Abstract
Hematological and coagulation profiles were studied in crossbred dogs experimentally infected with Angiostrongylus vasorum. Two groups of five dogs were experimentally inoculated with 50 and 100 third stage infective larvae (L3) of A. vasorum per kilogram of body weight. A third group of five uninfected animals was used as control. One sample of 10 ml of blood was collected from each animal on the 10, 20, 30, and 45 days after inoculation (dai) and at 30-day intervals thereafter for the remainder of the 210-day experimental period. The blood sample was used for the complete hemogram and platelet count, as well as measurements of prothrombin time, partial thromboplastin time and factors V and VIII. Anemia was observed in infected dogs, 6 weeks after the infection. The eosinophils presented peaks in four periods after infection. Thrombocytopenia became accentuated on the 72 dai. Decreased prothrombin time activity and increased partial thromboplastin time were observed at the 6 and 9 weeks after infection and decreased of factors VIII and V activities occurred from 4 to 6 weeks after infection. It may be conclude that infection by A. vasorum in dogs may cause a discrete anemia during the acute phase which is probably regenerative. In addition, important hemostatic alterations due to the infection suggest a chronic intravascular consumption coagulopathy.
Keywords: Angiostrongylus vasorum, Dog, Hematological profiles, Coagulation profiles
1. Introduction
The nematode Angiostrongylus vasorum (Baillet, 1866; Kamensky, 1905) is a helminth of the Protostrongylidae family (Leiper, 1926). Adult worms form are found in the right ventricle and the pulmonary artery (and its branches) of domestic dogs and wild carnivores. It is enzootic in dogs in the southwest of France (Guilhon and Cens, 1973), in the United Kingdom, Ireland (Dodd, 1973) and Uganda (Bwangamoi, 1972), with sporadic cases in the USA, Canada (Williams and Lindermann, 1995) and Brazil (Lima et al., 1985).
The life cycle involves aquatic and terrestrial snails of several genera, including Biomphalaria, Physa and Bradybaena (Rosen et al., 1970, Guilhon and Cens, 1973), which act as intermediate host. Dogs become infected by ingesting snails containing infecting third stage larvae. Larvae invade mesenteric lymph nodes where they undergo third and fourth molts. Young adult nematodes migrate via lymphatic or hepatic portal vessel to the right side of the heart and pulmonary arteries where they develop to sexual maturity. The reported prepatent period varies from 38 to 60 days (Rosen et al., 1970, Guilhon and Cens, 1973).
The severest clinical and pathological alterations happen in the lungs and heart, resulting in the pulmonary consolidation and cardiac insufficiency followed by weight loss and hemorrhagic diatheses (Dodd, 1973, Martin et al., 1993). A determinant factor in the pathology of the angiostrongilosis seems to be related to the location of the parasite in the host. Their presence inside the arteries and ramifications, promoting a mechanical and/or metabolic action on the wall of the vessels, may alter the hemostatic pathway, being able to induce to a coagulopathy of intravascular consumption, and changes in the hematological profile. Experimental studies suggest that infection with parasites, causes thrombocitopenia and changes of the coagulation profile developed subsequent to 6 weeks and paralleled the apparent host inflammatory response to the parasite (Schelling et al., 1986).
This paper discusses the mechanism through which the parasite promotes the hematological and coagulation alterations.
2. Material and methods
Fifteen, 6–8-month-old, crossbred dogs, were used in this experiment. Before dogs were infected the feces of these animals were collected and searched for helminth’s eggs and larvae. All the dogs were treated with anti-helminthic drug in two doses (nitroscanate, 50 mg/kg). Additional exams were performed from 7-day intervals to confirm that animals remained free of parasites. All of the dogs were immunized against parvovirus, coronavirus and distemper virus, in three doses at 1 month interval. The dogs were divided according to their weight in three groups of five animals. Groups A and B were inoculated, by oral route, with 50 and 100 infective larvae/kg of body weight, respectively. Group C was not inoculated and provided a control for the experiment. The third stage infective larvae (L3) were obtained by experimental infection of aquatic snail, Biomphalaria glabrata, maintained in laboratory, with 400 larvae of first stage/snails. After 25 days after inoculation (dai), feces were collected daily and examined by Baermann method to determine the prepatent period.
