Abstract
Bovine rotavirus (BRoV) and bovine coronavirus (BCoV) are major enteric viral pathogens responsible for calve diarrhoea. They are widespread both in dairy and beef cattle throughout the world and causing huge economic losses. The diagnosis of these agents is very difficult due to non-specific nature of lesions and the involvement of some intrinsic and extrinsic risk factors. We performed postmortem of 45 calves, which was below three months of age. Out of 45 necropscid calves, three (6.66%) cases were positive for BRoV and four (8.88%) cases were found positive for BCoV, screened by reverse transcriptase polymerase chain reaction (RT-PCR). Further RT-PCR positive cases were confirmed by immunohistochemistry (IHC) in paraffin-embedded intestinal tissue sections. Three cases of enteritis caused by BRoV showed the hallmark lesions of the shortening and fusion of villi, denudation and infiltration of mononuclear cells in the lamina propria. The BRoV antigen distribution was prominent within the lining epithelium of the villi, peyer's patches in the ileum and strong immunoreactions in the lymphocytes and some macrophages of the mesenteric lymph nodes. Four cases in which BCoV was detected, grossly lesions characterized by colonic mucosa covered with thick, fibrinous and diphtheritic membrane. Histopathologically, jejunum showed skipping lesion of micro-abscesses in crypts. The BCoV antigen distribution was prominent within the necrotic crypts in the jejunum and cryptic micro-abscesses in the colon and ileum. It is the first report of BRoV and BCoV antigen demonstration in the jejunum, colon, ileum, Peyer's patches and mesenteric lymph nodes of naturally infected calves from India by using IHC.
Keywords: Rotavirus, Coronavirus, RT-PCR, Immunohistochemistory
Highlights
-
•
The present study was to investigation of natural cases of BRoV and BCoV infection causing enteritis in dairy calves.
-
•
Out of 45 necropscid calves, 6.66% cases for BRoV and 8.88% cases for BCoV were found positive.
-
•
BRoV and BCoV antigen demonstration in the jejunum, colon, ileum, Peyer's patches and mesenteric lymph nodes of infected calves by using IHC.
1. Introduction
Livestock sector in India plays a pivotal role in supporting the livelihood of rural masses. This sector alone contributes 4.11% of the total GDP. Economics of any livestock farm dependent on the health of its neonates, which are the future herd [1]. Despite the fact, neonatal calves are abysmally cared, with the result significant morbidity and mortality of these calves incurred huge economic consequences. Amongst various causes, the calf diarrhoea (enteritis) is a commonly reported disease worldwide in cattle. Diarrhoea is a multi-factorial disease, which accounts for 57% of mortality in <1 month old calves [2]. In diarrhoeic calves, although co-infection observed frequently, but at times a single etiology can also be the cause in some cases. Major enteric viral pathogens responsible for calf diarrhoea are mainly Rotavirus and Coronavirus. Of late, newer emerging enteric pathogens such as Torovirus, Norovirus, Nebovirus, Enterovirus, Calcivirus and Parvovirus have also been added to the list of diarrhoea causing agents [3]. Co-infection with more than one pathogen is more frequent and often worsens the symptoms. The tetrad of Rotavirus, Coronavirus, Cryptosporidium, and Escherichia coli accounts for 75–95% of infection in neonatal calves worldwide, of which especially rotavirus and coronavirus account for 27–36% and 20–26% infections, respectively [[4], [5], [6], [7]]. Among all pathogens, rotaviruses are the leading cause of calf diarrhoea, and coronaviruses are a major contributor to it [4,5,8,9]. The coronavirus infect both small intestine and large intestine to cause severe disease [10]. In calves, group A rotavirus and coronavirus, either single or in combination, are predominately associated with neonatal (mostly up to 5–15 day old) diarrhoea [11]. These pathogens, if not causing death in claves, warrant extra care and sometimes intensive care of calves [12]. The diagnosis of diarrhoea (enteritis) cases is cumbersome due to non specific nature of clinical signs/lesions, interaction of polymicrobial agents and involvement of intrinsic and extrinsic risk factors [13].
