherel Y, Brugere-Picoux J, Naciri M. Effect of Cryptosporidium baileyi on the development of vaccinal immunity to Marek's disease in SPF chickens. In: 5th Avian Immunology Research Group Meeting. Turku, 1998, p. 7.4). Thus, the role this parasite plays in the pathogenesis of respiratory disease and the related production losses could be unexpectedly large. Therefore, avian cryptosporidiosis should not be neglected or overlooked during diagnosis.
6. Cryptosporidium infection in pigs and rabbits
Cryptosporidial infection was first reported in pigs by Bergeland[161]and Kennedy et al.[162]. Thereafter, naturally occurring cryptosporidiosis in pigs has been described worldwide[163]. Experimental infections of piglets resulted only in diarrhoeic problems when the animals were inoculated before 2 weeks of age164, 165, 166. In older piglets, experimental Cryptosporidium infection did not cause clinical manifestations167, 168, 169. The prevalence of cryptosporidiosis was reported to be very low in nursing piglets168, 170, 171. Several studies demonstrated that naturally occurring cryptosporidiosis is delayed until after weaning146, 169, 171, 172, 173. The infections are mostly asymptomatic and usually of low intensity, and no statistical association between infection and clinical symptoms has been found171, 174. Previous studies have confirmed that diarrhoea in suckling and weaned piglets is usually a multifactorial problem, where mixed infections between C. parvum and E. coli or rotavirus are frequent. It has been suggested that Cryptosporidium is not an important primary agent of diarrhoea in piglets but it may be a copathogen in the multifactorial aetiology[163]. Recently, genetic analysis of the Cryptosporidium isolates from pig herds revealed that the animals harboured two distinct genotypes: a porcine genotype and a bovine genotype with distinct virulence in nude mice[175]. The zoonotic potential of the porcine genotype is still uncertain and requires further study.
In commercial rabbits, digestive disorders are the predominant cause of mortality. Mainly weaned rabbits of 4 to 8 weeks of age are affected, although another peak may occur in suckling rabbits of 8 to 12 days old[176]. Experimental Cryptosporidium infection of rabbits resulted in high mortality and liquid diarrhoea in suckling 3-day-old rabbits, but no mortality and very discrete diarrhoea in weaned animals[177]. The parasite was found in 2–11% of weaned diarrhoeic Belgian rabbits[176]. In general, field outbreaks of cryptosporidiosis in suckling rabbits are rarely detected and in weaned rabbits the parasite causes only subclinical enteritis. When concurrent infections with immunosuppressive agents or respiratory pathogens occur, the impact of Cryptosporidium infection can be more significant (Peeters, personal communication).
7. Treatment and control
Aetiological treatment of cryptosporidiosis has only recently been made possible. Drugs demonstrated to be partially effective in the treatment and prophylaxis of cryptosporidiosis in ruminants include halofuginone lactate, paromomycin and decoquinate (Table 3 ). However, the commercial availability of some of these products is still a problem in many countries. In poultry, so far none of the tested drugs showed a satisfactory anti-cryptosporidial activity, unless at toxic dosage122, 125, 142, 160. Recently, it was shown that the oral infection of hens with C. baileyi at the beginning of their laying period, resulted in partial protection of their progeny[185].
Table 3.
Efficacy of different drugs against cryptosporidiosis in ruminants
| Best results |
||||||
| Drug | Animal species | Dose∗ | Administration period | Oocyst shedding | Episode of diarrhoea1 or stool consistence2 | Reference |
| Halofuginone lactate | lamb | 500 μg | 1–5 days | prevented | prevented1 | [178] |
| Halofuginone lactate | calf | 30–500 μg | 3–14 days | prevented | prevented1 | 41, 179, 180 |
| Paromomycin | calf | 25–100 mg | 11 days | prevented | reduced1, improved2 | [181] |
| Paromomycin | goat kid | 100 mg | 12 days | prevented | improved2 | [182] |
| Decoquinate | calf | 2.5–10 mg | 8 weeks | decreased | improved2 | [183] |
| Decoquinate | goat kid | 2.5 mg | 21 days | decreased | prevented1 | [184] |
∗Per kg body weight.
The importance of colostrum in protecting neonatal ruminants against infection by C. parvum is a very valuable point to address. In field conditions, passively acquired antibodies did not protect calves44, 186and lambs against naturally acquired infection[108]. However, both calves[187]and lambs[188]fed with colostrum from immunised mothers with high titres of specific antibodies were partially protected against infection. Immunoprophylaxis of cryptosporidiosis is more thoroughly dis-cussed in another contribution of this Special Issue of the International Journal for Parasitology [189].
From a perspective of disease control, preventive hygiene measures are the most important tools in the struggle against cryptosporidiosis in farm animals, the objective being to destroy external forms of the parasite and to prevent their transmission among animals and from the environment to the host[46]. In ruminant husbandry, the destruction of oocysts in the pens and buildings used for parturition by applying moist heat and/or chemical disinfectants, the use of abundant clean straw beds, avoidance of high stocking rates in the parturition area and the separation of healthy and ill animals during outbreaks of diarrhoea, in addition to the administration of appropriated supplies of colostrum to neonates, all help to prevent outbreaks of cryptosporidiosis and to minimise mortality and morbidity in infected herds.
8. Conclusion
Cryptosporidiosis is mainly a problem in neonatal ruminants. Cryptosporidium parvum is the most commonly found enteropathogen during the first weeks of the life of calves, lambs and goat kids and is considered to be an important agent in the aetiology of the neonatal diarrhoea syndrome. The parasite frequently acts alone, but the losses are more pronounced when concurrent enteropathogens are present. Economic losses associated with cryptosporidiosis are retarded growth and mortality, and a number of hard to estimate costs resulting from interventions necessitated by diarrhoeic problems. Especially in small ruminants, the direct losses due to mortality caused by cryptosporidiosis alone was reported to be high. Because of the limited availability of effective drugs, hygienic measures and good management are the most valid weapons in the struggle against this disease. Avian cryptosporidiosis is an emerging health problem. In chickens and other Galliformes, cryptosporidiosis is mostly manifest as respiratory disease, although in turkeys and quails enteric forms of the disease were also reported to be responsible for morbidity and mortality. It was alarming to ascertain the unexpectedly high prevalence of C. baileyi in chicken flocks and the enhanced pathogenicity when immunosuppresive or other respiratory pathogens are present, or when birds are subjected to vaccination against other pathogens. In pigs and rabbits, Cryptosporidium spp. were reported to cause only clinical disease when administered experimentally to nursing neonates, but in these farm animals naturally occurring cryptosporidiosis is mostly asymptomatic.
