Abstract
The wide occurrence of sialic acids (Sia) in various chemical forms linked as monomers or polymers in an outstanding position in a multitude of complex carbohydrates of animals and microorganisms renders them as most versatile function modulators in cell biology and pathology. A survey is presented of recent advances in the study of the influences that Sias have as bulky hydrophilic and electronegatively charged monosaccharides on animal cells and on their interaction with microorganisms. Some highlights are: sialylation leads to increased anti-inflammatory activity of IgG antibodies, facilitates the escape of microorganisms from the host's immune system, and in polymeric form is involved in the regulation of embryogenesis and neuronal growth and function. The role of siglecs in immunoregulation, the dynamics of lymphocyte binding to selectins and the interactions of toxins, viruses, and other microorganisms with the host's Sia are now better understood. N-Glycolylneuraminic acid from food is antigenic in man and seems to have pathogenic potential. Sia O-acetylation mediated by various eukaryotic and prokaryotic O-acetyltransferases modulates the affinity of these monosaccharides to mammalian and microbial receptors and hinders apoptosis. The functionally versatile O-acetylated ganglioside GD3 is an onco-fetal antigen.
Introduction
Sialic acids (Sia), a large family of neuraminic acid derivatives, are acidic monosaccharides common in higher animals and some microorganisms. They are found in cellular secretions and on the outer surface of cells, mostly as terminal components of glycoproteins and glycolipids (gangliosides) [1•]. Sia are exposed to the cellular environment functioning in intrinsic and extrinsic communication and in defense. The same holds for mucin secretions, in which Sia not only increase viscosity but also help to protect epithelia from harmful substances and pathogens [2, 3]. It is not easy to describe a general role of Sia because these monosaccharides participate directly or indirectly in multiple and diverse cellular events. However, in addition to their negative charge, it is useful to divide their functions into two groups that explain many phenomena. Firstly, Sia act as a biological mask, that is an antirecognition agent by shielding recognition sites such as penultimate monosaccharides of glycan chains or (antigenic) proteins and other macromolecules of cell membranes including receptor molecules. In this way Sia contribute to cells being ‘self’. This may be possible by their electronegative nature together with their bulky, hydrophilic chemical structure (Figure 1 ). Secondly, Sia operate in the opposite way by being biological recognition sites, that is ligands for a great variety of molecules such as hormones, lectins, antibodies, and inorganic cations. Especially the latter two protein classes have been recognized recently as being involved in most important phenomena of cellular and molecular interactions in both physiological and pathological processes. In this respect, polysialic acids (PSA) are presently drawing much attention [4]. Furthermore, many microorganisms infect cells by binding to Sia and thus exploit the host organism. Elucidation of the underlying mechanisms will enable the development of therapeutic or prophylactic strategies [5].
Figure 1.
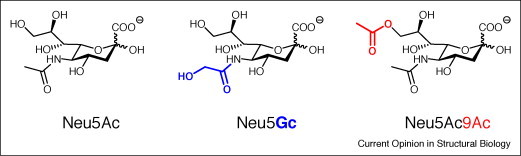
The three most frequent sialic acids N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and N-acetyl-9-O-acetylneuraminic acid (Neu5,9Ac2), in the figure denominated as Neu5Ac9Ac. Sia are biosynthesized in many steps from glucose leading to both N-acetylmannosamine-6-phosphate (ManNAc-6P) and phosphoenol pyruvate, which are condensed to Neu5Ac-9-phosphate followed by dephosphorylation [6, 7]. The formation of ManNAc-6P from UDP-N-acetylglucosamine (UDP-GlcNAc) is catalyzed by a bifunctional enzyme, the UDP-GlcNAc epimerase/ManNAc kinase (encoded by GNE), which is the key enzyme in Sia biosynthesis and can be feedback-inhibited by CMP-Neu5Ac. After the activation of Neu5Ac with CTP the resulting CMP-Neu5Ac is transported into the Golgi compartment and transferred to nascent glycoconjugates by a large family of monosialyltransferases and polysialyltransferases with varying specificities for glycoproteins and glycolipids [47]. Neu5Ac can be converted to Neu5Gc on the CMP-Neu5Ac level in the cytosol by the action of CMP-Neu5Ac hydroxylase (CMAH) [6]. The activity of this enzyme was lost during human evolution [38]. Enzymes O-acetylating Sia at C-4 of the pyranose ring or at C-7 to C-9 of the side-chain also seem to be frequent, studied best in animals in bovine submandibular gland [6, 17, 48]. Only prokaryotic sialate-O-acetyltransferase genes could be identified so far and some insight into the catalytic mechanism of OatC from Neisseria meningitidis was obtained [49•]. Hydroxylation and O-acetylation as well as other natural or artificial Sia modifications strongly influence the physiological properties of Sia.
These dual properties of Sia can be influenced strongly by different substituents on the neuraminic acid moiety. About 50 Sia types are known, with N-acetylneuraminic acid (Neu5Ac), followed by N-glycolylneuraminic acid (Neu5Gc) and O-acetylated derivatives, mostly N-acetyl-9-O-acetylneuraminic acid (Neu5,9Ac2) as most frequent forms [6] (Figure 1). This Sia variability can be increased by metabolic incorporation of non-natural substituents into cell membrane Sia, which also modulate their functions [7].
