Abstract
Diarrhoea among growing pigs (8–13 weeks old) is a significant problem in many herds. Nine herds with poor performance and diarrhoea among growing pigs were selected on the basis of their piglet mean age at a body weight of 25 kg, compared to the overall mean age in Swedish herds. In addition, four herds with good average performance and no problems with diarrhoea were selected. Pigs were necropsied and samples for histology and microbiology were collected. Based on the necropsy findings, the pigs from the good performing herds were all judged to be healthy. The presence of Brachyspira pilosicoli and Lawsonia intracellularis was significantly correlated to poor performing herds and the results indicate that these microbes are main pathogens involved in enteric diseases among Swedish grower pigs. In addition, concomitant infections with other presumptive pathogens were commonly found.
1. Introduction
Enteric diseases are a common problem in modern pig production worldwide (reviewed by Moxley and Duhamel, 1999). The diseases are often related to certain animal age categories or to certain periods during rearing, for example, “neonatal diarrhoea” and “post-weaning diarrhoea” (Holland, 1990; Lecce et al., 1982). Less attention has been paid to enteric diseases in young growing pigs that have passed the critical period of weaning (i.e., 8–13 weeks old), but diseases referred to as “grower scour” or “colitis” constitute an important problem in many herds (Taylor, 1987; Thomson et al., 1998).
Generally, studies on enteric disease have focused on a specific pathogen (Reed et al., 1986, Fellström et al., 1996). Several agents have been suggested as possible causes of diarrhoea in growing pigs, namely Brachyspira spp. (Taylor et al., 1980), Campylobacter spp. (Lawson and McOrist, 1993), Clostridium perfringens type A (Jestin et al., 1985), enterotoxic strains of Escherichia coli (Svensmark et al., 1989), Lawsonia intracellularis (McOrist et al., 1995), Salmonella spp. (Reed et al., 1986), Yersinia spp. (Taylor, 1999), coronavirus (Pritchard, 1987), rotavirus (Lecce et al., 1982), Isospora suis (Nilsson, 1988) and Trichuris suis (Batte et al., 1977). However, the importance and involvement of some of these organisms in clinical disease still remains to be clarified. It is often possible to relate a certain microbe to characteristic pathological lesions in the gut. For instance, the lesions caused by L. intracellularis are mainly located in the small intestines (McOrist et al., 1995), while lesions due to Brachyspira spp. are observed in the large intestines (Moxley and Duhamel, 1999; Taylor et al., 1980). B. hyodysenteriae causes necrosis in the intestinal mucosa, with mucohaemorrhagic exudate (Wilcock and Olander, 1979), whereas B. pilosicoli causes less prominent changes, with oedema and the development of a false brush border (Trott et al., 1996a). Few surveys have been conducted on the relationship between clinical disease and various pathogens (Johnston et al., 2001; Möller et al., 1998) or pathological lesions in the intestines (Jensen, 1995; Thomson et al., 1998).
The aim of this study was to investigate the presence of suspected or known pathogens in growing pigs with diarrhoea, and relate those findings to the clinical symptoms and to the pathological lesions in the intestines. Pigs with and without clinical diarrhoea were selected from herds with a history of “growing scour” and poor average performance. In addition, pigs were selected from herds with good performance and no such problems.
2. Materials and methods
2.1. Herds and animals
Herds were selected from two different geographical regions of Sweden (in the south-western and eastern parts of the country). From each region, five herds with poor performance and two with good performance were randomly selected among the 95% of the piglet-producing herds that are supervised by the Swedish Animal Health Service. The performances corresponded to the overall mean age in Swedish herds at 25 kg bw plus or minus one standard deviation, respectively. The poor performance herds were suffering from grower scour and had a poor average piglet growth rate, with a mean age of 85.6±3.6 days at a body weight (bw) of 25 kg. The good performance herds had an average mean age of 64.7±2.6 at 25 kg bw and no diarrhoea had occurred among the growers during the last year. All animals were weaned at approximately 5 weeks of age and no feed additives were used in the herds.
From each of the 10 poor performance herds three case and three control pigs were chosen, and from each of the four good performance herds three control pigs were chosen. One poor performance herd was later withdrawn from the study, hence altogether 66 pigs were included. All pigs had been weaned at least two weeks prior to entry into the study, but were less than 13 weeks old. They had not been treated with any antibiotics for the last two weeks. The case pigs had diarrhoea that had commenced within the last two days, but showed no clinical signs of other disease. The control pigs were matched to the case pigs for age and sex, but showed no signs of clinical disease. The pigs submitted from the good performance herds had a mean age of 67 days and a mean weight of 23.1 kg. Among the pigs submitted from the “poor herds” the controls had a mean age of 72 days and a mean weight of 18.7 kg, and the case pigs had a mean weight of 12.1 kg.
