Highlights
-
•
NURTURE is an ongoing study of nusinersen started in a presymptomatic stage of SMA.
-
•
All infants were ≥25 months old, and alive without permanent ventilation.
-
•
All infants achieved independent sitting and 88% (22/25) were walking alone.
-
•
Nusinersen demonstrated durability of effect with a median 2.9 years of follow up.
-
•
Nusinersen was well tolerated with no new safety concerns over extended follow up.
Keywords: Spinal muscular atrophy, Clinical trial, Neurofilament, Newborn screening, Nusinersen, Presymptomatic
Abstract
Spinal muscular atrophy (SMA) is a neurodegenerative disease associated with severe muscle atrophy and weakness in the limbs and trunk. We report interim efficacy and safety outcomes as of March 29, 2019 in 25 children with genetically diagnosed SMA who first received nusinersen in infancy while presymptomatic in the ongoing Phase 2, multisite, open-label, single-arm NURTURE trial. Fifteen children have two SMN2 copies and 10 have three SMN2 copies. At last visit, children were median (range) 34.8 [25.7–45.4] months of age and past the expected age of symptom onset for SMA Types I or II; all were alive and none required tracheostomy or permanent ventilation. Four (16%) participants with two SMN2 copies utilized respiratory support for ≥6 h/day for ≥7 consecutive days that was initiated during acute, reversible illnesses. All 25 participants achieved the ability to sit without support, 23/25 (92%) achieved walking with assistance, and 22/25 (88%) achieved walking independently. Eight infants had adverse events considered possibly related to nusinersen by the study investigators. These results, representing a median 2.9 years of follow up, emphasize the importance of proactive treatment with nusinersen immediately after establishing the genetic diagnosis of SMA in presymptomatic infants and emerging newborn screening efforts.
1. Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease associated with progressive and often severe muscle weakness and atrophy and is a leading cause of death in infants [1], [2], [3], [4]. SMA is caused by homozygous deletions (∼95% of SMA patients) or compound heterozygous mutations (∼5% of SMA patients) in the survival motor neuron 1 (SMN1) gene that prevent production of full-length functional SMN protein [5]. The paralogous gene SMN2 undergoes aberrant splicing and produces mostly truncated, dysfunctional protein (∼90%) [1]. Symptom onset most often occurs in infancy and early childhood, although onset can be in adulthood in the mildest form. Symptoms are generally more severe with earlier onset [1]. Most individuals who develop SMA have a symptom-free period after birth, which differs in duration for every individual. SMA is divided into four major subtypes based on age at symptom onset and maximum motor function achieved [1,6,7], but maximum motor function achievement can be influenced by SMA treatment. Many infants with infantile-onset SMA and children with later-onset SMA treated with nusinersen in the ENDEAR and CHERISH studies, respectively, achieved motor function incongruent with their expected SMA subtypes based on SMN2 copy number and age of symptom onset [8,9].
Prior to the onset of symptoms, other markers must be relied upon for diagnosis and classification of predicted SMA subtype. SMN2 gene copy number is roughly correlated with disease severity, as an increased number of SMN2 gene copies typically leads to an increased amount of functional SMN protein and a milder phenotype [1]. Among individuals who have SMN1 disruptions and are therefore expected to develop SMA symptoms, approximately 75% of individuals with two copies of the SMN2 gene are predicted to develop the Type I form of SMA, while approximately 80% of those with three copies of the SMN2 gene are predicted to develop SMA Type II [10,11]. Additionally, phosphorylated neurofilament heavy chain (pNF-H) levels have recently been shown to be a promising biomarker of disease activity and treatment response in individuals with SMA [12,13]. pNF-H is a neuron-specific cytoskeletal structural protein released into the plasma and cerebrospinal fluid (CSF) during axonal damage [14,15]. In the ENDEAR and CHERISH studies, concentrations of pNF-H were found to correlate with baseline clinical characteristics indicative of disease severity. Furthermore, pNF-H concentrations declined rapidly then stabilized at a lower level following nusinersen treatment in a manner not seen in the sham control group [13].
Nusinersen is an antisense oligonucleotide that alters the splicing of SMN2 pre-mRNA to promote expression of full-length SMN protein [7,[21], [22], [23] and is the first disease-modifying treatment approved for SMA [24]. In clinical studies in a range of symptomatic children across several SMA populations, nusinersen demonstrated significant and clinically meaningful benefit as assessed by achievement of motor milestones, measures of motor function, and survival in those with infantile-onset SMA, along with a favorable safety profile [8,9,25]. Importantly, subgroup analyses of the sham-controlled studies in infantile-onset (ENDEAR) and later-onset (CHERISH) SMA indicated that greater improvements in motor function following nusinersen treatment were observed in those with relatively shorter disease duration at treatment initiation [8,9], suggesting that earlier treatment may lead to better clinical outcomes.
NURTURE is an ongoing Phase 2, open-label study aimed to evaluate the safety and efficacy of nusinersen in preventing or profoundly attenuating the severity of SMA when initiated prior to the onset of symptoms. Infants enrolled in NURTURE had genetic confirmation of 5q SMA, were ≤6 weeks old at first dose, and were considered most likely to develop SMA Type I or Type II based upon the SMN2 gene copy number and the expected concordance with the phenotype of an affected sibling(s) based on previous studies [10,11,26]. In the absence of treatment, many of these infants would not be expected to achieve independent sitting (those likely to develop SMA Type I), and few if any would be expected to ever walk independently [6]. Most would also be expected to require respiratory intervention and some would not survive beyond their early years [27].
2. Patients and methods
2.1. Study design and participants
NURTURE (NCT02386553) is an ongoing, Phase 2, open-label, single-arm, multinational study to evaluate the long-term safety and efficacy of intrathecal nusinersen in infants who initiate treatment early, prior to the onset of clinical signs of SMA. Given the expectation that infants with genetically diagnosed SMA without a functioning copy of the SMN1 gene and with two or three copies of the SMN2 gene will develop severe or fatal symptoms during the first years of life, an internal control group was considered unnecessary and ethically unjustifiable.
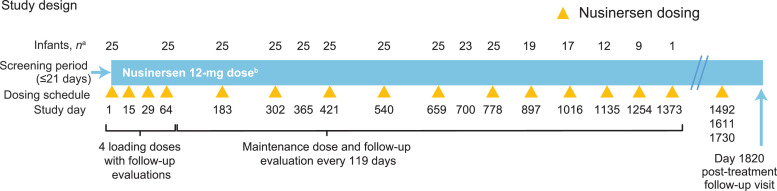
NURTURE consists of a 5-year treatment period and a post-treatment follow-up evaluation. Key eligibility criteria were age ≤6 weeks at first dose, genetic documentation of 5q SMA (biallelic deletion or protein disabling mutation of the SMN1 gene), two or three copies of the SMN2 gene, baseline compound muscle action potential (CMAP) amplitude ≥1 mV (protocol amended on 30 September 2015 [version 4] to baseline ulnar CMAP amplitude ≥1 mV), absence of hypoxemia, and no clinical signs or symptoms suggestive of SMA. Participants received nusinersen 12 mg administered as intrathecal injections by lumbar puncture. The nusinersen treatment regimen consists of four loading doses (administered on Days 1, 15, 29, and 64), followed by a maintenance dose every 119 days over five years (Fig. 1 ). This interim analysis from the ongoing NURTURE study reports data from the March 29, 2019, data cut. At the time of this interim analysis, the NURTURE infants were 25.7–45.4 (median 34.8) months of age and were past the expected age of symptom onset for SMA Types I or II [3,27].
Fig. 1.
NURTURE study design.
Intention-to-treat population is all infants who received ≥1 dose of study drug (n = 25).
aInfants who attended or had the opportunity to attend the visit.
bInfants treated with nusinersen 12 mg; some infants received a 12-mg scaled equivalent dose before the protocol was revised in March 2017.
The NURTURE trial is taking place at 15 active study sites in seven countries. The study was approved by the local ethics committee at each participating site and is being conducted in accordance with the International Council on Harmonization (ICH) guidelines for Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from the parent(s) or legal guardian(s) of each study participant in accordance with local practice and regulations.
2.2. Study endpoints reported in this interim analysis
2.2.1. Primary endpoints
The primary endpoint for NURTURE is time to death or respiratory intervention (invasive or non-invasive for ≥6 h per day continuously for ≥7 days or tracheostomy).
