Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) ORF2 contains an internal ORF that codes for a small non-glycosylated protein known as 2b. Previous work had identified the presence of a 10 kDa 2b protein in virus-infected cells and the induction of an anti-2b response in PRRSV-infected pigs, as well as a possible association of 2b with the virion (Wu et al., 2001, Virology 287:183–191). In this study, we utilized two experimental approaches, including the use of a 2b peptide-specific monoclonal antibody, to demonstrate that the PRRSV 2b protein is an integral component of the PRRSV virion. This study suggests that 2b in PRRSV is similar to the E protein in EAV and forms a minor structural component of the virion.
Keywords: Arterivirus, Porcine reproductive and respiratory syndrome virus (PRRSV), 2b protein
Porcine reproductive and respiratory syndrome virus (PRRSV), a member of the family Arteriviridae, causes reproductive disorders in swine breeding stock and severe respiratory disease in neonates (see Rossow, 1998, for review). Other members of the arterivirus family include lactate dehydrogenase-elevating virus (LDV) of mice, equine arteritis virus (EAV), and simian hemorrhagic fever virus (SHFV; for review see Plagemann, 1996, Snijder and Meulenberg, 1998). Arteriviridae, along with the Coronaviridae and the recently established Roniviridae family, are placed in a relatively new order, Nidovirales (Cavanagh, 1997, Cowley et al., 2000, Cowley et al., 2002).
PRRSV is an enveloped, positive polarity, non-segmented, single-stranded RNA virus possessing a 15 kb genome containing at least 9 open reading frames (ORFs) as well as two untranslated regions located on the 5′ and 3′ ends of the genome. The principal non-structural proteins, encoded by ORF1a and ORF1b, have replicase-associated activities. The 3′ end of the genome codes for at least seven structural proteins. The major structural proteins, GP5, matrix (M), and nucleocapsid (N) are derived from ORFs 5, 6, and 7, respectively (Benfield et al., 1992, Meulenberg et al., 1995, Bautista et al., 1996, Dea et al., 2000). The 15 kDa N protein accounts for approximately 20–40% of the total protein in the virion (Bautista et al., 1996). It forms the principal component of the nucleocapsid, and also localizes to the nucleolus during replication in cells (Rowland et al., 1999). The M protein is a non-glycosylated, triple membrane-spanning, integral envelope protein, which is disulfide bonded to the major envelope glycoprotein, GP5 (Mardassi et al., 1996). GP5 participates in the interaction with the viral receptor on macrophages and monkey kidney cell lines and is the principal target of neutralizing antibody (Vanderheijden et al., 2003, Plagemann et al., 2002). GP2a, GP3, and GP4 of PRRSV are considered minor structural proteins but their functions remain unclear (Drew et al., 1995, Meulenberg et al., 1995, Meulenberg and Petersen-den Besten, 1996, Wieczorek-Krohmer et al., 1996).
In 1999, Snijder et al. characterized a new structural protein in EAV and named it E, based on its structural similarities to the coronavirus envelope (E) protein (Godet et al., 1992, Yu et al., 1994, Raamsman et al., 2000). In EAV, the E protein is encoded by ORF2a and is found associated with virions prepared by sucrose-gradient centrifugation (Snijder et al., 1999). Inactivation of the E gene in EAV does not affect the assembly of viral particles but blocks the formation of infectious virions (Snijder et al., 1999, Wieringa et al., 2004). One potential role for E is in the integration of minor structural proteins into the mature virion. Minor structural proteins of EAV include GP2b, GP3 and GP4, encoded by ORF2b, ORF3 and ORF4, respectively (Wieringa et al., 2002). GP2b has been shown to associate covalently with GP4, while GP3 links to GP4 to form a heterotrimeric complex on the surface of the virion, which has been suggested to be involved in viral entry (Wieringa et al., 2003b, Wieringa et al., 2003a). In the absence of the E gene, the incorporation of GP2b/GP3/GP4 into the virion is blocked and conversely, deletion of one of the minor proteins decreases the amount of E incorporated into virions (Wieringa et al., 2003b, Wieringa et al., 2004).