The herd owners were responsible for the selection of pigs and transportation to the laboratory. On arrival, the animals were stunned with electricity, weighed and exsanguinated, and necropsy was immediately performed.
2.2. Necropsies
The necropsies were carried out at the Departments of Pathology at the National Veterinary Institute, Uppsala, or at AnalyCen, Skara, and started within a few minutes after euthanasia. All gross lesions were noted, and eight specimens for histological examination were taken from each pig: from the duodenum, the jejunum (3 and 6 m caudal to the pylorus), the ileum, the ileal papilla, the caecum, and from the colon (50 cm from the ileocaecal junction, and 1 m cranial to the anus). Additional specimens were taken from visible lesions. The samples were fixed in 10% buffered formalin and stained with haematoxylin and eosin according to standard protocols. The histological findings were judged as minor changes found at a single site in the specimen or moderate or major changes found at several sites in the specimen. The pH in the small and large intestines was measured with a litmus-paper pressed against the gut wall.
2.3. Microbial investigations
The samples collected for microbial investigation and the methods of analysis are summarised in Table 1 . The samples analysed for haemolytic E. coli were also analysed for the presence of O-antigens associated with pathogenic isolates. An additional swab specimen (Amies transport medium with charcoal) was collected from the rectum to determine the phenotypic diversity of the coliform flora and analysed as described by Kühn et al. (1993). The results were given on a scale from zero to one, where zero corresponds to no diversity of the coliform flora. Faeces were examined for the presence of rotavirus by a double antibody sandwich enzyme immuno-assay (the rotavirus/coronavirus EIA kit, SVANOVA Biotech, Uppsala, Sweden), for measuring antigen in faecal samples. Values less than 0.1 at A 450 were considered negative. Coronavirus was not included in the study, since Sweden has previously been shown to be free from transmissible gastroenteritis and porcine epidemic diarrhoea (Elvander et al., 1997).
Table 1.
Samples for microbiology were collected and analysed as recorded
| Microorganism | Sampling site | Sampling material | Analysis method | Reference |
|---|---|---|---|---|
| Brachyspira spp. | Caecum, proximal colon, rectum | Swab, W CHa | Culture | Fellström and Gunnarsson (1995) |
| Campylobacter spp. | Distal ileum | Swab, W CHa | Culture | NMKL (1990) |
| Clostridium perfringens | Distal colon or rectum | Faeces | Culture | Quinn et al. (1994) |
| Yoo et al. (1997) | ||||
| Escherichia coli | Distal jejunum | Swaba | Culture | Söderlind et al. (1988) |
| Lawsonia intracellularis | Ileum | Tissue specimen | PCR | Jacobson et al. (2000) |
| Salmonella spp. | Distal jejunum | Swaba | Culture | NMKL (1999) |
| Yersinia spp. | Distal colon | Faeces, ∼30 g | Culture | NMKL (1987)b |
| Parasites | Distal colon or rectum | Faeces, 30–50 g | Flotation | Anon (1986) |
| Rotavirus | Distal colon or rectum | Faeces | ELISA |
Swabs were rubbed against the gut wall and put in Amies transport medium with or without charcoal (Trans-system Amies W CH or W/O CH, Copan Italia, Brescia, Italy).
Selective enrichment in Rappaport broth was excluded.
2.4. Statistics
For statistical analyses Fischer’s exact test (SAS Institute, Cary, North Carolina, USA 1999) and the χ 2 test were used.
3. Results
3.1. Necropsies
3.1.1. Pigs from good performance herds (n=12)
All pigs were judged as clinically healthy and no gross lesions were observed (Table 2 ). Microscopically, the pigs were judged as normal. However, minor histological lesions were noted in terms of occasional crypt abscesses, moderate lymphocyte infiltration of the lamina propria of villi in the ileum, and moderate epithelial hyperplasia in all segments of the gut (Fig. 1 ).
Table 2.
The numbers of pigs with lesions at necropsy
| Poor performance herds |
Good performance herds |
||
|---|---|---|---|
| Case pigs (n=27) | Control pigs (n=27) | Control pigs (n=12) | |
| Watery content in the intestines | 21 | 5 | 0 |
| Gross lesions | 21 | 5 | 0 |
| Small intestines | 19 | 5 | |
| Large intestines | 11 | 0 | |
| Microscopical lesions | 22 | 17 | 0 |
| Small intestines | 21 | 14 | |
| Large intestines | 21 | 13 | |
The pigs were selected from herds with an average “poor performance” and problems with diarrhoea among growing pigs, and from herds without diarrhoea among growing pigs and with average “good performance”. The case pigs had current clinical diarrhoea, whereas the controls were judged as healthy.