2.2.2. Secondary endpoints
Secondary endpoints reported in this interim analysis include (1) proportion of participants alive; (2) attainment of motor milestones as assessed by World Health Organization (WHO) criteria; (3) attainment of motor milestones by Hammersmith Infant Neurologic Examination, Section 2 (HINE-2); (4) change from baseline in the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) motor function scale; (5) change from baseline in growth parameters: weight for age/length, head circumference, chest circumference, head-to-chest circumference ratio, and arm circumference; and (6) proportion of participants developing clinically manifested SMA at 13 and 24 months of age as defined by any of the following conditions: (a) age-adjusted weight <5th percentile or decrease of ≥2 major weight growth curve percentiles (3rd, 5th, 10th, 25th, or 50th) compared with baseline, or a percutaneous gastric tube placement for nutritional support at 13 or 24 months of age; (b) failure to achieve sitting without support, standing with assistance, and hands-and-knees crawling at age 13 months; or (c) failure to achieve the milestones defined at age 13 months and failure to achieve walking with assistance, standing alone, and walking alone at age 24 months.
Secondary safety endpoints include (1) incidences of adverse events (AEs) and serious adverse events (SAEs); (2) change from baseline in clinical laboratory parameters, electrocardiograms (ECGs), and vital signs; and (3) neurological examinations (HINE-1 [neurological status] and HINE-3 [behavior] for participants age ≤24 months; standard neurological exam for participants age >24 months).
2.2.3. Exploratory endpoints
Exploratory endpoints, including (1) change from baseline in CMAP amplitude; (2) time to death or permanent ventilation (defined as ≥16 h/day continuously for >21 days in the absence of an acute reversible event or tracheostomy); (3) time to death or ventilation for ≥6 h/day continuously for ≥1 day or tracheostomy; and (4) plasma and CSF pNF-H concentration and change over time were also assessed in this interim analysis.
2.3. Assessments
The following clinical assessments were performed to evaluate nusinersen efficacy: survival, respiratory events, WHO and HINE-2 motor milestone achievement, HINE-1 neurological evaluation, CHOP INTEND motor function scale, growth parameters, ulnar and peroneal CMAP amplitudes, and plasma/CSF pNF-H levels. A listing of study visits at which data was collected for the study assessments is provided in Supplementary Table 1.
Achievement of motor milestones was evaluated using WHO criteria [28] and the HINE-2 assessment tool [29]. WHO motor milestones are a set of six milestones (sitting without support, standing with assistance, hands-and-knees crawling, walking with assistance, standing alone, and walking alone) expected to be attained by age 24 months in healthy children [28]. WHO motor milestones were assessed by physical therapists; age of first achievement of each milestone was recorded by caregivers. HINE is a quantifiable, three-part assessment to evaluate neurological status (Section 1), development of motor function (Section 2), and behavior (Section 3) [29]. Neurological items assessed by the HINE-1 include sucking/swallowing ability during assessment of cranial nerve function [29]. Scores range from 0 to 3, with higher scores indicating better function. HINE-2 measures developmental progress in eight motor milestones: voluntary grasp, ability to kick in supine position, head control, rolling, sitting, crawling, standing, and walking. Total scores range from 0 to 26, with higher scores indicating better motor function [29]. The HINE-2 score was assessed until Day 778. CHOP INTEND is a 16-item, 64-point motor assessment developed to evaluate motor skills in infants with SMA Type I, with higher scores indicating better motor function [30,31]. The CHOP INTEND was assessed until a participant reached a maximum score of 64, after which it was not assessed. Motor function assessments were performed by trained clinical evaluators.
CMAP amplitude is an electrophysiological technique that reflects the mass of excitable muscle tissue depolarized with whole nerve stimulation, and in SMA relates to the approximate number of motor neurons innervating a muscle. Ulnar CMAP amplitude is a well-validated method for tracking disease progression in SMA [32,33].
Measurement of growth parameters included length, weight-for-age, length-for-age, weight-for-length, head circumference, chest circumference, head-to-chest circumference ratio, and arm circumference. WHO child growth standards were used to determine percentiles for each parameter [28].
pNF-H levels recently have been shown to be a potential biomarker of disease severity and response to treatment in individuals with SMA. Plasma and CSF samples were tested for pNF-H. pNF-H levels were summarized as geometric mean for NURTURE infants by SMN2 copy number and compared with levels reported for infants without SMA aged <1 year. Geometric mean was used to appropriately account for the underlying distribution of the data. Samples from 18 individuals were donated by Boston Children's Hospital (Boston, MA) and confirmed to be from individuals with no known neurological or musculoskeletal disorders or other chronic illness. pNF-H levels were measured using a pNF-H enzyme-linked lectin assay from ProteinSimple.
Safety assessments included treatment-emergent AE monitoring, neurological examinations, physical examinations, vital sign measurements, pulse oximetry, 12-lead ECGs, and laboratory testing (hematology, blood chemistry, urinalysis, and coagulation) and were performed by trained physicians and ancillary medical professionals.
2.4. Sibling assessments
When possible, comparisons in survival and motor milestone achievement were made between NURTURE participants and non-nusinersen-treated siblings with SMA. Only non-invasive data were collected from siblings, including SMN2 gene copy number, sibling SMA history, and sibling treatment history.
2.5. Statistical methods
Time to death or respiratory intervention, proportion of participants alive, proportion of participants achieving WHO motor milestones, proportion of participants developing clinically manifested SMA, and safety analyses were analyzed in all study participants who received ≥1 dose of nusinersen (intent-to-treat population). All other efficacy analyses were performed on interim efficacy sets that comprised all dosed participants who attended or had the opportunity to attend the targeted visit of the analysis (efficacy set). All secondary endpoints were assessed at ages 13 and 24 months. Proportion of participants alive and proportion who achieved WHO motor milestones at ages 13 and 24 months were estimated using the Kaplan-Meier method. CHOP INTEND total score, HINE-2 total motor milestone score, change in growth parameters, ulnar and peroneal CMAP amplitude, and pNF-H concentrations were summarized using descriptive statistics. The proportion of participants developing clinically manifested SMA at Day 365 (13-month assessment) or Day 700 (24-month assessment) were reported with a corresponding Wilson score confidence interval (CI) with continuity correction [34]. The age at which a WHO motor milestone was achieved was determined by using the caregiver-reported date if confirmed at the subsequent study visit by the physical therapist.
To identify early predictors of motor function, Spearman correlation coefficients were calculated. Specifically, the relationships between participant characteristics at baseline and at the end of the loading dose period on Day 64 (age at first dose of nusinersen, gestational age, plasma pNF-H levels, weight for age, CHOP INTEND total score, HINE-2 motor milestone total score, and ulnar CMAP amplitude) and future motor function (Day 302 total HINE-2 motor milestone score and age of achievement of walking alone) were evaluated. The statistical software SASⓇ version 9.4 (Cary, NC) or above was used for all summaries and statistical analyses.
3. Results
3.1. Study population
A total of 30 infants were screened; 25 infants (15 with two SMN2 copies and 10 with three SMN2 copies) met eligibility criteria and were enrolled in the study. Five infants were ineligible; one did not have a diagnosis of 5q SMA, one did not have two or three copies of the SMN2 gene, and three had an ulnar CMAP amplitude equal to or below one mV at screening (two of whom also had clinical signs or symptoms suggestive of SMA). The first participant's initial visit was in May 2015 and enrollment was complete in February 2017. As of data cutoff, no participants have withdrawn from the study or discontinued treatment.
The median (range) age at first dose of nusinersen among enrolled infants was 22.0 (3–42) days. At the time of the interim analysis, median (range) age at last visit was 34.8 (25.7–45.4) months and time on study was 33.9 (25.3–45.1) months. Baseline characteristics by SMN2 copy number are shown in Table 1 . Median (range) CHOP INTEND total score and total HINE-2 motor milestone scores were 45.0 (25.0–60.0) and 3.0 (0–5), respectively, in two-copy participants (n = 15) and 53.5 (40.0–60.0) and 3.0 (0–7), respectively, in three-copy participants (n = 10). Twenty-four participants consented to collection of plasma and CSF for future investigations of possible biomarkers of SMA disease. Baseline plasma values were unavailable in two participants, and baseline CSF values were unavailable in one participant. Geometric mean (95% CI) plasma pNF-H concentrations were 20,880.9 (9639.4–45,231.9) pg/mL in two-copy participants (n = 13), and 1870.7 (1152.9–3035.5) pg/mL in three-copy participants (n = 9). Geometric mean (95% CI) CSF pNF-H concentrations were 20,139.2 (10,075.0–40,256.7) pg/mL in two-copy participants (n = 14) and 951.5 (366.5–2470.2) pg/mL in three-copy participants (n = 9). Nineteen NURTURE participants had ≥1 sibling with SMA; in total, there were 22 full siblings and two half siblings. Of the 24 siblings, eight had two SMN2 copies, eight had three SMN2 copies, and eight had an undocumented number of SMN2 copies. One of the siblings was related to two NURTURE participants (a set of twins).
