Abstract
The microbiology of acute otitis media (AOM) is linked to the nasopharyngeal commensal flora. This respiratory ecosystem undergoes various selective pressures, such as antibiotic consumption and vaccine use. Socio-economic conditions also influence the bacterial composition of the nasopharynx. Streptococcus pneumoniae, non-encapsulated Haemophilus influenzae, Moraxella catarrhalis, and group A Streptococcus are the leading causes of bacterial AOM worldwide. This paper will discuss the causes and consequences of recent shifts in the underlying microbiology of AOM.
Keywords: Acute otitis media, Microbiology, Nasopharynx
1. Introduction
Otitis media (OM) is a very common childhood disease and a major concern for paediatricians. In a prospective, 7-year study performed in the USA, over three quarters (83%) of 498 children who completed the investigation experienced at least one episode of acute OM (AOM) by the age of 3 years and 45% had suffered from at least three episodes [1]. The Centers for Disease Control and Prevention has estimated that OM accounts for more than 20 million physician visits per annum in the USA [2]. Furthermore, in a recent multinational survey, paediatricians reported that they saw at least one patient with OM per day [3].
AOM is often preceded by a viral upper respiratory tract infection (URTI) – the most common infectious illness in the general population [4], and a very common illness in children. In a prospective, 1-year study following 201 children aged from 6 months to 3 years, a total of 1086 URTIs were recorded, with AOM reported in 341 (31%) children in the course of the viral infection, i.e. 1.7 episodes of AOM per child per year [5].
The underlying microbiology of infectious diseases is known to change in response to environmental factors, such as vaccination and antibiotic consumption. For example, the epidemiology of bacterial meningitis has changed in countries where Haemophilus influenzae type b [6], group C meningococcal, and pneumococcal conjugate vaccines have been introduced, with a dramatic reduction in the incidence of bacterial meningitis overall. However, non-vaccine serotypes of Streptococcus pneumoniae now account for a more significant proportion of the disease in the countries where the seven-valent pneumococcal conjugate vaccine (PCV7; Prevnar™/Prevenar™1 ) is widely used [7], [8]. Furthermore, S. pneumoniae isolates with reduced susceptibility to penicillin were recovered from human infections in the late 1960s in Australia and New Guinea [9]. Since then, penicillin non-susceptible S. pneumoniae strains have spread all over the world and their prevalence has dramatically increased in various countries [10]. Moreover, S. pneumoniae accumulated multiple resistance determinants in some strains and serotypes [11], [12], [13], and modified the epidemiological landscape in some regions, including the USA [14]. Although in most regions, penicillin remains active against S. pneumoniae despite increased minimal inhibitory concentrations and can be used safely to treat pneumococcal infections other than meningitis, some multiresistant strains have been described in infections such as AOM [15]. A recent report from the USA presented nine clinical failures in AOM due to S. pneumoniae resistant to amoxicillin, oral cephalosporins, macrolides, clindamycin, and co-trimoxazole, and required tube placement for drainage and the use of levofloxacin, a drug which is not licensed for paediatric use [16]. In another recent US study, an increased proportion of severe mastoiditis cases was observed, mostly due to multiresistant serotype 19A S. pneumoniae [14].
At the beginning of the 20th century, group A Streptococcus (GAS) was the most common pathogen leading to complications in AOM, but it is now rare in the Western world. A ‘new’ triad of AOM pathogens has emerged in the last century – S. pneumoniae, non-encapsulated H. influenzae (often called non-typable H. influenzae [NTHi]), and Moraxella catarrhalis – all of which are commensal bacteria found in the human nasopharynx. This review provides some insight into the microbiology of AOM in an era of antibiotic resistance and pneumococcal conjugate vaccine use.