Fig. 1.
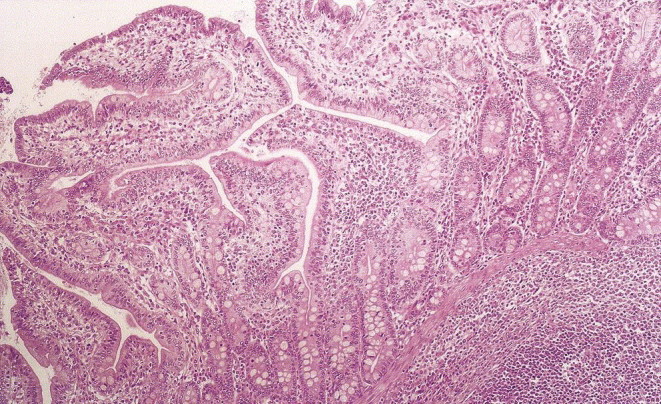
The ileum of a clinically healthy pig originating from a herd with an average good performance among growing pigs. The picture shows an example of a gut that is histologically judged as normal. No autolytic changes are present. H & E × 125.
3.1.2. Control pigs from poor performance herds (n=27)
Five pigs had loose to watery content in the large intestines (Table 2), but no gross lesions were found in four of those animals. Gross lesions were observed in altogether five pigs, and consisted of slightly thickened mucosa in the distal parts of the small intestine. Significant histological lesions were recorded in 17 pigs: Ten pigs had enterocolitis, with moderate numbers of crypt abscesses with neutrophils (n=8), a “false brush border” (Fig. 2 ) in the colon (n=1) and epithelial necrosis with neutrophilic infiltrations in the jejunum, ileum and caecum (n=4). Four pigs had lesions described as moderate numbers of crypt abscesses and neutrophilic infiltrations in the lamina propria in the small intestines. Two pigs had typhlitis or typhlocolitis and one pig had a parasitic colitis.
Fig. 2.
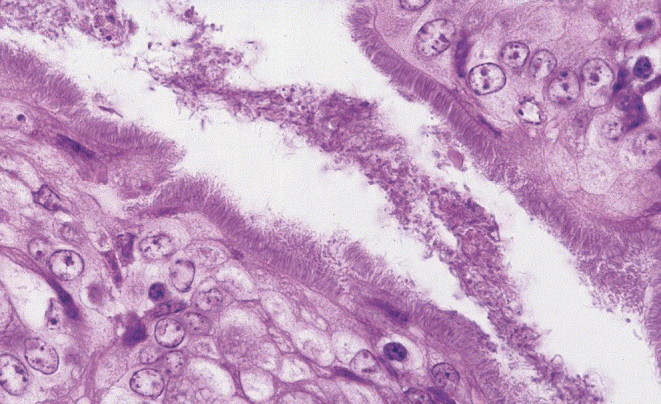
A false brush border consisting of spirochetes intimately attached to the apical membrane of surface enterocytes in proximal colon. A false brush border was found in caecum and proximal colon of two pigs infected with Brachyspira pilosicoli. The pigs originated from two herds suffering from diarrhoea and poor average performance in growing pigs. H & E × 500.
3.1.3. Case pigs from poor performance herds (n=27)
The intestinal content in the large intestine was “watery” in 17 pigs and “haemorrhagic” in four pigs. Gross lesions were observed in 21 pigs (Table 2). Nineteen of these pigs had a thickened mucosa in the distal jejunum, ileum, caecum or proximal colon, and in addition, necrotic enteritis (n=5), haemorrhages in the ileum (n=3) and pseudomembranes in the ileum, caecum and colon (n=1). Three of these pigs were also described as having a lustreless, velvet-like mucosa in the large intestine. Of the remaining two pigs, one had a necrotic-haemorrhagic colitis, and one had a parasitic colitis.
