Abstract
The Developing Countries Vaccine Manufacturers’ Network (DCVMN) gathered leaders in immunization programs, vaccine manufacturing, representatives of the Argentinean Health Authorities and Pan American Health Organization, among other global health stakeholders, for its 17th Annual General Meeting in Buenos Aires, to reflect on how vaccines are shaping global health. Polio eradication and elimination of measles and rubella from the Americas is a result of successful collaboration, made possible by timely supply of affordable vaccines. After decades of intense competition for high-value markets, collaboration with developing countries has become critical, and involvement of multiple manufacturers as well as public- and private-sector investments are essential, for developing new vaccines against emerging infectious diseases. The recent Zika virus outbreak and the accelerated Ebola vaccine development exemplify the need for international partnerships to combat infectious diseases. A new player, Coalition for Epidemic Preparedness Innovations (CEPI) has made its entrance in the global health community, aiming to stimulate research preparedness against emerging infections. Face-to-face panel discussions facilitated the dialogue around challenges, such as risks of viability to vaccine development and regulatory convergence, to improve access to sustainable vaccine supply. It was discussed that joint efforts to optimizing regulatory pathways in developing countries, reducing registration time by up to 50%, are required. Outbreaks of emerging infections and the global Polio eradication and containment challenges are reminders of the importance of vaccines’ access, and of the importance of new public-private partnerships.
Keywords: Vaccine access, Immunization, Developing countries, Quality, Regulatory convergence
1. Introduction
The Developing Countries Vaccine Manufacturers’ Network (DCVMN) is the world’s largest vaccine-industry alliance. In 2016, 50 corporate members are working to provide high-quality vaccines, and contribute to global health initiatives, ensuring uninterrupted vaccine supply to countries, to advance eradication of polio and facilitate response to emerging infectious diseases (EIDs) or outbreaks like the Zika outbreak [1].
The 17th Annual General Meeting of the DCVMN was held, in Buenos Aires, Argentina, hosted by Sinergium Biotech. Nearly 200 professionals working in vaccine research, development, manufacturing and supply attended, including representatives from the global health organizations such as the Pan-American Health Organization (PAHO), Gavi: the vaccine alliance, World Health Organization (WHO), United Nations International Children’s Fund (UNICEF), Biomedical Advanced Research and Development Authority (BARDA), Clinton Health Access Initiative (CHAI), International Vaccine Institute (IVI), Médecins Sans Frontières (MSF), PATH, Bill & Melinda Gates Foundation (BMGF), Institute for Translational Vaccinology (Intravacc), among other global health stakeholders, and representatives from 31 vaccine manufacturers from developing countries and 20 life sciences corporations.
M. Suhardono, DCVMN President, opened the meeting by thanking the Minister of Health of Argentina for attending, and Sinergium for hosting it. He provided a brief update on DCVMN: five new corporate members joined in 2016, including Amson Vaccines and Pharma from Pakistan, HLL Biotech, Green Signal Bio Pharma and Zydus Cadila from India, and Instituto Biologico Argentino (BIOL) from Argentina - the 50th member. He emphasized the importance of collaboration and partnerships among members for achieving sustainable supply of high-quality, affordable vaccines and to accomplish global health initiatives. One such initiative is the switch from trivalent (tOPV) to bivalent oral polio vaccine (bOPV) and eventually inactivated polio vaccine (IPV), in order to contain polioviruses better and, ultimately, eradicate polio.
J. Lemus, Minister of Health in Argentina, began with the reminder that vaccines and potable water are the most effective ways to reduce morbidity and mortality globally. The role of the Ministry of Health is to protect the Argentinian population and promote healthy living. The H1N1 outbreak in Argentina underscored the value of local vaccine manufacture. He also emphasized the importance of supporting international supply, and affirmed the Argentinian government’s and vaccine manufacturers’ commitment to work with PAHO to provide high-quality vaccines globally.
