Abstract
To combat variola virus in bioterrorist attacks, it is desirable to develop a noninvasive vaccine. Based on the vaccinia Tiantan (VTT) strain, which was historically used to eradicate the smallpox in China, we generated a modified VTT (MVTTZCI) by removing the hemagglutinin gene and an 11,944 bp genomic region from HindIII fragment C2L to F3L. MVTTZCI was characterized for its host cell range in vitro and preclinical safety and efficacy profiles in mice. Despite replication-competency in some cell lines, unlike VTT, MVTTZCI did not cause death after intracranial injection or body weight loss after intranasal inoculation. MVTTZCI did not replicate in mouse brain and was safe in immunodeficient mice. MVTTZCI induced neutralizing antibodies via the intranasal route of immunization. One time intranasal immunization protected animals from the challenge of the pathogenic vaccinia WR strain. This study established proof-of-concept that the attenuated replicating MVTTZCI may serve as a safe noninvasive smallpox vaccine candidate.
Keywords: Smallpox, Vaccinia, Mucosal vaccine, Tiantan, MVTTZCI
1. Introduction
Smallpox is a serious and contagious human infectious disease with a mortality rate of up to 50% [1], [2]. The causative agent of smallpox is variola virus, a member of the orthopoxvirus genus. In the aftermath of September 11, 2001 and the anthrax laced letters appearing in the United States, there is heightened concern that variola virus might be used as an agent of bioterrorism [3], [4]. Since there is no specific treatment for smallpox, the effective prevention strategy will still rely on vaccination. The currently stockpiled vaccines however have issues related to their adverse events including postvaccinal encephalitis (PVE) or encephalomyelitis (PVEM) [5], [6]. The invasive immunization procedure also adds additional difficulty for mass vaccination especially in developing countries. It therefore remains critical to determine whether or not a safe, effective and replicating vaccinia strain can be generated as a noninvasive smallpox vaccine [7], [8].
During the smallpox eradication campaign, the most extensively used smallpox vaccine in China was the vaccinia virus Tiantan (VTT) strain [9], [10], [11], [12]. VTT is also a member of the orthopoxvirus genus. Since vaccination with VTT protected against variola virus, it was used for hundreds of millions of Chinese people to prevent smallpox infection between 1920 and 1980 (http://www.who.int/emc/diseases/smallpox/Smallpoxeradication.html). This led to the eradication of variola in China before 1980. Logically, VTT remains the first choice to be stockpiled for the country. However, the clinical safety of this vaccine has neither been carefully studied nor clearly documented. It was reported that VTT caused larger lesions after intradermal vaccination and was likely more virulent than other widely used smallpox vaccines such as Lister or Wyeth [13]. We recently demonstrated that VTT remains virulent in mice after intranasal inoculation, which restricts its use as a noninvasive vaccine [14]. Moreover, the virulence of VTT implies significant risks not only to children but also to many immune compromised adult recipients (e.g. HIV/AIDS, Cancer, Leukemia, Lymphoma, Multiple Myeloma, etc). We therefore aimed to modify VTT to develop a safe, mucosal deliverable noninvasive smallpox vaccine or a safe vaccine vector for other pathogens.
2. Materials and methods
2.1. Cell lines and virus
The parental VTT strain and cell lines have been described previously [14], [15]. These cell lines were grown under conditions recommended by the American Type Culture Collection (ATCC, Rockville, MD, USA). The pathogenic vaccinia WR strain was purchased from ATCC (ATCC VR-1354) and propagated in Vero cells. Viral stocks were purified through a 36% sucrose cushion centrifugation. Virus for in vivo testing was further purified through sucrose density gradient centrifugation. The viral titer was determined by a traditional plaque-forming assay using crystal violet staining in Vero cells [14].
2.2. Construction of MVTTZCI
MVTTZCI was generated in Vero cells using a homologous recombination method [16]. Vero cells were infected with VTT and subsequently transfected with a shuttle vector ZCI containing a reporter green fluorescent protein (GFP) flanked with HA sequences. The homologous recombination also introduced an 84 bp deletion to disrupt the HA gene. The recombinant virus was obtained by picking up GFP-positive plaque. Seven rounds of clonal purification were applied to generate MVTTZCI. For comparison purpose, VTT and MVTTZCI was propagated, purified and titrated in Vero cells in parallel.
2.3. Western blot analysis
Vero cells were infected with VTT or MVTTZCI at a multiplicity of infection (MOI) of 10. Cell lysates were generated 48 h p.i. and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting were carried out with an anti-HA monoclonal antibody B2D10 (a generous gift of Dr H. Shida) and an anti-GFP polyclonal antibody (BD Biosciences, San Jose, CA, USA), respectively, as we previously described [16].
2.4. Analysis of VTT quasispecies
Vero cells were infected with each of 20 purified VTT clones at an MOI of 1. The infected cells were lysed by three freezing and thawing cycles. The cell lysates were treated with proteinase K at a final concentration of 50 ug/ml for 4 h and then cellular DNA was extracted by the conventional phenol–chloroform method. Individual VTT genes were amplified by PCR with each pair of specific primers as follows: C7LF (TTAATCCATGGACTCATAATCT) and C7LB (ATGGGTATACAGCACGAATTCG); C1LF (TCATTTCGACATTAATTCCTTT) and C1LB (ATGGTGAAAAATAATAAAATAA); N2.1LF (CAATTAGTACACCGCTATGTTT) and N2.1LB (TTAACAAAATAACATAAATATA); K1LF (TTAGTTTTTCTTTACACAATTG) and K1LB (ATGTTACAGGCTCTGTTCAAAT); K2LF (TTATTGGTGTTTGTCGACTGTC) and K2LB (ATGGATCTGTCACGAATTAATA); K4LF (TTATTGATGTCTACACATCCTT) and K4LB (ATGCTTGCATTTTGTTATTCGT); and K8RF (ATGGCGACTAAATTAGATTATG) and K8RB (CATCAATTCAATTTTTTTTCTAG). The PCR products were visualized in 1% Agarose after gel electrophoresis.