ORFs that code for proteins with characteristics similar to EAV E are found in most other arteriviruses (Snijder et al., 1999, Wu et al., 2001). However, Plagemann did not find evidence of an E-like protein in sucrose gradient-purified LDV (Plagemann, 2001). In previous work, we identified 2b, a 10 kD protein similar to EAV E, which is encoded by PRRSV ORF2b. Unlike the E protein of EAV, the translation of 2b is initiated two nucleotides downstream of the ORF2a start codon. The designation 2b is used to avoid confusion with the use of E by some to identify the major envelope glycoprotein of PRRSV, GP5 (Wu et al., 2001). A role for the 2b protein in the PRRSV virion and its structural morphology has not been determined. Even though PRRSV possesses proteins corresponding to GP2, GP3 and GP4, the formation of a heterotrimer has yet to be demonstrated experimentally. The 2b protein was found in preparations of infected cell lysates and in sucrose-gradient purified virus, while sera from pigs experimentally infected with PRRSV reacted with recombinant 2b protein expressed in baculovirus (Wu et al., 2001).
Our previous work, showing an association between PRRSV 2b and intact virus particles was based on locating the 2b protein in the same sucrose density fraction as infectious virus by Western immunoblot assay. However, these experiments could not rule out the possibility that the presence of 2b or another 10 kDa protein in the same fraction was a result of independent co-migration with the virions. Unlike Western immunoblotting, radioimmunoprecipitation (RIP) techniques using mAbs against specific PRRSV proteins can enhance the specific detection of viral proteins following infection and can be used to study interactions between molecules by co-precipitation properties.
We first examined [35S]-methionine/cysteine-labeled protein profiles in the sucrose gradient fractions that contained the highest concentrations of PRRSV virions. The sucrose-gradient-purified preparation yielded the expected major structural proteins, N (15 kDa), M (19 kDa) and GP5 (24–25 kDa) as well as small quality of a 10 kDa protein (Fig. 1A). The appearance of GP5 as a broad smear is consistent with its incorporation into virions as a glycoprotein. In order to demonstrate that the 2b protein was intimately associated with the PRRSV virion, we utilized a two-step purification process, consisting of sucrose-gradient purification of [35S]-labeled virus followed by incubation with monoclonal antibody (mAb) 2C12, which recognizes a matrix protein epitope exposed on the surface of the virus (Magar et al., 1997). The 2C12-virus complexes were immobilized onto protein A-sepharose CL-4B beads and washed extensively with TNE buffer. The radio-labeled matrix and other proteins included in the intact virions were analyzed by SDS-PAGE followed by autoradiography. In comparison to sucrose-gradient purified preparations, the second round of purification on immobilized 2C12 yielded similar protein profiles (Fig. 1A and B). To further confirm the identity of the radiolabeled bands as viral proteins, the immunoprecipitated preparation was dissociated in RIP buffer and immunoprecipitated a second time with high-titer immune serum from PRRSV-infected pigs (Wu et al., 2001). The result was the recovery of the three major viral proteins, N, M and GP5, and a small amount of the 10 kDa 2b protein (Fig. 1C). Collectively, these data indicate that the 10 kDa 2b protein is an integral, but minor, structural component of the PRRSV virion. In all experiments, the 10 kDa band was always much fainter than the 15 kDa N protein band. Given the fact that N and 2b are nearly the same size and possess a similar number of methionine and cysteine residues, we attribute this difference to a much larger number of N molecules incorporated into virions, as compared to 2b molecules. This result is consistent with the small quantities of E protein recovered from preparations of EAV and mouse hepatitis virus (Snijder et al., 1999, Raamsman et al., 2000, Wieringa et al., 2004).
Fig. 1.