References
- 1.Slavin D. Cryptosporidium meleagridis (sp.nov.) J Comp Pathol. 1955;65:262–266. doi: 10.1016/s0368-1742(55)80025-2. [DOI] [PubMed] [Google Scholar]
- 2.Tzipori S, Campbell I, Sherwood D, Snodgrass D.R, Whitelaw A. An outbreak of calf diarrhoea attributed to cryptosporidial infection. Vet Rec. 1980;107:579–580. [PubMed] [Google Scholar]
- 3.Angus K.W, Tzipori S, Gray E.W. Intestinal lesions in SPF lambs associated with Cryptosporidium from calves with diarrhoea. Vet Pathol. 1982;19:67–68. doi: 10.1177/030098588201900110. [DOI] [PubMed] [Google Scholar]
- 4.Nime F.A, Burek J.D, Page D.L, Holscher M.A, Yardley J.H. Acute enterocolitis in a human being infected with the protozoan Cryptosporidium. Gastroenterol. 1976;70:592–598. [PubMed] [Google Scholar]
- 5.Current W.L, Reese N.C, Ernst J.V, Bailey W.S, Heyman M.B, Weinstein W.M. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. New Engl J Med. 1983;308:1252–1257. doi: 10.1056/NEJM198305263082102. [DOI] [PubMed] [Google Scholar]
- 6.MacKenzie W.R, Hoxie N.J, Proctor M.E. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. New Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 7.Widmer G. Genetic heterogeneity and PCR detection of Cryptosporidium parvum. Adv Parasitol. 1998;40:223–239. doi: 10.1016/s0065-308x(08)60122-0. [DOI] [PubMed] [Google Scholar]
- 8.Fayer R, Speer CA, Dubey JP. General biology of Cryptosporidium. In: Dubey JP, Speer CA, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton: CRC Press, 1990;1–29
- 9.Fayer R, Leek R.G. The effects of reducing conditions, medium, pH, temperature, and time on in vitro excystation of Cryptosporidium. J Protozool. 1984;31:567–569. doi: 10.1111/j.1550-7408.1984.tb05504.x. [DOI] [PubMed] [Google Scholar]
- 10.Heine J, Moon H.W, Woodmansee D.B, Pohlenz J.F.L. Experimental tracheal and conjunctival infections with Cryptosporidium sp. in pigs. Vet Parasitol. 1984;17:17–25. doi: 10.1016/0304-4017(84)90061-x. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay D.S, Blagburn B.L, Hoerr F.J, Giambrone J.J. Experimental Cryptosporidium baileyi infections in chickens and turkeys produced by ocular inoculation of oocysts. Avian Dis. 1987;31:355–357. [PubMed] [Google Scholar]
- 12.Randall C.J. Conjunctivitis in pheasants associated with cryptosporidial infection. Vet Rec. 1986;118:211–212. doi: 10.1136/vr.118.8.211. [DOI] [PubMed] [Google Scholar]
- 13.Baskin G.B. Cryptosporidiosis of the conjunctiva in SIV-infected rhesus monkeys. J Parasitol. 1996;82:630–632. [PubMed] [Google Scholar]
- 14.Brady E, Margolis M.L, Korzeniowski O.M. Pulmonary cryptosporidiosis in acquired immuno deficiency syndrome. J Am Med Assoc. 1984;252:89–90. [PubMed] [Google Scholar]
- 15.Dhillon A.S, Thacker H.L, Dietzel A.V, Winterfield R.W. Respiratory cryptosporidiosis in broiler chickens. Avian Dis. 1981;25:747–751. [PubMed] [Google Scholar]
- 16.Glisson J.R, Brown T.P, Brugh M, Page R.K, Kleven S.H, Davis R.B. Sinusitus in turkeys associated with respiratory cryptosporidiosis. Avian Dis. 1984;28:783–790. [PubMed] [Google Scholar]
- 17.Harari M.D, West B, Dwyer B. Cryptosporidium as a cause of laryngotracheitis in an infant. Lancet. 1986;1:1207. doi: 10.1016/s0140-6736(86)91181-5. [DOI] [PubMed] [Google Scholar]
- 18.Tham V.L, Kniesberg S, Dixon B.R. Cryptosporidiosis in quails. Avian Pathol. 1982;11:619–626. doi: 10.1080/03079458208436138. [DOI] [PubMed] [Google Scholar]
- 19.Mascaro C, Arnedo T, Rosales M.J. Respiratory cryptosporidiosis in a bovine. J Parasitol. 1994;80:334–336. [PubMed] [Google Scholar]
- 20.Tzipori S, Griffiths J.K. Natural history and biology of Cryptosporidium parvum. Adv Parasitol. 1998;40:5–36. doi: 10.1016/s0065-308x(08)60116-5. [DOI] [PubMed] [Google Scholar]
- 21.Mawdsley J.L, Brooks A.E, Merry R.J, Pain B.F. Use of a novel soil tilting table apparatus to demonstrate the horizontal and vertical movement of the protozoan pathogen Cryptosporidium parvum in soil. Biol Fert Soils. 1997;23:215–220. [Google Scholar]
- 22.Goodrich J, Fox K. Small system control of cryptosporidium. Water Condit Purific. 1996;38:50–58. [Google Scholar]
- 23.Medema G.J, Bahar M, Schets F.M, Morris R, Grabow W.O.K, Jofre J. Survival of Cryptosporidium parvum, Escherichia coli, faecal enterococci and Clostridium perfringens in river water: influence of temperature and autochthonous microorganisms. Water Sci Technol. 1997;35:249–252. [Google Scholar]
- 24.Panciera R.J, Thomassen R.W, Garner F.M. Cryptosporidial infection in a calf. Vet Pathol. 1971;8:470–479. [Google Scholar]
- 25.Barker I.K, Carbonell P.L. Cryptosporidium agni sp. n. from lambs and Cryptosporidium bovis sp. n. from a calf with observations on the oocyst. Z Parasitenkd. 1974;44:289–298. doi: 10.1007/BF00366112. [DOI] [PubMed] [Google Scholar]
- 26.Meuten D.J, Van Kruiningen H.J, Lein D.H. Cryptosporidiosis in a calf. J Am Vet Med Assoc. 1974;165:910–914. [PubMed] [Google Scholar]
- 27.Tzipori S, Smith M, Halpin C, Angus K.W, Sherwood D, Campbell I. Experimental cryptosporidiosis in calves: clinical manifestations and pathological findings. Vet Rec. 1983;112:116–120. doi: 10.1136/vr.112.6.116. [DOI] [PubMed] [Google Scholar]
- 28.Heine J, Pohlenz J.F.L, Moon H.W, Woode G.N. Enteric lesions and diarrhea in gnotobiotic calves monoinfected with Cryptosporidium species. J Infect Dis. 1984;150:768–775. doi: 10.1093/infdis/150.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyzzer E.E. Cryptosporidium parvum (sp. nov.), a coccidium found in the small intestine of the common mouse. Arch Protistenkd. 1912;26:390–394. [Google Scholar]