Table 1.
Baseline characteristics.
| Characteristic | 2 SMN2 copies n = 15a | 3 SMN2 copies n = 10 | Total N = 25 |
|---|---|---|---|
| Age at first dose, days, n (%) | |||
| ≤14 | 6 (40) | 3 (30) | 9 (36) |
| >14 and ≤28 | 7 (47) | 5 (50) | 12 (48) |
| >28 | 2 (13) | 2 (20) | 4 (16) |
| Median (range) | 19.0 (8–41) | 23.0 (3–42) | 22.0 (3–42) |
| Mean (SD) | 19.5 (9.29) | 22.3 (12.45) | 20.6 (10.51) |
| Male, n (%) | 8 (53) | 4 (40) | 12 (48) |
| CHOP INTEND total score | n = 15 | n = 10 | n = 25 |
| Median (range) | 45.0 (25.0–60.0) | 53.5 (40.0–60.0) | 50.0 (25.0–60.0) |
| Mean (SD) | 47.0 (10.04) | 51.9 (6.10) | 49.0 (8.87) |
| HINE total motor milestones | n = 15 | n = 10 | n = 25 |
| Median (range) | 3.0 (0–5) | 3.0 (0–7) | 3.0 (0–7) |
| Mean (SD) | 2.7 (1.59) | 3.2 (1.87) | 2.9 (1.69) |
| Ulnar CMAP amplitude, mV | n = 14 | n = 10 | n = 24 |
| Median (range) | 2.30 (1.0–6.7) | 2.90 (1.8–4.9) | 2.65 (1.0–6.7) |
| Mean (SD) | 2.69 (1.516) | 3.11 (1.119) | 2.87 (1.354) |
| Peroneal CMAP amplitude, mV | n = 12 | n = 10 | n = 22 |
| Median (range) | 3.20 (1.1–9.7) | 4.00 (0.2–7.0) | 3.30 (0.2–9.7) |
| Mean (SD) | 3.52 (2.159) | 3.75 (2.188) | 3.62 (2.123) |
| Plasma pNF-H, pg/mLb | n = 13 | n = 9 | n = 22 |
| Geometric mean (95% CI) | 20880.9 (9639.4–45231.9) | 1870.7 (1152.9–3035.5) | 7782.7 (3828.6–15820.3) |
| Range | 845–52,900 | 959–7950 | 845–52,900 |
| CSF pNF-H, pg/mLb | n = 14 | n = 9 | n = 23 |
| Geometric mean (95% CI) | 20139.2 (10075.0–40256.7) | 951.5 (366.5–2470.2) | 6099.8 (2646.0–14062.0) |
| Range | 342–37,200 | 261–9140 | 261–37,200 |
CHOP INTEND, Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders; CMAP, compound muscle action potential; CSF, cerebrospinal fluid; HINE, Hammersmith Infant Neurologic Examination; pNF-H, phosphorylated neurofilament heavy chain; SD, standard deviation; SMN2, survival motor neuron 2.
Included one set of twins; each child had two SMN2 copies.
The number of decimal places reported in summary statistics is not indicative of biomarker assay precision or sensitivity.
3.2. Efficacy
3.2.1. Primary endpoint: survival and respiratory intervention
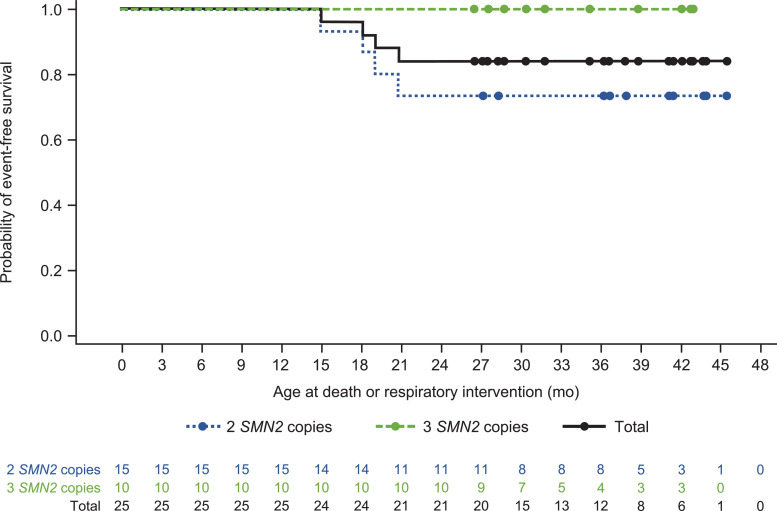
As of this interim analysis, all 25 NURTURE participants were alive and none required permanent ventilation. The median time to death or respiratory intervention (invasive or non-invasive ventilation for ≥6 h/day continuously for ≥7 days or tracheostomy) could not be estimated, as there were too few events. Four (16%) infants (all with two SMN2 copies) utilized respiratory intervention for ≥6 h per day continuously for ≥7 days (Fig. 2 ), all of whom initiated respiratory intervention during an acute, reversible illness. At the last study day prior to data cutoff, two of these infants no longer utilized respiratory intervention; these infants had previously received respiratory intervention for ≥6 h per day for totals of 20 and 266 days during the course of the study. The other two infants continued to receive respiratory intervention for two and 10 h per day, respectively, at the last study day prior to data cutoff; these infants received respiratory intervention for ≥6 h per day for totals of 236 and 644 days, respectively, over the course of the study. No other infants received respiratory intervention during the course of the study.
Fig. 2.
Kaplan–Meier plot for age at death or respiratory intervention.a
SMN2, survival motor neuron 2.
No participants have died or required tracheostomy or permanent ventilation (defined as ≥16 h/day continuously for >21 days in the absence of an acute reversible event or tracheostomy).
aRespiratory intervention was defined as ventilator use for ≥6 h per day for ≥7 days or tracheostomy.
3.2.2. Secondary endpoints
Achievement of motor milestones
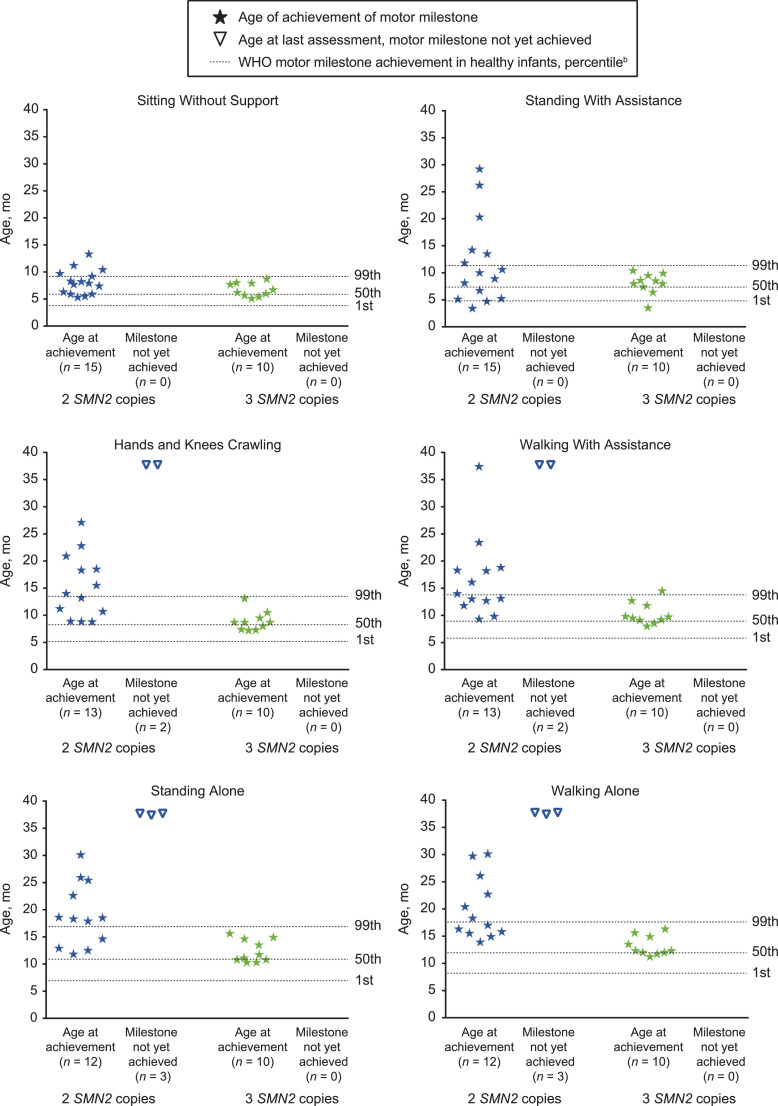
As of data cutoff, all (25/25; 100%) NURTURE infants achieved the WHO motor milestone “sitting without support”, while 23/25 (92%; 13/15 with two SMN2 copies and 10/10 with three SMN2 copies) achieved “walking with assistance”, and 22/25 (88%; 12/15 with two SMN2 copies and 10/10 with three SMN2 copies) achieved “walking alone”. Site- or caregiver-reported ages of first achievement for WHO motor milestones are shown in Fig. 3 . Most participants achieved these milestones within the window established by the WHO for healthy children; specifically, 21/25 [84%] achieved sitting without support, 15/23 [65%] achieved walking with assistance, and 16/22 [73%] achieved independent walking by the WHO 99th percentile age of achievement [28], [35]. The median (95% CI) ages for first achievement of sitting without support, walking with assistance, and walking alone in two SMN2 copy participants were 7.9 (5.9–9.2) months, 16.1 (11.8–18.8) months, and 20.4 (15.5, 29.7) months, respectively. In those with three SMN2 copies, the median (95% CI) ages for first achievement of sitting without support, walking with assistance, and walking alone were 6.4 (5.1–7.9) months, 9.6 (8.0–11.8) months, and 12.3 (11.2–14.9) months, respectively.