Significant microscopical lesions were observed in 22 pigs. In 15 cases, the changes were consistent with the gross lesions (Fig. 3 ). One pig had gross and microscopical lesions consistent with ileitis, and in addition a false brush border, consisting of spirochetes intimately attached to the apical membrane of surface enterocytes in the caecum and proximal colon (Fig. 2). One pig had colitis and histological signs of ileitis. Four pigs had suspected gross lesions which were judged as ileitis, but histologically, either only mild lesions in the ileum (n=3) or colitis (n=1) was found. Among the six pigs without gross lesions, the histological findings in four pigs consisted of enterocolitis (n=2), colitis (n=1) and ileitis (n=1), and two pigs were both macroscopically and histologically judged as normal.
Fig. 3.
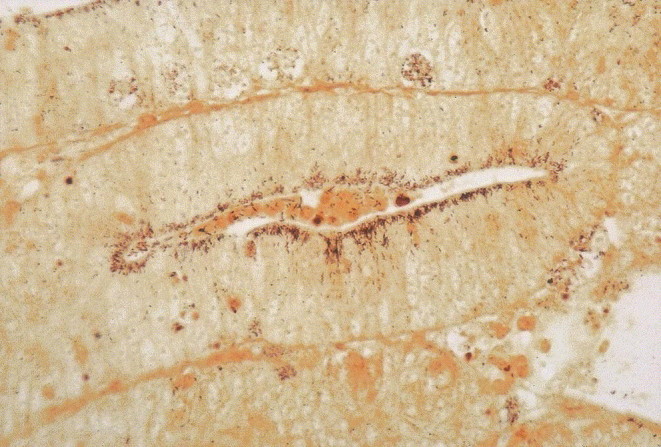
Ileum from a pig with clinical diarrhoea, selected from a herd suffering from diarrhoea and poor average performance in growing pigs. Intracellular organisms are seen in the apical part of the crypt enterocytes and the presence of Lawsonia intracellularis was confirmed by PCR. Macroscopically, the lesions consisted of necrotic enteritis in distal jejunum and ileum. W-S × 250.
In general, all pigs had some microscopical changes (villus atrophy and fusion) in the small intestines that were considered to be older changes that are usually observed in healthy pigs from commercial herds. In 20 pigs remnants of nematodes were seen in the ileum and on the surface of the large intestines. The mean pH values in the small intestines in the “case”, “poor control” and “good control” pigs were 7.3, 7.0 and 7.4, respectively. The corresponding values in the large intestines were 7.1, 6.9 and 6.5.
3.2. Microbiological investigations
Brachyspira pilosicoli, L. intracellularis, C. jejuni and Yersinia spp. were found only in the poor performance herds (Table 3 ) and of these pathogens L. intracellularis was more frequently observed (p⩽0.05) in the case pigs. Of the pigs infected with a single pathogen (findings of C. coli not included), four case and six control pigs were positive for L. intracellularis, and three case and two control pigs were positive for B. pilosicoli. One control pig was positive for C. jejuni and one case and one control pig for Y. enterocolitica. Sixteen case and 11 control pigs were infected with two or more pathogens (findings of C. coli not included), and among these, eight case pigs were concomitantly infected with B. pilosicoli and L. intracellularis.
Table 3.
The microbial findings in pigs from herds with diarrhoeic problems in growing pigs and poor average performance, and in pigs from herds without any such problems and good average performance
| Animals | Brachyspira pilosicoli | Campylobacter coli | Campylobacter jejuni | Clostridium perfringens | Escherichia coli | Lawsonia intracellularis | Salmonella spp. | Yersinia spp. | Coccidia | Parasites other | Rotavirus |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Case pigs, n=27 | 13 (48%) | 16 (59%) | 4 (15%) | 4 (15%) | 8 (30%) | 18 (67%) | 0 | 3 (11%) | 2 (7%) | 1 (4%) | 1 (4%) |
| B. Controls, “poor”, n=27 | 7 (26%) | 10 (37%) | 10 (37%) | 0 | 6 (22%) | 11 (41%) | 0 | 6 (22%) | 0 | 5 (19%) | 0 |
| C. Controls, “good”, n=12 | 0 | 11 (92%) | 0 | 2 (17%) | 1 (8%) | 0 | 0 | 0 | 1 (8%) | 0 | 0 |
| Comparison A vs B | NS | NS | NS | – | NS | * | – | NS | – | – | – |
| Comparison A+B vs C | ** | ** | * | – | NS | *** | – | NS | – | – | – |
The case pigs had diarrhoea, whereas the controls were judged as healthy. The number of pigs in each group (%) from which each enteropathogen was isolated.
NS, not significant; –, the number of observations were to few to allow statistical calculations.
p⩽0.05.
p⩽0.01.
p⩽0.001.