Samples of 10 ml of blood were obtained by atraumatic jugular venipuncture of each animal using vacutainer tubes. Blood samples were drawn before inoculation and on the 10, 20, 30 and 45 dai and thereafter 30-day intervals for the remainder of the 210-day experimental period. Five milliliters of the sample in EDTA were used to carry out the complete hemogram by the conventional technique of Dace and Lewis (1984). Red and white blood cell counts were also processed in an automatic cell counter (CELM CC510). The remaining 5 ml of citrated whole blood, was centrifuged at 100g during 5 min to obtain the plasma rich in platelets (PRP) and, afterwards, at 500g for 10 min obtaining the plasma poor in platelets (PPP). The PPP was used for prothrombin time (PT), partial thromboplastin time (PTT) and of V and VIII clot factors measurements, while the PRP was used for platelet aggregation test in which adenosine diphosphate (ADP) was employed as an agonist. In the coagulation evaluation, PT and PTT were assessed by using calcic thromboplastin (Pacific HemosthasisR-ThromboScreenR) and cephalin containing celite as an activator (Biolab, Merieux), respectively, following the manufacturer’s instructions. The ST4-Bio coagulometer (Stago, France) was used to read the results in seconds which were converted to percentage of activity by means of a standard calibration curve previously established.
Factors V and VIII—deficient plasmas (Diamed, Brazil) were used measuring Factors V and VIII activities, following the manufacturer’s instructions. The ST4-Bio coagulometer was used to read the results in seconds and the results were converted in percentage of activity a previously established standard calibration curve.
All the parameters analyzed were submitted to analysis of variance by using the STA statistical pack and the means were compared by the Student’s t-test. Differences were to be significant at p≤0.05.
3. Results
There was a reduction in the hemoglobin and hematocrit values in the infected group compared to the control group, mainly after the 45 dai when animals started to eliminate the larvae (prepatent period) and on the acute phase of the clinical manifestations. However, significant difference (p≤0.05) were observed from the 20 to 130 dai mainly group B and control (Fig. 1 A). A decrease was also observed in the hematocrit values on 45, 100 and 130 dai for groups A and B compared to control. No difference was observed on 72 dai between groups A and control (Fig. 1B).
Fig. 1.
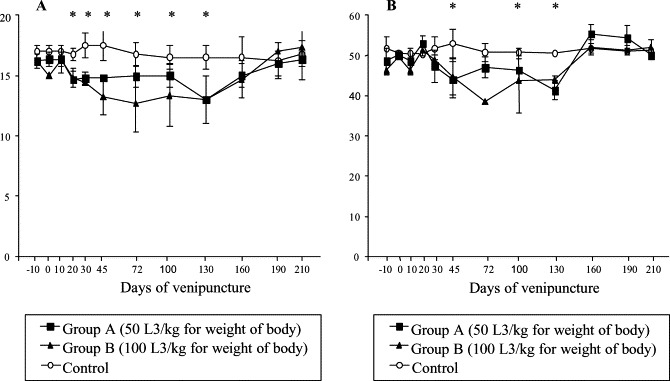
Mean values of hemoglobin (A) and hematocrit (B) in control and infected dogs with different number of larvae of A. vasorum; * represents significant difference between groups (p≤0.05).
The mean numbers of immature neutrophils from 45 to 160 dai, in the infected groups A and B compared to the control. Significant differences were observed between A and control group on 45, 100, 130 and 160 dai (p≤0.05); while between B and control groups significant differences were observed on 45 and 100 dai only (p≤0.05). Significant difference was also detected between the A and B groups on 100 dai (p≤0.05) (Fig. 2 A). Segmented neutrophils values did not show significant differences when the groups were compared, although a tendency to decrease was seen from 45 dai for group A (Fig. 2B).
