Abstract
The National Healthcare Safety Network’s new classification characterizes all adverse ventilator-associated events (VAE) into a tiered system designed to shift the focus away from ventilator-associated pneumonia as the only important cause or morbidity in ventilated patients. This new surveillance definition of VAE eliminates subjectivity by using clearly defined criteria and facilitates the automated collection of data. This allows for easier comparison and analysis of factors affecting rates of VAE. Numerous studies have been published that demonstrate its clinical application. This article presents the VAE criteria, contrasts the difference from the previous ventilator-associated pneumonia definition, and discusses its implementation over the past 5 years.
Keywords: Ventilator-associated pneumonia, Sepsis, Nosocomial infection, Critical care
Key points
-
•
New NHSN definition for ventilator-associated events (VAE) replaces previous definition of pneumonia.
-
•
Clear, defined objective criteria for each category of VAE eliminates subjectivity of previous definition.
-
•
Shifts focus of reportable events from pneumonia to broader classification of respiratory deterioration.
-
•
May underestimate the rate of clinical pneumonia, but captures other noninfectious causes of respiratory compromise in ventilated patients.
-
•
Definition may need to be modified to account for specific patient populations and alternative modes of ventilation.
Introduction
Ventilator-associated pneumonia (VAP) remains one of the most common nosocomial infections in the intensive care unit (ICU) affecting one-third of patients that require mechanical ventilation during a noninfectious admission.1 Despite having a significant attributable mortality (4.6%), VAP remains a single a component of a larger constellation of adverse events, such as aspiration, atelectasis, pulmonary edema, venous thromboembolic event, delirium, and acute respiratory distress syndrome (ARDS), which potentially increase the morbidity, mortality, hospital length of stay (LOS), and cost of care in mechanically ventilated patients. This broader view of complications that arise in patients requiring ventilator support provides the framework for the new quality metrics put forth by the Centers for Disease Control and Prevention (CDC) for ventilated patients.
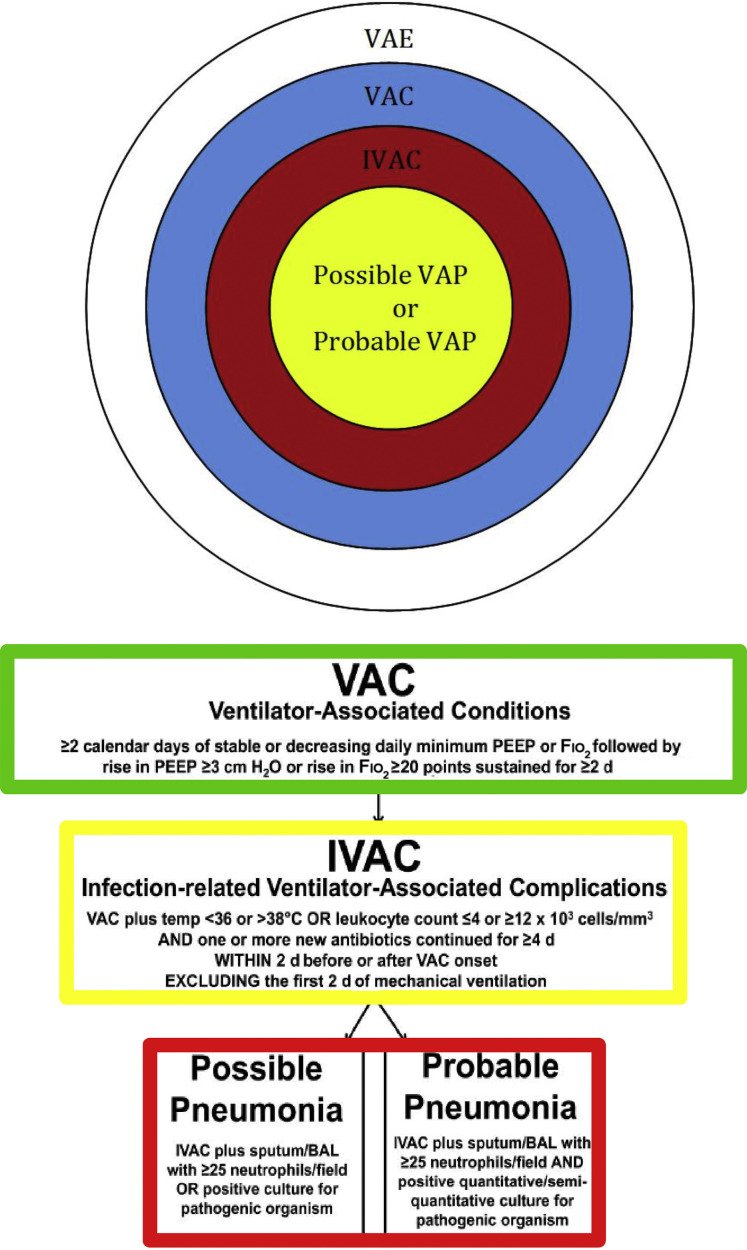
In 2011, CDC a workgroup encompassing physician leaders from multiple professional societies (eg, American College of Chest Physicians, American Thoracic Society, Society of Critical Care Medicine, Infectious Diseases Society of America) in conjunction with representatives of the US Department of Health and Human Services, Office of Disease Prevention and Heath Promotion, National Institutes of Health, and the CDC met to create a new definition of VAP that improves diagnosis, the reliability and validity of surveillance, and create a reporting algorithm for the National Healthcare Safety Network (NHSN).2 The final product of this workgroup resulted in a tiered system that encompasses the broader classification of ventilator-associated events (VAE), subcategorized by objective criteria for infection-related ventilator-associated condition (IVAC) and then more specifically by possible- and probable-VAP (Fig. 1 ).