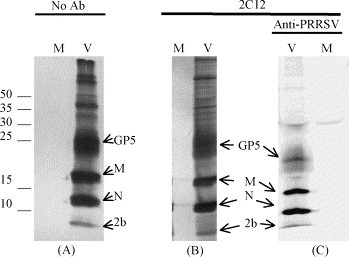
[35S]-labeled proteins in preparations of purified virions. (A) Autoradiograph of sucrose-gradient purified virus after SDS-PAGE separation of [35S]-labeled proteins on a 17% polyacrylamide gel. (B) SDS-PAGE after sucrose-gradient purification and immunoprecipitation of virions with 2C12 anti-matrix mAb. (C) Proteins from mAb 2C12-immunopurified virions followed by dissociation and immunoprecipitation with porcine immune serum. Experiments were performed on mock-infected (M) or virus-infected (V) MARC-145 cells at 3 days post-infection. The numbers indicate the location of molecular mass markers, in kDa. The letters identify the location of the major viral proteins, nucleocapsid (N), matrix (M) and the major envelope glycoprotein, GP5, as well as the 2b protein.
In order to confirm the identity of 2b, we prepared a 2b-specific mAb. A peptide containing the 14 carboxyl-terminal residues (amino acids 60–73) of PRRSV 2b from isolate SD-23983 was expressed in baculovirus as a GST fusion protein and injected into BALB/c mice. This peptide, predicted to be antigenic, reacted with immune sera from pigs experimentally infected with SD-23983 (Fig. 2 ). Hybridoma screening yielded a single mAb, 17C3, which was identified as an IgM. Specificity of the mAb for 2b and not GST was demonstrated by the capacity of 17C3 to strongly recognize the 10 kDa recombinant 2b protein by RIP but it did not recognize GST fused to another protein, GP5 (data not shown).
Fig. 2.
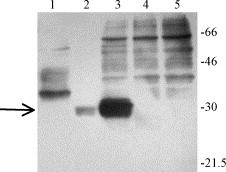
Recognition of baculovirus-expressed GST-ORF2b (60–73) products by immune pig serum. Reactivity of immune pig serum with recombinant GST-2b fusion protein is shown in Lane 1. Lanes 2 and 3 are GST-2b (60–73) fusion protein after and before purification on a glutathione-sepharose column, respectively. Lane 4 is GST alone and Lane 5 is a whole cell lysate prepared from uninfected insect cells. Western blots were probed with high titer anti-PRRSV pig immune serum. The same proteins demonstrated no antibody binding when probed with PRRSV-negative sera (data not shown). Molecular size markers, in kDa, are indicated on the right. The arrow identifies the predicted size for the GST-ORF2b (60–73) fusion protein.
The specificity of 17C3 for the 60–73 peptide was further assessed by a series of competitive ELISAs. Purified GST-2b (60–73) with a 6x histidine tag (6xhis) was coated onto nickel-covered ELISA plates and incubated with horseradish peroxidase conjugated 17C3 mAb (HRP-17C3). The antibody bound to the immobilized fusion protein but not to wells coated with GST. Since sera from PRRSV-infected pigs recognized the 2b peptide, the ability of immune pig serum to block recognition of 2b (60–73) by the 17C3 mAb was measured. GST-2b (60–73) coated wells were pre-incubated with dilutions of antisera from PRRSV-infected pigs. This treatment blocked the binding of 17C3. In the negative control, 17C3 blocking activity was absent in sera from PRRSV-negative pigs (data not shown). The 17C3 epitope was elucidated by another competitive ELISA in which a series of GST-bound peptide fragments were preincubated with 17C3 prior to incubation on wells coated with GST-2b (60–73). Two small peptides, 62–70 and 61–69, possessed 17C3 binding activity; whereas, peptides 60–68 and 63–71 failed to bind 17C3 in solution (Table 1 ). The overlapping region covered by peptides 62–70 and 61–69 is a peptide incorporating amino acids 62 through 69. These data demonstrate that 17C3 is specific for the 2b protein and indicate that the smallest linear epitope recognized by 17C3 is the 62–69 oligopeptide, PAIHPEQL.
Table 1.
