Abstract
Receptor–ligand interactions are fundamental to the regulation of cell physiology, enabling the communication between cells and their environment via signal transduction. Receptors are also exploited by toxins and infectious agents to mediate pathogenesis. Over the past 20 years, however, this bi-partite paradigm for cellular regulation, that is, receptors and their ligands, has been revised to include an unforeseen participant namely, soluble receptors or molecular decoys. Decoys function as nature's modifiers of potent responses such as inflammation, stimulation of cell proliferation and triggering apoptosis. Decoys not only provide the means to fine tune the regulation of these phenomena; they also serve as potential leads for the development of recombinant anti-toxins, anti-viral agents and novel therapeutics for combating cancer and inflammatory disease.
Introduction
One hundred years have past since Ehrlich [1, 2] and Langley [3, 4] proposed the existence of ‘receptors’ as the responsive cellular components targeted by toxins. The interplay of ligands binding to their cognate receptors as a means to regulate cell function, induce proliferation or trigger apoptosis has since become fundamental to our understanding of cell biology, immunology and neurobiology, as well as to the rational design of innovative therapeutic drugs, laying the foundations of modern pharmacology. During the past quarter of the 20th century, however, this paradigm of the receptor/ligand ‘duo’ has had to be revised so to incorporate a third player in this scene, soluble receptors, thus creating a triad of proteins whose balance regulates and fine tunes cellular function. Soluble receptors—molecular decoys, were first proposed as novel biologics, envisioned as receptor mimetics that would function to intercept and sequester a pathogen in solution, before it would have the chance to encounter its cellular target. However, as gene cloning, expression of recombinant proteins and genomics flourished it soon became obvious that artificial decoys were in fact lagging in comparison to what turned out to be nature's basic modus operandi. For almost every membrane receptor of cytokines, growth factors and cell adhesins, soluble versions were found to be naturally produced by cells; hence ‘natural decoys’ that function as modifiers of the potent stimulants and regulators of inflammation and immune response. Moreover, it was discovered that these same decoy receptors had been hijacked by viruses over the course of their co-evolution with their hosts.
In this review both recombinant and natural molecular decoys are described. Whereas sugar-based decoys [5•, 6, 7, 8] and oligonucleotide decoys [9, 10, 11, 12•, 13••] are certainly of importance, the focus here will be primarily on proteinaceous decoys, selecting illustrative examples of this class of cell regulator. Out of historical justice to Ehrlich and Langley, anti-toxin and anti-viral decoys will be the first to be discussed, then moving on to the natural decoys and their virally hijacked versions. At the end, examples of the biotech pipeline of recombinant decoys currently in development and production are provided.
Anti-toxin decoys
The nicotinic acetylcholine receptor (nAChR) is composed of five polypeptide subunits (α2βγδ, MW 300 kDa) that together form a ligand-regulated ion channel [14]. The neutrotoxins, such as d-tubocurarine and cobra toxin, are antagonists of the neurotransmitter, acetylcholine, whose binding opens the channel leading to membrane depolarization and ultimate muscle contraction. Ligand overlay of protein blots [15, 16] proved to be effective for delineating a major component of the cholinergic binding site within the extracellular domain of the receptor's alpha subunit. Recombinant fusion proteins expressing the ligand binding segment of the alpha subunit were found to efficiently bind alpha neurotoxins in vitro [17, 18]. The production of the recombinant cholinergic binding site, R4137, was ultimately tested in vivo and found to protect mice against lethal doses of both cobra toxin and d-tubocurarine [19]. With the in vivo proof of principle, the concept of ‘Decoyance’ was proposed as a general application of receptor derived soluble molecular mimetics—decoys1 , for the treatment and prevention of infectious disease.
Bacterial toxins are often the mediators of morbidity and death and their sequestration has been a target for decoy development. A case in point is Clostridium difficile Toxin A, a major cause of antibiotic associated diarrhoea and colitis [7]. The toxin is a large protein (308 kDa) that binds to the trisaccharide sequence Galα[1–3]Galβ[1–4]GlcNAc displayed on the luminal surface of the apical plasma membrane of the intestinal epithelium. Therefore, a sugar-based decoy was produced and tested by the Canadian Biotech company, Synsorb Biotech, who conjugated the trisaccharide onto an inert silicon-based support. This was then introduced orally to rats that were subsequently subjected to Toxin A. The decoy-treated rats did not present the typical Toxin A associated pathology of the ileal mucosa as compared with the controls. Hence the decoy was able to sequester the toxin. This decoy application is particularly attractive in the incidence where antibiotic treatment is in fact detrimental; antibiotics compromise the natural flora providing Clostridium with an opportunity to colonize. Telovamer, a soluble, high molecular weight anionic polymer represents a ‘functional decoy’ able to bind and neutralize both Toxin A and Toxin B of Clostridium difficile yet is not derived from the natural receptor for these toxins. Genzyme Corp has announced recently that a phase III clinical study proved less effective than earlier phase II results had indicated, yet further development is being pursued.
Another example where decoys become advantageous in light of negative effects of antibiotic treatment is hemolytic-uremic syndrome (HUS) caused by Shiga toxin-producing E. coli (STEC) infections [8]. Antibiotic therapy in such cases is contraindicated as it leads to the release of cell associated Shiga toxin (Stx) and induces toxin gene expression, thus leading to an increase in free toxin in the gut lumen. An elegant treatment of STEC infections has been proposed by Paton et al. who have constructed a recombinant probiotic E. coli strain that displays Stx receptor mimics on its surface. Stx binds the glycolipid receptor, globtriaosyl ceramide (Gb3) that has the structure Galα[1–4]Galβ[1–4]Glc ceramide. The glycosyl transferase genes IgtC and IgtE derived from Neisseria were introduced into E coli R1 rendering it able to produce a chimeric lipopolysaccharide (LPS) core terminating in Galα[1–4]Galβ[1–4]Glc. The ‘probiotic bacterial decoys’ were capable of preventing fatal systemic complications of STEC in mice treated by oral administration of these recombinant E. coli.