Rotaviruses mainly replicate in the mature villous enterocytes. Triple protein coat of virus helps them to escape unaffected from the acidic pH of the stomach and the digestive enzymes in the gut. The mature enterocytes of duodenum villi are the first to become infected to release significant number of virions, to favor more severe attack on enterocytes of mid and distal portion of small intestine [14]. Bovine coronavirus is capable of infecting mature epithelium of small intestine and large intestine. Villi of the affected small intestine and colonic crypts become atrophic, and the lamina propria becomes necrotic and lumen of hyperplastic crypts filled with necrotic debris [15]. Necrosis of mesenteric lymph node, payers patches of ileum and formation of cryptic abscesses in colon of calves also the histopathological lesions detected in coronavirus infection [16].The pathological data on bovine coronavirus and rotavirus in India are very limited.We used RT-PCR and IHC to investigate the pathological changes in the of natural cases of bovine rotavirus and coronavirus infected dairy calves.
2. Material and methods
2.1. Tissue samples
During the period from November 2016 to February 2018, total of 45 carcasses of calves (Vrindavani- 36, Tharparker – 4 and HFX -5) were necropsied at Post-mortem Facility of Division of Pathology, Indian Veterinary Research Institute, Izatnagar, Bareilly, India. These calves have history of enteritis. The calves were below three month of age (up to one month −20 calves, two months – 20 calves and up to three month −5 calves). Samples of intestinal contents and different tissues viz. intestine, mesenteric lymph nodes, spleen, liver, lungs, kidneys and heart were collected in 10% neutral buffered formalin (NBF) and in RNA later. The samples collected in RNA later were stored at −20 °C for molecular study.
2.2. Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from collected tissue (small intestine with content) samples using commercial TRIzol® Reagent (Thermo Fisher Scientific, USA) as per manufacture's protocol. All extracted RNA samples were quantified by NanoVue plus (Thermo Fisher Scientific, USA) and the purity of RNA was checked by A260/280 and A260/230 ratio. The cDNA was synthesized from total RNA by using High-capacity cDNA reverse transcription kits (Applied Biosystems). The synthesized cDNA was stored at −20 °C till further use. The amplification of N gene of coronavirus and VP6 gene of rotavirus was carried out via RT-PCR. The self designed primer Sequence(5'--3′)for bovine coronavirus BCoV; F-TGACGAGCCCCAGAAGGATGT and BCoV; R- GACCACCTGACGCTGTGGTT have amplicon size 127 bp and the primer Sequence(5'--3′) for rotavirus A,RVA; F-TTTGATCACTAATATTCACC and RVA; R- GGTCACATCCTCTCACTA have amplicon size 227 bp was used in the study [17]. PCR reaction was carried out in 0.2 ml PCR tubes containing reaction mixture of 6.0 μL of PCR Master Mix 2× (Takara, City), 0.5 μL of forward primer (10 pmol/μL) and reverse primer (10 pmol/μL), 1 μL of cDNA (200 ng/μL) 12.00 μl of Nuclease-free water. The tubes were then placed in a thermocycler and PCR cycling conditions were used with initial denaturation of 95 °C for 5 min, followed by 35 cycles [denaturation (94 °C, 10 s); annealing (48 °C, 20 s), extension on (72 °C, 30 s)] and a single cycle of final extension at 68 °C for 7 min. Amplified products were resolved by agarose gel electrophoresis (1% w/v) at 100 V for 1 h in TAE buffer with 0.5 μg/mL ethidium bromide and viewed under UV transilluminator (Geldoc, USA).
2.3. Histopathological examination
After proper fixation in 10% NBF, tissues were cut into small sections with thickness of 2–3 mm and embedded in the paraffin by standard procedures. The paraffin embedded tissues were cut into 5–6 μm thick section and stained with hematoxylin and eosin [18].
