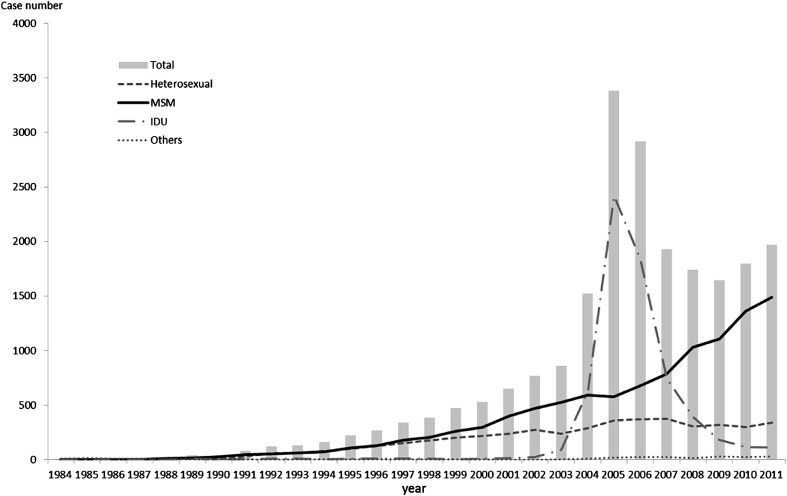
By the end of 2011, Taiwan had 22,020 reported cases of human immunodeficiency virus (HIV) infection.1 Fig. 1 shows the reported cases of HIV infection according to the year of report and different transmission routes in Taiwan. Before the severe acute respiratory syndrome epidemic struck Taiwan in 2003, the majority of new HIV infections occurred through sexual transmission, with men who have sex with men (MSM) accounting for the largest proportion (48.2%). However, the HIV outbreak among injecting drug users (IDUs) increased drastically after 2003 and peaked in 2005. In response, Taiwan's government endorsed harm reduction programs targeting IDUs. Soon after, the number of IDUs with HIV was down to around 400 and MSM came back to the top in 2008. The number of annually reported HIV cases among MSM and the proportion of all annually reported cases attributed to MSM from 2008 to 2011 were 1007 (58%), 1099 (67%), 1317 (73%), and 1474 (75%). A recent study revealed that the estimated HIV incidence among MSM in saunas went up from 7.8% in 2004 to 15% in 2007 in Taiwan.2 Another Taiwan study also showed MSM was the major contributing factor [odds ratio (OR) 11.54] for HIV infection in anonymous testing sites.3 Therefore, in Taiwan the recent HIV epidemic is mainly the result of unsafe sexual behaviors among MSM.
Figure 1.
The reported cases of human immunodeficiency virus (HIV) infection according to the year of report and different transmission routes in Taiwan.
Taiwan's government has provided free access to highly active antiretroviral therapy (HAART) for people with HIV since 1997. The concept of treatment as prevention is a hot issue among HIV/AIDS researchers. An ecological study showed a strong association between HAART coverage and the number of new HIV diagnoses.4 Furthermore, a recent randomized study indicated that early antiretroviral therapy for HIV-positive individuals can significantly reduce sexual transmission of HIV for discordant couples in the heterosexual population.5 Nevertheless, most developed countries with free HAART are facing a resurgence of HIV infections in MSM.6 In response to this challenge, positive prevention has gradually gained more attention as a HIV control strategy, particularly since the US Centers for Disease Control (CDC) launched the Sero-status Approach to Fighting the HIV Epidemic program (SAFE) for people with HIV.7 The SAFE approach seeks to: (1) encourage HIV-infected persons to know their serostatus; (2) increase the use of health care and preventive services; (3) increase high-quality care and treatment; (4) increase adherence to antiretroviral therapy; and (5) encourage individuals with HIV to adopt HIV/sexually-transmitted disease (STD) risk reduction behaviors.7 In addition, this program stresses that reduction in stigmatization must be integrated into strategies to avoid blaming an already stigmatized group.7
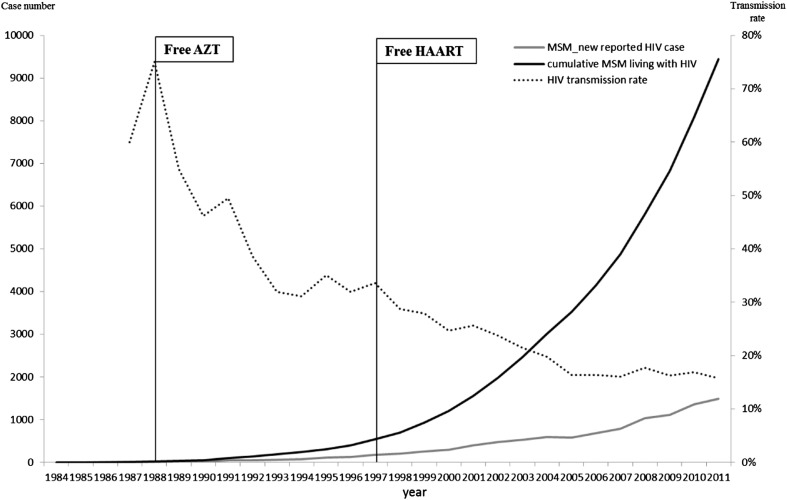
Regarding the HAART effect on the HIV epidemic among MSM in Taiwan, although serological testing algorithms such as the BED HIV-1 capture enzyme immunoassay can provide exact estimates of HIV incidence, national surveillance of Taiwan was not implemented until 2010. Therefore, an alternative method proposed by Cowan and her colleages was adopted to evaluate the effect of HAART on HIV trends among MSM: a ratio between the yearly number of new reported cases and the number of MSM living with HIV was used.8 Fig. 2 shows HIV transmission rates among MSM with HIV in Taiwan. After HAART implementation, HIV transmission rates dropped from 35% in 1997 to 16% in 2005, and continued to decrease more slowly toward 15% until 2011. This suggests that universal HAART for the infected had an immediate effect on HIV transmission rates. However, elimination of the residual rate is more difficult and will require complex efforts.
