Highlights
-
•
Lactobacillus S-layer protein plays an inhibitory role during PEDV infection.
-
•
In Vero cells infected with PEDV, apoptosis was mediated by caspase-8/3 activation.
-
•
Lactobacillus S-layer protein inhibited PEDV-induced apoptosis in Vero cells.
-
•
S-layer protein reduced caspase-8/3 activation against PEDV-induced apoptosis.
Keywords: L. acidophilus S-layer proteins, PEDV, Apoptosis, Vero cells
Abstract
To gain insight into the mechanism of Lactobacillus acidophilus (L. acidophilus) S-layer protein antiviral activity, we examined how S-layer protein impacts porcine epidemic diarrhea virus (PEDV) infection and PEDV-induced apoptosis of Vero cells. Pretreatment (exclusion assay), coincubation (competition assay), and post-treatment (displacement assay) of PEDV-infected Vero cells with the S-layer protein was examined. Interestingly, significant inhibition of PEDV by S-layer protein was only observed in the exclusion assay. In Vero cells infected with PEDV, we found that apoptosis was mediated by activation of caspase-8 and caspase-3 in the late stage of infection. When PEDV-infected Vero cells were pretreated with S-layer protein, rates of Vero cell apoptosis were markedly decreased and cell damage was significantly reduced, as evaluated by flow cytometry and microscopy. Detailed analyses showed that the S-layer protein inhibited caspase-8 and caspase-3 activity. Taken together, our results suggest that L. acidophilus S-layer protein plays an inhibitory role during PEDV infection of Vero cells, and that the antagonistic activity of the protein is not via competition with PEDV for binding sites. In addition, the findings suggest that L. acidophilus S-layer protein protects against PEDV-induced apoptosis through reduced caspase-8 and caspase-3 activation in the later stages of infection. This mechanism may represent a novel approach for antagonizing PEDV and other viruses.
1. Introduction
Surface layer (S-layer) proteins are crystalline arrays of proteinaceous subunits forming the outermost component of the cell wall in several Lactobacillus species. Studies have shown that the S-layer proteins of several Lactobacillus species have antimicrobial activity (Hynonen and Palva, 2013), with proteins from L. helveticus, L. crispatus, and L. kefir shown to inhibit Salmonella enteritidis (Golowczyc et al., 2007), Salmonella enterica serovar Typhimurium (Li et al., 2010), and enteropathogenic Escherichia coli (Zhang et al., 2017) infection of host epithelial cells. Martínez et al. also found that L. acidophilus ATCC 4356 surface protein extract inhibited Junin virus (JUNV) infection (Martinez et al., 2012).
Apoptosis is an innate host defense mechanism that disrupts bacterial or viral replication by eliminating infected cells. Bacteria can hijack a host’s apoptotic pathway to enhance their own pathogenesis in epithelial cells, resulting in a delayed apoptotic response and, subsequently, cell damage. The delay in onset of epithelial cell apoptosis may be critical for some intracellular pathogens, providing sufficient time for proliferation and adaptation to the intracellular environment and increasing the extent of cellular damage (Faherty and Maurelli, 2008; Kim et al., 1998; Philpott et al., 2001). Li et al. found that L. acidophilus S-layer proteins inhibit bacteria-induced apoptosis (Li et al., 2011), which is considered one of the most important antimicrobial functions of these proteins. Many viruses can actively induce apoptosis as a response to viral replication, thereby enabling the release and dissemination of viral progeny to neighboring cells. This apoptotic event is one of the cytolytic properties of viral infections causing cytopathic effects (CPE) in vitro and also contributes to viral pathogenesis in vivo, resulting in cell damage, tissue injury, and increased disease severity (DeDiego et al., 2011; Favreau et al., 2012; Lan et al., 2013). At present, it is unknown whether L. acidophilus S-layer proteins inhibit virus-induced cell apoptosis.
Porcine epidemic diarrhea virus (PEDV), the etiological agent of porcine epidemic diarrhea (PED), belongs to the family Coronaviridae and causes acute watery diarrhea, vomiting, dehydration, and high mortality rates in neonatal piglets (Lee, 2015). For the last three decades, PEDV infection has resulted in significant economic losses in the European, Asian, and North America pig industries. PEDV induces apoptotic cell death, and is associated with CPEs both in vitro and in vivo (Kim and Lee, 2014). Virus-induced apoptosis plays a critical role in PEDV replication and pathogenesis, suggesting that an anti-apoptotic approach may be an appropriate strategy for the development of PEDV-targeted therapy to combat PED.
The ability of a virus to hijack the host apoptotic pathway is an important component of infection. To date, it is not known whether L. acidophilus S-layer proteins can inhibit virus-induced cell apoptosis. In this study, we investigated the inhibitory effects of L. acidophilus S-layer protein on PEDV infection and on the ability of PEDV to induce host cell apoptosis. The findings of this study may help us to better understand how L. acidophilus S-layer proteins inhibit PEDV-induced apoptosis in Vero cells, and provide a rationale for the use of these proteins as potential agents for reducing the prevalence of PEDV infections.
2. Materials and methods
2.1. Cells and viruses
Vero cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Vero cells (passages 15–35) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) heat-inactivated fetal calf serum, 4.5 g/l d-glucose, 25 mM HEPES, 1% nonessential amino acids and 2 mM l-glutamine (Gibco, Carlsbad, CA, USA). The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 in air. Vero cells were cultured until they reached confluency (differentiated cells). PEDV strain CV777 was provided by our lab and propagated in Vero cells.
2.2. Cytotoxicity assay for S-layer proteins
S-layer protein was obtained from L. acidophilus ATCC 4356 as previously reported (Li et al., 2010). Cytotoxic effect of S-layer protein was accomplished by the MTT colorimetric assay. Vero cells in 96-well plate were treated with S-layer protein at a series of concentrations (0, 50, 100, 200, 500, 1000 μg/ml) in serum-free DMEM for 48 h. Mock-treated Vero cells served as a control. Thereafter, cell viability was determined by the MTT method using MTT Cell Proliferation and Cytotoxicity Assay Kit (Beyotime, China) as recommended by the manufacturer. Briefly, 20 μl (5 μg/μl) MTT solution was added into each well and the plate was incubated for 4 h in the dark. After medium was removed, 100 μl dimethyl sulfoxide (DMSO) solution was added to each well and the plate was vibrated to dissolve purple crystals. Then a microplate reader (Epoch, Bio Tek, USA) was applied to measure absorbance [optical density (OD) value] at 570 nm. Cell viability was expressed as the percentage of control. The same plate contained additional wells with media and chemical only (without cells), processed in parallel as reference blanks.