2. Aetiology of AOM
AOM is a multipathogen disease, and can be caused by a number of different viruses and bacteria. Viruses alone are found in only 20% of cases, while co-infection with bacteria is seen in 65% of cases [17]. Among the viruses, Coronavirus, Respiratory Syncytial Virus, and Adenovirus are most commonly associated with AOM [5]. S. pneumoniae and H. influenzae are by far the most common bacterial pathogens in AOM, being recovered in up to 80% of cases. M. catarrhalis is usually the third most frequent bacterium isolated (3–20%) and GAS makes up 1–5% of cases, although the incidence of GAS infection differs between countries, depending on when the study was performed, and whether severe cases of AOM were included (Fig. 1 ) [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29].
Fig. 1.
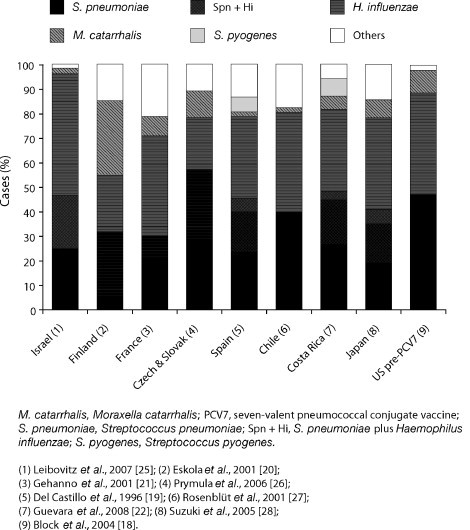
Worldwide distribution of the main otopathogens.
3. Clinical presentation and identification of otopathogens
The diagnosis of AOM is difficult as a number of symptoms, for example pain, fever, conjunctivitis, and headache, are shared with other infections of the upper respiratory tract. Furthermore, diagnosis in young children is hampered by the child's inability to describe their symptoms and the likelihood that they will be distressed and experiencing pain. For a clinical diagnosis of AOM, the key criteria that should be met are a history of acute onset of signs and symptoms (fever, distinct otalgia that precludes normal activity, or sleep), with signs of middle ear infection (a cloudy, bulging, or clearly immobile eardrum with red colouration of the eardrum and the presence of fluid in the middle ear or otorrhoea) [30], [31].
Some clinical signs have been associated with particular otopathogens (e.g. conjunctivitis is associated with H. influenzae, while more severe cases of AOM are more often caused by S. pneumoniae) [32], [33], [34]. However, accurate identification of underlying pathogens is not possible purely on clinical grounds. In the large, prospective Finnish trial, severe tympanic membrane findings (bulging tympanic membrane or spontaneous perforation) with concomitant high fever had a 53% positive predictive value and a 79% negative predictive value for a S. pneumoniae aetiology of the AOM episode. The presence of a purulent conjunctivitis gave a positive predictive value for H. influenzae AOM of 67% with an 86% negative predictive value. No useful predictors were found for M. catarrhalis AOM [34].
Even in the absence of definitive clinical signs for the identification of the underlying otopathogen(s), most guidelines do not advocate the systematic use of tympanocentesis, in which fluid is collected from behind the eardrum and analysed to identify the infectious organisms involved and perform antibiotic susceptibility testing. If an antibiotic treatment was to be prescribed, it would be chosen on empirical grounds based on local epidemiological data. However, in selected cases (antibiotic treatment failure and complicated AOM), it is essential to identify the causative otopathogen accurately and determine its antibiotic susceptibility. Tympanocentesis will, therefore, be recommended in order to ensure the most effective treatment course [31], [35], [36].
4. Incidence of AOM – microbiological patterns
All-cause AOM incidence peaks between the ages of 6 and 12 months, with one study reporting a peak incidence at 12 months of 17.8 AOM events/100 child months (Fig. 2 ) [23]. In this study, NTHi showed a distinct pattern of incidence compared with S. pneumoniae and M. catarrhalis. There was an increase in the incidence of NTHi AOM after the age of 15 months, peaking at 19 months (6.4 AOM events/100 child months). Moreover, H. influenzae was associated with recurrent AOM. It was recovered in middle ear fluid (MEF) in 12% of first AOM episodes compared with 25% of all subsequent OM episodes, and when only the first AOM episodes were considered, no peak in the incidence of H. influenzae AOM could be demonstrated in children over 1 year of age.