2.5. Viral replication in vitro and immunostaining of infected cells
Under multi-step growth conditions, cells were infected at a MOI of 0.05 in 100 μl of culture medium containing 3% fetal bovine serum (FBS). After 90 min of incubation at 37 °C, cells were washed three times with medium and replenished with fresh culture medium. Viral supernatant and infected cells were harvested at 0, 24, 48 and 72 h post-infection (p.i.). After freeze–thawing thrice, harvested samples were titrated in duplicate in Vero cells [16]. To determine the cell-to-cell spread of MVTTZCI, viral plaques were detected after immunostaining with a rabbit anti-VTT serum using a method previously described [15]. Briefly, target cells were grown to 90% confluence and then infected with 100 PFU MVTTZCI or VTT. After viral absorption for 90 min, cells were washed three times with culture medium and then incubated at 37 °C for additional 24, 48 or 72 h before antibody staining. Protein-A conjugated horseradish peroxidase (BOSTER, Wuhan, China) was used to detect bound rabbit antibodies. The color was developed with substrate solution containing 10 μl of 30% H2O2 and 0.2 ml of ethanol saturated dianisidine (Sigma, St. Louis, MO, USA) in 10 ml of PBS. Normal rabbit serum was used as a negative control. To determine the cytopathic effect (CPE), target cells were infected with MVTTZCI at a MOI of 5. After viral absorption for 90 min, cells were washed three times with culture medium and then incubated at 37 °C for additional 12 and 24 h for the detection of CPE [15].
2.6. The virulence of MVTTZCIin vivo
The inbred BALB/c mouse was chosen for the assessment of MVTTZCI virulence using a previously described method [17], [18]. Groups of five-week old mice were inoculated intranasally with 0, 104, 105 or 106 PFU of MVTTZCI in 20 ul of PBS. The viral virulence was subsequently determined by the daily measurement of animal body weight change for a period of 10 days [19], [20]. VTT was evaluated under the same conditions for comparison purpose. To evaluate the pathogenicity of MVTTZCI in immunodeficiency mice, groups of four SCID mice were infected intraperitoneally (i.p.) with 106 or 107 PFU of MVTTZCI, or with 103–106 PFU of VTT. Mice were weighted individually, and the averages were plotted. Mice that lose 25% of body weight were sacrificed according to the standard operating procedure of our animal facility. The number of animals that died of infection was also calculated. Uninfected mice were included as controls.
2.7. ICID50 measurement
Six groups of 3-week-old BALB/c mice (female and male half each) were inoculated intracranially with a series of diluted viruses of 102 to 106 PFU MVTTZCI in 10 μl of sterile PBS. The ICLD50 value was determined on mice that succumbed between 1 and 14 days p.i. by calculating the 50% end point using the Reed–Muench method [21].
2.8. Replication kinetics of virus in mouse brain with or without immunodeficiency
Groups of 3-week-old BALB/c mice were administrated with different doses of MVTTZCI or VTT via the intracranial route in 10 μl of PBS, respectively. Two mice in each group were sacrificed daily during the first 5 days p.i. for viral isolation. Infectious virions in the brains of inoculated mice were measured by culturing a serial diluted tissue homogenate in Vero cells. The viral titer was determined by counting plaque-forming units. Groups of 6-week-old SCID mice were injected intracranially with 106 or 104 PFU of MVTTZCI to access the replication profile of MVTTZCI in the brain of immunodeficiency mice.
2.9. Neutralization assay
To determine the serum neutralization against WR strain before challenge, a plaque reduction neutralization assay was used. Briefly, 100 PFU WR strain was mixed and incubated with serially diluted heat-inactivated mouse sera for 16 h at 37 °C. The mixture was transferred onto confluent monolayers of Vero cells in a 48-well plate and incubated for 90 min at 37 °C. On day 2, viral plaques were visualized by crystal violet staining and counted. IC50 or IC90 were determined by the highest dilution of mouse serum that generated 50% or 90% viral plaque reduction.
2.10. Immunogenicity and efficacy of MVTTZCIin vivo
To understand the immunogenicity of MVTTZCI in vivo, groups of five BALB/c mice (6-week-old) were immunized intramuscularly with 106 or 104 PFU of MVTTZCI or VTT, respectively. Control animals were given PBS. Two additional groups of mice were vaccinated intranasally with 104 or 106 PFU of MVTTZCI to understand the immunogenicity of MVTTZCI via a mucosal route. Three weeks after a single vaccination, serial 2-fold diluted heat-inactivated sera (2−1 to 2−7) specimens were subjected to the plaque reduction neutralization assay against WR strain. To determine the in vivo efficacy of MVTTZCI, we challenged the vaccinated animals 30 days after the single vaccination with a lethal challenge dose of 106 PFU WR strain via the intranasal route [22]. Animal body weight change was subsequently determined. All of our experimental protocols were approved by the committee on the use of live laboratory animals.
3. Results
3.1. Generation of the vaccinia MVTTZCI strain
To generate an attenuated viral variant of VTT, we sought to delete the HA gene because it is related to the attenuation of various vaccinia viruses [17], [23], [24], [25]. The new VTT variant named MVTTZCI was generated after a portion of the HA gene was deleted using a homologous recombination method [26], [27], [28]. To facilitate the purification of the MVTTZCI, we have introduced the gene of green florescence protein (GFP) into the deletion site. After serial rounds of plaque clonal purification in Vero cells, a homogenous MVTTZCI stock was obtained as determined by the co-expression of GFP and vaccinia specific protein in infected cells. The expression of HA was detected in cells infected with VTT, but not with MVTTZCI using a HA-specific monoclonal antibody B2D10 (a generous gift from Dr. H. Shida) (Fig. 1 ). We also performed overlapping PCR screening analysis of MVTTZCI genome. In comparison to VTT, we found that MVTTZCI contains a large 11,944 bp genomic deletion from C2L to F3L in the left terminal region that we did not intend to make (Fig. 1). This deletion was further confirmed by sequence analysis. The vaccinia gene names are based on the sequence of vaccinia Copenhagen strain.