Haemolytic E. coli of a known pathogenic O group was found in both the poor performance herds (n=14/54) and the good performance herds (n=1/12). In one case and in four control pigs this was the only pathogen observed. Also, Cl. perfringens type A was isolated from both case pigs (n=4/54) and from good performance herds (n=2/12). C. coli was frequently isolated in all groups and was the only finding in two case and two control pigs from the “poor performance herds”. Parasites and rotavirus were rarely demonstrated. Salmonella spp. was not isolated.
The mean phenotypic diversity of the coliform flora was 0.64±0.29 in the case pigs and 0.55±0.29 in the control pigs from the poor performance herds, and 0.60±0.19 in the pigs from the good performance herds.
4. Discussion
The results obtained indicate that the two pathogens B. pilosicoli and L. intracellularis are commonly involved in enteric diseases in Swedish grower pigs. Except for L. intracellularis, the microbiological findings were similar in the diarrhoeic and apparently healthy pigs from the poor performance herds. Demonstration of B. pilosicoli and L. intracellularis was significantly correlated to poor performance herds and compatible with the pathological lesions in the intestines (Lomax and Glocke, 1982; Trott et al., 1996b). This is in agreement with earlier studies showing that L. intracellularis and B. pilosicoli are the most commonly found pathogens in growing pigs (Möller et al., 1998; Thomson et al., 1998). These authors also report a frequent finding of concomitant infections with two or more pathogens, which is in conformity with our results. However, in the present study a concomitant infection with B. pilosicoli and L. intracellularis was found only in the case pigs. Thus, the risk of developing clinical disease might increase if both pathogens are present, since the damage in the intestines will probably be more extended. The extent of intestinal damage seems to be closely related to the clinical symptoms, since 78% of the case pigs but only 18% of the “poor controls” had gross lesions, despite the fact that most pigs had microscopic lesions in the intestines (Table 2).
The apparent significant finding (p⩽0.01) of C. coli in healthy pigs is probably caused by the absence of other Campylobacter spp. in the culture (NMKL, 1990). C. coli is often found in pigs irrespective of their clinical health status and is considered non-pathogenic to pigs (Lawson and McOrist, 1993). C. jejuni, Cl. perfringens type A, Y. enterocolitica, B. pilosicoli, L. intracellularis and/or pathogenic E. coli were identified in 49 of the 54 pigs from the poor performance herds. Except for B. pilosicoli and L. intracellularis, no correlation was found between the findings of these bacteria and the clinical symptoms. However, only one case of pathogenic E. coli and two cases of Cl. perfringens were found in the conventionally reared, good performance herds. It is possible that feed compounds, inadequate biosecurity, hygienic or other factors in a herd may facilitate the transmission, survival and growth of some enteropathogenic bacteria (Bertschinger et al., 1978,1979; Skjerve et al., 1998; Wathes et al., 1989). The increased number of potentially pathogenic species results in an increased risk of clinical enteric disease (Löfstedt et al., 2000). Thus, one or several risk factors may be present in the poor performance herds. Alternatively, the enteric diseases per se may alter the ecological balance of the gut flora or change the environmental conditions in the gut, which would favour some bacteria (Katouli et al., 1999; Kühn et al., 1995). Pathogenic strains of E. coli was found in four out of six samples from two of the poor performance herds. Both these herds had a case history of previous problems with post-weaning diarrhoea. As discussed above, this might indicate that these herds suffer from a high pathogen load, or that the post-weaning diarrhoea might predispose to outbreaks of other enteric diseases.
To be able to study alterations in the gut flora, it is important to identify a healthy flora. Several methods have been developed for the study of the gut flora, such as measurement of the diversity of the enteric coliform flora (Kühn et al., 1995), measurement of enzymatic activities (Microflora-associated characteristics, MAC) (Midtvedt, 1999), or determination of terminal restriction fragment length polymorphism profiles, i.e., T-RFLP profiles (Leser et al., 2000). Additionally, the presence of some of the microorganisms discussed above might serve as an indication of a disturbance. The applicability of these methods needs to be further evaluated. Measurement of the phenotypic diversity of the coliform flora is a useful marker in studies of post-weaning diarrhoea (Katouli et al., 1999; Melin et al., 2000). However, the results of the present study indicate that the coliform flora remains stable during the course of diarrhoea in growing pigs.
The results suggest that a high prevalence of enteric diseases in a herd might involve an increasing number of potential food pathogens in the gut. It might be suspected that if an animal harbours a large number of these bacteria at slaughter, the risk of contamination of the carcass will increase (Asplund et al., 1998; Nesbakken and Skjerve, 1996). However, our study has focused on enteric diseases in grower pigs and it is not known whether our findings will be applicable to finisher/slaughter pigs. In a Norwegian study, the prevalence of Y. enterocolitica in slaughter pigs differed between herds with different production systems (Skjerve et al., 1998). Interestingly, in the present study two case pigs and one control pig from the same herd showed an abundant growth of Y. enterocolitica.
