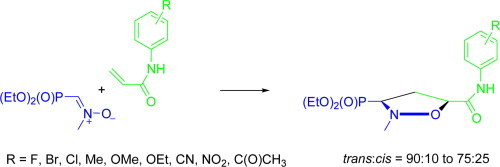
Graphical abstract
Keywords: 1,3-Dipolar cycloaddition; Isoxazolidines; Phosphonates; Antiviral; Cytostatic
Abstract
5-Arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates have been synthesised from N-methyl-C-diethoxyphosphorylnitrone and N-arylacrylamides in good yields. cis- and trans-isoxazolidine phosphonates obtained herein were evaluated for activity against a broad range of DNA and RNA viruses. None of the compounds were endowed with antiviral activity at subtoxic concentrations. Isoxazolidines having phenyl substituted with halogen (Ar = 2-F-C6H4; 3-Br-C6H4; and 4-Br-C6H4) have been found to inhibit proliferation of L1210, CEM as well as HeLa cells with IC50 in the 100–170 μM range.
1. Introduction
Nucleoside analogues are of great interest in medicinal chemistry due to their broad spectrum of biological activities. Extensive search for modified nucleosides has led to the discovery of many potent drugs for treatment of various viral infections1 and diverse types of neoplasms (Fig. 1 ).2 The adverse effects of the available therapies, low selectivity and the observed drug resistance have become a driving force in a search for new analogues with improved pharmacokinetic and pharmacodynamic properties. Numerous modifications of naturally occurring nucleosides have provided analogues with altered sugar and/or nucleobase subunits. Heterocycles containing one or more heteroatoms and carbocycles of various sizes as well as straight or branched chains also with heteroatoms have been applied as an alternative to the furanose ring.3, 4 So far less attention has been paid to the synthesis of analogues having modified nucleobase residues in comparison to sugar-modified analogues due to ensuring base pairing, but recently it has been proven that other aromatic rings are able to base-pair as well.5 A long list of nucleoside analogues continues to expand by incorporation of several linkers such as a 1,2,3-triazole group,6, 7, 8, 9 a carbamoyl10, 11, 12, 13, 14, 15, 16, 17 or an ureidyl function18 among others.
Figure 1.

Examples of clinically applied nucleoside analogues with anticancer or antiviral activity.
The isoxazolidine framework has successfully been applied as a surrogate for a furanose ring in the synthesis of nucleoside analogues with anticancer or antiviral activity (Fig. 2 ). Nucleoside analogue 1 [(−)-AdFU] having a fluorouracil residue attached to the isoxazolidine ring induces apoptosis on lymphoid and monocytoid cells and exhibits low level of cytotoxicity.19 Phosphonylated isoxazolidines 2 inhibit reverse transcriptase of HTLV-1 with activity comparable to that of AZT and protect human peripheral blood mononuclear cells against HTLV-1 transmission.20 Furthermore, compounds of general formula 3 show high cytotoxic activity against several cancer cell lines comparable to the known anticancer drugs, namely, Mitomycin C, Paclitaxel and 5-Fluorouracil, used as positive controls.21 Synthesis and promising antiproliferative properties of isoxazolidines 4 have been reported by Bortolini et al.22
Figure 2.
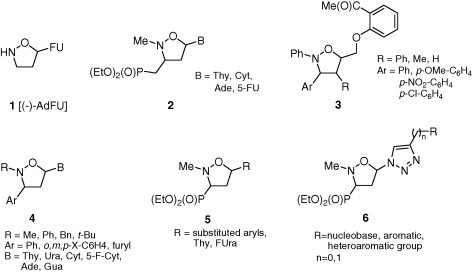
Examples of isoxazolidine nucleosides with cytotoxic and antiviral properties.
Recently, a series of 3,5-disubstituted isoxazolidine nucleosides 5 23, 24 as well as their further modifications 6 with an 1,2,3-triazole spacer25 have been obtained and their antiviral and cytotoxic properties were evaluated. Isoxazolidines 5 substituted with 1- and 2-naphthyl at C5 were found cytotoxic against HeLa and K562 cell lines (R = 1-naphthyl and 2-naphthyl; IC50 0.05 and 0.09 mM, respectively),23 while cis-configured 5-fluorouracil and 5-thymine derivatives 5 completely inhibited the reverse transcriptase activity of Avian Moloney Virus (AMV) and Human Immunodeficiency Virus (HIV).24 Although (1,2,3-triazolyl)isoxazolidinephosponates 6 did not show antiviral activity at subtoxic concentrations, derivatives of 6 having the unsubstituted and fluorosubstituted phenyl at C4 in the 1,2,3-triazole ring proved slightly cytostatic.25 Encouraged by these results a new series of 5-substituted (3-diethoxyphosphoryl)isoxazolidines 7 was designed (Scheme 1 ). Compounds 7 could be regarded as nucleotide prodrugs due to incorporation of a bioisosteric diethoxyphosphoryl function at C3 of the isoxazolidine ring which mimics nucleoside monophosphate.26 Moreover, it was anticipated that insertion of a carbamoyl linker between the isoxazolidine moiety and careful selection of mono-, di- or trisubstituted phenyl groups as nucleobase replacer would improve their interaction within DNA/RNA strands by forming stronger hydrogen bonds.
Scheme 1.

Retrosynthesis of isoxazolidynylphosphonates 7 with a carbamoyl linker.
2. Results and discussion
2.1. Chemistry
To synthesise the desired isoxazolidines 10 and 11 1,3-dipolar cycloaddition of N-methyl C-phosphorylnitrone 8 27, 28 with a series of acrylamides 9 was employed (Scheme 2 ). Most of substituted acrylamides 9 used in this paper have already been described in the literature. However, compounds 9ab, 9ad, 9an, 9az and 9ba were prepared according to the standard procedure from commercially available substituted anilines and acryloyl chloride in the presence of triethylamine.29 Cycloadditions of nitrone 8 with acrylamides 9aa–9ba were carried out in toluene at 70 °C and afforded mixtures of diastereoisomeric (3-diethoxyphosphoryl)isoxazolidines trans-10aa–10ba and cis-11aa–11ba (Scheme 2, Table 1 ). In all cases moderate to good trans/cis diastereoselectivities (de 50–80%) were observed. The crude mixtures of the respective cycloadducts were subjected to column chromatography and in almost all cases (except for 10ae, 10ah and 10aq) pure major trans-isomers 10 were separated in moderate to good yields (Table 1). Isolation of pure minor cis-isomers 11, which are very crucial for biological evaluation, was not a trivial task. However, several purifications of the enriched diastereoisomeric mixtures of the respective isoxazolidines 10/11 on silica gel columns proved fruitful for isoxazolidines 11aa, 11ab, 11ac, 11ai, 11aj, 11al, 11am, 11an, 11ap, 11aq, 11ar, 11as, 11at, 11av, 11aw and 11ba (Table 1) making minute quantities of cis-11 available.
Scheme 2.

Synthesis of compounds 10 and 11.
Table 1.
Isoxazolidines 10 and 11 obtained according to Scheme 2
| Entry | Acrylamide 9 | 10:11 ratio | Yield (%) |
|---|---|---|---|
| Ar | |||
| aa | 2-F-C6H4 | 88:12 | 10aa (43)a + 11aa (6)a + 10aa and 11aa (24)b |
| ab | 3-F-C6H4 | 78:22 | 10ab (55)a + 11ab (6)a + 10ab and 11ab (25)b |
| ac | 4-F-C6H4 | 80:20 | 10ac (48)a + 11ac (6)a + 10ac and 11ac (34)b |
| ad | 2,4-diF-C6H3 | 85:15 | 10ad (56)a + 10ad and 11ad (38)b |
| ae | 2-Br-C6H4 | 90:10 | 10ae and 11ae (94)b |
| af | 3-Br-C6H4 | 77:23 | 10af (37)a + 10af and 11af (51)b |
| ag | 4-Br-C6H4 | 78:22 | 10ag (68)a + 10ag and 11ag (16)b |
| ah | 2-Cl-C6H4 | 86:14 | 10ah and 11ah (91)b |
| ai | 3-Cl-C6H4 | 75:25 | 10ai (48)a + 11ai (3)a + 10ai and 11ai (39)b |
| aj | 4-Cl-C6H4 | 86:14 | 10aj (60)a + 11aj (8)a + 10aj and 11aj (20)b |
| ak | 2-NO2-C6H4 | 83:17 | 10ak (18)a + 10ak and 11ak (69)b |
| al | 3-NO2-C6H4 | 80:20 | 10al (26)a + 11al (8)a + 10al and 11al (57)b |
| am | 4-NO2-C6H4 | 80:20 | 10am (60)a + 11am (14)a |
| an | 3-CN-C6H4 | 78:22 | 10an (17)a+11an (6)a + 10an and 11an (67)b |
| ao | 4-CN-C6H4 | 80:20 | 10ao (10)a + 10ao and 11ao (85)b |
| ap | 2-CH3C(O)-C6H4 | 80:20 | 10ap (22)a + 11ap (7)a + 10ap and 11ap (55)b |
| aq | 3-CH3C(O)-C6H4 | 81:19 | 10aq (53)a+11aq (5)a + 10aq and 11aq (31)b |
| ar | 4-CH3C(O)-C6H4 | 83:17 | 10ar (51)a + 11ar (11)a |
| as | 3-CH3-C6H4 | 78:22 | 10as (40)a + 11as (7)a |
| at | 4-CH3-C6H4 | 77:23 | 10at (70)a + 11at (10)a |
| au | 3-CH3O-C6H4 | 76:24 | 10au (42)a + 10au and 11au (48)b |
| av | 4-C2H5O-C6H4 | 79:21 | 10av (38)a + 11av (4)a + 10av and 11av (47)b |
| aw | 3,4-diCH3O-C6H3 | 84:16 | 10aw (17)a+11aw (4)a + 10aw and 11aw (44)b |
| ax | 3,5-diCH3O-C6H3 | 80:20 | 10ax (25)a + 10ax and 11ax (70)b |
| ay | 3,4,5-triCH3O-C6H2 | 81:19 | 10ay (39)a+11ay (9)a + 10ay and 11ay (40)b |
| az | 4,5-diCH3O-2-CN-C6H2 | 80:20 | 10az (26)a + 10az and 11az (40)b |
| ba | 4,5-diCH3O-2-CH3C(O)-C6H2 | 80:20 | 10ba (10)a + 10ba and 11ba (74)b |
Yield of pure isomer.
Yield of pure mixture of cis- and trans-isomers.
Stereochemistry of the cycloaddition of N-substituted C-phosphorylated nitrones to various alkenes has already been described and the relative configurations of trans- and cis-isoxazolidine cycloadducts were established based on detailed conformational analyses.23, 24, 25, 27, 28 Indeed, the assignment of relative configurations in isoxazolidines has often been difficult due to conformational flexibility of substituted five-membered ring, but in the case of isoxazolidines containing the diethoxyphosphoryl group at C3 stereochemically valuable data are extended over PCCH 30, 31 and PCCC 31, 32, 33, 34 vicinal couplings, which appeared to be extremely useful in establishing the stereochemistries of phosphorus-labelled heterocycles.35, 36 For the major isomers of all obtained isoxazolidines 10aa–10ba trans-configuration was assigned taking advantage of our previous observations regarding stereochemistry of cycloaddition of N-methyl-C-phosphorylated nitrone 8 with terminal alkenes.27, 28 In this series a similar approach to configurational assignment was applied. Thus, based on the values of vicinal coupling constants [J CCCP = 8.6–9.5 Hz, J H3–H4α = 7.7–8.3 Hz, J H3–H4β = 8.5–9.2 Hz, J H4α–P = 8.0–9.8 Hz, J H4β–P = 15.4–16.1 Hz, J H4α–H5 = 5.0–5.7 Hz and J H4β–H5 = 8.5–9.2 Hz] extracted from the 1H and 13C NMR spectra of compounds 10al–10ao, 10aq, 10at and 10aw–10ba preferred 3 E conformation of the isoxazolidine ring (Fig. 3 ) was established. In this conformation the diethoxyphosphoryl group resides in the equatorial position of the isoxazolidine ring while carbamoyl substituents are located pseudoequatorially. Furthermore, a similar spectral pattern was previously observed for structurally related methyl trans-3-(diethoxyphosphoryl)-2-methylisoxazolidin-5-yl-5-carboxylate.27, 28
Figure 3.

The preferred conformations of trans-isoxazolidines 10.
To provide an additional piece of evidence for the already established relative configuration at C3 and C5 in isoxazolidines trans-10 and cis-11 2D NOE experiments were performed for trans-10ay and cis-11ay (Fig. 4 ). The occurrence of NOE signal between HC5 and HC3 was noticed for cis-11ay, while the spectrum of trans-10ay lacks such correlation.
Figure 4.

Observed NOEs for trans-10ay and cis-11ay.
2.2. Antiviral and cytostatic evaluation
5-Arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates trans-10 and cis-11 were evaluated for inhibitory activity against a wide variety of DNA and RNA viruses, using the following cell-based assays: (a) human embryonic lung (HEL) cells: herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus and herpes simplex virus-1 (TK− KOS ACVr); (b) HeLa cell cultures: vesicular stomatitis virus, Coxsackie virus B4 and respiratory syncytial virus; (c) Vero cell cultures: para-influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus; (d) MDCK cell cultures: influenza A virus (H1N1 and H3N2 subtypes) and influenza B virus; (e) CrFK cell cultures: feline herpes virus (FHV) and feline corona virus (FIPV) and (f) CEM cell cultures: human immunodeficiency virus type 1 (HIV-1) and HIV-2. Ganciclovir, cidofovir, acyclovir, brivudin, (S)-9-(2,3-dihydroxypropyl)adenine [(S)-DHPA], oseltamivir carboxylate, amantadine, rimantadine, ribavirin, dextran sulfate (molecular weight 5000, DS-5000), Hippeastrum hybrid agglutinin (HHA) and Urtica dioica agglutinin (UDA) were used as the reference compounds. The antiviral activity was expressed as the EC50: the compound concentration required to reduce virus plaque formation (VZV) by 50% or to reduce virus-induced cytopathogenicity by 50% (other viruses). Unfortunately, no inhibitory activity against any virus was detected for the evaluated compounds at 250 μM.