2.3. Effect of S-layer protein on viral infection
In order to investigate whether S-layer protein affects infection of PEDV to Vero cells, Vero cells were treated with a mixture of nontoxic concentrations of S-layer protein and PEDV [multiplicity of infection (MOI) 0.01] at 37 °C for 48 h. As a control, cells were infected with the same dose of PEDV without S-layer protein. Subsequently, the antiviral efficacy was evaluated by analysis of virus loads.
2.4. The dose screening and control experiment of S-layer protein against PEDV infection in Vero cells
Vero cells were seeded into 24-well and grown to confluency. Vero cells were preincubated with L. acidophilus S-layer protein at a series of concentrations in serum-free DMEM (0, 8, 16, 32, 64, 128, 256, 512 μg/ml) for 1 h at 37 °C, and then PEDV(MOI = 0.01) was added to each well and incubated for 1 h at 37 °C. After removing the unbound viruses by washing with cold DMEM, the cells were cultured at 37 °C for 48 h. The Vero cells were subjected to three freeze-thaw cycles in preparation for measuring virus loads. At the indicated time, the cellular lysates were collected, resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted using an antibody that recognizes PEDV N protein. The anti-PEDV N protein mAb was purchased from Medgene labs (1:1000).
The control experiment was investigated on Vero cell monolayers in 24-well plates. Vero cells were preincubated with the S-layer protein (256 μg/ml) or albumin of bovine serum (BSA, 256 μg/ml) for 1 h at 37 °C, and then PEDV (MOI 0.01) was added to each well and incubated for 1 h at 37 °C. After removing the unbound viruses by washing with cold DMEM, the cells were incubated at 37 °C for 48 h. The Vero cells were subjected to three freeze-thaw cycles in preparation for measuring virus loads.
2.5. PEDV association with S-layer protein assay
The association assays were investigated on Vero cell monolayers in 24-well plates. Different types of experiments were performed:
exclusion assay: Vero cells were preincubated with the S-layer protein (256 μg/ml) for 1 h at 37 °C, and then PEDV (MOI 0.01) was added to each well and incubated for 1 h at 37 °C. After removing the unbound viruses by washing with cold DMEM, the cells were incubated at 37 °C for 48 h.
competition assay: the S-layer protein (256 μg/ml) and PEDV (MOI 0.01) were mixed and coincubated simultaneously with Vero monolayers for 2 h at 37 °C. After washing with cold DMEM, the cells were incubated for 48 h at 37 °C;
displacement assay: PEDV (MOI 0.01) was added to Vero monolayers and incubated for 1 h at 37 °C before challenge with the S-layer protein (256 μg/ml); the plates were then incubated for 1 h at 37 °C. After washing with cold DMEM, the cells were incubated for 48 h at 37 °C.
The Vero cells were subjected to three freeze-thaw cycles in preparation for measuring virus loads in the three assays. Each treatment was carried out in triplicate wells. All assays were performed in triplicate on three consecutive cell passages.
2.6. Effect of S-layer protein on viral attachment
To evaluate the effect of S-layer protein on attachment of PEDV to Vero cells, Vero cells were pretreated with S-layer protein at 37 °C for 1 h, and then added PEDV (MOI = 1) at 4 °C for 1 h, which allowed the viruses to bind to the surface of cells but not enter the cells. As a control, cells were infected with the same dose of PEDV without S-layer protein treatment. After removing the S-layer protein and the unbound viruses by washing with cold DMEM, the Vero cells were subjected to three freeze-thaw cycles in preparation for measuring virus loads.
2.7. Effect of S-layer protein on viral entry and replication
To determine whether S-layer protein affects entry and replication of PEDV to Vero cells, the cells were incubated with S-layer protein at 37 °C for 1 h. Then Vero cells were infected with PEDV (MOI = 0.01) at 37 °C for 1 h. After removing the unbound viruses by washing with DMEM, the cells were cultured at 37 °C for 48 h. As a control, cells were infected with the same dose of PEDV without S-layer protein treatment. Subsequently, the antiviral efficacy was evaluated by analysis of virus loads.
2.8. Infection of Vero cells by PEDV
Vero cells were seeded into 24-well or 6-well plates and grown to confluency. Vero cells were preincubated with L. acidophilus S-layer protein (256 μg/ml) for 1 h at 37 °C, and then PEDV(MOI = 0.01) was added to each well and incubated for 1 h at 37 °C. After removing the unbound viruses by washing with cold DMEM, the cells were cultured at 37 °C for a different time. All assays were performed in triplicate on three consecutive cell passages.
2.9. Indirect immunofluorescence assay (IFA)
The IFA was performed to confirm the inhibitory effects of S-layer protein on PEDV infection. After washing with PBS, the Vero cells were fixed with 4% paraformaldehyde in PBS for 15 min and then permeabilized with 0.2% Triton X-100 in PBS for 10 min. After washing, the Vero cells were incubated with anti-PEDV mouse monoclonal antibody (1:100, obtained in our lab) for 1 h. Subsequently, FITC-conjugated goat antimouse IgG (1:100) (Boster, China) was used as secondary antibody. After washing, the coverslips were mounted on microscope glass slides in mounting buffer and cell staining was observed using a fluorescence microscope (AXIOVERT; Carl Zeiss, Jena, Germany).
