Abstract
The features of autoantibodies (autoAb) to liver fumarylacetoacetate hydrolase (FAH) elicited in mice infected with mouse hepatitis virus (MHV) were studied by ELISA and western-blot competition assays. All sera tested contained Ab to cryptic FAH epitopes according with results from western-blot tests, whereas ELISA data indicated that some of these same sera did recognize native epitopes of the autoantigen (autoAg). Such differences were detected in individual sera from various mouse strains, and were ascribed to the fact that proteins insolubilized on solid supports expose a variety of conformational and cryptic antigenic determinants.
On the other hand, whereas results from both experimental protocols showed that anti-MHV Ab did not cross-react with the soluble autoAg, the opposite situation did not show analogous results. Thus, binding of autoAb to insolubilized FAH could be inhibited by MHV depending on the mouse serum or the experimental protocol used.
Additionally, a set of synthetic homologous peptides from mouse FAH and various viral proteins was employed to analyze the Ab repertoire of MHV-infected mice. Results indicated that two homologous peptides were recognized by most Ab: the N-terminal sequences (1–10) from FAH and the nucleocapside, both sharing 50% of identity, and sequence 2317–2326 of the RNA polymerase, a peptide showing 30% of identity with FAH 11–20.
Results indicated that MHV-infection triggers at least three distinct Ab populations: anti-MHV, anti-FAH and cross-reacting Ab. This cross-reaction implies either sequential or conformational epitopes from both the viral proteins and the autoAg and may differ between individuals.
Keywords: Autoantibody, Autoimmune response, Cryptic epitopes, Fumarylacetoacetate hydrolase, Mouse hepatitis virus
1. Introduction
Viruses have been implicated in the generation of autoimmune disorders over the last 20 years [1], [2], [3]. It has been proposed that these infectious agents trigger an autoimmune humoral response by diverse mechanisms, including polyclonal B-lymphocyte activation, release of sequestered autoantigens (autoAg), antigenic mimicry, modification of self-antigen, epitope spreading of the anti-viral immune response or enhancement of major histocompatibility complex molecule expression [2], [4], [5].
Mouse hepatitis viruses (MHV) are known to be lymphotropic and, depending on the viral strain and also on the mouse genetic background, they induce diverse alterations of the immunologic system [6], [7], [8]. The MHV strain A59 (MHV-A59) is a coronavirus that triggers various pathologies in susceptible mice, including hepatitis and thymus involution, IgG2a-restricted hypergammaglobulinaemia and transient demyelination [8], [9].
In a previous paper we reported the presence of autoantibodies (autoAb) in sera from various mouse strains after MHV-infection [10]. The autoAb were directed to a 40-kDa protein present in mouse liver and kidney. The autoAg was isolated and identified as fumarylacetoacetate hydrolase (FAH), a soluble cytosolic enzyme that mediates the hydrolytic formation of fumarate and acetoacetate [10]. Furthermore, during FAH purification we found that the autoAb detected more weakly another liver protein, which turned to be the enzyme alcohol dehydrogenase (ADH). There was no correlation between the IgG titers and the presence of autoAb to liver FAH in CBA/Ht mice, suggesting that the autoAb production was not related to the non-specific polyclonal activation of B-lymphocytes produced after viral infection. Additionally, mice immunized with extracts of mouse liver did not develop autoAb, thus discarding the release of a self-antigen as the mechanism involved in the autoimmune response elicited by MHV-infection [10].
Since molecular mimicry of viral antigens with self determinants seemed to be the mechanism involved in the MHV-induction of autoAb to liver FAH, we explored the putative cross-reaction between the enzyme and MHV proteins. ELISA and western-blot competition assays, as well as Ab reactivity with synthetic peptides from both FAH and viral proteins, indicated that the autoAb recognized a wide range of cryptic and conformational epitopes of the antigen and that the cross-reaction showed by the anti-MHV Ab could be different between individuals.
2. Materials and methods
2.1. Mice
Female CBA/Ht and BALB/c mice were bred in isolators at the Ludwig Institute for Cancer Research (Brussels Branch) by Dr G. Warnier and used for experiments at the age of 8–10 weeks. Their microbiological status was described previously [11].
2.2. Viral infection
Mice were inoculated intraperitoneally with 104 50% tissue culture infectious doses (TCID50) of MHV A59, grown in NCTC 1469 cells [9]. Efficiency of MHV infection was checked by testing anti-viral Ab by ELISA [9].
2.3. Purification of rat liver FAH
The enzyme was prepared as previously described [10]. Briefly, livers from 90-day-old Wistar rats were homogenized in 5 vol (v/w) of chilled 0.3 M sucrose, 5 mM Tris/HCl buffer containing 0.5 mM CaCl2, 1 U/ml of trypsin inhibitor and 1 mM PMSF, pH 7.4. After centrifugation at 10,000g for 20 min and then at 100,000g for 1 h, ethyl alcohol was added to the supernatant as to obtain a final concentration of 50% ethanol. This mixture was allowed to stand overnight at 4 °C and then centrifuged at 16,300g for 15 min. The supernatant was mixed with 95% ethyl alcohol to yield a final alcohol concentration of 70%. After incubating overnight at 4 °C the precipitated enzyme was packed by centrifugation at 16,300g for 15 min.