Fig. 1.
Targeting VAE. Broad description of ventilator-related complications is more narrowly defined by each tier of the new CDC definition: VAC, IVAC, possible- or probable-VAP. Fio2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure.
This article reviews the criteria for ventilator-associated condition (VAC) and IVAC, including the classifications of probable- or possible-VAP; compares how the tiered definition of pneumonia contrasts to the previous NHSN definition; summarizes the studies validating its application; and explores its utility in surgical patients.
New definition
In 2013, NHSN supplanted the previous definition of pneumonia with the working group’s classification of VAE (Table 1 ). The intent is to cast a wider net using defined, objective criteria that captures all potentially preventable complications from data available in the electronic medical record (EMR) in most institutions. Automated surveillance directly from EMR is thought to decrease reporting bias by eliminating subjectivity from the analysis.
Table 1.
NHSN VAE criteria
| NHSN Surveillance Guidelines for Diagnosis of VAE | ||
|---|---|---|
| Name: Description | Dependent Qualification | Definition |
| VAC: new respiratory deterioration | ≥2 calendar days of stable or decreasing daily minimum PEEP or daily minimum Fio2 |
|
| iVAC: VAC + clinical signs of infection | Within 2 calendar days before or after onset of a VAC Excludes the first 2 d of mechanical ventilation |
Temperature: <36°C or >38°C OR Leukocyte count: ≤4000 or ≥12,000 cells/mm3 AND One or more new antibiotics continued for ≥4 d |
| Possible VAP: IVAC + qualitative evidence of pulmonary infection | Within 2 calendar days before or after onset of a VAC Excludes the first 2 d of mechanical ventilation |
Gram staining of endotracheal aspirate or BAL showing ≥25 neutrophils and ≤10 epithelial cells per low-power field OR Positive culture from sputum, endotracheal aspirate, BAL, lung tissue |
| Probable VAP: IVAC + quantitative evidence of pulmonary infection | Within 2 calendar days before or after onset of a VAC Excludes the first 2 d of mechanical ventilation |
|
Highlights the stepwise respiratory deterioration associated with VAC, iVAC, possible pneumonia, and probable pneumonia with specific, objective criteria that define each category.
Sputum cultures excludes the following: normal respiratory/oral flora, mixed respiratory/oral flora or equivalent; Candida species or yeast not otherwise specified; coagulase-negative Staphylococcus species; Enterococcus species.
Abbreviations: BAL, bronchoalveolar lavage; CFU, colony-forming unit; Fio2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure.
VAC is defined as a sustained increase in oxygen requirements in a ventilated patient over a period of 2 days. Sustained oxygen requirement is defined as an increase in the daily minimum positive end-expiratory pressure (PEEP) of greater than or equal to 3 cm H2O or an increase in the daily minimum fraction of inspired oxygen (Fio 2) of greater than or equal to 20 points for 2 days. To qualify as a VAC, the patient must have had a minimum of 2 days of mechanical ventilation with stable or decreasing oxygen requirements before the days of increased oxygenation.
The progression from VAC to IVAC depends on timing in relation to the increased oxygenation requirements that define a VAC, clinical signs of infection, and treatment of the patient with antibiotics by the ICU team. Patients must be mechanically ventilated a minimum of 3 days and have signs of infection in the 2 days before or 2 days after the diagnosis of VAC. In addition, the patient must have a low-grade fever (>38°C) or hypothermia (<36°C) or leukocytosis (≥12,000 cells/mm3) or leukopenia (≤4000 cells/mm3) and be started on a new antimicrobial agent for greater than or equal to 4 days. IVAC suggests a causal relationship between infectious cause and VAC.
In the new classification of VAE, patients that meet the criteria for VAC and IVAC are further characterized with the diagnosis of VAP according to the type of evidence available from their sputum assessment. Possible-VAP requires either a qualitative sputum analysis demonstrating purulent respiratory secretions defined as greater than or equal to 25 neutrophils and less than or equal to 10 squamous cells per low-power field or a positive qualitative, semiquantitative, or quantitative culture obtained from the lungs, bronchi, or trachea. Probable-VAP requires the presence of purulent secretions and specific cutoffs for the number of colony-forming units identified in culture that are determined by what level of the airway the sputum sample was obtained (see Table 1). Any of the following results may supplant the presence of purulent sputum in the diagnosis of probable-VAP: positive pleural fluid culture, positive lung histopathology, positive diagnostic test for legionella, or the presence of common respiratory viral pathogens in sputum.
The diagnosis of VAP is not easily established but is clearly defined within the new surveillance criteria of VAE. The mechanically ventilated patient must have a period of stability followed by deterioration and increased support (VAC), a suspected infectious cause (IVAC), and finally meet the confirmatory criteria for either possible-VAP or probable-VAP. Each tier has specific and clearly defined, objective criteria that must be met to qualify for the next level. This system is substantially different from the previous NHSN definition of VAP (PNU1), which was more subjective with many of the parameters not clearly stipulated. The PNU1 diagnosis was made when a patient met radiographic, systemic, and pulmonary function criteria. The guidelines were less precise allowing significant leeway in the interpretation, which led to high degree of variability between providers in defining when a patient had pneumonia. One ramification of the interobserver variability was that it limited the ability to compare reporting within institutions and across hospital systems. To highlight how the new diagnosis of VAP differs, we next review the three categories comprising the diagnosis of PNU1 (Box 1 ).
Box 1. NHSN PNU1 definition.
Radiologic criteria (≥2 serial radiographs with at least one of the following)
-
1.
New or progressive infiltrate
-
2.
Consolidation
-
3.
Cavitation
Systemic criteria (at least one of the following)
-
1.