The antirecognition effect of sialic acids
The first example of this phenomenon was the uptake of desialylated serum glycoproteins by hepatocytes observed by G. Ashwell and A.G. Morell 35 years ago (summarized in [1•, 6]). The reason for this was the exposure of Gal residues and trapping of unmasked glycoproteins by a Gal-recognizing receptor. This finding tremendously influenced not only sialobiology but also the whole area of protein (lectin)–carbohydrate interaction studies [8•]. In a similar way desialylation of erythrocytes, lymphocytes, and thrombocytes leads to sequestration from circulation by a galectin on liver and spleen macrophages [6]. Also tumor cells may be trapped in this way. Furthermore, interaction of CD44 on erythrocytes with hyaluronic acid on vascular endothelia leads to rolling and tethering of desialylated or aged erythrocytes thus facilitating sequestration [9]. In sepsis, the ‘Ashwell receptor’ normally modulating von Willebrand factor homeostasy mitigates the lethal coagulopathy by eliminating platelets desialylated by Streptococcus pneumoniae sialidase [10•]. A model of reversible interactions of cells regulated by Sia is depicted in Figure 2 .
Figure 2.
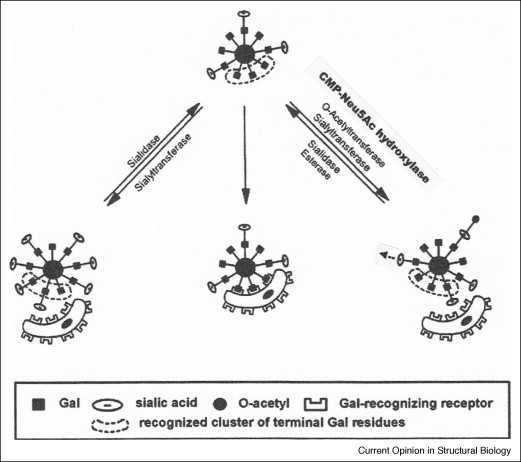
Model of the association and dissociation of cells regulated by the loss, restoration, and modification of Sia by the action of sialidases, sialyltransferases, sialate-O-acetyltransferases, CMP-Neu5Ac hydroxylase, and sialate-O-acetylesterases. Only after the unmasking of a sufficient number of galactose residues (a ‘cluster’), attachment to another cell, for example a macrophage, via galactose-recognizing receptors is possible. O-Acetyl (black dot) or N-glycolyl (black triangle) groups may inhibit the enzymatic release of Sia and the interaction of these monosaccharides with Sia-recognizing receptors, for example, siglecs, or with viruses and bacteria. These Sia modifications are involved in fine-tuning of recognition events or can even be considered as molecular switches. O-Acetylation may fortify masking of immunogenic epitopes on microorganisms and enable escape from immune defense [49•]. In this model symbols for Sia-recognizing receptors have not been drawn, and the symbol used for the Sia molecule could also represent oligosialic acids or polysialic acids, which may carry non-natural N-acyl or O-acyl modifications. The degradation of Sia is initiated by sialidases [6, 50, 51], which strongly influence the behavior of normal and malignant cells. Polysialic acids can be split into Sia oligosaccharides by endosialidases from phages [52]. For de-O-acetylation of Sia a variety of esterases from animals and microorganisms are known [6, 53].
Oversialylation, often observed in tumor cells or on placental syncytioblasts protects cells from humoral or cellular defense systems and can thus increase malignancy and metastatic potential of tumor cells [3]. A similar antirecognition strategy allowing better survival in the host and thus enhancing virulence is applied by microorganisms which coat themselves by colominic acid, a PSA, or express sialylated glycans mimicking mammalian structures (molecular mimicry). For example, group B Streptococcus presents terminal Sia α2,3Galβ1,4GlcNAc-units, like human neutrophils which interact in the cell membrane with siglec-9 in cis. It was shown that the bacterial oligosaccharide binds in trans to the same neutrophil siglec-9 in a Sia-dependent manner resulting in the weakening of the neutrophil immune response and thus demonstrating a novel mechanism of bacterial immune evasion [11•].
Bacteria often secrete sialidases to unmask galactose residues on host endothelia, which enables binding and spreading of these pathogens. Furthermore, virus sialidases, for example from influenza viruses, not only unmask antigens on cells but also facilitate colonization of, for example bronchial epithelia by opportunistic bacteria [1•, 3, 5, 12]. Sia can be considered as members of the innate immune system, rendering cells as ‘self’, since they can shield antigenic sites of cells and thus weaken immunoreactivity. Otherwise autoantibodies may be produced, for example, after bacterial and viral infection, possibly leading to chronic diseases such as neuronal disorders or glomerulonephritis [12].