The pellet was resuspended in 25 mM phosphate buffer pH 7.2 and stirred for 30 min. The supernatant fluid was recovered after centrifugation of the suspension at 16,300g for 10 min and solid ammonium sulfate was added so as to obtain a final salt concentration of 40%. After 1 h at 4 °C and centrifugation at 16,300g for 10 min the pellet was resuspended in 20 mM Tris/HCl pH 8.0 and dialyzed against the same buffer. An aliquot of this solution was chromatographied in a MonoQ HR 10/10 column (Pharmacia LKB Biotechnology) equilibrated with 20 mM Tris/HCl pH 8.0. Proteins were eluted at a flow rate of 1.0 ml/min by using a continuous 0–0.2 M NaCl gradient.
Effluent fractions containing the enzyme were concentrated and then applied to a Sephadex G-100 column (1.6 × 70 cm) equilibrated with 5 mM Tris/HCl, 0.15 M NaCl pH 7.4. Elution was carried out at a flow rate of 16 ml/h with the buffer cited before and the protein concentration in the effluent fractions was determined at 280 nm. Fractions were analysed by western-blot as indicated before [10].
2.4. Preparation of MHV stock
The NCTC 1469 adherent cell line derived from normal mouse liver was purchased from the American Type Culture Collection. Cells growing in T-75 bottles were inoculated with MHV A59 virus at a multiplicity of 1 to 5 TCID50/cell. After an adsorption period of 1 h at 37 °C, 15 ml of NCTC 135 medium with 10% fetal calf serum was added to each bottle and incubated at 37 °C. Several cycles of freezing and thawing were used to release the virus 24 h after inoculation. The harvested virus was centrifuged at 400g for 10 min to remove debris and the supernatant was frozen at −70 °C for storage (MHV stock). The same procedure, but without viral inoculation, was carried out to prepare a control cellular stock (NCTC stock).
Virus titration by endpoint method was performed by inoculating serial dilutions of the MHV stock onto cell monolayers in 96-multiwell. After 24 h wells with viral cytopathic effect were counted for each dilution and titer was expressed as 50% tissue infectious doses (TCID50). Before using in western-blot and ELISA assays the virus was inactivated by incubating the MHV stock 1 h at 56 °C [12]. Protein concentration in both MHV and NCTC stocks was determined by Lowry et al. [13].
2.5. Western-blot analysis
Viral and cellular stocks (25–30 μg) or purified FAH (1–2 μg) were subjected to 10% SDS-PAGE [14] and then transferred onto nitrocellulose sheets (Amersham, Buckingghamshire, UK). After reversible staining with Ponceau S to check satisfactory transfer, non-specific Ab-binding sites were blocked by incubating the sheets with 5% non-fat milk in 30 mM Tris, 0.14 M NaCl, 0.1% (v/v) Tween 20, pH 8.0 (TBS-M-T) for 1 h at room temperature with shaking. The strips were then incubated overnight at 4 °C with an Ab dilution in TBS-M-T. After several washings with TBS containing 0.1% Tween 20, bound Ab were revealed with peroxidase-labeled donkey anti-mouse IgG diluted 1:2500 in TBS-M-T (Jackson Immunoresearch Laboratories, Inc, West Grove, PA, USA) and ECL reagents (Amersham, Buckingghamshire, UK). The apparent molecular mass (kDa) of the detected bands was determined using a wide range protein standard (BDH Laboratory Supplies Poole BH15 1TD, UK).
To perform competition experiments the diluted Ab was incubated overnight at 4 °C with the strips in the presence of different concentrations of competitors, i.e., MHV or NCTC stocks, or the purified FAH. The intensity of the bands was quantified by densitometric scan of the autoradiograms and the results expressed as percent of control, i.e. band intensity in the absence of competitor.
2.6. Competition ELISA
ELISA microplates (Nunc Maxi-Sorb) were coated with 100 μl of purified FAH (5 μg/ml) in 0.1 M NaHCO3, pH 8.9, or MHV stock (2 mg/ml) in 50 mM glycine, 30 mM NaCl, pH 9.2. After overnight incubation at room temperature, the plates were washed with phosphate buffer saline (PBS) containing 0.01% Tween 20 (PBS-T) and blocked for 1 h at 37 °C with 0.01 M Tris, 0.13 M NaCl, pH 7.4 (TMS) containing 5% fetal calf serum (TMS-FCS). The plates were then incubated 2 h at 37 °C with the Ab diluted in TMS-FCS and different concentrations of the competitors. After washing with PBS-T, the bound Ab were revealed with peroxidase-labeled donkey IgG anti-mouse IgG (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) diluted 1:5000 in TMS-FCS. As a substrate, ortho-phenylene-diamine-dihydrochloride (OPD, Sigma Chemical Co, St. Louis, MO, USA) with freshly added H2O2 was used. The reaction was stopped after 10 min by addition of 1 M H2SO4. The absorption was measured by ELISA reader (Metertech Inc., Taipei, Taiwan) at 490 nm.