2.10. Apoptosis assays
Apoptosis of Vero cells were assessed with an Annexin V-FITC Apoptosis Detection Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cells were stained with annexin V-fluorescein isothiocyanate (Annexin V-FITC) and propidium iodide (PI) for analyses by flow cytometry (cells seeded into 6-well plates) or microscopy (cells differentiated on glass coverslips placed in 24-well plates). Early apoptotic cells showed positive staining for annexin V-FITC alone, while necrotic cells and late apoptotic cells showed positive staining for both annexin V-FITC and PI. Flow cytometry was performed using a FACSCanto II cytofluorimeter (Becton Dickinson, Mountain View, CA, USA) with excitation at 488 nm. Fluorescence emission by FITC was measured using a 530/30 bandpass filter while that of PI was measured using a 585/42 bandpass filter. In addition, the cells were observed under a fluorescence microscope (AXIOVERT; Carl Zeiss, Jena, Germany).
2.11. Caspase-8 and caspase-3 activity assays
In order to elucidate the activity of caspase-8 and caspase-3, we initially assessed whether the caspase-8 inhibitor, Z-IETD-FMK and the caspase-3 inhibitor, Z-DEVD-FMK block PEDV infection. To examine the effect of each inhibitor on PEDV infection, Vero cells were pretreated with Z-IETD-FMK (Selleck Chemicals, Houston, TX, USA; dissolved in DMSO) and Z-DEVD-FMK (Selleck Chemicals, Houston, TX, USA; dissolved in DMSO) for 1 h prior to PEDV infection for 48 h. The inhibitors were present during the entire period of infection. Viral production was measured by virus loads.
The activity of caspase-8 was determined using a Caspase-8 Activity Kit (Beyotime Institute of Biotechnology, Haimen, China), which is based on the ability of caspase-8 to change acetyl-Ile-Glu-Thr-Asp p-nitroanilide (Ac-IETD-pNA) into the yellow formazan product p-nitroaniline (pNA). Cell lysates were centrifuged at 12,000×g for 10 min, and the protein concentrations were determined by the Bradford protein assay. Cellular extracts (30 μg of protein) were incubated in a 96-well microtiter plate with 20 ng of Ac-IETD-pNA overnight at 37 °C. The absorbance values of pNA at 405 nm, OD405, were measured using a 96-well plate reader (BioTek, Santa Barbara, CA, USA). An increase in the OD405 indicated activation of caspase-8.
The activity of caspase-3 was determined using a Caspase-3 Activity Kit (Beyotime Institute of Biotechnology), based on the ability of caspase-3 to change acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) into the yellow formazan product pNA. The same method described for the caspase-8 assay was used to determine the activation of caspase-3.
2.12. Data analysis
The results were expressed as means ± SEM. ANOVA and an unpaired Student’s t-test were employed to determine the statistical significance of differences among multiple groups.
3. Results
3.1. Dose effects of S-layer protein against PEDV infection in Vero cells
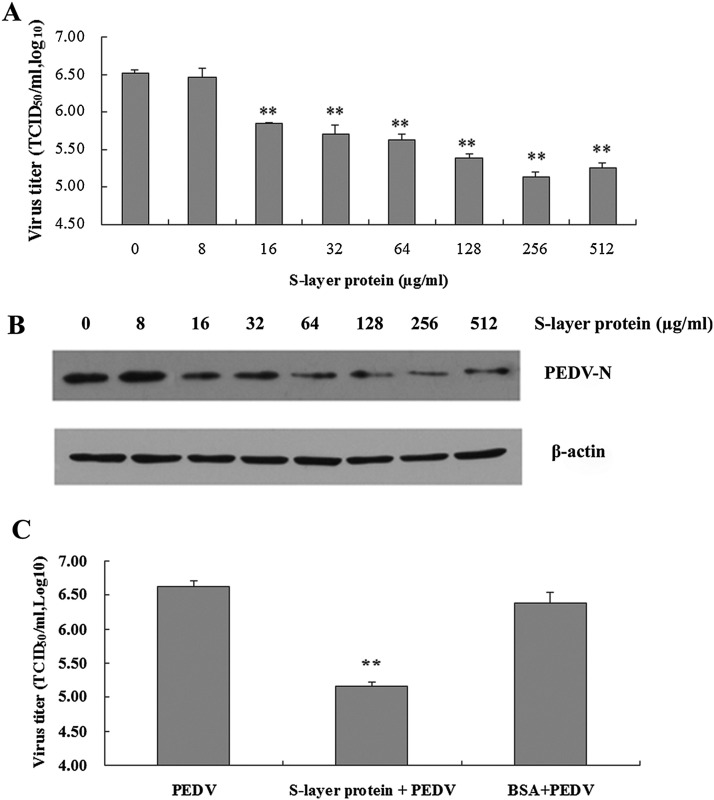
At an S-layer protein concentration of 1 mg/ml, no cytotoxicity was observed in Vero cells compared with mock-treated cells. In addition, S-layer protein had no discernable viricidal effects against PEDV (data not shown). However, as shown in Fig. 1 A, S-layer protein significantly decreased PEDV titers following infection of Vero cells. The lowest PEDV titer (5.13 ± 0.07 log10 TCID50/ml) was obtained following pretreatment of Vero cells with 256 μg/ml S-layer protein (relative to a PEDV titer without S-layer protein of 6.52 ± 0.04 log10 TCID50/ml; ANOVA: P < 0.01). Overall, viral titers in infected Vero cell cultures declined following pretreatment with 16, 32, 64, 128, and 256 μg/ml S-layer protein in a dose-dependent manner. A concentration of 256 μg/ml S-layer protein was therefore selected for use in the subsequent antiviral assays. Western blot data also showed the dose-dependent manner (Fig. 1B). BSA as a control protein has no the inhibition of S-layer protein against PEDV infection in Vero cells (Fig. 1C).
Fig. 1.
Dose screening and control experiment of L. acidophilus S-layer protein against PEDV infection in Vero cells. (A) The dose effects of S-layer protein against PEDV infection in Vero cells. **Analysis of variance (ANOVA) P < 0.01 compared with 0 μg/ml. (B) Western blot analysis of the corresponding viral protein levels. Immunoblot used an antibody that recognizes PEDV N protein. (C) The control experiment. BSA: albumin of bovine serum. “**”, P < 0.01 (compared with PEDV).