Sia are also involved in masking of cellular receptors. For example, the TrKA tyrosine kinase receptors known as signaling receptors for neurotrophin growth factors are activated only after the removal of α2,3-linked Sia from underlying Gal residues [13]. Sialylation functionally silences also the hyaluronan receptor LYVE-1 in lymphatic endothelium [14]. Sialylation of β1 integrins blocks cell adhesion to galectin-3 and in this way protects cells against apoptosis. This may explain why α2,6-linked Sia upregulation in β1 integrin and other glycoproteins of a number of tumors including adenocarcinoma correlates with tumor metastasis and poor prognosis [15]. Sialylation of β-integrins was found to be upregulated by higher expression of the ST6Gal I gene during exposure to ionising radiation [16•] resulting in a stronger radiation resistance of this protein. This may lead to the inhibition of apoptosis of tumor cells.
It is known that the ganglioside GD3 is involved in CD95-mediated and ceramide-mediated apoptosis. 9-O-Acetylation of the Neu5Ac moiety of GD3 (forming 9-OAc-GD3) suppresses this proapoptotic function of nonacetylated GD3 as summarized in [17]. 9-OAc-GD3 (Figure 3 ) is an important regulatory molecule being involved in signal transduction, regulation of growth and differentiation, especially of neuronal cells, apoptosis, representing a marker for tumors and inflammation and its concentration is increased in autoimmune lesions, T-cell activation, and T-cell keeping.
Figure 3.
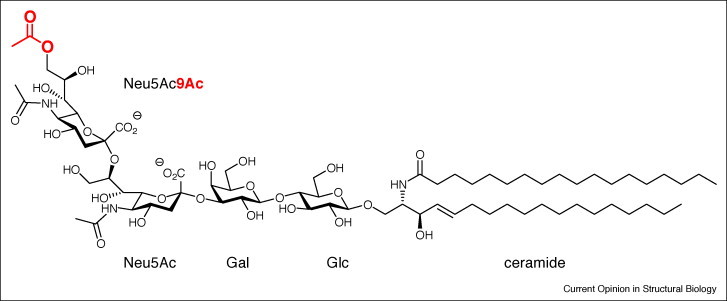
Structure of 9-O-acetylated disialo-ganglioside GD3 (9-OAcGD3; Neu5,9Ac2-α2,8Neu5Acα2,3Galβ1,4Glcβ1,1ceramide). The terminal Sia carries an O-acetyl (red) at C-9, which renders this frequent ganglioside an important regulator and biomarker of growth, differentiation, and malignancy of neuro-ectodermal tissues, as well as of lymphocyte function and apoptosis [17]. GD3 can directly be O-acetylated at C-7 by mammalian sialate-O-acetyltransferases, followed by migration of the O-acetyl to C-9. Therefore, both 7-OAcGD3 and 9-OAcGD3 occur in lymphocytes and tissues in various amounts.
Most importantly, Sia in IgG antibodies were found to lower their affinity to Fc-receptors and to increase their anti-inflammatory activities [18••]. Humans and mice with autoimmune disorders exhibit altered IgG glycosylation patterns with increased levels of antibodies lacking terminal Sia and galactose residues. Enrichment of Sia in intravenously applied immunoglobulin (IVIG) much increased its anti-inflammatory activity in man.
One of the most prominent topics in sialobiology, experiencing an explosion-like flood of literature, is PSAs, which are mainly expressed in developing and neuronal tissues and in cancer cells. In PSA of vertebrates and some microorganisms Sia are α2,8-connected and rarely α2,9-connected in homopolymers. In vertebrates PSA is attached to branched Asn-linked core glycans mostly of the neuronal cell-aggregation molecule NCAM (Figure 4 ) but also of other proteins like the voltage-sensitive sodium channel or neuropilin. Two polysialyltransferases (ST8SiaII and IV), highly selective for NCAM, assemble the polysaccharide chains (reviewed in [19, 20, 21, 22]). Sialyltransferases involved in α2,8-oligo-sialylation of glycoconjugates during (neuronal) development were discovered in zebrafish [23]. In starfish and sea urchin up to 30 Neu5Gc residues were found to be linked linearly in α2 → 5-Oglycolyl- between the glycosidic bond of one Neu5Gc and the hydroxyl of the N-glycolyl residue of another Neu5Gc molecule [4]. The function of this unusual molecule is unknown. The complex biosynthesis and export of PSA (colominic acid) of Escherichia coli K1 capsule, which is an important virulence determinant in many infectious diseases has been investigated by Steenbergen and Vimr [24].
Figure 4.
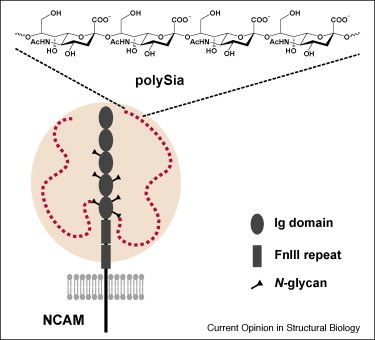
Polysialylation of the neuronal cell-aggregation molecule NCAM of mouse (modified from Mühlenhoff et al. [19]). The extracellular part of NCAM consists of five immunoglobulin (Ig)-like domains and two fibronectin type III (FnIII) repeats. Various isoforms exist, which are either attached to the plasma membrane by a glycosylphosphatidylinositol (GPI) anchor or are inserted into the membrane by trans-membrane protein domains. NCAM has six N-glycosylation sites, as indicated. In PSA–NCAM the inner (5th and 6th) N-glycans carry one or more PSA chains with up to 90 α2,8-linked Sia residues (red dots). The pink-shaped sphere represents the hydrodynamic radius of PSA.