Fever (>38°C or >100.4°F)
-
2.
Leukopenia (<4000 white blood cell/mm3) or leukocytosis (≥12,000 white blood cell/mm3)
-
3.
For adults ≥70 years old, altered mental status with no other recognized cause
Pulmonary criteria (at least two of the following)
-
1.
New onset of purulent sputum, or change in character of sputum, or increased respiratory secretions, or increased suctioning requirements
-
2.
Worsening gas exchange (eg, desaturations, increased requirements, or increased ventilator demands)
-
3.
New-onset or worsening cough, or dyspnea, or tachypnea
-
4.
Rails or bronchial breath sounds
Old versus new
PNU1 definition required radiologic evaluation with two or more serial studies that demonstrated a new, progressive, or persistent infiltrate, consolidation, or cavitation. The current VAE criteria do not have a radiographic component. Although radiographic evaluation may be helpful in identifying causes for worsening pulmonary function, a finding on plain chest radiograph may easily be interpreted as an infiltrate, atelectasis, effusion, or pneumonia between providers and frequently may not manifest until well after a patient with pulmonary dysfunction has clinically improved. Radiographic studies are an adjunct to the diagnosis of pneumonia but are not required and have been eliminated from VAE surveillance criteria.
The systemic component of the PNU1 definition required the patient to have at least one of the following criteria: fever with temperature greater than 38°C, leukopenia (≤4000 cells/mm3) or leukocytosis (≥12,000 cells/mm3), or altered mental status in patients who are greater than or equal to 70 years of age with no other identified cause. These criteria are similar to the standards that determine when a patient has an IVAC, except there is not a stipulation regarding hypothermia, temperature less than 36°C in the PNU1 definition. Conversely, the IVAC definition does not have a component that accounts for altered mental status in elderly patients.
To fulfill the pulmonary component of PNU1, patients must meet at least two of four criteria. New onset of purulent sputum, change in the character of the sputum, or need for more frequent suctioning were considered one element but there were no specific cutoffs leaving the evaluation of purulent, character, or frequency purely subjective. The new VAP definition has defined qualitative and quantitative values of neutrophil count and colony-forming units that must be met to establish possible-VAP and probable-VAP. The second of the four pulmonary criteria vaguely defined worsening gas exchange. However, these criteria were described as desaturations, increased oxygen requirements, or increased ventilator demand but did not define the increment or duration of time. In comparison the diagnosis of VAP is contingent on the patient having met the criteria of a VAC. The VAC criteria clearly establish minimum increases in Fio 2 and/or PEEP and stipulate the duration of time relative to a previous period of stability and to the diagnosis of VAP. The two remaining pulmonary criteria for PNU1 were comprised of patient symptoms: worsening cough, dyspnea, or tachypnea; and patient examination findings, such as rales or bronchial breath sounds. In contrast, the VAE surveillance definition does not use any symptoms or examination findings as criteria for defining VAP.
What’s the point?
The new characterization of VAE allows for multiple novel opportunities that were not accounted for by solely focusing on VAP and the PNU1 definition. Most (75%) patients that meet criteria for VAE have a noninfectious cause responsible for their respiratory setback (Fig. 2 ). Clinical complications, such as venous thromboembolic event, pulmonary edema, aspiration, and ARDS, can adversely affect the duration of mechanical ventilation; ICU LOS; increase mortality; and further convolute other important aspects of care, such as sedation, prevention of ICU delirium, and early mobility. Objectively identifying VAEs provides a wider vantage point that can promulgate performance improvement for all patients that require support with mechanical ventilation.
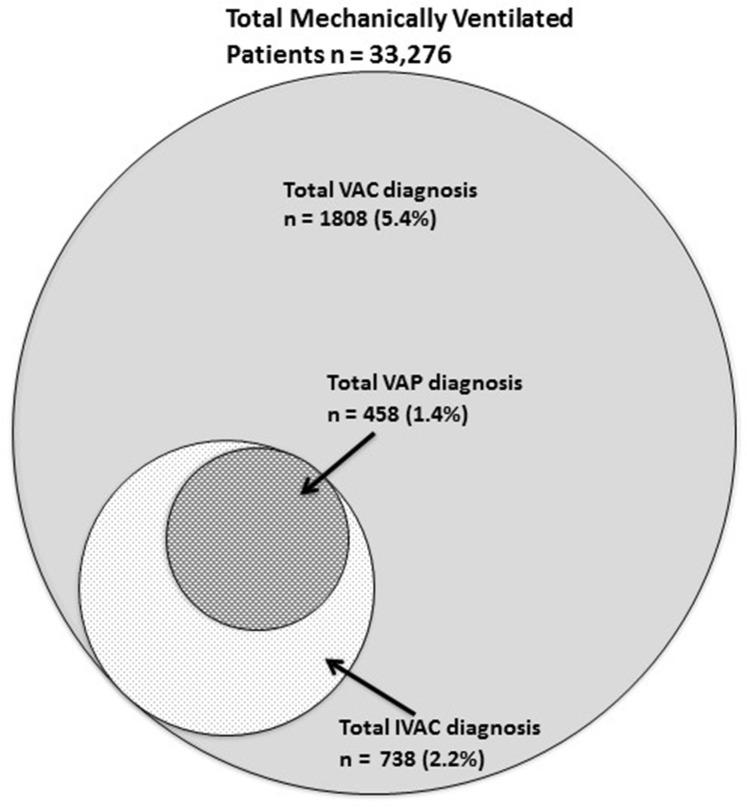
Fig. 2.
Diagram summarizing cumulative number of patients that met VAC, iVAC, and both possible- and probable-VAP criteria from the published studies summarized in Table 2.