- 30.Upton S.J, Current W.L. The species of Cryptosporidium (Apicomplexa: Cryptosporidiidae) infecting mammals. J Parasitol. 1985;71:620–625. [PubMed] [Google Scholar]
- 31.Anderson BC. Cryptosporidium spp. in cattle. In: Angus KW, Blewett DA, editors. Proc. 1st Int. Workshop on Cryptosporidiosis. Edinburgh, September 7 to 8, 1989;55–50
- 32.Bukhari Z, Smith H.V. Detection of Cryptosporidium muris oocysts in the faeces of adult dairy cattle in Scotland. Vet Rec. 1996;138:207–208. doi: 10.1136/vr.138.9.207. [DOI] [PubMed] [Google Scholar]
- 33.de J. Pena H.F, Kasai N, Gennari S.M. Cryptosporidium muris in dairy cattle in Brazil. Vet Parasitol. 1997;73:353–355. doi: 10.1016/s0304-4017(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 34.Anderson B.C. Abomasal cryptosporidiosis in cattle. Vet Pathol. 1987;24:235–238. doi: 10.1177/030098588702400307. [DOI] [PubMed] [Google Scholar]
- 35.Esteban E, Anderson B.C. Cryptosporidium muris: prevalence, persistency and detrimental effect on milk production in a drylot dairy. J Dairy Sci. 1995;78:1068–1072. doi: 10.3168/jds.S0022-0302(95)76723-6. [DOI] [PubMed] [Google Scholar]
- 36.Fayer R, Gasbarre L, Pasquali P, Canals A, Almeria S, Zarlenga D. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int J Parasitol. 1998;28:49–56. doi: 10.1016/s0020-7519(97)00170-7. [DOI] [PubMed] [Google Scholar]
- 37.Snodgrass D.R, Angus K.W, Gray E.W, Keir W.A, Clerihew L.W. Cryptosporidia associated with rotavirus and Escherichia coli in an outbreak of calf scour. Vet Rec. 1980;106:458–460. doi: 10.1136/vr.106.22.458-a. [DOI] [PubMed] [Google Scholar]
- 38.Xiao L, Herd R.P. Infection patterns of Cryptosporidium and Giardia in calves. Vet Parasitol. 1994;55:257–262. doi: 10.1016/0304-4017(93)00645-f. [DOI] [PubMed] [Google Scholar]
- 39.Quilez J, Sanchez-Acedo C, Del Cacho E, Clavel A, Causape A.C. Prevalence of Cryptosporidium and Giardia infections in cattle in Aragon (northeastern Spain) Vet Parasitol. 1996;66:139–146. doi: 10.1016/S0304-4017(96)01015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harp J.A, Woodmansee D.B, Moon H.W. Resistance of calves to Cryptosporidium parvum: effects of age and previous exposure. Infect Immun. 1990;58:2237–2240. doi: 10.1128/iai.58.7.2237-2240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villacorta I, Peeters J.E, Vanopdenbosch E, Ares-Mazas E, Theys H. Efficacy of halofuginone lactate against Cryptosporidium parvum in calves. Antimicrob Agents Chemother. 1991;35:283–287. doi: 10.1128/aac.35.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Current W.L. Cryptosporidiosis. J Am Vet Med Assoc. 1985;187:1334–1338. [PubMed] [Google Scholar]
- 43.Sanford S.A, Josephson G.K.A. Bovine cryptosporidiosis: clinical and pathological findings in forty-two scouring neonatal calves. Can Vet J. 1982;23:340–343. [PMC free article] [PubMed] [Google Scholar]
- 44.Peeters J.E, Villacorta I, Vanopdenbosch E. Cryptosporidium parvum in calves: kinetics and immunoblot analysis of specific serum and local antibody responses (immunoglobulin A (IgA), IgG and IgM) after natural and experimental infections. Infect Immun. 1992;60:2309–2316. doi: 10.1128/iai.60.6.2309-2316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pohlenz J, Moon H.W, Cheville N.F, Bemrick W.J. Cryptosporidiosis as a probable factor in neonatal diarrhea of calves. J Am Vet Med Assoc. 1978;172:452–457. [PubMed] [Google Scholar]
- 46.Angus KW. Cryptosporidiosis in ruminants. In: Dubey JP, Speer CA, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton: CRC Press, 1990;83–103
- 47.Abrahamsen M.S, Lancto C.A, Walcheck B, Layton W, Jutila M.A. Localization of α/β and γ/δ lymphocytes in Cryptosporidium parvum-infected tissues in naïve and immune calves. Infect Immun. 1997;65:2428–2433. doi: 10.1128/iai.65.6.2428-2433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanco J, Blanco M, Garabal J.I, Gonzalez E.A. Enterotoxins, colonization factors and serotypes of enterotoxigenic Escherichia coli from humans and animals. Microbiol. 1991;7:57–73. [PubMed] [Google Scholar]
- 49.Tzipori S. The aetiology and diagnosis of calf diarrhoea. Vet Rec. 1981;108:510. doi: 10.1136/vr.108.24.510. [DOI] [PubMed] [Google Scholar]
- 50.Moore D.A, Zeman D.H. Cryptosporidiosis in neonatal calves: 277 cases (1986–1987) J Am Vet Med Assoc. 1991;198:1969–1971. [PubMed] [Google Scholar]
- 51.McDonough S.P, Stull C.L, Osburn B.I. Enteric pathogens in intensively reared veal calves. Am J Vet Res. 1994;55:1516–1520. [PubMed] [Google Scholar]
- 52.de la Fuente R, Garcia A, Ruiz-Santa-Quiteria J.A. Proportional morbidity rates of enteropathogens among diarrheic dairy calves in central Spain. Prev Vet Med. 1998;36:145–152. doi: 10.1016/S0167-5877(98)00077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chanter N, Morgan J.H, Bridger J.C, Hall G.A, Reynolds D.R. Dysentery in gnotobiotic calves caused by atypical Escherichia coli. Vet Rec. 1984;114:71. doi: 10.1136/vr.114.3.71. [DOI] [PubMed] [Google Scholar]
- 54.Mainil J.G, Jacquemin E, Kaeckenbeeck A, Pohl P. Association between the effacing gene (eae) and the Shiga-like toxin-encoding genes in Escherichia coli isolates from cattle. Am J Vet Res. 1993;54:1064–1068. [PubMed] [Google Scholar]
