Abstract
Neonatal infections continue to cause morbidity and mortality in infants. Among approximately 400,000 infants followed nationally, the incidence rates of early-onset sepsis infection within 3 days of life are 0.98 cases per 1000 live births. Newborn infants are at increased risk for infections because they have relative immunodeficiency. This article provides evidence-based practical approaches to the diagnosis, management, and prevention of neonatal infections.
Keywords: Neonatal infections, Newborn sepsis, Early-onset sepsis, Late-onset sepsis, Respiratory viral infections in infants, Antibacterial therapy, Antiviral therapy, Neonatal antimicrobial stewardship
Key points
-
•
Neonatal infections continue to cause morbidity and mortality in infants. Group B streptococcus and Escherichia coli are the most common agents of early-onset sepsis, whereas coagulase-negative Staphylococcus is the predominant cause of late-onset sepsis.
-
•
Other important agents include Listeria monocytogenes, syphilis, Staphylococcus aureus, herpes simplex virus, cytomegalovirus, and Candida spp.
-
•
There is increasing recognition of respiratory viral infections contributing to ruling out sepsis in very young infants whose presentations are similar to bacterial infections.
-
•
Initial work up for neonatal infection consists of complete blood count and blood culture, with the option of performing cerebrospinal fluid analyses and culture if clinically indicated. Serial determinations of biomarkers (C-reactive protein, procalcitonin, or neutrophil CD64) may be used adjunctively in the diagnosis and management of neonatal infection.
-
•
Ampicillin and gentamicin remains the cornerstone of initial antimicrobial regimen for neonatal infections. Third-generation cephalosporins should be used judiciously.
-
•
The use of antiviral (acyclovir, ganciclovir, valganciclovir, and oseltamivir) and antifungal agents (fluconazole, amphotericin B, and voriconazole) may reduce mortality and morbidity due to specific viral and fungal disease.
-
•
Different strategies, such as group B streptococcal prophylaxis, hand hygiene, immunization and immunoprophylaxis, antimicrobial stewardship, probiotics, and prebiotics, and care bundles may be used in preventing infections in neonates.
Introduction
Neonatal infections continue to cause morbidity and mortality in infants. Among approximately 400,000 infants followed nationally, the incidence rates of early-onset sepsis (EOS) infection within 3 days of life were 0.98 cases per 1000 live births.1 More than two-thirds of the frequently isolated organisms were associated with group B streptococcus (GBS) (43%) and Escherichia coli (29%). Although 20% of the term infants were treated in the newborn nursery, 77% of the infected infants required intensive care management. Of those who survived beyond 3 days of life, about 21% had an episode of late-onset sepsis (LOS) infection after 3 days of life. The overall mortality rate of infected infants was 16%.
Newborn infants are at increased risk for infections because they have relative immunodeficiency. This may be due to decreased passage of maternal antibodies in preterm infants and to immaturity of the immune system in general.2, 3 The innate immune functions in infants are impaired with decreased production of inflammatory markers (interleukin 6 and tumor necrosis factor)4 and with decreased dendritic and neutrophil functions.5 The adaptive immune system is less than optimal with decreased cytotoxic functions,2 decreased cell mediated immunity,6 and delayed or lack of isotype switching.2, 3 Complement is important in opsonization and bacterial killing. In term infants, complement levels are approximately half compared with adults.2 Taken together, these predispose infants to severe, prolonged, or recurrent infections associated with bacterial, viral, or fungal infections.