The benefit of defining specific criteria that identify VAE and eliminates subjectivity is readily apparent. It allows for the creation of programs to identify these searchable elements from the EMR, which simplifies reporting and reduces variability. The data within institutions, between health care systems, and even across borders can now be collected and compared in an easy, efficient manner. This cross-comparison at multiple levels would allow for identification of effective patterns of care that could potentially be shared within and across health care systems.
Summary of studies evaluating the practicality of the ventilator-associated events criteria
Since the introduction of the VAE surveillance criteria multiple studies have been performed to validate its clinical applicability (Table 2 ). One of the first studies to evaluate how the surveillance criteria for VAE could be used to identify patient complications used retrospective data collected from three academic medical centers in different regions of the United States. Each unit submitted 100 patients that were mechanically ventilated 2 to 7 days and 100 patients that ventilated for greater than 7 days. Each patient was independently assessed using the VAC criteria and according to the PNU1 definition. This study demonstrated that patients who met criteria for VAC and PNU1 had a longer duration of mechanical ventilation, ICU LOS, and hospital LOS. VAC diagnosis was associated with increased mortality, whereas PNU1 diagnosis was not, and VAC assessment was considerably less time consuming for the assessors to perform compared with evaluation with the PNU1 criteria.3 Another early study performed in 11 ICUs in Canada and the United States prospectively evaluated 1320 patients using VAC, IVAC, and PNU1 criteria.4 This study demonstrated that patients meeting criteria for VAC and IVAC had increased mortality compared with patients diagnosed with VAP using PNU1. The results also highlight the importance of the subjective surveillance definition, because there was a poor correlation agreement between patients that met the criteria for VAC or IVAC and patients diagnosed with VAP using the PNU1 definition. Similarly, another retrospective study from a tertiary, teaching hospital in Australia evaluated 543 patients intubated greater than or equal to 2 days, using the VAC surveillance criteria, and demonstrated patients with VAC had increased duration of mechanical ventilation, ICU LOS, and duration of antibiotic therapy.5
Table 2.
Summary of studies that evaluated patients using NHSN VAE criteria
| Emphasis | Design/Population | Summary | VAE Rates n (%) | |
|---|---|---|---|---|
| Klompas et al,7 2014 | Epidemiology and morbidity of VAE | Retrospective review at an academic medical center studying adult patients on MV over 5 y | 20,356 patients studied over 5 y showing VAEs corresponded with three times longer to extubate patients, increased hospital LOS by 50%, and doubled the risk of death compared with those that did not meet the criteria for VAE. Most frequent organisms were Staphylococcus aureus (29%), Pseudomonas aeruginosa (14%), and Enterobacter species (7.9%). | VAC 1141 (5.61) IVAC 431 (2.12) pVAP 139 (0.68) prVAP 127 (0.62) |
| Klouwenberg et al,6 2014 | Novel electronic surveillance mechanism to identify VAE | Multi-institutional prospective cohort study on mixed ICU patients | 2080 patients ventilated for >2 d. The VAE algorithm detected 32% of clinical VAP patients. Most VAC patients had volume overload and infections, but not necessarily VAP. Concordance between VAE algorithm and clinical VAP was poor. | VAC 158 (7.60) IVAC 66 (3.17) p/prVAP 51 (2.45) |
| Boyer et al,11 2015 | Prospectively evaluate VAE and VAC rates and preventability | Prospective cohort study at an academic medical center studying adult medical and surgical ICU patients | 1209 patients studied. Most common cause of VAC were IVACs (50.7%), ARDS (16.4%), pulmonary edema (14.9%), and atelectasis (9%). 37.3% of VACs were determined to be potentially preventable. The sensitivity of NHSN criteria for detecting VAP determined to be 25.9%. | VAC 77 (6.37) iVAC 34 (2.81) pVAP 6 (0.49) prVAP 15 (1.24) |
| Stoeppel et al,14 2014 | Applicability of NHSN VAE definitions in surgical ICU patients | Prospective cohort study at an academic medical center studying adult surgical and ICU patients | 437 surgical ICU patients of which only 37 met VAE criteria. Of the 400 other patients who did not meet VAE criteria, 111 (28%) had respiratory deterioration, 99 patients had clinical pneumonia. Most of these patients (58%) had <2 d of respiratory deterioration. Agreement between prVAP and clinical VAP was 77.3%. | VAC 37 (8.47) IVAC 31 (7.09) p/prVAP 22 (5.03) |
| Lewis et al,17 2014 | Evaluate risk factors for VAE | Retrospective case-controlled study at an academic medical center studying adult medical, surgical, cardiac, and neuroscience patients | 2990 patients analyzed and 110 case matched to control subjects showing significant risk factors in developing a VAE were mandatory modes of ventilation, positive fluid balance, starting benzodiazepines before intubation, total opioid exposure, and use of paralytic medications. | VAC 172 (5.75) IVAC 70 (2.34) |
| Lilly et al,9 2014 | Prevalence and characteristics of VAE | Prospective cohort study at two academic medical centers studying adult medical, surgical, cardiovascular, and neurologic ICU patients | 8408 MV patients discharged from ICU. NHSN VAE guidelines had a poor predictive value (0.07) of patients with clinically determined VAP. Most patients (71%) who met VAE/VAC criteria were diagnosed with ARDS. | VAC - 13.8/1000 MV days IVAC – 8.8/1000 MV days VAP – 2.96/1000 MV days |