- 55.China B, Pirson V, Mainil J. Prevalence and molecular typing of attaching and effacing Escherichia coli among calf populations in Belgium. Vet Microbiol. 1998;63:249–259. doi: 10.1016/S0378-1135(98)00237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohammad A, Peiris J.S.M, Wijewanta E.A, Mahalingam S, Gunasekara G. Role of verocytotoxigenic Escherichia coli in cattle and buffalo calf diarrhoea. FEMS Microbiol Lett. 1985;26:281–283. [Google Scholar]
- 57.Wieler L.H, Vieler E, Erpenstein C. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J Clin Microbiol. 1996;34:2980–2984. doi: 10.1128/jcm.34.12.2980-2984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orden J.A, Ruiz-Santa-Quiteria J.A, Cid D, Garcia S, Sanz R, de la Fuente R. Verotoxin-producing Escherichia coli (VTEC) and eae-positive non-VETEC in 1–30-days-old diarrhoeic dairy calves. Vet Microbiol. 1988;11:239–248. doi: 10.1016/s0378-1135(98)00218-1. [DOI] [PubMed] [Google Scholar]
- 59.Vanopdenbosch E, Wellemans G. Bovine birna type virus: a new etiological agent of neonatal calf diarrhoea? Vl Dierg Tijdschr. 1990;59:137–140. [Google Scholar]
- 60.Woode G.N, Bridger J.C. Isolation of small viruses resembling astrovirus and caliciviruses from acute enteritis of calves. J Med Microbiol. 1978;11:441–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]
- 61.Orr J.P. Necrotizing enteritis in a calf infected with adenovirus. Can Vet J. 1984;25:72–74. [PMC free article] [PubMed] [Google Scholar]
- 62.Van de Maaten M.J, Packer R.A. Isolation and characterization of bovine enteric viruses. J Am Vet Med Assoc. 1967;28:677–684. [PubMed] [Google Scholar]
- 63.Snodgrass D.R, Stewart J, Taylor J, Krautl F.L, Smith M.L. Diarrhoea in dairy calves reduced by feeding colostrum from cows vaccinated with rotavirus. Res Vet Sci. 1982;32:70–73. doi: 10.1016/S0034-5288(18)32440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bellinzoni R.C, Blackhall J, Terzolo H.R. Microbiology of diarrhoea in young beef and dairy calves in Argentina. Rev Argent Microbiol. 1990;22:130–136. [PubMed] [Google Scholar]
- 65.de Visser N.A, Breukink H.J, van Zijderveld F.G, de Leeuw P.W. Enteric infections in veal calves: a longitudinal study on four veal calf units. Vet Q. 1987;9:289–296. doi: 10.1080/01652176.1987.9694116. [DOI] [PubMed] [Google Scholar]
- 66.Lorenzo-Lorenzo M.J, Ares-Mazas E, Villacorta-Martinez de Maturana I. Detection of oocysts and IgG antibodies to Cryptosporidium parvum in asymptomatic adult cattle. Vet Parasitol. 1993;47:9–15. doi: 10.1016/0304-4017(93)90171-i. [DOI] [PubMed] [Google Scholar]
- 67.Scott C.A, Smith H.V, Gibbs H.A. Excretion of Cryptosporidium parvum by a herd of beef suckler cows. Vet Rec. 1994;134:172. doi: 10.1136/vr.134.7.172. [DOI] [PubMed] [Google Scholar]
- 68.Scott C.A, Smith H.V, Mtambo M.M.A, Gibbs H.A. An epidemiological study of Cryptosporidium parvum in two herds of adult beef cattle. Vet Parasitol. 1995;57:277–288. doi: 10.1016/0304-4017(94)00694-8. [DOI] [PubMed] [Google Scholar]
- 69.Atwill E.R, Harp J.A, Jones T, Jardon P.W, Checel S, Zylstra M. Evaluation of periparturient dairy cows and contact surfaces as a reservoir of Cryptosporidium parvum for calfhood infection. Am J Vet Res. 1998;59:1116–1121. [PubMed] [Google Scholar]
- 70.Reynolds D.J, Morgan J.H, Chanter N. Microbiology of calf diarrhoea in southern Britain. Vet Rec. 1986;119:34–39. doi: 10.1136/vr.119.2.34. [DOI] [PubMed] [Google Scholar]
- 71.Garber L.P, Salman M.D, Hurd H.S, Keefe T, Schlater J.L. Potential risk factors for Cryptosporidium infection in dairy calves. J Am Vet Med Assoc. 1994;205:86–91. [PubMed] [Google Scholar]
- 72.Mann E.D, Sekla L.H, Nayer G.P.S. Infection with Cryptosporidium spp. in humans and cattle in Manitoba. Can J Vet Res. 1986;50:174–178. [PMC free article] [PubMed] [Google Scholar]
- 73.Vanopdenbosch E, Wellemans G, Dekegel X, Strobbe R. Neonatal calf diarrhoea: a complex etiology. Vl Dierg Tijdschr. 1979;48:512–526. [Google Scholar]
- 74.Snodgrass D.R, Angus K.W, Gray E.W. Experimental cryptosporidiosis in germ-free lambs. J Comp Pathol. 1984;94:141–152. doi: 10.1016/0021-9975(84)90016-1. [DOI] [PubMed] [Google Scholar]
- 75.Matos-Fernandez M.J, Ortega-Mora L.M, Pereira-Bueno J. Epidemiologia de la criptosporidiosis en el ganado ovino y caprino de la montana de León. Med Vet. 1994;11:147–152. [Google Scholar]
- 76.Munoz-Fernandez M, Alvarez M, Lanza I, Carmenes P. Role of enteric pathogens in the aethiology of neonatal diarrhoea in lambs and goat kids in Spain. Epidemiol Infect. 1996;117:203–211. doi: 10.1017/s0950268800001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mason R.W, Martley W.J, Tilt L. Intestinal cryptosporidiosis in a kid goat. Aust Vet J. 1981;57:386–388. doi: 10.1111/j.1751-0813.1981.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 78.Polack B, Chermette R, Savey M, Bussieras J. Les cryptosporidies en France. Techniques usuelles d'identification et résultats préliminaires d'enquêtes épidémiologiques. Point Vet. 1983;15:41. [Google Scholar]
- 79.Nagy B, Bozso M, Palfi V, Nagy G, Sahibi MA. Studies on cryptosporidial infection of goat kids. In: Yvoré P, Perrini G, editors. Les maladies de la chèvre. INRA Publ, 1984;443–451
- 80.Matos-Fernandez M.J, Pereira-Bueno J, Ortega-Mora L.M, Pilar-Izquierdo M, Ferre I, Rojo-Vazquez F.A. Prevalencia de la infeccion por Cryptosporidium parvum en corderos, cabritos y terneros en la provincia de León. Acta Paras Port. 1993;1:211. [Google Scholar]
- 81.Koudela B, Modry D, Vitovec J. Infectivity of Cryptosporidium muris isolated from cattle. Vet Parasitol. 1998;76:181–188. doi: 10.1016/s0304-4017(97)00217-3. [DOI] [PubMed] [Google Scholar]