2.4. Immunohistochemistory
The immunohistochemistory (IHC) was carried out in paraffin sections after deparaffinisation and rehydration. The slides were treated with 0.3% Hydrogen peroxide in methanol solution for 20 min to quench the endogenous peroxides, thoroughly rinsed thrice with 1× PBS (pH7.2), 5 min each. Antigen unmasking was performed by subjecting the tissue sections to microwave irradiation in a coplin jar containing 0.01 M citrate buffer (pH 6.0) for 15 min. Non-specific binding was blocked with 5% normal goat serum (200–400 μl) (Sigma Chemicals,USA) in PBS for 30 min at room temperature in a humidifier chamber. The slides were washed thrice (5 min each) using PBS (pH 7.2). For immunostaining, antibody was titrated for optimum concentration and sections were covered with 100–150 μl of primary monoclonal antibodies (1:100, mouse monoclonal IgG2a RVA capsid protein and 1:100, mouse monoclonal IgG2a coronavirus capsid antigen, Santa Cruz Biotechnology, USA), in PBS containing 1% BSA (Sigma Chemicals, USA). The negative controls were covered with 1% BSA in PBS only. The sections were tilted in two different directions before being incubated overnight at 4 °C in a humid chamber. The slides were washed with PBS for 3 times (5 min each) with slow and continuous stirring. After wiping around the section carefully, Goat anti-mouse IgGs secondary antibodies HRP conjugated (Invitrogen) diluted at 1:500 were applied to cover the moist section and incubated for 1 h in a humid chamber. Following three PBS (pH 7.2) washing of 5 min each, the section was covered with 3, 3-diaminobenzidine (DAB, Sigma Chemicals, USA) as a substrate, which gave brick red (reddish brown) colour. DAB substrate solution was prepared by adding 1 drop of DAB chromogen to 1 ml of DAB buffer. The reaction was terminated before generalized background staining appeared in the negative controls by rinsing in double DW. Sections were then counter stained for 1–2 min with Mayer's haematoxylin. After dehydrating in ascending grades of alcohol, sections were mounted with cover slips using DPX mountant.
3. Results
In 45 necropscid calves, three (6.66%) cases were positive for rotavirus and four (8.88%) cases were found positive for coronavirus by employing RT-PCR (Fig. 1, Fig. 2 respectively) and IHC. All seven cases (rotavirus-3 and coronavirus-4) were found positive in below one month of age of calves. In three cases, where rotavirus was identified, grossly the serosa of small and large intestine was severely congested and the lumen filled with blood mixed catarrhal contents. The pin head sized hemorrhagic spots were found on the mucosal surfaces of the jejunum and the ileum. Mesenteric lymph nodes were swollen, severely congested and edematous. Histopathological examination of the ileum showed fusion and sloughing of villi enterocytes and severe engorgement of capillary plexus and mononuclear cells (mainly lymphocytes and few macrophages) infiltration in the lamina propria (Fig. 3 , a).The crypt epithelium was found proliferated. The Peyer's patches were edematous and congested and lymphoid cells were depleted and necrosed (Fig. 3, b). The mesenteric lymph nodes showed engorged vessels and depletion of lymphoid follicles and with moderate deposition of fibrin in the cortex and the medulla (Fig. 3, c).We observed immunohistochemical staining for rotavirus in all three calves which were positive from RT-PCR. The rotavirus antigen distribution (dark brown signals) was prominent within the lining epithelium of the villi along with peyer's patches of the ileum (Fig. 4 c). We also detected strong immunohistochemical staining for rotavirus in the lymphocytes and some macrophages of mesenteric lymph nodes (Fig. 4b).
Fig. 1.
PCR amplification of VP6 gene of RVA showing 227 bp size amplicon in 1.5% agrose gel. Lane 1 loaded 1 kb plus DNA ladder; samples in lane 2, 3 and 4 are positive samples, lane 5 as non template control.
Fig. 2.
PCR amplification of N gene of bovine coronavirus showing 172 bp size amplicon in 1.5% agrose gel. Lane 2 loaded 1 kb plus DNA ladder, samples in lane 3, 4, 5 and 6 are positive samples and lane 1 as positive control plasmid 172 bp.
Fig. 3.
Histopathology of rotavirus infected calves: Paraffin sections of a calf (41A/17, 537A/16) stained with H & E stain showing exfoliation of villi, capillary plexus engorgement, moderate infiltration in the lamina propria (a) and Peyer's patches depletion (b) in the ileum. The section of mesenteric lymph node showing edema/congestion, medullary cords and sinuses infiltrated with MNCs (c). H&E.
Fig. 4.
Immunohistochemistry of bovine rotavirus infection in bovine calves:a) Antibody control; b)The RVA antigen distribution in calves (41A/17, 537A/16) is seen as reddish brown signals (DAB as chromogen, arrows) in paraffin sections of mesenteric lymph nodes(b) (MLN) (Inset, 400X) and c) Peyer's patches (PP), Mayer's haematoxylin used as counter stain.
