Abstract
Ethnopharmacological relevance
Houttuynia cordata Thunb. (Family: Saururaceae) is an herbaceous perennial plant that grows in moist and shady places. The plant is well known among the people of diverse cultures across Japan, Korea, China and North-East India for its medicinal properties. Traditionally the plant is used for its various beneficial properties against inflammation, pneumonia, severe acute respiratory syndrome, muscular sprain, stomach ulcer etc.
Oxidative stress and inflammation were found to be linked with most of the diseases in recent times. Many ancient texts from Chinese Traditional Medicine, Ayurveda and Siddha, and Japanese Traditional medicine have documented the efficacy of H. cordata against oxidative stress and inflammation.
Aim of the study
This review aims to provide up-to-date and comprehensive information on the efficacy of H. cordata extracts as well as its bioactive compounds both in vitro and in vivo, against oxidative stress and inflammation
Materials and methods
Relevant information on H. cordata against oxidative stress and inflammation were collected from the established scientific databases such as NCBI, Web of Science, ScienceDirect, Elsevier, and Springer. Additionally, a few books and magazines were also consulted to get the important information.
Results
Herbal medicines or plant products were traditionally being used for treating the oxidative stress and inflammation related diseases in diverse communities across the world. Scientifically, H. cordata has shown to target several signaling pathways and found to effectively reduce the oxidative stress and inflammation. Phyto-constituents such as afzelin, hyperoside and quercitrin have shown to reduce inflammation both in vitro and in vivo models. These molecules were also shown to have strong antioxidant properties both in vivo and in vitro models.
Conclusions
H. cordata extracts and its bioactive molecules were shown to have both anti-inflammatory and anti-oxidative properties. As both in vitro and in vivo studies were shown that H. cordata did not have any toxicity on the various model systems used, future clinical studies will hopefully make an impact on the future direction of treating inflammation-related diseases.
Abbreviations: NCBI, National Centre for Biotechnology Information; SARS, Severe acute respiratory syndrome; NO, Nitric Oxide; LPS, Lipopolysaccharide; NF-κB, Nuclear factor kappa B; ROS, Reactive Oxygen Species; TNF-α, Tumor Necrosis Factor-α; IL, Interleukin; MCP-1, Monocyte chemotactic protein 1 (CCL2); LTB4, Leukotriene B4; HSV, Herpes simplex virus; PGE2, Prostaglandin E2; COX, Cyclooxygenase; TLR, Toll like receptor; AMPK, 5' AMP-activated protein kinase; GSH, Reduced glutathione; GPx, Glutathione peroxide; SOD, Superoxide dismutase; MDA, Malondialdehyde; STZ, Streptozotocin; DPPH, 2,2-diphenyl-1-picrylhydrazyl; ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid); FRAP, Ferric-reducing antioxidant power; NOS, Nitric oxide synthase; GDH, Glutamate dehydrogenase; DNP-BSA, 2,4-Dinitrophenol-Bovine Serum Albumin; CYP, Cytochrome p450; NLRP3, NACHT, LRR and PYD domains-containing protein 3; GSK-3β, Glycogen synthase kinase 3 beta
Keywords: Houttuynia cordata Thunb, Inflammation, Oxidative stress, Therapy
Graphical abstract
1. Introduction
Houttuynia cordata Thunb. (HC) is an herbaceous, rhizomatous and a perennial plant primarily found in Japan, Korea, China and Southeast Asia. HC is usually grown as leaf and root vegetables especially in moist and shady places (Chopra et al., 2002). Traditionally people of these regions were using this plant extract for the treatment of a number of diseases.
A large number of current scientific evidence have supported the efficacy of HC extract, which has been used by several communities as folk medicines for thousands of years. Different solvent extracts of HC has been used to unveil the scientific knowledge underlying its medicinal value (Fu et al., 2013). HC extracts were shown to be effective against various ailments including cancer, diabetes, obesity, lung fibrosis, skin diseases and severe acute respiratory syndrome (SARS) (Chang et al., 2001, Du et al., 2012, Kumar et al., 2014, Kwon and Kim, 2014, Lu et al., 2006, Miyata et al., 2010). In addition, HC extracts were also shown to have both anti-bacterial and anti-viral activities against a host of pathogens such as methicillin-resistant Staphylococcus aureus, corona and dengue viruses etc (Chiow et al., 2016, Lau et al., 2008, Li et al., 2017, Sekita et al., 2016, Verma et al., 2017). Based upon HC efficacy in improving the immune system of patients infected with the severe acute respiratory syndrome, Chinese experts have also enlisted HC for their national SARS program (Lu et al., 2006).
Oxidative stress and inflammation were considered to be the central mechanisms involved in various disease pathologies. Several studies have provided scientific evidence about the interlinking pathways governing oxidative stress-induced inflammation and vice versa. Thus it became prerogative to selectively target these mechanisms in order to develop therapeutics against many diseases (Khansari et al., 2009, Reuter et al., 2010, Salzano et al., 2014).
Traditional knowledge on medicinal plants and its active molecules were always been the favorite choice of researchers to develop modern drugs. With minimal or no toxicity, medicinal plants have found utmost priority for screening and developing new therapeutics against a number of diseases. Based on traditional knowledge several investigators have studied the efficacy of HC against both oxidative stress and inflammation indued diseases using both in vitro and in vivo models organisms. With both genomic and proteomic approaches, investigators have able to validate the efficacy of HC against several diseases.
In addition to the excellent review presented by Fu et al. (2013), this review presents an up-to-date comprehensive understanding of the anti-inflammatory and antioxidant properties of HC and provides a scientific basis for future research directions.
2. Traditional uses of H. cordata
HC leaves have a unique taste of fishy where it earned its name as fish mint. Chinese people were the first to discover the medicinal properties of this plant and used it for medical purposes as Traditional Chinese Medicine. In Traditional Chinese Medicine it is used for treating pneumonia and SARS (Lau et al., 2008). In North Eastern states of India, it is used as a garnish over ethnic side dishes and leaf salad to lower the blood sugar level. Leaf juices were also known to treat cholera, dysentery, anemia, and purification of blood (Laloo and Hemalatha, 2011). During the growing season, the stem and leaves are harvested and used for preparing a decoction. Traditionally the decoction is used both internally and externally. Internally it is good for the treatment of many ailments including cancer, coughs, dysentery, enteritis and fever and externally it is applied to treat snake bites and skin diseases (Vent, 1987). In Japan, a beverage made from an infusion of the leaves of HC herbs called Dokudami Cha mixed in with other herbal remedies were being used for removing free radicals, reducing inflammation and supporting the immune system.
3. Potential role of H. cordata against inflammation
Inflammation is basically a cellular protective response against various stimuli such as foreign pathogens, irritants or damaged cells. Inflammation has been classified in two principal categories, acute inflammation such as in case of vasodilation or edema, and chronic inflammation as in case of rheumatoid arthritis, periodontitis or atherosclerosis etc (Kumar et al., 2004). The inflammation mechanism involves various types of cells including basophils, eosinophils, neutrophils, mononuclear cells and fibroblast (Kerschensteiner et al., 1999, Pober and Cotron, 1990).