The threat of infectious disease took on a more sinister reality since 11 September 2001, where bio-warfare has become an ever growing concern [20]. The following two examples of anti-toxin decoys are thus especially relevant.
Staphylococcal enterotoxin B (SEB) [21] acts as a super-antigen that can indiscriminately activate a broad population of T cells triggering the massive release of inflammatory cytokines that can escalate to toxic shock syndrome and death. The mode of action of SEB is to bind the Vβ region of the T cell receptor (TCR). Despite the relatively low affinity between SEB and Vβ (Kd = 144 μM), the potency of this toxin is very high (lethal doses can be as low as nanograms per kilogram body weight). Buonpane et al. [22••] have systematically optimized the murine Vβ8.2 domain using serial mutagenesis and yeast-display followed by increasingly stringent screens against biotinylated SEB. The highest-affinity mutant isolated had an affinity of 48 pM that is a three million fold improvement over the wild type Vβ8.2–SEB interaction. This Vβ decoy proved highly effective in neutralizing the toxin, even in rabbits that had already developed early signs of toxic shock syndrome. This not only illustrates the power of decoys but emphasizes that their efficacy is apparently directly correlated with their affinity for their target.
Anthrax toxin certainly plays a major role in the arsenal of the bioterrorist. Bacillus anthracis secretes a tripartite toxin composed of: Protective antigen (PA, 83 kDa) that binds macrophages; Lethal factor (LF), a zinc metalloproteinase; and a Ca+2/calmodulin-dependent adenylate cyclase called Edema factor (EF) [23]. PA83 binding to the integrin-like I domain of the macrophage surface protein; capillary morphogenesis protein 2 (CMG2), leads to its cleavage by furin endoprotease producing PA63. The oligomerization of PA63 into a heptamer provides the binding site for LF and EF that are then endocytosed and eventually kill the cell. Various approaches have been developed to counteract anthrax toxin of which anti-PA antibodies have proven particularly effective. The concern however, is that weaponized strains of B. anthracis may be engineered to produce antigenically altered versions of PA that would escape neutralization by existing anti-PA antibodies. Here the attribute of decoys is apparent as one assumes that the receptor binding surfaces of PA would not be amenable to mutagenesis and epitopic modification. Scobie et al. have been able to demonstrate that soluble CMG2 can function as a potent decoy capable of protecting rats against lethal toxin challenge making this decoy one of the most effective anthrax anti-toxins known [24].
Anti-viral decoys
The first bona fide anti-viral decoy to be developed was soluble CD4 (sCD4) for the treatment of AIDS.
CD4 is naturally an integral membrane glycoprotein protein that traverses the plasma membrane once. Its extracellular portion is composed of four immunoglobulin (Ig) domains (D1–D4) where D1 binds HIV-1 gp120 [25].
The fact that CD4 is a member of the immunoglobulin superfamily has had a profound effect on its development as a decoy and on the development of decoys in general. Immunoglobulins are naturally presented as membrane bound cell surface receptors (B cell receptors, BCR) yet mature into soluble serum antibodies as the result of alternative splicing. Antibodies are as efficient in antigen recognition as are their cognate membrane bound BCRs. Isotype switching is fundamental to antibody biology and as such, swapping V domains for other Ig domains makes perfect sense. Moreover, antibodies naturally are polyvalent; as in bivalent IgGs, tetravalent IgAs and decavalent IgMs and therefore are avid binders of their targets.
Capon et al. [26] were the first to engineer an ‘immunoadhesin’, grafting the ligand binding Ig domains D1–D2 of CD4 onto a human IgG1 Fc scaffold that proved to increase the serum half-life of the decoy markedly. So much so, this strategy for decoy design has been adopted by most working on soluble receptors/decoys in general [27•] Figure 1 .
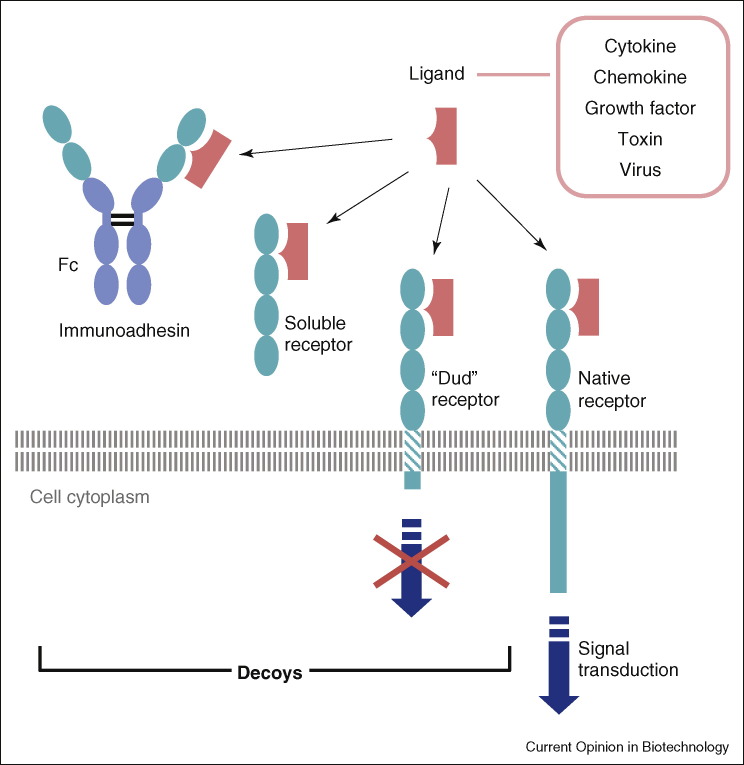
Figure 1.