Fig. 1.
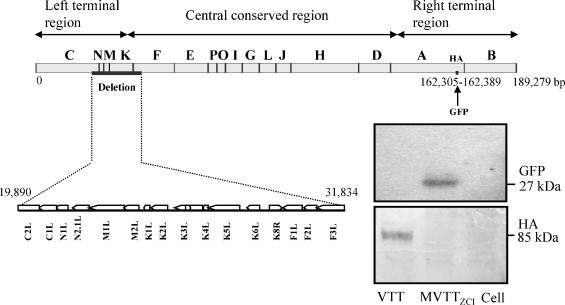
Schematic representation of the MVTTZCI genome. The 11,944 bp genomic deletion was found in the left terminal region of the viral genome. This deletion was confirmed by sequence analysis. The letters indicate the HindIII fragments. The vaccinia gene names are based on the sequence of vaccinia Copenhagen strain. The inset shows the results of Western blot analysis, which confirms the loss of the HA gene.
To understand the origin of the 11,944 bp genomic deletion, we studied 20 randomly selected viral clones purified from VTT. We hypothesized that the smallpox vaccine VTT was probably consisted of a pool of variants namely quasispecies. We used a PCR-scanning method to analyze the VTT quasispecies. As shown in Fig. 2 , we found that VTT genes are highly variable within the C2L to F3Lregion in the left terminal region. Nine out of the 20 clones contained various gene deletions. The results suggested that that MVTTZCI was likely derived from one of VTT variants containing the 11,944 bp genomic deletion. However, since none of these 20 clones contain the identical 11,944 bp deletion, we cannot exclude the possibility that our in vitro clonal selection process in Vero cells may also contributed to the emergence of MVTTZCI.
Fig. 2.
Analysis of VTT quasispecies. To understand the source of the 11,944 bp genomic deletion, 20 randomly selected clones were purified from VTT. A PCR-scanning method was used to analyze the VTT quasispecies using 7 pairs of primers. Each pair of primers specific target one VTT gene open reading frame in the left terminal flank region from C7L to K8R. If a deletion occurs, the PCR product specific for the gene will be absent. We found that nine out of the 20 clones contained various gene deletions including 1, 2, 6, 13, 14, 15, 16, 17 and 20.
3.2. Reduced host cell range of MVTTZCI
Due to the significant loss of viral genomic fragments, we sought to determine the host cell range and replication capacity of MVTTZCI in vitro. Thirteen cell types, which have been previously used to determine the host cell range of VTT [14], were tested for MVTTZCI. We found that MVTTZCI replicates in mouse NIH3T3 cells in a semi-permissive way suggesting that its replication is not completely restricted in mouse cells (Table 1 ). It however displayed a rather limited replication capacity in three cell lines including RK13, MDCK, and C6, which were derived from rabbit, canine and rat, respectively (Table 1 and Fig. 3A). MVTTZCI did not seem to replicate in RK13 and MDCK cells at all even in the single round replication experiment with a MOI of 5. There was no viral particle formation in RK13 cells infected with MVTTZCI by electron microscopy analysis (data not shown). To further confirm this observation, we determined the level of viral spread among cells using an immunohistochemical staining method [15]. As shown in Fig. 3B, no cell-to-cell spread of MVTTZCI could be found in RK13 and MDCK cells. Since only singly infected cells were found up to 72 h (h) post-infection (p.i.), the data suggested that the cell-to-cell spread was likely absent in these two cell lines. Moreover, only a low level of viral spread was found in C6 cells with small clusters of infected cells identified 48–72 h p.i. (Fig. 3B). As controls, the replication and spread of MVTTZCI is indistinguishable to those of VTT in Vero cells, suggesting that the HA gene and the genes in the C2L-F3L region are non-essential for MVTTZCI replication and propagation in Vero cells (Table 1 and Fig. 3A and B).
Table 1.
Host cell range, cell-to-cell spread, CPE and replication of vaccinia MVTTZCI in vitro.
| Cell line | ATCC code | Species | Organ | Morphology | Virala spread | CPEb |
Viral replicationc | |
|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | |||||||
| HeLa | CCL-2 | Human | Cervix | Epithelial | ++ | ++++ | ++++ | 61.36 (P) |
| MRC-5 | CCL-171 | Human | Lung | Fibroblast | +++ | ++++ | ++++ | 52.50 (P) |
| 293T | CRL-11268 | Human | Kidney | Epithelial | ++ | ++++ | ++++ | 25.45 (P) |
| WISH | CCL-25 | Human | Amnion | Epithelial | +++ | ++++ | ++++ | 66.67 (P) |
| RK13 | CCL-37 | Rabbit | Kidney | Epithelial | + | + | + | 0.0031(NP) |
| MDCK | CCL-34 | Canine | Kidney | Epithelial | + | + | + | 0.035 (NP) |
| C6 | CCL-1 07 | Rat | Brain; glial cell; glioma | Fibroblast | ++ | + | ++ | 3.83 (SP) |
| CHO-K1 | CCL-61 | Hamster, Chinese | Ovary | Epithelial | − | ++++ | ++++ | 0.0229 (NP) |
| BHK-21 | CCL-10 | Hamster, Syrian | Kidney | Fibroblast | +++ | ++ | +++ | 5.69 (SP) |
| Vero | CCL-81 | African green monkey | Kidney | Epithelial | +++ | ++++ | ++++ | 275 (P) |
| COS-7 | CRL-1657 | African green monkey | Kidney | Fibroblast | +++ | ++++ | ++++ | 89.58 (P) |
| CEF | Primary | Chick embryo | Assorted | Fibroblast | +++ | ++ | +++ | 112.5(P) |
| NIH3T3 | CRL-1658 | Mouse embryo | Embryo | Fibroblast | +++ | ++ | +++ | 6.8(SP) |
The biological properties of the parental VTT has been previously described (Fang et al. [14]).