M-P. Kieny reviewed evolving collaborative innovation in vaccine development. Historically, one scientist, like Edward Jenner, could develop a vaccine. Then came small groups, such as Louis Pasteur and colleagues. Innovation subsequently became the work of a large team, sometimes spanning several corporations. Now, after decades of intense competition for high-value markets, collaboration with developing countries has become critical, and involvement of multiple manufacturers as well as public- and private-sector investments are essential. The 10-year Meningitis A project and the 30-year malaria vaccine development illustrate the efforts for developing vaccines for African epidemics. The accelerated Ebola vaccine development in 2014 required urgent collaboration between manufacturers, regulatory authorities and funding agencies to respond to and contain the emerging outbreak [2]. Following the registration of the first dengue vaccine in 2015, the most advanced dengue vaccine, a collaboration between Butantan and National Institutes of Health (NIH) [3], encourages partnerships with DCVMs and public-sector entities. Full participation of DCVMN members in future innovative collaboration will require increased development capability, novel production processes and regulatory skills, along with equal partnerships, justified investments and national regulatory agencies (NRAs) capabilities. She concluded that commitment to public health as a joint responsibility is essential.
H. Sigman, Chairman of Group InSud and Sinergium welcomed meeting participants. He introduced Sinergium’s technology transfer capabilities, providing an example of partnership between public and private sectors, and confirmed the importance of collaboration among vaccine manufacturers.
2. Access to vaccines
The Director of PAHO, C.F. Etienne, presented via video. PAHO has undertaken to make national health programs sustainable and affordable, encouraging improvements in quality and availability of vaccines from both private and public sectors. Rubella was eliminated from the WHO Americas region in 2015, and it is now the first region to be declared free of measles [4]. She thanked DCVMN members for their contribution to national immunization programs. PAHO is committed to continue collaboration with DCVMN members and welcome innovative suggestions, to ensure that the Americas have sustainable access to life-saving vaccines.
I. Danel, Deputy Director from PAHO, outlined achievements of public health goals in the Americas, including extension of the reach of national immunization programs, new vaccine introductions, strengthening of regulatory pathways and improving financing and forecasting mechanisms. Elimination of measles from the Americas is a result of countries working together successfully. This was made possible by timely supply of measles-rubella (MR) and measles-mumps-rubella (MMR) vaccines by DCVMN members. In 2015, DCVM’s vaccines represented 74% of the total volume of 250 million doses procured through PAHO’s Revolving Fund. PAHO is constantly seeking alternative sources of vaccines and adopting strategies to enable manufacturers to find new opportunities. She encouraged DCVMN members to get involved in fast-track development of vaccines, to enhance sustainability of supply and to introduce required vaccines.
K. Owen from BMGF reviewed the number of deaths averted through immunization by 2015 [5], then discussed the challenge of creating stable vaccine supply in developing countries. Stable supply requires high-quality manufacturing capacity, committed financing, strong regulatory systems and predictable demand. BMGF’s efforts have focused on partnering with manufacturers, stimulating procurement, and optimizing regulatory pathways in developing countries, to reduce registration time by up to 50%. Challenges around predictability remain. A recent example of challenge is the transition in demand from OPV to IPV. It is essential that DCVMs have strategies to sustain their business in a competitive market.
R. Bright presented BARDA’s role in tackling emerging and re-emerging infectious diseases. The large number of potential EIDs and unpredictability of emergence requires multi-hazard strategies and investment in a broad spectrum of vaccine platforms. Emerging threats require a rapid and coordinated response which integrates diagnostics for early detection, therapeutics and vaccines. Collaboration between multiple groups to develop and evaluate vaccines and new supply and funding models are becoming increasingly necessary to respond efficiently. There are currently 38 companies developing Zika vaccine, including DCVMN members Bharat, Butantan, and Sinergium. He urged DCVMs who are working on Zika candidate vaccines to meet and foster possible partnerships.
F. Kristensen provided an overview about the new Coalition for Epidemic Preparedness Innovations (CEPI) [6]. CEPI aims to prioritize, stimulate, finance, coordinate and advance vaccine development against EIDs with epidemic potential, especially where market incentive alone will not achieve this. The Ebola response showed that it is possible to advance the clinical development of vaccines in an emergency. Manufacturing capability and capacity for vaccines is a bottle-neck during crises, thus the effort will focus on driving vaccine manufacturing pipelines, while also funding diagnostics and therapeutics. CEPI seeks multi-year donor contributions of up to one billion dollars between 2017 and 2021 from those aligned with the strategic objectives and mission. The operating principles are: equitable access, cost coverage and shared risks and benefits. Funding from CEPI will be available to vaccine producers or consortia with a track record of bringing vaccine candidates to human clinical trials. The founders of CEPI are the governments of India and Norway, the Bill and Melinda Gates Foundation, Wellcome Trust and The World Economic Forum (WEF). CEPI was, in fact, launched at the WEF Meeting in Davos, in January 2017.