2.7. Immunization of mice with rat liver FAH
Ten-week-old BALB/C mice were immunized subcutaneously on day 0 with 20 μg of FAH in 50 μl of saline, emulsified in an equal volume of complete Freund's adjuvant (DIFCO Laboratories, USA). The animals were boosted on day 15 with the same amount of FAH in incomplete Freund's adjuvant (DIFCO Laboratories, USA) and bled 15 days after the last inoculation.
2.8. Alignment of peptide sequences
LALIGN program (http://www.ch.embnet.org/software/LALIGN_form.html) using two different algorithms or matrices (pam120.mat, blosum80.mat) was utilized to locate multiple matching sub-segments in two protein sequences. Sequences of MHV A59 surface glycoprotein (E2), membrane glycoprotein (E1), nucleocapside (N), RNA-direct RNA polymerase (RNA), hemagglutinin-esterase and 30 kDa non-structural protein were aligned with the mouse liver FAH amino acid sequence. Our minimum criterion for homology was the existence of at least 20% of sequence identity between FAH and each viral protein.
2.9. Peptide synthesis and sera reactivity
Homologous peptides (10 mers) from FAH and viral proteins were synthesized according to the method of Geysen et al. [15] onto activated polyethylene pins, in a standard 96-well microtiter plate format (Mimotopes, San Diego, CA, USA).
Serum reactivity with synthetic peptides was determined by ELISA as follows: immobilized pins were blocked for 1 h at room temperature with PBS, pH 7.2, containing 2% BSA and 0.1% Tween 20. After washing with PBS, pH 7.2, for 10 min at room temperature, pins were incubated overnight at 4 °C in 150 μl of each serum, diluted 1:300 in the above-described blocking buffer. Pins were then washed four times with PBS, pH 7.2, and incubated for 1 h at room temperature with peroxidase-labeled donkey IgG anti-mouse IgG diluted 1:1500 in PBS, pH 7.2, containing 1% FCS and 0.1% Tween 20. After several washes, the bound Ab were detected by incubating the pins for 45 min at room temperature in 200 μl of 0.5 mg/ml 2,2′-azino-bis(3-ethylbenthiazoline-6-sulfonic acid) (ABTS) dissolved in 0.1 M Na2HPO4, 0.1 M citric acid, pH 4.0, containing 0.01% H2O2. The absorption was measured by ELISA reader at 405 nm.
3. Results
3.1. Time course of Ab elicited in MHV-infected mice
As described previously [10], because mouse and rat liver FAH share 97% of sequence identity, we were able to use the purified rat liver enzyme as a substitute of the murine protein. Additionally, trace amounts of rat ADH co-purified with FAH, accounting by about 2% of total proteins [10]. Thus, for the sake of simplicity hereafter we are going to refer only to Ab recognizing rat liver FAH as autoAg.
It was shown that the autoAb appeared as soon as 10 days after MHV-infection and persisted up to 90 days post-infection [10], [16]. Accordingly, Ab directed to the different viral proteins could be detected in sera from MHV-infected mice [8]. Under our experimental conditions, after 15 days of MHV-infection only a band corresponding to the MW of the nucleocapside protein (N) was detected in western-blot assays. Ab to proteins of MW analogous to surface glycoprotein E2 (also called spike glycoprotein, peplomer protein or protein S) and membrane glycoprotein E1 (also termed matrix glycoprotein or protein M) appeared 30 days post-infection and continued to be secreted up to 90 days after the virus inoculation (data not shown).
3.2. AutoAb to native or cryptic FAH epitopes in MHV-infected mice
In a previous work we communicated that the autoAb occurring in MHV-infected mice were directed to cryptic FAH epitopes, since western-blot experiments indicated that simultaneous incubation of sera with either mouse liver extracts or purified rat liver FAH did not affect the autoAb binding to the insolubilized autoAg [10]. In the present paper we further explored this fact adding competition ELISA experiments and testing individual sera from BALB/c and CBA/Ht mouse strains.
Results from Fig. 1 illustrate the different behaviors of the autoAb when tested either by competition ELISA or by western-blot assays. Thus, data from both experimental protocols obtained with a serum from a BALB/c mouse indicated that soluble rat liver FAH did not alter the binding of autoAb to the insolubilized enzyme, corroborating that the autoAb were mainly directed to cryptic epitopes of the autoAg (Fig. 1A and B). However, the soluble enzyme behaved as a good competitor in ELISA when a serum from a CBA/Ht mouse was tested, suggesting the presence of some autoAb to native FAH epitopes (Fig. 1A). In contrast, results from western-blot competition experiments performed with this same mouse serum showed that incubation with FAH in solution failed to inhibit autoAb binding to the insolubilized autoAg (Fig. 1B). Results similar to those presented in Fig. 1 for the BALB/c serum were obtained with three BALB/c and one CBA/Ht sera tested, whereas autoAb from one BALB/c and two CBA/Ht sera behaved as that of the CBA/Ht mouse presented in the same figure.
Fig. 1.