Fig. 3.
Site- or caregiver-reporteda age at first WHO motor milestone achievement.
SMN2, survival motor neuron 2; WHO, World Health Organization.
aIf caregiver-reported, achievement was confirmed by the study site at the next study visit with a yes or no response.
bWHO motor milestone windows of achievement were determined based on the WHO Multicenter Growth Reference Study windows of achievement in healthy children [28].
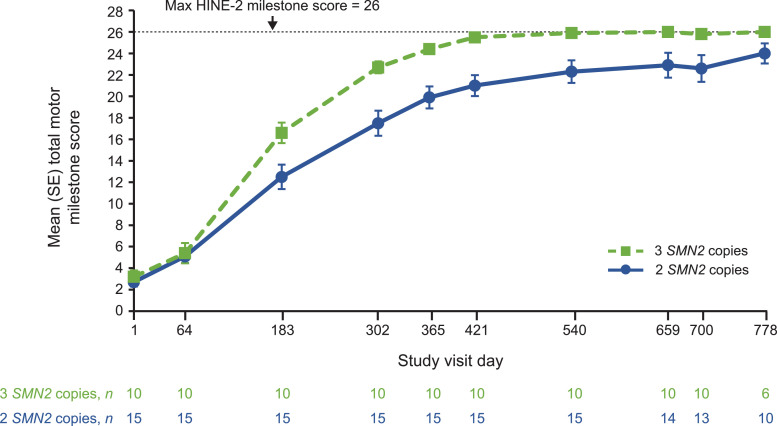
HINE-2 motor milestone total scores also increased over time for all participants regardless of SMN2 copy number. Mean (range) total scores increased from a baseline of 2.7 (0–5) to 23.9 (16–26) at the last observed visit, up to and including Day 778, for participants with two SMN2 copies and from 3.2 (0–7) to 26.0 (26–26) for those with three SMN2 copies. Mean scores for infants with two or three SMN2 copies approached the scale maximum of 26 points; three-copy participants approached the maximum earlier than participants with two SMN2 copies (Fig. 4 ).
Fig. 4.
Mean HINE-2 motor milestone scores over time.
HINE-2, Hammersmith Infant Neurologic Examination, Section 2; SE, standard error; SMN2, survival motor neuron 2.
Time points with n ≥ 5 included. HINE-2 score was assessed in NURTURE participants up until the Day 778 study visit.
Motor function
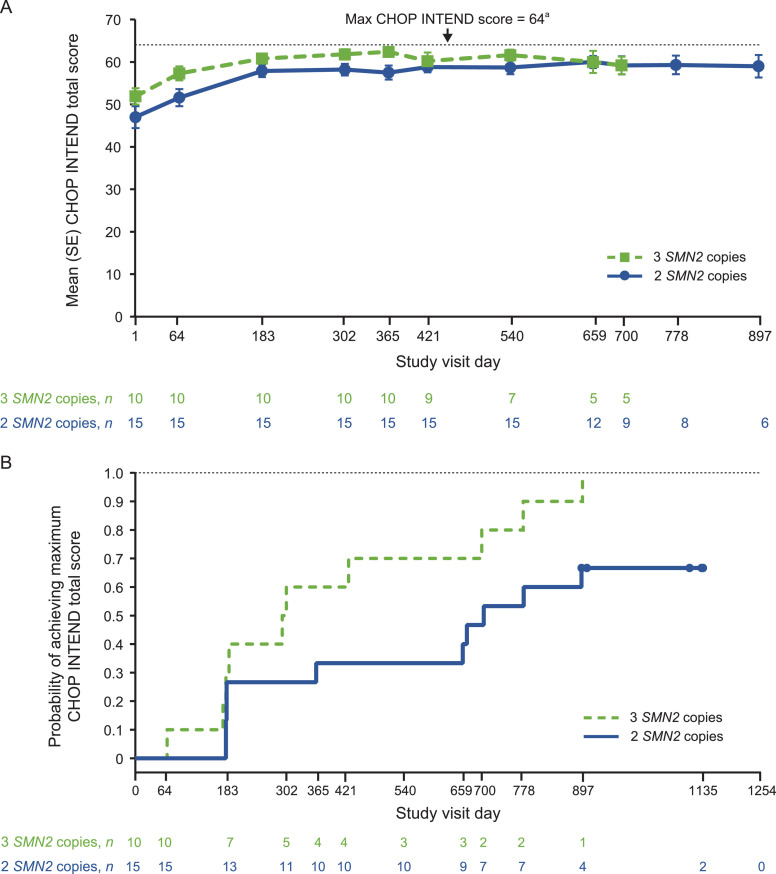
Mean CHOP INTEND total scores rose steadily from baseline until approximately Day 183 and then remained stable over time (Fig. 5 (A)). At the last visit, mean (range) CHOP INTEND total score was 62.1 (48–64) in those with two SMN2 copies and 63.4 (58–64) in those with three SMN2 copies. At the time of this interim analysis, 10/15 (67%) of participants with 2 SMN2 copies and 10/10 (100%) of those with 3 SMN2 copies had achieved a maximum score of 64 (Fig. 5(B)). Beyond Day 183, CHOP INTEND total scores appear to be constrained by a plateau ceiling effect of ∼60 points and was no longer assessed after participants had achieved the maximum score of 64; however, continued improvement was observed in HINE-2 and WHO milestones outcomes.
Fig. 5.
(A) Mean CHOP INTEND score over time, (B) Kaplan–Meier plot for time to first achievement of maximum CHOP INTEND score of 64 points.a
CHOP INTEND, Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders; SE, standard error; SMN2, survival motor neuron 2.
For Fig. 5(A), time points with n ≥ 5 included. aIn the original protocol, CHOP INTEND was to be assessed in participants at each visit up to Day 778; however, this was amended to be until they had a maximum score of 64. Once a score of 64 was achieved, CHOP INTEND was no longer assessed.
At the last observed visit up to and including Day 778, all NURTURE participants had the ability to suck and swallow as measured by the HINE-1 neurological assessment. Twenty-two participants (12/15 with two SMN2 copies, 10/10 with three SMN2 copies) achieved the maximum score of 3 (good sucking and swallowing) on the HINE-1. Three infants (all with two SMN2 copies) achieved a score of 1 (poor sucking and/or swallowing); each of these three had gastrostomy tubes placed.
Clinically manifested SMA
The proportions of two SMN2 copy participants who had protocol-defined symptoms of SMA were 0.67 (95% CI 0.39–0.87; n = 10 of 15 total) at age 13 months and 0.47 (95% CI 0.22–0.73; n = 7 of 15 total) at age 24 months. The proportions of three SMN2 copy participants who had protocol-defined symptoms of SMA were 0.20 (95% CI 0.04–0.56; n = 2 of 10 total) at age 13 months and 0.00 (95% CI 0.00–0.34; n = 0 of 10 total) at age 24 months. The seven infants who developed protocol-defined symptoms of SMA by 24 months (all with two SMN2 copies) were all continuing to grow and achieve WHO motor milestones inconsistent with Type I SMA and with the milestone attainment of their siblings with SMA. All seven were sitting without support, five were walking with or without assistance, and four were walking alone. Six of these seven participants had siblings with SMA; none of the siblings achieved sitting independently and 5/6 required tracheostomy and/or died by 16 months of age.