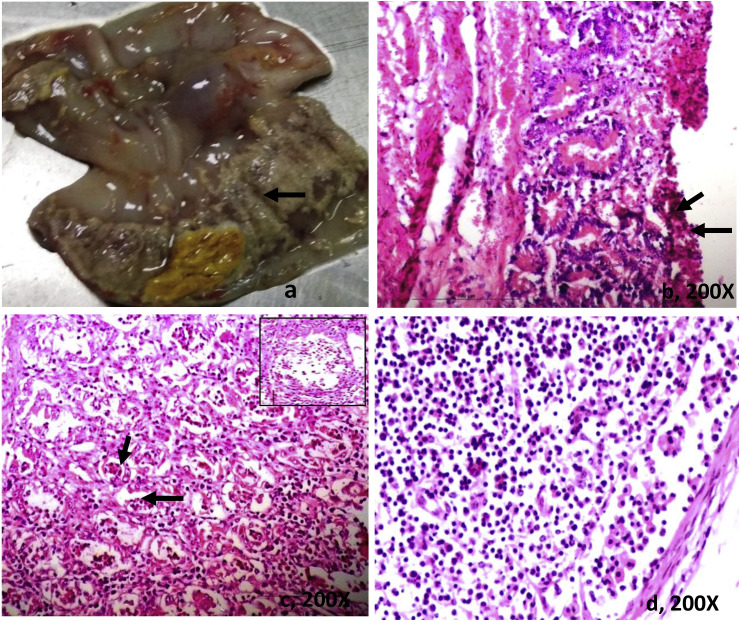
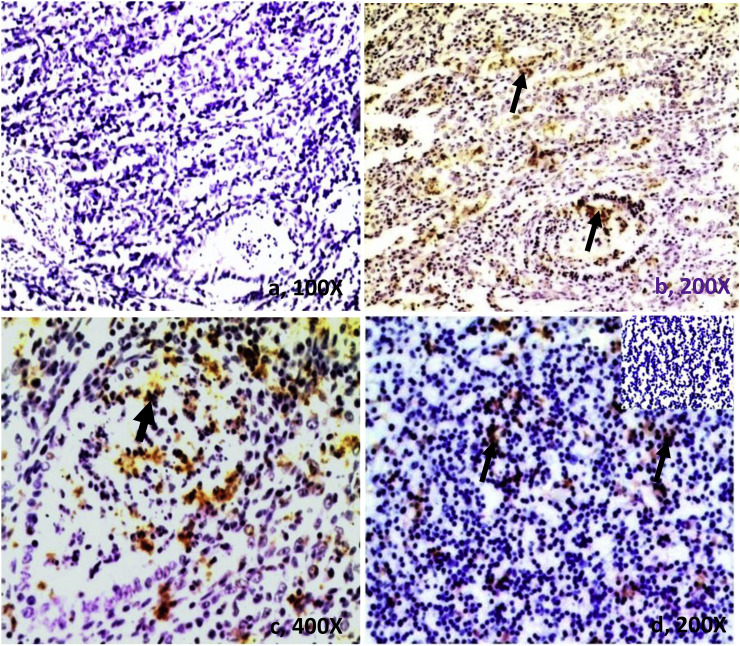
Four cases in which coronavirus was detected, grossly reddish white necrotic ulcers were seen on serosal surface of the colon. The ileum and mesenteric lymph nodes were markedly congested and swollen. The colonic mucosa was covered with thick, fibrinous diphtheric membrane (Fig. 5 , a). Microscopically, the villi of the ileum were sloughed off at several places and their lamina propria was severely engorged/hemorrhagic and infiltrated with mononuclear cells. There was thick homogeneous necrotic material adhered upon upper mucous surface of the colon (Fig. 5, b). Mesenteric lymph nodes showed moderate depletion of many cortical follicles (Fig. 3, d). In other one case, grossly, whole small and large intestinal tract was moderately congested and filled with yellowish faecal material. Mesenteric lymph nodes were juicy, enlarged and slightly congested. Histopathological examination of the jejunum showed severs infiltration of mononuclear cells in the lamina propria and crypts showed skipping lesion of microabscesses (Fig. 5, c). Crypt lining cells were slightly hyperplastic. The capsule of mesenteric lymph nodes was thick and lymphoid follicles were necrosed. We observed immunohistochemical staining for coronavirus antigen in all four calves which was positive from RT-PCR. The BCoV antigen distribution was prominent within the lining epithelium of the villi, necrotic crypts in the jejunum and cryptic microabscesses in the colon and ileum (Fig. 6 ). We also detected strong immunoreactions in the mononuclear cells of mesenteric lymph nodes (Fig. 6d).