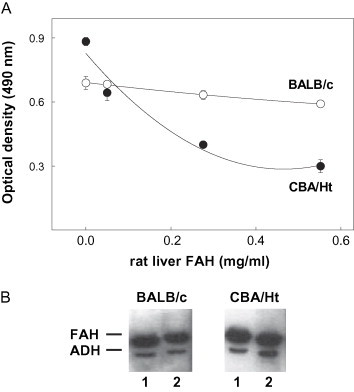
Reactivity of soluble rat liver FAH with the autoAb induced in MHV-infected mice. (A) Competition ELISA assays. Plates coated with rat liver FAH were incubated with 1:100 diluted serum from MHV-infected mice in the presence of different concentrations of the soluble enzyme. Bound Ab were revealed with peroxidase-labeled IgG anti-mouse IgG and OPD. (B) Western-blot competition assays. Rat liver FAH (1 μg) was run in 10% SDS-PAGE, transferred onto nitrocellulose sheets and incubated with a 1:200 diluted serum from MHV-infected mice in the absence (lane 1) or in the presence (lane 2) of soluble FAH (100 μg/ml). Densitometric values, expressed as percentage of intensity in lane 1, were 90% and 99% for sera from BALB/c and CBA/Ht mice, respectively. Results presented in the figure were obtained with sera from a BALB/c or a CBA/Ht mouse 30 days post MHV-infection.
3.3. Reactivity of anti-MHV Ab with rat liver FAH
MHV was insolubilized on ELISA microplates or nitrocellulose sheets and allowed to react with sera from MHV-infected mice in the presence of different concentrations of soluble rat liver FAH. Results obtained with sera from five BALB/c and three CBA/Ht mice showed that the enzyme did not compete for Ab binding to MHV neither in ELISA nor in western-blot competition assays, suggesting that the anti-MHV Ab did not recognize the native epitopes exposed in the soluble autoAg (see representative results in Fig. 2).
Fig. 2.
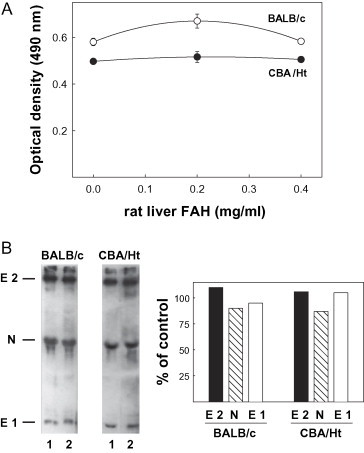
Reactivity of soluble rat liver FAH with anti-MHV Ab. (A) ELISA competition assay: the plates were coated with 2 mg/ml of MHV stock and incubated with a 1:1000 dilution of the indicated mouse serum and different concentrations of FAH in solution. Bound Ab were detected with peroxidase-labeled donkey anti-mouse IgG. (B) Left panel: western-blot competition assay. MHV stock (25 μg) was run in 10% SDS-PAGE, transferred onto nitrocellulose sheets and incubated with a 1:200 dilution of the indicated mouse serum in the absence (lane 1) or presence (lane 2) of 200 μg/ml of rat liver FAH. Right panel: western-blot densitometric analysis. Values for each viral protein are expressed as percentage of band intensity in lane 1. Results shown were obtained with the same mouse sera used in Fig. 1.
3.4. Reactivity of the autoAb with MHV
When the binding of the autoAb to insolubilized rat liver FAH was tested in the presence of MHV as competitor, three different patterns of autoAb reactivity could be distinguished ( Fig. 3). For instance, when sera from three different CBA/Ht mice were tested, results indicated that the virus did not inhibit autoAb binding to the insolubilized enzyme neither in ELISA nor in western-blot experiments (see representative results in Fig. 3A). Conversely, the MHV did compete for autoAb binding to the Ag in both procedures when a serum from a BALB/c mouse was used (Fig. 3B). Lastly, results obtained with four sera from BALB/c mice indicated that the virus could behave as a poor competitor in ELISA but a very good one in western-blot experiments (see representative results in Fig. 3C). In no case NCTC stock, used as a control, produced any effect (data not shown).
Fig. 3.
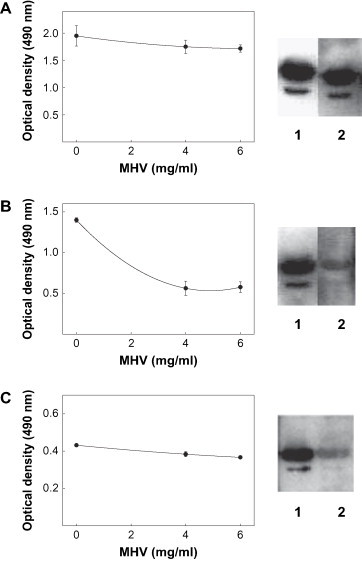
Reactivity of MHV with the autoAb to liver FAH induced in MHV-infected mice. Left panel: competition ELISA. Plates coated with 5 μg/ml of rat liver FAH were incubated with 1:100 diluted serum from MHV-infected mice and different concentrations of MHV stock in solution. Bound Ab were revealed as indicated in Section 2. Right panel: western-blot competition. Rat liver FAH (1 μg) was run in 10% SDS-PAGE, transferred onto nitrocellulose sheets and incubated with a 1:200 diluted serum from MHV-infected mice in the absence of competitor (lane 1) or in the presence of 2.0 mg/ml of protein from MHV stock (lane 2). Densitometric values, expressed as percentage of intensity in lane 1, were 85%, 20% and 25% for, A, B and C, respectively. A: serum from a CBA/Ht mouse 30 days post-infection. B and C: sera from two different BALB/c mice 30 days post-infection.