The cytotoxicity of the tested compounds toward the uninfected host cells was defined as the minimum compound concentration (MCC) that caused a microscopically detectable alteration of normal cell morphology. The 50% cytostatic inhibitory concentration (IC50), causing a 50% decrease in cell proliferation was determined against murine leukemia L1210, human lymphocyte CEM and human cervix carcinoma HeLa cells. None of the tested compounds affected cell morphology of HEL, HeLa, Vero, MDCK and CrFK cells at concentrations up to 100 μM. However, several compounds, having phenyl residue substituted with F, Br, Cl, NO2 and CH3C(O) groups, were able to inhibit cell proliferation by 50% (CC50) at concentrations ranging from 116 to 228 μM for L1210 cells, and from 102 to 227 μM for CEM and HeLa cells (Table 2 ).
Table 2.
Inhibitory effect of several 5-arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates against the proliferation of murine leukemia (L1210), human T-lymphocyte (CEM) and human cervix carcinoma cells (HeLa)
| Compound | Ar | IC50a (μM) |
||
|---|---|---|---|---|
| L1210 | CEM | HeLa | ||
| 10aa | 2-F-C6H4 | >250 | >250 | ⩾250 |
| 11aa | 2-F-C6H4 | 130 ± 24 | 145 ± 30 | 177 ± 42 |
| 10ab | 3-F-C6H4 | >250 | >250 | >250 |
| 11ab | 3-F-C6H4 | 228 ± 12 | ⩾250 | ⩾250 |
| 10af | 3-Br-C6H4 | >250 | ⩾250 | 180 ± 35 |
| 11af/10af (77:23) | 3-Br-C6H4 | 156 ± 1 | 140 ± 16 | 136 ± 11 |
| 10ag | 4-Br-C6H4 | 177 ± 20 | 227 ± 32 | ⩾250 |
| 11ag/10ag (87:13) | 4-Br-C6H4 | 116 ± 2 | 102 ± 9 | 136 ± 11 |
| 10aj | 4-Cl-C6H4 | >250 | >250 | ⩾250 |
| 11aj | 4-Cl-C6H4 | 170 ± 27 | ⩾250 | >250 |
| 10ak | 2-NO2-C6H4 | 168 ± 24 | ⩾250 | >250 |
| 11ak | 2-NO2-C6H4 | Not available | ||
| 10ar | 4-CH3C(O)-C6H4 | >250 | >250 | >250 |
| 11ar | 4-CH3C(O)-C6H4 | 150 ± 9 | 227 ± 18 | 211 ± 55 |
50% Inhibitory concentration or compound concentration required to inhibit tumor cell proliferation by 50%.
Structure–activity relationship studies for a series of 5-arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates trans-10 and cis-11 described in this paper revealed that, in general, cis-isomers 11 are more cytostatic toward tested tumour cell lines than the respective trans-10 (11aa vs 10aa, 11ab vs 10ab, 11af vs 10af, 11ag vs 10ag, 11aj vs 10aj and 11ar vs 10ar). Isoxazolidines 11ab (Ar = 3-F-C6H4), 10ag (Ar = 4-Br-C6H4), 11aj (Ar = 4-Cl-C6H4), 10ak (Ar = 2-NO2-C6H4) and 11ar (Ar = 4-CH3C(O)-C6H4) slightly inhibit cell proliferation of murine leukemia (L1210), while they are less active or inactive toward human T-lymphocyte (CEM) and human cervix cells (HeLa) at 250 μM. Compounds containing phenyl substituted with halogen, namely, cis-11aa (Ar = 2-F-C6H4), cis-11af (Ar = 3-Br-C6H4), and cis-11ag (Ar = 4-Br-C6H4) appeared to be the most active toward L1210, CEM as well as HeLa cells at IC50’s consistently ranging between 100 and 200 μM against all three tumor cell lines. 5-Arylcarbamoyl derivatives 10 and 11 substituted with CN, Me, MeO or EtO did not show any appreciable cytostatic activity on the tested tumour cell lines. Among 5-arylcarbamoyl derivatives substituted with fluorine atom, compound 11aa (Ar = 2-F-C6H4) is the most cytostatic. According to Kool, a 2,4-fluorophenyl group could be regarded as an uracil non-polar isoster due to the steric and electrostatic similarities.37, 38, 39, 40 To our surprise introduction of additional fluorine atoms into the aromatic ring resulted in loss of cytostatic activity (10ad/11ad; Ar = 2,4-diF-C6H3).
3. Conclusions
A new series of 5-arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates have been efficiently obtained from N-methyl-C-diethoxyphoshporylnitrone and the respective N-arylacrylamides via the 1,3-dipolar cycloaddition. All synthesised isoxazolidineposphonates trans-10 and cis-11 were evaluated against a variety of DNA and RNA viruses but were not active at 250 μM.
Cytostatic activity of trans-10 and cis-11 compounds were performed on three tumor cell lines (L1210, CEM and HeLa) and showed that cis-configurated isoxazolidines containing phenyl substituted with halogen [cis-11aa (Ar = 2-F-C6H4), cis-11af (Ar = 3-Br-C6H4), and cis-11ag (Ar = 4-Br-C6H4)] are the most active toward all tested cancerous cell lines (IC50 100–180 μM).
Further studies on isoxazolidinephosphonates of general formula 7 containing natural or modified nucleobases instead of aryl groups are in progress and will be published in due course.
4. Experimental section
1H NMR spectra were taken in CDCl3 or CD3OD on the following spectrometers: Varian Mercury-300 and Bruker Avance III (600 MHz) with TMS as internal standard. 13C NMR spectra were recorded for CDCl3 solution on the Varian Mercur-300 machine at 75.5 MHz. 31P NMR spectra were performed in CDCl3 solution on the Varian Mercury-300 at 121.5 MHz. IR spectra were measured on an Infinity MI-60 FT-IR spectrometer. Melting points were determined on a Boetius apparatus and are uncorrected. Elemental analyses were performed by the Microanalytical Laboratory of this Faculty on Perkin–Elmer PE 2400 CHNS analyzer. The following adsorbents were used: column chromatography, Merck silica gel 60 (70–230 mesh); analytical TLC, Merck TLC plastic sheets silica gel 60 F254.
Starting materials: All solvents were dried according to the literature methods. Nitrone 8 was previously reported.27
4.1. General procedure for the preparation of acrylamides 9
To a solution of substituted aniline (1.00 mmol) in dichloromethane (2 mL) triethylamine (1.10 mmol) was added. The mixture was cooled in an ice bath and acryloyl chloride (1.05 mmol) was added dropwise. The reaction mixture was stirred for 24 h at room temperature and extracted with water (3 × 3 mL). Subsequently, the inorganic layer was extracted with ethyl ether (3 × 5 mL). The combined organic layers were dried over anhydrous MgSO4 and filtered. After evaporation of solvents the residue was purified on a silica column with chloroform:methanol mixtures (100:1, 50:1 v/v) as eluents to afford the respective acrylamides 9.
4.1.1. N-(3-Fluorophenyl)acrylamide (9ab)
Yield: 46%; white amorphous solid (crystallised from chloroform/hexane) mp 125–126 °C; IR (KBr, cm−1) ν max: 3277, 1666, 1611, 1549, 1491, 1443, 1223, 774, 677; 1H NMR (300 MHz, CDCl3) δ: 7.52–7.48 (m, 1H), 7.34 (br s, 1H, NH), 7.24–7.11 (m, 2H), 6.79–6.73 (m, 1H), 6.38 (dd, 1H, J = 16.9, 1.2 Hz, CH CH 2), 6.17 (dd, 1H, J = 16.9, 10.1 Hz, CH CH2), 5.73 (dd, 1H, J = 10.1, 1.2 Hz, CH CH 2); 13C NMR (75.5 MHz, CDCl3) δ: 164.68 (s, C(O)), 162.73 (d, J = 243.3 Hz, C3), 139.62 (d, J = 10.9 Hz, C1), 130.89 (s, CH CH2), 129.89 (d, J = 9.2 Hz, C5), 127.85 (s, CH CH2), 115.52 (s, C6), 110.96 (d, J = 21.2 Hz, C4), 107.53 (d, J = 26.1 Hz, C2). Anal. Calcd for C9H8FNO: C, 65.45; H, 4.88; N, 8.48; found: C, 65.24; H, 4.63; N, 8.56.
4.1.2. N-(2,4-Difluorophenyl)acrylamide (9ad)
Yield: 56%; white plates (crystallised from chloroform/hexane) mp 105–106 °C; IR (KBr, cm−1) ν max: 3277, 1668, 1549, 1503, 1214, 1142, 1099, 847, 808, 699; 1H NMR (300 MHz, CDCl3) δ: 8.41–8.33 (m, 1H), 7.36 (br s, 1H, NH), 6.94–6.85 (m, 2H), 6.47 (dd, 1H, J = 16.9, 1.2 Hz, CH=CH 2), 6.28 (dd, 1H, J = 16.9, 10.2 Hz, CH CH2), 5.83 (dd, 1H, J = 10.2, 1.2 Hz, CH CH 2); 13C NMR (75.5 MHz, CDCl3) δ: 163.87 (s, C(O)), 158.84 (dd, J = 246.3, 11.7 Hz, C4), 153.02 (dd, J = 245.4, 10.6 Hz, C2), 130.66 (s, CH CH2), 128.41 (s, CH CH2), 123.65 (d, J = 9.8 Hz, C6), 122.36 (dd, J = 10.7, 3.8 Hz, C5), 111.21 (dd, J = 21.8, 3.7 Hz, C1), 103.69 (dd, J = 26.6, 23.5 Hz, C3). Anal. Calcd for C9H7F2NO: C, 59.02; H, 3.85; N, 7.65; found: C, 58.83; H, 3.94; N, 7.68.
4.1.3. N-(3-Cyanophenyl)acrylamide (9an)
Yield: 86%; yellow amorphous solid; mp 125–126 °C; IR (KBr, cm−1) ν max: 3253, 3077, 2230, 1665, 1606, 1556, 1484, 1415, 1328, 1212; 1H NMR (600 MHz, CDCl3) δ: 8.02 (s, 1H), 7.84–7.83 (m, 1H), 7.58 (br s, 1H, NH), 7.48–7.42 (m, 2H), 6.50 (dd, 1H, J = 16.9, 0.8 Hz, CH CH 2), 6.29 (dd, 1H, J = 16.9, 10.3 Hz, CH CH2), 5.87 (dd, 1H, J = 10.3, 0.8 Hz, CH CH 2); 13C NMR (151.0 MHz, CDCl3) δ: 164.40, 139.13, 130.77 (s, CH CH2), 129.87, 128.64 (s, CH CH2), 127.68, 124.38, 123.26, 118.65 (s, CN), 112.66. Anal. Calcd for C10H8N2O: C, 69.76; H, 4.68; N, 16.27; found: C, 69.83; H, 4.85; N, 16.31.
4.1.4. N-(2-Cyano-4,5-dimethoxyphenyl)acrylamide (9az)
Yield: 91%; yellow amorphous solid mp 158–162 °C; IR (KBr, cm−1) ν max: 3247, 2225, 1663, 1610, 1514, 1450, 1356, 1283, 1226, 1109; 1H NMR (600 MHz, CDCl3) δ: 8.22 (s, 1H), 7.65 (br s, 1H, NH), 6.99 (s, 1H), 6.51 (dd, 1H, J = 16.8, 0.9 Hz, CH CH 2), 6.34 (dd, 1H, J = 16.8, 10.3 Hz, CH CH2), 5.90 (dd, 1H, J = 10.3, 0.9 Hz, CH CH 2), 3.99 (s, 3H, CH 3O), 3.91 (s, 3H, CH 3O); 13C NMR (151.0 MHz, CDCl3) δ: 163.65 (s, C(O)), 153.71, 145.68, 136.33, 130.59 (s, CH CH2), 129.07 (s, CH CH2), 116.80 (s, CN), 112.91, 104.97, 92.54, 56.32 (s, CH3O), 56.17 (s, CH3O). Anal. Calcd for C12H12N2O3: C, 62.06; H, 5.21; N, 12.06; found: C, 62.07; H, 5.28; N, 11.97.
4.1.5. N-(2-Acethyl-4,5-dimethoxyphenyl)acrylamide (9ba)
Yield: 88%; yellow amorphous solid; mp 89–90 °C; IR (KBr, cm−1) ν max: 3447, 3120, 2938, 1683, 1615, 1522, 1366, 1253, 1207, 1153; 1H NMR (600 MHz, CDCl3) δ: 8.67 (s, 1H), 7.32 (s, 1H), 6.45 (dd, 1H, J = 16.9, 1.0 Hz, CH CH 2), 6.34 (dd, 1H, J = 16.9, 10.2 Hz, CH CH2), 5.81 (dd, 1H, J = 10.2, 1.0 Hz, CH CH 2), 4.02 (s, 3H, CH 3O), 3.94 (s, 3H, CH 3O), 2.65 (s, 3H, CH 3–C(O)); 13C NMR (151.0 MHz, CDCl3) δ: 200.82 (s, C(O)), 164.63 (s, C(O)NH), 154.74, 143.76, 137.88, 132.53 (s, CH CH2), 127.22 (s, CH CH2), 114.50, 113.86, 103.65, 56.43 (s, CH3O), 56.21 (s, CH3O), 28.37 (s, CH3C(O)). Anal. Calcd for C13H15NO4: C, 62.64; H, 6.07; N, 5.62; found: C, 62.69; H, 6.16; N, 5.74.