Fig. 2.
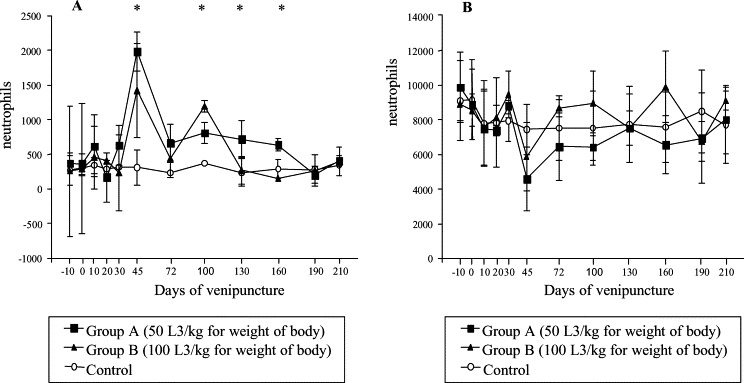
Absolute mean count of immature (A) and segmented neutrophils (B) in control and infected dogs with different number of larvae of A. vasorum; * represents significant difference between groups (p≤0.05).
Eosinophils counts increased in groups A and B compared to control from 10 dai at the end of the experimental period. Significant differences were observed except on 30 and 100 dai (p≤0.05) (Fig. 3 ).
Fig. 3.
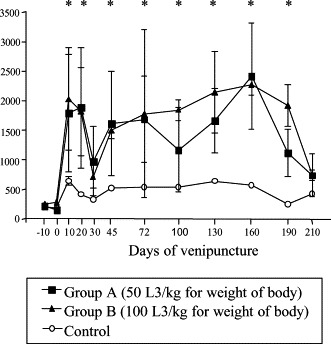
Absolute mean of eosinophils count in control and experimentally infected dogs with different number of larvae of A. vasorum; * represents significant difference between groups (p≤0.05).
The main qualitative alterations of the blood films were hypochromia and elongated platelets which were visualized from the 30 dai in both groups A and B.
The platelet counts decreased in the infected groups A and B related to control from the 20 dai becoming accentuated on the 72 dai. Significant difference between infected groups and controls were observed from the 20 to 190 dai (p≤0.05) (Fig. 4 ), this being consistent with platelets aggregation results, which was also reduced on those days.
Fig. 4.
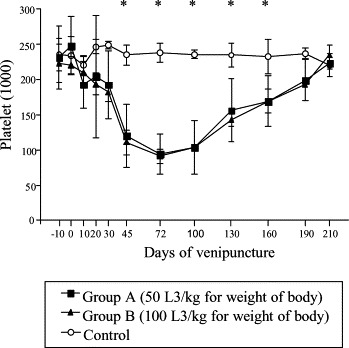
Mean platelet in control and experimentally infected dogs with different number of larvae of A. vasorum; * represents significant difference between groups (p≤0.05).
Average values of plasma PT activity in inoculated dogs were reduced from 45 to the 130 dai, returning to normal after this period. Significant difference between groups A and B compared to control were seen from 45 to 100 dai (p≤0.05) (Fig. 5 ). Significantly higher PTT values for A and B compared to the control were observed from the 10 to 130 dai (p≤0.05) (Fig. 6 ). Factors V and VIII activities were reduced mainly at 30 and 45 dai. Significant difference between infected groups related to control to factor V activity from 10 to 45 dai and factor VIII activity from 30 to 130 dai to groups B and C and on the 45 and 72 dai to groups A and C (p≤0.05) (Fig. 7 ).
Fig. 5.
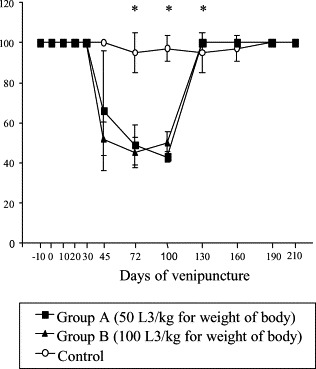
Mean values of PT percentage of activity in control and experimentally infected dogs with different number of larvae of A. vasorum; * represents significant difference between groups (p≤0.05).