Recognition of 2b peptide fragments by 17C3 antibody
| GST-fusion peptide used for competitiona | Amount of GST-peptide (μg/ml) required to inhibit 50% mAb bindingb | |
|---|---|---|
| 1 | GST alone | >0.50 |
| 2 | 66–72 | >0.50 |
| 3 | 65–73 | >0.50 |
| 4 | 64–72 | >0.50 |
| 5 | 63–71 | >0.50 |
| 6 | 62–70 | 0.20 |
| 7 | 61–69 | 0.12 |
| 8 | 60–68 | >0.50 |
Numbers refer to the peptide region in the 2b protein. Peptides were expressed as GST fusion proteins, expressed in E. coli and purified on glutathione-sepharose.
The indicated GST-peptides were incubated with HRP-17C3 prior to transfer to GST-(60–73)-coated plates. Values >0.50 indicate weak or no peptide binding.
The 17C3 mAb and anti-PRRSV immune serum from infected pigs was used to immunoprecipitate [S35]-labeled proteins from whole cell lysates and sucrose-gradient purified virions. The method for immunoprecipitation was the same as described for the experiments in Fig. 1, except that immunoprecipitation was performed after RIP buffer disruption of sucrose-gradient purified virions. Results, presented in Fig. 3 , show that 17C3 immunoprecipitated a 10 kDa protein from sucrose-gradient purified virus (Fig. 3B), but not from a mock-infected preparation. The same results were found when 17C3 was used to immunoprecipitate proteins from lysates prepared from PRRSV-infected cells (Fig. 3C). The 10 kDa protein immunoprecipitated with 17C3 corresponded in size to the 10 kDa protein found in sucrose-gradient purified virus after immunoprecipitation with PRRSV immune sera (compare Fig. 3A and B). In addition to 2b, a protein migrating at approximately 15 kDa was frequently recovered by 17C3 immunoprecipitation. Since this 15 kDa protein was not found in mock-infected cells and migrated the same as the N protein, we assume that it is a viral protein. This preliminary result suggested that 2b and N may interact or form a complex. Therefore, we performed several reciprocal immunoprecipitation experiments, attempting to co-precipitate 2b using anti-N mAbs. In all cases we were able to immunoprecipitate [S35]-labeled N from virions and from lysates of infected cells, but were not able to recover detectable quantities of 2b. One possibility is that 2b, in association with N, blocks recognition of N by these particular conformation-dependent mAbs. The anti-N mAbs may precipitate free N protein but not N bound to 2b. Another possibility is that N forms a non-specific interaction with the 2b-17C3 immune complex or with the protein A-sepharose used for immobilization. The N protein of EAV is known to specifically bind to formalin-fixed Staphylococcus aureus cells in immunoprecipitation studies (van Berlo et al., 1983). However, under the experimental conditions used in this study, we were not able to demonstrate precipitation of the PRRSV N protein with protein A sepharose CL-4B alone or when using it bound to another IgM isotype mAb directed against an unrelated protein (data not shown). The co-precipitation of the N protein with 2b appears to be an inconsistent event, possibly related to specific experimental conditions and possibly due to some non-covalent interaction between the N and 2b proteins.
Fig. 3.
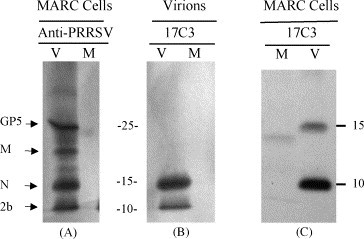
Immunoprecipitation of [35S]-labeled proteins from PRRSV infected cells and purified virions. (A) SDS-PAGE separation of MARC-145 cell lysates after immunoprecipitation with PRRSV-immune pig sera. (B) Immunoprecipitation of sucrose-gradient purified virus with 17C3. (C) Immunoprecipitation of MARC-145 cell lysates with 17C3. MARC-145 cells were PRRSV (V) or mock-infected (M) and labeled 24 h after infection with [35S]-methionine/cysteine. At 60 h after infection, cell monolayers and media were harvested for immunoprecipitation or sucrose-gradient purification followed by immunoprecipitation.