3.1. Role of H. cordata in inflammation-related molecular mechanism in vitro
Different solvent extracts of HC and their active biomolecules have been shown to be effective against inflammation caused various inflammagens ( Table 1). The major macrophage-derived pro-inflammatory cytokine TNF-α, various interleukins (IL) and the reactive free radical NO synthesized by inducible NOS (iNOS) were widely been recognized to be involved in the development of inflammatory diseases (Freeman and Natanson, 2000). In the study done by Park et al., 2005 has shown the aqueous extract from HC were found to inhibit NO and TNF- α (up to 30%) production at 0.06 and 0.12 mg/ml in a dose-dependent manner in LPS induced mouse macrophages (Park et al., 2005). Moreover, the ethanolic extract of HC was found to inhibit the inflammatory biomarkers IL-6 and NO in LPS treated lung epithelial cells (A549) and alveolar macrophages (MH-S) (Lee et al., 2015). Furthermore, the ethanolic extracts were shown to inhibit the NF-κB signaling pathway with inhibited phosphorylation of IκBα, and reduction in TNF- α, IL-6 and IL-8 level in phorbolmyristic acetate (PMA)/calcium ionophore (A23187) induced human mast cells (HMC-1) (Lee et al., 2013). These studies have sufficiently provided evidence of the anti-inflammatory role of HC extracts against both bacterial and ionic toxins.
Table 1.
Inhibition of inflammation by different solvent extracts H. cordata in vivo and in vitro models.
| Extracts | Doses | Standard drug | Inflammagen used | Model used | Time period | Minimal active concentration | Most potent biomolecule | Reference |
|---|---|---|---|---|---|---|---|---|
| Afzelin | 50,100,200 mg/kg | None | LPS (40 μg·kg1) /D-galactosamine (800 mg·kg1) | Mice | 5 h | 200 mg/kg | Afzelin | (Lee et al., 2017) |
| Ethanol (100%) | 0.1,0.5,1% | None | LTA(1 µg/ml) | RT-7 | 24hrs | 0.5% | Not mentioned | (Sekita et al., 2016) |
| Ethanol (80%) | 400,600,1000 mg/kg Per day | None | Oxaliplatin (6 mg/kg) | Male Sprague Dawley rat (Th17 and Tregs cells) | 15 days | 1000 mg/kg/day | Not mentioned | (Wan et al., 2016) |
| Becatamide | 0,0.05,0.25,0.5 µm | NS-398 | None | Swiss Webster mice | 10 min | 0.25 µm | Houttuynamide A | (Park, 2015) |
| Ethanol (70%) | 30, 100, 300 µg/ml 100, 400 mg/kg | Dexomethasone and 2-Amino− 5,6-dihydro− 6-methyl-4H− 1,3-thiazine hydrochloride (AMT) | LPS | A549, MH-S and Mouse | 16 h | 100 mg/kg | Quercitrin, Hyperoside, Afzelin | (Lee et al., 2015) |
| Polysaccharides | 40,80,160 mg/kg | Dexomethasone | LPS | BALB/cA mice | 24 h | 40 mg/kg | Not mentioned | (Xu et al., 2015) |
| Polysaccharides | 1, 10, and 100 μg/ml | Dexomethasone | LPS | Macrophage | 24 h | 10 μg/ml | Not mentioned | (Xu et al., 2015) |
| Essential oil (Sodium houttuyfonate and 2 undecanone) | 0.1, 1, 10, 20 μg/ml | None | LPS (1 µg/ml) | RAW 264.7 | 24hrs |
|
Sodium houttuyfonate | (Chen et al., 2014a) |
| Essential oil (Sodium houttuyfonate and 2-Undecanone) | 100,200,400 mg/kg | Aspirin | Xylene | Mouse | 30 min | 200 mg/kg | sodium houttuyfonate | (Chen et al., 2014a) |
| Ethyl acetate | 25,50,100,200 μg/ml | None | LPS(1 µg/ml) | RAW 264.7 | 20hrs | 25 µg/ml | Chlorogenic acid, hyperoside, quercitrin | (Chun et al., 2014) |
| Aqueous | 1 and 2 g/L | None | Acetaminophen (350 mg/kg body weight) | BALB/cA mice | 4 weeks | 2 g/L | Not mentioned | (Chen et al., 2014b) |
| Ethanol | 0.05,0.1,0.2, mg/ml | None | PMA + Ca+ ionophore (A23187) | HMC-1 | 5hrs | 0.2 mg/ml | Not mentioned | (Lee et al., 2013) |
| Volatile oil | 1,10,100,1000 μg/ml | None | LPS | Mouse peritoneal macrophage | 24hrs | 100 μg/ml | 2-undecane, n-Decanoic acid, Hexadecanoic acid- methyl ester, 1-Octadecanol, Phytol | (Li et al., 2013) |
| Volatile oil | 20,40 mg/kg | Dexamethasone | Xylene, Formaldehyde, Carrageenan | Mouse | 7days | 20 mg/kg | 2-undecane, n-Decanoic acid, Hexadecanoic acid- methyl ester, 1-Octadecanol, Phytol | (Li et al., 2013) |
| Essential oil | 0.01, 0.1, 1, 10, 100 µg/ml | NS- 398 | LPS(1 µg/ml) | Mouse peritoneal macrophage | 24hrs | 10 µg/ml | Not mentioned | (Li et al., 2011) |
| Hot water | 45, 150, 450 μg/ml | Acyclovir | HSV-2 | HeLa 229 | 6,12,24hrs | 450 μg/ml | quercetin, quercitrin or isoquercitrin, | (Chen et al., 2011) |
| Supercritical solution dissolved in soyabean oil | 65,200 mg/kg | Dexomethasone/ Indomethacin | Carrageenan (1 ml) | Male ICR mice | 1hrs | 200 mg/kg | Not mentioned | (Shin et al., 2010) |
| Supercritical solution dissolved in soyabean oil | 0.001, 0.01, 0.1,1% | None | LPS(2.5 µg/ml) | RAW 264.7 | 24hrs | 0.01% | Not mentioned | (Shin et al., 2010) |
| Sodium houttuyfonate | 60, 120 mg/kg | None | C-BSA | BALB/cMice | 66days | 120 mg/kg | Sodium houttuyfonate | (Pan et al., 2010) |
| Aqueous | 0.5 – 3 g/kg | None | DNP-BSA | ICR Mouse | 2 h | 0.5 g/kg | Not mentioned | (Han et al., 2009) |
| Aqueous | 1, 10, 20 µg/ml | None | DNP-BSA | RBL-2H3 | 30 min | 1 µg/ml | Not mentioned | (Han et al., 2009) |
The mast cell-mediated anaphylactic shock is considered to be one of the important molecular mechanisms involved in allergy associated inflammatory pathophysiology. The histamine released from de-granulated mast cells and changes in intercellular Ca+ are the main factors behind such acute anaphylactic reactions. In a study Li et. al., investigated the anti-allergic activity of HCWE against both systemic and acute anaphylactic reactions in rat peritoneal mast cells (RPMC). The HCWE (0, 2.5, 25, or 250 μg/ml) has shown to inhibit the histamine release and Ca+ uptake in a dose-dependent manner (Li et al., 2005). The possible mechanism of inhibition of histamine release and lower Ca+ uptake was thought to be due to increase in adenylate cyclase activity and subsequent increase in intracellular cAMP level in mast cells. The IgE mediated allergic reactions were also found to play important role in inflammatory pathophysiology. The human basophilic cell expresses a high-affinity IgR receptor known as Fc epsilon RI receptor that can potentially activate allergic reactions. In a study, Shim et al. showed that the HC water extract reduced the IgE binding activity and m RNA expression of both α- and γ- chains of Fc epsilon RI receptor in human KU812F cells. Furthermore, the HC extract was also shown to reduce the Fc epsilon RI mediated histamine release in KU812F cells (Shim et al., 2009). In a yet another study, Han et al. provided evidence of the anti-inflammatory role of HC water extract against IgE mediated allergic response in rat mast RBL-2H3 cells. The HC water extract was shown to suppress the DNP-BSA mediated release of beta-hexosaminidase, histamine, ROS, TNF- α and IL-6 in IgE-sensitized RBL-2H3 cells. Moreover, it suppressed DNP-BSA-induced phosphorylation of Syk, Lyn, LAT, Gab2, PLC γ2, Akt and MAP kinases (Han et al., 2009).