4.2. General procedure for preparation of isoxazolidines 10 and 11
A mixture of nitrone 8 (1.00 mmol), acrylamide 9 (1.00 mmol) and toluene (2 mL) was stirred at 70 °C for 24 h or until disappearance of the starting nitrone. After evaporation of the solvent under reduced pressure the crude products were purified by silica gel chromatography with chloroform/methanol mixtures as eluents.
4.2.1. Diethyl (3RS,5SR)-5-(2-fluorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10aa)
Colourless oil; IR (film, cm−1) ν max: 3396, 2983, 1700, 1531, 1456, 1238, 1053, 1025, 757; 1H NMR (300 MHz, CDCl3) δ: 8.56 (br s, 1H, NH), 8.34–8.29 (m, 1H), 7.18–7.08 (m, 3H), 4.65 (dd, 1H, J = 8.8, 4.9 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.15–2.93 (m, 2H, H βC4 and HC3), 3.05 (s, 3H, CH 3N), 2.90–2.80 (m, 1H, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.13 (s, C(O)), 152.61 (d, J = 243.6 Hz, C2′), 125.37 (d, J = 10.0 Hz, C1′), 125.04 (d, J = 7.7 Hz, C4′), 124.67 (d, J = 3.7 Hz, C6′), 121.51 (s, C5′), 115.03 (d, J = 18.9 Hz, C3′), 76.51 (d, J = 9.4 Hz, C5), 63.59 (d, J = 6.6 Hz, CH2OP), 63.51 (d, J = 168.6 Hz, C3), 62.78 (d, J = 6.9 Hz, CH2OP), 47.08 (s, CH3N), 36.90 (s, C4), 16.76 (d, J = 5.4 Hz, CH3CH2OP), 16.69 (d, J = 5.2 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.20. Anal. Calcd for C15H22FN2O5P: C, 50.00; H, 6.15; N, 7.77; found: C, 49.83; H, 6.04; N, 7.96.
4.2.2. Diethyl (3RS,5RS)-5-(2-fluorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11aa)
Colourless oil; IR (film, cm−1) ν max: 3397, 2983, 1699, 1619, 1531, 1457, 1236, 1053, 1024, 970; 1H NMR (300 MHz, CDCl3) δ: 8.95 (br s, 1H, NH), 8.37–8.28 (m, 1H), 7.15–7.06 (m, 3H), 4.63–4.58 (m, 1H, HC5), 4.16–4.05 (m, 4H, 2 × CH 2OP), 3.02 (s, 3H, CH 3N), 3.10–2.82 (m, 3H, H 2C4 and HC3), 1.28 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.17 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR signals of cis-11aa were extracted from the spectrum of a 65:35 mixture of trans-10aa and cis-11aa, 13C NMR (75.5 MHz, CDCl3) δ: 170.50 (s, C(O)), 152.58 (d, J = 243.7 Hz, C2′), 125.89 (d, J = 10.1 Hz, C1′), 124.54 (d, J = 3.8 Hz, C6′), 124.46 (d, J = 8.3 Hz, C4′), 121.26 (s, C2′), 114.89 (d, J = 19.1 Hz, C3′), 75.70 (d, J = 8.0 Hz, C5), 63.80 (d, J = 169.3 Hz, C3), 63.58 (d, J = 6.0 Hz, CH2OP), 62.75 (d, J = 6.8 Hz, CH2OP), 45.99 (s, CH3N), 36.97 (s, C4), 16.56 (d, J = 6.1 Hz, CH3CH2OP), 16.41 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.51. Anal. Calcd for C15H22FN2O5P: C, 50.00; H, 6.15; N, 7.77; found: C, 49.97; H, 6.01; N, 7.94.
4.2.3. Diethyl (3RS,5SR)-5-(3-fluorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ab)
White amorphous solid (crystallised from ether/hexane); mp 85–87 °C. IR (KBr, cm−1) ν max: 3267, 3084, 3051, 2995, 1698, 1623, 1562, 1445, 1215, 1021, 877, 583; 1H NMR (300 MHz, CDCl3) δ: 8.24 (br s, 1H, NH), 7.56–7.51 (m, 1H), 7.33–7.25 (m, 1H), 7.20–7.17 (m, 1H), 6.88–6.82 (m, 1H), 4.62 (dd, 1H, J = 8.8, 5.2 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.10–2.90 (m, 2H, H βC4 and HC3), 3.02 (s, 3H, CH 3N), 2.89–2.77 (m, 1H, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.88 (s, C(O)), 162.87 (d, J = 244.5 Hz, C3′), 138.39 (d, J = 10.9 Hz, C1′), 130.18 (d, J = 9.2 Hz, C5′), 115.14 (d, J = 2.9 Hz, C6′), 111.53 (d, J = 21.5 Hz, C4′), 107.37 (d, J = 26.3 Hz, C2′), 76.45 (d, J = 8.9 Hz, C5), 63.52 (d, J = 167.8 Hz, C3), 63.51 (d, J = 6.3 Hz, CH2OP), 62.78 (d, J = 6.9 Hz, CH2OP), 46.83 (s, CH3N), 36.51 (s, C4), 16.72 (d, J = 5.4 Hz, CH3CH2OP), 16.65 (d, J = 5.2 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.16. Anal. Calcd for C15H22FN2O5P: C, 50.00; H, 6.15; N, 7.77; found: C, 49.99; H, 5.92; N, 7.98.
4.2.4. Diethyl (3RS,5RS)-5-(3-fluorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11ab)
Colourless oil; IR (film, cm−1) ν max: 3276, 2984, 2931, 1690, 1613, 1536, 1445, 1230, 1052, 1026, 966; 1H NMR (300 MHz, CDCl3) δ: 8.92 (br s, 1H, NH), 7.59–7.55 (m, 1H), 7.29–7.20 (m, 2H), 6.84–6.78 (m, 1H), 4.61 (dd, 1H, J = 8.7, 5.7 Hz, HC5), 4.23–4.02 (m, 4H, 2 × CH 2OP), 3.17–2.99 (m, 2H, HC3 and H βC4), 3.02 (s, 3H, CH 3N), 2.90–2.74 (m, 1H, H αC4), 1.31 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.21 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR signals of cis-11ab were extracted from the spectrum of a 68:32 mixture of trans-10ab and cis-11ab, 13C NMR (75.5 MHz, CDCl3) δ: 170.09 (s, C(O)), 162.96 (d, J = 245.0 Hz, C3′), 139.04 (d, J = 10.9 Hz, C1′), 130.09 (d, J = 9.5 Hz, C5′), 115.08 (d, J = 3.1 Hz, C6′), 111.08 (d, J = 21.2 Hz, C4′), 107.18 (d, J = 26.3 Hz, C2′), 76.06 (d, J = 6.7 Hz, C5), 63.78 (d, J = 169.8 Hz, C3), 63.31 (d, J = 6.8 Hz, CH2OP), 63.14 (d, J = 6.8 Hz, CH2OP), 46.21 (s, CH3N), 36.48 (s, C4), 16.70 (d, J = 5.9 Hz, CH3CH2OP), 16.57 (d, J = 5.8 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.08. Anal. Calcd for C15H22FN2O5P: C, 50.00; H, 6.15; N, 7.77; found: C, 50.03; H, 6.24; N, 7.82.
4.2.5. Diethyl (3RS,5SR)-5-(4-fluorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ac)
White amorphous solid (crystallised from ether/hexane) mp 59–62 °C. IR (KBr, cm−1) ν max: 3278, 3084, 2999, 1701, 1512, 1211, 1021, 834, 582; 1H NMR (300 MHz, CDCl3) δ: 8.17 (br s, 1H, NH), 7.54–7.48 (m, 2H), 7.07–7.00 (m, 2H), 4.60 (dd, 1H, J = 8.7, 5.4 Hz, HC5), 4.26–4.13 (m, 4H, 2 × CH 2OP), 3.15–2.90 (m, 2H, HC3 and H βC4), 3.01 (s, 3H, CH 3N), 2.87–2.76 (m, 1H, H αC4), 1.36 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.34 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.65 (s, C(O)), 159.43 (d, J = 243.6 Hz, C4′), 132.92 (d, J = 2.8 Hz, C1′), 121.67 (d, J = 7.7 Hz, C2′ and C6′), 115.66 (d, J = 22.6 Hz, C3′ and C5′), 76.40 (d, J = 9.2 Hz, C5), 63.45 (d, J = 168.9 Hz, C3), 63.42 (d, J = 6.3 Hz, CH2OP), 62.71 (d, J = 6.9 Hz, CH2OP), 46.86 (s, CH3N), 36.48 (s, C4), 16.66 (d, J = 5.4 Hz, CH3CH2OP), 16.59 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.38. Anal. Calcd for C15H22FN2O5P: C, 50.00; H, 6.15; N, 7.77; found: C, 50.04; H, 5.94; N, 8.00.
4.2.6. Diethyl (3RS,5RS)-5-(4-fluorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11ac)
Colourless oil; IR (film, cm−1) ν max: 3279, 2983, 1688, 1532, 1510, 1227, 1052, 1025, 971; 1H NMR (300 MHz, CDCl3) δ: 8.81 (br s, 1H, NH), 7.58–7.54 (m, 2H), 7.05–6.98 (m, 2H), 4.64–4.60 (m, 1H, HC5), 4.21–4.06 (m, 4H, 2 × CH 2OP), 3.12–2.97 (m, 2H, HC3 and H βC4), 2.96 (s, 3H, CH 3N), 2.88–2.73 (m, 1H, H αC4), 1.31 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.21 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR signals of cis-11ac were extracted from the spectrum of a 48:52 mixture of trans-10ac and cis-11ac, 13C NMR (75.5 MHz, CDCl3) δ: 169.77 (s, C(O)), 159.28 (d, J = 242.9 Hz, C4′), 133.56 (d, J = 2.8 Hz, C1′), 121.38 (d, J = 7.9 Hz, C2′ and C6′), 115.82 (d, J = 22.5 Hz, C3′ and C5′), 75.99 (d, J = 6.8 Hz, C5), 63.78 (d, J = 169.7 Hz, C3), 63.56 (d, J = 6.5 Hz, CH2OP), 63.09 (d, J = 6.8 Hz, CH2OP), 46.28 (s, CH3N), 36.69 (s, C4), 16.69 (d, J = 5.9 Hz, CH3CH2OP), 16.57 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.14. Anal. Calcd for C15H22FN2O5P: C, 50.00; H, 6.15; N, 7.77; found: C, 49.98; H, 6.16; N, 7.82.
4.2.7. Diethyl (3RS,5SR)-5-(2,4-difluorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ad)
Colourless oil; IR (film, cm−1) ν max: 3397, 2984, 1698, 1534, 1432, 1236, 1054, 1025, 964, 848; 1H NMR (300 MHz, CDCl3) δ: 8.43 (s, 1H, NH), 8.31–8.23 (m, 1H), 6.93–6.87 (m, 2H), 4.63 (dd, 1H, J = 8.5, 4.6 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.20–2.95 (m, 2H, HC3 and H βC4), 3.04 (s, 3H, CH 3N), 2.95–2.78 (m, 1H, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.06 (s, C(O)), 158.82 (dd, J = 246.5, 11.5 Hz, C4′), 152.69 (dd, J = 246.6, 11.8 Hz, C2′), 122.57 (dd, J = 9.1, 2.2 Hz, C6′), 121.70 (dd, J = 10.3, 3.7 Hz, C5′), 111.34 (dd, J = 21.7, 3.8 Hz, C1′), 103.79 (dd, J = 26.6, 23.2 Hz, C3′), 76.36 (d, J = 9.4 Hz, C5), 63.54 (d, J = 6.6 Hz, CH2OP), 63.48 (d, J = 167.8 Hz, C3), 62.72 (d, J = 6.9 Hz, CH2OP), 46.98 (s, CH3N), 36.85 (s, C4), 16.73 (d, J = 5.2 Hz, CH3CH2OP), 16.66 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.25. Anal. Calcd for C15H21F2N2O5P: C, 47.62; H, 5.60; N, 7.40; found: C, 47.38; H, 5.66; N, 7.31.
4.2.8. Diethyl (3RS,5SR)-5-(2-bromophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ae)
Colourless oil; IR (film, cm−1) ν max: 3354, 2980, 1698, 1524, 1439, 1245, 1052, 1025, 755; (signals of trans-10ae were extracted from the spectra of a 86:14 mixture of trans-10ae and cis-11ae); 1H NMR (300 MHz, CDCl3) δ: 8.94 (br s, 1H, NH), 8.42–8.38 (m, 1H), 7.58–7.55 (m, 1H), 7.36–7.31 (m, 1H), 7.04–6.99 (m, 1H), 4.64 (dd, 1H, J = 8.8, 4.6 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.14–2.90 (m, 2H, HC3 and H βC4), 3.09 (s, 3H, CH 3N), 2.90–2.77 (m, 1H, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.24 (s, C(O)), 134.84, 132.36, 128.41, 125.61, 121.34, 113.59, 76.39 (d, J = 9.7 Hz, C5), 63.52 (d, J = 6.6 Hz, CH2OP), 63.44 (d, J = 168.6 Hz, C3), 62.67 (d, J = 7.2 Hz, CH2OP), 47.07 (s, CH3N), 37.09 (s, C4), 16.71 (d, J = 5.2 Hz, CH3CH2OP), 16.64 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.15. Anal. Calcd for C15H22BrN2O5P: C, 42.77; H, 5.26; N, 6.65; found: C, 42.52; H, 5.07; N, 6.71 (obtained on a 86:14 mixture of trans-10ae and cis-11ae).