Virus spread as visualized by immunostaining after 72 h. −, no stained cells; +, foci of 1–4 stained cells; ++, foci of 5–25 stained cells; +++, foci of >25 stained cells (Carroll and Moss, [15]).
CPE was categorized by the following criteria: −, no difference from control; +, <25% CPE; ++,25–50% CPE; +++, >50–75% CPE; ++++, >75–100% or high level cell detachment.
Virus replication (fold increase in virus titer) determined by dividing the virus yield at 72 h by the practical input titer. Cell lines were therefore categorized into permissive (P, >25-fold increase), semi-permissive (SP, 1-fold to 25-fold increase) and non-permissive (NP, <1-fold increase) cells.
Fig. 3.
Host cell range of MVTTZCIin vitro. (A) The replication kinetics of MVTTZCI was determined in the five cell lines indicated. Confluent cells were infected at a MOI of 0.05 with MVTTZCI or VTT viruses. The viral replication titer was measured on permissive Vero cells after absorption (0), 24, 48, and 72 h p.i. The experiments were repeated twice with similar results obtained. (B) Cell-to-cell spread of MVTTZCI was determined in the five cell lines tested. The indicated cells were infected with 100 PFU of MVTTZCI or VTT, fixed at 48 h p.i. and then immunostained with anti-VTT specific polyclonal antibody. The panels show representative fields at an approximately 100× magnification.
3.3. In vivo virulence of MVTTZCI
The inbred BALB/c mouse was chosen for the assessment of MVTTZCI virulence using an intranasal (i.n.) inoculation model described previously by others [17], [18]. As shown in Fig. 4A, none of the mice in either of the experimental groups died during the experimental period. However, in contrast to VTT, which caused a clear dose-dependent pattern of body weight loss in mice, those infected with MVTTZCI did not show signs of weight loss even in the 106 PFU group. These results demonstrate that MVTTZCI is attenuated with respect to its parent VTT.
Fig. 4.
Virulence of MVTTZCI in mice after intranasal and intracranial inoculations. (A) Groups of five BALB/c mice (5-week-old) were inoculated intranasally with 106, 105 and 104 PFU of MVTTZCI or VTT in 30 μl of PBS on day 0 (arrow), respectively. Mice inoculated with PBS served negative controls. The body weight changes were represented by the mean values of each group of mice p.i. overtime. The error bar indicates the standard deviation (SD) of animals from each group. (B) Six mice per dilution group (3-week-old) were inoculated intracranially with 5-fold diluted MVTTZCI or VTT, respectively. The percentage of animals surviving was determined over 30 days observation p.i. None of mice died in MVTTZCI inoculated groups. (C) Groups of SCID mice (4 mice each group) were infected i.p. with MVTTZCI (106 and 107 PFU) or VTT (103, 104, 105 and 106 PFU). Mice inoculated with PBS were included as controls. The body weights of mice were measured individually and the mean values of each group were plotted. “†” indicates the loss of mice from infectious mortality or sacrifice due to 25% of body weight loss.
To further investigate the neurovirulence of MVTTZCI, young BALB/c mice were infected via the intracranial (i.c.) route and the 50% lethal dose (ICLD50) determined using the Reed–Muench method [21]. The results of the ICLD50 are consistent with the intranasal infection model, MVTTZCI being a significantly attenuated virus (Fig. 4B). Mice neither developed signs of encephalitis nor were there any deaths during the 30 days of observation, even in the group given the highest dose (3.5 × 106 PFU/per mouse, data not shown). Since the ICLD50 of the parent VTT is 3.1 × 103 PFU, these results demonstrate that MVTTZCI is attenuated by at least 1000-fold, and is essentially non-neurovirulent.
3.4. MVTTZCI is safe in severe combined immunodeficiency disease (SCID) mice
To further determine the safety profile of MVTTZCI, we inoculated SCID mice with 106 and 107 PFU of MVTTZCI via the intraperitoneal (i.p.) route using a previously published method [29]. For controls, groups of mice received 103–106 PFU of the parental VTT or PBS. As shown in Fig. 4C, all animals that received 103–106 PFU of VTT died or were euthanized due to significant body weight loss (over 25%) according to the standard operating procedure. Fig. 4C shows that the average survival times of the VTT infected SCID mice are approximately dose-dependent with the animals eventually succumbing to as little as 103 PFU of VTT. In contrast, all of the mice that received 106 and 107 PFU of MVTTZCI survived and continued gaining their body weight during the experimental period. This 10,000-fold difference in dose represents a significant attenuated phenotype of MVTTZCI.
3.5. Deficient replication of MVTTZCI in mouse brains
To further investigate the underlying mechanism that may account for the attenuated phenotype of MVTTZCI, we analyzed the replication kinetics of MVTTZCI in the brains of mice. A clear dose-dependent response was found in mice infected with the parental VTT (Fig. 5A), viral replication appearing to peak around 4 days p.i. Interestingly, MVTTZCI did not seem to replicate in the brains of the mice even when a dose of 106 PFU was used and titers declined with time, which might suggest that the inoculated infectious MVTTZCI was somehow effectively cleared by the animals (Fig. 5B). Similar results were obtained when SCID mice were tested in an independent experiment (Fig. 5C).
Fig. 5.
Replication kinetics of MVTTZCI in mouse brain. Groups of 10 BALB/c mice (3-week-old) were inoculated intracranially with the indicated doses of VTT (A) or MVTTZCI (B), respectively. Another group of 10 SCID mice were given MVTTZCI (C). Two mice in each group were sacrificed daily during the first 5 days p.i. The titer of virus in brain homogenates was determined in Vero cells using a plaque-forming assay. The error bar indicates the standard deviation (SD) of animals from each group.