The seven infants with clinically manifested SMA at age 24 months included the three participants with a poor suck/swallow reflex who had gastrostomy tubes placed. While these three infants undoubtedly exceeded their expected two SMN2 copy Type I predicted phenotype by achieving the ability to sit independently, they achieved motor milestones at a slower pace than other participants with two SMN2 copies in the study. All three participants were able to stand with assistance, two were able to walk with assistance, and one achieved walking alone; two were infants who received respiratory intervention at last visit.
3.2.3. Exploratory endpoints
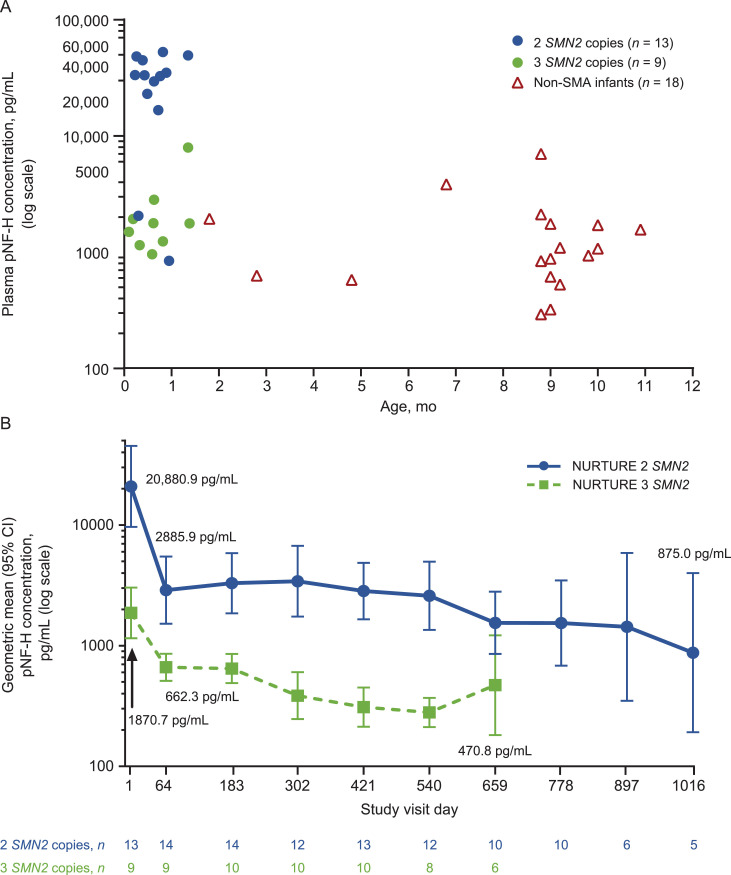
Change in pNF-H over time
Baseline pNF-H levels were higher in most presymptomatic infants with SMA compared with non-SMA infants and were significantly higher in most participants with two SMN2 copies compared with those with three SMN2 copies (plasma P = 0.0050; CSF P = 0.0020). The geometric mean plasma pNF-H level in healthy infants <1 year of age was 1090.8 pg/mL (median 1007.4 [range 293–7033] pg/mL; n = 18; Fig. 6 (A)). In presymptomatic infants with SMA, geometric mean plasma pNF-H levels declined rapidly during the loading phase of nusinersen treatment and then stabilized (Fig. 6(B)). A similar pattern of decline followed by stabilization was observed for geometric mean CSF pNF-H levels (Supplementary Fig. 1).
Fig. 6.
(A) Plasma pNF-H levels at baseline in NURTURE infants and infants <1 year of age without SMA, (B) plasma pNF-H levels in NURTURE infants by study visit.a
CI, confidence interval; ELLA, enzyme-linked lectin assay; pNF-H, phosphorylated neurofilament heavy chain; SMA, spinal muscular atrophy; SMN2, survival motor neuron 2.
pNF-H levels were evaluated using a pNF-H ELLA from ProteinSimple. 7.46 pg/mL was used as the imputed value if the pNF-H concentration was below the limit of quantification. Baseline pNF-H values in NURTURE infants were obtained on Study Visit Day 1, either prior to nusinersen administration or four h post-dose. Samples from infants without SMA were provided by Boston Children's Hospital.
aTime points with n ≥ 5 included. The number of decimal places reported in summary statistics is not indicative of biomarker assay precision or sensitivity.
In Panel A, some data points have an x-value offset of +/−0.2 months for better visualization.
CMAP
Mean (standard deviation [SD]) ulnar nerve CMAP amplitudes at baseline were 2.69 (1.52) mV and 3.11 (1.12) mV in participants with two and three SMN2 copies, respectively (P = 0.2895), and remained stable over time (Supplementary Fig. 2(A)). Similar results were observed for peroneal nerve to tibialis anterior CMAP amplitude (baseline peroneal CMAP amplitude in two versus three SMN2 copy participants, P = 0.4755; Supplementary Fig. 2(B))
3.3. Predictors of future motor function achievements
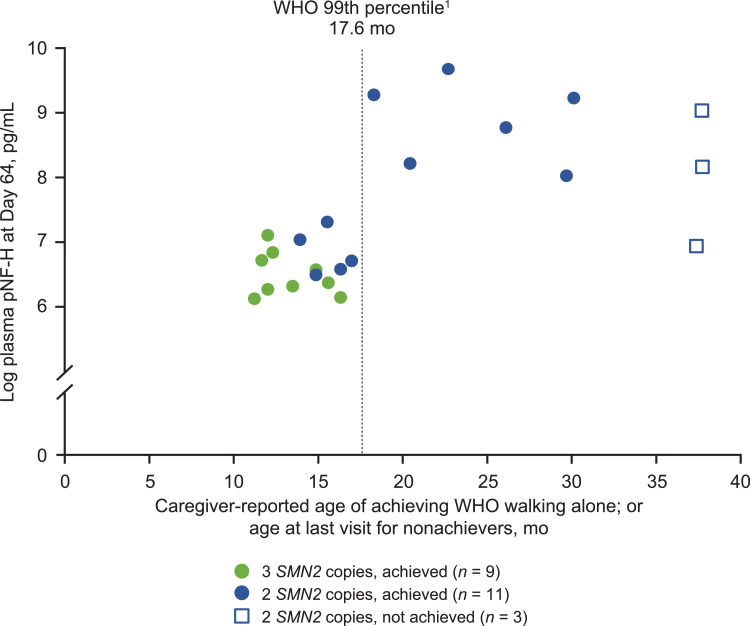
Correlations were calculated to identify the earliest, strongest predictors of motor function. Among the baseline characteristics analyzed, baseline plasma pNF-H level was the strongest predictor of HINE-2 total motor milestone score at Day 302 (rs=−0.53; P = 0.0120; n = 22) and age of achievement of WHO walking alone (rs=0.55; P = 0.0147; n = 19). In the analysis of participant characteristics at Day 64, plasma pNF-H levels at Day 64 were the best predictor of future motor function achievement in the overall population. Plasma pNF-H levels at Day 64 were significantly correlated with the total HINE-2 motor milestone score at Day 302 (rs = −0.67; P = 0.0005; n = 23) and with achievement of the WHO motor milestone walking alone (rs = 0.64; P = 0.0025; n = 20). Lower plasma pNF-H levels at day 64 were associated with earlier achievement of walking alone (Fig. 7 ). In participants with 2 SMN2 copies, Day 64 weight for age (rs = 0.72; P = 0.0027; n = 15) and Day 64 CMAP amplitude (rs = 0.66; P = 0.0098; n = 14) were correlated with total HINE-2 motor milestone achievement at Day 302, and similar results were observed for age at achievement of WHO walking alone. The associations between Day 64 weight for age or Day 64 CMAP amplitude and achievement of WHO walking alone are shown in Supplementary Figs. 3(A) and 3(B).
Fig. 7.
Plasma pNF-H levels at Day 64 and achievement of WHO motor milestone of walking alone
pNF-H, phosphorylated neurofilament heavy chain; SMN2, survival motor neuron 2; WHO, World Health Organization.
1WHO 99th percentile for age of achievement for development in healthy children [28].