4.2.9. Diethyl (3RS,5SR)-5-(3-bromophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10af)
White amorphous solid (crystallised from ether/hexane); mp 111–112 °C. IR (KBr, cm−1) ν max: 3254, 3107, 3070, 2995, 1699, 1612, 1546, 1422, 1215, 1022, 582; 1H NMR (300 MHz, CDCl3) δ: 8.16 (br s, 1H, NH), 8.20–7.81 (m, 1H), 7.49–7.45 (m, 1H), 7.29–7.18 (m, 2H), 4.61 (dd, 1H, J = 8.5, 5.2 Hz, HC5), 4.27–4.13 (m, 4H, 2 × CH 2OP), 3.10–2.92 (m, 2H, HC3 and H βC4), 3.02 (s, 3H, CH 3N), 2.89–2.76 (m, 1H, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.86 (s, C(O)), 138.20, 130.35, 127.71, 122.74, 122.63, 118.33, 76.45 (d, J = 8.9 Hz, C5), 63.51 (d, J = 167.5 Hz, C3), 63.47 (d, J = 6.9 Hz, CH2OP), 62.78 (d, J = 7.2 Hz, CH2OP), 46.94 (s, CH3N), 36.50 (s, C4), 16.73 (d, J = 5.4 Hz, CH3CH2OP), 16.66 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.27. Anal. Calcd for C15H22BrN2O5P: C, 42.77; H, 5.26; N, 6.65; found: C, 42.88; H, 4.99; N, 6.90.
4.2.10. Diethyl (3RS,5SR)-5-(4-bromophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ag)
White amorphous solid (crystallised from ether/hexane); mp 81–82 °C. IR (KBr, cm−1) ν max: 3260, 3057, 2988, 1705, 1547, 1489, 1219, 1025, 827, 577; 1H NMR (300 MHz, CDCl3) δ: 8.22 (br s, 1H, NH), 7.52–7.38 (m, 4H), 4.59 (dd, 1H, J = 8.8, 5.5 Hz, HC5), 4.25–4.12 (m, 4H, 2 × CH 2OP), 3.15–2.90 (m, 2H, H-C3 and H β-C4), 3.00 (s, 3H, CH 3N), 2.89–2.75 (m, 1H, H α-C4), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.34 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.77 (s, C(O)), 135.99, 132.06, 121.44, 117.47, 76.48 (d, J = 9.2 Hz, C5), 63.67 (d, J = 161.4 Hz, C3), 63.54 (d, J = 6.6 Hz, CH2OP), 62.79 (d, J = 6.9 Hz, CH2OP), 46.91 (s, CH3N), 36.57 (s, C4), 16.76 (d, J = 5.4 Hz, CH3CH2OP), 16.69 (d, J = 5.2 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 20.63. Anal. Calcd for C15H22BrN2O5P: C, 42.77; H, 5.26; N, 6.65; found: C, 42.95; H, 5.06, N, 6.86.
4.2.11. Diethyl (3RS,5SR)-5-(2-chlorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ah)
Colourless oil; IR (film, cm−1) ν max: 3477, 3368, 2982, 1699, 1593, 1528, 1442, 1242, 1055, 1027, 756; (signals of trans-10ah were extracted from the spectra of a 81:19 mixture of trans-10ah and cis-11ah); 1H NMR (300 MHz, CDCl3) δ: 8.95 (br s, 1H, NH), 8.43–8.40 (m, 1H), 7.41–7.38 (m, 1H), 7.32–7.27 (m, 1H), 7.11–7.05 (m, 1H), 4.65 (dd, 1H, J = 8.8, 4.6 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.07 (s, 3H, CH 3N), 3.15–2.78 (m, 3H, H 2C4 and HC3), 1.37 (t, 3H, J = 6.9 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.27 (s, C(O)), 133.75, 129.18, 127.82, 125.19, 123.19, 121.15, 76.51 (d, J = 9.7 Hz, C5), 63.59 (d, J = 6.6 Hz, CH2OP), 63.52 (d, J = 165.8 Hz, C3), 62.76 (d, J = 6.9 Hz, CH2OP), 47.13 (s, CH3N), 37.07 (s, C4), 16.76 (d, J = 5.2 Hz, CH3CH2OP), 16.68 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.17. Anal. Calcd for C15H22ClN2O5P: C, 47.82; H, 5.89; N, 7.44; found: C, 47.52; H, 5.60; N, 7.53 (obtained on a 81:19 mixture of trans-10ah and cis-11ah).
4.2.12. Diethyl (3RS,5SR)-5-(3-chlorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ai)
White amorphous solid (crystallised from ether/hexane); mp 106–109 °C. IR (KBr, cm−1) ν max: 3256, 3071, 2963, 1699, 1597, 1548, 1425, 1258, 1216, 1022, 583; 1H NMR (300 MHz, CDCl3) δ: 8.24 (br s, 1H, NH), 7.70–7.68 (m, 1H), 7.42–7.40 (m, 1H), 7.27–7.23(m, 1H), 7.14–7.11 (m, 1H), 4.63 (dd, 1H, J = 8.8, 4.6 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 2.95–3.20 (m, 2H, HC3 and H βC4), 3.03 (s, 3H, CH 3N), 2.90–2.75 (m, 1H, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.87 (s, C(O)), 138.07, 134.62, 130.04, 124.75, 119.92, 117.84, 76.45 (d, J = 8.9 Hz, C5), 63.52 (d, J = 167.8 Hz, C3), 63.45 (d, J = 6.6 Hz, CH2OP), 62.56 (d, J = 6.9 Hz, CH2OP), 46.89 (s, CH3N), 36.49 (s, C4), 16.71 (d, J = 5.2 Hz, CH3CH2OP), 16.64 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.17. Anal. Calcd for C15H22ClN2O5P: C, 47.82; H, 5.89; N, 7.44; found: C, 47.92; H, 5.75; N, 7.63.
4.2.13. Diethyl (3RS,5RS)-5-(3-chlorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11ai)
Colourless oil; IR (film, cm−1) ν max: 3256, 3071, 2908, 1698, 1596, 1547, 1426, 1258, 1217, 1050, 1022, 973; 1H NMR (300 MHz, CDCl3) δ: 8.87 (br s, 1H, NH), 7.72–7.71 (m, 1H), 7.44–7.40 (m, 1H), 7.26–7.21 (m, 1H), 7.09–7.06 (m, 1H), 4.61 (dd, 1H, J = 9.0, 5.4 Hz, HC5), 4.23–4.01 (m, 4H, 2 × CH 2OP), 3.12–2.94 (m, 2H, HC3 and H βC4), 2.96 (s, 3H, CH 3N), 2.88–2.72 (m, 1H, H αC4), 1.31 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.21 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR signals of cis-11ai were extracted from the spectrum of a 70:30 mixture of trans-10ai and cis-11ai, 13C NMR (75.5 MHz, CDCl3) δ: 170.05 (s, C(O)), 138.52, 134.44, 129.85, 124.25, 119.62, 117.63, 75.88 (d, J = 6.9 Hz, C5), 63.50 (d, J = 167.2 Hz, C3), 63.17 (d, J = 6.9 Hz, CH2OP), 63.01 (d, J = 6.9 Hz, CH2OP), 46.12 (s, CH3N), 36.31 (s, C4), 16.62 (d, J = 5.7 Hz, CH3CH2OP), 16.51 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.97. Anal. Calcd for C15H22ClN2O5P: C, 47.82; H, 5.89; N, 7.44; found: C, 47.84; H, 5.61; N, 7.56.
4.2.14. Diethyl (3RS,5SR)-5-(4-chlorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10aj)
White amorphous solid (crystallised from ether/hexane); mp 75–76 °C. IR (KBr, cm−1) ν max: 3259, 3059, 1705, 1549, 1493, 1302, 1219, 1027, 832, 577; 1H NMR (300 MHz, CDCl3) δ: 8.18 (br s, 1H, NH), 7.55–7.49 (m, 2H), 7.33–7.25 (m, 2H), 4.60 (dd, 1H, J = 8.7, 5.1 Hz, HC5), 4.26–4.13 (m, 4H, 2 × CH 2OP), 3.15–2.89 (m, 2H, HC3 and H βC4), 3.01 (s, 3H, CH 3N), 2.88–2.76 (m, 1H, H αC4), 1.36 (t, 3H, J = 7.2 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.50 (s, C(O)), 135.31, 129.39, 128.75, 120.88, 76.20 (d, J = 9.2 Hz, C5), 63.28 (d, J = 167.2 Hz, C3), 63.18 (d, J = 6.6 Hz, CH2OP), 62.48 (d, J = 6.9 Hz, CH2OP), 46.58 (s, CH3N), 36.21 (s, C4), 16.45 (d, J = 5.1 Hz, CH3CH2OP), 16.38 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.31. Anal. Calcd for C15H22ClN2O5P: C, 47.82; H, 5.89; N, 7.44; found: C, 47.93; H, 5.82; N, 7.58.
4.2.15. Diethyl (3RS,5RS)-5-(4-chlorophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11aj)
Colourless oil; IR (film, cm−1) ν max: 3286, 3264, 2983, 2924, 1691, 1537, 1494, 1234, 1051, 1025, 971; 1H NMR (300 MHz, CDCl3) δ: 8.86 (br s, 1H, NH), 7.58–7.53 (m, 2H), 7.31–7.26 (m, 2H), 4.61 (dd, 1H, J = 9.0, 4.8 Hz, HC5), 4.23–4.01 (m, 4H, 2 × CH 2OP), 3.12–2.99 (m, 2H, HC3 and H βC4), 2.95 (s, 3H, CH 3N), 2.88–2.72 (m, 1H, H αC4), 1.31 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.20 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR signals of cis-11aj were extracted from the spectrum of a 51:49 mixture of trans-10aj and cis-11aj, 13C NMR (75.5 MHz, CDCl3) δ: 169.91 (s, C(O)), 136.12, 129.21, 128.9, 120.93, 76.04 (d, J = 6.9 Hz, C5), 63.56 (d, J = 168.4 Hz, C3), 63.25 (d, J = 6.9 Hz, CH2OP), 63.13 (d, J = 6.0 Hz, CH2OP), 46.89 (s, CH3N), 36.40 (s, C4), 16.67 (d, J = 6.8 Hz, CH3CH2OP), 16.58 (d, J = 7.1 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.16. Anal. Calcd for C15H22ClN2O5P: C, 47.82; H, 5.89; N, 7.44; found: C, 47.84; H, 5.81; N, 7.40.
4.2.16. Diethyl (3RS,5SR)-5-(2-nitrophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ak)
Colourless oil; IR (film, cm−1) ν max: 3320, 2982, 1704, 1503, 1278, 1238, 1051, 1023, 969, 745; 1H NMR (300 MHz, CDCl3) δ: 8.84–8.80 (m, 1H), 8.28–8.25 (m, 1H), 7.71–7.66 (m, 1H), 7.28–7.22 (m, 1H), 4.66 (dd, 1H, J = 8.9, 5.6 Hz, HC5), 4.28–4.15 (m, 4H, 2 × CH 2OP), 3.14–2.98 (m, 2H, HC3 and H βC4), 3.12 (s, 3H, CH 3N), 2.89–2.75 (m, 1H, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.36 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 170.43 (s, C(O)), 136.77, 135.86, 133.74, 125.85, 123.86, 122.00, 76.30 (d, J = 9.7 Hz, C5), 63.43 (d, J = 166.8 Hz, C3), 63.37 (d, J = 6.4 Hz, CH2OP), 62.63 (d, J = 6.9 Hz, CH2OP), 46.57 (s, CH3N), 37.27 (s, C4), 16.49 (d, J = 6.1 Hz, CH3CH2OP), 16.41 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.06. Anal. Calcd for C15H22N3O7P: C, 46.51; H, 5.73; N, 10.85; found: C, 46.32; H, 5.82; N, 10.95.
4.2.17. Diethyl (3RS,5SR)-5-(3-nitrophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10al)
Yellowish amorphous solid (crystallised from ether/hexane); mp 139–142 °C; IR (KBr, cm−1) ν max: 3271, 3103, 2982, 1704, 1608, 1530, 1353, 1227, 1050, 1040, 977, 738; 1H NMR (300 MHz, CDCl3) δ: 8.54 (s, 1H, NH), 8.47–8.45 (m, 1H), 8.03–7.98 (m, 2H), 7.56–7.50 (m, 1H), 4.68 (dd, 1H, J = 8.5, 5.7 Hz, HC5), 4.28–4.15 (m, 4H, 2 × CH 2OP), 3.10–3.08 (br m, 1H, HC3), 3.05 (s, 3H, CH 3N), 3.00 (dddd, 1H, J = 15.9, 13.0, 8.5, 8.5 Hz, H βC4), 2.86 (dddd, 1H, J = 13.0, 9.5, 8.3, 5.7 Hz, H αC4), 1.38 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.36 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.29 (s, C(O)), 148.41, 138.36, 129.86, 125.60, 119.16, 114.71, 76.61 (d, J = 8.6 Hz, C5), 63.52 (d, J = 168.6 Hz, C3), 63.43 (d, J = 6.6 Hz, CH2OP), 62.94 (d, J = 6.9 Hz, CH2OP), 46.79 (s, CH3N), 36.20 (s, C4), 16.70 (d, J = 5.4 Hz, CH3CH2OP), 16.63 (d, J = 5.2 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 20.93. Anal. Calcd for C15H22N3O7P: C, 46.51; H, 5.73; N, 10.85; found: C, 46.51; H, 5.73; N, 10.87.