Suspected sepsis, presumed infection, and ruling out sepsis remain the most common diagnoses in the nursery intensive care unit (NICU). The American Academy of Pediatrics (AAP) Committee on Fetus and Newborn7 has published a clinical report extensively discussing clinically relevant challenges: identifying newborns with signs of sepsis with high likelihood of EOS requiring antimicrobial regimen and identifying healthy-appearing newborns with high likelihood of EOS requiring antimicrobial regimen. The committee concluded that, although these guidelines are evidence-based, they may be modified by the clinical judgment of the provider. The primary reason is that the clinical presentation of neonatal infection may be subtle and nonspecific, and may overlap with noninfectious causes.7, 8 Many clinicians empirically start broad spectrum antimicrobial regimen for infants considered at risk for sepsis but antibiotics are occasionally continued despite a negative blood culture. This practice may be detrimental to the infant8 because it increases the risk of invasive fungal infections,9 necrotizing enterocolitis (NEC), or death,10, 11 which increases the pressure for selecting multidrug-resistant organisms12 and even the risk of LOS.11
The purpose of this article is to provide evidence-based practical approaches to the diagnosis, management, and prevention of neonatal infections.
Microbiologic agents
The timing of transmission is one of the factors contributing to the cause of neonatal infections. Different pathogens may be acquired during pregnancy (prenatal), during delivery (perinatal), or after delivery (postnatal). Table 1 shows the different periods of transmission of various neonatal pathogens.
Table 1.
Periods of transmission in neonatal infections
| Pathogens | During Pregnancy | During Delivery | After Delivery |
|---|---|---|---|
| Bacteria | |||
| Chlamydia trachomatis | — | +a | — |
| GBS | ++b | ++ | ++ |
| Enterococcus spp | — | + | + |
| Enterobacteriaceae | — | ++ | ++ |
| Listeria monocytogenes | + | + | + |
| Neisseria gonorrhea | — | + | — |
| Staphylococcus spp | — | — | ++ |
| Treponema pallidum | + | — | — |
| Ureaplasma urealyticum | — | + | — |
| Viruses | |||
| Coronavirus | — | — | + |
| Cytomegalovirus | + | + | + |
| Enterovirus | + | + | + |
| Hepatitis B virus | + | + | — |
| Herpes simplex virus | + | — | — |
| Human immunodeficiency virus | + | + | + |
| Human metapneumovirus | — | — | + |
| Influenza | — | — | + |
| Parainfluenza virus | — | — | + |
| Parvovirus B19 | + | — | — |
| Respiratory syncytial virus | — | — | + |
| Rhinovirus | — | — | + |
| Rubella virus | + | — | — |
| Varicella-zoster virus | + | + | + |
| Fungi | |||
| Candida spp | — | + | + |
| Aspergillus spp | — | — | + |
| Protozoa | |||
| Toxoplasma gondii | + | — | — |
The introduction of new molecular-based assays, such as quantitative real-time polymerase chain reaction (PCR),13 has paved the way for increasing recognition of respiratory viral infections contributing to ruling out sepsis in late-onset infections.14 Table 1 includes respiratory viral infections (coronavirus, enterovirus, human metapneumovirus, influenza, parainfluenza virus, respiratory syncytial virus [RSV], and rhinovirus) as possible causes of postnatal infections in infants.14, 15, 16
Clinical presentations
Early-Onset Infections
EOS is arbitrarily defined as infection within the first 3 days of life. The most common organisms associated with EOS include GBS and E coli.1, 19 In general, the risk of bacterial infection in a healthy-appearing newborn remains relatively low.20 The most common clinical findings include hypoglycemia (<40 mg/dL, 22%) and hypothermia (<36.5°C, 20%), followed by hyperglycemia (>140 mg/dL, 19%) and apnea (18%).19
Edwards and Baker21 summarized that newborn infants with sepsis manifest similar clinical signs as those with meningitis, including hyperthermia; hypothermia; respiratory distress; anorexia or vomiting; jaundice; and lethargy. Hypotension may be more frequently found in infants with sepsis, whereas irritability, convulsions, and bulging or full fontanel is found in those with meningitis. However, they cautioned that absence of any of the aforementioned signs do not exclude central nervous system involvement. Furthermore, it was suggested to evaluate infants for various foci of infections such as acute otitis media, conjunctivitis, osteomyelitis, pyogenic arthritis, and skin soft-tissue infections.