Native receptors and their decoys. Binding of ligands to their native receptors triggers a signal transduction cascade (for cytokines, chemokines and growth factors). Often, toxins and viruses exploit existing receptors as alternative ligands and thereby elicit morbidity or gain entry into the cell. In order to prevent toxin or viral pathogenesis, or to modify the effects of the natural ligands, decoys can intercept the ligands before they reach the native receptor. Three types of decoys are portrayed: ‘Dud’ receptors are membrane associated decoys that bind the native ligands yet are unable to signal transduce. Soluble receptors can be produced naturally either by alternative splicing or proteolytic cleavage. Recombinant soluble receptors provide research tools and leads for the development of novel pharmaceuticals. The preferred modality for such therapeutic decoys is ‘immunoadhesins’, receptor binding domains grafted onto an Fc scaffold.
Despite the optimism, however, it turned out that CD4-based decoys simply did not have the clout required to keep HIV in check. It was quickly realized that the neutralizing potency of sCD4 was preferentially greater for lab adapted strains of HIV than for the field isolates of this virus [28, 29]. The mechanism for neutralization by the decoy required induced gp120 shedding that turned out to be much more demanding for the field isolates [30]. All attempts to optimize and improve sCD4 decoys [31, 32, 33, 34, 35] proved insufficient and for the moment sCD4 decoys have not progressed beyond phase I/II clinical trials.
Since the introduction of sCD4 as an AIDS therapeutic, soluble receptors for various viruses (e.g. Rhinovirus [36, 37], poliovirus [38], Foot and Mouth disease virus [39], SARS coronavirus [40] and Hepatitis A virus [41]) have been reported to have neutralizing activity and thus form the basis for novel therapeutics. However, to date, a commercial decoy-product effective in the prevention or treatment of viral infections has still not appeared on the market. This may be due to the fact that viruses are replicating pathogens and demand enormous efficiency for viral clearance in order for a decoy to be truly therapeutic.
Decoys as modifiers of regulation on the contrary, turn out to be natural components in the fine tuning of inflammation and immune responses.
Natural decoys
In 1984 Ullrich et al. published the cloning of the human epidermal growth factor receptor (EGFR, MW 138 kDa) from A431 epidermoid carcinoma cells [42]. Curiously, they also discovered that these cells contained a 2.8 kb mRNA that when expressed produced a 70 kDa truncated version of the EGF extracellular domain. The authors concluded that this is ‘particularly intriguing in view of [their] earlier observation that the v-erb-B oncogene encodes what seems to be a truncated avian receptor polypeptide corresponding to the transmembrane and cytoplasmic domains’. Thus, the focus and motivation in 1984 were not neutralizing soluble receptors, but rather truncated constitutive signal transducing receptors that could explain the out-of-control cell proliferation associated with cancer.
Nonetheless, shortly there after reports appeared describing a diversity of naturally existing soluble receptors, most of which could be associated with one of two gene families, the tumour necrosis factor (TNF) superfamily [43•] and the Ig superfamily [44, 45•]. It immediately became apparent that by proteolytic shedding of cell surface proteins (by metallo-proteases coined ‘Sheddases’) or by alternative splicing, truncated versions of otherwise membrane associated receptors are readily generated [46•]. Nature produces such soluble receptors in order to crucially regulate immune responses towards cancer and infection as well as inflammation in general (Figure 1).
Mantovani and his colleagues have pioneered the concept of natural decoy receptors as nature's solution for the fine tuning of its most potent defense mechanisms [47••]. The specific example of the soluble interleukin 1 receptor type II (IL-1RII) is illustrated here as a case in point.
Interleukin I was one of the first cytokines to be discovered and is responsible for triggering a diversity of physiological effects such as fever, augmentation of lymphocyte responses and induction of degenerative changes in joints [48]. Owing to this diversity, there was even question as to whether a single molecule could be responsible for such a variety of functions. The ultimate cloning of IL-1α and IL-1β in 1984, laid this debate to rest as it became clear that these cytokines function as a ‘master switch’ of sorts, responsible for the expression and release of numerous other cytokines (e.g. IL-6 and many chemokines). Availability of recombinant IL1 also promptly led to the discovery of its receptors, first IL-1R type 1 that is a member of the Ig superfamily (contains three Ig-like extracellular domains) and signal tranduces through a Toll-like cytoplasmic domain. This receptor is expressed on most cells and functions in a complex with its ‘associated protein’—AcP. A second IL1-R (type II), expressed primarily on B cells, monocytes and polymorphonuclear cells (PMN), also exists yet is unable to signal transduce (its cytoplasmic tail is only 29aa long). Colotta et al. were able to show that IL-1R type II functions as a decoy receptor and is present in both membrane associated and soluble forms [49]. Furthermore, its expression is regulated by IL-4. Together, these illustrate the complexity and elaborate regulatory devices that exist for the control of the IL-1 response. The action of IL-1, mediated by its association to IL-1R type I, can be moderated by sequestration of the cytokine by soluble IL-1R type II, which is itself upregulated by IL-4. Moreover, as IL-1R type II also binds AcP, the effect of binding of decoy IL-1R type II to the complex—IL-1/IL-R type I/AcP causes a dominant negative shut down of signal transduction.