4.2.18. Diethyl (3RS,5RS)-5-(3-nitrophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11al)
Colourless oil; IR (film, cm−1) ν max: 3222, 3095, 2988, 1708, 1570, 1509, 1330, 1231, 1024, 974; 1H NMR (600 MHz, CDCl3) δ: 9.28 (s, 1H, NH), 8.52–8.51 (m, 1H), 8.01–7.98 (m, 2H), 7.53–7.50 (m, 1H), 4.69 (dd, 1H, J = 9.2, 4.5 Hz, HC5), 4.27–4.08 (m, 4H, 2 × CH 2OP), 3.15–3.06 (m, 2H, H βC4 and HC3), 2.98 (s, 3H, CH 3N), 2.87–2.79 (m, 1H, H αC4), 1.35 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.25 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 170.39 (s, C(O)), 148.63, 138.85, 129.70, 125.39, 118.79, 114.49, 76.09 (d, J = 6.4 Hz, C5), 63.56 (d, J = 170.8 Hz, C3), 63.23 (d, J = 7.1 Hz, CH2OP), 62.92 (d, J = 7.1 Hz, CH2OP), 45.94 (s, CH3N), 36.02 (s, C4), 16.42 (d, J = 5.7 Hz, CH3CH2OP), 16.31 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 21.14. Anal. Calcd for C15H22N3O7P: C, 46.51; H, 5.73; N, 10.85; found: C, 46.58; H, 5.66; N, 10.89.
4.2.19. Diethyl (3RS,5SR)-5-(4-nitrophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10am)
Yellowish amorphous solid (crystallised from ether/hexane); mp 98–99 °C; IR (KBr, cm−1) ν max: 3221, 3095, 2990, 2910, 1710, 1600, 1570, 1510, 1332, 1300, 1270, 1230, 1050, 1025, 974; 1H NMR (300 MHz, CDCl3) δ: 8.56 (s, 1H, NH), 8.25–8.21 (m, 2H), 7.79–7.76 (m, 2H), 4.67 (dd, 1H, J = 8.8, 5.5 Hz, HC5), 4.28–4.15 (m, 4H, 2 × CH 2OP), 3.10–3.08 (m, 1H, HC3), 3.04 (s, 3H, CH 3N), 3.01 (dddd, 1H, J = 15.7, 12.7, 8.8, 8.8 Hz, H βC4), 2.84 (dddd, 1H, J = 12.7, 8.8, 8.3, 5.5 Hz, H αC4), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.36 (t, 3H, J = 6.9 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.30 (s, C(O)), 143.78, 142.85, 125.04, 119.41, 76.57 (d, J = 8.6 Hz, C5), 63.52 (d, J = 166.3 Hz, C3), 63.49 (d, J = 6.3 Hz, CH2OP), 62.93 (d, J = 7.2 Hz, CH2OP), 46.79 (s, CH3N), 36.30 (s, C4), 16.73 (d, J = 5.2 Hz, CH3CH2OP), 16.66 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 20.97. Anal. Calcd for C15H22N3O7P: C, 46.51; H, 5.73; N, 10.85; found: C, 46.60; H, 5.66; N, 10.94.
4.2.20. Diethyl (3RS,5RS)-5-(4-nitrophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11am)
Colourless oil; IR (film, cm−1) ν max: 3215, 3085, 2920, 1715, 1607, 1580, 1500, 1260, 1250, 1057, 1020; 1H NMR (300 MHz, CDCl3) δ: 9.49 (s, 1H, NH), 8.24–8.19 (m, 2H), 7.83–7.78 (m, 2H), 4.68 (dd, 1H, J = 9.2, 4.7 Hz, HC5), 4.28–4.03 (m, 4H, 2 × CH 2OP), 3.20–3.00 (m, 2H, H βC4 and HC3), 2.95 (s, 3H, CH 3N), 2.87–2.71 (m, 1H, H αC4), 1.26 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.23 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 170.35 (s, C(O)), 142.85, 142.85, 125.14, 119.33, 76.58 (br s, C5), 63.69 (d, J = 6.9 Hz, CH2OP), 63.65 (d, J = 171.2 Hz, C3), 63.16 (d, J = 6.9 Hz, CH2OP), 46.11 (s, CH3N), 36.14 (s, C4), 16.73 (d, J = 5.2 Hz, CH3CH2OP), 16.66 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.20. Anal. Calcd for C15H22N3O7P: C, 46.51; H, 5.73; N, 10.85; found: C, 46.59; H, 5.73; N, 10.96.
4.2.21. Diethyl (3RS,5SR)-5-(3-cyanophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10an)
White amorphous solid (crystallised from ether/hexane); mp 85–86 °C; IR (KBr, cm−1) ν max: 3268, 3081, 2984, 2231, 1695, 1590, 1537, 1485, 1432, 1305, 1231, 1050, 1026, 971, 796; 1H NMR (600 MHz, CDCl3) δ: 8.36 (br s, 1H, NH), 8.02–8.01 (m, 1H), 7.79–7.77 (m, 1H), 7.48–7.43 (m, 2H), 4.64 (dd, 1H, J = 8.8, 5.6 Hz, H-C5), 4.26–4.18 (m, 4H, 2 × CH 2OP), 3.13–3.08 (m, 1H, HC3), 3.04 (s, 3H, CH 3N), 3.02 (dddd, 1H, J = 16.0, 12.7, 8.8, 8.8 Hz, H βC4), 2.84 (dddd, 1H, J = 12.7, 9.8, 8.3, 5.6 Hz, H αC4), 1.39 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 169.19 (s, C(O)), 137.88, 129.94, 128.12, 123.89, 122.99, 118.26 (s, CN), 113.19, 76.35 (d, J = 8.8 Hz, C5), 63.55 (d, J = 165.5 Hz, C3), 63.33 (d, J = 6.5 Hz, CH2OP), 62.63 (d, J = 7.2 Hz, CH2OP), 46.62 (s, CH3N), 36.28 (s, C4), 16.54 (d, J = 2.0 Hz, CH3CH2OP), 16.52 (d, J = 4.8 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.11. Anal. Calcd for C16H22N3O5P: C, 52.31; H, 6.04; N, 11.44; found: C, 52.52; H, 6.07; N, 11.44.
4.2.22. Diethyl (3RS,5RS)-5-(3-cyanophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11an)
Colourless oil; IR (film, cm−1) ν max: 3212, 3079, 2980, 2228, 1703, 1594, 1432, 1211, 1048, 1015, 961; 1H NMR (600 MHz, CDCl3) δ: 9.20 (s, 1H, NH), 8.07–8.06 (m, 1H), 7.82–7.80 (m, 1H), 7.45–7.40 (m, 2H), 4.67 (dd, 1H, J = 9.4, 4.4 Hz, HC5), 4.26–4.08 (m, 4H, 2 × CH 2OP), 3.15–3.05 (m, 2H, H βC4 and HC3), 2.96 (s, 3H, CH 3N), 2.83–2.76 (m, 1H, H αC4), 1.35 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.25 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 170.23 (s, C(O)), 138.59, 129.78, 127.64, 123.79, 122.78, 118.49 (s, CN), 113.03, 76.14 (d, J = 5.8 Hz, C5), 63.58 (d, J = 171.3 Hz, C3), 63.29 (d, J = 7.0 Hz, CH2OP), 62.90 (d, J = 6.8 Hz, CH2OP), 45.96 (s, CH3N), 35.98 (s, C4), 16.43 (d, J = 5.8 Hz, CH3CH2OP), 16.32 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 21.22. Anal. Calcd for C16H22N3O5P: C, 52.31; H, 6.04; N, 11.44; found: C, 52.50; H, 6.18; N, 11.46.
4.2.23. Diethyl (3RS,5SR)-5-(4-cyanophenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ao)
Colourless oil; IR (film, cm−1) ν max: 3261, 2985, 2224, 1701, 1600, 1521, 1410, 1311, 1233, 1025, 970, 842; 1H NMR (300 MHz, CDCl3) δ: 8.36 (s, 1H, NH), 7.73–7.71 (m, 2H), 7.67–7.65 (m, 2H), 4.64 (dd, 1H, J = 8.8, 5.6 Hz, HC5), 4.27–4.18 (m, 4H, 2 × CH 2OP), 3.13–3.08 (m, 1H, HC3), 3.04 (s, 3H, CH 3N), 3.03 (dddd, 1H, J = 16.1, 13.0, 8.8, 8.8 Hz, H βC4), 2.84 (dddd, 1H, J = 13.0, 9.8, 8.3, 5.6 Hz, H αC4), 1.39 (t, 3H, J = 6.9 Hz, CH 3CH2OP), 1.38 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 169.28 (s, C(O)), 141.11, 133.22, 119.60, 118.64 (s, CN), 107.62, 76.46 (d, J = 8.6 Hz, C5), 63.42 (d, J = 167.6 Hz, C3), 63.29 (d, J = 6.5 Hz, CH2OP), 62.75 (d, J = 6.8 Hz, CH2OP), 46.61 (s, CH3N), 36.04 (s, C4), 16.46 (d, J = 5.8 Hz, CH3CH2OP), 16.40 (d, J = 6.1 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.00. Anal. Calcd for C16H22N3O5P: C, 52.31; H, 6.04; N, 11.44; found: C, 52.39; H, 6.11; N, 11.67.
4.2.24. Diethyl (3RS,5SR)-5-(2-acetylphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ap)
Colourless oil; IR (film, cm−1) ν max: 3472, 3217, 2981, 2912, 1691, 1660, 1580, 1518, 1451, 1251, 1050, 1023, 964; 1H NMR (300 MHz, CDCl3) δ: 8.78–8.74 (m, 1H), 7.93–7.90 (m, 1H), 7.59–7.54 (m, 1H), 7.19–7.14 (m, 1H), 4.60 (dd, 1H, J = 9.1, 5.0 Hz, HC5), 4.27–4.12 (m, 4H, 2 × CH 2OP), 3.16 (s, 3H, CH 3N), 3.12–2.95 (m, 2H, H βC4 and HC3), 2.80–2.68 (m, 1H, H αC4), 2.67 (s, 3H, CH 3C(O)), 1.36 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.34 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 201.92 (s, C(O)), 170.60 (s, C(O)NH), 139.52, 134.75, 131.54, 122.98, 122.66, 120.76, 76.51 (d, J = 9.7 Hz, C5), 63.28 (d, J = 6.5 Hz, CH2OP), 63.27 (d, J = 167.2 Hz, C3), 62.49 (d, J = 6.9 Hz, CH2OP), 46.42 (s, CH3N), 37.26 (s, C4), 28.36 (s, CH3C(O)), 16.40 (d, J = 5.7 Hz, CH3CH2OP), 16.33 (d, J = 5.8 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.55. Anal. Calcd for C17H25N2O6P: C, 53.12; H, 6.56; N, 7.29; found: C, 52.86; H, 6.80; N, 7.35.
4.2.25. Diethyl (3RS,5RS)-5-(2-acetylphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11ap)
Colourless oil; IR (film, cm−1) ν max: 3482, 2980, 2915, 1690, 1670, 1585, 1520, 1450, 1250, 1050, 1025; 1H NMR (300 MHz, CDCl3) δ: 8.82–8.79 (m, 1H), 7.91–7.87 (m, 1H), 7.56–7.51 (m, 1H), 7.15–7.10 (m, 1H), 4.54 (dd, 1H, J = 8.5, 5.2 Hz, HC5), 4.15–3.92 (m, 4H, 2 × CH 2OP), 3.10 (s, 3H, CH 3N), 2.96–2.88 (m, 3H, H 2C4 and HC3), 2.65 (s, 3H, CH 3C(O)), 1.27 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.08 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 201.49 (s, C(O)), 172.25 (s, C(O)NH), 139.94, 134.68, 131.45, 122.83, 122.62, 120.57, 75.51 (d, J = 8.5 Hz, C5), 63.83 (d, J = 166.3 Hz, C3), 63.44 (d, J = 6.0 Hz, CH2OP), 62.15 (d, J = 7.1 Hz, CH2OP), 45.36 (s, CH3N), 36.79 (s, C4), 28.44 (s, CH3C(O)), 16.33 (d, J = 6.2 Hz, CH3CH2OP), 16.11 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.95. Anal. Calcd for C17H25N2O6P: C, 53.12; H, 6.56; N, 7.29; found: C, 53.38; H, 6.68; N, 7.37.