3.5. Reactivity of Ab from MHV-infected mice with synthetic peptides
Various decapeptides displaying at least 20% identity between the mouse liver FAH sequence and the viral surface glycoprotein E2, the nucleocapside, the membrane glycoprotein E1 or RNA polymerase were prepared using the PEPSCAN method ( Fig. 4).
Fig. 4.
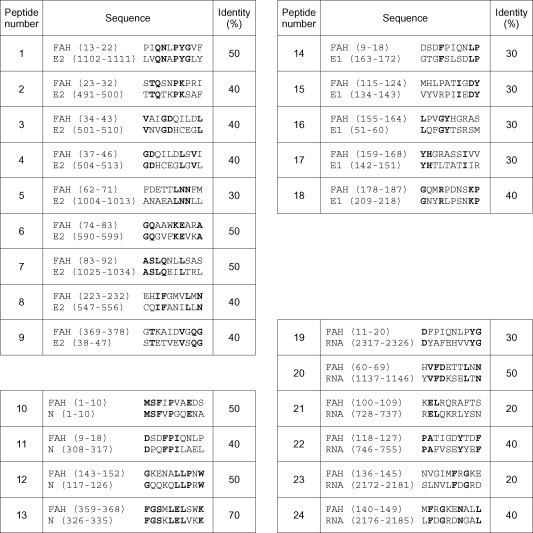
Homologous synthetic peptides from mouse liver FAH and various MHV A59 proteins. Alignment of mouse liver FAH amino acid sequence with MHV A59 proteins was realized with LALIGN program. Left, upper panel: surface glycoprotein E2; left, lower panel: nucleocapside (N); right, upper panel: membrane glycoprotein E1; right, lower panel: RNA-direct RNA polymerase (RNA). Identical residues are shown in bold.
As indicated in Section 2, Ab binding to the insolubilized peptides was determined by ELISA. Fig. 5 shows representative results from three of 10 experiments. All sera tested recognized the nucleocapside sequence 1–10, whatever the time post-infection studied, and most of the Ab showed cross-reaction with the homologous FAH portion, i.e., N-terminal sequence 1–10 (Fig. 4, Fig. 5). Furthermore, Ab from sera 30 and 45 days post-infection displayed quite strong reactivity with peptides from RNA polymerase (sequence 2317–2326) and the FAH peptides (11–20) (Fig. 4, Fig. 5). Results obtained with two homologous peptides from the hemagglutinin-esterase and one from the 30 kDa non-structural MHV-proteins are not shown because of their lack of reactivity with all sera tested.
Fig. 5.
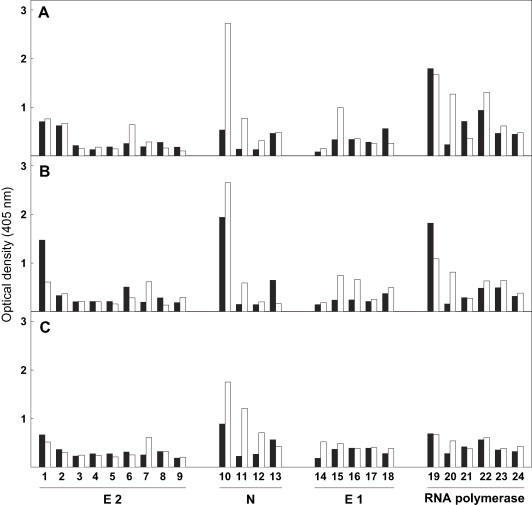
Reactivity of sera from MHV-infected mice with synthetic peptides. Ab binding to the decapeptides showed in Fig. 4 was determined by ELISA as indicated in Section 2. Results are expressed as specific optical density values for Ab binding to sequences corresponding to rat liver FAH (black bars) or the indicated different MHV proteins (white bars). Peptides synthesized on pins were numbered according to Fig. 4. Results presented in A, B and C were obtained with individual sera from three different BALB/c mice after 30, 45 and 90 days of MHV infection, respectively.
3.6. Reactivity of Ab from mice immunized with rat liver FAH
Sera from 10 BALB/c mice immunized with rat liver FAH exhibited strong reactivity towards the insolubilized enzyme when tested by ELISA, but the anti-FAH Ab did not recognize MHV proteins neither in ELISA nor in western-blot assays (data not shown).
Results from ELISA competition assays showed that soluble rat liver FAH strongly inhibited the binding of anti-FAH Ab to the insolubilized Ag, whereas only 50% of competition was observed in western-blot experiments ( Fig. 6). On the other hand, the presence of MHV did not affect the Ab binding in any procedure (Fig. 6).
Fig. 6.