Only a few pigs shed parasite eggs (Oesophagostomum spp or Hyostrongylus spp), I. suis or rotaviruses. The number of positive samples was too small for the findings to be conclusive regarding the involvement in clinical disease. Additionally, parasitic remnants were found in 20 pigs, but except in one case they were not related to the clinical disease. Salmonella spp. and Y. pseudotuberculosis were not found in this study, but have been reported as a cause of colitis from other studies (Thomson et al., 1998). The results confirm the very low prevalence of Salmonella in Swedish pig herds reported previously (Wahlström et al., 1998; Wahlström et al., 2000). The overall occurrence of Y. pseudotuberculosis in Swedish pig herds is unknown.
In conclusion, L. intracellularis and B. pilosicoli are main pathogens associated with enteric disease in Swedish grower pigs, but these infections can also appear subclinically. However, the risk of developing clinical disease might increase if a concomitant infection with both microbes occurs. Further, several other potentially pathogenic microbes were found in pigs from herds suffering from “grower scour” and with poor piglet performance. Whether this finding represents a cause or a sign of disease is obscure, but it indicates a problem of a complex ecological nature.
Acknowledgements
This study was financed by grants from the Swedish Council for Forestry and Agricultural Research. We also wish to express our gratitude to the farmers who willingly and promptly supported us with animals. We wish to thank PhD. Marie Sterning for her assistance with the SAS analyses, Dr. Anders Linder for skilful performance of the necropsies, and Ms. Anna-Karin Florman, Mr. Anders Sigurd and Associate Professor Nils Holmgren for providing us with production figures and herd data.
References
- Anon . Reference Book 418. third ed. Her Majesty’s Stationary Office; London: 1986. Manual of veterinary parasitological laboratory techniques; pp. 11–12. [Google Scholar]
- Asplund K., Hakkinen M., Okkonen T., Vanhala P., Nurmi E. Effects of growth- promoting antimicrobials on inhibition of Yersinina enterocolitica O:3 by porcine ileal microflora. Journal of Applied Microbiology. 1998;85:164–170. doi: 10.1046/j.1365-2672.1998.00488.x. [DOI] [PubMed] [Google Scholar]
- Batte E.G., McLamb R.D., Muse K.E., Tally S.D., Vestal T.J. Pathophysiology of swine trichuriasis. American Journal of Veterinary Research. 1977;38:1075–1079. [PubMed] [Google Scholar]
- Bertschinger H.U., Eggenberger E., Jucker H., Pfirter H.P. Evaluation of low nutrient, high fibre diets for the prevention of porcine Escherichia coli enterotoxaemia. Veterinary Microbiology. 1978/1979;3:281–290. [Google Scholar]
- Elvander, M., Larsson, B., Engvall, A., Klingeborn, B., Gunnarsson, A., 1997. Nation-wide surveys of TGE/PRCV, CSF, PRRS, SVD, L. pomona and B. suis in pigs in Sweden. Epidémiologie santé animale 31–32
- Fellström C., Gunnarsson A. Phenotypic characterisation of intestinal spirochaetes isolated from pigs. Research in Veterinary Science. 1995;59:1–4. doi: 10.1016/0034-5288(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Fellström C., Pettersson B., Johansson K.-E., Lundeheim N., Gunnarsson A. Prevalence of Serpulina species in relation to diarrhea and feed medication in pig-rearing herds in Sweden. American Journal of Veterinary Research. 1996;57:807–811. [PubMed] [Google Scholar]
- Holland R.E. Some infectious causes of diarrhea in young farm animals. Clinical Microbiology Reviews. 1990;3:345–375. doi: 10.1128/cmr.3.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M., Fellström C., Heldtander M., Gunnarsson A. The16th International Pig Veterinary Society Congress, Melbourne, Australia. 2000. The prevalence of Lawsonia intracellularis in Swedish pigs submitted for autopsy; p. 74. [Google Scholar]
- Jensen, T.K., 1995. Diarré hos slagtesvin. Patoanatomiske studier over colitis. (Patho-anatomical studies on colitis). Thesis. Copenhagen: The Royal Veterinary and Agricultural University, Institution for Pharmacology and Pathology, 138