NF-κB and MAPK pathway predominantly regulates inflammation. Similar to water extracts, ethyl acetate fraction of HC was also shown to suppress nuclear translocation of NF-κB p65 subunit and attenuated the activation of MAPKs (p38 and JNK) in LPS primed RAW 264.7 cells. Furthermore, the extract showed to significantly reduce the NO, PGE2, TNF- α and IL-6 levels (Chun et al., 2014).
Similarly, the HSV-2 induced inflammation was also shown to be down-regulated by hot water extract of HC via blocking the NF-κB activation (Chen et al., 2011).
Apart from the HC whole plant solvent extracts, the volatile and essential oils, polysaccharides and bioactive molecules such as sodium houttuyfonate and 2-undecanone extracted from HC were also shown to have anti-inflammatory properties against different inflammations. Concentration-dependent increase in HC volatile oil is found to be associated with suppression of LPS stimulated the production of NO and TNF- α in mouse resident peritoneal macrophages. The volatile oil treatment at a dose of 1, 10, 100, and 1000 µg/ml has shown to inhibit the NO and TNF-α level in a dose-dependent manner. The oil treatment was also found to significantly inhibit the iNOS activity in LPS primed macrophages (IC50 = 562.3 mg/ml). Furthermore, the iNOS and TNF-α level were found to be regulated by HC volatile oil at both translational and transcriptional levels (Li et al., 2013).
The essential oils extracted from HC were shown to significantly reduce the LPS induced inflammation in RAW 264.7 cells. HC extracted via supercritical carbon dioxide at a concentration of 1% solution have shown to reduce the NO and PGE2 level by 98% and 80.4% respectively in LPS treated RAW 264.7 cells (Shin et al., 2010).
The HC polysaccharides, when administered, prevented complement activation and macrophage migration antagonizing nitric oxide and pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) (Xu et al., 2015). HC were found to have the same effect like non-steroidal anti-inflammatory drug (NSAID) and specific COX-2 inhibitor NS-398, in inhibiting LPS induced PGE2 (IC50 value: 44.8 μg/ml) and COX-2 enzyme activity (IC50 value: 30.9 μg/ml) in mouse peritoneal macrophages (Li et al., 2011).
The active biomolecules sodium houttuyfonate and 2-undecanone isolated from HC essential oil were shown to have marked anti-inflammatory effect in LPS primed RAW 264.7 cells. Sodium houttuyfonate were found to more effectively reduce the TNF-α (p < 0.001) and IL-1β level than 2-undecanone at the same concentration (Chen et al., 2014a).
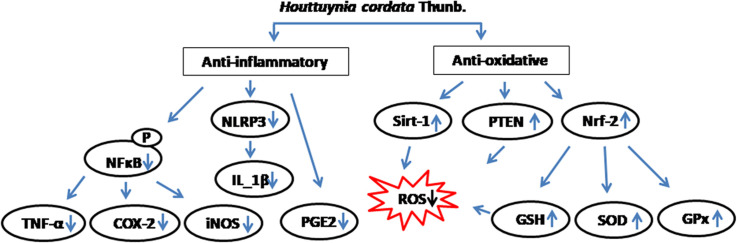
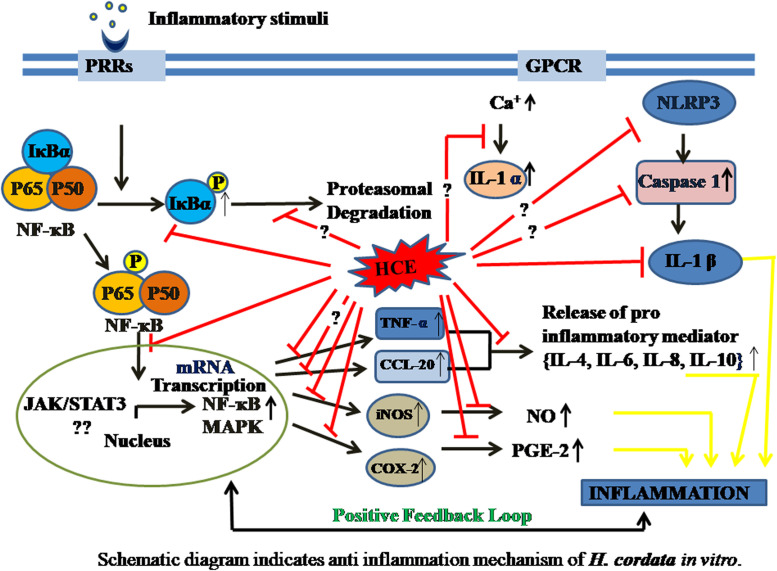
These studies have certainly provided ample evidence of anti-inflammatory properties of HC against several inflammagens at different cell lines. Based on the studies a schematic pathway through which HC acts in different in vitro models were represented in Fig. 1. With different types of immunogens used for the studies, it is apparent that H. cordata can reduce the level both pro- and anti-inflammatory cytokines (TNF-α, IL-1β, IL-4, IL-6, IL-8), and free radicals (NO) known to be involved in inflammatory pathophysiology. Moreover, H. cordata was found to act by inhibiting the iNOS/NF-κB/Ca+/MAPK/Akt signaling cascade.
Fig. 1.
Schematic diagram indicates anti inflammation mechanism of H. cordata in vitro.
3.2. Role of H. cordata in inflammation-related molecular mechanism in vivo
In addition to anti-inflammatory effect in various in vitro models, HC extracts were also shown to have potent anti-inflammatory effect in various in vivo model organisms (Table 1).
In a study done by Lee et al. showed that the ethanolic extract of aerial parts H. cordata possesses an anti-inflammatory effect on LPS induced airway inflammation in male ICR mice models. With the doses of 100 and 400 mg/kg body weight they showed that the total cell count in LPS induced model was found to reduce by 46.1% and 66.5% respectively, which were found to be as effective as dexamethasone (30 mg/kg, 66.1% reduction) (Lee et al., 2015). Further, the oral administration of HC supercritical extracts (200 mg/kg), when tested against carrageenan- air pouce model showed to suppress the exudation and albumin leakage, as well as inflammatory cell infiltration in ICR mice models. Further, when the investigators compared the efficacy of supercritical extracts with standard drugs such as dexamethasone (2 mg/kg, i.p.) and indomethacin (2 mg/kg, i.p.), the extracts showed to more effectively reduce both TNF-α/NO and cyclooxygenase 2/PGE2 pathways (Shin et al., 2010).
Similarly, the aqueous extract of HC was also shown to ameliorate inflammation in acetaminophen-treated Balb/cA mice. In this study, the investigators primarily mixed the aqueous extract with drinking water before acetaminophen was induced. The extract was found to diminish the acetaminophen-induced elevation of TNF-α, IL-6, and IL-10 levels significantly (Chen et al., 2014b).