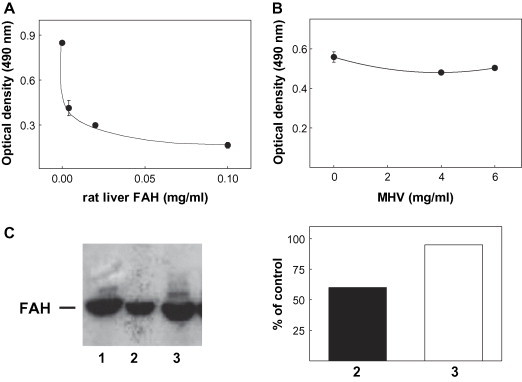
Reactivity of soluble rat liver FAH and MHV stock with anti-FAH Ab induced in mice immunized with rat liver FAH. Upper panel: competition ELISA. Plates coated with rat liver FAH were incubated with 1:100 diluted serum from a mouse immunized with rat liver FAH, in the presence of different concentrations of the soluble enzyme (A) or MHV-stock (B). Bound Ab were revealed with peroxidase-labeled IgG anti-mouse IgG and OPD. Lower panel: Western-blot competition assay. C: Rat liver FAH (1 μg) was run in 10% SDS-PAGE, transferred onto nitrocellulose sheets and incubated with a 1:200 diluted serum from a mouse immunized with rat liver FAH, in the absence (lane 1) or in the presence of soluble FAH (100 μg/ml) (lane 2) or MHV stock (2 mg/ml of total protein) (lane 3). Densitometric values for FAH and MHV stock are expressed as percentage of intensity in lane 1.
In order to investigate the fine specificity of the anti-FAH Ab, sera from six mice immunized with the enzyme were tested for their reactivity towards the homologous FAH/MHV peptides displayed in Fig. 4. Representative results from three different experiments shown in Fig. 7 indicated that the individual responses were diverse. However, although a unique pattern of Ab reactivity could not be found, anti-FAH Ab consistently recognized sequences 13–22 and 359–368 of the enzyme and, depending on the serum tested, bound to various MHV peptides, mainly sequences from E1 and RNA polymerase proteins (Fig. 7).
Fig. 7.
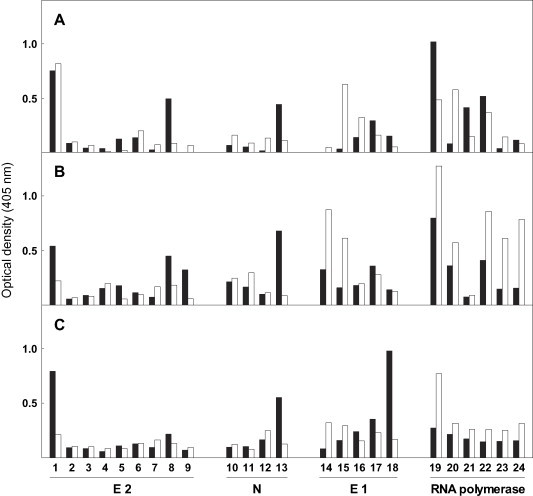
Reactivity of synthetic peptides with sera from mice immunized with rat liver FAH. Ab binding to the homologous decapeptides showed in Fig. 4 was determined by ELISA as indicated in Section 2. Results are expressed as specific optical density values for Ab binding to sequences corresponding to rat liver FAH (black bars) or the indicated different MHV proteins (white bars). Peptides synthesized on pins were numbered according to Fig. 4. Results presented in A, B and C were obtained with individual sera from three different BALB/c mice immunized with rat liver FAH as indicated in Section 2.
4. Discussion
As stated before, mice infected with MHV developed autoAb to mouse liver and kidney fumarylacetoacetate hydrolase (FAH). These Ab bound to liver FAH from various origins, i.e., the rat, sheep and human enzyme, and also recognized rat liver alcohol dehydrogenase (ADH) [10]. In addition, western-blot competition experiments suggested that the autoAb elicited in MHV-infected mice were directed to cryptic epitopes of the Ag [10].
Since hypergammaglobulinaemia or tissue damage as mechanisms triggering the MHV-elicited autoimmune process appeared less likely than molecular mimicry, we undertook competition experiments.
Because individual sera were tested, it could be found that results using ELISA and western-blot competition assays were sometimes different, and that such differences did not depend on the mouse strain utilized. Thus, whereas western-blot assays indicated that autoAb were directed to cryptic FAH epitopes in all sera tested, ELISA results indicated that some of these same sera did recognize native epitopes of the autoAg (Fig. 1). These results could be explained taking into consideration that insolubilized proteins expose cryptic epitopes while still retaining some conformational features [17], [18]. Since proteins submitted to SDS-PAGE and then transferred to nitrocellulose should be more denatured than those insolubilized on plastic surface, it is possible that the autoAb detected both conformational and cryptic epitopes in ELISA and only cryptic determinants in western-blot assays. So, sera showing lack of competition by the soluble autoAg in both protocols should have autoAb directed mostly to cryptic epitopes of the FAH, whereas those displaying dissimilar reactivity may have a mixture of autoAb directed to both hidden and conformational antigenic determinants of the enzyme.
On the other hand, results from ELISA as well as from western-blot competition assays showed that Ab to MHV did not cross-react with the soluble autoAg (Fig. 2). However, results from the opposite situation were not the same. In fact, when MHV stock was used as competitor for binding of autoAb to insolubilized FAH different patterns of reactivity were obtained. ELISA as well as western-blot competition tests showed lack of effect of MHV in solution or, depending on the mouse serum used, both procedures did show an inhibitory activity of the virus (Fig. 3). Furthermore, some sera displayed different behavior in both procedures, MHV being inhibitory in western-blot experiments but not in ELISA assays (Fig. 3).