Fig. 6.
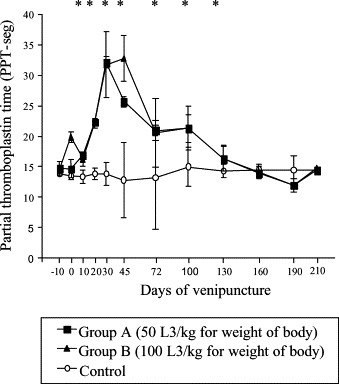
Mean values of PTT in seconds in control and experimentally infected dogs with different number of larvae of A. vasorum; * represents significant difference between groups (p≤0.05).
Fig. 7.
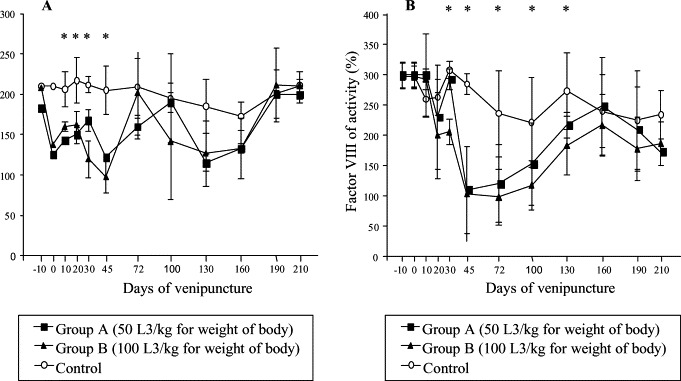
Factors V (A) and VIII (B) mean value in control and experimentally infected dogs with different number of larvae of A. vasorum; * represents significant difference between groups (p≤0.05).
4. Discussion
Based on the results of the red blood cell data, we observed that the infection with A. vasorum infection caused a slight anemia, mainly from the 45 to 72 dai when there was the first larvae elimination and the beginning of the clinical manifestations, respectively. The hypochromic red blood cells present in the infected animals suggest that the parasite interferes in the synthesis of hemoglobin and in the iron release by the cells of the reticulum endothelial system (RES). The insufficient iron release may compromise the synthesis of hemoglobin in the erythroblasts resulting in hypochromia and reduction of the cellular volume. This fact could explain the reduction of the hemoglobin and hematocrit values with no decrease in the counting of red blood cells.
While blood cell counts have been used to investigate possible temporal changes in the bloodstream caused by A. vasorum. With regard to the differential leukocyte count, the kinetic of the immature and segmented neutrophils presented inverse profile on the 45 dai, suggesting migration of the segmented neutrophils to the tissues and a rapid release of immature neutrophils to the peripheral blood. Neutrophils that have migrated into tissue do not return to the bloodstream, normally, they migrate into the lung, gastrointestinal tract, liver and spleen (Osgood, 1954). Since A. vasorum is known to have a pulmonary phase and this may cause an inflammatory process in the lungs. Neutrophils are attracted to the tissues in response to stimuli from inflamed area and may secrete cytokynes that further influence the number of these cells in the bloodstream, as well as the release of immature neutrophils. Traumatic injury produced by adult worms, causing tissue necrosis, larvae elimination and migration, would justify the mobilization of immature neutrophils from bone marrow to the bloodstream and subsequently to the tissues. It is possible that constituents of tissue necrosis may result in enhanced cytokynes production by lymphocytes that promote myelopoiesis (Nimer and Goldi, 1995).