Fig. 2.
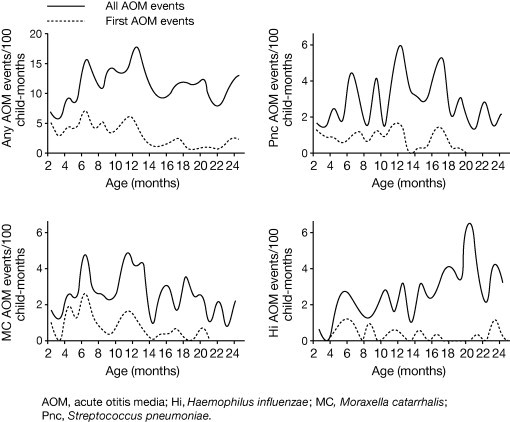
Age-specific incidence rates of AOM [23]. Reproduced with permission from Kilpi et al. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr Infect Dis J 2001;20:654-62.
5. Complications arising from AOM – impact of otopathogens
Despite the use and availability of antibiotics and appropriate medical access, AOM can often lead to recurrences and, in rare cases, severe intratemporal (facial paralysis, labyrinthitis, and acute petrosistis, which are extremely uncommon) and intracranial complications, such as mastoiditis, meningitis, intracranial abscesses, and sinus thrombosis. Although mastoiditis has become infrequent in industrialized countries (incidences from 0.3 to 6/100,000 child-year) [37], [38], [39], it is still a common belief that antibiotic treatment should be prescribed in AOM to prevent its occurrence. The prevention of mastoiditis by systematic antibiotic treatment of AOM has never been established [40], [41]. Many factors can account for variations in the incidence of mastoiditis in different countries: socio-economic and living conditions, antibiotic prescribing rates, exhaustiveness of the epidemiological surveillance systems, and differences in complication rates by pathogens [29], [38], [42]. Indeed, not all bacterial otopathogens have the same propensity to cause complications of AOM. GAS is associated with the most frequent and severe complications, such as mastoiditis (Table 1 ), while severe complications of H. influenzae are uncommon and those of M. catarrhalis infection are rare [29].
Table 1.
The risk for development of mastoiditis following AOM caused by different bacterial otopathogens [29].
| Bacterial otopathogen | Incidence | Cases of mastoiditis/1000 episodes of AOM (95% CI) |
|---|---|---|
| Group A Streptococcus | 4/346 | 11.6 (3.2–29.3) |
| Streptococcus pneumoniae | 8/3651 | 2.2 (0.9–4.3) |
| Haemophilus influenzae | 1/3999 | 0.3 (0.0–1.4) |
| Moraxella catarrhalis | 0/394 | 0.0 (0.0–3.0) |
As previously discussed, evidence suggests that S. pneumoniae is more common in severe episodes of OM [33], while NTHi is more commonly associated with recurrent OM (ROM) (Fig. 2) [23], [43]. One recent study assessed the underlying microbiology of ROM (defined as three acute episodes in the previous 6 months or four in the past 12 months) and AOM treatment failure (defined as persisting signs and symptoms of AOM after ≥48 h of antibiotic therapy or within 30 days of completing an antibiotic treatment course) in US children following the widespread introduction of PCV7 in 2000 [43]. Although there was a slight increase in the proportion of S. pneumoniae isolates present during the 2005–2006 season, H. influenzae was the most frequently isolated pathogen (51% of all isolates across three respiratory seasons, 2003–2006) in this difficult-to-treat patient group during a time of increasing and widespread use of PCV7 (Fig. 3 ).
Fig. 3.
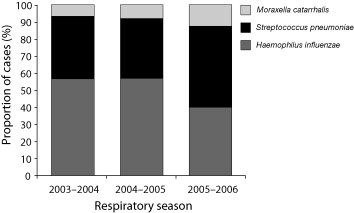
AOM pathogens identified in the PCV7 era using tympanocentesis in US children failing initial antibiotic therapy or with recurrent infection [43]. Pichichero et al. Clin Pediatr (Phila); June 16, 2008 [Epub ahead of print], © 2008 by SAGE. Reprinted by permission of SAGE publications.