| Resetar et al,18 2014 | Use of automated electronic surveillance to detect VAE from EMR data | Retrospective review at an academic medical center studying adult medical, surgical, cardiac, cardiothoracic, and neurologic ICU patients | 3691 patients with 19,105 MV days. Electronic VAE surveillance is a significant clinical and technical investment. The greatest cost was implementation and testing. | VAC 62 (1.67) IVAC 35 (0.94) pVAP 14 (0.38) prVAP 10 (0.27) |
| Stevens et al,8 2014 | Validation of automated algorithm to detect VAE | Retrospective cohort analysis at a tertiary care hospital studying adult medical and surgical ICU patients | 426 patients validated by human abstractor. The electronic algorithm had a net sensitivity of 93.5% and specificity of 100% and accuracy of 99.5% compared with the human reviewer. Algorithm took 0.16 s per patient compared with 17–30 min per patient for the human reviewer. | VAC 19 (4.46) IVAC 3 (0.70) pVAC 6 (1.41) prVAC 0 (0) |
| McMullen et al,15 2015 | Retrospective evaluation with an automated algorithm compared with prospective clinical evaluation | Retrospective review and prospective cohort study at an academic medical center studying adult medical and surgical patients | 1209 patients evaluated with both automated algorithms and prospective clinical evaluation showed good agreement between clinicians using NHSN definitions and automated algorithms to detect VAEs. | VAC 37 (3.06) IVAC 19 (1.57) pVAC 8 (0.66) prVAC 5 (0.41) |
| Nuckchady et al,19 2015 | Accuracy of automated surveillance techniques for VAE | Retrospective review at an academic medical center studying MV patients >48 h | 192 patients identified by billing records who were analyzed with an automated algorithm to detect VAE per the NHSN definitions. Sensitivity, specificity, PPV, and NPV all >93% and reduced the time spent on detection of VAEs by >90%. | VAC 44 (22.92) IVAC 22 (11.46) pVAC 12 (6.25) prVAC 1 (0.52) |
| Zhu et al,20 2015 | Impact of VAE surveillance on clinical outcomes | Multi-institutional prospective cohort study on adult medical and surgical patients | 2356 patients received MV for 8438 d. Compared with patients without VAEs those with VACs had longer ICU LOS (6.2 d), longer duration of MV (7.7 d), and higher hospital mortality rate (50% vs 27.3%). Patients with IVAC had longer duration of MV and increased LOS compared with those with VAC alone. | VAC 94 (3.99) IVAC 31 (1.32) pVAC 16 (0.68) prVAP 0 (0) |
| Klompas et al,3 2011 | Validation of a novel surveillance paradigm to identify complications of MV | Retrospective review at an academic medical center studying adult medical and surgical patients | 597 patients evaluated showing that VAP and VAC patients had prolonged intubation, ICU LOS, and hospital LOS. VAC was associated with increased mortality, but not VAP. | VAC 137 (23) VAP 56 (9.3)a |
| Prospero et al,21 2012 | Characterizing VAE rates | Prospective cohort study at an academic medical center studying adult MV patients | 127 patients analyzed with a significant increase in days of MV, ICU LOS, and mortality for those patients diagnosed with VAC compared VAC-negative patients. VAP patients showed increased mortality compared with non-VAP patients. | VAC 19 (15) VAP 2 (1.57)a |
| Muscedere et al,4 2013 | Impact and preventability of VAC | Multi-institutional retrospective study on adult medical, surgical, and trauma ICU patients | 1320 patients studied with an agreement between clinically diagnosed VAP and VAC of 0.18, VAP and IVAC and 0.19. Patients with VAC or IVAC had more ventilator days, hospital days, antibiotic days, and higher hospital mortality. Although the agreement between clinically diagnosed VAP and VAC/IVAC is poor these NHSN criteria define potential useful quality indicators. | VAC 139 (10.53) IVAC 65 (4.92) VAP 26 (1.97)a |
| Dessap et al,22 2014 | Evaluation of depletive fluid management on rates of VAC | Multi-institutional randomized controlled trial of adult ICU patients | 304 patients evaluated from the B-type Natriuretic Peptide for the Fluid Management of Weaning (BMC) trial showing that depletive fluid management was associated with significantly reduced rates of VAC and VAP. | VAC 40 (13.16) VAP 17 (5.60)a |
Abbreviations: MV, mechanical ventilation; NPV, negative predictive value; PPV, positive predictive value; prVAP, probable VAP; pVAP, possible VAP.
VAP diagnosis made using additional clinical findings not specified in the NHSN surveillance guidelines.
In 2014, a group from the Netherlands published results from a prospective evaluation of the EMR from patients in two tertiary care centers from 2011 to 2012. This study reviewed ventilator settings, microbiology, and clinical data from 2080 patients who were mechanically ventilated for greater than 2 consecutive days. In addition to screening the EMR with the VAE surveillance criteria, the authors also developed clinical definition of prospective VAP, which used the VAE criteria for possible-VAP and the radiographic evidence from PNU1. The results of this study demonstrated that patients with VAC diagnosis have an increased mortality compared with those without VAC. This was similar to the patients diagnosed with prospective VAP. VAE surveillance criteria only detected 32% of prospectively diagnosed VAP.6
The Dutch study was the first to demonstrate the complexity involved in applying the surveillance criteria to EMR data. The authors identified that using the minimum Fio 2 and PEEP settings recorded daily in the respiratory flow-rows frequently identified the values recorded during the spontaneous breathing trial (SBT) and were not the true baseline levels of oxygen support. Transitions from the Fio 2 and PEEP during SBT back to the baseline levels when patient completed their trial but were not extubated resulted in an erroneous identification of VAE. The authors realized that establishing a surveillance programs that does not accommodate for similar variations in support will likely result in overestimating patients with respiratory deterioration and can significantly effect VAE reporting.