3.2. Ability of S-layer protein to inhibit PEDV infection in Vero cells
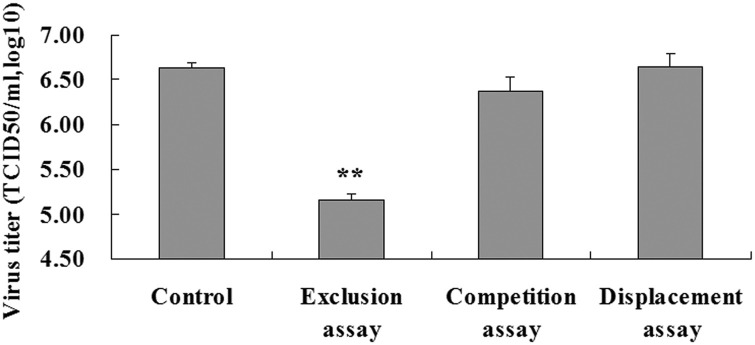
To investigate the antiviral activity of S-layer protein against PEDV, pretreatment (exclusion), co-incubation (competition), and post-treatment (displacement) assays using PEDV-infected Vero cells and S-layer protein were conducted. Significant inhibition of PEDV by S-layer protein was only observed in the exclusion assays. In the competition and displacement assays, no inhibition of PEDV infection by S-layer protein was noted (Fig. 2 ). In the exclusion assay, the PEDV viral titers in infected Vero cells were significantly reduced by the S-layer protein (6.63 ± 0.07 log10 TCID50/ml reduced to 5.15 ± 0.07 log10 TCID50/ml) relative to PEDV-infected cells without S-layer protein (ANOVA: P < 0.01; Fig. 2).
Fig. 2.
Pretreatment of Vero cells with L. acidophilus S-layer protein reduces PEDV infection. Exclusion assay: pretreatment; Competition assay: coincubation; Displacement assay: post-treatment. **Analysis of variance (ANOVA) P < 0.01 compared with the control.
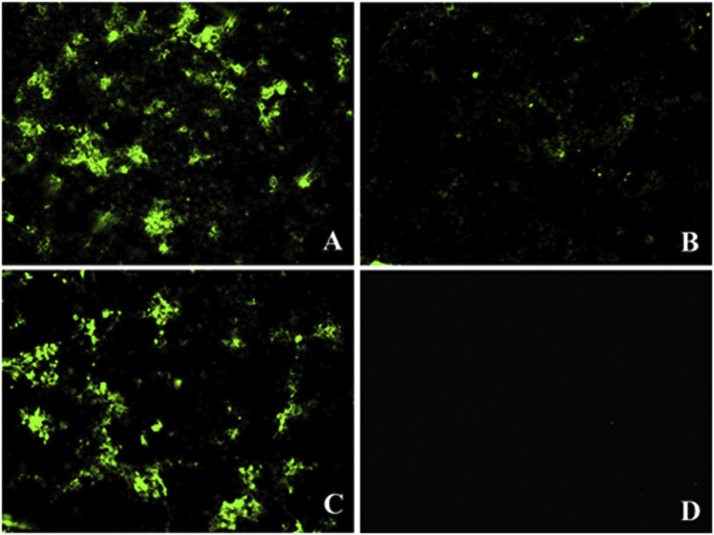
In indirect immunofluorescence assays (IFA), mock-treated Vero cells produced significantly stronger fluorescent signals at 48 h post-infection with PEDV (Fig. 3 A) compared with signals produced by cells pre-treated with S-layer protein (Fig. 3B). When Vero cells were coincubated with PEDV and S-layer protein, the fluorescent signals were no changes compared with PEDV infection alone (Fig. 3C). There were no fluorescent signals in control cells (Fig. 3D).
Fig. 3.
L. acidophilus S-layer protein-mediated inhibition of PEDV infection observed by IFA (×100). (A) PEDV infection alone. (B) PEDV-infeced Vero cells were pretreated with S-layer protein. (C) Vero cells were coincubated with PEDV and S-layer protein (D) Control cells.
3.3. S-layer protein inhibits PEDV entry and replication
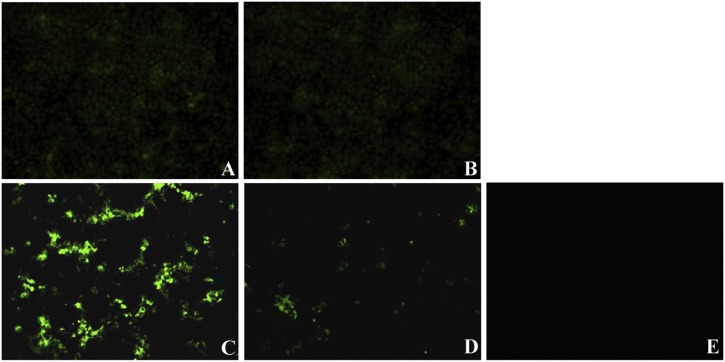
To determine which step in the viral life cycle is affected by S-layer protein, viral attachment, entry, and replication assays were performed in Vero cells. As shown in Fig. 4 , the fluorescent signals show the corresponding viral titers. In viral attachment assays, the viral titers of mock-treated cells and those treated with 256 μg/ml S-layer protein were 4.25 ± 0.17 (Fig. 4A) and 4.05 ± 0.21 (Fig. 4B) log10 TCID50/ml, respectively, suggesting that S-layer protein had no effect on PEDV attachment to Vero cells. In viral entry and replication assays, the viral titers of mock-treated cells and those treated with 256 μg/ml S-layer protein were 6.65 ± 0.17 (Fig. 4C) and 5.15 ± 0.08 (Fig. 4D) log10 TCID50/ml, respectively, indicating that S-layer protein inhibited PEDV entry and replication in Vero cells.
Fig. 4.
Effect of L. acidophilus S-layer protein on PEDV attachment, entry and replication observed by IFA (×100). The fluorescent signals show the corresponding viral titers. (A) PEDV infection alone at 4 °C for 1 h (attchment assay). (B) PEDV-infeced Vero cells at 4 °C for 1 h were pretreated with S-layer protein at 37 °C for 1 h (attchment assay). (C) PEDV infection alone at 37 °C for 1 h (entry and replication assay). (D) PEDV-infeced Vero cells were pretreated with S-layer protein at 37 °C for 1 h (entry and replication assay). (E) Blank cells.