3.6. Immunogenicity of MVTTZCI
To explore the potential of using MVTTZCI as a smallpox vaccine, we evaluated the immunogenicity of the virus in terms of inducing neutralizing antibodies against the pathogenic vaccinia WR strain. As shown in Table 2 , one time i.n. immunization of MVTTZCI induced systemic neutralizing antibodies (Nabs) using the dose of 106 PFU per mouse. The lower dose of 104 PFU MVTTZCI, was not sufficient to elicit a detectable level of systemic Nabs via either route of inoculation. When compared with VTT in parallel (Table 2), a higher dose of MVTTZCI is apparently needed to achieve equivalent levels of systemic Nab response.
Table 2.
Neutralization antibody titer in murine sera.
| Vaccine | Dose (PFU) | Routea | IC50 | IC90 |
|---|---|---|---|---|
| MVTTZCI | 106 | i.m. | 23.8 | 22.3 |
| 104 | i.m. | <2 | <2 | |
| 106 | i.n. | 24.8 | 21 | |
| 104 | i.n. | <2 | <2 | |
| VTTb | 106 | i.m. | 26.1 | 22.7 |
| 104 | i.m. | 24.5 | 21.8 | |
i.m., intramuscular inoculation; i.n., intranasal inoculation.
Since VTT kills mice via i.n., the i.n. Nab titer was not determined in this group. Neutralization antibody titer was calculated by determining the highest serum dilution (1:2 serial dilution) to achieve 50% or 90% viral inhibition.
3.7. Protection of vaccinated mice against the pathogenic vaccinia WR strain challenge
To understand the in vivo efficacy of MVTTZCI, we challenged the vaccinated animals with a lethal challenge dose of 106 PFU WR strain via the i.n. route 30 days after the single intramuscular (i.m.) vaccination [22]. The body weight change was monitored for 14 days after the viral challenge (Fig. 6 ). Unvaccinated mice began to lose their body weight three days post challenge and they all died after infection or were sacrificed due to 25% of total body weight loss. On the contrary, both MVTTZCI and VTT vaccinated animals that received the dose of 106 PFU were completely protected (<3% of weight loss) (Fig. 6A). Moreover, for the lower dose groups (104 PFU), VTT vaccinated animals were completely protected while MVTTZCI also conferred significant protection (<8% of weight loss). None of these MVTTZCI vaccinated mice died during the experimental period (Fig. 6B).
Fig. 6.
Protection of mice against pathogenic vaccinia WR strain challenge. Groups of five BALB/c mice (5-week-old) were immunized once with 106 PFU (A) or 104 PFU (B) of MVTTZCI or VTT strain via indicated routes, respectively. Mice received PBS were included as controls. Thirty days post-immunization, mice were challenged intranasally with a lethal dose (106 PFU, equivalent to over 100 LD50) of WR strain. Mice that lose 25% of body weight were sacrificed according to the standard operating procedure. The body weight changes were represented by the mean values of each group of mice p.i. overtime. The error bar indicates the standard deviation (SD) of animals from each group.
Since VTT is lethal via the i.n. vaccination, it was impossible to use it as a noninvasive mucosal vaccine. This option, however, is possible for MVTTZCI. Two groups of mice were vaccinated once through the i.n. route with 104 or 106 PFU of MVTTZCI. Thirty days after the single vaccination, animals were challenged with 106 PFU of the pathogenic WR strain via the same route. Fig. 6A and B show that MVTTZCI is equally effective in protection of mice against challenge with the WR strain following vaccination via the i.n. route when compared with the i.m. vaccination. Again, vaccinated animals that received 106 PFU i.n. were completely protected (<3% of weight loss), while mice that received the low dose of 104 PFU were also significantly protected (<8% of weight loss). None of these MVTTZCI vaccinated mice died during the experimental period.
4. Discussion
MVTTZCI is an attenuated variant of VTT. Moderate to severe adverse reactions are associated with smallpox vaccines currently stockpiled in several nations [6], [30]. Recent studies, therefore, suggested that none or low virulent vaccinia strains should be selected for vaccination against smallpox [31]. In China, VTT is the licensed smallpox vaccine. Despite its application in smallpox eradication, whether or not VTT should be stockpiled to respond to a possible bioterrorist attack has attracted some debate. We previously demonstrated that VTT is less virulent when compared with the pathogenic WR strain [14]. VTT however remains neurovirulent and not feasible for noninvasive mucosal vaccination. Here, we report the generation of MVTTZCI, a variant of VTT. This variant remains replication-competent in several mammalian cells (Table 1) but does not cause body weight loss after intranasal inoculation or death after intracranial injection in mice (Fig. 4A and B). The significantly attenuated phenotype of MVTTZCI is also evident in immunodeficient SCID mice after a high dose of viral infection (Fig. 4C). Furthermore, we showed that the non-neurovirulent nature of MVTTZCI is likely due to the loss of the viral replication capacity in the brains of two mouse species (Fig. 5B and C). Therefore, the much improved safety profile of MVTTZCI has made it an attractive noninvasive smallpox vaccine candidate or a safe vaccine vector for other pathogens.