Late-Onset Infections
LOS is arbitrarily defined as infection after 3 days of life. The most common organisms isolated with LOS include coagulase-negative staphylococci in more than a third of the cases, which may or may not be associated with a medical device.19 Yeast or Candida spp infection is another important pathogen.19 Also, there is increasing recognition of viral respiratory infections as a possible cause in LOS.14 The most common clinical findings include hypothermia (41%), hyperglycemia (38%), apnea (38%), and bradycardia (30%).19
There are several factors that may increase the risk for LOS. There are significantly more infants with LOS who have an indwelling central vascular catheter at the time of infection than those infants with EOS (78% vs 10%, P<.0001).19 Additionally, there are more infants with LOS who had a surgical procedure before infection (8% vs 1%, P<.0001).
Diagnostic evaluations
The clinical presentations of infections may overlap with noninfectious causes in newborns. It has been previously demonstrated that relying on symptoms alone may not be sufficient in diagnosing neonatal infections.22 Bacteremia has been reported in infants without clinical signs of sepsis.23 There are several diagnostic tests and principles that may guide clinicians in evaluating infants with infections.
Algorithm-Based Guideline
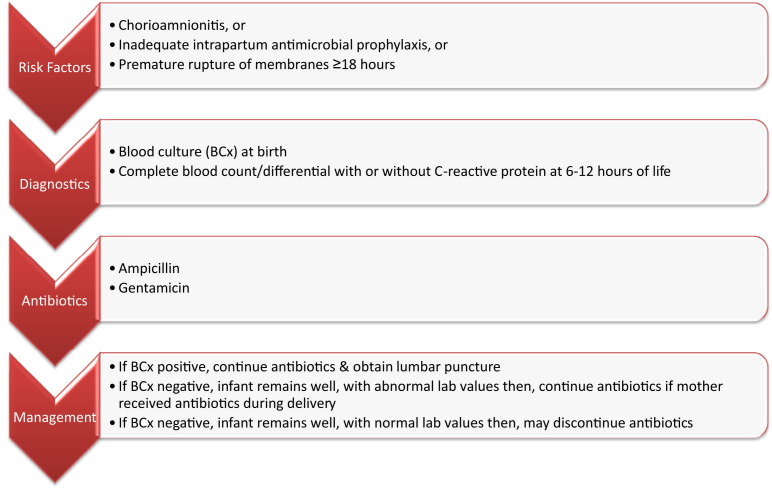
The AAP Committee on Fetus and Newborn have published a clinical report on the evaluation of asymptomatic infants (<37 and ≥37 week gestation) with risk factors for sepsis.7 Evaluation of asymptomatic preterm infants (<37-week) with risk factors for sepsis is shown in Fig. 1 .7 Similar algorithms for the evaluation of asymptomatic term infants (≥37 week gestation) are available from the AAP Committee on Fetus and Newborn. (http://www.ncbi.nlm.nih.gov/pubmed/22547779).7
Fig. 1.
Evaluation of asymptomatic preterm infants (<37-week gestation) with risk factors for sepsis.
(Adapted from Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006–15.)
Additional principles in the evaluation of infants with risk factors for sepsis7 follow:
-
•
Major risk factors for neonatal sepsis include chorioamnionitis, prolonged rupture of membrane 18 or more hours, and colonization of GBS with inadequate intrapartum antimicrobial prophylaxis (IAP).
-
•
Chorioamnionitis usually presents as maternal fever greater than 38°C (100.4°F) and its diagnosis should be discussed with the obstetric providers. Maternal fever may be the only abnormal finding in chorioamnionitis.
-
•
Adequate IAP means maternal treatment with penicillin, ampicillin, or cefazolin at or earlier than 4 hours before delivery.
-
•
At least 1 mL of blood may be sufficient for a single blood culture from a peripheral vein. Blood culture from umbilical artery catheter or umbilical vein may be a reliable alternative following aseptic techniques
-
•
Screening blood cultures have not been proven of value and are not recommended.