Fig. 5.
Gross and histopathological lesions of corona virus infected calf: Gross image of the colon (40A/18) showing thick necrotic bran -like diphtheritic deposits over the mucosa (a, arrow). Microscopically, thick homogeneous necrotic material is seen over the ulcerated mucosa of the colon (b, arrows). Crypt lining cells are necrotic (c, arrows) and the lamina propria infiltrated with MNCs. Microabscesses in the crypt (c, inset). Mesenteric lymph nodes showed moderate depletion of many cortical follicles with thick capsule (d). H & E.
Fig. 6.
Immunohistochemistry of bovine coronavirus in tissues section of calves: a) antibody control). The bovine coronavirus (BCoV) antigen distribution is seen as dark brown (DAB as chromogen) positive signals in lining epithelial cells of villi and necrotic crypts in the jejunum (thin arrows, b) and microabscesses (thick arrow, c) respectively and d) In MNCs of mesenteric lymph nodes (MLN) also showed positive signals of BCoV antigen (arrows) (inset, antibody control no signals).
4. Discussion
Diarrhoea in calves is a multifactorial disease that results into hypersecretion of fluid, malabsorption due to disruption of enterocytes, exudation of protein due to increased capillary/epithelial permeability or hypermotility leading to approximately 75% of the calf mortality under 3-weeks-age [19]. The recovered cases of diarrhoea might suffer with production losses even more than anticipated. The diarrhoea due to rotavirus and coronavirus in infants of humans, neonates of mammalian and avian species is a worldwide problem. The age has been found to be the greatest influencing factor in causing rotavirus and coronavirus infections. In our study calves below 1-month of age suffered with these pathogens. The same has been described by previous workers for occurrence of rotaviral and coronavirus diarrhoea in neonatal calves [20]: [21,22]. The reason for high occurrence of these pathogens below one-month of age could be due to less developed immune system in neonates and the lack of adequate amount of maternal antibodies in the colostrum [23], so the free sites are available for attachment of virus to sialic acid or galactosidase receptors of enterocytes. These receptors disappear with the increasing age of neonates [24].
Among 45 deaths of cattle calves, 3 cases of enteritis were of due to rotavirus as demonstrated by the hallmark lesions of the shortening and fusion of villi, denudation, and the presence of cuboidal enterocytes, these lesions were very similar to those described by earlier workers in rotavirus infected calves [8,25,26]. Similarly, the extra-intestinal lesions in mesenteric lymph nodes (lymphocytes depletion and antigen detection by IHC) were also matched with the findings of previous workers, who described the depletion of lymphocytes from the germinal centers of lymphoid follicles of mesenteric lymph nodes and detection of rotavirus antigen by direct immunofluorescence technique in calves [27,28]: [29]. The rotavirus replication in macrophages have also been demonstrated in such studies, indicating a possible mechanism of rotaviral spread via the lymphatic of lamina propria to extra-intestinal organs.
In four cases of coronavirus necrotic enteritis, the jejunum lesions included sever capillary bed engorgement/hemorrhage, chronic cells infiltration in villi, scattered crypt microabscesses, crypt lining cells hyperplasia, and lymphoid necrosis/depletion in draining mesenteric lymph nodes were akin to the lesions described by others in coronavirus enteritis in calves [15,16,30]. The histopathological lesions of coronavirus were colocalized with viral capsid protein in the jejunum and mesenteric lymph nodes, indicated that the lesions observed were due to coronavirus infection and replication. In present study the bovine coronavirus antigen distribution was prominent within the lining epithelium of the villi, jejunum, ileum, colon and mesenteric lymph nodes which was accordance with [31]; who demonstrated coronavirus antigen in intestine of calves by immunohistochemistory, which was negative for RT-PCR. It is the first report of BRoV and BCoV antigen demonstration in the jejunum, colon, ileum, Peyer's patches and mesenteric lymph nodes of naturally infected calves from India by using immunohistochemistory. Overall, the findings suggest that, the BRoV and BCoV caused calf diarrhoea with specific lesions in the intestines (enteritis) and mesenteric lymph nodes. These lesions might have resulted into morbidity and mortality in bovine calves below 3 weeks of age. Further studies are needed to elucidate the pathogenesis by which BRoV and BCoV spread beyond the intestine and cause disease other than enteritis. Understanding the immuno-pathogenesis at subtle level would further enhance our knowledge in adopting better measures to control the BRoV and BCoV associated diarrhoea in bovine calves.