The attenuated phenotype of MVTTZCI is not solely determined by the HA gene. It have been previously demonstrated that the vaccinia genomic deletions may result in the generation of highly attenuated viral variants. For example, MVA contains six major genomic deletions which rendered the virus replication incompetent in mammalian cells [32]. Similar situation applies to vaccinia strain NYVAC [25]. MVTTZCI is different from MVA and NYVAC in that it remains replication-competent in some mammalian cells tested (Table 1). HA is found on the plasma membrane of infected cells and the envelope of extracellular virus (EEV) but is absent from intracellular mature virus (IMV), the most abundant infectious forms of infectious virions [33], [34]. Several studies showed that the loss of HA is associated with the reduced virulence of vaccinia viruses [17], [23], [35]. For example, MVA has the major deletion III in the HA promoter region [36]. The inactivation of HA gene was able to attenuate the neurovirulence of vaccinia WR strain by 104-fold as determined by the ICLD50 value but did not render the virus replication incompetent in mouse brains [23]. To our surprise, the removal of HA gene of VTT has resulted in the MVTTZCI with diminished neurovirulence (Fig. 4, Fig. 5). We speculated that other factors could have played a role. For this reason, we performed an overlapping PCR-scanning analysis of MVTTZCI genomic DNA to search for potential mutations especially corresponding to unstable deletion regions identified in MVA. We found that MVTTZCI contains a major 11,944 bp genomic deletion from HindIII fragment C2L to F3L in the left terminal region of viral genome that we did not intend to introduce. This deletion is located near the DelII site of MVA [32]. Besides HA, several genes in the deletion region could have contributed to the attenuated phenotype of MVTTZCI. First, it is known that N1L enhances virulence and replication of vaccinia virus in vivo [37]. Second, K1L is recognized as a host range gene (hr) that is essential to support the replication of vaccinia virus in RK13 cells [27], [38], [39], which are consistent to our finding that MVTTZCI becomes replication incompetent in RK13 cells (Fig. 3). Third, M2L is probably involved in the regulation of host NF-kB responses in virus infection which may potentially influence inflammation and the severity of vaccinia infection [40]. Fourth, K3L gene is an immunomodulatory and anti-apoptotic gene that inhibits IFN intracellular signaling pathway by preventing RNA-dependent protein kinase (PKR) activation [41], [42], [43]. Thus, deletion of K3L in vaccinia virus may render the virus sensitive to the antiviral effects of IFN and probably limit disease progression in vivo. Lastly, F1L is a mitochondrial-localized protein that functions to protect cells from apoptosis and inhibits cytochrome c release, which may affect viral propagation in vivo [44]. Therefore, the attenuated phenotype of MVTTZCI is likely due to the loss of HA as well as of several critical genes in the 11,944 bp deletion region. We found that the parental VTT consists of many viral variants (Fig. 2). We cannot exclude the possibility that other genes may also contribute to the attenuated phenotype of MVTTZCI. Future study will require the full genomic analysis of MVTTZCI.
MVTTZCI is an attractive noninvasive smallpox vaccine candidate or a safe vaccine vector for other pathogens for further development. Several studies have demonstrated that MVA and LC16m8 induced comparable protective immune response against pathogenic vaccinia virus infection in small animal models when compared with replicating vaccine strains [7], [8], [22], [45], [46]. A recent study also indicated that the longevity of protection induced by MVA is comparable to that induced by Lister strain [47]. Furthermore, vaccination with two doses of MVA was safe and well tolerated compared to the currently available smallpox vaccine Dryvax in humans. The vaccination also produced comparable cellular and humoral immune responses to one dose of Dryvax [47]. These attenuated vaccine candidates however are not available in China. Here, we demonstrated that MVTTZCI protects mice effectively from the challenge of a pathogenic vaccinia WR strain with an efficacy profile similar to that of VTT. Apparently, higher levels of neutralizing antibodies induced by either VTT or MVTTZCI contributed to the complete protection of animals against the lethal challenge of pathogenic WR strain. This finding should not be a surprise because major neutralizing determinants in B5R, H3L and other proteins were not affected by the deletions in MVTTZCI, which correlate with the protection of animals in previous studies [48], [49]. Interestingly, although the low dose of MVTTZCI (104 PFU) did not induce detectable systemic neutralizing antibodies against WR strain, vaccinated animals were all significantly protected (Fig. 6B). We reason that the low dose of MVTTZCI had probably induced other protective immune mechanism effectively such as protective cell-mediated and mucosal immune responses, which will require further investigations. Since intranasal inoculation of MVTTZCI was equally effective compared to the intramuscular injection, our study has established proof-of-concept that the attenuated replicating MVTTZCI may serve as a safe and noninvasive smallpox vaccine candidate, which is a critical element for the mass vaccination program in developing countries including China, the nation with the world's largest human population. A recent study indicated that aerosol immunization with non-replicating NYVAC and MVA vectored vaccines is safe, simple, and immunogenic [50]. It would be interesting to determine whether or not MVTTZCI will do better. Since a replicating MVTT-vectored vaccine induced higher level of neutralizing antibodies (∼100-fold) against SARS-CoV infection via i.n. or i.o. (intraoral) inoculation when compared with the non-replicating MVA vector [51], [52], the replication-competence likely offers greater advantage for improving the immunogenicity of MVTT-based vaccines. Moreover, the replication-competence may offer greater advantage for reducing the dose and frequency of smallpox vaccination. Further prime-boost studies should also be conducted to determine whether or not MVTTZCI vaccination prior to VTT or Dryvax would help to minimize the primary cutaneous lesion or other side efforts related to the currently stockpiled vaccines. In addition, MVTTZCI is useful as a safe and replicating vector for the development of mucosal deliverable vaccines against infections of human immunodeficiency virus and avian influenza virus, etc. We have recently constructed an MVTTSIVgpe to express simian immunodeficiency virus gag/pol/env using the MVTTZCI as a vaccine vector. We inoculated 108 PFU of MVTTSIVgpe into four rhesus macaques through intranasal inoculation, respectively. All four macaques tolerated the vaccination well without showing clinical signs of disease. There is therefore no evidence to indicate that the virulence of MVTTZCI is quite different in non-human primates when compared with mice. To proof the safety profile of MVTTZCI in humans, however, a careful clinical trial remains necessary. To this end, due to the replication-competency of MVTTZCI, it is necessary to carefully evaluate its use in immunocompromized non-human primate models and individuals if MVTTZCI-based vaccine will be used for immune therapy of AIDS patients. The GFP gene in MVTTZCI serves nicely as a negative selection marker for generating recombinant viruses and should not be included in vaccines developed for human use.
Acknowledgment
This work was supported by Hong Kong research grant council (HK-RGC762208 and HK-RGC762209 to ZC), the National Basic Research Program of China (the 973 project 2006CB504208), the National 11th 5-year project 2008ZX10O01-011 and the University Development Fund of the University of Hong Kong to its AIDS Institute. We thank Drs. D. Ho and K.Y. Yuen for scientific advice and Dr. M. Mackett for critical reading of the manuscript.