4.2.26. Diethyl (3RS,5SR)-5-(3-acetylphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10aq)
Yellowish amorphous solid (crystallised from ether/hexane); mp 99–103 °C; IR (KBr, cm−1) ν max: 3244, 3084, 2974, 1701, 1596, 1433, 1212, 1047, 1016, 953; 1H NMR (300 MHz, CDCl3) δ: 8.32 (s, 1H, NH), 8.05–8.04 (m, 1H), 7.94–7.91 (m, 1H), 7.75–7.72 (m, 1H), 7.49–7.44 (m, 1H), 4.62 (dd, 1H, J = 8.8, 5.3 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.13–3.08 (m, 1H, HC3), 3.04 (s, 3H, CH 3N), 3.04 (dddd, 1H, J = 16.0, 12.7, 8.8, 8.8 Hz, H βC4), 2.86 (dddd, 1H, J = 12.7, 8.9, 8.3, 5.3 Hz, H αC4), 2.62 (s, 3H, CH 3C(O)), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 197.64 (s, C(O)), 169.08 (s, C(O)NH), 137.85, 137.40, 129.53, 124.75, 124.44, 119.33, 76.42 (d, J = 9.2 Hz, C5), 63.42 (d, J = 167.6 Hz, C3), 63.58 (d, J = 6.6 Hz, CH2OP), 62.81 (d, J = 6.9 Hz, CH2OP), 47.00 (s, CH3N), 36.72 (s, C4), 26.99 (s, CH3C(O)), 16.80 (d, J = 5.4 Hz, CH3CH2OP), 16.73 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.30. Anal. Calcd for C17H25N2O6P: C, 53.12; H, 6.56; N, 7.29; found: C, 52.97; H, 6.52; N, 7.23.
4.2.27. Diethyl (3RS,5RS)-5-(3-acetylphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11aq)
Colourless oil; IR (film, cm−1) ν max: 3274, 2981, 1700, 1680, 1617, 1564, 1237, 1046, 1016, 972; 1H NMR (300 MHz, CDCl3) δ: 8.93 (s, 1H, NH), 8.11 (s, 1H), 7.93–7.90 (m, 1H), 7.72–7.69 (m, 1H), 7.46–7.41 (m, 1H), 4.63 (dd, 1H, J = 8.9, 4.9 Hz, H-C5), 4.20–4.06 (m, 4H, 2 × CH 2OP), 2.98 (s, 3H, CH 3N), 3.15–2.92 (m, 2H, H βC4 and HC3), 2.90–2.76 (m, 1H, H αC4), 2.62 (s, 3H, CH 3C(O)), 1.30 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.22 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR signals of cis-11aq were extracted from the spectrum of a 65:35 mixture of trans-10aq and cis-11aq, 13C NMR (151.0 MHz, CDCl3) δ: 197.77 (s, C(O)), 170.34 (s, C(O)NH), 137.99, 137.87, 129.27, 124.23, 124.12, 119.28, 75.88 (d, J = 7.4 Hz, C5), 63.49 (d, J = 169.1 Hz, C3), 63.16 (d, J = 5.9 Hz, CH2OP), 62.88 (d, J = 7.2 Hz, CH2OP), 46.70 (s, CH3N), 36.50 (s, C4), 26.63 (s, CH3C(O)), 16.39 (d, J = 5.6 Hz, CH3CH2OP), 16.29 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.01. Anal. Calcd for C17H25N2O6P: C, 53.12; H, 6.56; N, 7.29; found: C, 52.90; H, 6.54; N, 7.22.
4.2.28. Diethyl (3RS,5SR)-5-(4-acetylphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ar)
White amorphous solid (crystallised from ether/hexane); mp 66–67 °C; IR (KBr, cm−1) ν max: 3258, 3188, 3103, 1707, 1678, 1600, 1642, 1269, 1226, 1050, 1017, 957; 1H NMR (300 MHz, CDCl3) δ: 8.39 (s, 1H, NH), 7.95–7.92 (m, 2H), 7.67–7.64 (m, 2H), 4.44 (dd, 1H, J = 8.5, 5.5 Hz, HC5), 4.24–4.11 (m, 4H, 2 × CH 2OP), 3.06–2.91 (m, 2H, H βC4 and HC3), 3.01 (s, 3H, CH 3N), 2.87–2.78 (m, 1H, H αC4), 2.56 (s, 3H, CH 3C(O)), 1.34 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.33 (t, 3H, J = 6.9 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 196.77 (s, C(O)), 169.05 (s, C(O)NH), 141.16, 133.33, 129.29, 119.64, 76.42 (d, J = 9.2 Hz, C5), 63.40 (d, J = 167.5 Hz, C3), 63.52 (d, J = 6.3 Hz, CH2OP), 62.81 (d, J = 6.9 Hz, CH2OP), 47.00 (s, CH3N), 36.54 (s, C4), 26.99 (s, CH3C(O)), 16.80 (d, J = 5.4 Hz, CH3CH2OP), 16.73 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.16. Anal. Calcd for C17H25N2O6P: C, 53.12; H, 6.56; N, 7.29; found: C, 53.37; H, 6.47; N, 7.10.
4.2.29. Diethyl (3RS,5RS)-5-(4-acetylphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11ar)
Colourless oil; IR (film, cm−1) ν max: 3258, 3188, 3060, 3001, 1710, 1679, 1599, 1542, 1270, 1230, 1051, 1025; 1H NMR (300 MHz, CDCl3) δ: 9.11 (s, 1H, NH), 7.96–7.92 (m, 2H), 7.72–7.69 (m, 2H), 4.64 (dd, 1H, J = 8.4, 5.4 Hz, HC5), 4.23–4.05 (m, 4H, 2 × CH 2OP), 3.11–3.02 (m, 2H, H βC4 and HC3), 2.97 (s, 3H, CH 3N), 2.85–2.79 (m, 1H, H αC4), 2.58 (s, 3H, CH 3C(O)), 1.31 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.19 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 196.87 (s, C(O)), 170.32 (s, C(O)NH), 141.88, 133.04, 129.68, 118.97, 76.07 (d, J = 6.6 Hz, C5), 63.64 (d, J = 170.3 Hz, C3), 63.07 (d, J = 6.7 Hz, CH2OP), 63.03 (d, J = 7.4 Hz, CH2OP), 45.99 (s, CH3N), 36.24 (s, C4), 26.38 (s, CH3C(O)), 16.41 (d, J = 5.6 Hz, CH3CH2OP), 16.30 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.94. Anal. Calcd for C17H25N2O6P: C, 53.12; H, 6.56; N, 7.29; found: C, 52.91; H, 6.43; N, 7.18.
4.2.30. Diethyl (3RS,5SR)-5-(m-tolylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10as)
White amorphous solid (crystallised from ether/hexane); mp 96–97 °C; IR (KBr, cm−1) ν max: 3273, 3104, 2899, 1698, 1621, 1600, 1566, 1293, 1262, 1214, 1055, 1020, 959; 1H NMR (300 MHz, CDCl3) δ: 8.09 (s, 1H, NH), 7.33–7.26 (m, 2H), 7.18–7.13 (m, 1H), 6.91–6.88 (m, 1H), 4.55 (dd, 1H, J = 8.7, 5.4 Hz, HC5), 4.20–4.06 (m, 4H, 2 × CH 2OP), 3.00–2.88 (m, 2H, H βC4 and HC3), 2.96 (s, 3H, CH 3N), 2.85–2.76 (m, 1H, H αC4), 2.28 (s, 3H, CH 3), 1.30 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.28 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.62 (s, C(O)), 138.89, 136.73, 128.80, 125.51, 120.38, 116.88, 76.45 (d, J = 9.4 Hz, C5), 63.47 (d, J = 167.5 Hz, C3), 63.38 (d, J = 6.6 Hz, CH2OP), 62.61 (d, J = 6.9 Hz, CH2OP), 46.84 (s, CH3N), 36.58 (s, C4), 21.56 (s, CH3), 16.65 (d, J = 5.2 Hz, CH3CH2OP), 16.58 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.22. Anal. Calcd for C16H25N2O5P: C, 53.93; H, 7.07; N, 7.86; found: C, 54.16; H, 7.30; N, 7.99.
4.2.31. Diethyl (3RS,5RS)-5-(m-tolylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11as)
Colourless oil; IR (film, cm−1) ν max: 3216, 2971, 2900, 1700, 1600, 1565, 1453, 1290, 1210, 1050, 1020; 1H NMR (600 MHz, CDCl3) δ: 8.66 (s, 1H, NH), 7.44 (s, 1H), 7.39–7.38 (m, 1H), 7.24–7.22 (m, 1H), 6.96–6.94 (m, 1H), 4.61 (dd, 1H, J = 8.6, 5.1 Hz, HC5), 4.22–4.06 (m, 4H, 2 × CH 2OP), 3.08–2.99 (m, 2H, H βC4 and HC3), 3.00 (s, 3H, CH 3N), 2.90–2.81 (m, 1H, H αC4), 2.37 (s, 3H, CH 3), 1.33 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.23 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 169.91 (s, C(O)), 138.84, 137.36, 128.77, 125.13, 120.29, 116.82, 75.83 (d, J = 7.6 Hz, C5), 63.86 (d, J = 168.7 Hz, C3), 63.24 (d, J = 6.6 Hz, CH2OP), 62.68 (d, J = 6.8 Hz, CH2OP), 46.09 (s, CH3N), 36.56 (s, C4), 21.45 (s, CH3), 16.40 (d, J = 5.6 Hz, CH3CH2OP), 16.30 (d, J = 5.8 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 21.82. Anal. Calcd for C16H25N2O5P: C, 53.93; H, 7.07; N, 7.86; found: C, 54.07; H, 7.20; N, 7.98.
4.2.32. Diethyl (3RS,5SR)-5-(p-tolylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10at)
White amorphous solid (crystallised from ether/hexane); mp 62–64 °C; IR (KBr, cm−1) ν max: 3264, 3125, 2974, 1699, 1614, 1550, 1516, 1299, 1264, 1210, 1050, 1017, 952, 819; 1H NMR (300 MHz, CDCl3) δ: 8.16 (br s, 1H, NH), 7.46–7.43 (m, 2H), 7.17–7.14 (m, 2H), 4.63 (dd, 1H, J = 9.0, 5.4 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.10–3.04 (m, 1H, HC3), 3.03 (s, 3H, CH 3N), 2.96 (dddd, 1H, J = 15.7, 13.0, 9.0, 9.0 Hz, H βC4), 2.85 (dddd, 1H, J = 13.0, 8.3, 7.8, 5.4 Hz, H αC4), 2.33 (s, 3H, CH 3), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.59 (s, C(O)), 134.55, 134.28, 129.60, 120.22, 76.45 (d, J = 9.5 Hz, C5), 63.50 (d, J = 167.5 Hz, C3), 63.58 (d, J = 6.3 Hz, CH2OP), 62.76 (d, J = 6.9 Hz, CH2OP), 46.73 (s, CH3N), 36.71 (s, C4), 20.91 (s, CH3), 16.70 (d, J = 5.5 Hz, CH3CH2OP), 16.55 (d, J = 5.5 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.24. Anal. Calcd for C16H25N2O5P: C, 53.93; H, 7.07; N, 7.86; found: C, 53.95; H, 7.09; N, 7.92.
4.2.33. Diethyl (3RS,5RS)-5-(p-tolylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11at)
Colourless oil; IR (film, cm−1) ν max: 3260, 2970, 2890, 1700, 1615, 1300, 1210, 1045, 1020; 1H NMR (300 MHz, CDCl3) δ: 8.65 (br s, 1H, NH), 7.47–7.44 (m, 2H), 7.13–7.11 (m, 2H), 4.60 (dd, 1H, J = 8.6, 5.4 Hz, HC5), 4.21–4.03 (m, 4H, 2 × CH 2OP), 3.07–3.01 (m, 2H, H βC4 and HC3), 2.97 (s, 3H, CH 3N), 2.89–2.79 (m, 1H, H αC4), 2.31 (s, 3H, CH 3), 1.30 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.20 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 168.62 (s, C(O)), 134.48, 134.32, 129.56, 119.87, 76.39 (d, J = 9.1 Hz, C5), 63.52 (d, J = 169.5 Hz, C3), 63.34 (d, J = 6.4 Hz, CH2OP), 62.53 (d, J = 7.1 Hz, CH2OP), 46.74 (s, CH3N), 36.53 (s, C4), 20.85 (s, CH3), 16.40 (d, J = 5.6 Hz, CH3CH2OP), 16.31 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.96. Anal. Calcd for C16H25N2O5P: C, 53.93; H, 7.07; N, 7.86; found: C, 53.87; H, 7.18; N, 7.87.
4.2.34. Diethyl (3RS,5SR)-5-(3-methoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10au)
White amorphous solid; mp 83–84 °C; IR (KBr, cm−1) ν max: 3265, 3212, 3088, 2896, 1700, 1600, 1559, 1453, 1213, 1048, 1018, 957; 1H NMR (300 MHz, CDCl3) δ: 8.20 (s, 1H, NH), 7.40–7.35 (m, 1H), 7.35–7.31 (m, 1H), 7.04–7.01 (m, 1H), 6.72–6.69 (m, 1H), 4.61 (dd, 1H, J = 9.1, 5.5 Hz, HC5), 4.27–4.14 (m, 4H, 2 × CH 2OP), 3.82 (s, 3H, CH 3O), 3.07–2.92 (m, 2H, H βC4 and HC3), 3.03 (s, 3H, CH 3N), 2.89–2.82 (m, 1H, H αC4), 1.38 (t, 3H, J = 6.9 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 6.9 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.56 (s, C(O)), 159.78, 138.01, 129.40, 111.86, 110.22, 105.44, 76.35 (d, J = 8.9 Hz, C5), 63.24 (d, J = 167.9 Hz, C3), 63.13 (d, J = 6.5 Hz, CH2OP), 62.49 (d, J = 6.9 Hz, CH2OP), 55.11 (s, CH3O), 46.57 (s, CH3N), 36.17 (s, C4), 16.42 (d, J = 5.3 Hz, CH3CH2OP), 16.37 (d, J = 5.2 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.17. Anal. Calcd for C16H25N2O6P: C, 51.61; H, 6.77; N, 7.52; found: C, 51.72; H, 6.88; N, 7.54.