J. Kalil discussed emerging and re-emerging diseases in tropical regions. Butantan is developing Dengue and Zika vaccines, currently in phase III clinical trials, and shows results comparable to the NIH vaccine [3]. Development of a Zika candidate vaccine was undertaken as an urgent response to the recent outbreak in South America. The ideal situation would have been to develop a combination vaccine against both Zika and Dengue. Butantan is willing to collaborate with other companies developing Zika vaccines to test candidate vaccines in an endemic area.
3. Vaccine markets, supply and procurement
M. Malhame reviewed the Gavi 2016–2020 strategy, which was informed by lessons of previous periods. It includes fostering of innovative products, further strengthening of collaboration though engagement with industry, clearer inclusion criteria for vaccines and improved, consistent market analysis and forecasting. Securing multiple vaccine suppliers has a positive impact on the effectiveness of immunization programs. Gavi’s new strategy has three priorities: 1. Taking a long-term view of markets to help countries and manufacturers prepare for transition to self-financing. 2. Aligning product innovation priorities across market-shaping partners to match needs. 3. Adopting a higher tolerance of risk in markets that require it. Lastly, M. Malhame extended Gavi’s gratitude to DCVMN members contributing to the supply of vaccines to poor countries. Gavi will continue to shape healthy vaccine supply globally.
S. Rautio noted that more than 50% of the total UNICEF procurement of $3.4 billion was dedicated to vaccines in 2015. A total of 2.8 billion doses of vaccines were procured by UNICEF, reaching 45% of the world’s children. 60% of the doses were sourced from DCVMs at a total value of $612 million, providing an important market opportunity for DCVMs. Vaccine security is the sustained, uninterrupted supply of affordable vaccines of assured quality. To achieve it, accurate forecasting, availability of funding and appropriate contracting with a diverse supplier base is critical. Therefore, continued engagement with DCVMs is essential. Beyond securing Expanded Programme on Immunization (EPI) vaccines, UNICEF is engaged in supply of pipeline vaccines, during outbreaks or health and humanitarian emergencies, and improving access to affordable new vaccines in middle-income countries (MICs). UNICEF supports the development of healthy markets through leveraging its procurement, transparency on pricing and building procurement capacity in countries. Pentavalent vaccine is an example of a market, which is now considered healthy, 16 years after procurement of the first dose.
J. Fitzsimmons provided an overview of PAHO’s technical cooperation strategies with countries in the Americas, including considerations for national decision-making on introducing new vaccines in national immunization programs. PAHO’s sustainability model for immunization consists of national vaccine forecasting, national budgetary financing, legislation and the Revolving Fund mechanism which together facilitate access to vaccines. After four decades of experience, this model has proven to be effective: the Americas enjoy high immunization coverage for traditional and new vaccines. The success of the PAHO Revolving Fund has been enabled by the high-level commitment from member countries, its business model and the high-quality vaccines supplied by DCVMs and other suppliers (Fig. 1 ).
Fig. 1.