6. Otopathogens and resistance to treatment
Difficulties encountered in the treatment of OM are not only due to the existence of antibiotic resistance in otopathogens, but are also attributable to the biofilm nature of bacterial OM infections.
6.1. Biofilms
In natural environments, the majority of bacteria exist as a biofilm (a structured community of microorganisms embedded within a polymeric matrix that is attached to an inert or living surface) rather than in a planktonic state. In contrast to planktonic bacteria, biofilm bacteria are characterized by slow rates of cell division and a tolerance to very high concentrations of antibiotics. Biofilm infections are, therefore, difficult to treat effectively with currently available antibiotic agents, which rely on the rapid metabolic and divisional rates of planktonic bacteria for their mode of action.
The presence of bacterial biofilms in OM was first suspected owing to the persistence of infection despite treatment with antibiotics and the absence of positive cell culture specimens. Definitive evidence for the biofilm nature of OM has been provided by a number of studies. For example, one study used polymerase chain reaction techniques to detect H. influenzae DNA and mRNA in MEF from children with chronic OM with effusion. The presence of the short-lived mRNA molecules, even in the absence of positive culture specimens, indicated the presence of viable bacteria in these specimens [44]. Additionally, the three major bacterial pathogens of OM have been proven to form biofilms in vitro and in vivo [45], [46], [47], [48], [49], while one study has reported the direct detection of bacterial biofilms on middle ear mucosa biopsies from children with chronic OM [50].
6.2. Antibiotic resistance
Antibiotic use results in the selection of strains resistant to antibiotics. This was demonstrated in vitro for S. pneumoniae by Alexander Fleming shortly after he discovered penicillin [51]. More recently, the correlation between antibiotic consumption and resistance was demonstrated in a European study comprising 26 countries. Outpatient antibiotic use was correlated with resistance for all antibiotic–pathogen combinations, and more specifically for S. pneumoniae [52]. The nasopharynx constitutes a wide reservoir where resistant bacteria (S. pneumoniae but also Streptococcus viridans, H. influenzae, and M. catarrhalis) can easily be selected whenever antibiotic selective pressure is applied [53].
Antibiotic resistance in S. pneumoniae and H. influenzae has become a major public health issue and a European Union priority for research and action. It is expected that the prevalence of chronic obstructive pulmonary disease will increase in the coming years in Europe, and both S. pneumoniae and H. influenzae have major infectious roles for this condition [54]. Similarly, the World Health Organization (WHO) has set antimicrobial resistance containment as a research priority, particularly regarding S. pneumoniae [55].
All three major otopathogens cause antibiotic resistance concerns. Penicillin and multidrug resistance in S. pneumoniae has already been described above. In Europe, dual erythromycin and penicillin non-susceptibility varies widely between countries, from less than 1% to more than 25% [56]. While amoxicillin resistance in M. catarrhalis is universally seen in approximately 90% of the strains, it is still a limited occurrence in NTHi (a mean of 13% β-lactamase production in one international study) [57]. However, some regions, such as France, the USA, Japan, and other Southern Asian regions have high rates of amoxicillin resistance in NTHi [58], [59]. Several mechanisms cause this resistance, the most common being β-lactamase production, which is detected in most laboratories. However, other resistance mechanisms are increasingly being described in France and Japan, which confer additional resistance to amoxicillin-clavulanate, cefuroxime, and sometimes to third-generation cephalosporins, and which are usually not investigated in routine microbiology [59], [60]. In a recent study conducted in Japan, only 44% of the NTHi strains isolated from children with URTIs were amoxicillin susceptible and 4% were β-lactamase producers; the others were also resistant to amoxicillin-clavulanate and to a various degree to cephalosporins [61].