4.2.35. Diethyl (3RS,5SR)-5-(4-ethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10av)
Colourless oil; IR (film, cm−1) ν max: 3270, 2980, 1683, 1512, 1237, 1050, 1026, 969, 826; 1H NMR (300 MHz, CDCl3) δ: 7.46–7.43 (m, 2H), 8.09 (s, 1H, NH), 6.88–6.85 (m, 2H), 4.61 (dd, 1H, J = 8.8, 5.2 Hz, HC5), 4.27–4.13 (m, 4H, 2 × CH 2OP), 4.02 (q, 2H, J = 6.9 Hz, CH3CH 2O), 3.15–2.92 (m, 2H, H βC4 and HC3), 3.02 (s, 3H, CH 3N), 2.90–2.80 (m, 1H, H αC4), 1.41 (t, 3H, J = 6.9 Hz, CH 3CH2O), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.35 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 168.22 (s, C(O)), 155.77, 129.59, 121.37, 114.53, 76.21 (d, J = 9.2 Hz, C5), 63.53 (s, CH2CH3), 63.29 (d, J = 168.6 Hz, C3), 63.25 (d, J = 6.6 Hz, CH2OP), 62.45 (d, J = 6.9 Hz, CH2OP), 46.67 (s, CH3N), 36.44 (s, C4), 16.49 (d, J = 5.4 Hz, CH3CH2OP), 16.42 (d, J = 5.4 Hz, CH3CH2OP), 14.78 (s, CH3CH2O); 31P NMR (121.5 MHz, CDCl3) δ: 21.37. Anal. Calcd for C17H27N2O6P: C, 52.84; H, 7.04; N, 7.25; found: C, 52.61; H, 7.01; N, 7.26.
4.2.36. Diethyl (3RS,5RS)-5-(4-ethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11av)
Colourless oil; IR (film, cm−1) ν max: 3270, 2981, 1680, 1607, 1513, 1298, 1234, 1050, 1024, 970; 1H NMR (300 MHz, CDCl3) δ: 8.61 (s, 1H, NH), 7.49–7.46 (m, 2H), 6.87–6.84 (m, 2H), 4.60 (dd, 1H, J = 8.7, 5.5 Hz, HC5), 4.19–4.06 (m, 4H, 2 × CH 2OP), 4.01 (q, 2H, J = 6.9 Hz, CH3CH 2O), 3.07–2.99 (m, 2H, H βC4 and HC3), 2.97 (s, 3H, CH 3N), 2.85–2.77 (m, 1H, H αC4), 1.40 (t, 3H, J = 6.9 Hz, CH 3CH2O), 1.30 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.21 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR signals of cis-11av were extracted from the spectrum of a 55:45 mixture of trans-10av and cis-11av, 13C NMR (75.5 MHz, CDCl3) δ: 169.57 (s, C(O)), 155.71, 130.49, 121.36, 114.75, 75.90 (d, J = 7.2 Hz, C5), 63.84 (d, J = 168.7 Hz, C3), 63.79 (s, CH2CH3), 63.40 (d, J = 6.6 Hz, CH2OP), 62.75 (d, J = 6.6 Hz, CH2OP), 46.26 (s, CH3N), 36.60 (s, C4), 16.62 (d, J = 6.2 Hz, CH3CH2OP), 16.54 (d, J = 6.4 Hz, CH3CH2OP), 15.10 (s, CH3CH2O); 31P NMR (121.5 MHz, CDCl3) δ: 22.06. Anal. Calcd for C17H27N2O6P: C, 52.84; H, 7.04; N, 7.25; found: C, 52.83; H, 7.07; N, 7.15.
4.2.37. Diethyl (3RS,5SR)-5-(3,4-dimethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10aw)
White amorphous solid (crystallised from ether/hexane); mp 83–84 °C; IR (KBr, cm−1) ν max: 3274, 2981, 2934, 2837, 1682, 1608, 1515, 1453, 1234, 1050, 969, 806, 765; 1H NMR (600 MHz, CDCl3) δ: 8.09 (br s, 1H, NH), 7.38–7.37 (m, 1H), 6.95–6.93 (m, 1H), 6.85–6.83 (m, 1H), 4.61 (dd, 1H, J = 9.0, 5.3 Hz, HC5), 4.25–4.19 (m, 4H, 2 × CH 2OP), 3.91 (s, 3H, CH 3O), 3.88 (s, 3H, CH 3O), 3.12–3.07 (m, 1H, HC3), 3.04 (s, 3H, CH 3N), 3.01 (dddd, 1H, J = 15.9, 12.9, 9.0, 9.0 Hz, H βC4), 2.86 (dddd, 1H, J = 12.9, 8.9, 8.3, 5.3 Hz, H αC4), 1.38 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 168.54 (s, C(O)), 149.25, 146.31, 130.52, 111.86, 111.50, 104.82, 76.37 (d, J = 9.2 Hz, C5), 63.53 (d, J = 166.3 Hz, C3), 63.34 (d, J = 6.4 Hz, CH2OP), 62.54 (d, J = 7.0 Hz, CH2OP), 56.16 (s, CH3O), 55.98 (s, CH3O), 46.72 (s, CH3N), 36.52 (s, C4), 16.49 (d, J = 5.7 Hz, CH3CH2OP), 16.43 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.33. Anal. Calcd for C17H27N2O7P: C, 50.74; H, 6.76; N, 6.96; found: C, 50.53; H, 6.93; N, 6.90.
4.2.38. Diethyl (3RS,5RS)-5-(3,4-dimethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11aw)
Colourless oil; IR (film, cm−1) ν max: 3263, 2965, 2928, 1690, 1618, 1513, 1447, 1232, 1212, 1055, 1021, 972; 1H NMR (300 MHz, CDCl3) δ: 8.62 (br s, 1H, NH), 7.41–7.40 (m, 1H), 6.97–6.90 (m, 1H), 6.82–6.79 (m, 1H), 4.60 (dd, 1H, J = 8.6, 5.1 Hz, HC5), 4.22–4.06 (m, 4H, 2 × CH 2OP), 3.89 (s, 3H, CH 3O), 3.86 (s, 3H, CH 3O), 2.98 (s, 3H, CH 3N), 3.12–2.92 (m, 2H, H βC4 and HC3), 2.90–2.76 (m, 1H, H αC4), 1.31 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.22 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR signals of cis-11aw were extracted from the spectrum of a 79:21 mixture of trans-10aw and cis-11aw, 13C NMR (151.0 MHz, CDCl3) δ: 169.64 (s, C(O)), 149.11, 145.91, 131.15, 111.70, 111.45, 104.62, 75.83 (d, J = 7.2 Hz, C5), 63.52 (d, J = 168.2 Hz, C3), 63.21 (d, J = 6.9 Hz, CH2OP), 62.74 (d, J = 6.7 Hz, CH2OP), 56.13 (s, CH3O), 55.95 (s, CH3O), 46.73 (s, CH3N), 36.53 (s, C4), 16.41 (d, J = 5.3 Hz, CH3CH2OP), 16.35 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.06. Anal. Calcd for C17H27N2O7P: C, 50.74; H, 6.76; N, 6.96; found: C, 50.83; H, 6.94; N, 6.99.
4.2.39. Diethyl (3RS,5SR)-5-(3,5-dimethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ax)
White amorphous solid; mp 118–118.5 °C. IR (KBr, cm−1) ν max: 3272, 3213, 3113, 3002, 2892, 1704, 1615, 1563, 1480, 1452, 1264, 1250, 1212, 1160, 1050, 1017, 966, 581; 1H NMR (600 MHz, CDCl3) δ: 8.13 (br s, 1H, NH), 6.81–6.80 (m, 2H), 6.30–6.29 (m, 1H), 4.61 (dd, 1H, J = 8.9, 5.3 Hz, HC5), 4.27–4.18 (m, 4H, 2 × CH 2OP), 3.82 (s, 6H, 2 × CH 3O), 3.12–3.07 (m, 1H, HC3), 3.04 (s, 3H, CH 3N), 3.01 (dddd, 1H, J = 15.9, 13.0, 8.9, 8.9 Hz, H βC4), 2.86 (dddd, 1H, J = 13.0, 8.9, 8.2, 5.3 Hz, H αC4), 1.39 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.38 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 168.81 (s, C(O)), 161.17, 138.58, 98.12, 97.21, 76.41 (d, J = 9.2 Hz, C5), 63.48 (d, J = 170.9 Hz, C3), 63.47 (d, J = 6.5 Hz, CH2OP), 62.55 (d, J = 7.1 Hz, CH2OP), 55.40 (s, 2 × CH3O), 46.72 (s, CH3N), 36.46 (s, C4), 16.48 (d, J = 5.7 Hz, CH3CH2OP), 16.45 (d, J = 4.0 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.27. Anal. Calcd for C17H27N2O7P: C, 50.74; H, 6.76; N, 6.96; found: C, 50.88; H, 6.97; N, 7.01.
4.2.40. Diethyl (3RS,5SR)-5-(3,4,5-trimethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ay)
White amorphous solid (crystallised from ether/hexane); mp 123–124 °C; IR (KBr, cm−1) ν max: 3277, 3143, 2982, 2938, 1688, 1606, 1540, 1505, 1453, 1415, 1231, 1127, 1050, 1023, 969; 1H NMR (600 MHz, CDCl3) δ: 8.09 (br s, 1H, NH), 6.87 (s, 2H), 4.61 (dd, 1H, J = 8.9, 5.4 Hz, HC5), 4.26–4.17 (m, 4H, 2 × CH 2OP), 3.88 (s, 6H, 2 × CH 3O), 3.83 (s, 3H, CH 3O), 3.14–3.07 (m, 1H, HC3), 3.04 (s, 3H, CH 3N), 3.00 (dddd, 1H, J = 16.0, 12.9, 8.9, 8.9 Hz, H βC4), 2.85 (dddd, 1H, J = 12.9, 9.1, 8.2, 5.4 Hz, H αC4), 1.38 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 168.69 (s, C(O)), 153.46, 135.26, 132.91, 97.66, 76.37 (d, J = 9.1 Hz, C5), 63.49 (d, J = 169.1 Hz, C3), 63.36 (d, J = 6.5 Hz, CH2OP), 62.58 (d, J = 6.8 Hz, CH2OP), 60.94 (s, CH3O), 56.20 (s, 2 × CH3O), 46.72 (s, CH3N), 36.46 (s, C4), 16.50 (d, J = 3.0 Hz, CH3CH2OP), 16.47 (d, J = 3.8 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.27. Anal. Calcd for C18H29N2O8P: C, 50.00; H, 6.76; N, 6.48; found: C, 50.06; H, 6.92; N, 6.45.
4.2.41. Diethyl (3RS,5RS)-5-(3,4,5-trimethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-11ay)
Colourless oil; IR (film, cm−1) ν max: 3278, 2984, 1697, 1620, 1560, 1510, 1235, 1124, 1054, 1015, 971; 1H NMR (300 MHz, CDCl3) δ: 8.64 (br s, 1H, NH), 6.89 (s, 2H), 4.60 (dd, 1H, J = 8.6, 5.5 Hz, HC5), 4.20–4.07 (m, 4H, 2 × CH 2OP), 3.86 (s, 6H, 2 × CH 3O), 3.82 (s, 3H, CH 3O), 3.06–2.99 (m, 2H, H βC4 and HC3), 2.98 (s, 3H, CH 3N), 2.94–2.74 (m, 1H, H αC4), 1.32 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.23 (t, 3H, J = 6.9 Hz, CH 3CH2OP); 13C NMR signals of cis-11ay were extracted from the spectrum of a 74:26 mixture of trans-10ay and cis-11ay, 13C NMR (151.0 MHz, CDCl3) δ: 169.79 (s, C(O)), 153.32, 134.87, 133.54, 97.56, 75.86 (d, J = 7.5 Hz, C5), 63.50 (d, J = 170.6 Hz, C3), 63.17 (d, J = 6.7 Hz, CH2OP), 62.78 (d, J = 6.8 Hz, CH2OP), 60.92 (s, CH3O), 56.16 (s, 2 × CH3O), 46.77 (s, CH3N), 36.50 (s, C4), 16.41 (d, J = 5.9 Hz, CH3CH2OP), 16.35 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.04. Anal. Calcd for C18H29N2O8P: C, 50.00; H, 6.76; N, 6.48; found: C, 50.23; H, 6.97; N, 6.48.
4.2.42. Diethyl (3RS,5SR)-5-(2-cyano-4,5-dimethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10az)
White amorphous solid (crystallised from ether/hexane); mp 79–80 °C; IR (KBr, cm−1) ν max: 3342, 2982, 2207, 1696, 1593, 1521, 1451, 1223, 1023, 965, 751; 1H NMR (600 MHz, CDCl3) δ: 8.87 (br s, 1H, NH), 8.10 (s, 1H), 6.99 (s, 1H), 4.65 (dd, 1H, J = 9.1, 5.0 Hz, HC5), 4.27–4.19 (m, 4H, 2 × CH 2OP), 3.98 (s, 3H, CH 3O), 3.91 (s, 3H, CH 3O), 3.13 (s, 3H, CH 3N), 3.16–3.10 (m, 1H, HC3), 3.08 (dddd, 1H, J = 15.4, 12.7, 9.1, 9.1 Hz, H βC4), 2.84 (dddd, 1H, J = 12.7, 8.7, 7.7, 5.0 Hz, H αC4), 1.39 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.38 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 169.59 (s, C(O)), 153.67, 145.87, 135.42, 116.46 (s, CN), 112.94, 104.44, 93.12, 76.58 (d, J = 9.5 Hz, C5), 63.35 (d, J = 167.7 Hz, C3), 63.31 (d, J = 6.4 Hz, CH2OP), 62.62 (d, J = 6.8 Hz, CH2OP), 56.23 (s, CH3O), 56.19 (s, CH3O), 46.89 (s, CH3N), 37.07 (s, C4), 16.46 (d, J = 5.6 Hz, CH3CH2OP), 16.41 (d, J = 5.9 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.03. Anal. Calcd for C17H23N3O7P: C, 49.52; H, 5.62; N, 10.19; found: C, 49.39; H, 5.83; N, 9.98.