- 82.Tzipori S, Angus K.W, Campbell I, Clerihew L.W. Diarrhea due to Cryptosporidium infection in artificially reared lambs. J Clin Microbiol. 1981;14:100–105. doi: 10.1128/jcm.14.1.100-105.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koudela B, Jiri V. Experimental cryptosporidiosis in kids. Vet Parasitol. 1997;71:273–281. doi: 10.1016/s0304-4017(97)00024-1. [DOI] [PubMed] [Google Scholar]
- 84.Blewett D.A, Wright S.E, Casemore D.P, Booth N.Z, Jones C.E. Infective dose size studies on Cryptosporidium parvum using gnotobiotic lambs. Wat Sci Tech. 1993;27:61–64. [Google Scholar]
- 85.Ortega-Mora L.M, Wright S.E. Age-related resistance in ovine cryptosporidiosis: patterns of infection and humoral immune response. Infect Immun. 1994;62:5003–5009. doi: 10.1128/iai.62.11.5003-5009.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tzipori S, Larsen J, Smith M, Luefl R. Diarrhea in goat kids attributed to Cryptosporidium infection. Vet Rec. 1982;111:35–36. doi: 10.1136/vr.111.2.35. [DOI] [PubMed] [Google Scholar]
- 87.Anderson B.C. Cryptosporidiosis in Idaho lambs: natural and experimental infections. J Am Vet Med Assoc. 1982;181:151–153. [PubMed] [Google Scholar]
- 88.Angus K.W, Appleyard W.T, Menzies J.D, Campbell I, Sherwood D. An outbreak of diarrhoea associated with cryptosporidiosis in naturally reared lambs. Vet Rec. 1982;110:129–130. doi: 10.1136/vr.110.6.129. [DOI] [PubMed] [Google Scholar]
- 89.Hill B.D, Blewett D.A, Dawson A.M, Wright S. Analysis of kinetics, isotype and specificity of serum and coproantibody in lambs infected with Cryptosporidium parvum. Res Vet Sci. 1990;48:76–81. [PubMed] [Google Scholar]
- 90.Naciri M, Yvoré P, Leieux D. Cryptosporidiose expérimentale du chèvreau. Influence de la prise du colostrum. Essais de traitements. In: Yvoré P, Perrini G, editors. Les maladies de la chèvre. I. NRA Publ, 1984;465–471
- 91.Blanco J, Cid D, Blanco J.E, Ruiz-Santa-Quiteria J.A, de la Fuente R. Serogroups, toxins and antibiotic resistance of Escherichia coli strains isolated from diarrhoeic lambs in Spain. Vet Microbiol. 1996;49:209–217. doi: 10.1016/0378-1135(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 92.Cid D, Blanco M, Blanco J.E, Ruiz-Santa-Quiteria J.A, de la Fuente R, Blanco J. Serogroups, toxins and antibiotic resistance of Escherichia coli strains isolated from diarrhoeic goat kids in Spain. Vet Microbiol. 1996;53:349–354. doi: 10.1016/s0378-1135(96)01222-9. [DOI] [PubMed] [Google Scholar]
- 93.Forey W.J. Coccidiosis and cryptosporidiosis in sheep and goats. Vet Clin N Am Food Anim Pract. 1990;6:655–669. doi: 10.1016/s0749-0720(15)30838-0. [DOI] [PubMed] [Google Scholar]
- 94.Xiao L. Giardia infection in farm animals. Parasitol Today. 1994;10:436–438. doi: 10.1016/0169-4758(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 95.Olson M.E, Thorlakson C.L, Deselliers L, Morck D.W, McAllister T.A. Giardia and Cryptosporidium in Canadian farm animals. Vet Parasitol. 1997;68:375–381. doi: 10.1016/s0304-4017(96)01072-2. [DOI] [PubMed] [Google Scholar]
- 96.Nouri M, Ahdfavi S. Effect of nomada shepherds and their sheep on the incidence of cryptosporidiosis in an adjacent town. J Infect. 1993;26:105–106. doi: 10.1016/0163-4453(93)97224-l. [DOI] [PubMed] [Google Scholar]
- 97.Rossanigo E.G, Gialletti L, Grelloni V, Floroni A, Rivero V.B. Diagnosi di criptosporidiosi in alcuni allevamenti dell'Italia Centrale. Riv Zoot Vet. 1987;15:9–15. [Google Scholar]
- 98.Pilar-Izquierdo M, Ortega-Mora L.M, Pereira-Bueno J, Rojo-Vazquez F.A. Participacion de Cryptosporidium parvum en brotes de diarrea en corderos en al NO de Castilla y Leon. Acta Paras Port. 1993;1:223. [Google Scholar]
- 99.Kaminjolo J.S, Adesiyun A.A, Loregnard R, Kitson-Piggott W. Prevalence of Cryptosporidium oocysts in livestock in Trinidad and Tobago. Vet Parasitol. 1993;45:209–213. doi: 10.1016/0304-4017(93)90076-y. [DOI] [PubMed] [Google Scholar]
- 100.Xiao L, Herd R.P, Rings D.M. Diagnosis of Cryptosporidium on a sheep farm with neonatal diarrhea by immunofluorescence assays. Vet Parasitol. 1993;47:17–23. doi: 10.1016/0304-4017(93)90172-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caver J.A, Hill J.E, Thompson S.J. Surveillance of cryptosporidia in a veterinary diagnostic laboratory. J Vet Diagn Invest. 1996;8:497–500. doi: 10.1177/104063879600800420. [DOI] [PubMed] [Google Scholar]
- 102.Yvoré P, Esnault A, Naciri M, et al. Enquête épidémiologique sur les diarrhées néonatales des chevreaux dans les élevages de Touraine. In: Yvoré P, Perrini G, editors. Les maladies de la chèvre. INRA Publ, 1984;437–442
- 103.Thamsborg S.M, Jorgensen R.J, Henriksen S.A. Cryptosporidiosis in kids of dairy goats. Vet Rec. 1990;127:627–628. [PubMed] [Google Scholar]
- 104.Vieira L.S, Silva M.B.O, Tolentino A.C.V, Lima J.D, Silva A.C. Outbreak of cryptosporidiosis in dairy goats in Brazil. Vet Rec. 1997;140:427–428. doi: 10.1136/vr.140.16.427. [DOI] [PubMed] [Google Scholar]
- 105.Xiao L, Herd R.P, McClure K.E. Periparturient rise in the excretion of Giardia sp. cysts and Cryptosporidium parvum oocysts as a source of infection for lambs. J Parasitol. 1994;80:55–59. [PubMed] [Google Scholar]
- 106.Bukhari Z, Smith H.V. Cryptosporidium parvum: oocyst excretion and viability patterns in experimentally infected lambs. Epidemiol Infect. 1997;119:105–108. doi: 10.1017/s0950268897007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klesius P.H, Haynes T.B, Malo L.K. Infectivity of Cryptosporidium sp. isolated from mice for calves and mice. J Am Vet Med Assoc. 1986;189:192–193. [PubMed] [Google Scholar]