PSA are dealt with in this section, because their main function is masking of recognition sites. The bulky, electronegatively charged, much hydrated and ‘slippery’ PSA have an unstable conformation and therefore are not suited as a receptor [21]. PSA occupies large spaces between cells and thus regulates membrane–membrane apposition and blocks cell contacts and NCAM function. NCAM enables adhesion, signaling, and transport of synaptic vesicles resulting in differentiation, maturation, and function of neuronal cells. Therefore, PSA are mainly found during tissue and tumor development. PSA seem to reflect the primitive stage of differentiation and to enhance the metastatic potential of malignant cells. They are believed to reduce cell interactions through a form of permissive regulation, thus allowing only certain factors to influence a cell or through ‘insulative’ regulation in order to protect a cell from inappropriate or premature actions [21]. PSA seem to guarantee that NCAM contacts take place in a highly organized, time-specific and site-specific manner. Correspondingly, the PSA level decreases during embryogenesis with the progression of neuronal differentiation, synaptogenesis, and circuit maturation in neonatal and adult animals. In adults, it persists only at sites that maintain neuronal plasticity like the hippocampus, thalamus, amygdala, and the olfactory bulb. In summary, PSA facilitate migration and maturation of neuronal progenitor cells, are involved in myelination of axons, learning and memory, regulation of circadian rhythms, secretion of hypophyseal hormones, chronic pain, emotional reactions such as anxiety and aggression, neuronal repair, and psychiatric diseases such as schizophrenia. Correspondingly, efforts are undertaken to apply or regenerate PSA for adult tissue repair (summarized in [21]). As expected, blocking PSA biosynthesis in ST8SiaII and IV double knockout mice leads to severe defects especially in the brain [19, 20]. Remarkably, however, additional deletion of NCAM in mice was found to largely prevent these defects, demonstrating that the untimely unmasking of NCAM from PSA after the deletion of polysialyltransferase action causes the fatal developmental phenotype observed.
Another example for the antirecognition effect of Sia is the sialylation of unglycosylated recombinant proteins leading to improved pharmaceutical properties owing to longer lifetime in circulation as shown, for example, for erythropoietin and asparaginase [25]. This seems mainly to be because of the reduction of unspecific binding in blood circulation and the antiproteolytic effect of Sia on the neoglycoproteins. Care should be taken that all galactose residues are masked by Sia to avoid clearance by the Ashwell–Morell pathway.
The ligand function of sialic acids
In contrast to masking, Sia participate in many recognition processes by interacting as ligands with specific proteins (lectins or other receptors) of vertebrate cells, protozoa, bacteria, mycoplasma, viruses and with hormones, toxins, lectins, and antibodies [1•, 12, 26]. This versatility renders them important regulators of physiological events, although pathogens may exploit them for their own purposes. Among the numerous virus species recognizing Sia the oldest and most prominent example are influenza viruses where the role of Sia in their binding and spreading has been well investigated and led to the development of antivirals, based on the inhibition of the sialidase or ‘receptor-destroying enzyme’ involved in the infection mechanism [5]. At present attention is given to the study of the binding specificity of influenza and other viruses. A most prominent result is the difference between the human viruses (e.g. H3N2, recognizing N-acetylneuraminyl galactose that is α2,6-linked) and avian viruses (e.g. H5N1, showing α2,3-linkage preference). However, the efficiency of a virus to infect also depends on the distribution of such Sia glycosides on cells and on additional properties of cell membranes [27, 28]. Reconstitution of the 1918 influenza virus which had caused a fatal pandemic showed that this strain, in contrast to contemporary human strains, can overactivate the innate immune system of the host and thus lead to a deadly ‘cytokine storm’ [29].
Neu5Ac is the most common ligand for virus binding but Neu5Gc and 9-O-acetylated Sia were also found to be specific ligands, the latter, for example for influenza C and corona viruses [12, 17, 26]. X-ray crystallography has greatly improved our understanding of the detailed structural basis of virus receptors by, for example, GM1 ganglioside recognition by simian virus 40 (SV40) [30].
A large number of bacteria like E. coli, Streptococcus and Helicobacter species, as well as the malaria parasite Plasmodium falciparum attach to cells via Sia [12, 26]. Currently H. pylori draws special attention, because its Sia-specific adhesin SabA is involved in its binding to oral and gastric mucins [31]. A combination of Sia and catechins in the diet was shown to prevent H. pylori infection or to decrease the bacterial load and inflammation in mouse stomach [32•]. This observation may be of clinical implications, since H. pylori strains are getting increasingly drug-resistant.
Toxins from Vibrio and Clostridium species as well as from E. coli and Bordetella pertussis also bind to Sia, mostly of gangliosides [26]. The attachment of botulinum neurotoxin to Sia of vascular endothelia cells may play a role for foodborne botulism [33]. AB5 toxin secreted by Shiga toxigenic E. coli causes serious gastrointestinal disease in humans [34]. Interestingly, it binds with a strong preference to Neu5Gc apparently derived from food on gut epithelia and kidney vasculature.