In the present study, two experimental approaches were utilized to demonstrate that the PRRSV 2b protein is an integral structural component of the PRRS virion. The first was the analysis of protein in preparations of highly purified virions obtained using sucrose-gradient centrifugation followed by immunopurification of whole virions with an anti-matrix protein mAb. The second approach involved the preparation and use of an anti-2b peptide mAb, 17C3, to immunoprecipitate 2b from sucrose-gradient purified virus. These experiments indicate that the 2b protein of PRRSV is an integral viral protein, similar to the E protein of EAV. An association of 2b with N is suggested, but has yet to be confirmed. Thus, the specific role for 2b in PRRSV replication and virion formation remains to be determined.
Acknowledgments
This work was supported by the USDA National Research Initiative Competitive Grants Program Grants 9502254, 9702754, 0002114 and 2003-35204-13704; National Science Foundation Grant OSR-9108773, South Dakota Future Fund and the South Dakota Agricultural Experiment Station. We thank Dr. Ronald Magar for the generous gift of monoclonal antibody 2C12.
Footnotes
This is South Dakota Experiment Station paper 3477.
References
- Bautista E.M., Meulenberg J.J., Choi C.S., Molitor T.W. Structural polypeptides of the American (VR-2332) strain of porcine reproductive and respiratory syndrome virus. Arch. Virol. 1996;141:1357–1365. doi: 10.1007/BF01718837. [DOI] [PubMed] [Google Scholar]
- Benfield D.A., Nelson E.A., Collins J.E., Harris L., Goyal S.M., Robison D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Spann K.M., Walker P.J. Gill-associated virus of Penaeus monodon prawns: an invertebrate virus with ORF1a and ORF1b genes related to arteri- and coronaviruses. J. Gen. Virol. 2000;81:1473–1484. doi: 10.1099/0022-1317-81-6-1473. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Walker P.J. Gill-associated nidovirus of Penaeus monodon prawns transcribes 3′-coterminal subgenomic mRNAs that do not possess 5′-leader sequences. J. Gen. Virol. 2002;83:927–935. doi: 10.1099/0022-1317-83-4-927. [DOI] [PubMed] [Google Scholar]
- Dea S., Gagnon C.A., Mardassi H., Pirzadeh B., Rogan D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: comparison of the North American and European isolates. Arch. Virol. 2000;145:659–688. doi: 10.1007/s007050050662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T.W., Meulenberg J.J., Sands J.J., Paton D.J. Production, characterization and reactivity of monoclonal antibodies to porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1995;76:1361–1369. doi: 10.1099/0022-1317-76-6-1361. [DOI] [PubMed] [Google Scholar]
- Godet M., L’Haridon R., Vautherot J.F., Laude H. TGEV corona virus ORF4 encodes a membrane protein that is incorporated into virions. Virology. 1992;188:666–675. doi: 10.1016/0042-6822(92)90521-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magar R., Larochelle R., Nelson E.A., Charreyre C. Differential reactivity of a monoclonal antibody directed to the membrane protein of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 1997;61:69–71. [PMC free article] [PubMed] [Google Scholar]
- Mardassi H., Massie B., Dea S. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5–7 of porcine reproductive and respiratory syndrome virus. Virology. 1996;221:98–112. doi: 10.1006/viro.1996.0356. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J., Petersen-den Besten A. Identification and characterization of a sixth structural protein of Lelystad virus: the glycoprotein GP2 encoded by ORF2 is incorporated in virus particles. Virology. 1996;225:44–51. doi: 10.1006/viro.1996.0573. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J., Petersen-den Besten A., De Kluyver E.P., Moormann R.J., Schaaper W.M., Wensvoort G. Characterization of proteins encoded by ORFs 2–7 of Lelystad virus. Virology. 1995;206:155–163. doi: 10.1016/S0042-6822(95)80030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P.G. Lactate dehydrogenase-elevating virus and related viruses. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology, third ed. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 1105–1120. [Google Scholar]