- 108.Ortega-Mora L.M, Troncoso J.M, Rojo-Vazquez F.A, Gomez-Bautista M. Serum antibody response in lambs naturally and experimentally infected with Cryptosporidium. Vet Parasitol. 1993;50:45–54. doi: 10.1016/0304-4017(93)90006-9. [DOI] [PubMed] [Google Scholar]
- 109.Molina J.M, Rodriguez-Ponce E, Ferrer O, Gutierrez A.C, Hernandez S. Biopathological data of goat kids with cryptosporidiosis. Vet Rec. 1994;135:67–68. doi: 10.1136/vr.135.3.67. [DOI] [PubMed] [Google Scholar]
- 110.Tyzzer E.E. Coccidiosis in gallinaceous birds. Am J Hyg. 1929;10:269–383. [Google Scholar]
- 111.Current W.L, Upton S.V, Haynes T.B. The life cycle of Cryptosporidium baileyi n. sp. (Apicmplexa Cryptosporidiidae) infecting chickens. J Parasitol. 1986;33:289–296. doi: 10.1111/j.1550-7408.1986.tb05608.x. [DOI] [PubMed] [Google Scholar]
- 112.Lindsay D.S, Blagburn B.L, Sundermann C.A. Morphometric comparison of the oocysts of Cryptosporidium meleagridis and Cryptosporidium baileyi from birds. Proc Helminthol Soc Wash. 1989;56:91–92. [Google Scholar]
- 113.Ritter G.D, Ley D.H, Levy M, Guy J, Barnes H.J. Intestinal cryptosporidiosis and reovirus isolation from bobwhite quail (Colinus virginianus) with enteritis. Avian Dis. 1986;30:603–608. [PubMed] [Google Scholar]
- 114.Guy J.S, Levy M.G, Ley D.H, Barnes H.J, Geric T.M. Experimental reproduction of entiritis in bobwhite quail (Colinus virginianus) with Cryptosporidium and reovirus. Avian Dis. 1987;31:713–722. [PubMed] [Google Scholar]
- 115.Gajadhar A.A. Host specificity studies and oocyst description of a Cryptosporidium sp. isolated from ostriches. Parasitol Res. 1994;80:316–319. doi: 10.1007/BF02351873. [DOI] [PubMed] [Google Scholar]
- 116.O'Donoghue P.J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 117.Fayer R, Speer CA, Dubey JP. Avian cryptosporidiosis. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton: CRC Press, 1997;1–33
- 118.Bermudez A.J, Ley D.H, Levy M.G, Ficken M.D, Guy J.S, Gerig T.M. Intestinal and bursal cryptosporiosis in turkeys following inoculation with Cryptosporidium sp. isolated from commercial poults. Avian Dis. 1988;32:445–450. [PubMed] [Google Scholar]
- 119.Goodwin M.A, Steffens W.L, Russell I.D, Brown J. Diarrhea associated with intestinal crypotosporidiosis in turkeys. Avian Dis. 1988;32:63–67. [PubMed] [Google Scholar]
- 120.Lindsay D.S, Blagburn B.L, Sundermann C.A, Hoerr F.J, Ernest J.A. Experimental Cryptosporidium infections in chickens: oocyst structure and tissue specificity. Am J Vet Res. 1986;47:876–879. [PubMed] [Google Scholar]
- 121.Blagburn B.L, Lindsay D.S, Giambrone J.J, Sunderman C.A, Hoerr F.J. Experimental cryptosporidiosis in broiler chickens. Poultry Sci. 1987;66:442–449. doi: 10.3382/ps.0660442. [DOI] [PubMed] [Google Scholar]
- 122.Lindsay D.S, Blagburn B.L, Hoerr F.J. Experimental induced infections in turkeys with Cryptosporidium baileyi isolated from chickens. Am J Vet Res. 1987;48:104–108. [PubMed] [Google Scholar]
- 123.Lindsay D.S, Blagburn B.L, Sundermann C.A, Hoerr F.J. Experimental infections in domestic ducks with Cryptosporidium baileyi isolated from chickens. Avian Dis. 1989;33:69–73. [PubMed] [Google Scholar]
- 124.Lindsay DS, Blagburn BL. Cryptosporidiosis in birds. In: Dubey JP, Speer CA, Fayer R, editors. Cryptosporidiosis in man and animals. Boca Raton: CRC Press, 1990;133–148
- 125.Hoerr F.J, Current W.L, Haynes T.B. Fatal cryptosporidiosis in quail. Avian Dis. 1986;30:421–425. [PubMed] [Google Scholar]
- 126.Itakura C, Goryo M, Umemura T. Cryptosporidial infection in chickens. Avian Pathol. 1984;13:487–499. doi: 10.1080/03079458408418550. [DOI] [PubMed] [Google Scholar]
- 127.Goodwin M.A, Latimer K.S, Brown J, Steffens W.L, Martin P.W, Resurreccion R.S, Smeltzer M.A, Dickson T.G. Respiratory cryptosporidiosis in chicken. Poultry Sci. 1988;67:1684–1693. doi: 10.3382/ps.0671684. [DOI] [PubMed] [Google Scholar]
- 128.Current W.L, Snyder D.B. Development of an serologic evaluation of acquired immunity to Cryptosporidium baileyi by broiler chickens. Poultry Sci. 1988;67:720–729. doi: 10.3382/ps.0670720. [DOI] [PubMed] [Google Scholar]
- 129.Lindsay D.S, Blagburn B.L, Sundermann C.A, Giambrone J.J. Effect of broiler chicken age on susceptibility to experimentally induced Cryptosporidium baileyi infection. Am J Vet Res. 1988;49:1412–1414. [PubMed] [Google Scholar]
- 130.Taylor M.A, Catchpole J, Norton C.C, Green J.A. Variations in oocyst output associated with Cryptosporidium baileyi infections in chickens. Vet Parasitol. 1994;53:7–14. doi: 10.1016/0304-4017(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 131.Sréter T, Varga I, Bikisi L. Age-dependent resistance to Cryptosporidium baileyi infection in chickens. J Parasitol. 1995;81:827–829. [PubMed] [Google Scholar]
- 132.Goodwin M.A, Brown J. Effect on Cryptosporidium baileyi on broilers infected at 26 days of age. Avian Dis. 1990;34:458–462. [PubMed] [Google Scholar]
- 133.Fayer R, Ungar B.L.P. Cryptosporidium sp. and cryptosporidiosis. Microbiol Rev. 1986;50:458–483. doi: 10.1128/mr.50.4.458-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goodwin M.A, Brown J. Histologic incidence and distribution of Cryptosporidium sp. infection in chickens: 68 cases in 1986. Avian Dis. 1988;32:365–369. [PubMed] [Google Scholar]