In another study, using Sprague dawki rats, Wan et al. showed that the ethanolic extract of HC can decrease the expression level of IL-6, macrophage inflammatory protein-1 α (MIP-1α) in oxaliplatin induced neuropathic models (Wan et al., 2016). They showed that the ethanolic extract reduces the inflammation via PI3K/Akt/mTOR signaling pathway.
Similar to HC solvent extracts, the isolated flavonoids, bioactive molecules, polysaccharides and volatile oils extracted from HC has also shown to profound anti-inflammatory effect in various in vivo models.
Upon oral administration of isolated flavonoids (afzelin, hyperoside, and quercitrin) at 100 mg/kg body weight, quercitrin showed the maximum inhibition of up to 81.3% in total cell number in the bronchoalveolar fluid in LPS induced male ICR mouse (Lee et al., 2015).
The afzelin isolated from a methanolic fraction of HC when administered intra-peritoneally (200 mg/kg) in D-galactosamine/LPS induced mice model, it decreased the level of inflammatory cytokines (TNF-α and IL-6) and AMPK expression and increased the Sirtuin-1 (Sirt-1) expression (Lee et al., 2017).
Cyclooxygenase is an important regulator of inflammation regulating PGE2. Presence of unmodified 3-hydroxyl and 4-hydroxyl groups is critical for inhibition of COX 1/2 as an important therapeutic target for inflammation (Park, 2015). Among derivative from H. cordata, becatamide (houttuynamide A) showed the most potent inhibitor of the COX-1 enzyme by (IC50 = 0.27 μM), COX-2 enzyme (IC50 = 0.78 μM) in mice. Upon comparison with specific COX-1 inhibitor aspirin (IC50 = 3.57 μM), becatamide was found to be more potent in inhibiting COX-1. Evidently, becatamide was also found to inhibit collagen-induced production of TXB2 (thromboxane) by 35% and the P-selectin expression on mice platelets by 28% (Park, 2015). Furthermore, becatamide were also shown to reduce the basal P-selectin (unactivated) at a very low concentration of 0.25 μM (Park, 2015).
The complement system, a part of the innate immune mechanism, provides an effective defense of the body against the foreign pathogens. However, an over-activation of complement system has also been found to play a major role in the inflammatory pathogenesis of acute lung injury. The polysaccharides extracted from HC were shown to effectively inhibit the complement deposition in acute lung injured Balb/c mice. The polysaccharides (40, 60, 120 mg/kg) were shown to effectively attenuate the acute lung injury induced by LPS in a dose dependent manner. The level of TNF-α and TLR-4 expression was found to be effectively reduced by the polysaccharides (Xu et al., 2015).
To explain how HC is linked to anti-inflammation against multiple inflammagens such as xylene, formaldehyde and carrageenan, Li et. al. treated Kunming mice with volatile oils extracted from HC. Upon treatment with volatile oils (20 and 40 mg/ml/kg) in xylene-induced ear edema models, the formation of edema was found to be significantly inhibited in a dose dependent manner (35.3% and 67.6% respectively). While dexamethasone (5 mg/kg) was shown to inhibit edema formation by 44.1%. The volatile oil treatment at a dose of 20 mg/ml and 40 mg/ml also showed to inhibit the formaldehyde-induced paw edema by 24.6% and 11.9% respectively. However, in this case, the efficacy of volatile oils was found to be less than dexamethasone (5 mg/ml; 35.4%). Similarly, the efficacy of volatile oil extracts was also found to reduce the carrageenan-induced paw edema (Li et al., 2013).
In a study, Chen et al. tested the active components of volatile oils such as sodium houttuyfonate and 2-undecanone on xylene-induced ear edema model. They found that the volatile oils inhibit the xylene-induced ear edema at a dose of 100 mg/kg body weight (Chen et al., 2014a). In another study, sodium houttuyfonate were shown to reduce the toxicity of cationic bovine serum albumin (cBSA) by inhibiting the cytokine production via down-regulating NF-κB and MCP expressions (Pan et al., 2010).
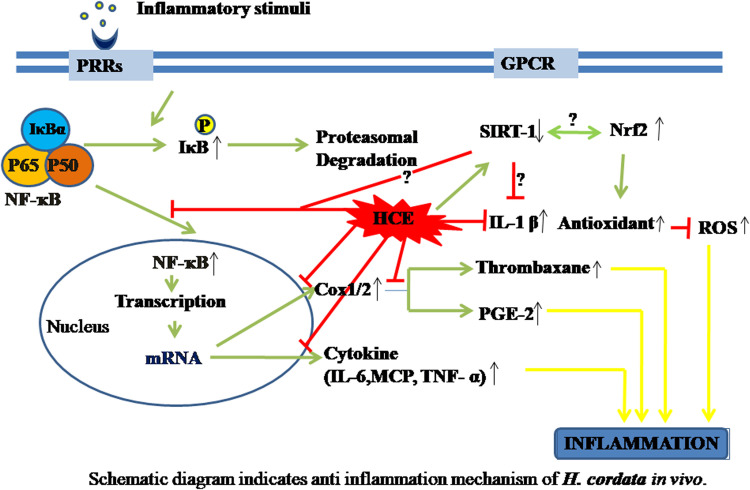
With different in vivo models along with different inflammagens including acetaminophen, xylene, formaldehyde, carrageenan, D-galactosamine, and LPS, several investigators have provided ample scientific evidence of anti-inflammatory activity of HC. Similar to in vitro model studies, HC has also shown to effectively reduce inflammation in several in vivo models. Based on the in vivo model studies a schematic pathway through which HC acts were shown in Fig. 2. The volatile oils extracted from HC were found to be most effective at a very low concentration of 20 mg/ml. The polysaccharides, as well as sodium houttuyfonate and 2-undecanone, were also found to effectively reduce inflammation at a dose of 40 mg/kg, 100 mg/kg, and 100 mg/kg respectively.
Fig. 2.
Schematic diagram indicates anti inflammation mechanism of H. cordata in vivo.
4. Potential role of H. cordata against oxidative stress
Oxidative stress plays a major role in the pathology of several inflammatory diseases. Oxidative stress is mainly viewed as the imbalance between the productions of reactive oxygen and their elimination mechanism from the body (Biswas, 2016). Several investigators have provided evidence of antioxidant properties of HC and its bioactive molecules ( Table 2). By using multiple antioxidant assay method, diverse studies have illustrated the strong antioxidant properties of HC both in vitro and in vivo models.
Table 2.