Conflict of interest statement: The authors declare no financial or commercial conflict of interest.
Contributions: Q.F. and W.Y. contribute equally to this work. Z.C., W.Y. and Q.F. designed study. Q.F., W.Y., W.Z., H.W. and Z.C. conducted research. P.T., L.Z. and Z.C. contributed to reagents, facility and analytic tools. Q.F., W.Y. and Z.C. conducted data analysis. Z.C., W.Y. and Q.F. wrote the paper.
Contributor Information
Qing Fang, Email: qfang@adarc.org.
Zhiwei Chen, Email: zchenai@hku.hk, zchenai@hkucc.hku.hk.
References
- 1.Fulginiti V.A., Papier A., Lane J.M., Neff J.M., Henderson D.A. Smallpox vaccination: a review, part II adverse events. Clin Infect Dis. 2003;37(July (2)):251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 2.Nafziger S.D. Smallpox. Crit Care Clin. 2005;21(October (4)):739–746. doi: 10.1016/j.ccc.2005.06.004. vii. [DOI] [PubMed] [Google Scholar]
- 3.Berche P. The threat of smallpox and bioterrorism. Trends Microbiol. 2001;9(January (1)):15–18. doi: 10.1016/s0966-842x(00)01855-2. [DOI] [PubMed] [Google Scholar]
- 4.Drazen J.M. Smallpox and bioterrorism. N Engl J Med. 2002;346(April (17)):1262–1263. doi: 10.1056/NEJM200204253461702. [DOI] [PubMed] [Google Scholar]
- 5.Eckart R.E., Shry E.A., Atwood J.E., Brundage J.F., Lay J.C., Bateson T.F. Smallpox vaccination and ischemic coronary events in healthy adults. Vaccine. 2007;25(December (50)):8359–8364. doi: 10.1016/j.vaccine.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 6.Kretzschmar M., Wallinga J., Teunis P., Xing S., Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 2006;3(August (8)):e272. doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belyakov I.M., Wyatt L.S., Ahlers J.D., Earl P., Pendleton C.D., Kelsall B.L. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J Virol. 1998;72(October (10)):8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belyakov I.M., Earl P., Dzutsev A., Kuznetsov V.A., Lemon M., Wyatt L.S. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci USA. 2003;100(August (16)):9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrahams B.C., Kaufman D.M. Anticipating smallpox and monkeypox outbreaks: complications of the smallpox vaccine. Neurologist. 2004;10(September (5)):265–274. doi: 10.1097/01.nrl.0000138998.11209.88. [DOI] [PubMed] [Google Scholar]
- 10.Fulginiti V.A. Risks of smallpox vaccination. JAMA. 2003;290(September (11)):1452. doi: 10.1001/jama.290.11.1452-a. author reply. [DOI] [PubMed] [Google Scholar]
- 11.Jin Q., Chen L.H., Chen S.X., Huang J., Feng Z.H., Yuan J.S. Characterization of the complete genomic sequence of the vaccinia virus Tiantan strain. Sci China (Series) 1997;27(6):562–567. [Google Scholar]
- 12.Jin Q., Chen N.H., Yao E.M., Huang J., Hou Y.D. Analysis of open reading frames located in Hind III-C and -B fragments of vaccinia virus strain Tiantan. Biomed Environ Sci. 1997;10(4):462. [Google Scholar]
- 13.Tscharke D.C., Reading P.C., Smith G.L. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. J Gen Virol. 2002;83(August (Pt 8)):1977–1986. doi: 10.1099/0022-1317-83-8-1977. [DOI] [PubMed] [Google Scholar]
- 14.Fang Q., Yang L., Zhu W., Liu L., Wang H., Yu W. Host range, growth property, and virulence of the smallpox vaccine: vaccinia virus Tiantan strain. Virology. 2005;335(May (2)):242–251. doi: 10.1016/j.virol.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Carroll M.W., Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a non-human mammalian cell line. Virology. 1997;238(November (2)):198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W., Fang Q., Zhuang K., Wang H., Yu W., Zhou J. The attenuation of vaccinia Tiantan strain by the removal of the viral M1L-K2L genes. J Virol Methods. 2007;(April) doi: 10.1016/j.jviromet.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M.S., Roos J.M., McGuigan L.C., Smith K.A., Cormier N., Cohen L.K. Molecular attenuation of vaccinia virus: mutant generation and animal characterization. J Virol. 1992;66(May (5)):2617–2630. doi: 10.1128/jvi.66.5.2617-2630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson J.D., Reith R.W., Jeffrey L.J., Arrand J.R., Mackett M. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J Gen Virol. 1990;71(November (Pt 11)):2761–2767. doi: 10.1099/0022-1317-71-11-2761. [DOI] [PubMed] [Google Scholar]
- 19.Betakova T., Wolffe E.J., Moss B. The vaccinia virus A14.5L gene encodes a hydrophobic 53-amino-acid virion membrane protein that enhances virulence in mice and is conserved among vertebrate poxviruses. J Virol. 2000;74(May (9)):4085–4092. doi: 10.1128/jvi.74.9.4085-4092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W.H., Wilcock D., Smith G.L. Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J Virol. 2000;74(December (24)):11654–11662. doi: 10.1128/jvi.74.24.11654-11662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Hygiene. 1938;27:493–497. [Google Scholar]
- 22.Kidokoro M., Tashiro M., Shida H. Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc Natl Acad Sci USA. 2005;102(March (11)):4152–4157. doi: 10.1073/pnas.0406671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flexner C., Hugin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987;330(November (6145)):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- 24.Shida H., Hinuma Y., Hatanaka M., Morita M., Kidokoro M., Suzuki K. Effects and virulences of recombinant vaccinia viruses derived from attenuated strains that express the human T-cell leukemia virus type I envelope gene. J Virol. 1988;62(December (12)):4474–4480. doi: 10.1128/jvi.62.12.4474-4480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tartaglia J., Perkus M.E., Taylor J., Norton E.K., Audonnet J.C., Cox W.I. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992;188(May (1)):217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- 26.Sutter G., Moss B. Non-replicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89(November (22)):10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutter G., Wyatt L.S., Foley P.L., Bennink J.R., Moss B. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12(August (11)):1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 28.Mackett M., Smith G.L. Vaccinia virus expression vectors. J Gen Virol. 1986;67(October (Pt 10)):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- 29.Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci USA. 2004;101(March (13)):4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser J. Smallpox vaccine. A tame virus runs amok. Science. 2007;316(June (5830)):1418–1419. doi: 10.1126/science.316.5830.1418. [DOI] [PubMed] [Google Scholar]