Eosinophils are present in the blood and some tissues, predominantly the skin, lungs, intestines and genitourinary tract. They migrate from bone marrow to peripheral blood, both in marginal pools and the circulation, as well as to the tissues. Eosinophils levels depend on the activity of different eosinophilopoietic factors and the contributions of margination and recirculation. The most common causes of blood and tissue eosinophilia are hypersensibility reactions and parasite infection. It is known that acute pulmonary eosinophilic infiltrates with blood eosinophilia may be caused by immune reactions to antigens localized in the lungs including drugs and parasites, frequently (Galli and Goetzl, 1995). Based on the host/parasite interaction, it is possible that the peaks in four periods, observed for eosinophils in the present work (on 10, 20, 72 and 160 dai) to be associated to the maturation of the L3 larvae to the early L5 stage within mesenteric lymph nodes and subsequent migration of the latter via the hepatic portal system to the right side of the heart and pulmonary arteries during the initial periods of infection. During the growing of adults worms and patency, immunogenic particles released from the parasites may produce eosinophilia by multiple interactions involving macrophages and lymphocytes. These results are similar to those of Mishra and Cens (1971), who observed peaks, associated the with infective larvae migration, growing of the adults worms and patency. Eosinophils alterations were reported by Rosen et al. (1970), Bwangamoi (1972), Perry et al. (1991), however, Prestwood et al. (1981) and Cury (1995) did not find any consistent hematological abnormalities in dogs infected experimentally with A. vasorum.
The thrombocytopenia observed in inoculated dogs may have been triggered by the likely antigenic dissemination resulting from the adults worms, gravid female deposited eggs in terminal arterioles, present in the lungs of dogs which would promote an intense cellular response, in addition to vascular lesions, by the presence of adults and larvaes in the vessels. Due to the possible presence of vascular lesion, which would be caused by parasites and/or antigen–antibody complexes deposition, a greater afflux of platelets to the area could happen resulting in the reduction of the number of circulating platelets. The lower number of platelets resulted in a decreased platelet aggregation. In the present study, platelet regeneration was evident from the gradual increase in their numbers during the chronic phase of the infection.
The reduction of the PT activity and the prolongated PTT occurred between the 6th and the 9th weeks after the infection, being associated with the time expected for onset of eggs deposition and their hatching. It was observed that these alterations coincided with the occurrence of thrombocytopenia, which may indicate a role for the parasite in the process of intravascular coagulation as previously suggested (Schelling et al., 1986). In addition, possible factor released from the eggs deposition and hatching area could be able to inhibit thrombocytopoiesis.
The reduction of the factor V and factor VIII activities, thrombocytopenia and prolonging of the PT and PTT characterize a chronic coagulopathy of consumption. This is more evident at times when the adult parasites are expected to exist in the pulmonary artery or eliminating larvae and when larvae migrate. The findings of the present study with regard to the reduction in factor VIII activity did not corroborate those of Schelling et al. (1986), who found that it increases. However, they agreed with respect to the moment when the alterations arise, suggesting a role for larval migration in the development of the coagulopathy of consumption. Although elevated plasma levels of factor VIII are found in inflammatory processes. However, the coagulopathy of consumption would justify the lower factor VIII levels found in this study. It is possible that the normal to increased levels of factor VIII mentioned by Schelling et al. (1986) be consistent with lower consumption of factors than that found in the present study.
The alterations observed allows us to conclude that the infection by A. vasorum in dogs is able to cause a slight regenerative anemia in the acute phase of the disease, confirmed by the presence of polychromatophilic red blood cells. Furthermore, important hemostatic alterations resulting from the infection indicate a chronic intravascular coagulopathy of consumption. However, it is important to emphasize that these hematological and coagulation alterations are not evident only in the angiostrongilosis, being able to occur in other infections as in the Dirofilaria immitis, for instance.
Although the real prevalence of the infection of the A. vasorum in Brazil is not known, however, considering its clinical importance, the use of the Baermann method is suggested for searching parasite’s larvae in cases of dogs presenting hematological and coagulation alterations of obscure cause.
Acknowledgements
Thanks to S.A. Braswey for supplying dog food and Dr. J.P. Graan for English review.