The PAHO Revolving Fund vaccines supplied by DCVMs and other suppliers as of September 2016. Different colours indicate different vaccine groups: traditional vaccines are represented in orange; new and underutilized vaccines are represented in green; seasonal influenza vaccines are represented in blue; and trending vaccines are represented in yellow, as shown at the bottom of the figure. Circle size represents the number of countries purchasing the vaccine; from 1–5 countries (smallest circle), to 20–40 countries (largest circle). Acronyms: aP combination = combination of acellular Pertussis vaccines; BCG = Bacillus Calmette-Guérin vaccine; DT Adult = Diphtheria and Tetanus vaccines for adult; DT Ped = Diphtheria and Tetanus vaccines for pediatric use; DTP = Diphtheria-Pertussis-Tetanus vaccine; HPV = Human Papillomavirus vaccine; IPV = Inactivated Polio Vaccine; Meningo ACYW135 = Meningococcal meningitis vaccine against serotypes A, C, Y and W-135; MMR = Measles, Mumps, Rubella vaccine; MR = Measles and Rubella vaccine; PCV = Pneumococcal Conjugate Vaccine; Pentavalent = Pentavalent vaccines (DTP-Haemophilus influenzae type b and Hepatitis B vaccine); Pneumo 23 valent = Pneumococcal 23-valent vaccine; Rabies = Rabies vaccine; Rota = Rotavirus vaccine; Seasonal Flu NH = Seasonal influenza vaccine in North Hemisphere; Seasonal Flu SH = Seasonal influenza vaccine in South Hemisphere; TdaP = Tetanus-Diphtheria-acellular Pertussis vaccine (booster); Varicella = Varicella vaccine; Yellow fever = Yellow fever vaccine. Note that some countries are purchasing different combinations of acellular Pertussis vaccines (aP combo), such as trivalent TdaP, rather than tetravalent and pentavalent aP. This figure is a courtesy of J. Fitzsimmons. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
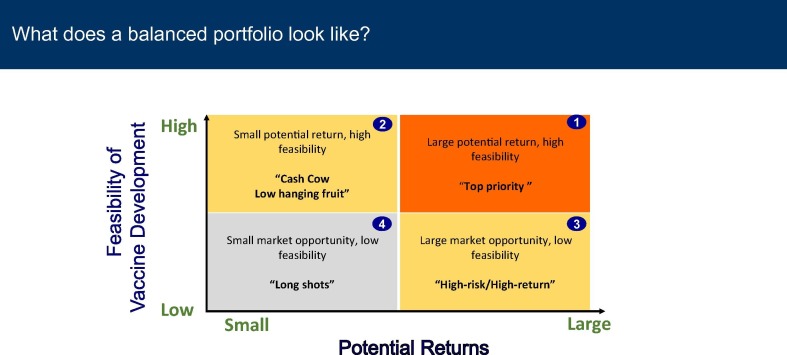
A panel discussion moderated by J. Chu, from CHAI, focused on management decisions that can contribute to increased success and greater commercial viability of vaccines. Vaccine manufacturing is a critical component of global health, but it comes with risks. One example is the decline in success rate of clinical trials for pharmaceuticals, including vaccines, over the past 20 years [7]. Both scientific and management factors contribute to four risk categories: 1 – Large potential return/High Feasibility e.g. innovative Respiratory Syncytial Virus (RSV) and Group B Streptococcus (GBS) vaccines; 2 – Small potential return/ High feasibility; 3 – Large market opportunity/ Low feasibility; 4 – Small market opportunity/ Low feasibility (Fig. 2 ) and emphasized that a balanced portfolio is critical to ensure that companies remain viable.
Fig. 2.
A balanced vaccine product portfolio as a risk assessment tool. A representative of vaccine products portfolio shows that products can be classified into 4 main categories: 1. Large potential return, high feasibility (top right quadrant/orange): 2. Small potential return, high feasibility (top left quadrant/yellow); 3. Large market opportunity, low feasibility (bottom right quadrant/yellow); 4. Small market opportunity, low feasibility (bottom left quadrant/grey), where X-axis represents potential financial returns, and Y-axis represents the degree of feasibility of vaccine development. This figure is a courtesy of J. Chu.
M. Datla said it is increasingly important to choose the vaccine portfolio wisely. Biological E, for example, takes pride in supporting EPI needs of India as well as Gavi countries. There are also few follow-on vaccines and limited new product introductions, so many companies end up with similar pipeline. Both under-capacity and over capacity of supply influence sustainability for customers and manufacturers respectively. Pentavalent vaccine is a prime example of over-capacity, as the demand is now divided among a larger group of manufacturers. Reduction in market shares will challenge manufactures’ sustainability as their business models depend on reasonable market shares. MICs have unique characteristics, such as size of birth cohort, ability to procure, local registration requirements and reluctance to join pooled procurement. However, they are seeking vaccines at Gavi prices posing another challenge to manufacturers’ sustainability. High-income countries have different barriers to entry, requiring heavy investments, due to the need for more clinical trials. Access to funding for vaccine development is also getting increasingly difficult as it takes about 6 years for a vaccine to reach the market, and most investors prefer faster and higher returns.
J. Khalil noted that partnerships help to reduce the financial risks. Butantan has a history of forging successful partnerships with multinational companies with the primary objective of domestic supply. Access to HPV vaccines was only possible after local government enabled a technology transfer agreement. Butantan now prioritizes innovative products and products for valuable markets. If manufacturing capacity exceeds demand, lower pricing would be possible.