Soluble decoy receptors are not always antagonistic to their ligands as is illustrated by soluble IL-6 receptor (sIL-6R). This decoy binds IL-6 and in doing so prolongs its half-life. Furthermore, binding of IL-6/sIL-6R to membrane bound gp130 triggers signal transduction via a process of ‘trans-signaling’ [50•].
Finally, decoy action can be mediated by membrane bound receptors as well. This is particularly relevant for chemokine receptors whose decoys persist as membrane proteins that are effective in ligand binding but ‘handicapped’ in signal transduction. Thus, ‘dud’ receptors serve as functional decoys to fine tune inflammation [51•] (Figure 1).
This strategy is also employed in the regulation of programmed cell death mediated by death receptors and their decoys [52]. Decoy receptors 1 and 2 are membrane bound ‘dud’ receptors unable to signal transduce. Decoy receptor 3 (DcR3) [53] however, is a potent soluble decoy for Fas ligand that tends to be overexpressed in various cancers illustrating how tipping the balance of the regulation of apoptosis can have a profound effect in cancer pathogenesis (see Figure 1).
Viral hijacked-decoys
It is not surprising that the ability to intervene in immuno-regulatory processes through decoy receptors has been exploited by the large DNA viruses, thus creating ‘viroceptors’ [54, 55, 56, 57]. The poxviruses produce a diversity of soluble cytokine receptors as well as their own versions of cytokine binding proteins. Viral decoy receptors for TNF, IL-1 and interferon γ (IFNγ) provide poxviruses the means to counter act and evade the immune response, contributing directly to the virulence of the virus [58]. Thus for example, the deletion of the viral IFNγ binding protein has no effect on viral replication in cell culture yet dampens its virulence dramatically in vivo. This point might be important in the design of safer vaccines in which viral decoy receptors can be deleted, rendering a more tolerated virus, without compromising its antigenic repertoire [59••].
Viral decoys, although derived from host receptors, have evolved to enhance their activity in immune evasion [56]. Thus for example, the vaccinia viral receptor for IL-1 tends to be much more specific for IL-1β than its mammalian cell homologue. For the viral IFNγR, which is highly stringent in the host, the opposite occurs and is broadly cross reactive as a soluble viral decoy. This decoy may actually be the result of capture of not only the receptor domain from the host but also of a helix-turn-helix (HTH) derived from the TFIIA host transcription factor [60]. As in the transcription factor, this HTH domain allows oligomerization and is the structural element that enables the viral decoy to form tetramers and thus benefit from more avid binding. The multi-domain nature of viral decoys is found in the vTNFR as well [61]. The pox TNFRs are coded by four genes named cytokine response modifiers B (CrmB), CrmC, CrmD and CrmE. In CrmB, in addition to its TNF binding domain, its carboxy terminus has developed the capacity to bind chemokines. Thus, CrmB can simultaneously sequester TNF and bind chemokines as well.
Chemokine binding receptors are particularly well developed in the Herpes viruses [62•]. A case in point is the human cytomegalovirus (HCMV) open reading frame US28, which is closely homologous to CC chemokine receptors and binds CC chemokines at the nanomolar range [63]. Whereas US28 is effective in chemokine sequestration, its expression is not required for viral replication in cultured cells. On the contrary this membrane bound receptor may have a different role where it may enable the virus-infected cells to follow natural chemokine gradients and thus assist in the dissemination of these cells to ‘preferred’ tissues in the host. The viroceptors and the interplay between viruses and their hosts may actually provide new insights for the design of future therapies [64•].
The decoy ‘Pipeline’
Undoubtedly, the concept of exploiting the ligand binding domains of receptors as leads for therapeutic decoys is extremely attractive. However, the fact is, that for the moment, only few decoys have been FDA approved as bona fide commercially viable products. Nonetheless, there has been progress in this effort and the greatest promise seems to be for counter acting chronic inflammation and treating cancer.
Tumour necrosis factor (TNF) is a central pro-inflammatory cytokine that triggers the production of other mediators of inflammation and tissue destruction, such as IL-1β and IL-6 [65]. TNF is directly associated with diseases of chronic and severe inflammation such as rheumatoid arthritis (RA), Crohn's disease and psoriasis and therefore has been the target for the production of specific therapeutic antagonists. Etanercept (Enbrel marketed by Amgen and Wyeth) is a dimeric immunoadhesin (see above) consisting of the extracellular ligand binding domain of the TNF receptor linked to an IgG1 Fc scaffold [66]. In contrast to two other antagonists based on TNF-specific monoclonal antibodies (infliximab and adalimumab), etanercept binds both TNFα and lymphotoxin (formerly TNFβ) thus illustrating the advantage of a decoy over antibodies that tend to be more restrictive for their binding. Etanercept also shows higher potency for RA therapy although does not seem to be as effective as the antibodies in treatment of Crohn's disease ([65], see also [67•]). Most certainly there are numerous off label indications that are currently being evaluated for this drug that should prove to be extremely important for the treatment of inflammatory disease and may find application in the treatment of cancer as well.
Indeed, cancer appears to be directly associated with chronic inflammation [68•]. Chronic inflammatory bowel disease, for example, is often a prelude to colon cancer. In the recent study of Popivanova et al. [69••], sequestration of TNF is shown to reduce the development of colorectal cancer in a mouse model for ulcerative colitis. Treatment of these mice with etanercept markedly reduces progression to colorectal cancer and extends survival dramatically. These results not only demonstrate the association of TNF mediated inflammation with colon cancer but the potential use of etanercept as an anti-cancer drug.