- 135.Current WL. Cryptosporidiosis. In: Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, editors. Diseases of poultry. Ames: Iowa State University Press, 1997;883–890
- 136.Goodwin M.A. Cryptosporidiosis in birds—a review. Avian Pathol. 1989;18:365–384. doi: 10.1080/03079458908418612. [DOI] [PubMed] [Google Scholar]
- 137.Kichou F, Saghir F, El Hamidi M. Infection naturelle de Cryptosporidium sp. chez le poulet de chair au Maroc. Avian Pathol. 1996;25:103–111. doi: 10.1080/03079459608419124. [DOI] [PubMed] [Google Scholar]
- 138.Tzipori S, Campbell I. Prevalence of Cryptosporidium antibodies in 10 animal species. J Clin Microbiol. 1981;14:455–456. doi: 10.1128/jcm.14.4.455-456.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Randall C.J. Cryptosporidiosis of the bursa of Fabricius and trachea in broilers. Avian Pathol. 1982;11:95–102. doi: 10.1080/03079458208436084. [DOI] [PubMed] [Google Scholar]
- 140.Gorham S.L, Mallinson E.T, Synder D.B, Odor E.M. Cryptosporidia in the bursa of Fabricius—a correlation with mortality rates in broiler chickens. Avian Pathol. 1987;16:205–211. doi: 10.1080/03079458708436369. [DOI] [PubMed] [Google Scholar]
- 141.Snyder D.B, Current W.L, Russek-Cohen E. Serologic incidence of Cryptosporidium in Delmarva broiler flocks. Poultry Sci. 1988;67:730–735. doi: 10.3382/ps.0670730. [DOI] [PubMed] [Google Scholar]
- 142.Papadopoulou C, Xylouri E, Zisides N. Cryptosporidial infection in broiler chikens in Greece. Avian Dis. 1988;32:842–843. [PubMed] [Google Scholar]
- 143.Ley D.H, Levy M.G, Hunter L, Barnes H.J. Cryptosporidia-positive rates of avian necropsy accessions determined by examination of auramine O-stained fecal smears. Avian Dis. 1988;32:108–113. [PubMed] [Google Scholar]
- 144.Woodmansee D.B, Pavlasek I, Pohlenz J.F.L, Moon H.W. Subclinical cryptosporidiosis of turkeys in Iowa. J Parasitol. 1988;74:898–900. [PubMed] [Google Scholar]
- 145.Goodwin M.A, Brown J, Fletcher O. The relationship of Cryptosporidium sp. infection on the bursa of Fabricius, intestinal tract, and respiratory system of chickens in Georgia, 1974–1988. Avian Dis. 1990;34:701–703. [PubMed] [Google Scholar]
- 146.Rhee J.K, Seu Y.S, Park B.K. Isolation and identification of Cryptosporidium from various animals in Korea. I. Prevalence of Cryptosporidium in various animals. Korean J Parasitol. 1991;29:139–148. doi: 10.3347/kjp.1991.29.2.139. [DOI] [PubMed] [Google Scholar]
- 147.Goodwin M.A, Brown J. Incidence of respiratory cryptosporidiosis in Georgia broilers: 1987–1992. Avian Dis. 1994;38:358–360. [PubMed] [Google Scholar]
- 148.Goodwin M.A, Brown J, Resurreccion R.S, Smith J.A. Respiratory coccidiosis (Cryptosporidium baileyi) among Northern Georgia broilers in one company. Avian Dis. 1996;40:572–575. [PubMed] [Google Scholar]
- 149.Naciri M, Mazella O. Association cryptosporidiose et maladie de Marek chez des poulets nains. Rec Med Vet. 1988;164:311–312. [Google Scholar]
- 150.Fletcher O.J, Munnell J.F, Page R.K. Cryptosporidiosis of the bursa of Fabricius of chickens. Avian Dis. 1975;19:630–639. [PubMed] [Google Scholar]
- 151.Goodwin M.A. Small-intestinal cryptosporidiosis in a chicken. Avian Dis. 1988;32:844–848. [PubMed] [Google Scholar]
- 152.Dobos-Kovacs M, Varga I, Bikisi L, Drin CsN, Nimeth I, Farkas T. Concurrent cryptosporidiosis and chicken anemia virus infection in broiler chickens. Avian Pathol. 1994;23:365–368. doi: 10.1080/03079459408419005. [DOI] [PubMed] [Google Scholar]
- 153.Guy J.S, Levy M.G, Ley D.H, Barnes H.J, Gerig T.M. Interaction of reovirus and Cryptosporidium baileyi in experimentally infected chickens. Avian Dis. 1988;32:881–890. [PubMed] [Google Scholar]
- 154.Levy M.G, Ley D.H, Barnes J, Gerig T.M, Corbett W.T. Experimental cryptosporidiosis and infectious bursal disease virus infection of specific pathogen-free chickens. Avian Dis. 1988;32:803–811. [PubMed] [Google Scholar]
- 155.Hornok S, Heijmans J.F, Bikisi, Peek H.W, Dobos-Kovas M, Varga I. Interaction of chickens anaemia virus and Cryptosporidium baileyi in experimentally infected chickens. Vet Parasitol. 1998;76:43–55. doi: 10.1016/s0304-4017(97)00046-0. [DOI] [PubMed] [Google Scholar]
- 156.Goodwin M.A. Esophageal and proventricular cryptosporidiosis in a chicken. Avian Dis. 1995;39:643–645. [PubMed] [Google Scholar]
- 157.Blagburn B.L, Lindsay D.S, Hoerr F.J, Davis J.F, Giambrone J.J. Pathobiology of cryptosporidiosis (C. baileyi) in broiler chickens. J Protozool. 1991;38:25S–28S. [PubMed] [Google Scholar]
- 158.Rhee J.K, Kim H.C, Park B.K. Effect of Cryptosporidium baileyi infection on antibody response to sRBC in chickens. Korean J Parasitol. 1998;36:33–36. doi: 10.3347/kjp.1998.36.1.33. [DOI] [PubMed] [Google Scholar]
- 159.Rhee J.K, Kim H.C, Lee S.B, Yook S.Y. Immunosuppresive effect of Cryptosporidium baileyi infection on vaccination against Newcastle disease in chicks. Korean J Parasitol. 1998;36:121–125. doi: 10.3347/kjp.1998.36.2.121. [DOI] [PubMed] [Google Scholar]
- 160.Varga I, Sréter T, Bikisi L. Potentiation of ionophorous anticoccidials with Duokvin: battery trials against Cryptosporidium baileyi in chickens. J Parasitol. 1995;81:777–780. [PubMed] [Google Scholar]
- 161.Bergeland M.E. Necrotic enteritis in nursing piglets. Proc Am Assoc Vet Lab Diagn. 1977;20:151–158. [Google Scholar]
- 162.Kennedy G.A, Kreitner G.L, Strafuss A.C. Cryptosporidiosis in three pigs. J Am Vet Med Assoc. 1977;170:348–350. [PubMed] [Google Scholar]
- 163.Kim CW. Cryptosporidiosis in pigs and horses. In: Dubey JP, Speer CA, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton: CRC Press, 1990;104–111