M. Malhame explained how Gavi assists suppliers to understand the competitive and commercial risks involved by publishing strategic demand forecasts and roadmaps, and analyzing how countries and donors select products. Companies also need to develop their own strategy. Gavi also analyzes the cost of administration and calculates the weighted average prices of vaccines. There is a need to expand the conversation from price per dose to cost per child immunized.
S. Rautio commented that UNICEF’s focus is on security of supply. UNICEF hosts a conference for vaccine manufacturers annually at which market forecasts are provided. UNICEF works closely with Ministries of Health in various countries to estimate demand. No supplier, however, should rely only on UNICEF; similarly, no market should be reliant only on one supplier.
J. Fitzsimmons commented that another risk for manufacturers is the different product requirements in different markets, such Gavi and PAHO markets. Networking opportunities, such as this meeting, offer a forum to discuss these issues and develop a more common target product profile relevant across public-health markets.
G. Widmyer acknowledged that BMGF is in a unique position to shape global health. While financing agencies seek the lowest pricing, suppliers need support for vaccine development. BMGF has a diverse portfolio, funding projects on various diseases. Agendas may overlap, as is the case where investment in a manufacturer may result in excess capacity available for Gavi markets, in addition to the domestic market. Companies must focus on financial viability. The foundation is keen to engage with suppliers seeking investment who can show a portfolio that reflects a strategic and balanced vision. Trust and transparency in interactions are critical.
4. Regulatory convergence
J.L. Di Fabio opened the session on regulatory convergence efforts to facilitate registration and improve access to health products.
P. Aprea, Director of Biologicals Evaluation and Control at the National Administration of Medicines, Food and Technologies, Argentina (ANMAT), remarked that convergence is currently the biggest challenge for regulatory authorities. They require confidence, clarity and competitiveness, and must apply regulatory science through solid knowledge gained from academic and other collaborations.
D. Decina presented the draft WHO Good Regulatory Practices (GRP) guidelines [8] for NRAs. The guideline is intended to assist regulators and regulated organizations, irrespective of resource levels. It provides a framework for improving practices and principles on which regulatory systems can be established. Key concepts are impact analysis and stakeholder consultation. The nine major principles: legality, impartiality, consistency, proportionality, flexibility, effectiveness, efficiency, clarity and transparency are important for sharing information. She presented the types of international regulatory cooperation that help to avoid redundancy in regulatory work, for example, reliance or mutual recognition.
A. Porrás shared the regional approach to regulatory harmonization used by the Pan American Drug Regulatory Harmonization (PANDRH) Network. The technical cooperation approach for Regulatory System Strengthening (RSS) in the Americas encompasses three work streams: 1. Facilitating development of context-specific national regulatory systems; 2. Promoting regulatory convergence and harmonization; 3. Supporting efficient use of resources by leveraging the work of others. The program relies on peer-assessment of NRAs using standardized indicators of regulatory capacity, and the adoption of institutional development plans based on identified gaps. Participation implies commitment to undergo assessment. Assessment results are shared with participating NRAs, and aggregated data is published online on the Regional Platform on Access and Innovation for Health Technologies (PRAIS [9]). She concluded that this effort represents a new trend towards convergence in the regulatory systems in the Americas.
N. Dellepiane discussed how manufacturers can contribute to dialogue on global regulatory convergence. She reviewed existing approaches to regulatory harmonization, including reliance within Regulators’ Networks, provision of mutual support, and alignment of requirements, procedures and standards among regulatory agencies. Continued reliance and mutual recognition of NRAs through networking initiatives, improving technical and scientific expertise through joint review activities and partnerships between NRAs remain important. Alignment of NRAs, by agreeing and following a common list of essential documents to fulfil country-specific requirements for registration files may also foster regulatory convergence. She encouraged DCVMN members to collect data related to various registration approaches and contribute to the dialogue between regulatory groups and industry.
5. Future vaccines and biological products
M.E. Bottazzi, from Sabin Vaccine Institute Product Development Partnership, gave an overview on advances in hookworm and schistosomiasis vaccine development. The strategy is to collaborate with academic, public and private sectors to leverage expertise and promote open-source research, capacity building and knowledge sharing. Both vaccine candidates have now completed phase I clinical trials [10], conducted with international collaboration in Brazil, Gabon and the USA. Other vaccine development projects against Chagas disease, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) are ongoing.