Plasma pNF-H levels at Day 302 were evaluated in relationship to those from individuals without SMA and to motor milestone achievement and other outcomes. Plasma samples were analyzed from 18 non-SMA individuals who ranged in age from 0.15 to 0.91 years. These individuals had plasma pNF-H levels ranging from 293 to 7033 pg/mL (Q1, 616 pg/mL; Q3, 1753 pg/mL). NURTURE Day 302 was selected for this analysis based on the age range of non-SMA individuals. Twenty-two NURTURE participants had a recorded measurement for Day 302 plasma pNF-H level, 13 of whom (3 with 2 SMN2 copies; 10 with 3 SMN2 copies) had a result below Q3 (≤1753 pg/mL) for non-SMA individuals <1 year of age. Among these 22 NURTURE participants, all children who initiated ventilation support (n = 4), met the protocol definition of clinically manifested SMA at Day 700 (n = 6), and/or had delayed (or no) achievement of sitting or walking alone (per WHO 99th percentile window) had a Day 302 plasma pNF-H level above the Q3 (>1753 pg/mL) for non-SMA individuals <1 year of age.
3.4. Safety
AEs were reported in 25/25 (100%) NURTURE participants (Table 2 ). Twenty of 25 (80%) participants had an AE that was mild or moderate in severity. There were no AEs considered to be definitely related to study drug by the investigators. All AEs considered by the investigator to be possibly related to study drug (8/25 infants; 32%) resolved despite continued treatment, with the exception of one case each of proteinuria and clonus, which were ongoing at the time of data cutoff. A total of 33 SAEs were reported in 12/25 (48%) participants. Treatment-emergent SAEs in those 12 participants included tendon disorder and dehydration (n = 1); bronchitis, choking, pneumonia (n = 1); pneumonia (n = 1); mycoplasmal pneumonia (n = 1); viral upper respiratory tract infection (n = 1); abdominal distension, respiratory distress, dehydration, enterovirus infection, corona virus infection, respiratory syncytial virus bronchiolitis, bacterial pneumonia, acute respiratory failure, respiratory failure, tachycardia, viral gastroenteritis, pneumonia (n = 1); respiratory distress, respiratory syncytial virus bronchiolitis, aspiration pneumonia, pneumonia (n = 1); failure to thrive (n = 1); urinary tract infection (n = 1); pyrexia, pneumonia, pneumococcal pneumonia, pseudomonal pneumonia, upper respiratory tract infection (n = 1); respiratory syncytial virus infection (n = 1); upper respiratory tract infection (n = 1). There were no SAEs related to study drug.
Table 2.
Summary of safety.
| AE, n (%) | 2 SMN2 copies n = 15 | 3 SMN2 copies n = 10 | Total N = 25 |
|---|---|---|---|
| Any AE | 15 (100) | 10 (100) | 25 (100) |
| Serious AE | 9 (60) | 3 (30) | 12 (48) |
| Severe AE | 5 (33) | 0 | 5 (20) |
| AE related to study druga | 0 | 0 | 0 |
| AE possibly related to study druga | 4 (27) | 4 (40) | 8 (32) |
| Proteinuria | 1 (7) | 1 (10) | 2 (8) |
| Increased alkaline phosphatase and calcium | 1 (7) | 0 | 1 (4) |
| Muscular weakness, weight-bearing difficulty, extensor plantar response, clonus | 1 (7) | 0 | 1 (4) |
| Hyperreflexia and tachycardia | 0 | 1 (10) | 1 (4) |
| Pyrexia, increased ALT, increased AST, increased eosinophil, lymphocyte, and WBC counts | 1 (7) | 0 | 1 (4) |
| Increased platelet count | 0 | 1 (10) | 1 (4) |
| Rash | 0 | 1 (10) | 1 (4) |
| Serious AE related to study druga | 0 | 0 | 0 |
| AE possibly related to lumbar puncture procedureb | 6 (40) | 2 (20) | 8 (32) |
| AE leading to treatment discontinuation or study withdrawal | 0 | 0 | 0 |
| Incidence of AEs by MedDRA PT occurring in ≥5 participants | |||
| Pyrexia | 14 (93) | 7 (70) | 21 (84) |
| Upper respiratory tract infection | 12 (80) | 7 (70) | 19 (76) |
| Cough | 8 (53) | 5 (50) | 13 (52) |
| Nasopharyngitis | 9 (60) | 4 (40) | 13 (52) |
| Vomiting | 7 (47) | 3 (30) | 10 (40) |
| Fall | 4 (27) | 5 (50) | 9 (36) |
| Rhinorrhea | 5 (33) | 4 (40) | 9 (36) |
| Nasal congestion | 5 (33) | 3 (30) | 8 (32) |
| Otitis media | 4 (27) | 4 (40) | 8 (32) |
| Diarrhea | 2 (13) | 5 (50) | 7 (28) |
| Influenza | 4 (27) | 3 (30) | 7 (28) |
| Seasonal allergy | 3 (20) | 4 (40) | 7 (28) |
| Tremor | 7 (47) | 0 | 7 (28) |
| Anemia | 3 (20) | 3 (30) | 6 (24) |
| Constipation | 5 (33) | 1 (10) | 6 (24) |
| Dehydration | 5 (33) | 1 (10) | 6 (24) |
| Pneumonia | 6 (40) | 0 | 6 (24) |
| Diaper dermatitis | 3 (20) | 2 (20) | 5 (20) |
| Viral gastroenteritis | 3 (20) | 2 (20) | 5 (20) |
| Muscular weakness | 5 (33) | 0 | 5 (20) |
| Tachycardia | 4 (27) | 1 (10) | 5 (20) |
AE, adverse event; ALT, alanine aminotransferase, AST, aspartate aminotransferase; MedDRA PT, Medical Dictionary for Regulatory Activities Preferred Term; SMN2, survival motor neuron 2.
Values refer to numbers and percentages of study participants.
Assessed by the investigator.
Assessed by the investigator; includes events that are possibly related or related to the LP procedure.
The lumbar puncture procedure was generally well tolerated. Eight participants had an event determined by investigators to be possibly related or definitely related to the lumbar puncture procedure: traumatic lumbar puncture (n = 1); vomiting (n = 1); subdural hematoma, post-procedure discomfort (n = 1); headache (n = 1); post-lumbar puncture procedure syndrome, lower lumbar spinal canal hematoma, hypertension (n = 1); post-procedural swelling (n = 1); extradural hematoma, tachycardia, weight-bearing difficulty, muscular weakness (n = 1); and epidural hemorrhage, spinal subarachnoid hemorrhage, tachycardia, hyperreflexia (n = 1). Five hemorrhages near the thecal space in four participants occurred in the setting of multiple lumbar puncture attempts; all events occurred when participants were <6 weeks old, and none were in the context of thrombocytopenia. The only lumbar puncture-related event classified as an SAE was one case of post-lumbar puncture syndrome that occurred before the first dose of study drug following a failed dosing attempt.
No clinically relevant trends with respect to thrombocytopenia, coagulation abnormalities, abnormal liver and kidney function tests, or proteinuria were observed in the NURTURE cohort. There were no observed cases of heart disease, liver failure, bacterial meningitis, aseptic meningitis, hypersensitivity, or hydrocephalus. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase, creatinine, cystatin C, and platelet levels remained stable over time (Supplementary Figs. 4–8).
4. Discussion
To our knowledge, NURTURE is the first study to investigate a treatment targeting the underlying cause of the SMA disease in a presymptomatic period and one of the first two studies to investigate any treatment for presymptomatic SMA [36]. The results of this interim analysis, representing a median 2.9 years of follow up, demonstrate substantial clinical benefit in infants with two or three copies of the SMN2 gene (considered most likely to develop Type I or II SMA) as a result of early initiation of nusinersen treatment. NURTURE infants, who at the time of this interim analysis were age ≥25 months and past the age of symptom onset [3,27], were all alive without requirement for permanent ventilation. The contrast to the natural history of untreated SMA is dramatic: those with SMA Type I (the expected phenotype for approximately half our cohort based on SMN2 copy number) die or require permanent ventilation, on average, by 13.5 months of age [27]. That early treatment is key to this rescue arises from comparison of the NURTURE experience with that of the ENDEAR study, in which treatment with nusinersen was initiated in a symptomatic period. By the end of the ENDEAR study, 31/80 (39%) nusinersen-treated infants with infantile-onset SMA died or required permanent ventilation [8]. All NURTURE participants are alive without permanent ventilation and able to sit independently; nearly all children (23/25) can walk with assistance or independently, and all continued to gain motor skills throughout the study interval. In both groups of children with two and three SMN2 copies, nusinersen showed rapid onset of improvement and durability of effect on mean CHOP INTEND motor function scores. These results are clearly incongruent with the natural history of individuals with two or three copies of SMN2 [6]. These results demonstrate that treatment with nusinersen of genetically diagnosed infants with SMA in the presymptomatic period allows for gains in motor function closer to normal development than that expected in individuals with SMA Type I or II.