- Jestin A., Popoff M.R., Mahé S. Epizootiologic investigations of a diarrheic syndrome in fattening pigs. American Journal of Veterinary Research. 1985;46:2149–2151. [PubMed] [Google Scholar]
- Johnston W.T., Dewey C.E., Friendship R.M., Smart N., McEwen B.J., Stalker M., de Lange C.F.M. An investigation of the etiology of a mild diarrhea observed in a group of grower/finisher pigs. Canadian Veterinary Journal. 2001;42:33–37. doi: 10.4141/cjas62-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katouli M., Melin L., Jensen-Waern M., Wallgren P., Möllby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. Journal of Applied Microbiology. 1999;87:564–573. doi: 10.1046/j.1365-2672.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Kühn I., Katouli M., Lund A., Wallgren P., Möllby R. Phenotypic diversity and stability of the intestinal coliform flora in piglets during the first 3 months of age. Microbial Ecology in Health and Disease. 1993;6:101–107. [Google Scholar]
- Kühn I., Katouli M., Wallgren P., Söderlind O., Möllby R. Biochemical fingerprinting as a tool to study the diversity and stability of intestinal microfloras. Microecology and Therapy. 1995;23:140–148. [Google Scholar]
- Lawson G.H.K., McOrist S. The enigma of the proliferative enteropathies: A review. Journal of Comparative Pathology. 1993;108:41–46. doi: 10.1016/s0021-9975(08)80225-3. [DOI] [PubMed] [Google Scholar]
- Lecce J.G., Balsbaugh R.K., Clare D.A., King M.W. Rotavirus and hemolytic enteropathogenic Escherichia coli in weanling diarrhea of pigs. Journal of Clinical Microbiology. 1982;16:715–723. doi: 10.1128/jcm.16.4.715-723.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leser T.D., Lindecrona R.H., Jensen T.K., Jensen B.B., Möller K. Changes in bacterial community structure in the colon of pigs fed different experimental diets and after infection with Brachyspira hyodysenteriae. Applied and Environmental Microbiology. 2000;66:3290–3296. doi: 10.1128/aem.66.8.3290-3296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax L.G., Glocke R.D. Naturally occurring porcine proliferative enteritis: Pathologic and bacteriologic findings. American Journal of Veterinary Research. 1982;43:1608–1614. [PubMed] [Google Scholar]
- Löfstedt M., Holmgren N., Lundeheim N. The 16th International Pig Veterinary Society Congress, Melbourne, Australia. 2000. Risk factors for post weaning diarrhoea in Swedish pig herds; p. 367. [Google Scholar]
- McOrist S., Gebhart C.J., Boid R., Barns S.M. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. International Journal of Systematic Bacteriology. 1995;45:820–825. doi: 10.1099/00207713-45-4-820. [DOI] [PubMed] [Google Scholar]
- Melin L., Mattsson M., Wallgren P. The 16th International Pig Veterinary Society Congress, Melbourne, Australia. 2000. Challenge with pathogenic strains of E. coli at weaning. II. Monitoring of the faecal coliform flora; p. 23. [Google Scholar]
- Midtvedt T. Microbial functional activities. In: Hanson L.A., Yolkenm R.H., editors. Probiotics. Other Nutritional Factors, and Intestinal Microflora. Nestec Ltd., Vevey/Lippincott-Raven Publishers; Philadelphia: 1999. [Google Scholar]
- Moxley R.A., Duhamel G.E. Comparative pathology of bacterial enteric diseases of swine. In: Francis P.A., editor. International Rushmore Conference on Mechanisms in the Pathogenesis of Enteric Diseases 2. Kluwer Academic/ Plenum Publishers; New York: 1999. pp. 83–101. [Google Scholar]
- Möller K., Jensen T.K., Jorsal S.E., Leser T.D., Carstensen B. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta-haemolytic intestinal spirochaetes, Salmonella enterica, and haemolytic Escherichia coli from swine herds with and without diarrhoea among growing pigs. Veterinary Microbiology. 1998;62:59–72. doi: 10.1016/s0378-1135(98)00199-0. [DOI] [PubMed] [Google Scholar]
- Nesbakken T., Skjerve E. Interruption of microbial cycles in farm animals from farm to table. Meat Science. 1996;43:S47–S57. doi: 10.1016/0309-1740(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Nilsson O. Isospora suis in pigs with post weaning diarrhoea. The Veterinary Record. 1988;122:310–311. doi: 10.1136/vr.122.13.310. [DOI] [PubMed] [Google Scholar]