V. Brizzio presented Sinergium’s progress on the development of Zika vaccine in partnership with Protein Sciences, Liomont, Mundo Sano and UMN Pharma. The process uses a Baculovirus Expression Vector system. The project is currently in pre-clinical phase. Phase I clinical trials are planned in 2017.
J. Iyer presented the view from Access to Medicine Foundation on the role of manufacturers in promoting access. Their goal is to gather insights into how pharmaceutical companies improve access to medicines, by ranking corporations based on criteria such as research and development capacity, affordability, manufacturing and supply. An Access to Vaccines Index [11] has been developed to help understand how companies make vaccines more accessible in low and middle-income countries. Vaccine manufacturers can assess, monitor and track progress based on the analysis provided by the index and benchmark their international business strategy.
D. Atherly presented a study by PATH’s Center for Vaccine Innovation and Access on the dynamics of vaccine uptake in developing-country markets. The project goal is to accelerate the development and introduction of products with high health impact. PATH collaborated with DCVMN member Chengdu Institute of Biological Products (CDIBP) on development and introduction of Japanese Encephalitis (JE) vaccine. This collaboration helped to obtain WHO prequalification, the first WHO-prequalified vaccine from China. From 2003 to 2017, production capacity increased by 180%. Approximately 308 million children outside of China will be vaccinated by CDIBP’s JE vaccine. It is envisioned that by 2018, eight additional countries will introduce JE vaccine as part of the national immunization program.
Y. Lim from CHAI discussed the risks and potential of future vaccine markets. She advised companies to understand the market and existing competition, then compare and select candidate products based on potential risks and returns. The resultant optimized and balanced product portfolio should be responsive to changes in vaccine market dynamics.
6. Polio eradication, innovation and endgame strategy
J. Fournier-Caruana gave an update on the WHO Global Action Plan for containment of polioviruses (GAPIII, [12]) and the Containment Certification Scheme (CCS) endorsed by the Strategic Advisory Group of Experts (SAGE) in 2014 and October 2016 [13], respectively. Eradication of poliovirus is a global endeavour and can only be achieved by engaging all stakeholders. The globally-synchronized switch from trivalent (type 1, 2, 3) OPV to bivalent OPV (type 1, 3) in April 2016 [14] started the poliovirus type-2 containment period to eliminate vaccine-derived type 2 poliovirus (VDPV2) infection. However, there have been delays: poliovirus production facilities, research facilities and repositories should have demonstrated implementation of GAPIII, and National Authorities for Containment (NACs) should have certified containment. This has not been realized in many countries due to lack of national regulatory frameworks to implement containment. WHO and other stakeholders are supporting national containment certification efforts.
UNICEF’s role in the endgame, outlined by A. Ottosen, includes securing the supply of polio vaccines as well as synchronized withdrawal of tOPV and roll out of bOPV. From December 2015 to March 2016, 650 million doses of OPV (tOPV and bOPV) were delivered in a timely manner for planned activities. Towards tOPV cessation, however, perceptions of risk increased among both suppliers and programme managers. An industry survey emphasized the necessity for involving suppliers from early stages of decision-making for final OPV cessation to allow for integration into their strategies and ensure sufficient supply. The recent polio outbreak in Nigeria required 200 million vaccine doses. DCVMs provided a large quantity through flexibility, stockpiles and response capacity. Due to a shortage of IPV, UNICEF is not currently able to meet the WHO recommendation of at least one IPV dose in all countries. Therefore, in October 2016, SAGE recommended a fractional-dose IPV (fIPV: one-fifth of the normal IPV intramuscular dose delivered intradermally) [15], and catch-up on IPV vaccination when it becomes available. Currently 43 countries do not have access to IPV [16].
S. Boyle from BMGF elaborated on IPV use and challenges, and the rationale behind the switch from tOPV to bOPV. Since 1999, no indigenous transmission of type-2 wild poliovirus has been detected, while the type-2 component in the tOPV makes up more than 90% of the vaccine-derived polioviruses, and causes 40% of vaccine-associated paralytic polio cases [17]. Further, it also interferes with immune responses to type 1 and 3 polioviruses. In response to the shortage of IPV, WHO has recommended two doses of fIPV delivered intradermally at 6 and 14 weeks, along with routine bOPV as well as campaign immunization. IPV supply constraints are expected to remain an issue until 2018. BMGF priorities include reliable supply of bOPV, introducing and maintaining IPV in routine immunization, and assessing the potential of new mucosal-immunity enhancing adjuvants and more genetically stable OPVs.