The motor milestone and motor function achievements of many NURTURE infants are also drastically discordant from their untreated siblings with the same SMN2 copy number. This discordance is notable, given that a previous study found that 86.8% of 265 sibling pairs with SMA had a concordant phenotype [26], further emphasizing the clinically meaningful benefit of nusinersen treatment in the presymptomatic period. CHOP INTEND scores for NURTURE infants were similar to those observed for healthy infants over the first three study months, the interval for which natural history data on healthy infants are available [37]. NURTURE infants’ CHOP INTEND scores greatly exceed those observed in a natural history cohort of symptomatic infants with SMA with two copies of SMN2 over the course of the study, who had a mean (SD) decline of 10.71 (9.43) points over 6–12 months [37]. Similarly, the mean ulnar CMAP amplitude of NURTURE infants remained stable over time in participants with two and three SMN2 copies in NURTURE, while amplitude fell rapidly and was never higher than 0.6 mV in a natural history cohort of infants with SMA aged ≥6 months with two SMN2 copies [37].
Overall, the NURTURE infants who initiated treatment before the appearance of clinical symptoms had earlier and substantially greater improvement in HINE-2 total scores than did infants and children who initiated treatment after symptom onset in the ENDEAR and CS3a trials [8,25]. NURTURE infants also had higher baseline CHOP INTEND scores and were able to attain maximum or near-maximum CHOP INTEND scores more quickly than did infants who initiated treatment after symptom onset [8,25]. While the differences in population ages and study designs between studies must be kept in mind when interpreting these data, the differences in HINE-2 score trajectories and CHOP INTEND maximum scores indicate that treatment with nusinersen can have a meaningful impact on clinical outcome beyond that seen when treatment is initiated after symptom onset.
The functional outcomes of NURTURE participants with three SMN2 copies were generally better than those observed in participants with two copies of SMN2. Specifically, all participants with three SMN2 copies have achieved all of the more advanced WHO motor milestones, including walking independently; whereas three participants with two SMN2 copies have yet to achieve these milestones. Participants with three SMN2 copies also achieved higher mean CHOP INTEND and HINE-2 total scores by last observed visit than participants with two SMN2 copies. Baseline CHOP INTEND and HINE-2 scores were also higher in participants with three SMN2 copies versus two SMN2 copies. While slight differences in the outcomes of patients with three SMN2 copies and two SMN2 copies were observed, all far exceed outcomes predicted by SMA Type I and II natural history.
These findings highlight the substantial benefit of early therapy, and thus point to the value of early diagnosis. Population-based newborn screening (NBS) is a method for identifying presymptomatic individuals with treatable diseases that aims to provide the opportunity for early intervention [16]. In the United States, NBS for homozygous deletion of exon 7 in the SMN1 gene was added to the Recommended Uniform Screening panel (RUSP) on July 16th, 2018. Other NBS programs are in development globally [17], [18], [19], [20]. Presymptomatic individuals with SMA are now being identified in several US states through NBS programs, and many additional states will begin screening later in 2019. Additional NBS efforts for SMA are ongoing globally and are supported by the general population in several countries [17], [18], [19],38,39]. A treatment algorithm based on SMN2 copy number for SMA positive infants identified though NBS was recently developed by a group of SMA experts [40]. The recommendation was for infants with two or three SMN2 copies to receive immediate treatment following confirmatory testing. An additional recommendation by other SMA experts suggests immediate treatment for presymptomatic infants with up to four SMN2 copies [41]. NURTURE results support immediate treatment of infants with two or three SMN2 copies and support the notion that earlier treatment across the spectrum improves eventual outcome as was demonstrated by the ENDEAR and CHERISH studies [8,9].
pNF-H levels recently have been shown to be a potential biomarker of disease severity and response to treatment in individuals with SMA. Concentrations of pNF-H in both the ENDEAR and CHERISH studies were found to correlate with several baseline clinical characteristics indicative of disease severity and decline rapidly after treatment with nusinersen before stabilizing at lower plateau levels [13]. To our knowledge, NURTURE is the first study to evaluate pNF-H levels in a presymptomatic SMA population. Geometric mean plasma and CSF levels of pNF-H were substantially higher in NURTURE participants than in infants without SMA and, within NURTURE participants, plasma and CSF pNF-H levels were significantly higher in participants with two versus three SMN2 copies. After nusinersen treatment initiation, pNF-H levels declined rapidly during the loading period before apparently stabilizing at lower levels. The rapid decline of pNF-H levels suggests the value of pNF-H as a potential biomarker of treatment response to SMN-enhancing therapies. Levels of pNF-H in plasma at the end of the nusinersen loading dose period may predict future motor function such as walking alone by the WHO 99th percentile for expected age of achievement.
The very high pNF-H levels measured before onset of clinical symptoms suggest that pathophysiologic processes precede the onset of clinical symptoms; whether release of pNF-H into interstitial fluids marks a treatment-reversible pathology, or instead an early degeneration that is not yet of sufficient magnitude to be clinically apparent, is yet unknown. In either case, pre-treatment elevation of pNF-H levels in these presymptomatic infants further emphasizes the value of NBS initiatives worldwide and stress the importance of proactive intervention in the presymptomatic phase of SMA, consistent with other chronic progressive neurological diseases [42], [43], [44], [45].
Nusinersen demonstrated a favorable benefit-risk profile consistent with data from previous studies [8,9] and no new safety concerns were identified. The lumbar puncture procedure was generally well tolerated. A small number of hemorrhages near the thecal space occurred in the setting of multiple LP attempts in infants who were <6 weeks old; none were in the context of thrombocytopenia. Careful monitoring of hematology, blood chemistry, urinalysis, coagulation, vital signs, and ECGs demonstrated no clinically relevant trends related to nusinersen treatment. Levels of creatine kinase and transaminases (ALT and AST) remained stable over time, suggesting no predisposition to muscle injury and no hepatic abnormalities.
Though the NURTURE outcomes are dramatic, key limitations of the study should be noted. NURTURE is an open-label study with a relatively small number of participants. While several participants had untreated siblings with SMA that allowed for the comparison of motor function achievement with natural history, formal assessments were not performed in the siblings with SMA, and the study has no sham-control group. It should be noted that infants had variable presentation across clinical measures at baseline (notably tendon reflexes, ulnar CMAP, and CHOP-INTEND). However, although there is no uniformly established definition of SMA symptom onset, all infants met the entry criteria and were considered presymptomatic at enrollment according to the investigator. CHOP INTEND score was assessed until a participant achieved the maximum score of 64, after which time it was no longer assessed, but a ceiling effect of the CHOP INTEND score was apparent at a score of approximately 60. HINE-2 score was assessed until Day 778 but was not assessed at later study visits. Additionally, comparisons of changes in CHOP INTEND and HINE-2 scores over time across the nusinersen clinical development program must be made cautiously; differences in study populations and study designs should be taken into consideration when interpreting the data. Caregiver-reported versus study visit documentation of achievement of motor milestones could lead to variation in the reported timing of achievements. Additionally, time of follow-up varies among participants, and first achievement of motor milestones may occur between study visits where these are assessed and documented.
5. Conclusions
Results from the NURTURE study demonstrate the potential benefits of initiating nusinersen during the presymptomatic period in infants with SMA. In this interim analysis, many infants and children treated during the presymptomatic period achieved motor milestones in timelines consistent with normal development. Data demonstrate durability of effect over a median of 2.9 years of follow up, with children continuing to make progress throughout the study with no evidence of sustained regression. These results not only exceed expectations based on the natural history of SMA and the phenotypes of participants’ siblings with SMA, but also represent treatment benefits exceeding those observed when treatment is initiated in a symptomatic period. Additionally, pNF-H data from NURTURE demonstrate that the underlying SMA disease is biologically active in the presymptomatic period and merits treatment. Results from NURTURE strongly emphasize the need for early identification of infants with SMA through NBS and support the value of treatment initiation in presymptomatic infants, genetically diagnosed with SMA with two or three copies of the SMN2 gene, immediately after SMA diagnosis. This conclusion is consistent with current recommendations [40,41].
Acknowledgments
Acknowledgment
The authors thank the patients who are participating in this study and their parents/guardians and family members, without whom this effort cannot succeed. The authors also thank the people who are contributing to this study, including the study site principal investigators, clinical monitors, study coordinators, physical therapists, and laboratory technicians. Biogen provided funding for medical writing support in the development of this manuscript; Susan Chow, PhD, and Allison Green, PhD, from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Nathaniel Hoover from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. The authors had full editorial control of the manuscript and provided their final approval of all content.
Data availability
Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nmd.2019.09.007.