- NMKL, Nordic Comittee on Food Analysis, 1987. Yersinia enterocolitica. Detection in foods. No 117, 2nd ed
- NMKL, Nordic Comittee on Food Analysis, 1990. Campylobacter jejuni/coli. Detection in foods. No 119, 2nd ed
- NMKL, Nordic Comittee on Food Analysis, 1999. Salmonella. Detection in foods. No 71, 5th ed
- Pritchard G.C. Transmissible gastroenteritis in endemically infected breeding herds of pigs in East Anglia, 1981–1985. Veterinary Record. 1987;120:226–230. doi: 10.1136/vr.120.10.226. [DOI] [PubMed] [Google Scholar]
- Quinn P.J., Carter M.E., Markey B.K., Carter G.R. Clinical Veterinary Microbiology. 1994. Clostridium species; pp. 191–208. (Mosby International, London, England). [Google Scholar]
- Reed W.M., Olander H.J., Thacker H.L. Studies on the pathogenesis of Salmonella typhimurium and Salmonella cholerasuis var kunzendorf infection in weanling pigs. American Journal of Veterinary Research. 1986;47:75–83. [PubMed] [Google Scholar]
- Skjerve E., Lium B., Nielsen B., Nesbakken T. Control of Yersinia enterocolitica in pigs at herd level. International Journal of Food Microbiology. 1998;45:195–203. doi: 10.1016/s0168-1605(98)00162-7. [DOI] [PubMed] [Google Scholar]
- Svensmark B., Nielsen K., Willeberg P., Jorsal S.E. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. II. Post-weaning diarrhoea. Acta Veterinaria Scandinavica. 1989;30:55–62. doi: 10.1186/BF03548068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlind O., Thafvelin B., Möllby R. Virulence factors in Escherichia coli strains isolated from Swedish piglets with diarrhea. Journal of Clinical Microbiology. 1988;26:879–884. doi: 10.1128/jcm.26.5.879-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.J. Grower scour and non-specific colitis. Veterinary Record. 1987;14:479–480. doi: 10.1136/vr.121.20.479. [DOI] [PubMed] [Google Scholar]
- Taylor D.J. Miscellaneous bacterial infections. In: Straw B.E., D’Allaire S., Mengeling W.L., Taylor D.J., editors. Diseases of Swine. Blackwell Science Ltd; Ames, Iowa: 1999. pp. 630–633. [Google Scholar]
- Taylor D.J., Simmons J.R., Laird H.M. Production of diarrhoea and dysentery in pigs by feeding pure cultures of a spirochaete differing from Treponema hyodysenteriae. Veterinary Record. 1980;106:326–332. doi: 10.1136/vr.106.15.326. [DOI] [PubMed] [Google Scholar]
- Thomson J.R., Smith W.J., Murray B.P. Investigations into field cases of porcine colitis with particular reference to infection with Serpulina pilosicoli. Veterinary Record. 1998;7:235–239. doi: 10.1136/vr.142.10.235. [DOI] [PubMed] [Google Scholar]
- Trott D.J., Huxtable C.J., Hampson D.J. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infection and Immunity. 1996;64:4648–4654. doi: 10.1128/iai.64.11.4648-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott D.J., Stanton T.B., Jensen N.S., Duhamel G.E., Johnson J.L., Hampson D.J. Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis. International Journal of Systemic Bacteriology. 1996;46:206–215. doi: 10.1099/00207713-46-1-206. [DOI] [PubMed] [Google Scholar]
- Wahlström H., Bergström K., Engvall A., Gunnarsson A., Lindqvist H., Berge C., Wierup M. The 15th International Pig Veterinary Society Congress, Birmingham, England. 1998. The Swedish Salmonella control of pig and pork production; p. 73. [Google Scholar]
- Wahlström H., Eriksson E., Noll B., PlymForsell L., Wierup M., Wollin R. The 16th International Pig Veterinary Society Congress, Melbourne, Australia. 2000. The Swedish control of pig and pork production during 1999; p. 215. [Google Scholar]
- Wathes C.M., Miller B.G., Bourne F.J. Cold stress and post-weaning diarrhoea in piglets inoculated orally or by aerosol. Animal Production. 1989;49:483–496. [Google Scholar]
- Wilcock B.P., Olander H.J. Studies on the pathogenesis of swine dysentery. Veterinary Pathology. 1979;16:450–465. doi: 10.1177/030098587901600409. [DOI] [PubMed] [Google Scholar]
- Yoo H.S., Lee S.U., Park K.Y., Park Y.H. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. Journal of Clinical Microbiology. 1997;35:228–232. doi: 10.1128/jcm.35.1.228-232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