Two large retrospective studies were published in 2014 that used automated data analysis to review the EMR of patients, based on VAE surveillance criteria, and provide descriptive epidemiology of VAE. The first study reviewed the charts of 20,356 mechanically ventilated patients and found a low overall rate of VAC (5.6%) with low rates of IVAC (2.1%) and even lower rates of possible- (0.7%) and probable-VAP (0.6%). The median day to the onset of VAC was 6, and was more common in medical, surgical, and thoracic units.7 The other study reviewed the records from 10,998 patients and identified a lower overall rate of VAE (3%). This study focused on the reliability of automated data evaluation compared with manual extraction. The automated method of identifying VAE was more reliable than using a human abstractor. Both studies demonstrated patients with VAE had longer duration of mechanical ventilation, hospital LOS, and mortality compared with patients without VAE, and suggest in their discussions that the future performance improvement initiatives should be targeted on the prevention of VAE rather than VAP.8
One of the important selling points of the new VAE surveillance criteria is that it is based on objectivity and eliminates subjective criteria, thus reducing variability and increases validity of data and outcomes. Several recently published studies have argued that the nested definitions of possible-VAP and probable-VAP underperform, because they do not account for radiographic findings common in patients with VAP.9, 10 Lilly and colleagues9 reviewed the charts of 8408 mechanically ventilated patients and identified a total of 83 incidences of clinical VAP. In this group, 27 patients met criteria for VAC, which means most patients (n = 56) that were diagnosed with clinical pneumonia in this study did not have any evidence of increased oxygen requirements. An even smaller number of patients in this cohort had either qualitative (possible-VAP, n = 18) or quantitative (probable-VAP, n = 4) microbiologic data supporting the VAP diagnosis. Another study by Chang and colleagues10 demonstrated similar results; only one-third of the 165 episodes of clinically diagnosed VAP would have met VAC criteria with even fewer possible-VAP (12.1%) and probable-VAP (1.2%). Although this study demonstrated that patients with VAC had higher mortality than patients with clinical VAP, it is one of the few that demonstrates patients with possible- and probable-VAP have increased hospital mortality compared with the patients with clinical VAP.
Two studies published in 2015 attempted to better characterize VAC events. One study prospectively evaluated all patients admitted to an ICU and mechanically ventilated 2 or more days using the VAC surveillance criteria during a 12-month period from 2013 to 2014 in large teaching hospital. They identified 67 VACs in 1209 patients. They substantiated the finding that the mortality in patients with VAC was significantly higher than patients without VAC, and characterized the most common causes of VAC: IVAC (50.7%), ARDS (16.4%), pulmonary edema (14.9%), and atelectasis (9.0%). Probable-VAP and possible-VAP accounted for 44.1% and 17.6% of the IVACs, whereas the remaining cases did not have microbiologic data to delineate the cause. This study also attempted to determine which cases of VAC were preventable. Two investigators reviewed each case and identified cases where medical error contributed to the respiratory complication or whether the VAC was a result of the patient’s underlying disease process. They determined that 25 (37.3%) were potentially preventable events.11 The other study was a large retrospective review of 16 years of data from a French national ICU database (OUTCOMEREA). This study evaluated the data for patients who were intubated a minimum of 5 consecutive days. They identified 3028 patients, 77% of them had VAC and 29% had IVAC. Patients with either possible-VAP or probable-VAP accounted for only 14.5% of VAC events and only 27.6% of the IVAC events. Other significant causes of VAC and IVAC were attributed to other nosocomial infections, failed extubation requiring reintubation, derecruitment during patient transport, and overresuscitation. The authors attribute the high rate of VAC to the study group; patients that require mechanical ventilation a minimum of 5 days are higher risk for adverse events. Both of these studies are important because they begin to characterize the frequent causes of respiratory deterioration in mechanically ventilated patients. By understanding how VACs occur clinicians can identify methods to prevent future events.12
These 15 studies discussed in this review are concisely summarized in Table 2. The studies are organized in alphabetical order by first author for each year published. The study design, patient population, and type of institution or database used and a succinct description of the each study findings are included. Additionally, the rates of the different categories of VAE identified for each study are presented. Four studies used the clinical definition of pneumonia to calculate their rate of VAP and are included in the table for comparison. The cumulative number of patients that were diagnosed with VAC, iVAC, possible- and probable-VAP from the studies that evaluated patients using the new NHSN VAE criteria is presented in Fig. 2. The sizes of the circles are proportional to the percentage of patients that meet the different categories of VAE. The total number of patients evaluated using VAE criteria was 33,276. VAC was diagnosed in 1808 (5.4%) of the patients and of these patients 738 (2.2%) met the iVAC criteria. The total number of patients with either possible- or probable-VAP was 458, representing 1.5% of all mechanically ventilated patients.
A recent report compared VAP rates by the NHSN and the Medicare Patient Safety Monitoring System (MPSMS).13 Between 2006 and 2012, the rate of VAP per 1000 ventilator days reported by NHSN decreased from 3.1 to 0.9 (71% decline) in medical ICUs and 5.2 to 2.0 (62% decline) in surgical ICUs. In contrast, MPSMS VAP rates were stable over time: 10.8% during 2005 to 2006; 7.5% from 2007 to 2009; 10.4% from 2010 to 2011; and 10.2% from 2012 to 2013. This significant dichotomy between VAP rates reported to NHSN and measured in MPSMS is of significant concern, and is likely related to the different VAP definitions used by each group. Future studies must address this concern.