- 31.Brandt T., Heck M.C., Vijaysri S., Jentarra G.M., Cameron J.M., Jacobs B.L. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not induction of a protective immune response. Virology. 2005;333(March (2)):263–270. doi: 10.1016/j.virol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Antoine G., Scheiflinger F., Dorner F., Falkner F.G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244(May (2)):365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 33.Payne L.G. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J Virol. 1979;31(July (1)):147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payne L.G., Kristensson K. Extracellular release of enveloped vaccinia virus from mouse nasal epithelial cells in vivo. J Gen Virol. 1985;66(March (Pt 3)):643–646. doi: 10.1099/0022-1317-66-3-643. [DOI] [PubMed] [Google Scholar]
- 35.Shida H., Tochikura T., Sato T., Konno T., Hirayoshi K., Seki M. Effect of the recombinant vaccinia viruses that express HTLV-I envelope gene on HTLV-I infection. Embo J. 1987;6(November (11)):3379–3384. doi: 10.1002/j.1460-2075.1987.tb02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antoine G., Scheiflinger F., Holzer G., Langmann T., Falkner F.G., Dorner F. Characterization of the vaccinia MVA hemagglutinin gene locus and its evaluation as an insertion site for foreign genes. Gene. 1996;177(October (1–2)):43–46. doi: 10.1016/0378-1119(96)00267-3. [DOI] [PubMed] [Google Scholar]
- 37.Bartlett N., Symons J.A., Tscharke D.C., Smith G.L. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J Gen Virol. 2002;83(August (Pt 8)):1965–1976. doi: 10.1099/0022-1317-83-8-1965. [DOI] [PubMed] [Google Scholar]
- 38.Gillard S., Spehner D., Drillien R. Mapping of a vaccinia host range sequence by insertion into the viral thymidine kinase gene. J Virol. 1985;53(January (1)):316–318. doi: 10.1128/jvi.53.1.316-318.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkus M.E., Goebel S.J., Davis S.W., Johnson G.P., Limbach K., Norton E.K. Vaccinia virus host range genes. Virology. 1990;179(November (1)):276–286. doi: 10.1016/0042-6822(90)90296-4. [DOI] [PubMed] [Google Scholar]
- 40.Gedey R., Jin X.L., Hinthong O., Shisler J.L. Poxviral regulation of the host NF-kappaB response: the vaccinia virus M2L protein inhibits induction of NF-kappaB activation via an ERK2 pathway in virus-infected human embryonic kidney cells. J Virol. 2006;80(September (17)):8676–8685. doi: 10.1128/JVI.00935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beattie E., Tartaglia J., Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991;183(July (1)):419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- 42.Carroll K., Elroy-Stein O., Moss B., Jagus R. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2 alpha-specific protein kinase. J Biol Chem. 1993;268(June (17)):12837–12842. [PubMed] [Google Scholar]
- 43.Davies M.V., Chang H.W., Jacobs B.L., Kaufman R.J., The E3L K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J Virol. 1993;67(March (3)):1688–1692. doi: 10.1128/jvi.67.3.1688-1692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor J.M., Quilty D., Banadyga L., Barry M. The vaccinia virus protein F1L interacts with Bim and inhibits activation of the pro-apoptotic protein Bax. J Biol Chem. 2006;281(December (51)):39728–39739. doi: 10.1074/jbc.M607465200. [DOI] [PubMed] [Google Scholar]
- 45.Empig C., Kenner J.R., Perret-Gentil M., Youree B.E., Bell E., Chen A. Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine. 2006;24(April (17)):3686–3694. doi: 10.1016/j.vaccine.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 46.Parrino J., McCurdy L.H., Larkin B.D., Gordon I.J., Rucker S.E., Enama M.E. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine. 2007;25(February (8)):1513–1525. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phelps A.L., Gates A.J., Hillier M., Eastaugh L., Ulaeto D.O. Comparative efficacy of modified vaccinia Ankara (MVA) as a potential replacement smallpox vaccine. Vaccine. 2007;25(January (1)):34–42. doi: 10.1016/j.vaccine.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Berhanu A., Wilson R.L., Kirkwood-Watts D.L., King D.S., Warren T.K., Lund S.A. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol. 2008;82(April (7)):3517–3529. doi: 10.1128/JVI.01854-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawrence S.J., Lottenbach K.R., Newman F.K., Buller R.M., Bellone C.J., Chen J.J. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J Infect Dis. 2007;196(July (2)):220–229. doi: 10.1086/518793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corbett M., Bogers W.M., Heeney J.L., Gerber S., Genin C., Didierlaurent A. Aerosol immunization with NYVAC and MVA vectored vaccines is safe, simple, and immunogenic. Proc Natl Acad Sci USA. 2008;105(February (6)):2046–2051. doi: 10.1073/pnas.0705191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z., Fang Q., Lu B., Zhuang K., Yu W., Wang H. A novel mucosal vacinia vector for inducing high level anti-viral immunity. Antiviral Ther. 2006;11(Suppl 2):0A09–5. [Google Scholar]
- 52.Huang X., Lu B., Yu W., Fang Q., Liu L., Zhuang K. A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS One. 2009;4(1):e4180. doi: 10.1371/journal.pone.0004180. [DOI] [PMC free article] [PubMed] [Google Scholar]