Tumour vascularization is a pivotal step in the progression to metastatic cancer [70]. So long as the tumour is not ‘hooked-up’ to the vasculature, oxygen and nutrients are limited, growth is restricted and the shedding of malignant cells able to ‘seed’ other organs is not possible. Folkman was the first to propose anti-angiogenic drugs for the treatment of solid tumours [71, 72••]. Indeed, a central target for drug development is the regulator of angiogenesis, vascular endothelial growth factor, VEGF [73•]. VEGF exists as a number of molecular variants and related factors such as placental growth factor, and elicits its effect by binding to its receptors VEGFR1 (flt-1), VEGFR2 (flk-1) and VEGFR3. The first biologic to be FDA approved as an anti-VEGF cancer therapeutic is the monoclonal antibody bevacizumab (Avastin). Since then a highly potent VEGF decoy, ‘VEGF trap’ (Aflibercept, being developed by Regeneron Pharmaceuticals and Sanofi-Aventis2 ), has been produced [74, 75•]. VEGF-trap is a recombinant immunoadhesin composed of the D2 Ig domain of VEGFR1 linked to the D3 Ig domain of VEGFR2 fused to the Fc of IgG1 thus creating a dimeric decoy. In preclinical mouse models for non-small cell lung cancer [76] and renal cell cancer [77] VEGF-trap has proven to be substantially more potent than bevacizumab. In view of these results VEGF-trap is currently the subject of four Phase III clinical studies in patients with non-small cell lung cancer, pancreatic cancer, colorectal cancer and prostrate cancer [75•].
Conclusions
Decoy receptors are clearly a basic component in the regulatory machinery of the cell and function in the fine tuning of proliferation and death of cells and especially in immune responses. They also play a role in immune evasion of viruses. The basic concept of recombinant soluble receptors as potent therapeutics actually preceded the realization of the existence of their natural homologues. The decoy neutralization of toxins appears to be very effective and should provide genuine biologic anti-dotes. The prospects for anti-virals seem more complex as the demand for sterilizing efficiency for the moment is beyond the potency of existing decoys. Markedly increasing the affinity of decoys for their cognate viruses might be necessary to make this class of therapeutic effective. For the moment the greatest success in decoys as biologic drugs is in the immunoadhesin variants of the natural cell regulators such as TNF and VEGF. Similar products for IL-1 [78] and cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) [79] have also been approved, indicating that we should be seeing multiple decoys on the market in the very near future.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The author acknowledges Anna Roitburd-Berman, Natalia Tarnovitski Freund, Yael Weiss and Gilad Kaplan for their considerable help in putting this article together, their thoughtful reading of the Drafts, their critical constructive comments and most of all, for making my lab a fun place to come to each morning—TODA. Jonathan M. Gershoni is the incumbent of the David Furman Chair in Immunobiology of Cancer.
Footnotes
The term ‘decoy’ was coined from the Hebrew word for suppression ( ) pronounced ‘Decooy’.
) pronounced ‘Decooy’.
Bayer HealthCare is collaborating with Regeneron in the development and commercialization of Aflibercept for the treatment of eye disorders.
References
- 1.Ehrlich P: On immunity with special reference to cell life. The Croonian Lecture 1900 pp 428–448.
- 2.Prull C.R. Part of a scientific master plan? Paul Ehrlich and the origins of his receptor concept. Med Hist. 2003;47:332–356. [PMC free article] [PubMed] [Google Scholar]
- 3.Langley JN: On nerve endings and on special excitable substances in cells. The Croonian Lecture 1906 pp 170–194.
- 4.Maehle A.-H. ‘Receptive substances’: John Newport Langley (1852–1925) and his path to receptor theory of drug action. Med Hist. 2004;48:153–174. doi: 10.1017/s0025727300000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta. 2006;1760:527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]; A comprehensive review of the role of carbohydrate ‘receptors’ and bacterial lectin-like molecules that mediate adhesion. The structures of the glycomoeities and their recognition are described as well as a discussion of the potential applications for anti-adhesion therapy.
- 6.Svanborg Eden C., Hagberg L., Leffler H., Lomberg H. Recent progress in the understanding of the role of bacterial adhesion in the pathogenesis of urinary tract infection. Infection. 1982;10:327–332. doi: 10.1007/BF01640890. [DOI] [PubMed] [Google Scholar]
- 7.Castagliuolo I., LaMont J.T., Qiu B., Nikulasson S.T., Pothoulakis C. A receptor decoy inhibits the enterotoxic effects of Clostridium difficile toxin A in rat ileum. Gastroenterology. 1996;111:433–438. doi: 10.1053/gast.1996.v111.pm8690209. [DOI] [PubMed] [Google Scholar]
- 8.Paton A.W., Morona R., Paton J.C. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat Med. 2000;6:265–270. doi: 10.1038/73111. [DOI] [PubMed] [Google Scholar]
- 9.Sullenger B.A., Gallardo H.F., Ungers G.E., Gilboa E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 1990;63:601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- 10.Mann M.J., Dzau V.J. Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest. 2000;106:1071–1075. doi: 10.1172/JCI11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita N., Morishita R., Tomita T., Ogihara T. Potential therapeutic applications of decoy oligonucleotides. Curr Opin Mol Ther. 2002;4:166–170. [PubMed] [Google Scholar]
- 12•.Moriyama I., Ishihara S., Rumi M.A., Aziz M.D., Mishima Y., Oshima N., Kadota C., Kadowaki Y., Amano Y., Kinoshita Y. Decoy oligodeoxynucleotide targeting activator protein-1 (AP-1) attenuates intestinal inflammation in murine experimental colitis. Lab Invest. 2008;88:652–663. doi: 10.1038/labinvest.2008.38. [DOI] [PubMed] [Google Scholar]; A comparative analysis of AP-1 vs NFkB decoys and their application in suppressing intestinal inflammation in a mouse model.