Figure 2.
The human immunodeficiency virus (HIV) transmission rates among men who have sex with men (MSM) with HIV in Taiwan.
In considering policy options, it is worth noting that studies have shown that individuals tend to decrease sexual risk behaviors in the initial years after HIV notification.9 A study by Lo showed that late HIV diagnosis was associated with irregular HIV testing and missed opportunities for HIV testing after seeking medical attention in Taiwan.10 Therefore, strategies such as routine HIV testing in clinical settings and increased accessibility of voluntary HIV testing to help individuals with HIV to learn their serostatus are necessary in Taiwan. Besides, 10,420 Taiwan HIV cases had received HAART, and of these, 6408 were MSM in 2011. The percentage of cases on HAART among the 9298 MSM cases with HIV was 69%, and the coverage rate of those deemed in need of antiretroviral therapy (CD4 < 350 cells/μL) was 94% in 2011. However, 25% of MSM with HIV were inconsistent in adherence to treatment, as defined by dropping out of HAART for more than 2 months in 2011. Additionally, 35% of MSM with HIV were diagnosed with syphilis after HIV notification. Therefore, programs should be implemented to offer effective HIV case management, such as skill training for treatment adherence and behavior interventions to promote condom use.
In conclusion, the concept that early treatment can reduce HIV transmission was proven, but Taiwan is facing a budget shortage for HAART. Therefore, in light of this emerging HIV epidemic among MSM in Taiwan, effective implementations beyond HAART are preferential to ensure that individuals with HIV learn their serostatus and change risky behaviors.
References
- 1.Centers for Disease Control, Taiwan. HIV/AIDS monthly report. Taiwan CDC website, http://www2.cdc.gov.tw/np.asp?ctNode=2694&mp=220. Published 2012. [Accessed 20.06.12] [in Chinese].
- 2.Ko N.Y., Lee H.C., Hung C.C., Tseng F.C., Chang J.L., Lee N.Y. Trends of HIV and sexually transmitted infections, estimated HIV incidence, and risky sexual behaviors among gay bathhouse attendees in Taiwan: 2004–2008. AIDS Behav. 2011;15:292–297. doi: 10.1007/s10461-010-9748-2. [DOI] [PubMed] [Google Scholar]
- 3.Wu H., Wu P.Y., Li S.Y., Chang S.Y., Liu W.C., Wu C.H. Maximising the potential of voluntary counselling and testing for HIV: sexually transmitted infections and HIV epidemiology in a population testing for HIV and its implications for practice. Sex Transm Infect. 2012;88:612–616. doi: 10.1136/sextrans-2011-050354. [DOI] [PubMed] [Google Scholar]
- 4.Montaner J., Lima V.D., Barrios R., Yip B., Wood E., Kerr T. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen M.S., Chen Y.Q., McCauley M., Gamble T., Hosseinipour M.C., Kumarasamy N. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan P.S., Hamouda O., Delpech V., Geduld J.E., Prejean J., Semaille C. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol. 2009;19:423–431. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Janssen R.S., Holtgrave D.R., Valdiserri R.O., Shepherd M., Gayle H.D., De Cock K.M. The serostatus approach to fighting the HIV epidemic: prevention strategies for infected individuals. Am J Public Health. 2001;91:1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan S, Christiansen AH, Haff J. New paradigm for positive prevention: “Test and treat” testing for and treating HIV has lowered transmission rate in Denmark in spite of increased unsafe sex among MSM. 18th International AIDS conference website, http://pag.aids2010.org/Session.aspx?s=456. Published 2010. [Accessed 01.06.12].
- 9.Marks G., Crepaz N., Senterfitt J.W., Janssen R.S. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 10.Lo Y.C., Wu P.Y., Hsieh C.Y., Chen M.Y., Sheng W.H., Hsieh S.M. Late diagnosis of Human Immunodeficiency Virus infection in the era of highly active antiretroviral therapy: role of socio-behavioral factors and medical encounters. J Formos Med Assoc. 2011;110:306–315. doi: 10.1016/S0929-6646(11)60046-6. [DOI] [PubMed] [Google Scholar]