- 164.Tzipori S, McCartney E, Lawson G.H.K, Rowland A.C, Campbell I. Experimental infection of piglets with Cryptosporidium. Res Vet Sci. 1981;31:358–368. [PubMed] [Google Scholar]
- 165.Moon H.W, Bemrick W.J. Fecal transmission of calf cryptosporidia between calves and pigs. Vet Pathol. 1981;18:248–255. doi: 10.1177/030098588101800213. [DOI] [PubMed] [Google Scholar]
- 166.Vitovec J, Koudela B. Pathogenesis of intestinal cryptosporidiosis in conventional and gnotobiotic piglets. Vet Parasitol. 1992;43:25–36. doi: 10.1016/0304-4017(92)90045-b. [DOI] [PubMed] [Google Scholar]
- 167.Tzipori S, Smith M, Makin T, Halpin C. Enterocolitis in piglets caused by Cryptosporidium spp. purified from calf faeces. Vet Parasitol. 1982;11:121–126. doi: 10.1016/0304-4017(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 168.Trotti G.C, Pampliglione S, Visconti S. Cryptosporidium sp. and Isospora suis in pigs in Italy. Parassitologia. 1984;26:299–304. [PubMed] [Google Scholar]
- 169.Ayeni A.O, Olubunmi P.A, Abe J.O. The occurrence and effect of Cryptosporidium species on livestock in Ile-Ife, Nigeria. Trop Vet. 1985;3:96–100. [Google Scholar]
- 170.Cabrera J.F, Garcia M.G. Intestinal cryptosporidiosis of diarrhoeic piglets in our environment. Rev Cubana Cienc Vet. 1985;16:259–264. [Google Scholar]
- 171.Quilez J, Sanchez-Acedo C, Clavel A, del Cacho E, Lopez-Bernad F. Prevalence of Cryptosporidium infections in pigs in Aragon (Northeastern Spain) Vet Parasitol. 1996;67:83–88. doi: 10.1016/s0304-4017(96)01026-6. [DOI] [PubMed] [Google Scholar]
- 172.Tacal J.V, Sobieh M, El-Ahraf A. Cryptosporidium in market pigs in southern California, USA. Vet Rec. 1987;120:615–617. doi: 10.1136/vr.120.26.615. [DOI] [PubMed] [Google Scholar]
- 173.Kaminjolo J.S, Adesiyun A.A, Loregnard R, Kison-Piggott W. Prevalence of Cryptosporidium oocysts in livestock in Trinidad and Tobago. Vet Parasitol. 1993;45:209–213. doi: 10.1016/0304-4017(93)90076-y. [DOI] [PubMed] [Google Scholar]
- 174.Adesiyun A.A, Kaminjolo J.S. Prevalence and epidemiology of selected enteric infections of livestock in Trinidad. Prev Vet Med. 1994;19:151–165. [Google Scholar]
- 175.Morgan U.M, Buddle J.R, Armson A, Elliot A, Thompson R.C.A. Molecular and biological characterisation of Cryptosporidium in pigs. Aust Vet J. 1999;77:44–47. doi: 10.1111/j.1751-0813.1999.tb12428.x. [DOI] [PubMed] [Google Scholar]
- 176.Peeters JE. Recent advances in intestinal pathology of rabbits and further perspectives. In: 4th Congress of the World Rabbit Science Association. Budapest, Hungary, 1988; 293–313
- 177.Peeters JE, Charlier GJ, Dussart P. Pouvoir pathogène de Cryptosporidium sp. chez les lapereaux avant et apres sevrage. In: 4émes journées de la recherche cunicole en France. Paris, France, 1986; 371–378
- 178.Naciri M, Yvoré P. Efficacité du lactate d'halofuginone dans le traitement de la cryptosporidiose chez l'agneau. Rec Med Vet. 1989;165:823–826. [Google Scholar]
- 179.Naciri M, Mancassola R, Yvoré P, Peeters J.E. The effect of halofuginone lactate on experimental Cryptosporidium parvum infections in calves. Vet Parasitol. 1993;45:199–207. doi: 10.1016/0304-4017(93)90075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peeters J.E, Villacorta I, Naciri M, Vanopdenbosch E. Specific serum and local antibody responses against Cryptosporidium parvum during medication of calves with halofuginone lactate. Infect Immun. 1993;61:4440–4445. doi: 10.1128/iai.61.10.4440-4445.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fayer R, Ellis W. Paromomycin is effective as prophylaxis for cryptosporidiosis in dairy calves. J Parasitol. 1993;79:771–774. [PubMed] [Google Scholar]
- 182.Mancassola R, Répérant J.M, Naciri M, Chartier C. Chemoprophylaxis of Cryptosporidium parvum infection with paromomycin in kids and immunological study. Antimicrob Agents Chemother. 1995;39:75–78. doi: 10.1128/aac.39.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Redman D.R, Fox J.E. The effect of varying levels D. ECCOX® on experimental Cryptosporidia infections in Holstein bull calves. Bovine Proc. 1994;26:157–159. [Google Scholar]
- 184.Mancassola R, Richard A, Naciri M. Evaluation of decoquinate to treat experimental cryptosporidiosis in kids. Vet Parasitol. 1997;69:31–37. doi: 10.1016/s0304-4017(96)01094-1. [DOI] [PubMed] [Google Scholar]
- 185.Hornok S, Bitay Z, Szill Z, Varga I. Assessment of maternal immunity to Cryptosporidium baileyi in chickens. Vet Parasitol. 1998;79:203–212. doi: 10.1016/s0304-4017(98)00170-8. [DOI] [PubMed] [Google Scholar]
- 186.Harp J.A, Woodmansee D.B, Moon H.W. Effect of colostral antibody on susceptibility of calves to Cryptosporidium parvum infection. Am J Vet Res. 1989;50:2117–2119. [PubMed] [Google Scholar]
- 187.Fayer R, Andrews C, Ungar B.L.P, Blagburn B. Efficacy of hyperimmune bovine colostrum for prophylaxis of cryptosporidiosis in neonatal calves. J. Parasitol. 1989;75:393–397. [PubMed] [Google Scholar]
- 188.Naciri M, Mancassola R, Répérant J.M, Canivez O, Quinque B, Yvoré P. Treatment of experimental ovine crytosporidiosis with ovine or bovine hyperimmune colostrum. Vet Parasitol. 1994;53:173–190. doi: 10.1016/0304-4017(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 189.de Graaf DC, Spano F, Petry F, Sagodira S, Bonnin A. Speculation on whether a vaccine against cryptosporidiosis is a reality or fantasy. Int J Parasitol (in press) [DOI] [PMC free article] [PubMed]