- 13••.Azuma H., Tomita N., Sakamoto T., Kiyama S., Inamoto T., Takahara K., Kotake Y., Segawa N., Morishita R., Takahara S. Marked regression of liver metastasis by combined therapy of ultrasound-mediated NF kappaB-decoy transfer and transportal injection of paclitaxel, in mouse. Int J Cancer. 2008;122:1645–1656. doi: 10.1002/ijc.23280. [DOI] [PubMed] [Google Scholar]; A major problem in the application of oligonucleotide decoys is the method of delivery. Here the application of ultrasound is described as a means to deliver NFkB decoys in the treatment of liver disease.
- 14.Changeux J.P., Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med. 2008;14:93–102. doi: 10.1016/j.molmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Gershoni J.M., Hawrot E., Lentz T.L. Binding of alpha-bungarotoxin to isolated alpha subunit of the acetylcholine receptor of Torpedo californica: quantitative analysis with protein blots. Proc Natl Acad Sci U S A. 1983;80:4973–4977. doi: 10.1073/pnas.80.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gershoni J.M., Palade G.E. Protein blotting: principles and applications. Anal Biochem. 1983;131:1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- 17.Barkas T., Mauron A., Roth B., Alliod C., Tzartos S.J., Ballivet M. Mapping the main immunogenic region and toxin-binding site of the nicotinic acetylcholine receptor. Science. 1987;235:77–80. doi: 10.1126/science.2432658. [DOI] [PubMed] [Google Scholar]
- 18.Gershoni J.M. Expression of the alpha-bungarotoxin binding site of the nicotinic acetylcholine receptor by Escherichia coli transformants. Proc Natl Acad Sci U S A. 1987;84:4318–4321. doi: 10.1073/pnas.84.12.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gershoni J.M., Aronheim A. Molecular decoys: ligand-binding recombinant proteins protect mice from curarimimetic neurotoxins. Proc Natl Acad Sci U S A. 1988;85:4087–4089. doi: 10.1073/pnas.85.11.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnett J.C., Henchal E.A., Schmaljohn A.L., Bavari S. The evolving field of biodefence: therapeutic developments and diagnostics. Nat Rev Drug Discov. 2005;4:281–297. doi: 10.1038/nrd1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick J.K., Yarwood J.M., Schlievert P.M. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 22••.Buonpane R.A., Churchill H.R., Moza B., Sundberg E.J., Peterson M.L., Schlievert P.M., Kranz D.M. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat Med. 2007;13:725–729. doi: 10.1038/nm1584. [DOI] [PubMed] [Google Scholar]; An excellent example of optimization of an anti-toxin decoy. Systematic mutagenesis leads to a marked enhancement of affinity between the Vβ-based decoy and its target. This illustrates the correlation between affinity and decoy efficacy.
- 23.Bradley K.A., Young J.A. Anthrax toxin receptor proteins. Biochem Pharmacol. 2003;65:309–314. doi: 10.1016/s0006-2952(02)01455-7. [DOI] [PubMed] [Google Scholar]
- 24.Scobie H.M., Thomas D., Marlett J.M., Destito G., Wigelsworth D.J., Collier R.J., Young J.A., Manchester M. A soluble receptor decoy protects rats against anthrax lethal toxin challenge. J Infect Dis. 2005;192:1047–1051. doi: 10.1086/432731. [DOI] [PubMed] [Google Scholar]
- 25.Sweet R.W., Truneh A., Hendrickson W.A. CD4: its structure, role in immune function and AIDS pathogenesis, and potential as a pharmacological target. Curr Opin Biotechnol. 1991;2:622–633. doi: 10.1016/0958-1669(91)90089-n. [DOI] [PubMed] [Google Scholar]
- 26.Capon D.J., Chamow S.M., Mordenti J., Marsters S.A., Gregory T., Mitsuya H., Byrn R.A., Lucas C., Wurm F.M., Groopman J.E. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989;337:525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- 27•.Ashkenazi A., Chamow S.M. Immunoadhesins as research tools and therapeutic agents. Curr Opin Immunol. 1997;9:195–200. doi: 10.1016/s0952-7915(97)80135-5. [DOI] [PubMed] [Google Scholar]; Sets the foundations of what has become the standard platform for production of soluble receptors.