Fungi, plants, and animals express many Sia-recognizing lectins [12, 26, 35] and the mammalian siglecs and selectins are attracting increasing attention. Fifteen siglecs were identified in mammals [36, 37, 38]. These membrane-bound I-type lectins belong to the immunoglobulin superfamily and occur in various cell types of the immune and hematopoietic systems of primates and rodents or in the central nervous system as is the case of the myelin-associated glycoprotein (MAG; Siglec-4). Each Siglec has a distinct preference for the Sia and linkage type. O-Acetylation can mask the ligand function of Sia in the case of sialoadhesin (Siglec-1), CD22 (Siglec-2), and MAG [17, 36]. Furthermore, the degree of O-acetylation of the capsular polysaccharide of Group B Streptococcus affects the interaction between the bacteria and CD33-related siglecs of leukocytes [37]. While mouse and ape CD22 preferentially bind to Neu5Gc, the human one prefers Neu5Ac, which parallels the loss of Neu5Ac hydroxylation in man. The evolutionary significance of this difference is still mysterious [38].
Siglecs are able to mediate both cell–cell interactions and signaling functions by cis-interactions and trans-interactions with Sia-capped glycans (i.e. within a cell membrane or between cells [36, 39]) and in this way modulate the immune system, for example by dampening inflammatory gene activation. For example, CD22 is an inhibitory receptor of B-cells that regulates multiple B-cell functions and lymphocyte survival. A future aim for the modulation of B-cell function and reduction of inflammation is to design CD22-specific inhibitors by synthesis of novel sialosides with hydrophobic substituents at C-9 of Sia [40]. Most human siglecs are related to CD33. They undergo rapid evolution and regulate leukocyte functions during inflammatory and immune responses [41]. Many questions are open regarding the function of MAG localized in periaxonal Schwann cells and oligodendroglial membranes, which binds to α2,3-linked Sia of gangliosides and is involved in glia–axon interactions [42]. This selectin is a target antigen in autoimmune peripheral neuropathy including multiple sclerosis.
Another target of intensive research is the selectins which according to their preferred location on endothelial cells, leukocytes, and blood platelets have been named E-selectins, L-selectins, and P-selectins, respectively [12, 26, 43]. They bind to the tetrasaccharides sialyl-Lewisx and sialyl-Lewisa. Crystallographic studies revealed the formation of ‘catch bonds’ for selectins during the interaction with immobilized ligands, whereby large regions of the lectin domain switch between two distinct states [44]. A bond is called a catch bond if its lifetime increases with force up to a critical level. Such mechanosensitive bonds formed under nonequilibrium conditions can explain the threshold observed for leukocyte rolling. Lymphocyte L-selectin specifically recognizes also the sulphated version of sialyl-Lewisx found on the high-endothelial venules of peripheral lymph nodes. The selectins are involved in rolling and migration through the endothelium of lymphocytes and thus in immunological and inflammatory events. Tumor cells often bearing sialyl-Lewisx residues can also bind to selectins, which provide a route for tumor cell migration and metastasis formation [3]. Sia O-acetylation may impair this interaction [17]. Normal cellular human prion protein (PrPc) contains both sialyl-Lewisx and Lewisx glycans. It was found to bind to selectins, but surprisingly only by the Lewisx moiety [45•]. In that case sialylation prevents selectin interaction, which can be reversed by sialidase treatment.
A last important example for protein–Sia interactions is the recognition of nonhuman, most likely diet-derived, Neu5Gc by anti-Neu5Gc antibodies regularly found in normal humans [46••]. Most of these are of the IgG class and exhibit a diverse spectrum against a variety of Neu5Gc epitopes. The authors discuss that Neu5Gc-antibody reactions could contribute to chronic inflammation and cancer and that this threat may be reduced by limiting the uptake of foods, such as ‘red’ meat of cows and pigs that contain Neu5Gc.
Conclusions
The concept of arranging most of the Sia functions as either masking biological recognition sites or representing recognition epitopes (ligands) has been solidified by an impressive amount of publications during recent years. There is more evidence for Sia's positive or negative modulation of molecular and cellular interactions, thus rendering cells ‘self’, affecting immune reactions, regulating apoptosis or influencing receptor function, growth, differentiation, and aging. In particular, intensive research on PSA and siglecs has shed light on cell and neuronal tissue differentiation and immune regulation. Knowledge how tumor cells and microorganisms, especially viruses, can exploit the properties of Sia also increased appreciably. Still, more work is necessary to fully understand these phenomena, and it is necessary to obtain more information about the regulation of Sia biosynthesis, degradation, and modification on the gene and protein levels, how, for example hormones influence these events, as well as on structural aspects of Sia–receptor interactions and supramolecular arrangement of sialylated glycans in cell membranes. This will increase the sialo-pharmaceutical possibilities to fight against the many infectious, immunological, malignant, psychiatric, and degenerative diseases in which Sia are involved.
References and recommended reading
Papers of particular interest, published within the biannual/triannual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Because of space limitations many valuable original contributions could not be included and the author acknowledges the impressive advances in this field.