3.4. S-layer protein-mediated inhibition of PEDV-induced apoptosis of Vero cells
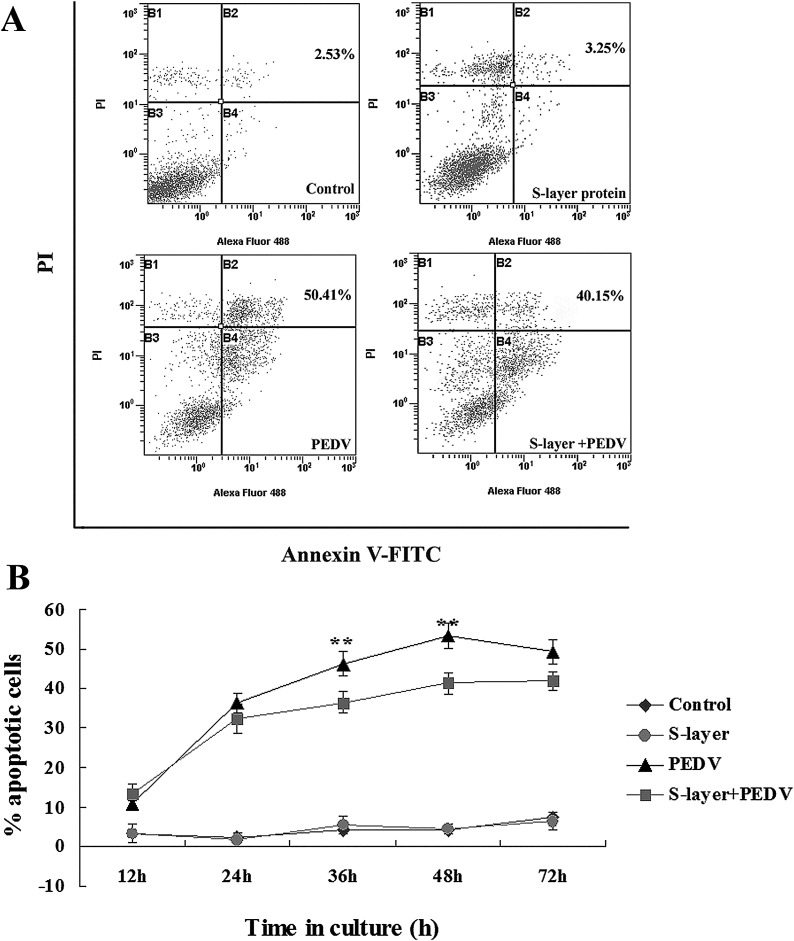
Flow cytometry revealed that apoptosis was induced in PEDV-infected Vero cells. As shown in Fig. 5 A, the percentage of apoptotic cells was increased to 50.41 ± 1.24% following PEDV infection for 48 h, compared with 2.53 ± 0.95% of uninfected control cells. The number of apoptotic cells progressively and significantly increased in a time-dependent manner. S-layer protein alone did not affect Vero cell apoptosis. However, when Vero cells pre-treated with S-layer protein were then infected with PEDV for 36 h or 48 h, the number of apoptotic cells was significantly decreased (P < 0.01; Fig. 5B).
Fig. 5.
Apoptosis of PEDV-infected Vero cells determined by flow cytometry after pretreatment with L. acidophilus S-layer protein. The cell distribution was analyzed by Annexin V-FITC and PI uptake. (A) Apoptosis of Vero cells was observed at 48 h after infection with PEDV. The numbers indicate the percentages of cells present in the sums of the B2 and B4 areas. (B) Time courses of apoptosis in Vero cells after PEDV infection with S-layer protein. The data shown are the means ± SEM of three independent experiments on three consecutive cell passages. The control cells received no treatment.
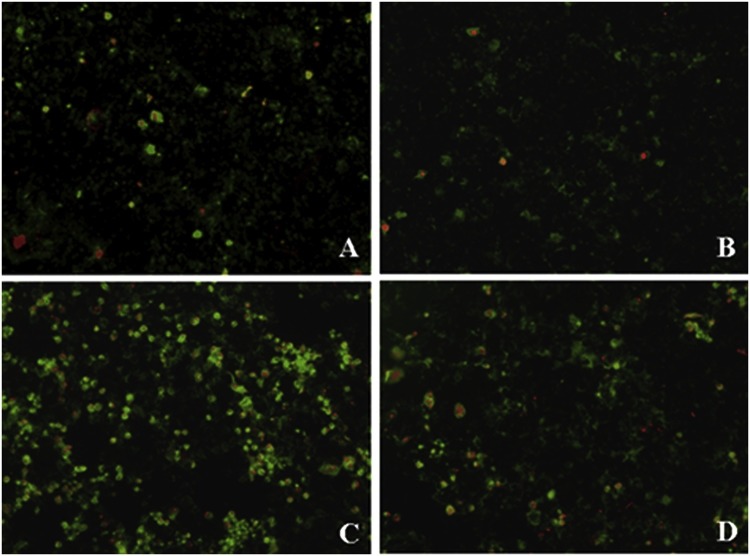
Data obtained from morphological assessments of apoptosis by microscopy were in agreement with the flow cytometry data. When Vero cells were infected with PEDV for 48 h, a large proportion of cells showed both green and red fluorescence, indicating apoptosis or necrosis (Fig. 6 C). Untreated Vero cells or those treated with S-layer protein alone showed little or no green fluorescence, indicating the presence of few apoptotic cells (Fig. 6A, B). As shown in Fig. 6D, infected Vero cells pre-treated with S-layer protein showed a significant reduction in fluorescence compared with the infected cells without S-layer protein, indicating that S-layer protein markedly inhibits apoptosis-induced cell injury.
Fig. 6.