It is noteworthy to mention than anti-MHV Ab elicited in all mice tested were directed to native epitopes of the viral proteins, because MHV strongly inhibited Ab binding to the insolubilized virus in ELISA as well as in western-blot competition experiments (data not shown).
Molecular mimicry between viral proteins and self-Ag is one of the most probable mechanisms that explain autoimmune responses induced by viral infections [2], [3]. Murine adenovirus, Semliki forest virus, lactate dehydrogenase-elevating virus, herpes simplex virus type-1, hepatitis B virus, encephalomyocarditis virus, Theiler's murine encephalomyelitis virus, Coxsackievirus and cytomegalovirus have been found to mimic physiologically important host proteins [2]. Thus, in order to analyze the Ab repertoire of MHV-infected mice a set of 10 mers homologous peptides corresponding to the sequence of mouse FAH and viral proteins E2, nucleocapside, E1 and RNA polymerase was employed (Fig. 4).
Most Ab recognized the N-terminal sequence (1–10) of both FAH and the nucleocapside, two peptides that share 50% of identity. In addition, Ab from 30 and 45 days post-infection mice bound to the sequence 2317–2326 of the RNA polymerase, a peptide showing 30% of identity with FAH 11–20. Thus, recognition of the FAH sequence 1–20 by Ab elicited by MHV infection could be at least partially responsible of the autoimmune response described herein. This observation should also explain the lack of reactivity of anti-FAH Ab with viral stock. In fact, sera from FAH-immunized mice did not react with the N-terminal sequence 1–10 of the enzyme, and only showed binding to sequence 11–20 or 13–22 (Fig. 4, Fig. 7).
The crystal structure of FAH showed that the protein folds into a 120-residue N-terminal domain of unknown function and a 300-residue C-terminal domain defined by a novel β-sandwich roll structure [19]. In that structure, sequence 1–20 appears as a coil exposed to the solvent, suggesting that autoAb could recognize this structure in the enzyme either insolubilized or in solution.
We cannot discard that sequences other than FAH 1–20 were recognized by the autoAb elicited in MHV-infected mice because we did not test peptides spanning the entire FAH sequence. Moreover, it was reported that similarity of sequences is sometimes not sufficient to mimic epitopes and that structural considerations contribute significantly to the underlying mechanisms of molecular mimicry [3]. Accordingly, results presented in this work indicate that MHV-infection triggers a cross-reaction of either sequential or conformational epitopes from both the viral proteins and the autoAg. Data also suggest that MHV-infected mice could develop at least three different Ab populations: Ab directed either to viral proteins or to the autoAg, and cross-reacting Ab.
The observation that immune responses undergo determinant spreading is a major finding shaping current theories regarding autoimmunity and molecular mimicry [20]. Therefore, immune diversification originated from only a single autoreactive determinant, i.e., a common sequence, could probably provide a pathway for the generation of the multifaceted autoimmune response described in this paper.
The absence of autoimmune disease in our model remains to be explained. In fact, mice infected with MHV-A59 develop acute hepatitis but liver regeneration may take place as early as 10–14 days after infection [6], [8]. Additionally, other MHV-A59 effects, such as thymus involution, the enlargement of the spleen and production of great amounts of IgG2a, are also transient [9], [10], [21]. Thus, in spite of the presence of the autoAb reported in this work, no signs of autoimmune hepatitis were evident.
Our findings remind the response to allo-HPPD (allotype of the hepatocyte enzyme 4-hydroxy-phenylpyruvate dioxigenase, alias F liver protein) that has been long studied [22], [23], [24]. The mouse F antigen is expressed mainly in the liver (10−5 M) but leaks into body fluids at a concentration of ≈10−9 M. It has been proposed that this protein concentration is just sufficient to induce complete tolerance of T cells, whereas B cells are not tolerized [24], [25]. Although the concentration of liver FAH in serum is not known, its similarity in MW and localization with HPPD [10], [24], [25] enabled us to speculate that a T cell effect similar to that proposed by N.A. Mitchinson and colleagues [22], [23], [24], [25] may take place in our model.
Acknowledgements
The authors are indebted to Drs. Pierre L. Masson (ICP, Brussels, Belgium) and Leonor P. Roguin (IQUIFIB, Buenos Aires, Argentina) for helpful discussions and critical revision of the manuscript. This work was supported by grants from CONICET, FONCYT and Universidad de Buenos Aires, Argentina, and Fonds National de la Recherche Scientifique (FNRS), Fonds de la Recherche Scientifique Médicale (FRSM), the State-Prime Minister's Office—S.S.T.C. (interuniversity attraction poles, grant n°44) and the French Community (concerted actions, grant n° 99/04-239), Belgium. J.-P. Coutelier is a research director with the FRNS.