References
- Bwangamoi O. Angiostrongylus vasorum and other worms in dogs in Uganda. Vet. Rec. 1972;19(11):267. doi: 10.1136/vr.91.11.267. [DOI] [PubMed] [Google Scholar]
- Cury, M.C., 1995. Angiostrongylus vasorum (Baillet, 1866) Kamensky, 1905: aspectos clı́nicos hematológicos e imunológicos em cães experimentalmente infectados. Instituto de Ciências Biológicas, UFMG, Belo Horizonte, MG, Brasil, 137 pp. (Tese, Mestrado em Parasitologia).
- Dace, J.V., Lewis, S.M., 1984. Practical Haematology, Vol. 6. J&A Churchill Ltd., Livingstone, London, 536 pp.
- Dodd K. Angiostrongylus vasorum (Baillet, 1866) infection in a Greyhound Kennel. Vet. Rec. 1973;92:195–197. doi: 10.1136/vr.92.8.195. [DOI] [PubMed] [Google Scholar]
- Galli, S.J., Goetzl, E.J., 1995. Eosinophils, basophils and mast cells. In: Hardin, R.I., Lux, S.E., Stossel, T.P. (Eds.), Blood—Principles and Practice of Hematology, 1st Edition. Lippincott, Philadelphia, PA, pp. 621–640.
- Guilhon J., Cens B. Angiostrongylus vasorum(Baillet, 1866). Étude biologique et morfologique. Ann. Parasitol. Hum. Comp. 1973;48:567–569. [PubMed] [Google Scholar]
- Lima W.S., Costa H.M.A., Guimarães M.P., Leite A.C.R. Angiostrongylus vasorum (Baillet, 1866) Nematoda: Prothostrongylidae em cães de Minas Gerais. Brasil. Mem. Inst. Oswaldo Cruz, Rio de Janeiro. 1985;80:233–235. doi: 10.1590/s0074-02761985000200015. [DOI] [PubMed] [Google Scholar]
- Martin N.W.S., Ashton G., Simpson B.R. Angiostrongylosis in Cornwall clinical presentations of eight cases. J. Small Pract. 1993;34:20–25. [Google Scholar]
- Mishra G.S., Cens B. Eosinophilic variations and electrophoretic analysis of plasma of dogs infected with Angiostrongylus vasorum (Baillet, 1866) Indian Vet. J. 1971;48(6):633–640. [PubMed] [Google Scholar]
- Nimer, S.D., Goldi, D.W., 1995. Molecular, cellular, and clinical biology of phagocytic cells. In: Hardin, R.I., Lux, S.E., Stossel, T.P. (Eds.), Blood–Principles and Practice of Hematology, 1st Edition. Lippincott, Philadelphia, PA, pp. 513–541.
- Osgood E.E. Number and distribution of human hemic cells. Blood. 1954;9(1141):544–549. [PubMed] [Google Scholar]
- Perry A.W., Hertling R., Kennedy M.J. Angiostrongylosis with disseminated larval infection associated with signs of ocular and nervous disease in an imported dog. Can. Vet. J. 1991;32(7):430–431. [PMC free article] [PubMed] [Google Scholar]
- Prestwood A.K., Greene C.E., Mahhaffey E.A., Burges D.E. Experimental canine angiostrongylosis. I. Pathologic manifestations. J. Am. Anim. Hosp. Assoc. 1981;17(3):491–497. [Google Scholar]
- Rosen L., Ash L.R., Wallance G.D. Life history of the canine lungworm Angiostrongylus vasorum (Baillet, 1866) Am. J. Vet. Res. 1970;186(10):1101–1103. [PubMed] [Google Scholar]
- Schelling C.G., Greene C.E., Prestwood A.K. Coagulation abnormalities associated with acute Angiostrongylus vasorum (Baillet, 1866), infection in dogs. Am. J. Vet. Res. 1986;47(12):2669–2673. [PubMed] [Google Scholar]
- Williams J.F., Lindermann B. Angiostrongylus in Greyhound. J. Am. Vet. Med. Ass. 1995;186:1101–1103. [Google Scholar]