Declaration of competing interest
All the authors declare no conflict of interest.
Acknowledgements
The authors are thankful to the Director and Joint Director (Research) of the Institute for providing the funds and facilities to carry out this work.
References
- 1.Razzaque M.A., Bedair M., Abbas S. Performance of pre-weaned female calves confined in housing and open environment hutches in Kuwait. Pak. Vet. J. 2009;29:1–4. [Google Scholar]
- 2.USDA.Dairy . USDA-APHIS-VS, CEAH; Fort Collins: 2007. Part II: Changes in the U.S. Dairy Cattle Industry, 1991-2007; pp. 57–61. 2008. [Google Scholar]
- 3.Cho Y.I., Yoon K.J. An overview of calf diarrhea-infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014;15(1):1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhama K., Chauhan R.S., Mahendran M., Malik S.V.S. Rotavirus diarrhea in bovines and other domestic animals. Vet. Res. Commun. 2009;33(1):1–23. doi: 10.1007/s11259-008-9070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumusova S.O., Yazici Z., Albayrak H., Meral Y. Rotavirus and coronavirus prevalence in healthy calves and calves with diarrhea. Med. Vet. 2007;63:62–64. [Google Scholar]
- 6.Malik S.V.S., Barbuddhe S.B., Rawool D.B., Vaidya V.M., Sahare A.M. CAB International; Wallingford, UK: 2005. Data Sheet on Rotaviruses (Global Status of Rotavirus Infections in Man and Animals). Animal Health and Production Compendium. [Google Scholar]
- 7.Singh S., Singh R., Kamdi B.P., Kasyap G., Singh R., George N., Kumar P., Singh V. Occurrence and pathology of cryptosporidium in bovine calves of North and Central India. J. Anim. Res. 2018;8(5):925. [Google Scholar]
- 8.Kashyap G., Singh R., Malik Y.S., Agrawal R.K., Singh K.P., Kumar P., Singh R., Gupta D. Rotavirus A associated pathology of intestine and mesenteric lymph nodes and occurrence in bovine calves of Gwalior and Bareilly regions. Indian J. Anim. Res. 2018;52(1):99–104. [Google Scholar]
- 9.Uhde F.L., Kaufmann T., Sager H., Albini S., Zanoni R., Schelling E., Meylan M. Prevalence of four enteropathogens in the faeces of young diarrhoeic dairy calves in Switzerland. Vet. Rec. 2008;163(12):362–366. doi: 10.1136/vr.163.12.362. [DOI] [PubMed] [Google Scholar]
- 10.Boileau M.J., Kapil S. Bovine coronavirus associated syndromes. Vet. Clin. N. Am. 2010;26(1):123–146. doi: 10.1016/j.cvfa.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry A.F., Alfieri A.F., Stipp D.T., Alfieri A.A. Bovine coronavirus detection in a collection of diarrheic stool samples positive for group a bovine rotavirus. Braz. Arch. Biol. Technol. 2009;52:45–49. [Google Scholar]
- 12.Gay C.C., Hodgson J.C., Lofstedt J., Boli N.S.R. 2012. Diarrhoea in Neonatal Ruminants: Intestinal Diseases in Ruminants: Merck Veterinary Manual.http://www.merckmanuals.com/vet/print/digestive_system/intestinal_diseases_in_ruminants/diarrhea_in_neonatal_ruminants.html [online]available from: [Google Scholar]
- 13.Athanassious R., Marsolais G., Assaf R., Dea S., Descoteaux J.P., Dulude S., Montpetit C. Detection of bovine coronavirus and type A rotavirus in neonatal calf diarrhea and winter dysentery of cattle in Quebec: evaluation of three diagnostic methods. Can. Vet. J. 1994;35(3):163–169. [PMC free article] [PubMed] [Google Scholar]
- 14.Ramig R.F. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultze B., Gross H.J., Brossmer R., Herrler G. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J. Virol. 1991;65:6232–6237. doi: 10.1128/jvi.65.11.6232-6237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jubb K., Palmers . vol. 1. 2007. pp. 546–550. (Patholoy of Domestic Animals). [Google Scholar]