The author thanks Thisbe K Lindhorst and Martina Mühlenhoff for help in preparing the figures.
References
- 1•.Kamerling J.P., Boons G.-J., Lee Y.C., Suzuki A., Taniguchi N., Voragen A.G.J., editors. vols. 1–4. Elsevier; Oxford: 2007. (Comprehensive Glycoscience—From Chemistry to Systems Biology). [Google Scholar]; This multiauthor, well-edited book series comprises all aspects of structure, analysis, chemical and biochemical synthesis, catabolism, molecular biology and cell biological, and pathophysiological aspects of complex carbohydrates. The reader's attention is drawn to the following chapters: Kobata A: Glycoprotein glycan structures, vol. 1, 39–72; Yu RK, Yanagisawa M, Ariga T: Glycosphingolipid structures, vol. 1, 73–122; Miyagi T, Yamaguchi K: Sialic acids, vol. 3, 297–323.
- 2.Royle L., Matthews E., Corfield A., Berry M., Rudd P.M., Dwek R.A., Carrington S.D. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25:763–773. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 3.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janas T., Janas T. Polysialic acids: structure and properties. In: Dumitriu S., editor. Polysaccharides — Structural Diversity and Functional Versatility. edn 2. Marcel Dekker; New York: 2005. pp. 707–727. [Google Scholar]
- 5.von Itzstein M., Thomson R. Anti-influenza drugs: the development of sialidase inhibitors. In: Kräusslich H.-G., Bartenschlager R., editors. vol. 189. Springer-Verlag; Berlin, Heidelberg: 2009. pp. 111–154. (Antiviral Strategies. Handbook Exp Pharmacol). [DOI] [PubMed] [Google Scholar]
- 6.Varki A., Schauer R. Sialic acids. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York: 2009. pp. 199–217. [PubMed] [Google Scholar]
- 7.Kontou M., Weidemann W., Bork K., Horstkorte R. Beyond glycosylation: sialic acid precursors act as signaling molecules and are involved in cellular control of differentiation of PC12 cells. Biol Chem. 2009;390:575–579. doi: 10.1515/BC.2009.058. [DOI] [PubMed] [Google Scholar]
- 8•.Cummings R., Liu F.-T. Galectins. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York: 2009. pp. 475–487. [PubMed] [Google Scholar]; In addition to this article describing various galectins and their functions it is recommended to turn also to other chapters of the Section “Glycan-Binding Proteins” of this book and to corresponding chapters of Ref. [1].
- 9.Kerfoot S.M., McRae K., Lam F., McAvoy E.F., Clark S., Brain M., Lalor P.F., Adams D.H., Kubes P. A novel mechanism of erythrocyte capture from circulations in humans. Exp Hematol. 2008;36:111–118. doi: 10.1016/j.exphem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 10•.Grewal P.K., Uchiyama S., Ditto D., Varki N., Le D.T., Nizet V., Marth J.D. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14:648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]; The paper reports an important function of the Ashwell Gal-recognizing receptor: it retards the onset and impairs the severity of disseminated intravascular coagulation during sepsis and thus favors survival of the host by trapping desialylated thrombocytes.
- 11•.Carlin A.F., Uchiyama S., Chang Y.-C., Lewis A.L., Nizet V., Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sialylated capsular polysaccharide of group B Streptococcus engages siglec-9 of neutrophils and in this way impairs the defense functions of these white blood cells. A reduction of the neutrophil oxidative burst, diminished formation of neutrophil extracellular DNA traps, and increased bacterial survival were observed.
- 12.Schauer R. The diversity of sialic acids and their interplay with lectins. In: Sansom C., Markman O., editors. Glycobiology. Scion Publ. Ltd.; Bloxham: 2007. pp. 136–149. [Google Scholar]
- 13.Woronowicz A., Amith S.R., De Vusser K., Laroy W., Contreras R., Basta S., Szewczuk M.R. Dependence of neurotrophic factor activation of Trk tyrosine kinase receptors on cellular sialidase. Glycobiology. 2006;17:10–24. doi: 10.1093/glycob/cwl049. [DOI] [PubMed] [Google Scholar]
- 14.Nightingale T.D., Frayne M.E.F., Clasper S., Banerji S., Jackson D.G. A mechanism of sialylation functionally silences the hyaluronan receptor LYVE-1 in lymphatic endothelium. J Biol Chem. 2009;284:3935–3945. doi: 10.1074/jbc.M805105200. [DOI] [PubMed] [Google Scholar]
- 15.Zhuo Y., Chammas R., Bellis S.L. Sialylation of β1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem. 2008;283:22177–22185. doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Lee M., Lee H.-J., Bae S., Lee Y.-S. Protein sialylation by sialyltransferase involves radiation resistance. Mol Cancer Res. 2008;6:1316–1325. doi: 10.1158/1541-7786.MCR-07-2209. [DOI] [PubMed] [Google Scholar]; Ionizing radiation stimulated the expression of ST6GalI followed by increased protein sialylation, especially cell membrane integrin β1. This is an important observation and the resulting higher tumor resistance to radiation should be considered during cancer treatment.