L. acidophilus S-layer protein-mediated inhibition of PEDV-induced apoptosis observed by microscopy (×100). (A) Control. (B) S-layer protein alone. Control cells and cells treated with S-layer protein alone show little green fluorescence or no fluorescence, indicating few apoptotic cells. (C) Among Vero cells infected with PEDV for 48 h, many apoptotic cells and necrotic cells show green and red fluorescence. (D) When PEDV-infected Vero cells were pretreated with S-layer protein, the fluorescence was significantly reduced. The results indicate that S-layer protein significantly inhibit PEDV-induced apoptosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.5. Treatment with caspase inhibitor partly inhibit PEDV infection
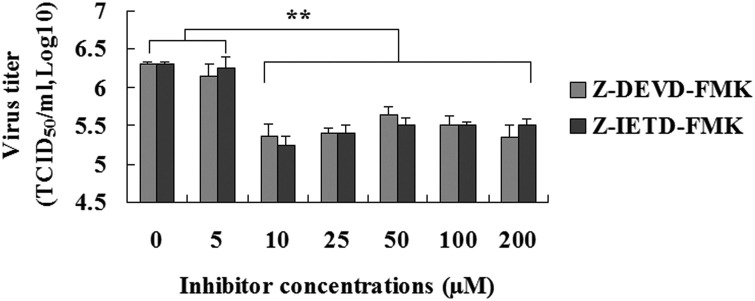
As shown in Fig. 7 , When the concentration of inhibitor was more than 10 μM, PEDV infection was reduced by the inhibitor of caspase-8 (Z-IETD-FMK) and caspase-3 (Z-DEVD-FMK) treatment, respectively, compared to vehicle-treated control cells (the maximum concentration, P < 0.01). However, there were none of the doses of Z-IETD-FMK (≥10 μM) and Z-DEVD-FMK (≥10 μM) tested in the present study resulted in any change in PEDV infection (Fig. 7). The results indicated that activation of caspases is still relevant to PEDV infection.
Fig. 7.
Effect of PEDV infection in the presence of Z-IETD-FMK and Z-DEVD-FMK. At the different concentrations after PEDV infection 48 h, culture supernatants were harvested and viral titers were measured. The data shown are the means ± SEM of three independent experiments for each protocol. “*”, P < 0.05; “**”, P < 0.01.
3.6. Effects of S-layer protein on caspase-8 activity
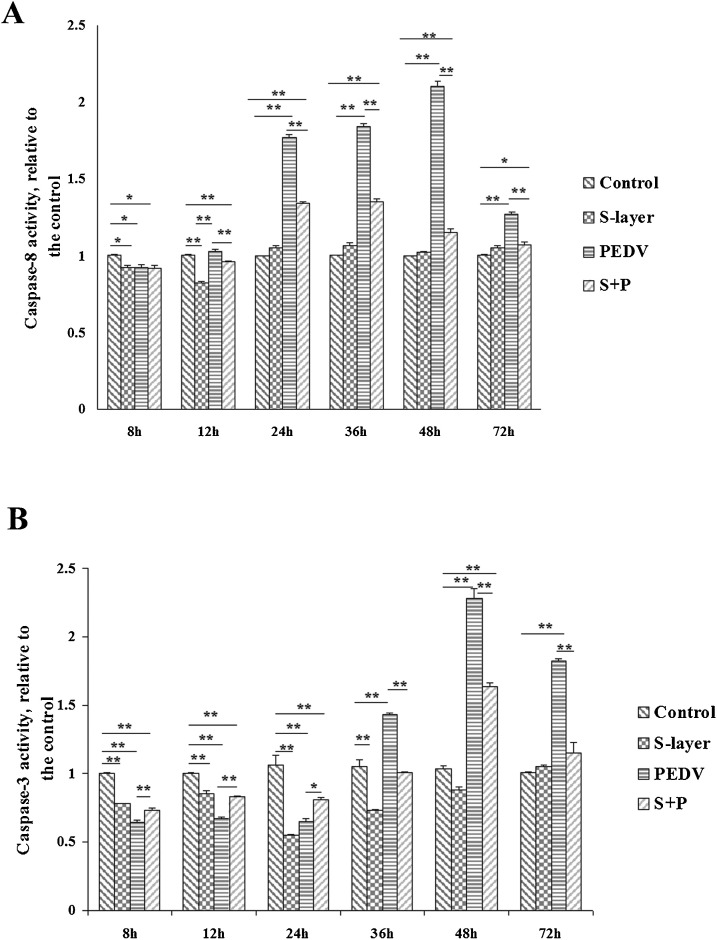
We first analyzed whether PEDV affected the activation of caspase-8 in Vero cells, as assessed by cleavage of the caspase-8 substrate, Ac-IETD-pNA. As shown in Fig. 8 A, there was a significant decrease in caspase-8 activity in PEDV-infected Vero cells at 8 h post-infection (7.53 ± 1.24% reduction compared with uninfected cells, P < 0.05). However, significant increases in caspase-8 activity were observed at 24 h (76.85 ± 2.17% increase, P < 0.01), 36 h (84.22 ± 2.32% increase, P < 0.01), and 48 h (100.1 ± 3.15% increase, P < 0.01) post-infection compared with uninfected cells. PEDV-induced caspase-8 activity reached a peak at 48 h post-infection, and although levels had decreased by 72 h post-infection, caspase-8 activity remained higher in infected cells compared with uninfected cells at this time point (27 ± 2.15% increase, P < 0.01).
Fig. 8.
Effects on caspase-8 and caspase-3 activities in PEDV-infected Vero cells pretreated with S-layer protein. (A) Caspase-8 activity assays. (B) Caspase-3 activity assays. The relative caspase-8 and caspase-3 activities were calculated as ratios of the cleavage of their substrates in treated cells relative to control cells, and the values of the control cells were set to 1. The data shown are the means ± SEM of three independent experiments for each protocol. “*”, P < 0.05; “**”, P < 0.01.
S-layer protein alone also had a significant effect on caspase-8 activation (Fig. 8A). There was a significant decrease in caspase-8 activity at 8 h (7.42 ± 0.84% reduction, P < 0.05) and 12 h (17.76 ± 1.01% reduction, P < 0.01) post-treatment with S-layer protein compared with untreated control cells. When PEDV-infected Vero cells were pretreated with S-layer protein, significant decreases in caspase-8 activity were observed at 12 h, 24 h, 36 h, 48 h, and 72 h post-infection (7.10 ± 1.12%, 42.68 ± 1.10%, 48.89 ± 1.25%, 95.21 ± 2.22%, and 19.83 ± 1.91% reduction, respectively, P < 0.01) compared with PEDV-infected cells alone.