- 17.Mondal A., Sharma K., Malik Y.S., Joardar S.N. Detection of group a rotavirus in faeces of diarrhoeic bovine porcine and human population from eastern India by reverse transcriptase–polymerase chain reaction. Adv. Anim. Vet. Sci. 2013;1(1S):18–19. [Google Scholar]
- 18.Luna L.G. 3 edn. McGraw Hill Book Co. 12-17; New York: 1972. Histological Staining Methods of the Armed Forces Institute of Pathology; pp. 32–37. 159. [Google Scholar]
- 19.McGavin M.D., Zachary J.F. fourth ed. Mosby Elsevier; St. Louise, Missouri: 2007. Pathological Basis of Veterinary Disease. [Google Scholar]
- 20.Bellinzoni R.C., Mattion N.M., Burrone O., Gonzalez A., La Torre J.L., Scodeller E.A. Isolation of group A swine rotaviruses displaying atypical electropherotypes. J. Clin. Microbiol. 1987;25(5):952–954. doi: 10.1128/jcm.25.5.952-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dash S.K., Tewari A., Kumar K., Goel A., Bhatia A.K. Detection of Rotavirus from diarrhoeic cow calves in Mathura, India. Vet. World. 2011;4(12):554–556. [Google Scholar]
- 22.Radostits O.M., Gay C.C., Hinchcliff K.W., Constable P.D. tenth ed. Saunders; Philadelphia: 2007. Veterinary Medicine. [Google Scholar]
- 23.Windeyer M.C., Leslie K.E., Godden S.M., Hodgins D.C., Lissemore K.D., LeBlanc S.J. The effects of viral vaccination of dairy heifer calves on the incidence of respiratory disease, mortality, and growth. J. Dairy Sci. 2012;95(11):6731–6739. doi: 10.3168/jds.2012-5828. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan R.S., Dhama K., Mahendran M. Pathobiology of rotaviral diarrhea in calves and its diagnosis and control: a review. J. Immunol. Immunopathol. 2008;10(1):1–13. [Google Scholar]
- 25.Gruenberg W. Diarrhoea in neonatal ruminants. In: Kahn C., L S., editors. The Merck Veterinary Manual. 2014. (Whitehouse Station, NJ, USA) [Google Scholar]
- 26.Reynolds D.J., Hall G.A., Debney T.G., Bunch K.J., Parsons K.R. Pathology of natural rotavirus infection in clinically normal calves. Res. Vet. Sci. 1985;38:264–269. doi: 10.1016/S0034-5288(18)31791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashyap G., Singh R., Malik Y.S., Agrawal R.K., Singh K.P., Kumar P., Sahoo M., Gupta D., Singh R. Experimental bovine rotavirus-A (RV-A) infection causes intestinal and extra-intestinal pathology in suckling mice. Microb. Pathog. 2018;121:22–26. doi: 10.1016/j.micpath.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.J., Park J.G., Matthijnssens J., Lee J.H., Bae Y.C., Alfajaro M.M., Park S.I., Kang M.I., Cho K.O. Intestinal and extra-intestinal pathogenicity of a bovine reassortant rotavirus in calves and piglets. Vet. Microbiol. 2011;152(3–4):291–303. doi: 10.1016/j.vetmic.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Crawford S.E., Patel D.G., Cheng E., Berkova Z., Hyser J.M., Ciarlet M., Finegold M.J., Conner M.E., Estes M.K. Rotavirus viremia and extraintestinal viral infection in the neonatal Rat model. J. Virol. 2006;80(10):4820–4832. doi: 10.1128/JVI.80.10.4820-4832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.J., Kim G.Y., Choy H.E., Hong Y.J., Saif L.J., Jeong J.H., Park S.I., Kim H.H., Kim S.K., Shin S.S., Kang M.I. Dual enteric and respiratory tropisms of winter dysentery bovine coronavirus in calves. Arch. Virol. 2007;152(10):1885–1900. doi: 10.1007/s00705-007-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Field P.M., Newsletter A.H.L. Bovine coronavirus (BCoV) and rotavirus (BRV) – on the increase? AHL Newsl. 2015;19:3. [Google Scholar]