- 28.Daar E.S., Li X.L., Moudgil T., Ho D.D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orloff S.L., Kennedy M.S., Belperron A.A., Maddon P.J., McDougal J.S. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J Virol. 1993;67:1461–1471. doi: 10.1128/jvi.67.3.1461-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore J.P., Klasse P.J. Thermodynamic and kinetic analysis of sCD4 binding to HIV-1 virions and of gp120 dissociation. AIDS Res Hum Retroviruses. 1992;8:443–450. doi: 10.1089/aid.1992.8.443. [DOI] [PubMed] [Google Scholar]
- 31.Traunecker A., Schneider J., Kiefer H., Karjalainen K. Highly efficient neutralization of HIV with recombinant CD4-immunoglobulin molecules. Nature. 1989;339:68–70. doi: 10.1038/339068a0. [DOI] [PubMed] [Google Scholar]
- 32.Van Oijen M.G., Preijers F.W. Rationale for the use of immunotoxins in the treatment of HIV-infected humans. J Drug Target. 1998;5:75–91. doi: 10.3109/10611869808995861. [DOI] [PubMed] [Google Scholar]
- 33.Dey B., Del Castillo C.S., Berger E.A. Neutralization of human immunodeficiency virus type 1 by sCD4-17b, a single-chain chimeric protein, based on sequential interaction of gp120 with CD4 and coreceptor. J Virol. 2003;77:2859–2865. doi: 10.1128/JVI.77.5.2859-2865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyuhas R., Noy H., Montefiori D.C., Denisova G., Gershoni J.M., Gross G. HIV-1 neutralization by chimeric CD4-CG10 polypeptides fused to human IgG1. Mol Immunol. 2005;42:1099–1109. doi: 10.1016/j.molimm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher C.V., DeVille J.G., Samson P.M., Moye J.H., Jr., Church J.A., Spiegel H.M., Palumbo P., Fenton T., Smith M.E., Graham B. Nonlinear pharmacokinetics of high-dose recombinant fusion protein CD4-IgG2 (PRO 542) observed in HIV-1-infected children. J Allergy Clin Immunol. 2007;119:747–750. doi: 10.1016/j.jaci.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greve J.M., Forte C.P., Marlor C.W., Meyer A.M., Hoover-Litty H., Wunderlich D., McClelland A. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J Virol. 1991;65:6015–6023. doi: 10.1128/jvi.65.11.6015-6023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicodemou A., Petsch M., Konecsni T., Kremser L., Kenndler E., Casasnovas J.M., Blaas D. Rhinovirus-stabilizing activity of artificial VLDL-receptor variants defines a new mechanism for virus neutralization by soluble receptors. FEBS Lett. 2005;579:5507–5511. doi: 10.1016/j.febslet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Belnap D.M., McDermott B.M., Jr., Filman D.J., Cheng N., Trus B.L., Zuccola H.J., Racaniello V.R., Hogle J.M., Steven A.C. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc Natl Acad Sci U S A. 2000;97:73–78. doi: 10.1073/pnas.97.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duque H., LaRocco M., Golde W.T., Baxt B. Interactions of foot-and-mouth disease virus with soluble bovine alphaVbeta3 and alphaVbeta6 integrins. J Virol. 2004;78:9773–9781. doi: 10.1128/JVI.78.18.9773-9781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H., Gramberg T., Pohlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun. 2004;319:1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silberstein E., Xing L., van de Beek W., Lu J., Cheng H., Kaplan G.G. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobin-and mucin-like regions. J Virol. 2003;77:8765–8774. doi: 10.1128/JVI.77.16.8765-8774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ullrich A., Coussens L., Hayflick J.S., Dull T.J., Gray A., Tam A.W., Lee J., Yarden Y., Libermann T.A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 43•.Ware C.F. The TNF Superfamily-2008. Cytokine Growth Factor Rev. 2008;19:183–186. doi: 10.1016/j.cytogfr.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; An editorial preceding a special issue on the TNF superfamily including comprehensive tables of family members.
- 44.Peggs K.S., Allison J.P. Co-stimulatory pathways in lymphocyte regulation: the immunoglobulin superfamily. Br J Haematol. 2005;130:809–824. doi: 10.1111/j.1365-2141.2005.05627.x. [DOI] [PubMed] [Google Scholar]
- 45•.Aricescu A.R., Jones E.Y. Immunoglobulin superfamily cell adhesion molecules: zippers and signals. Curr Opin Cell Biol. 2007;19:543–550. doi: 10.1016/j.ceb.2007.09.010. [DOI] [PubMed] [Google Scholar]; A comprehensive overview of the IG family.
- 46•.Levine S.J. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–5348. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]; A systematic analysis of various mechanisms used to generate soluble receptors including ‘Sheddase’ metallo-proteanases.
- 47••.Mantovani A., Bonecchi R., Martinez F.O., Galliera E., Perrier P., Allavena P., Locati M. Tuning of innate immunity and polarized responses by decoy receptors. Int Arch Allergy Immunol. 2003;132:109–115. doi: 10.1159/000073711. [DOI] [PubMed] [Google Scholar]; A landmark review describing the general concept of natural decoy receptors as a means to regulate and fine tune cytokine activity.
- 48.Dinarello C.A. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 49.Colotta F., Re F., Muzio M., Bertini R., Polentarutti N., Sironi M., Giri J.G., Dower S.K., Sims J.E., Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 50•.Dominitzki S., Fantini M.C., Neufert C., Nikolaev A., Galle P.R., Scheller J., Monteleone G., Rose-John S., Neurath M.F., Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]; Trans-signaling provides a means to enhance the effect of a decoy rather than suppress the signal as illustrated for the case of soluble IL-6.
- 51•.Mantovani A., Bonecchi R., Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]; Incorporation of Chemokine decoys into the equation of mechanisms for effective regulation of immune response. A comprehensive review bolstered with informative tables and charts.