3.7. S-layer protein inhibits caspase-3 activation
To assess the effects of PEDV on caspase-3 activation in Vero cells, we determined the time course of caspase-3 activation after infection. PEDV-induced apoptosis was accompanied by changes in caspase-3 activity, as assessed by cleavage of the caspase-3 substrate, Ac-DEVD-pNA. As shown in Fig. 8B, there was a significant decrease in caspase-3 activity in PEDV-infected cells at 8 h, 12 h, and 24 h post-infection (35.71 ± 1.77%, 32.92 ± 0.91%, and 35.19 ± 1.78% reduction, respectively, P < 0.01) compared with uninfected control cells. There was then a significant increase in caspase-3 activity at 36 h post-infection (43.28 ± 1.29% increase, P < 0.01), which reached a peak at 48 h post-infection (128.39 ± 6.95% increase, P < 0.01). Caspase-3 activity remained higher in PEDV-infected cells compared with uninfected cells at 72 h post-infection (82.22 ± 1.91% increase, P < 0.01).
As shown in Fig. 8B, there was a significant decrease in caspase-3 activity in Vero cells in the presence of S-layer protein compared with untreated cells at 8 h, 12 h, 24 h, and 36 h post-treatment (22.13 ± 0.61%, 14.41 ± 2.13%, 45.35 ± 0.93%, and 26.72 ± 2.51% reduction, respectively, P < 0.01). When PEDV-infected Vero monolayers were pretreated with S-layer protein for 1 h, caspase-3 activity was significantly reduced at 4 h, 12 h, and 24 h post-infection, but increased by 48 h and returned to normal levels at 72 h post-infection.
4. Discussion
Lactobacilli are used as probiotic bacteria in both functional foods and antimicrobial agents (Vadillo-Rodriguez et al., 2005), with L. acidophilus S-layer proteins playing an important role in the antimicrobial activity of probiotic strains (Hynonen and Palva, 2013). In this study, L. acidophilus S-layer protein was shown to have an inhibitory effect on PEDV infection in Vero cells. Three association assays (exclusion, competition, and displacement assays) were conducted to investigate the underlying mechanism of how S-layer proteins inhibit PEDV infection. A previous study found that L. acidophilus S-layer protein has significant antagonistic activity against S. Typhimurium infection of Caco-2 cells (Li et al., 2012). The resulting data supported the proposed antimicrobial mechanisms of L. acidophilus S-layer protein, including competition for binding sites on the surface of host epithelial cells and direct interaction between S-layer protein and the S. Typhimurium cell surface. Interestingly, inhibition of PEDV infection of Vero cells was only observed in the exclusion assay in the current study. These results further demonstrate that L. acidophilus S-layer protein can antagonize the entry and replication, but not attachment, of PEDV in Vero cells. Simultaneous infection and post infection treatments with S-layer did not have an effect in viral infection, leading to the idea that the inhibition caused by S-layer may be due to an early effect during viral replication cycle or PEDV entry in Vero cells. Consequently, we concluded that the antiviral activity of S-layer protein is not based on competition with PEDV for binding sites on the host cell. However, S-layer protein has previously been shown to bind to the C-type lectin DC-specific ICAM-3-grabbing non-integrin receptor (DC-SIGN, CD209) (Prado Acosta et al., 2016). DC-SIGN is a cell-surface adhesion factor that enhances the entry of viruses belonging to several different families into the host cell. S-layer protein inhibits JUNV infection via direct interaction with the DC-SIGN receptor (Martinez et al., 2012). Whether the S-layer proteins inhibit PEDV infection by binding to DC-SIGN receptors needs to be examined further.
Caspase-8 is an initiator caspase, with the released active dimer known to cleave a variety of downstream targets, including the executioner caspase, caspase-3 (Keller et al., 2018). Caspase-3 is activated by both extrinsic and intrinsic apoptotic pathways, and is responsible for the morphological features of apoptosis (Lee and Lee, 2018). Apoptotic factors caspase-3 and caspase-8 are up-regulated during PEDV infection, especially in the later stages (Ming-Ming et al., 2014). PEDV coronavirus induces HEK 293 T apoptosis via activation of caspase-3 by papain-like protease (Qian et al., 2015). Interestingly, Kim et al. could not detect any cleaved caspase-3 in PEDV-infected cells up to 48 h post-infection. A small amount of activated caspase-3 appeared at 48 h post-infection, which is likely to be the basal level normally present in the late stage of infection (Kim and Lee, 2014). In the present study, our results clearly indicated that PEDV can mediate cellular apoptosis by inducing caspase-8 and caspase-3 activation. Caspase-3 activity in Vero cells was decreased at 8, 12, and 24 h post-infection and then significantly increased late in the infection cycle. Our findings suggest that the delayed onset of PEDV-induced apoptosis may be the result of inhibition of caspase-3 activation. Likewise, chemical suppression of caspase activation was found to be partly abrogating PEDV infetion. Kim et al. found that PEDV induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor (Kim and Lee, 2014). Our results indicated that caspase may play a part in the late stage of PEDV infection. The phenomenon of none of the doses of inhibitor affected in PEDV infection needs further study.
In the present study, we found that the inhibition of PEDV-induced apoptosis was mediated by L. acidophilus S-layer protein in Vero cells. The underlying mechanism may involve S-layer protein inhibition of caspase-8 and caspase-3 activation. We have previously shown that S-layer proteins inhibit caspase-3 activity and activate the extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling pathway in Caco-2 cells (Li et al., 2011). In the present study, S-layer protein alone inhibited the activity of both caspase-8 and caspase-3 in Vero cells. We found that caspase-3 activity was significantly decreased at 24 h post-infection with PEDV, while S-layer proteins could also inhibit the activity of caspase-3. Interestingly, when PEDV-infected Vero cells were pretreated with S-layer proteins for 1 h, caspase-3 activity was increased compared with that in cells treated with PEDV or S-layer protein alone. This might be related to the reduction in the number of invading viruses caused by the S-layer proteins. In addition, the S-layer proteins antagonized the upregulation of caspase-3 activity caused by PEDV in the later stages of infection. This antagonistic activity of the S-layer proteins may be directly involved in their inhibition of caspase-3 activation. S-layer proteins may counteract cellular changes induced by PEDV infection by affecting host cell apoptosis in vitro. In other words, S-layer proteins are involved in active defense rather than frontal attack against PEDV-induced apoptosis.