- 17.Schauer R, Srinivasan GV, Wipfler D, Kniep B, Schwartz-Albiez R: O-Acetylated sialic acids and their role in immune defense. In Molecular Immunology of Complex Carbohydrates-3 (MICC-3), Edited by Wu AM. Adv Exp Med Biol, Kluwer Academic/Plenum Publishers, New York; 2009, in press. [DOI] [PMC free article] [PubMed]
- 18••.Nimmerjahn F., Anthony R.M., Ravetch J.V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci U S A. 2007;104:8433–8437. doi: 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]; IgG antibodies contain a branched glycan residue at the asparagine residue 297 of the Fc portion, which is essential for maintaining a functional Fc structure. Antibody activity is much influenced by variation of the glycan composition. Patients with autoimmune disorders, for example rheumatoid arthritis, have increased levels of antibodies lacking terminal Sia and Gal residues. Antibodies with high levels of terminal Sia show reduced affinity to cellular receptors and have anti-inflammatory effects. Accordingly, Sia seem to be crucial regulators of antibody activity in vivo, thus reducing inflammatory reactions.
- 19.Mühlenhoff M., Oltmann-Norden I., Weinhold B., Hildebrandt H., Gerardy-Schahn R. Brain development needs sugar: the role of polysialic acid in steering NCAM functions. Biol Chem. 2009;390:567–574. doi: 10.1515/BC.2009.078. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrandt H., Mühlenhoff M., Weinhold B., Gerardy-Schahn R. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 2007;103(Suppl 1):56–64. doi: 10.1111/j.1471-4159.2007.04716.x. [DOI] [PubMed] [Google Scholar]
- 21.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- 22.Conboy L., Bisaz R., Markram K., Sandi C. Role of NCAM in emotion and learning. Neurochem Res. 2008 doi: 10.1007/978-1-4419-1170-4_18. [DOI] [PubMed] [Google Scholar]
- 23.Chang L.-Y., Mir A.-M., Thisse C., Guérardel Y., Delannoy P., Thisse B., Harduin-Lepers A. Molecular cloning and characterization of the expression pattern of the zebrafish α2,8-sialyltransferases (ST8Sia) in the developing nervous system. Glycoconj J. 2009;26:263–275. doi: 10.1007/s10719-008-9165-1. [DOI] [PubMed] [Google Scholar]
- 24.Steenbergen S.M., Vimr E.R. Biosynthesis of the Escherichia coli K1 group 2 polysialic acid capsule occurs within a protected cytoplasmic compartment. Mol Microbiol. 2008;68:1252–1267. doi: 10.1111/j.1365-2958.2008.06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne B., Donohoe G.G., O’Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discov Today. 2007;12:319–326. doi: 10.1016/j.drudis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann F., Tiralongo E., Tiralongo J. Sialic acid-specific lectins: occurrence, specificity and function. Cell Mol Life Sci. 2006;63:1331–1354. doi: 10.1007/s00018-005-5589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao L., Korteweg C., Hsueh W., Gu J. Avian influenza receptor expression in H5N1-infected and noninfected human tissues. FASEB J. 2008;22:733–740. doi: 10.1096/fj.06-7880com. [DOI] [PubMed] [Google Scholar]
- 28.Kumari K., Gulati S., Smith D.F., Gulati U., Cummings R.D., Air G.M. Receptor binding specificity of recent human H3N2 influenza viruses. Virol J. 2007;4:42–53. doi: 10.1186/1743-422X-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loo Y.-M., Gale M., Jr. Fatal immunity and the 1918 virus. Nature. 2007;445:267–268. doi: 10.1038/445267a. [DOI] [PubMed] [Google Scholar]
- 30.Neu U., Woellner K., Gauglitz G., Stehle T. Structural basis of GM1 ganglioside recognition by simian virus 40. Proc Natl Acad Sci U S A. 2008;108:5219–5224. doi: 10.1073/pnas.0710301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindén S.K., Wickström C., Lindell G., Gilshenan K., Carlstedt I. Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter. 2008;13:81–93. doi: 10.1111/j.1523-5378.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- 32•.Yang J.-C., Shun C.-T., Chien C.-T., Wang T.-H. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J Nutr. 2008;138:2084–2090. doi: 10.3945/jn.108.090985. [DOI] [PubMed] [Google Scholar]; Helicobacter pylori infection of cell cultures and mice was considerably reduced or prevented by combined application of sialyllactose and catechins, which hinder binding of the bacteria and diminish oxidative stress, respectively. In this connection, see Ref. [31].