Inhibition of oxidative stress by different solvent extracts of H. cordata in vivo and in vitro models.
| Extracts | Doses | Standard drug | Inflammagen used | Model used | Time period | Minimal concentration | Most potent biomolecule | Reference |
|---|---|---|---|---|---|---|---|---|
| Afzelin | 50,100,200 mg/kg | None | LPS/D galactosamine | Mice | 1 h | 200 mg/kg | Afzelin | (Lee et al., 2017) |
| Ethanol | 100,200,400 mg/kg | Glibenclamide | Streptozotocin | Albino rat | 7, 14, 21 days | 200 mg/kg | Quercetin | (Kumar et al., 2014) |
| 50% Ethanol | 50 µg/ml | None | Palmitate | HAECS | 3 h | 50 µg/ml | chlorogenic acid, rutin, and quercitrin, | (Yang et al., 2015) |
| Aqueous | 1, 2 g/L | None | acetaminophen | BALB/cA mice | 4 weeks | 2 g/L | Not mentioned | (Chen et al., 2014b) |
| Methanol | 50,100,200,400 mg/kg | Silymarin | Carbon tetrachloride | Mice | 7 weeks | 200 mg/kg | Not mentioned | (Kang and Koppula, 2014) |
| 50% Methanol | 25,50,100 μg/ml | None | H2O2 | Human peripheral blood lymphocytes | 30 mins | 25 µg/ml | Quercitin, Myricetin, Kaempferol | (Lin et al., 2013) |
| 80% Methanol | 500,1000 mg/kg/day (Pharmacologically/ Therapeutically irrelevant) | None | Gentamicin sulphate | Sprague Dawley rat | 12 days | 500 mg/kg/day | Not mentioned | (Kang et al., 2013) |
| Ethyl acetate | 250,500,1000 mg/kg (Pharmacologically/ Therapeutically irrelevant) | None | Carbon tetrachloride | Kunming Mice | 8days | 500 mg/kg | Quercitrin, quercetin, hyperoside | (Tian et al., 2012) |
| Aqueous | 1, 10, 20 µg/ml | None | DNP-BSA | RBL-2H3 | 30 mins | 1 µg/ml | Not mentioned | (Han et al., 2009) |
| Aqueous | 1 g/10 ml/kg | None | Bleomycin | Male Wistar rats | 5 weeks | 1 g/10 ml/kg | Not mentioned | (Ng et al., 2007) |
| Aqueous | 0, 2, 5% | None | Oxidized Fried Oil | Sprague Dawley rat | 28 days | 2% | Not mentioned | (Chen et al., 2003) |
4.1. Role of H. cordata in oxidative stress-related molecular mechanism in vitro
Nitric oxide (NO) plays a predominant role in maintaining homeostasis especially in the vascular systems as well as a part of the immune system. Vascular oxidative stress leads to lower NO production and endothelial dysfunction. In a study, Yang et al. showed the antioxidative properties of 50% methanolic extract of HC against palmitate-induced oxidative stress in human aortic endothelial cells. The authors showed that HC extract increased NO production through insulin-mediated eNOS phosphorylation in human aortic endothelial cells (Yang et al., 2015).
In another study by Doi et al. showed HC extract to have a positive effect on ROS related photoaging and barrier-disrupted skin problems. HC extract inhibited the generation of ROS in TNF-α/ benzo (α) pyrene stimulated human keratinocyte cells. In addition, the extract activated aryl hydrocarbon receptor (AHR) and nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2), with subsequent induction of the antioxidative-enzyme quinone oxidoreductase 1 in human keratinocytes (Doi et al., 2014). The water extract was also shown to reduce the production of ROS in DNP-BSA mediated oxidative stress in IgE sensitized RBL-2H3 cells (Han et al., 2009). Interestingly, the aqueous extracts of HC was also found to have potent anti-lipid peroxidation activity (IC50 = 1.02 mg/ml) similar to vitamin E (IC50 = 0.94 mg/ml) (Ng et al., 2007).
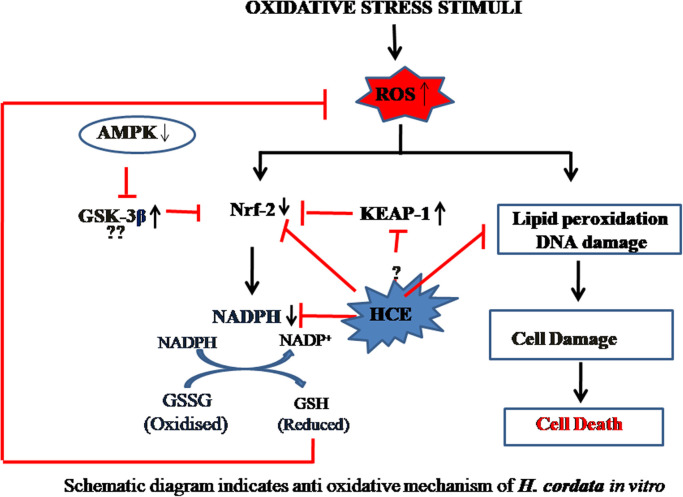
Thus these studies validate the efficacy of HC extracts against oxidative stress induced by ROS in different in vitro models. Similar to whole plant extracts, the active bioactive molecules isolated form HC plant were also shown to have an antioxidant effect on various cell models. Moreover, the active molecules were also shown to have a protective effect against oxidative stress-induced protein fragmentation. In a study done by Toda et al., showed the polyphenolic compounds derived from aqueous extract inhibits bovine serum albumin fragmentation assaulted by copper–hydrogen peroxide in a dose dependent manner (Toda, 2005). The active polyphenol, quercitin were shown to protect DNA damage from H2O2 induced oxidative stress in lymphocytes at a very lower 10 µM concentration (Lin et al., 2013). Whereas, at the higher concentrations 100 µM and above, it was shown to induce DNA breaks in lymphocytes. Based on the in vitro studies, a summary diagram of various interactive pathways of H. cordata anti-inflammatory activity was shown in Fig. 3.
Fig. 3.
Schematic diagram indicates anti oxidative mechanism of H. cordata in vitro.
4.2. Role of H. cordata in oxidative stress-related molecular mechanism in vivo
The enzymes responsible for metabolizing xenobiotic compounds plays a very important role in oxidative stress-related diseases. Among them, the cytochrome p450, glutathione S-transferase, superoxide dismutase and catalase plays a major role in scavenging free radicals. It is known that CYP2E1 augment oxidative stress with excessive production of reactive electrophile and free radicals such as reactive oxygen species. Cells attempt to counteract the toxicity of oxidative stress and redox balance by activating defense antioxidant. With the treatment of HC aqueous extract (2 g/l) showed suppressing CYP2E1 activity and reversing of antioxidant enzymes like hepatic GSH, catalase, SOD, GPx activity along with decrease ROS, lipid peroxidation, oxidized glutathione (GSSG) in acetaminophen-induced BALB/cA mice (Chen et al., 2014b). This report was also supported by the studies by Hsu et al. in diabetic mice. The extract (2%) was shown to restore the glutathione level while suppressing the ROS and protein carbonyl level in heart and kidney cells of diabetic rats (Hsu et al., 2016). In addition, biochemical analysis of lung in Wistar rats have demonstrated that HC aqueous extract (1 g/10 ml/kg), have an ability to inhibit pulmonary fibrosis caused by bleomycin with a reduction in superoxide dismutase activity and increased catalase activity (Ng et al., 2007).
Mitochondrial oxidative stress is characterized by oxidative damage and decreased antioxidant enzymes activity. In a study done by Kumar et al. showed that HC extract (200 and 400 mg/kg) attenuate the streptozotocin-induced mitochondrial oxidative stress and stabilized the mitochondrial function and integrity in the pancreatic β-cells and reversed diabetes-induced MDA levels significantly. Moreover, administration of HC (200 and 400 mg/kg) significantly increased the SOD activity in liver, pancrease and adipose tissue (Kumar et al., 2014).
In a study Kang et al. examined antioxidant properties of HC and its protective effect on gentamicin-induced oxidative stress in rats. Methanolic extract of HC (500/1000 mg/kg) significantly increased the level of GSH, SOD and catalase activity in kidney tissues in a gentamicin-induced rat model (Kang et al., 2013).