- 52.Ashkenazi A., Dixit V.M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 53.Pitti R.M., Marsters S.A., Lawrence D.A., Roy M., Kischkel F.C., Dowd P., Huang A., Donahue C.J., Sherwood S.W., Baldwin D.T. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 54.McFadden G., Murphy P.M. Host-related immunomodulators encoded by poxviruses and herpesviruses. Curr Opin Microbiol. 2000;3:371–378. doi: 10.1016/s1369-5274(00)00107-7. [DOI] [PubMed] [Google Scholar]
- 55.Alcami A., Koszinowski U.H. Viral mechanisms of immune evasion. Mol Med Today. 2000;6:365–372. doi: 10.1016/S1357-4310(00)01775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat Rev Immunol. 2003;3:36–50. doi: 10.1038/nri980. [DOI] [PubMed] [Google Scholar]
- 57.Sodhi A., Montaner S., Gutkind J.S. Viral hijacking of G-protein-coupled-receptor signalling networks. Nat Rev Mol Cell Biol. 2004;5:998–1012. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- 58.Johnston J.B., McFadden G. Poxvirus immunomodulatory strategies: current perspectives. J Virol. 2003;77:6093–6100. doi: 10.1128/JVI.77.11.6093-6100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Sakala I.G., Chaudhri G., Buller R.M., Nuara A.A., Bai H., Chen N., Karupiah G. Poxvirus-encoded gamma interferon binding protein dampens the host immune response to infection. J Virol. 2007;81:3346–3353. doi: 10.1128/JVI.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; The interplay between Pox viruses and INFγ. Whereas the cytokine ‘viroceptor’ is not required for the replication of the virus, it does effect its virulence. The authors propose that modification of viruses by deletion of their viroceptors may provide for safer vaccines.
- 60.Nuara A.A., Walter L.J., Logsdon N.J., Yoon S.I., Jones B.C., Schriewer J.M., Buller R.M., Walter M.R. Structure and mechanism of IFN-gamma antagonism by an orthopoxvirus IFN-gamma-binding protein. Proc Natl Acad Sci U S A. 2008;105:1861–1866. doi: 10.1073/pnas.0705753105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alejo A., Ruiz-Arguello M.B., Ho Y., Smith V.P., Saraiva M., Alcami A. A chemokine-binding domain in the tumor necrosis factor receptor from variola (smallpox) virus. Proc Natl Acad Sci U S A. 2006;103:5995–6000. doi: 10.1073/pnas.0510462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Vischer H.F., Leurs R., Smit M.J. HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks. Trends Pharmacol Sci. 2006;27:56–63. doi: 10.1016/j.tips.2005.11.006. [DOI] [PubMed] [Google Scholar]; A critical analysis of the chemokine decoys produced in HCMV thus illustrating this mode of virus decoy action for Herpes viruses.
- 63.Lalani A.S., Barrett J.W., McFadden G. Modulating chemokines: more lessons from viruses. Immunol Today. 2000;21:100–106. doi: 10.1016/s0167-5699(99)01556-x. [DOI] [PubMed] [Google Scholar]
- 64•.Dagna L., Lusso P. Virus-encoded chemokines, chemokine receptors and chemokine-binding proteins: new paradigms for future therapy. Future Virol. 2007;2:353–368. [Google Scholar]; An insightful analysis of the potential for novel anti-viral therapies based on viroceptors.
- 65.Tracey D., Klareskog L., Sasso E.H., Salfeld J.G., Tak P.P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Goldenberg M.M. Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis. Clin Ther. 1999;21:75–87. doi: 10.1016/S0149-2918(00)88269-7. discussion 71–72. [DOI] [PubMed] [Google Scholar]
- 67•.Gladman D.D. Adalimumab, etanercept and infliximab are equally effective treatments for patients with psoriatic arthritis. Nat Clin Pract Rheumatol. 2008;4(10):510–511. doi: 10.1038/ncprheum0880. [DOI] [PubMed] [Google Scholar]; A careful comparative analysis of anti-TNF drugs in patients suffering from psoriatic arthritis. Enanercept as compared to the two mAb-based drugs was found equally effective and thus it is concluded that patients should be allowed to choose the therapy best suited to them.
- 68•.Lin W.W., Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review linking inflammation to the development of malignant disease.
- 69••.Popivanova B.K., Kitamura K., Wu Y., Kondo T., Kagaya T., Kaneko S., Oshima M., Fujii C., Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a mouse model for inflammatory bowel disease and progression to colon cancer the authors illustrate the effect of Etanercept. This not only demonstrates the involvement of TNF in the progression to colon cancer but also the potency of its decoy in treatment.
- 70.Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 71.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]; An extremely comprehensive and insightful review on the role of angiogenesis in cancer and its treatment. The article not only reviews the field in detail, it is exceptionally well illustrated and provides Prof Folkman's personal perspectives only months before his untimely death (January 2008).
- 73•.Roskoski R., Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]; A comprehensive review of a central component in the biology of tumour progression.
- 74.Holash J., Davis S., Papadopoulos N., Croll S.D., Ho L., Russell M., Boland P., Leidich R., Hylton D., Burova E. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Aflibercept: VEGF Trap. Drugs R D. 2008;9:261–269. doi: 10.2165/00126839-200809040-00006. [DOI] [PubMed] [Google Scholar]; A useful survey of the latest developments and clinical trials related to VEGF-trap
- 76.Riely G.J., Miller V.A. Vascular endothelial growth factor trap in non small cell lung cancer. Clin Cancer Res. 2007;13:s4623–s4627. doi: 10.1158/1078-0432.CCR-07-0544. [DOI] [PubMed] [Google Scholar]
- 77.Verheul H.M., Hammers H., van Erp K., Wei Y., Sanni T., Salumbides B., Qian D.Z., Yancopoulos G.D., Pili R. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13:4201–4208. doi: 10.1158/1078-0432.CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 78.Hoffman H.M., Throne M.L., Amar N.J., Sebai M., Kivitz A.J., Kavanaugh A., Weinstein S.P., Belomestnov P., Yancopoulos G.D., Stahl N. Efficacy and safety of rilonacept (interleukin-1 trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 79.Schiff M., Keiserman M., Codding C., Songcharoen S., Berman A., Nayiager S., Saldate C., Li T., Aranda R., Becker J.C. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]