Appendix. Supplementary materials
References
- 1.Darras B.T., Monani U.R., De Vivo D.C. Genetic disorders affecting the motor neuron: spinal muscular atrophy. In: Swaiman KF, editor. Swaiman's pediatric neurology: principles and practice. Elsevier; Edinburgh: 2017. pp. 1057–1064. [Google Scholar]
- 2.Mercuri E., Bertini E., Iannaccone S.T. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11:443–452. doi: 10.1016/S1474-4422(12)70061-3. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz J.A., Singh P., Darras B.T. Spinal muscular atrophy: a clinical and research update. Pediatr Neurol. 2012;46:1–12. doi: 10.1016/j.pediatrneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Farrar M.A., Kiernan M.C. The genetics of spinal muscular atrophy: progress and challenges. Neurotherapeutics. 2015;12:290–302. doi: 10.1007/s13311-014-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubo Y., Nishio H., Saito K. A new method for SMN1 and hybrid SMN gene analysis in spinal muscular atrophy using long-range PCR followed by sequencing. J Hum Genet. 2015;60:233–239. doi: 10.1038/jhg.2015.16. [DOI] [PubMed] [Google Scholar]
- 6.Finkel R., Bertini E., Muntoni F., Mercuri E. 209th ENMC International Workshop: Outcome Measures and Clinical Trial Readiness in Spinal Muscular Atrophy 7–9 November 2014, Heemskerk, the Netherlands. Neuromuscul Disord. 2015;25:593–602. doi: 10.1016/j.nmd.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Talbot K., Tizzano E.F. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017;24:529–533. doi: 10.1038/gt.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel R.S., Mercuri E., Darras B.T., Connolly A.M., Kuntz N.L., Kirschner J. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 9.Mercuri E., Darras B.T., Chiriboga C.A., Day J.W., Campbell C., Connolly A.M. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 10.Calucho M., Bernal S., Alías L., March F., Venceslá A., Rodríguez-Álvarez F.J. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. 2018;28:208–215. doi: 10.1016/j.nmd.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Feldkötter M., Schwarzer V., Wirth R., Wienker T.F., Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time LightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford T., Sumner C., Finkel R., De Vivo D.C., Oskoui M., Tizzano E. Phosphorylated neurofilament heavy chain (pNF-H) levels in infants and children with SMA: evaluation of pNF-H as a potential biomarker of sma disease activity. Neuromuscul Disord. 2018;28:S110–S111. [Google Scholar]
- 13.Darras B., Finkel R., Mercuri E., Sumner C., Oskoui M., Tizzano E. Association of phosphorylated neurofilament heavy chain (pNF-H) with nusinersen treatment of SMA: analyses from the ENDEAR and CHERISH studies. Neuromuscul Disord. 2018;28(Suppl 2):S31. [Google Scholar]
- 14.Yuan A., Rao M.V., Veeranna, Nixon R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233:183–198. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 16.El-Hattab A.W., Almannai M., Sutton V.R. Newborn screening: history, current status, and future directions. Pediatr Clin North Am. 2018;65:389–405. doi: 10.1016/j.pcl.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Taylor J.L., Lee F.K., Yazdanpanah G.K., Staropoli J.F., Liu M., Carulli J.P. Newborn blood spot screening test using multiplexed real-time PCR to simultaneously screen for spinal muscular atrophy and severe combined immunodeficiency. Clin Chem. 2015;61:412–419. doi: 10.1373/clinchem.2014.231019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien Y.H., Chiang S.C., Weng W.C., Lee N.C., Lin C.J., Hsieh W.S. Presymptomatic diagnosis of spinal muscular atrophy through newborn screening. J Pediatr. 2017;190:124–129. doi: 10.1016/j.jpeds.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 19.Kraszewski J.N., Kay D.M., Stevens C.F., Koval C., Haser B., Ortiz V. Pilot study of population-based newborn screening for spinal muscular atrophy in New York state. Genet Med. 2018;20:608–613. doi: 10.1038/gim.2017.152. [DOI] [PubMed] [Google Scholar]
- 20.Lopes JM. SMA added to list of recommended screenings for disease given to newborns in US. 2018[cited February 5, 2019]; Available from: https://smanewstoday.com/2018/07/16/sma-added-to-us-list-of-diseases-recommended-for-newborn-screening/.
- 21.Hua Y., Sahashi K., Hung G., Rigo F., Passini M.A., Bennett C.F. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010;24:1634–1644. doi: 10.1101/gad.1941310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passini M.A., Bu J., Richards A.M., Kinnecom C., Sardi S.P., Stanek L.M. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med. 2011;3:72ra18. doi: 10.1126/scitranslmed.3001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiriboga C.A., Swoboda K.J., Darras B.T., Iannaccone S.T., Montes J., De Vivo D.C. Results from a Phase 1 study of nusinersen (ISIS-SMNRx) in children with spinal muscular atrophy. Neurology. 2016;86:890–897. doi: 10.1212/WNL.0000000000002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoy S.M. Nusinersen: first global approval. Drugs. 2017;77:473–479. doi: 10.1007/s40265-017-0711-7. [DOI] [PubMed] [Google Scholar]
- 25.Finkel R.S., Chiriboga C.A., Vajsar J., Day J.W., Montes J., De Vivo D.C. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a Phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–3026. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- 26.Jones C.C., Cook S.F., Hobby K., Jarecki J. Presented at: 2016 Annual Spinal Muscular Atrophy Conference, June 16–19, 2016. Anaheim, CA; 2016. SMA subtype concordance in siblings: findings from the Cure SMA cohort. (unpublished results) [Google Scholar]
- 27.Finkel R.S., McDermott M.P., Kaufmann P., Darras B.T., Chung W.K., Sproule D.M. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Multicentre Growth Reference Study Group. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450:86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 29.Haataja L., Mercuri E., Regev R., Cowan F., Rutherford M., Dubowitz V. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135:153–161. doi: 10.1016/s0022-3476(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 30.Glanzman A.M., Mazzone E., Main M., Pelliccioni M., Wood J., Swoboda K.J. The Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20:155–161. doi: 10.1016/j.nmd.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glanzman A.M., McDermott M.P., Montes J., Martens W.B., Flickinger J., Riley S. Validation of the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) Pediatr Phys Ther. 2011;23:322–326. doi: 10.1097/PEP.0b013e3182351f04. [DOI] [PubMed] [Google Scholar]
- 32.Lewelt A., Krosschell K.J., Scott C., Sakonju A., Kissel J.T., Crawford T.O. Compound muscle action potential and motor function in children with spinal muscular atrophy. Muscle Nerve. 2010;42:703–708. doi: 10.1002/mus.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swoboda K.J., Prior T.W., Scott C.B., McNaught T.P., Wride M.C., Reyna S.P. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julious S.A. Two-sided confidence intervals for the single proportion: comparison of seven methods by Robert G. Newcombe, Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. . Stat Med 2005;24:3383-4. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Child growth standards. [cited December 16, 2018]; Available from:https://www.who.int/childgrowth/en/.
- 36.ClinicalTrials.gov. Study to evaluate sodium phenylbutyrate in pre-symptomatic infants with spinal muscular atrophy (STOPSMA). 2015[cited February 5, 2019]; NCT00528268]. Available from:https://clinicaltrials.gov/ct2/show/NCT00528268.
- 37.Kolb S.J., Coffey C.S., Yankey J.W., Krosschell K., Arnold W.D., Rutkove S.B. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82:883–891. doi: 10.1002/ana.25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boardman F.K., Sadler C., Young P.J. Newborn genetic screening for spinal muscular atrophy in the UK: the views of the general population. Mol Genet Genomic Med. 2018;6:99–108. doi: 10.1002/mgg3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin P.J., Yeh W.S., Neumann P.J. Willingness to pay for a newborn screening test for spinal muscular atrophy. Pediatr Neurol. 2017;66:69–75. doi: 10.1016/j.pediatrneurol.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Glascock J., Sampson J., Haidet-Phillips A., Connolly A., Darras B., Day J. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5:145–158. doi: 10.3233/JND-180304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darras B.T., De Vivo D.C. Precious SMA natural history data: a benchmark to measure future treatment successes. Neurology. 2018;91:337–339. doi: 10.1212/WNL.0000000000006026. [DOI] [PubMed] [Google Scholar]
- 42.Benatar M., Wuu J. Presymptomatic studies in ALS: rationale, challenges, and approach. Neurology. 2012;79:1732–1739. doi: 10.1212/WNL.0b013e31826e9b1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisen A., Kiernan M., Mitsumoto H., Swash M. Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry. 2014;85:1232–1238. doi: 10.1136/jnnp-2013-307135. [DOI] [PubMed] [Google Scholar]
- 44.Paulsen J.S., Langbehn D.R., Stout J.C., Aylward E., Ross C.A., Nance M. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com).