In another study, Chen et al. investigated the antioxidant effect of HC on oxidized fried oil fed rat model. The fried oil fed rat when supplemented with aqueous extract of HC(2–5%), the plasma TBARS and protein carbonyl content was observed to be lower than the control groups (Chen et al., 2003).
Apart from the HC extracts, the active bio-molecules were also shown to have potent anti-oxidative properties in various in vivo models. The polyphenolic compounds such as quercetin, quercitrin, and hyperoside extracted from ethyl acetate fraction of HC (1000 mg/kg) were shown to inhibit the increase in GSH level; SOD and CAT activity in CCl4 induced oxidative stress in mouse liver in a dose dependent manner (Tian et al., 2012).
Mitochondria are considered to be the primary source of oxidative stress as it utilizes oxygen to produce energy. Alternatively, excessive oxidative stress has been found to be associated with mitochondrial dysfunction. The afzelin (200 mg/kg) isolated from the methanol extract of HC were shown to regulate both mitophagy and mitochondrial biogenesis through Rev‐Erb‐α/phospho‐AMPK/SIRT1 signaling. Afzelin was found to attenuate the increased gene expression of mitophagy related PINK1 and perkin proteins. In addition, it also inhibited mitochondrial dysfunction by attenuating reduction of mitochondrial GDH activity and hepatic ATP production in D-galactosamine/LPS induced hepatic injury (Lee et al., 2017).
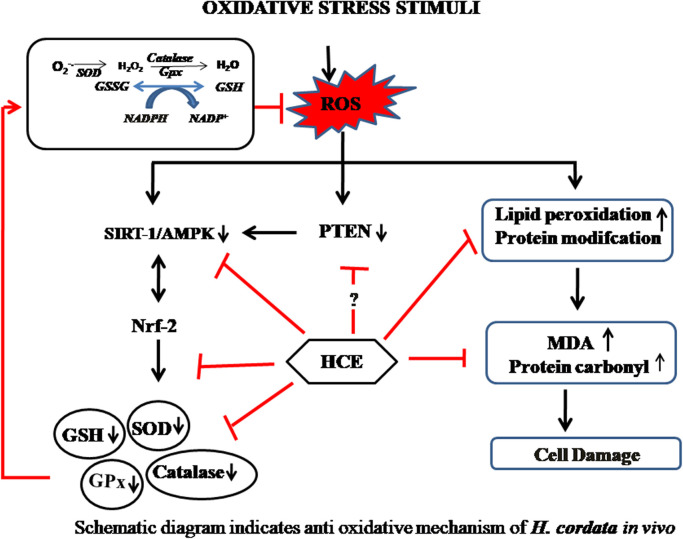
Thus, both the in vitro and in vivo data provides sufficient evidence of anti-oxidative properties of HC extracts and its bioactive molecules. Based upon the various in vivo studies, a schematic has been drawn on the anti-inflammatory effect of HC ( Fig. 4).
Fig. 4.
Schematic diagram indicates anti oxidative mechanism of H. cordata in vivo.
5. Toxicity of H. cordata
Till date, HC was found to be non-toxic to various in vitro and in vivo models used for different investigations (Yoshino et al., 2005, Zhang et al., 2010). The study conducted by Yoshino et al. reported that dietary level of 1.5% (999 mg/kg body weight per day) for males and 0.5% (350 mg/kg body weight per day) for females showed No Observed Adverse Effect Level (NOAEL) of HC extract in rats (Yoshino et al., 2005). Though it may not show any side effects, some Chinese study found that HC may induce an allergic reaction in some people. However some studies have suggested that HC injection should not be given with common cold only to children, pregnant women and patients as interaction with other medicines may induce allergic reaction (Chen et al., 2006, Ji et al., 2009, Yang et al., 2007). However, the reasons behind such allergic reactions have not been deciphered yet. Due to less known side effects, HC as extracts and its bioactive molecules may be used for medical implication.
6. Conclusion and future perspectives
HC has the ability to influence regulatory mechanism involved in inflammation and oxidative stress both in vivo and in vitro. The studies based on the action mechanism of HC, through which it acts as an anti-inflammatory agent, shows that it inhibits mainly the NF-κB/MAPK pathway and decreases the level of inflammatory cytokines and chemokines. The anti-inflammatory and anti-oxidant activities of HC were thought to be due to the presence of several polyphenols (quercitrin, quercetin, and hyperoside) and volatile oils (2-undecane, n-decanoic acid, hexadecanoic acid- methyl ester, 1-octadecanol, and phytol).
In a few studies, it has been mentioned that they have used HC extract at a dose of more than 400 mg/kg body weight and showed it has very high antioxidant activities. However, it seems to be pharmacologically or rather therapeutically not feasible to use any drug molecules at these high doses. Moreover, a large number of studies showed in silico anti-oxidant assays pertaining to find out the antioxidant activity of HC which also seems to be not at par with contemporary scientific understandings. In addition, a large of plant bioactive molecules tested having strong anti-oxidant potential in silico, seems to have either very low or no anti-oxidative activity in either various animal models tested or in clinical trials. Thus it will be very important to go for more in-depth studies in order to establish the anti-inflammatory and anti-oxidant properties of HC.
In addition, there are still some research gaps to be fulfilled for a better understanding of the mechanistic pathways behind its activity. Firstly, the routes of entry of HC constituents into the cell will be of utmost importance, in order to understand the basic characteristics of the drug molecules interact with the cellular membrane. Secondly, in order to validate the possibility of the molecules of HC as a drug, further studies on its ability to cross blood brain barrier, skin permeability, lipophilicity as well as its pharmacodynamics properties will be useful. Thirdly, the role of HC extract/active molecules in calcium signaling is yet to be studied. Though a few studies have shown HC to inhibit the calcium uptake, still its role has to identify in order to comprehend information on its effect in cAMP/calcium signaling. Recent studies on inflammation have opened up the area of NLRP3 signaling. NLRP3 is found to be predominantly expressed in macrophages and is a component of inflammasome complex. Thus fourthly, elucidation of HC role in NLRP3 inflammasome is needed to understand its anti-inflammatory activities. Furthermore, studies concerning the role played by HC in GSK3β signaling will be required in order to understand its role in oxidative stress-induced diseases. Thus due to its less toxic nature and having both anti-inflammatory and antioxidant properties, HC may find its utility as a lead plant to treat inflammatory and oxidative stress-related diseases in near future.
Acknowledgment
This work was supported by the Council of Scientific and Industrial Research (CSIR), New Delhi (31/25(128)/2017-EMR-I and 19/06/2016(i)EU-V). PM is thankful to DBT, Govt. of India (GAP-0733) for providing the Ramalingaswami Re-entry Fellowship. KS and TD are thankful to the CSIR for providing the JRF and SRF respectively. Authors would like to thank the Director, CSIR-North East Institute of Science & Technology, Jorhat for all-round support to carry out the work.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Equal contribution
KS and TD have contributed equally to this manuscript.
Authors contribution
KS and TD: Planned, reviewed the previous literature and wrote the manuscript.
JK and PM: Edited and reviewed the manuscript.