7. Impact of vaccination on otopathogens
The introduction of conjugate vaccines that impact on the commensal flora creates ‘epidemiological niches’ for alternative potential pathogens that are not included in the vaccine. The introduction of PCV7 in the USA resulted in rapid shifts in the microbiology of OM [18], [43], [62], [63]. PCV7 vaccination was followed by rapid replacement with non-vaccine S. pneumoniae serotypes in the nasopharynx of vaccinated children and their siblings and, as a result, the proportion of AOM caused by vaccine serotypes has fallen and disease caused by non-vaccine serotypes and other pathogens, such as NTHi, has risen. For example, in one US study, significant increases in the percentage of AOM cases due to non-PCV7 pneumococcal serogroups occurred between 1999 and 2002 (from 12% to 32%, respectively; p < 0.01). However, no decline was observed in the penicillin non-susceptible S. pneumoniae strains [63]. As mentioned previously, multidrug-resistant replacement serotypes may arise [16], stressing the need for reduced antibiotic prescribing in order to maximize the benefits of the vaccine in eliminating the most prevalent antibiotic-resistant serotypes. Additionally, the trend for an increase in persistent AOM and AOM treatment failure attributable to H. influenzae observed from 2001 to 2003 in a single US study centre [62] was also reported in three US centres for the period 2003–2006 [43].
8. Conclusions
OM is a common disease that affects approximately three quarters of children before their third birthday. Currently, S. pneumoniae and NTHi are responsible for approximately 80% of all bacterial AOM cases, with S. pneumoniae generally causing more severe episodes, and NTHi responsible for recurrent episodes.
The underlying microbiology of OM, which is inherently linked to the nasopharyngeal commensal flora, is changing over time in response to various selective pressures, such as vaccine use and antibiotic consumption. Consequently, continuous monitoring of the changes in underlying OM microbiology is required in order to provide the most effective preventative and treatment strategies for combating this common and distressing childhood disease.
Conflict of interest
A.V. has received speaker fees from GlaxoSmithKline, Sanofi and Wyeth, consultant fees from GlaxoSmithKline and Wyeth, and research fees from Wyeth.
Acknowledgements
I would like to thank Philippe Lepage and Pierre Smeesters for the critical proofreading of this manuscript.
Footnotes
Prevnar/Prevenar are trademarks of Wyeth Lederle Vaccines S.A., Pearl River, NY, USA.
References
- 1.Teele D.W., Klein J.O., Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Pneumococcal disease. In: Epidemiology and prevention of vaccine-preventable diseases: “The Pink Book”. 10th ed.; 2007. p. 257–70.
- 3.Arguedas A, Lefebre C, Dean C, Maudrich J, Vercruysse S., Paediatricians’ awareness of and attitudes about otitis media: results of a multinational survey. Presented at 25th International Congress of Pediatrics, 25-30 August, Athens, Greece, 2007.
- 4.Woodwell DA, Cherry DK. Advance data from vital and health statistics. In: National ambulatory medical care survey: 2002 summary, No. 346. Hyattsville (MD): National Center for Health Statistics. http://www.cdc.gov/nchs/data/ad/ad346.pdf [accessed 28.07.2008].