New definition, new focus
Although the NHSN surveillance definition of VAE has been shown to be an effective method of characterizing respiratory deterioration, several areas may require further clarification. The current definition requires a 2-day period of stability with subsequent sustained increases in either PEEP or Fio 2. This requirement necessarily eliminates any patient with a primary process that adversely affects the pulmonary status at the time of admission to the ICU. Stoeppel and colleagues14 demonstrated a large number of surgery patients (77%) were excluded from meeting VAE criteria, because they never had a period of stability before their respiratory deterioration. In these patients, this deterioration was an evolution of their inciting process. Similarly, several studies have discussed that VAE criteria does not account for airway pressure release ventilation or similar modes that do not use PEEP, which results in underreporting the number of patients that would otherwise have VAE.11, 14, 15 Potential solutions that would accommodate the use of these modes would be to include increasing requirements of mean airway pressure or potentially using changes in the P/ ratio. Although the goal of tracking VAE may be to track and trend preventable causes of respiratory failure; it is important for the surveillance criteria to have the broadest view including modes that directly affect the mean airway pressure so as to capture all events so that even these patterns of care may be analyzed for possible improvement.
Several studies in this article have criticized the new VAE surveillance definition for underidentifying clinical pneumonia. In these studies, the possible-VAP and probable-VAP identified using the VAE surveillance criteria were compared with clinical pneumonias. Frequently the clinical pneumonias were characterized using a hybrid of the objective probable-/possible-VAP criteria but allows the inclusion of the subjective radiographic criteria of PNU1. The comparisons have demonstrated that using the objective VAE surveillance criteria to identify pneumonia correlates poorly with the diagnosis of clinical pneumonia. Nearly all of the studies demonstrated that ventilator days, ICU LOS, and hospital LOS correlates more closely with VAC compared with clinical VAP. These findings support the decision of NHSN to place emphasis on identifying the broader range of VAEs even at the expense of missing some clinical VAPs.
As the focus of prevention shifts from VAP to VAC, more studies need to be designed to identify methods to reduce the duration of mechanical ventilation and subsequently the opportunity for VAC to occur. The Wake Up and Breathe Collaborative study evaluated how adherence to spontaneous awakening trials (SATs) and SBTs affects the incidence of VAE and VAP.16 This collaborative was really an education initiative that prospectively tracked the incidence of daily SAT, SBT, and VAEs in participating ICUs over 2 years from 2011 to 2013. Each participating ICU designated a physician, nurse, and respiratory therapist to champion the education and data collection. The results of the study were impressive. Initial compliance with SAT and SBT in most centers was low but through the course of the study rates of daily SAT and SBT significantly improved while the rate of VAC and IVAC per episode of mechanical ventilation was significantly decreased. During the study, the duration of mechanical ventilation decreased by 2.4 days, ICU LOS by 3.0 days, and hospital LOS by 6.3 days. There were no changes in the rate of hospital morality or VAP. Not surprisingly, the rate of self-extubations per episode of mechanical ventilation increased but there was no change in the rate of reintubation within 24 hours. This paper brings to the forefront VAP is not an adequate surrogate for quality.
Dr Klompas, the architect of the new criteria, outlines future efforts directed at minimizing VAE in a recent review.16 He summarizes several strategies to reduce or prevent VAEs. Intrinsic to the efforts are approaches that minimize the duration of mechanic ventilation and coincide with the Society of Critical Care Medicine’s initiative Awakening, Breathing, Coordination, Delirium monitoring/management and Early exercise/mobility bundle. This bundle has been shown to improve patient outcomes by eliminating sedative and narcotic infusions, decreasing delirium, increasing patient participation in SATs and SBTs, and promoting early participation with occupational and physical therapy. All of these efforts, in turn, reduce duration of mechanical ventilation, and translate to decreased incidence of VAE.16 Other efforts that may play a role in reducing VAE include using lung-protective ventilation and preventing overaggressive fluid or blood product resuscitation. Although there have not been any studies to date demonstrating the effects of these strategies on VAE, each has been shown to mitigate lung injury, which may lead to deterioration of pulmonary function in ventilated patients.
Future directions
The application of NHSN Surveillance Definition of VAE to surgical patients has not been tested. Many of the publications reviewed in this article were performed in mixed populations and so how the criteria apply to surgical patients is unclear. Many surgical patients require mechanical ventilation secondary to another insult or injury and not from a primary pulmonary process as in many of the medicine patients. There is a bimodal distribution of pulmonary dysfunction in surgical patients. Some patients have early progressively worsening respiratory failure as a consequence of their initial injury or process and other patients develop late pulmonary dysfunction as a consequence of pneumonia or aggressive resuscitation secondary to the treatment of their primary event. The VAE criteria do not readily identify the first group because they never have a period of stability, but readily identify the other group. The appropriate resuscitation of a surgical patient with abdominal sepsis or the trauma patient with acute hemorrhage may secondarily exacerbate pulmonary dysfunction. Future studies that investigate VAE in these subsets of patients may help identify new end points for resuscitation that minimize pulmonary dysfunction while providing correction of acidosis and restoration of end-target organ perfusion.
Summary
The new NHSN definition for VAE replaces the previous PNU1 definition of pneumonia. The VAE criteria stratify respiratory compromise of ventilated patient into a tiered system that includes VAC, iVAC, possible-VAP, and probable-VAP. Each classification has defined objective criteria that eliminate the subjectivity of previous PNU1 definition. The implementation of VAE criteria is intended to better characterize and quantitate the broader causes of respiratory deterioration in ventilated patients, and to shift the focus away from VAP as the only relevant ventilator-associated complication. This new definition eliminates radiographic finding as a component of the definition and, as a result, may underestimate the rate of clinical pneumonia. Instead these categories are intended to also capture all of the other noninfectious causes of respiratory compromise in ventilated patients. These objective criteria facilitate the automated collection of data from the EMR and reduce variability in reporting, which allows for easier comparison of data within institutions, between health care systems, and even across international borders. The current definition has been well studied in medicine patients but has not been well vetted in the surgical population. Additionally, these criteria may need to be amended to account for nonconventional modes of ventilation, such as airway pressure release ventilation. Using the new VAE surveillance definition serves as a quality indicator proxy. Adherence to the best practices standards of daily awakening and breathing trials and initiating early mobilization reduces the duration of ventilator dependence and the subsequent incidence of all classifications of VAE.