References
- Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016;2016:9. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.S., Chiang L.C., Chen C.C., Liu L.T., Wang K.C., Lin C.C. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am. J. Chin. Med. 2001;29(2):303–312. doi: 10.1142/S0192415X01000320. [DOI] [PubMed] [Google Scholar]
- Chen J., Wang W., Shi C., Fang J. A comparative study of sodium houttuyfonate and 2-undecanone for their in vitro and in vivo anti-inflammatory activities and stabilities. Int. J. Mol. Sci. 2014;15(12):22978–22994. doi: 10.3390/ijms151222978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.T., Yang C.L., Yin M.C. Protective effects from Houttuynia cordata aqueous extract against acetaminophen-induced liver injury. Biomedicine (Taipei) 2014;4:5. doi: 10.7603/s40681-014-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang H.Y., Liu Y.C., Xi H.T., Xi R.R. A foreign patient with anaphylactic shock caused by intravenous Houttuynia cordata injection. Chin. Pharm. Aff. 2006;3:153. [Google Scholar]
- Chen X., Wang Z., Yang Z., Wang J., Xu Y., Tan R.X., Li E. Houttuynia cordata blocks HSV infection through inhibition of NF-kappaB activation. Antivir. Res. 2011;92(2):341–345. doi: 10.1016/j.antiviral.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Y., Liu J.F., Chen C.M., Chao P.Y., Chang T.J. A study of the antioxidative and antimutagenic effects of Houttuynia cordata Thunb. using an oxidized frying oil-fed model. J. Nutr. Sci. Vitaminol. (Tokyo) 2003;49(5):327–333. doi: 10.3177/jnsv.49.327. [DOI] [PubMed] [Google Scholar]
- Chiow K.H., Phoon M.C., Putti T., Tan B.K., Chow V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016;9(1):1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R.N., Nayar S.L., Chopra I.C. National Institute of Science Communication and Information Resources; New Delhi: 2002. Glossary of Indian Medicinal Plants; p. 330. [Google Scholar]
- Chun J.M., Nho K.J., Kim H.S., Lee A.Y., Moon B.C., Kim H.K. An ethyl acetate fraction derived from Houttuynia cordata extract inhibits the production of inflammatory markers by suppressing NF-small ka, CyrillicB and MAPK activation in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement. Altern. Med. 2014;14:234. doi: 10.1186/1472-6882-14-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K., Mitoma C., Nakahara T., Uchi H., Hashimoto-Hachiya A., Takahara M., Tsuji G., Nakahara M., Furue M. Antioxidant Houttuynia cordata extract upregulates filaggrin expression in an aryl hydrocarbon-dependent manner. Fukuoka Igaku Zasshi. 2014;105(11):205–213. [PubMed] [Google Scholar]
- Du S., Li H., Cui Y., Yang L., Wu J., Huang H., Chen Y., Huang W., Zhang R., Yang J., Chen D., Li Y., Zhang S., Zhou J., Wei Z., Chow N.T. Houttuynia cordata inhibits lipopolysaccharide-induced rapid pulmonary fibrosis by up-regulating IFN-gamma and inhibiting the TGF-beta1/Smad pathway. Int. Immunopharmacol. 2012;13(3):331–340. doi: 10.1016/j.intimp.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B.D., Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin. Investig. Drugs. 2000;9(7):1651–1663. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- Fu J., Dai L., Lin Z., Lu H. Houttuynia cordata. Thunb: a Review of Phytochemistry and Pharmacology and Quality Control. Chin. Med. 2013;4(3):23. [Google Scholar]
- Han E.H., Park J.H., Kim J.Y., Jeong H.G. Houttuynia cordata water extract suppresses anaphylactic reaction and IgE-mediated allergic response by inhibiting multiple steps of FcepsilonRI signaling in mast cells. Food Chem. Toxicol. 2009;47(7):1659–1666. doi: 10.1016/j.fct.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Hsu C.C., Yang H.T., Ho J.J., Yin M.C., Hsu J.Y. Houttuynia cordata aqueous extract attenuated glycative and oxidative stress in heart and kidney of diabetic mice. Eur. J. Nutr. 2016;55(2):845–854. doi: 10.1007/s00394-015-0994-y. [DOI] [PubMed] [Google Scholar]
- Ji, K.M., Li, M., Chen, J.J., Zhan, Z.K., Liu, Z.G., 2009. Anaphylactic shock and lethal anaphylaxis caused by Houttuynia cordata injection, a herbal treatment in The State Administration of Traditional Chinese Medicine of People’s Republic of China (SATCM) Bulletin. [DOI] [PubMed]
- Kang C., Lee H., Hah D.Y., Heo J.H., Kim C.H., Kim E., Kim J.S. Protective effects of Houttuynia cordata Thunb. on Gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicol. Res. 2013;29(1):61–67. doi: 10.5487/TR.2013.29.1.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Koppula S. Hepatoprotective effect of Houttuynia cordata Thunb extract against carbon tetrachloride-induced hepatic damage in mice. Indian J. Pharm. Sci. 2014;76(4):267–273. [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M., Gallmeier E., Behrens L., Leal V.V., Misgeld T., Klinkert W.E.F., Kolbeck R., Hoppe E., Oropeza-Wekerle R.-L., Bartke I., Stadelmann C., Lassmann H., Wekerle H., Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory Brain Lesions: a neuroprotective role of inflammation? J. Exp. Med. 1999;189(5):865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3(1):73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- Kumar M., Laloo D., Prasad S.K., Hemalatha S. Antihyperglycemic activity of Houttuynia cordata Thunb. in Streptozotocin-induced diabetic rats. Adv. Pharmacol. Sci. 2014;2014:809438. doi: 10.1155/2014/809438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Clermont G., Vodovotz Y., Chow C.C. The dynamics of acute inflammation. J. Theor. Biol. 2004;230(2):145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Kwon T.K., Kim J.C. In vitro skin permeation and anti-atopic efficacy of lipid nanocarriers containing water soluble extracts of Houttuynia cordata. Drug Dev. Ind. Pharm. 2014;40(10):1350–1357. doi: 10.3109/03639045.2013.819883. [DOI] [PubMed] [Google Scholar]
- Laloo D., Hemalatha S. Ethnomedicinal plants used for diarrhea by tribals of Meghalaya, Northeast India. Pharmacogn. Rev. 2011;5(10):147–154. doi: 10.4103/0973-7847.91108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.M., Lee K.M., Koon C.M., Cheung C.S., Lau C.P., Ho H.M., Lee M.Y., Au S.W., Cheng C.H., Lau C.B., Tsui S.K., Wan D.C., Waye M.M., Wong K.B., Wong C.K., Lam C.W., Leung P.C., Fung K.P. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118(1):79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Seo H.S., Kim G.J., Jeon C.Y., Park J.H., Jang B.H., Park S.J., Shin Y.C., Ko S.G. Houttuynia cordata Thunb inhibits the production of pro-inflammatory cytokines through inhibition of the NFkappaB signaling pathway in HMC-1 human mast cells. Mol. Med. Rep. 2013;8(3):731–736. doi: 10.3892/mmr.2013.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Ahn J., Kim J.W., Lee S.G., Kim H.P. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch. Pharm. Res. 2015;38(7):1304–1311. doi: 10.1007/s12272-015-0585-8. [DOI] [PubMed] [Google Scholar]