References
- 1.Cohen A.D., Shoenfeld Y. The viral–autoimmunity relationship. Viral Immunol. 1995;8:1–9. doi: 10.1089/vim.1995.8.1. [DOI] [PubMed] [Google Scholar]
- 2.Lawson C.M. Evidence for mimicry by viral antigens in animal models of autoimmune disease including myocarditis. Cell Mol Life Sci. 2000;57:552–560. doi: 10.1007/PL00000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohm A.P., Fuller K.G., Miller S.D. Mimicking the way to autoimmunity: an evolving theory of sequence and structural homology. Trends Microbiol. 2003;11:101–105. doi: 10.1016/s0966-842x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 4.Coutelier J.-P., Coulie P.G., Wauters P., Heremans H., van der Logt J.T.M. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol. 1990;64:5383–5388. doi: 10.1128/jvi.64.11.5383-5388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose N.R. The role of infection in the pathogenesis of autoimmune disease. Semin Immunol. 1998;10:5–13. doi: 10.1006/smim.1997.0100. [DOI] [PubMed] [Google Scholar]
- 6.Kraft L.M. Viral diseases of the digestive system. In: Foster H.L., Small J.D., Fox J.G., editors. The mouse in biomedical research. II Diseases. Academic Press; New York: 1982. pp. 159–191. [Google Scholar]
- 7.Godfraind C., Havaux N., Holmes K.V., Coutelier J.-P. Role of virus receptor-bearing endothelial cells of the blood–brain barrier in preventing the spread of mouse hepatitis virus-A59 into the central nervous system. J Neurovirol. 1997;3:428–434. doi: 10.3109/13550289709031188. [DOI] [PubMed] [Google Scholar]
- 8.Godfraind C., Coutelier J.-P. Morphological analysis of mouse hepatitis virus A59-induced pathology with regard to viral receptor expression. Histol Histopathol. 1998;13:181–199. doi: 10.14670/HH-13.181. [DOI] [PubMed] [Google Scholar]
- 9.Lardans V., Godfraind C., van der Logt J.T.M., Heessen F.W.A., Gonzalez M.-D., Coutelier J.-P. Polyclonal B lymphocyte activation induced by mouse hepatitis virus A59 infection. J Gen Virol. 1996;77:1005–1009. doi: 10.1099/0022-1317-77-5-1005. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu P.A., Gómez K.A., Coutelier J.-P., Retegui L.A. Identification of two liver proteins recognized by autoantibodies elicited in mice infected with mouse hepatitis virus A59. Eur J Immunol. 2001;31:1447–1455. doi: 10.1002/1521-4141(200105)31:5<1447::AID-IMMU1447>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutelier J.-P., van der Logt J.T.M., Heessen F.W.A., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapikian A.Z. The coronavirus. Dev Biol Stand. 1975;28:42–64. [PubMed] [Google Scholar]
- 13.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Geysen H.M., Rodda S.J., Mason T.J., Tribbick G., Schoofs P.G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987;102:259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 16.Mathieu P.A., Gómez K.A., Coutelier J.-P., Retegui L.A. Detection of mouse hepatitis virus infection by assay of anti-liver autoantibodies. J Virol Methods. 2002;106:159–166. doi: 10.1016/s0166-0934(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 17.Gómez K.A., Coutelier J.-P., Mathieu P.A., Lustig L., Retegui L.A. Autoantibodies to cryptic epitopes elicited by infection with lactate dehydrogenase-elevating virus. Scand J Immunol. 2000;51:447–453. doi: 10.1046/j.1365-3083.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- 18.Forastiero R.R., Martinuzzo M.E., Carreras L.O. Binding properties of antibodies to prothrombin and β2-glycoprotein I (β2-GPI) assayed by ELISA and dot blot. Clin Exp Immunol. 1999;118:480–486. doi: 10.1046/j.1365-2249.1999.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timm D.E., Mueller H.A., Bhanumoorthy P., Harp P., Bunick G.J. Crystal structure and mechanism of a carbon–carbon bond hydrolase. Structure. 1999;7:1023–1033. doi: 10.1016/s0969-2126(99)80170-1. [DOI] [PubMed] [Google Scholar]
- 20.Farris A.D., Keech C.L., Gordon T.P., McCluskey J. Epitope mimics and determinant spreading: pathways to autoimmunity. Cell Mol Life Sci. 2000;57:569–578. doi: 10.1007/PL00000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godfrain C., Holmes K.V., Coutelier J.-P. Thymus involution induced by mouse hepatitis virus A59 in BALB/c mice. J Virol. 1995;69:6541–6547. doi: 10.1128/jvi.69.10.6541-6547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukic M.L., Mitchison N.A. Self- and allo-specific suppressor T cells evoked by intravenous injection of F protein. Eur J Immunol. 1984;14:766–768. doi: 10.1002/eji.1830140820. [DOI] [PubMed] [Google Scholar]
- 23.Schneider S.C., Mitchison N.A. Self-reactive T cell hybridomas and tolerance. J Immunol. 1995;154:3796–3805. [PubMed] [Google Scholar]
- 24.Brunner M.C., Mitchison N.A. Regulation by non-major histocompatibility complex genes of the allo-4-hydroxy-phenylpyruvate dioxygenase (F liver protein) response. Immunology. 1996;88:452–455. doi: 10.1046/j.1365-2567.1996.d01-670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nardi N.B., Freitas A.A., Coutinho A. Selection of anti-F protein B-cell repertoires in normal mice. Res Immunol. 1990;141:711–721. doi: 10.1016/0923-2494(90)90002-g. [DOI] [PubMed] [Google Scholar]