B. Giersing presented the role of WHO’s Initiative for Vaccine Research (IVR) in accelerating vaccine development and access to vaccines in LMIC. Challenges around new vaccine development include investments being driven by high-income countries, lack of robust regulatory pathways and differences in health priorities among stakeholders. Since 2014, 35 pathogens, including RSV, were evaluated by IVR. IVR also evaluates new delivery strategies. An example is the microarray patch (MAP, [18]) for administration of MR vaccines. The MAP requires minimal training, is light-weight and thermo-stable, yet provides the same efficiency as traditional vaccine. IVR wishes to provide neutral evidence on the value of new technologies to overcome challenges in vaccination.
D. Zehrung reviewed new vaccine delivery technologies, including MAPs for IPV or MR, blow-fill-seal tubes for rotavirus, integrated reconstitution packaging and intradermal delivery technology. New delivery technologies are likely to reduce commodity and system costs, resulting in greater access and health impact worldwide.
M. Zaffran reported on the current situation of the Global Polio Endgame Strategy. In 2016, the lowest number of cases of polio was reported [19], with cases in Pakistan and Afghanistan. Four new cases of wild-type 1 in Nigeria and 19 VDPV2 events caused concern. Restricted access to conflict zones contributed to lack of surveillance and access to vaccines. In April 2016, WHO announced the switch from tOPV to bOPV, removing type-2 OPV. By the end of August 2016, 173 countries had introduced IPV [20]. Priorities for the next six months are to interrupt transmission in the three endemic countries, maintain active surveillance in security- and IPV-deprived countries, and containment to maintain a polio-free status.
7. Conclusion
The 17th DCVMN Annual General Meeting provided an occasion for professionals in the vaccine industry and global health organizations to discuss challenges, innovations, risks and gain deeper understanding of regulatory practices and convergence opportunities. The Zika outbreak and polio endgame are reminders of the importance of vaccines, but also the importance of collaboration, collective effort and global partnerships. The DCVMN aims to foster networking opportunities to support members in shaping global health.
Conflict of interest
The authors are employees of the respective indicated organizations, and have no conflict of interest to declare. DCVMN International did not provide any financial or travel support to speakers or moderators to participate at this meeting. N. Dellepiane is a consultant, partly with DCVMN.
Acknowledgements
We are grateful to all speakers and moderators whose contribution made the agenda invaluable. We thank corporate partners for supporting DCVMN with unrestricted educational grants: Merck (MilliporeSigma), Temptime Corporation, GE Healthcare, Bioengineering, GEA, Bosch, OMPI (Stevanato Group), Applikon Biotechnology, Alfa Wasserman, Munters. We are indebted to the experts who graciously served as rapporteurs of specific sessions: D. Kristensen, S. Sobti, and J. Hendriks. We thank S. Villasenor for administrative assistance and M. Dennehy for editorial support. This conference was partly supported by a grant from the Bill & Melinda Gates Foundation, Grant no. OPP1157021.
Footnotes
IMPORTANT NOTE: This report summarizes the views of an international group of experts as presented at a scientific conference in a given time and context, and does not necessarily represent the decisions or the stated policy of any institution or corporation. Report of the Annual General Meeting of the Developing Countries Vaccine Manufacturers Network, 24–26 October 2016, Buenos Aires, Argentina.
Contributor Information
the DCVMN Executive Committee Group:
Mahima Datla, Steven Gao, Akira Homma, Li Meng, Mahendra Suhardono, Rajinder Suri, and Patrick Tippoo
References
- 1.WHO statement on the first meeting of the International Health Regulations (2005) (IHR2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. Media centre of World Health Organization. 1 February 2016. Available at: <http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/>.
- 2.Ebola virus disease in Guinea. Outbreak news. Regional office for Africa of World Health Organization. 23 March 2014. Available at: <http://www.afro.who.int/en/disease-outbreaks/outbreak-news/4063-ebola-virus-disease-in-guinea.html>.