In conclusion, L. acidophilus S-layer protein plays an inhibitory role during PEDV infection of Vero cells. The antiviral effect of S-layer protein appears to manifest during PEDV entry into and replication within Vero cells. The present results suggest that in Vero cells infected with PEDV, apoptosis is mediated by caspase-8 and caspase-3 activation in the later stages of infection. We have demonstrated that the antagonistic activity of S-layer protein may be partly involved in the process by reducing downstream caspase-8 and caspase-3 activation. Further research is required to explore whether S-layer protein has a direct antiviral effect and to discover the mechanism of the antiviral effect of S-layer protein on PEDV infection in host animals.
Declarations of interest
None.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (No. 31502105).
References
- DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Regla-Nava J.A., Alvarez E., Oliveros J.C., Zhao J., Fett C., Perlman S., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faherty C.S., Maurelli A.T. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 2008;16:173–180. doi: 10.1016/j.tim.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau D.J., Meessen-Pinard M., Desforges M., Talbot P.J. Human coronavirus-induced neuronal programmed cell death is cyclophilin d dependent and potentially caspase dispensable. J. Virol. 2012;86:81–93. doi: 10.1128/JVI.06062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowczyc M.A., Mobili P., Garrote G.L., Abraham A.G., De Antoni G.L. Protective action of Lactobacillus kefir carrying S-layer protein against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2007;118:264–273. doi: 10.1016/j.ijfoodmicro.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Hynonen U., Palva A. Lactobacillus surface layer proteins: structure, function and applications. Appl. Microbiol. Biotechnol. 2013;97:5225–5243. doi: 10.1007/s00253-013-4962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller N., Ozmadenci D., Ichim G., Stupack D. Caspase-8 function, and phosphorylation, in cell migration. Semin. Cell Dev. Biol. 2018;82:105–117. doi: 10.1016/j.semcdb.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Kim Y., Lee C. Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology. 2014;460(461):180–193. doi: 10.1016/j.virol.2014.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Eckmann L., Savidge T.C., Lowe D.C., Witthoft T., Kagnoff M.F. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Invest. 1998;102:1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Zhao K., Wang G., Dong B., Zhao J., Tang B., Lu H., Gao W., Chang L., Jin Z., Gao F., He W. Porcine hemagglutinating encephalomyelitis virus induces apoptosis in a porcine kidney cell line via caspase-dependent pathways. Virus Res. 2013;176:292–297. doi: 10.1016/j.virusres.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus. Virol. J. 2015;12:193. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Lee C. Porcine deltacoronavirus induces caspase-dependent apoptosis through activation of the cytochrome c-mediated intrinsic mitochondrial pathway. Virus Res. 2018;253:112–123. doi: 10.1016/j.virusres.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Ye X., Wang Z., Yu Q., Yang Q. Effects of S-layer proteins from lactobacillus against Salmonella typhimurium adhesion and invasion on Caco-2 cells. Acta Microbiol. Sin. 2010;50:1226–1231. [PubMed] [Google Scholar]
- Li P., Yin Y., Yu Q., Yang Q. Lactobacillus acidophilus S-layer protein-mediated inhibition of Salmonella-induced apoptosis in Caco-2 cells. Biochem. Biophys. Res. Commun. 2011;409:142–147. doi: 10.1016/j.bbrc.2011.04.131. [DOI] [PubMed] [Google Scholar]
- Li P., Ye X., Yang Q. Antagonistic activity of Lactobacillus acidophilus ATCC 4356 S-layer protein on Salmonella enterica subsp. enterica serovar Typhimurium in Caco-2 cells. Ann. Microbiol. 2012;62:905–909. [Google Scholar]
- Martinez M.G., Prado Acosta M., Candurra N.A., Ruzal S.M. S-layer proteins of Lactobacillus acidophilus inhibits JUNV infection. Biochem. Biophys. Res. Commun. 2012;422:590–595. doi: 10.1016/j.bbrc.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming-Ming H., Hai-Dong Y., Long-Jun G., Jian-Fei C., Li F., Yu-E W., Hui-Ying R. Induction of apoptosis in Vero-E6 cells infected with porcine epidemic diarrhea virus. Chin. J. Prev. Vet. Med. 2014;36:926–929. [Google Scholar]
- Philpott D.J., Girardin S.E., Sansonetti P.J. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr. Opin. Immunol. 2001;13:410–416. doi: 10.1016/s0952-7915(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Prado Acosta M., Ruzal S.M., Cordo S.M. S-layer proteins from Lactobacillus sp. Inhibit bacterial infection by blockage of DC-SIGN cell receptor. Int. J. Biol. Macromol. 2016;92:998–1005. doi: 10.1016/j.ijbiomac.2016.07.096. [DOI] [PubMed] [Google Scholar]
- Qian Z., Xiao-Juan C., Ya-Ling X., Qing X., Zhong-Bin C. PEDV coronavirus induces apoptosis through activation of Caspase-3 by papain-like protease. Chin. J. Biochem. Mol. Biol. 2015;31:1171–1178. [Google Scholar]
- Vadillo-Rodriguez V., Busscher H.J., van der Mei H.C., de Vries J., Norde W. Role of lactobacillus cell surface hydrophobicity as probed by AFM in adhesion to surfaces at low and high ionic strength. Colloids Surf. B Biointerfaces. 2005;41:33–41. doi: 10.1016/j.colsurfb.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Zhang J., Gao J., Guo Y., Wu Z., Pan D. Extraction of Lactobacillus acidophilus CICC 6074 S-Layer proteins and their ability to inhibit enteropathogenic Escherichia coli. Curr. Microbiol. 2017;74:1123–1129. doi: 10.1007/s00284-017-1291-1. [DOI] [PubMed] [Google Scholar]