4.2.43. Diethyl (3RS,5SR)-5-(2-acetyl-4,5-dimethoxyphenylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10ba)
White amorphous solid (crystallised from ether/hexane); mp 108–112 °C; IR (KBr, cm−1) ν max: 3535, 3148, 2973, 2916, 1649, 1586, 1529, 1272, 1066, 1028, 963; 1H NMR (600 MHz, CDCl3) δ: 8.57 (s, 1H), 7.33 (s, 1H), 4.61 (dd, 1H, J = 9.2, 5.0 Hz, HC5), 4.28–4.18 (m, 4H, 2 × CH 2OP), 4.01 (s, 3H, CH3O), 3.94 (s, 3H, CH 3O), 3.17 (s, 3H, CH 3N), 3.16–3.12 (m, 1H, HC3), 3.05 (dddd, 1H, J = 15.8, 13.0, 9.2, 9.2 Hz, H βC4), 2.82 (dddd, 1H, J = 13.0, 8.0, 8.0, 5.0 Hz, H αC4), 2.64 (s, 3H, CH 3C(O)), 1.39 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 199.98 (s, C(O)), 170.77 (s, C(O)NH), 154.37, 144.02, 136.31, 115.31, 113.87, 103.77, 76.58 (d, J = 9.5 Hz, C5), 63.37 (d, J = 165.7 Hz, C3), 63.30 (d, J = 6.3 Hz, CH2OP), 62.44 (d, J = 7.0 Hz, CH2OP), 56.43 (s, CH3O), 56.16 (s, CH3O), 46.39 (s, CH3N), 37.41 (s, C4), 28.28 (s, CH3C(O)), 16.48 (d, J = 5.7 Hz, CH3CH2OP), 16.42 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.68. Anal. Calcd for C19H29N2O8P: C, 51.35; H, 6.58; N, 6.30; found: C, 51.23; H, 6.47; N, 6.22.
4.3. Antiviral activity assays
The compounds were evaluated against the following viruses: herpes simplex virus type 1 (HSV-1) strain KOS, thymidine kinase-deficient (TK−) HSV-1 KOS strain resistant to ACV (ACVr), herpes simplex virus type 2 (HSV-2) strains Lyons and G, vaccinia virus Lederle strain, respiratory syncytial virus (RSV) strain Long, vesicular stomatitis virus (VSV), Coxsackie B4, Parainfluenza 3, Influenza virus A (subtypes H1N1, H3N2), influenza virus B, Reovirus-1, Sindbis, Punta Toro, human immunodeficiency virus type 1 strain IIIB and human immunodeficiency virus type 2 strain ROD. The antiviral, other than anti-HIV, assays were based on inhibition of virus-induced cytopathicity or plaque formation in human embryonic lung (HEL) fibroblasts, African green monkey cells (Vero), human epithelial cells (HeLa) or Madin-Darby canine kidney cells (MDCK). Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) in the presence of varying concentrations of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. Antiviral activity was expressed as the EC50 or compound concentration required to reduce virus-induced cytopathogenicity or viral plaque formation by 50%.
4.4. Anti-HIV activity assays
Inhibition of HIV-1(IIIB)- and HIV-2(ROD)-induced cytopathicity in CEM cell cultures was measured in microtiter 96-well plates containing 3 × 105 CEM cells/mL infected with 100 CCID50 of HIV per milliliter and containing appropriate dilutions of the test compounds. After 4−5 days of incubation at 37 °C in a CO2-controlled humidified atmosphere, CEM giant (syncytium) cell formation was examined microscopically. The EC50 (50% effective concentration) was defined as the compound concentration required to inhibit HIV-induced giant cell formation by 50%.
4.5. Cytostatic activity assays
All assays were performed in 96-well microtiter plates. To each well were added (5−7.5) × 104 tumor cells and a given amount of the test compound. The cells were allowed to proliferate for 48 h (murine leukemia L1210 cells) or 72 h (human lymphocytic CEM and human cervix carcinoma HeLa cells) at 37 °C in a humidified CO2-controlled atmosphere. At the end of the incubation period, the cells were counted in a Coulter counter. The IC50 (50% inhibitory concentration) was defined as the concentration of the compound that inhibited cell proliferation by 50%.
Acknowledgments
The authors wish to express their gratitude to Mrs. Jolanta Płocka, Mrs. Leentje Persoons, Mrs. Frieda De Meyer, Mrs. Leen Ingels and Mrs. Lizette van Berckelaer for excellent technical assistance. The preliminary experiments in this project was conducted by Miss Katarzyna Raj, M.Sc. The synthetic part of this work was supported by the Medical University of Łódź internal funds (503/3-014-1/503-01 and 502-03-/3-014-01/502-34-020). The biological part of this work was supported by the KU Leuven (GOA 10/014).
References and notes
- 1.Mehellou Y., De Clercq E. J. Med. Chem. 2010;53:521. doi: 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- 2.Galmarini C.M., Mackey J.R., Dumontet C. Lancet Oncol. 2002;3:415. doi: 10.1016/s1470-2045(02)00788-x. [DOI] [PubMed] [Google Scholar]
- 3.Romeo G., Chiacchio U., Corsaro A., Merino P. Chem. Rev. 2010;110:3337. doi: 10.1021/cr800464r. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa E., Kato K. Curr. Med. Chem. 2001;8:385. doi: 10.2174/0929867013373471. [DOI] [PubMed] [Google Scholar]
- 5.Krueger A.T., Kool E.T. Chem. Biol. 2009;16:242. doi: 10.1016/j.chembiol.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chittepu P., Sirivolu V., Seela F. Bioorg. Med. Chem. 2008;16:8427. doi: 10.1016/j.bmc.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary P.M., Chavan S.R., Shirazi F., Razdan M., Nimkar P., Maybhate S.P., Likhite A.P., Gonnade R., Hazara B.G., Deshpande M.V., Deshpande S.R. Bioorg. Med. Chem. 2009;17:2433. doi: 10.1016/j.bmc.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Park S.M., Yang H., Park S.-K., Kim H.M., Kim B.H. Bioorg. Med. Chem. Lett. 2010;20:5831. doi: 10.1016/j.bmcl.2010.07.126. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y.-S., Park S.M., Kim H.M., Park S.-K., Lee K., Lee C.W., Kim B.H. Bioorg. Med. Chem. Lett. 2009;19:4688. doi: 10.1016/j.bmcl.2009.06.072. [DOI] [PubMed] [Google Scholar]
- 10.Manfredini S., Solaroli N., Angusti A., Nalin F., Durini E., Vertuani S., Pricl S., Ferrone M., Spadari S., Focher F., Verri A., De Clercq E., Balzarini J. Antiviral Chem. Chemother. 2003;14:183. doi: 10.1177/095632020301400403. [DOI] [PubMed] [Google Scholar]
- 11.Guianvarc’h D., Fourrey J.-L., Maurisse R., Suna J.-S., Benhid R. Bioorg. Med. Chem. 2003;11:2751. doi: 10.1016/s0968-0896(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 12.Rodenko B., Detz R.J., Pinas V.A., Lambertucci C., Brun R., Wanner M.J., Koomen G.-J. Bioorg. Med. Chem. 2006;14:1618. doi: 10.1016/j.bmc.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Devine S.M., Gregg A., Figler H., McIntosh K., Urmaliya V., Linden J., Pouton C.W., White P.J., Bottle S.E., Scammells P.J. Bioorg. Med. Chem. 2010;18:3078. doi: 10.1016/j.bmc.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Beattie D., Brearley A., Brown Z., Charlton S.J., Cox B., Fairhurst R.A., Fozard J.R., Gedeck P., Kirkham P., Meja K., Nanson L., Neef J., Oakman H., Spooner G., Taylor R.J., Turner R.J., West R., Woodward H. Bioorg. Med. Chem. Lett. 2010;20:1219. doi: 10.1016/j.bmcl.2009.11.131. [DOI] [PubMed] [Google Scholar]
- 15.Brunschweiger A., Iqbal J., Umbach F., Scheiff A.B., Munkonda M.N., Sévigny J., Knowles A.F., Müller C.E. J. Med. Chem. 2008;51:4518. doi: 10.1021/jm800175e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargsyan G., MacLeod B.L., Tohgha U., Balaz M. Tetrahedron. 2012;68:2093. [Google Scholar]
- 17.Frazer J.D., Horner S.M., Woski S.A. Tetrahedron Lett. 1998;39:1279. [Google Scholar]
- 18.Shelton J.R., Burt S.R., Peterson M.A. Bioorg. Med. Chem. Lett. 2011;21:1484. doi: 10.1016/j.bmcl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Chiacchio U., Corsaro A., Iannazzo D., Piperno A., Pistarà V., Rescifina A., Romeo R., Valveri V., Mastino A., Romeo G. J. Med. Chem. 2003;46:3696. doi: 10.1021/jm0308186. [DOI] [PubMed] [Google Scholar]
- 20.Chiacchio U., Balestrieri E., Macchi B., Iannazzo D., Piperno A., Rescifina A., Romeo R., Saglimbeni M., Sciortino M.T., Valveri V., Mastino A., Romeo G. J. Med. Chem. 2005;48:1389. doi: 10.1021/jm049399i. [DOI] [PubMed] [Google Scholar]
- 21.Singh R., Bhella S.S., Sexana A.K., Shanmugavel M., Faruka A., Ishar M.P.S. Tetrahedron. 2007;63:2283. [Google Scholar]
- 22.Bortolini O., De Nino A., Eliseo T., Gavioli R., Maiuolo L., Russo B., Sforza F. Bioorg. Med. Chem. 2010;19:6970. doi: 10.1016/j.bmc.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Piotrowska D.G., Cieślak M., Królewska K., Wróblewski A.E. Arch. Pharm. Chem. Life Sci. 2011;11:301. doi: 10.1002/ardp.201000282. [DOI] [PubMed] [Google Scholar]
- 24.Piperno A., Giofrè S.V., Iannazzo D., Romeo R., Romeo G., Chiacchio U., Rescifina A., Piotrowska D.G. J. Org. Chem. 2010;75:2798. doi: 10.1021/jo902485m. [DOI] [PubMed] [Google Scholar]
- 25.Piotrowska D.G., Balzarini J., Głowacka I.E. Eur. J. Med. Chem. 2012;47:501. doi: 10.1016/j.ejmech.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecker S.J., Erion M.D. J. Med. Chem. 2008;51:2328. doi: 10.1021/jm701260b. [DOI] [PubMed] [Google Scholar]
- 27.Piotrowska D.G. Tetrahedron Lett. 2006;47:5363. [Google Scholar]
- 28.Piotrowska D.G. Tetrahedron. 2006;62:12306. [Google Scholar]
- 29.Fernández F., García-Mera X., Rodríguez G., Urrutia A. Chem. Pharm. Bull. 1999;47:1006. doi: 10.1248/cpb.47.1006. [DOI] [PubMed] [Google Scholar]
- 30.Benezra C. J. Am. Chem. Soc. 1973;95:6890. [Google Scholar]
- 31.Neeser J.-R., Tronchet J.M.J., Charollais E.J. Can. J. Chem. 1983;61:2112. [Google Scholar]
- 32.Adiwidjaja G., Meyer B., Thiem J.Z. Naturforsch. 1979;34:1547. [Google Scholar]
- 33.Buchanan G.W., Bourque K., Seeley A. Magn. Reson. Chem. 1986;24:360. [Google Scholar]
- 34.Lapper R.D., Mantsch H.H., Smith I.C.P. J. Am. Chem. Soc. 1973;95:2878. doi: 10.1021/ja00790a024. [DOI] [PubMed] [Google Scholar]
- 35.Bentrude W.G., Setzer W.N. In: Phosphorus-31 NMR Spectroscopy in Stereochemical Analysis. Verkade J.G., Quin L.D., editors. VCH; Deerfield: 1987. Chapter 11. [Google Scholar]
- 36.Quin L.D. In: Phosphorus-31 NMR Spectroscopy in Stereochemical Analysis. Verkade J.G., Quin L.D., editors. VCH; Deerfield: 1987. Chapter 12. [Google Scholar]
- 37.Kool E.T., Sintim H.O. Chem. Commun. 2006:3575. doi: 10.1039/b605414e. [DOI] [PubMed] [Google Scholar]
- 38.Somoza A., Chelliserrykattil J., Kool E.T. Angew. Chem., Int. Ed. 2006;45:4994. doi: 10.1002/anie.200601311. [DOI] [PubMed] [Google Scholar]
- 39.Somoza A., Silverman A.P., Miller R.M., Chelliserrykattil J., Kool E.T. Chem. Eur. J. 2008;14:7978. doi: 10.1002/chem.200800837. [DOI] [PubMed] [Google Scholar]
- 40.Killelea T., Ghosh S., Tan S.S., Heslop P., Firbank S.J., Kool E.T., Connolly B.A. Biochemistry. 2010;49:5772. doi: 10.1021/bi100421r. [DOI] [PMC free article] [PubMed] [Google Scholar]