- 3.Precioso A.R., Palacios R., Thomé B., Mondini G., Braga P., Kalil J. Clinical evaluation strategies for a live attenuated tetravalent dengue vaccine. Vaccine. 2015;33(50):7121–7125. doi: 10.1016/j.vaccine.2015.09.105. [DOI] [PubMed] [Google Scholar]
- 4.La región de las Américas es declarada libre de sarampión. PAHO/WHO Regional office for the Americas. Washington, 27 September 2016. Available at: <http://www.paho.org/hq/index.php?option=com_content&view=article&id=12528%3Aregion-americas-declared-free-measles>.
- 5.Gavi, The Vaccine Alliance. Results & Evidence. Gavi Progress Report. Gavi Progress Report 2015. Available at: <http://gaviprogressreport.org/2015/#page=11>.
- 6.Cohen J. Science 2016, Sep 2nd, commentary. New vaccine coalition aims to ward off epidemics. Available at: <http://www.sciencemag.org/news/2016/09/new-vaccine-coalition-aims-ward-epidemics>.
- 7.Pammolli F., Magazzini L., Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 2011;10(6):428–438. doi: 10.1038/nrd3405. [DOI] [PubMed] [Google Scholar]
- 8.Good regulatory practices: guidelines for national regulatory authorities for medical products. WHO Working document QAS/16.686. October 2016, Draft for comment. Prepared by EMP/RSS. Available at: <http://www.who.int/medicines/areas/quality_safety/quality_assurance/GoodRegulatory_PracticesPublicConsult.pdf?ua=1>.
- 9.Regional Platform on Access and Innovation for Health Technologies. PRAIS.PAHO/WHO Regional office for the Americas. Available at: <http://prais.paho.org/>.
- 10.Hotez P.J., Strych U., Lustigman S., Bottazzi M.E. Human anthelminthic vaccines: Rationale and challenges. Vaccine. 2016;34(30):3549–3555. doi: 10.1016/j.vaccine.2016.03.112. Jun 24. [DOI] [PubMed] [Google Scholar]
- 11.Access to Vaccines Index. Available at: <https://accesstomedicinefoundation.org/news/access-to-vaccines-index-the-data-is-in-analysis-can-begin/#about-atvi>.
- 12.WHO Global Action Plan to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of oral polio vaccine use. GAP III. WHO/POLIO/15.05. Global Polio Eradication Initiative. Available at: <http://polioeradication.org/wp-content/uploads/2016/09/GAPIII_2014.pdf>.
- 13.GAP III Containment Certification Scheme (CCS). Polio Today. Global Polio Eradication Initiative. 2016. Available at: <http://polioeradication.org/wp-content/uploads/2016/10/CCS.pdf>.
- 14.Replacing trivalent OPV with bivalent OPV. Vaccines and diseases. Immunization, Vaccines and Biologicals. WHO. Available at: <http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/oral_polio_vaccine/en/>.
- 15.Meeting of the Strategic Advisory Group of Experts on Immunization, October 2016 – conclusions and recommendations. Weekly Epidemiological Record, 2 December 2016, vol. 91, pp. 561–584. Programmes and projects. WHO. Available at: <http://apps.who.int/iris/bitstream/10665/251810/1/WER9148.pdf?ua=1>.
- 16.Inactivated Polio Vaccine: Supply Update. UNICEF Supply Division. UNICEF. September 2016. Available at: <https://www.unicef.org/supply/files/Inactivated_Polio_Vaccine_(IPV)_-_september_2016.pdf>.
- 17.Preparing for the withdrawal of all oral polio vaccines (OPVs): replacing trivalent OPV with bivalent OPV. Frequently Asked Questions. February 2015. EPI. Global Polio Eradication Initiative. Available at: <http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/oral_polio_vaccine/OPVswitch-overviewFAQs-Feb2015.pdf>.
- 18.Jacoby E., Jarrahian C., Hull H.F., Zehrung D. Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine. 2015;33(37):4699–4704. doi: 10.1016/j.vaccine.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 19.GPEI. Global Polio Eradication Initiative. Polio Now. Available at: <http://polioeradication.org/polio-today/polio-now/>.
- 20.Cessation of use of trivalent oral polio vaccine and introduction of inactivated poliovirus vaccine worldwide, 2016. Weekly epidemiological record. 9 September 2016, vol. 91, pp. 421–432. Programmes and projects. WHO. Available at: <http://apps.who.int/iris/bitstream/10665/250045/1/WER9136_37.pdf>. [PubMed]