- 5.Chonmaitree T., Revai K., Grady J.J., Clos A., Patel J.A., Nair S. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46(6):815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuchat A., Robinson K., Wenger J.D., Harrison L.H., Farley M., Reingold A.L. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337(14):970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 7.Hicks L.A., Harrison L.H., Flannery B., Hadler J.L., Schaffner W., Craig A.S. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196(9):1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 8.Nigrovic L.E., Kuppermann N., Malley R. Children with bacterial meningitis presenting to the emergency department during the pneumococcal conjugate vaccine era. Acad Emerg Med. 2008;15(6):522–528. doi: 10.1111/j.1553-2712.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- 9.Hansman D., Glasgow H., Sturt J., Devitt L., Douglas R. Increased resistance to penicillin of pneumococci isolated from man. N Engl J Med. 1971;284(4):175–177. doi: 10.1056/NEJM197101282840403. [DOI] [PubMed] [Google Scholar]
- 10.Baquero F. Pneumococcal resistance to beta-lactam antibiotics: a global geographic overview. Microb Drug Resist. 1995;1(2):115–120. doi: 10.1089/mdr.1995.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Geslin P., Buu-Hoi A., Fremaux A., Acar J.F. Antimicrobial resistance in Streptococcus pneumoniae: an epidemiological survey in France, 1970–1990. Clin Infect Dis. 1992;15(1):95–98. doi: 10.1093/clinids/15.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum P.C. Epidemiology and in vitro susceptibility of drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 1996;15(10):932–934. doi: 10.1097/00006454-199610000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Klugman K.P., McGee L. Resurgence of the multiresistant pneumococcus in the United States: a commentary. Pediatr Infect Dis J. 2007;26(6):473–474. doi: 10.1097/INF.0b013e3180517b8b. [DOI] [PubMed] [Google Scholar]
- 14.Ongkasuwan J., Valdez T.A., Hulten K.G., Mason E.O., Jr., Kaplan S.L. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics. 2008;122(1):34–39. doi: 10.1542/peds.2007-2703. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs M.R. Clinical significance of antimicrobial resistance in Streptococcus pneumoniae. S Afr Med J. 2007;97(11):1133–1140. [PubMed] [Google Scholar]
- 16.Pichichero M.E., Casey J.R. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298(15):1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 17.Segal N., Leibovitz E., Dagan R., Leiberman A. Acute otitis media-diagnosis and treatment in the era of antibiotic resistant organisms: updated clinical practice guidelines. Int J Pediatr Otorhinolaryngol. 2005;69(10):1311–1319. doi: 10.1016/j.ijporl.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Block S.L., Hedrick J., Harrison C.J. Community-wide vaccination with heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829–833. doi: 10.1097/01.inf.0000136868.91756.80. [DOI] [PubMed] [Google Scholar]
- 19.del Castillo F., Garcia-Perea A., Baquero-Artigao F. Bacteriology of acute otitis media in Spain: a prospective study based on tympanocentesis. Pediatr Infect Dis J. 1996;15(6):541–543. doi: 10.1097/00006454-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Eskola J., Kilpi T., Palmu A., Jokinen J., Haber M., Herva E. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 21.Gehanno P., Panajotopoulos A., Barry B., Nguyen L., Levy D., Bingen E. Microbiology of otitis media in the Paris, France, area from 1987 to 1997. Pediatr Infect Dis J. 2001;20(6):570–573. doi: 10.1097/00006454-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Guevara S., Soley C., Arguedas A., Porat N., Dagan R. Seasonal distribution of otitis media pathogens among Costa Rican children. Pediatr Infect Dis J. 2008;27(1):12–16. doi: 10.1097/INF.0b013e3181468643. [DOI] [PubMed] [Google Scholar]
- 23.Kilpi T., Herva E., Kaijalainen T., Syrjänen R., Takala A.K. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr Infect Dis J. 2001;20:654–662. doi: 10.1097/00006454-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Leibovitz E., Satran R., Piglansky L., Raiz S., Press J., Leiberman A. Can acute otitis media caused by Haemophilus influenzae be distinguished from that caused by Streptococcus pneumoniae? Pediatr Infect Dis J. 2003;22(6):509–515. doi: 10.1097/01.inf.0000069759.79176.e1. [DOI] [PubMed] [Google Scholar]