- Plagemann P.G. An ORF-2a protein is not present at a significant level in virions of the arterivirus lactate dehydrogenase-elevating virus. Virus. Res. 2001;74:47–52. doi: 10.1016/S0168-1702(00)00247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P.G., Rowland R.R., Faaberg K.S. The primary neutralization epitope of porcine reproductive and respiratory syndrome virus strain VR-2332 is located in the middle of the GP5 ectodomain. Arch. Virol. 2002;147:2327–2347. doi: 10.1007/s00705-002-0887-2. [DOI] [PubMed] [Google Scholar]
- Raamsman M.J., Locker J.K., de Hooge A., de Vries A.A., Griffiths G., Vennema H., Rottier P.J. Characterization of the coronavirus mouse hepatitis virus strain A59 small membrane protein E. J. Virol. 2000;74:2333–2342. doi: 10.1128/jvi.74.5.2333-2342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Rowland R.R., Kervin R., Kuckleburg C., Sperlich A., Benfield D.A. The localization of porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus of infected cells and identification of a potential nucleolar localization signal sequence. Virus. Res. 1999;64:1–12. doi: 10.1016/s0168-1702(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Meulenberg J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., van Tol H., Pedersen K.W., Raamsman M.J., de Vries A.A. Identification of a novel structural protein of arteriviruses. J. Virol. 1999;73:6335–6345. doi: 10.1128/jvi.73.8.6335-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo M.F., Zeegers J.J.W., Horzinek M.C., van der Zeijst B.A.M. Antigenic comparison of equine arteritis virus (EAV) and lactic dehydrogenase virus (LDV); binding of staphylococcal protein A to the nucleocapsid protein of EAV. Zentralbl. Veterinaermed. Reihe B. 1983;30:297–304. doi: 10.1111/j.1439-0450.1983.tb01846.x. [DOI] [PubMed] [Google Scholar]
- Vanderheijden N., Delputte P.L., Favoreel H.W., Vandekerckhove J., Damme J., Woensel P.A., Nauwynck H.J., Van Damme J., van Woensel P.A. Involvement of siaoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 2003;77:8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek-Krohmer M., Weiland F., Conzelmann K., Kohl D., Visser N., van Woensel P., Thiel H.J., Weiland E. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and US isolates. Vet. Microbiol. 1996;51:257–266. doi: 10.1016/0378-1135(96)00047-8. [DOI] [PubMed] [Google Scholar]
- Wieringa R., de Vries A.A., Raamsman M.J., Rottier P.J. Characterization of two new structural glycoproteins, GP(3) and GP(4), of equine arteritis virus. J. Virol. 2002;76:10829–10840. doi: 10.1128/JVI.76.21.10829-10840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa R., de Vries A.A., Rottier P.J. Formation of disulfide-linked complexes between the three minor envelope glycoproteins (GP2b, GP3, and GP4) of equine arteritis virus. J. Virol. 2003;77:6216–6226. doi: 10.1128/JVI.77.11.6216-6226.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa R., De Vries A.A., Post S.M., Rottier P.J. Intra- and intermolecular disulfide bonds of the GP2b glycoprotein of equine arteritis virus: relevance for virus assembly and infectivity. J. Virol. 2003;77:12996–13004. doi: 10.1128/JVI.77.24.12996-13004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa R., de Vries A.A., van der Meulen J., Godeke G.J., Onderwater J.J., van Tol H., Koerten H.K., Mommaas A.M., Snijder E.J., Rottier P.J. Structural protein requirements in equine arteritis virus assembly. J. Virol. 2004;78:13019–13027. doi: 10.1128/JVI.78.23.13019-13027.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.H., Fang Y., Farwell R., Steffen-Bien M., Rowland R.R., Christopher-Hennings J., Nelson E.A. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology. 2001;287:183–191. doi: 10.1006/viro.2001.1034. [DOI] [PubMed] [Google Scholar]
- Yu X., Bi W., Weiss S.R., Leibowitz J.L. Mouse hepatitis virus gene 5b protein is a new virion envelope protein. Virology. 1994;202:1018–1023. doi: 10.1006/viro.1994.1430. [DOI] [PubMed] [Google Scholar]