- Lee S.B., Kang J.W., Kim S.J., Ahn J., Kim J., Lee S.M. Afzelin ameliorates D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure by modulating mitochondrial quality control and dynamics. Br. J. Pharmacol. 2017;174(2):195–209. doi: 10.1111/bph.13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.Z., Chai O.H., Lee M.S., Han E.H., Kim H.T., Song C.H. Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biol. Pharm. Bull. 2005;28(10):1864–1868. doi: 10.1248/bpb.28.1864. [DOI] [PubMed] [Google Scholar]
- Li T., Liu L., Wu H., Chen S., Zhu Q., Gao H., Yu X., Wang Y., Su W., Yao X., Peng T. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antivir. Res. 2017;144:273–280. doi: 10.1016/j.antiviral.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Li W., Fan T., Zhang Y., Fan T., Zhou P., Niu X., He L. Houttuynia cordata Thunb. volatile oil exhibited anti-inflammatory effects in vivo and inhibited nitric oxide and tumor necrosis factor-α production in LPS-stimulated mouse peritoneal macrophages in vitro. Phytother. Res. 2013;27(11):1629–1639. doi: 10.1002/ptr.4905. [DOI] [PubMed] [Google Scholar]
- Li W., Zhou P., Zhang Y., He L. Houttuynia cordata, a novel and selective COX-2 inhibitor with anti-inflammatory activity. J. Ethnopharmacol. 2011;133(2):922–927. doi: 10.1016/j.jep.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.H., Yang Y.Y., Yang C.M., Huang M.Y., Lo H.F., Liu K.C., Lin H.S., Chao P.Y. Antioxidant activity of herbaceous plant extracts protect against hydrogen peroxide-induced DNA damage in human lymphocytes. BMC Res. Notes. 2013;6:490. doi: 10.1186/1756-0500-6-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.M., Liang Y.Z., Yi L.Z., Wu X.J. Anti-inflammatory effect of Houttuynia cordata injection. J. Ethnopharmacol. 2006;104(1–2):245–249. doi: 10.1016/j.jep.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M., Koyama T., Yazawa K. Water extract of Houttuynia cordata Thunb. leaves exerts anti-obesity effects by inhibiting fatty acid and glycerol absorption. J. Nutr. Sci. Vitaminol. (Tokyo) 2010;56(2):150–156. doi: 10.3177/jnsv.56.150. [DOI] [PubMed] [Google Scholar]
- Ng L.T., Yen F.L., Liao C.W., Lin C.C. Protective effect of Houttuynia cordata extract on bleomycin-induced pulmonary fibrosis in rats. Am. J. Chin. Med. 2007;35(3):465–475. doi: 10.1142/S0192415X07004989. [DOI] [PubMed] [Google Scholar]
- Pan P., Wang Y.J., Han L., Liu X., Zhao M., Yuan Y.F. Effects of sodium houttuyfonate on expression of NF-kappaB and MCP-1 in membranous glomerulonephritis. J. Ethnopharmacol. 2010;131(1):203–209. doi: 10.1016/j.jep.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Park E., Kum S., Wang C., Park S.Y., Kim B.S., Schuller-Levis G. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. Am. J. Chin. Med. 2005;33(3):415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]
- Park J.B. Becatamide found in Houttuynia cordata suppresses P-selectin expression via inhibiting COX enzyme, not increasing cAMP in platelets. Phytother. Res. 2015;29:1381–1387. doi: 10.1002/ptr.5391. [DOI] [PubMed] [Google Scholar]
- Pober J.S., Cotron R.S. The role of endothelial cells in inflammation. Transplantation. 1990;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzano S., Checconi P., Hanschmann E.-M., Lillig C.H., Bowler L.D., Chan P., Vaudry D., Mengozzi M., Coppo L., Sacre S., Atkuri K.R., Sahaf B., Herzenberg L.A., Herzenberg L.A., Mullen L., Ghezzi P. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA. 2014;111(33):12157–12162. doi: 10.1073/pnas.1401712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekita Y., Murakami K., Yumoto H., Mizuguchi H., Amoh T., Ogino S., Matsuo T., Miyake Y., Fukui H., Kashiwada Y. Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci. Biotechnol. Biochem. 2016;80(6):1205–1213. doi: 10.1080/09168451.2016.1151339. [DOI] [PubMed] [Google Scholar]
- Shim S.Y., Seo Y.K., Park J.R. Down-regulation of FcepsilonRI expression by Houttuynia cordata Thunb extract in human basophilic KU812F cells. J. Med. Food. 2009;12(2):383–388. doi: 10.1089/jmf.2007.0684. [DOI] [PubMed] [Google Scholar]
- Shin S., Joo S.S., Jeon J.H., Park D., Jang M.J., Kim T.O., Kim H.K., Hwang B.Y., Kim K.Y., Kim Y.B. Anti-inflammatory effects of a Houttuynia cordata supercritical extract. J. Vet. Sci. 2010;11(3):273–275. doi: 10.4142/jvs.2010.11.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Shi X., Yu L., Zhu J., Ma R., Yang X. Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. J. Agric. Food Chem. 2012;60(18):4641–4648. doi: 10.1021/jf3008376. [DOI] [PubMed] [Google Scholar]
- Toda S. Antioxidative effects of polyphenols in leaves of Houttuynia cordata on protein fragmentation by copper-hydrogen peroxide in vitro. J. Med. Food. 2005;8(2):266–268. doi: 10.1089/jmf.2005.8.266. [DOI] [PubMed] [Google Scholar]
- Vent, W., 1987. Duke, J. A. & Ayensu, E. S., Medicinal Plants of China. 2 Vols. 705 S., 1300 Strichzeichnungen. Reference Publ., Inc., Algonac. Michigan, 1985. Feddes Repertorium 98(7-8), 398-398.
- Verma R.S., Joshi N., Padalia R.C., Singh V.R., Goswami P., Kumar A., Iqbal H., Verma R.K., Chanda D., Chauhan A., Saikia D. Chemical composition and allelopathic, antibacterial, antifungal, and antiacetylcholinesterase activity of Fish-mint (Houttuynia cordataThunb.) from India. Chem. Biodivers. 2017;14:e1700189. doi: 10.1002/cbdv.201700189. [DOI] [PubMed] [Google Scholar]
- Wan C.F., Zheng L.L., Liu Y., Yu X. Houttuynia cordata Thunb reverses oxaliplatin-induced neuropathic pain in rat by regulating Th17/Treg balance. Am. J. Transl. Res. 2016;8(3):1609–1614. [PMC free article] [PubMed] [Google Scholar]
- Xu Y.Y., Zhang Y.Y., Ou Y.Y., Lu X.X., Pan L.Y., Li H., Lu Y., Chen D.F. Houttuynia cordata Thunb. polysaccharides ameliorates lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2015;173:81–90. doi: 10.1016/j.jep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Zhang X.X., Wan N. Comments on the temporary prohibition of Yuxingcao (Houttuynia cordata) herbal injection use in China. J. Jianxi Univ. TCM. 2007;19(1):81–83. [Google Scholar]
- Yang U.J., Maeng H., Park T.S., Shim S.M. Houttuynia cordata extract improves physical endurance performance by regulating endothelial production of nitric oxide. J. Med. Food. 2015;18(9):1022–1031. doi: 10.1089/jmf.2014.3371. [DOI] [PubMed] [Google Scholar]
- Yoshino H., Imai N., Nabae K., Doi Y., Tamano Seiko, Ogawa K., Shirai T. Thirteen-week oral toxicity study of Dokudami extract (Houttuynia cordata Thunb.) in F344/DuCrj rats. J. Toxicol. Pathol. 2005;18:175–182. [Google Scholar]
- Zhang M.-Y., Li L.-D., Li Y.-K., Zhang J., Hao W. Anaphylactic reactions of new Houttuynia cordata injection. Chin. J. New Drugs. 2010 (2010-09) [Google Scholar]