- 25.Leibovitz E., Asher E., Piglansky L., Givon-Lavi N., Satran R., Raiz S. Is bilateral acute otitis media clinically different than unilateral acute otitis media? Pediatr Infect Dis J. 2007;26(7):589–592. doi: 10.1097/INF.0b013e318060cc19. [DOI] [PubMed] [Google Scholar]
- 26.Prymula R., Peeters P., Chrobak V. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 27.Rosenblüt A., Santolaya M.E., Gonzáles P., Corbalán V., Avendanõ L.F., Martínez M.A. Bacterial and viral etiology of acute otitis media in Chilean children. Pediatr Infect Dis J. 2001;20:501–507. doi: 10.1097/00006454-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki A., Watanabe O., Okamoto M., Endo H., Yano H., Suetake M. Detection of human metapneumovirus from children with acute otitis media. Pediatr Infect Dis J. 2005;24(7):655–657. doi: 10.1097/01.inf.0000168755.01196.49. [DOI] [PubMed] [Google Scholar]
- 29.Segal N., Givon-Lavi N., Leibovitz E., Yagupsky P., Leiberman A., Dagan R. Acute otitis media caused by Streptococcus pyogenes in children. Clin Infect Dis. 2005;41(1):35–41. doi: 10.1086/430605. [DOI] [PubMed] [Google Scholar]
- 30.Klein J.O. Otitis media. Clin Infect Dis. 1994;19(5):823–833. doi: 10.1093/clinids/19.5.823. [DOI] [PubMed] [Google Scholar]
- 31.Subcommittee on Management of Acute Otitis Media Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 32.Coffey J.D., Jr. Otitis media in the practice of pediatrics. Bacteriological and clinical observations. Pediatrics. 1966;38(1):25–32. [PubMed] [Google Scholar]
- 33.Howie V.M., Ploussard J.H., Lester R.L., Jr. Otitis media: a clinical and bacteriological correlation. Pediatrics. 1970;45(1):29–35. [PubMed] [Google Scholar]
- 34.Palmu A.A., Jokinen J.T., Kaijalainen T., Leinonen M., Karma P., Kilpi T.M. Association of clinical signs and symptoms with pneumococcal acute otitis media by serotype – implications for vaccine effect. Clin Infect Dis. 2004;40(1):52–57. doi: 10.1086/426446. [DOI] [PubMed] [Google Scholar]
- 35.Paradise J.L. Commentary. Pediatrics. 1998;102(1 Pt 2):221–224. [PubMed] [Google Scholar]
- 36.Hoberman A., Paradise J.L. Acute otitis media: diagnosis and management in the year 2000. Pediatr Ann. 2000;29(10):609–620. doi: 10.3928/0090-4481-20001001-06. [DOI] [PubMed] [Google Scholar]
- 37.Leskinen K., Jero J. Complications of acute otitis media in children in southern Finland. Int J Pediatr Otorhinolaryngol. 2004;68(3):317–324. doi: 10.1016/j.ijporl.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Van Zuijlen D.A., Schilder A.G., van Balen F.A., Hoes A.W. National differences in incidence of acute mastoiditis: relationship to prescribing patterns of antibiotics for acute otitis media? Pediatr Infect Dis J. 2001;20(2):140–144. doi: 10.1097/00006454-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Katz A., Leibovitz E., Greenberg D., Raiz S., Greenwald-Maimon M., Leiberman A. Acute mastoiditis in Southern Israel: a twelve year retrospective study (1990 through 2001) Pediatr Infect Dis J. 2003;22(10):878–882. doi: 10.1097/01.inf.0000091292.24683.fc. [DOI] [PubMed] [Google Scholar]
- 40.Molstad S., Erntell M., Hanberger H., Melander E., Norman C., Skoog G. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis. 2008;8(2):125–132. doi: 10.1016/S1473-3099(08)70017-3. [DOI] [PubMed] [Google Scholar]
- 41.Cizman M., Srovin T., Pokorn M., Cad P.S., Battelino S. Analysis of the causes and consequences of decreased antibiotic consumption over the last 5 years in Slovenia. J Antimicrob Chemother. 2005;55(5):758–763. doi: 10.1093/jac/dki098. [DOI] [PubMed] [Google Scholar]
- 42.Petersen I., Johnson A.M., Islam A., Duckworth G., Livermore D.M., Hayward A.C. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi: 10.1136/bmj.39345.405243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichichero ME, Casey JR, Hoberman A, Schwartz R. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003–2006. Clin Pediatr (Phila); June 16, 2008 [Epub ahead of print]. [DOI] [PubMed]