Footnotes
Disclosure Statement: This paper comprises original work created by the authors and they have no commercial or financial conflicts of interest to disclose.
References
- 1.Van Vught L.A., Klowenberg P.M., Spitoni C., for the MARS Consortium Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315(14):1469–1479. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 2.Magill S.S., Klompas M., Balk R. Developing a new, national approach to surveillance for ventilator-associated events. Am J Crit Care. 2013;22(6):469–473. doi: 10.4037/ajcc2013893. [DOI] [PubMed] [Google Scholar]
- 3.Klompas M., Khan Y., Kleinman K. Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One. 2011;6:e18062. doi: 10.1371/journal.pone.0018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muscedere J., Sinuff T., Heyland D.K., on behalf of for the Canadian Critical Care Trials Group The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest. 2013;144(5):1453–1460. doi: 10.1378/chest.13-0853. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi Y., Morisawa K., Klompas M. Towards improved surveillance: the impact of ventilator-associated complications (VAC) on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis. 2012;56(4):471–477. doi: 10.1093/cid/cis926. [DOI] [PubMed] [Google Scholar]
- 6.Klouwenberg P.M., van Mourik M.S., Ong D.S. Electronic implementation of a novel surveillance paradigm for ventilator-associated events: feasibility and validation. Am J Respir Crit Care Med. 2014;189(8):947–955. doi: 10.1164/rccm.201307-1376OC. [DOI] [PubMed] [Google Scholar]
- 7.Klompas M., Kleinman K., Murphy M.V., for the CDC Prevention Epicenters Program Descriptive epidemiology and attributable morbidity of ventilator-associated events. Infect Control Hosp Epidemiol. 2014;35(5):502–510. doi: 10.1086/675834. [DOI] [PubMed] [Google Scholar]
- 8.Stevens J.P., Silva G., Gillis J. Automated surveillance for ventilator-associated events. Chest. 2014;146(6):1612–1618. doi: 10.1378/chest.13-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lilly C.M., Landry K.E., Sood R.N. Prevalence and test characteristics of national health safety network ventilator-associated events. Crit Care Med. 2014;42(9):2019–2028. doi: 10.1097/CCM.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 10.Chang H.C., Chen C.M., Kung S.C. Differences between novel and conventional surveillance paradigms of ventilator-associated pneumonia. Am J Infect Control. 2015;43(2):133–136. doi: 10.1016/j.ajic.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Boyer A.F., Schoenberg N., Babcock H. A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest. 2015;147(1):68–81. doi: 10.1378/chest.14-0544. [DOI] [PubMed] [Google Scholar]
- 12.Boudma L., Sonneville R., Garrouste-Orgeas M. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015;43(9):1798–1806. doi: 10.1097/CCM.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 13.Metersky M.L., Wang Y., Klompas M. Trend in ventilator-associated pneumonia rates between 2005 and 2013. JAMA. 2016;316(22):2427–2429. doi: 10.1001/jama.2016.16226. [DOI] [PubMed] [Google Scholar]
- 14.Stoeppel C.M., Eriksson E.A., Hawkins K. Applicability of the National Healthcare Safety Network’s surveillance definition of ventilator-associated events in the surgical intensive care unit: a 1-year review. J Trauma Acute Care Surg. 2014;77(6):934–937. doi: 10.1097/TA.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 15.McMullen K.M., Boyer A.F., Schoenberg N. Surveillance versus clinical adjudication: differences persist with new ventilator-associated event definition. Am J Infect Control. 2015;43(6):589–591. doi: 10.1016/j.ajic.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Klompas M., Anderson D., Trick W. The preventability of ventilator-associated events. The CDC prevention epicenters wake up and breathe collaborative. Am J Respir Crit Care Med. 2015;191(3):292–301. doi: 10.1164/rccm.201407-1394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis S.C., Li L., Murphy M.V. Risk factors for ventilator-associated events: a case-control multivariable analysis. Crit Care Med. 2014;42(8):1839–1848. doi: 10.1097/CCM.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resetar E., McMullen K.M., Russo A.J. Development, implementation and use of electronic surveillance for ventilator-associated events (VAE) in adults. AMIA Annu Symp Proc. 2014;2014:1010–1017. [PMC free article] [PubMed] [Google Scholar]
- 19.Nuckchady D., Heckman M.G., Diehl N.N. Assessment of an automated surveillance system for detection of initial ventilator-associated events. Am J Infect Control. 2015;43(10):1119–1121. doi: 10.1016/j.ajic.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Zhu S., Cai L., Ma C. The clinical impact of ventilator-associated events: a prospective multi-center surveillance study. Infect Control Hosp Epidemiol. 2015;36(12):1388–1395. doi: 10.1017/ice.2015.200. [DOI] [PubMed] [Google Scholar]
- 21.Prospero E., Illuminati D., Marigliano A. Learning from Galileo: ventilator-associated pneumonia surveillance. Am J Respir Crit Care Med. 2012;186(12):1308–1309. doi: 10.1164/ajrccm.186.12.1308. [DOI] [PubMed] [Google Scholar]
- 22.Dessap A.M., Katsahian S., Roche-Campo F. Ventilator-associated pneumonia during weaning from mechanical ventilation: role of fluid management. Chest. 2014;146(1):58–65. doi: 10.1378/chest.13-2564. [DOI] [PubMed] [Google Scholar]