- 33.Yoneyama T., Miyata K., Chikai T., Mikami A., Suzuki T., Hasegawa K., Ikeda T., Watanabe T., Ohyama T., Niwa K. Clostridium botulinum serotype D neurotoxin and toxin complex bind to bovine aortic endothelial cells via sialic acid. FEMS Immunol Med Microbiol. 2008;54:290–298. doi: 10.1111/j.1574-695X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 34.Byres E., Paton A.W., Paton J.C., Löfling J.C., Smith D.F., Wilce M.C.J., Talbot U.M., Chong D.C., Yu H., Huang S. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature. 2008;456:648–652. doi: 10.1038/nature07428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 36.Varki A., Angata T. Siglecs — the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 37.Carlin A.F., Lewis A.L., Varki A., Nizet V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 2007;189:1231–1237. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varki A. Multiple changes in sialic acid biology during human evolution. Glycoconj J. 2009;26:231–245. doi: 10.1007/s10719-008-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crocker P.R., Paulson J.C., Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 40.Abdu-Allah H.H.M., Tamanaka T., Yu J., Zhuoyuan L., Sadagopan M., Adachi T., Tsubata T., Kelm S., Ishida H., Kiso M. Design, synthesis, and structure — affinity relationships of novel series of sialosides as CD22-specific inhibitors. J Med Chem. 2008;51:6665–6681. doi: 10.1021/jm8000696. [DOI] [PubMed] [Google Scholar]
- 41.McMillan S.J., Crocker P.R. CD33-related sialic-acid-binding immunoglobulin-like lectins in health and disease. Carbohydr Res. 2008;343:2050–2056. doi: 10.1016/j.carres.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Quarles R.H. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 43.Taylor M.E., Drickamer K. Paradigms for glycan-binding receptors in cell adhesion. Curr Opin Cell Biol. 2007;19:572–577. doi: 10.1016/j.ceb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Thomas W.E. Mechanochemistry of receptor–ligand bonds. Curr Opin Struct Biol. 2009;19:50–55. doi: 10.1016/j.sbi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 45•.Li C., Wong P., Pan T., Xiao F., Yin S., Chang B., Kang S.-C., Ironside J., Sy M.-S. Normal cellular prion protein is a ligand of selectins: binding requires Lex but is inhibited by sLex. Biochem J. 2007;406:333–341. doi: 10.1042/BJ20061857. [DOI] [PMC free article] [PubMed] [Google Scholar]; Although information is available about the role of selectins in the vascular system, especially in inflammation, the function of these receptors in other systems, particularly the brain, is largely unknown. Human brain-derived PrPc contains Lex and/or sLex epitopes, only the former of which was found to bind to selectin.
- 46••.Padler-Karavani V., Yu H., Cao H., Chokhawala H., Karp F., Varki N., Chen X., Varki A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]; Normal human serum contains a diverse spectrum of anti-Neu5Gc antibodies, directed against the xenoantigen Neu5Gc, most likely derived from food. Since this observation may have severe clinical implications, this publication will greatly stimulate further, especially clinical, investigations. See Ref. [34]: Neu5Gc mediates human susceptibility to Shiga toxin.
- 47.Lehmann F., Kelm S., Dietz F., von Itzstein M., Tiralongo J. The evolution of galactose α2,3-sialyltransferase: Ciona intestinalis ST3GAL I/II and Takifugu rubripes ST3GAL II sialylate Galβ1,3GalNAc structures on glycoproteins but not glycolipids. Glycoconj J. 2008;25:323–334. doi: 10.1007/s10719-007-9078-4. [DOI] [PubMed] [Google Scholar]
- 48.Lrhorfi L.A., Srinivasan G.V., Schauer R. Properties and partial purification of sialate-O-acetyltransferase from bovine submandibular glands. Biol Chem. 2007;388:297–306. doi: 10.1515/BC.2007.033. [DOI] [PubMed] [Google Scholar]
- 49•.Bergfeld A.K., Claus H., Lorenzen N.K., Spielmann F., Vogel U., Mühlenhoff M. The polysialic acid-specific O-acetyltransferase OatC from Neisseria meningitidis serogroup C evolved apart from other bacterial sialate O-acetyltransferases. J Biol Chem. 2009;284:6–16. doi: 10.1074/jbc.M807518200. [DOI] [PubMed] [Google Scholar]; In mutagenesis studies of this O-acetyltransferase that belongs to the α/β-hydrolase fold family a catalytic triad composed of Ser-286, Asp-376, and His-399 was discovered. A covalent acetyl-enzyme intermediate is formed on catalysis.
- 50.Miyagi T. Aberrant expression of sialidase and cancer progression. Proc Jpn Acad, Ser B. 2008;84:407–418. doi: 10.2183/pjab/84.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chavas L.M.G., Tringali C., Fusil P., Venerando B., Tettamanti G., Kato R., Monti E., Wakatsuki S. Crystal structure of the human cytosolic sialidase Neu2. J Biol Chem. 2005;280:469–475. doi: 10.1074/jbc.M411506200. [DOI] [PubMed] [Google Scholar]
- 52.Schwarzer D., Stummeyer K., Haselhorst T., Freiberger F., Rode B., Grove M., Scheper T., von Itzstein M., Mühlenhoff M., Gerardy-Schahn R. Proteolytic release of the intramolecular chaperone domain confers processivity to endosialidase f. J Biol Chem. 2009;284:9465–9474. doi: 10.1074/jbc.M808475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schauer R., Shukla A.K. Isolation and properties of two sialate-O-acetylesterases from horse liver with 4- and 9-O-acetyl specificities. Glycoconj J. 2008;25:625–632. doi: 10.1007/s10719-008-9109-9. [DOI] [PubMed] [Google Scholar]