- 44.Rayner M.G., Zhang Y., Gorry M.C., Chen Y., Post J.C., Ehrlich G.D. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279(4):296–299. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 45.Allegrucci M., Hu F.Z., Shen K., Hayes J., Ehrlich G.D., Post J.C. Phenotypic characterization of Streptococcus pneumoniae biofilm development. J Bacteriol. 2006;188(7):2325–2335. doi: 10.1128/JB.188.7.2325-2335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jurcisek J., Greiner L., Watanabe H., Zaleski A., Apicella M.A., Bakaletz L.O. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect Immun. 2005;73(6):3210–3218. doi: 10.1128/IAI.73.6.3210-3218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy T.F., Kirkham C. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2002;2:7. doi: 10.1186/1471-2180-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearson M.M., Laurence C.A., Guinn S.E., Hansen E.J. Biofilm formation by Moraxella catarrhalis in vitro: roles of the UspA1 adhesin and the Hag hemagglutinin. Infect Immun. 2006;74(3):1588–1596. doi: 10.1128/IAI.74.3.1588-1596.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starner T.D., Zhang N., Kim G., Apicella M.A., McCray P.B., Jr. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med. 2006;174(2):213–220. doi: 10.1164/rccm.200509-1459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall-Stoodley L., Hu F.Z., Gieseke A., Nistico L. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zajicek G. The antibiotic paradox. http://www.tribunes.com/tribune/art96/zaji.htm [accessed 26.09.2008].
- 52.Goossens H., Ferech M., Vander S.R., Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs M.R. Streptococcus pneumoniae: epidemiology and patterns of resistance. Am J Med. 2004;117:3S–15S. doi: 10.1016/j.amjmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 54.European Academies Science Advisory Council. Tackling antibacterial resistance in Europe. http://www.easac.eu/document.asp?id=31 [June 2007].
- 55.WHO Policy Perspectives on Medicines. Containing antimicrobial resistance. Geneva: World Health Organization. http://www.who.int/management/anmicrobialresistance.pdf [April 2005].
- 56.European Antimicrobial Resistance Surveillance System (EARSS). http://www.rivm.nl/earss/ [accessed 21.10.2008].
- 57.Beekmann S.E., Heilmann K.P., Richter S.S., Garcia-de-Lomas J., Doern G.V. Antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and group A beta-haemolytic streptococci in 2002–2003. Results of the multinational GRASP Surveillance Program. Int J Antimicrob Agents. 2005;25(2):148–156. doi: 10.1016/j.ijantimicag.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Doern G.V., Jones R.N., Pfaller M.A., Kugler K. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY antimicrobial Surveillance Program (United States and Canada, 1997) Antimicrob Agents Chemother. 1999;43(2):385–389. doi: 10.1128/aac.43.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamanaka N., Hotomi M., Billal D.S. Clinical bacteriology and immunology in acute otitis media in children. J Infect Chemother. 2008;14(3):180–187. doi: 10.1007/s10156-007-0599-3. [DOI] [PubMed] [Google Scholar]
- 60.Dabernat H., Seguy M., Faucon G., Delmas C. [Epidemiology of Haemophilus influenzae strains collected in 2004 in France and in vitro assessment of their susceptibility to antibiotics] Med Mal Infect. 2007;37(6):320–324. doi: 10.1016/j.medmal.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Harimaya A., Yokota S., Sato K., Himi T., Fujii N. Remarkably high prevalence of fts I gene mutations in Haemophilus influenzae isolates from upper respiratory tract infections in children of the Sapporo district, Japan. J Infect Chemother. 2008;14(3):223–227. doi: 10.1007/s10156-008-0604-5. [DOI] [PubMed] [Google Scholar]
- 62.Casey J.R., Pichichero M.E. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23:824–828. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 63.McEllistrem M.C., Adams J.M., Patel K., Mendelsohn A.B., Kaplan S.L., Bradley J.S. Acute otitis media due to penicillin-nonsusceptible Streptococcus pneumoniae before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2005;40(12):1738–1744. doi: 10.1086/429908. [DOI] [PubMed] [Google Scholar]