-
•
Complete blood count with differential has poor positive predictive value and it is suggested waiting 6 to 12 hours after birth to avoid falsely normal values at birth.
-
•
Platelet counts remain low days to weeks after sepsis; thus this cannot be used in following response to treatment.
-
•
The sensitivity of C-reactive protein (CRP) improves if done 6 to 12 hours after birth. Bacterial sepsis is unlikely if CRP remains normal.
-
•
Lumbar puncture may be indicated in infants whom sepsis is highly suspected, those infants with bacteremia, and in infants who fail to respond to antimicrobial therapy.
-
•
Urinary tract infection in newborns is associated with episodes of bacteremia; thus urine culture should not be part of routine sepsis workup.
-
•
Microbiologic evaluation using gastric aspirates, tracheal aspirates, or superficial body sites cultures are of limited value and are not routinely recommended for sepsis.
Biomarkers
Several acute-phase reactants or biomarkers (neutrophil CD64 [nCD64], procalcitonin [PCT], or CRP) may be used adjunctively in the evaluation and management of neonatal infection. The diagnostic usefulness of the various surrogate markers depends on the phases of neonatal sepsis: early phase or 2 to 12 hours (nCD64), mid phase or 12–24 hours (PCT), and late phase or greater than 24 hours (CRP).24
nCD64 is a high-affinity Fc receptor that increases with exposure to bacterial or fungal agents.2, 24 The usefulness of nCD64 is related to its high negative predictive value as well as decreasing concentration on serial determinations on infants undergoing antimicrobial treatment of bacteremia.24 However, there is a scarcity of medical evidence to recommend nCD64 for routine evaluation of neonatal infection and this may not be readily available.
Procalcitonin released from tissues increases with infection at around 2 hours and peaks at 12 hours.7 It may also increase with noninfectious causes such as in respiratory distress syndrome and a physiologic increase during the first 24 hours of birth.7 PCT may not be readily available and the turnaround time varies in different institutions from 20 minutes to 5 hours.24
CRP increases around 6 hours associated with an inflammatory response with release of interleukin-6 and peaks at 24 hours.7 CRP has been used in the algorithm-based guideline from the AAP Committee on Fetus and Newborn for the evaluation of asymptomatic term and preterm infants with a risk factor for sepsis.7 It is best used as part of a group of diagnostic tests2 together with blood culture and white blood cell with differential in the evaluation of neonatal infection.7 However, there is not enough medical evidence at this time to recommend serial determinations of CRP in guiding duration of antimicrobial therapy in infants.7, 24 Further studies are needed to evaluate the usefulness of sequential determination of CRP and biomarkers for an antimicrobial stewardship program (ASP) in the NICU setting.
Molecular-Based Tests
In 2013, the Infectious Disease Society of America, in collaboration with the American Society for Microbiology, affirmed the importance of close collaboration and positive working relationships between clinicians and microbiologists25 to better serve patients. The most up-to-date edition of the Red Book provides contact information for expert advice and national collaborative study groups that give guidance on diagnostic assays regarding specific agents causing mother-to-child transmission. It is important to know the various microbiologic resources available locally, which include but are not limited to PCR and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF).
Rapid antigen tests for respiratory viruses may lack sensitivity,25 which is important in the NICU setting in controlling local outbreaks. There are several nucleic acid amplification test platforms currently available that differ in the number of analytes detected.25 It is important to obtain adequate specimens and to use suitable viral transport media following manufacturer instructions.
MALDI-TOF is a valuable alternative to the conventional microbiologic assays; however, it may not be a readily available resource for diagnostic testing in most institutions. However, if it is available, it has several practical applications that may benefit clinical management even in the NICU settings:
-
•
Earlier and accurate diagnosis of neonatal sepsis due to various bacteria26
-
•
Rapid identification of highly virulent GBS that causes meningitis and LOS in infants27
-
•
Identification of maternal-to-child transmissions (chorioamnionitis and neonatal infections) of opportunistic pathogen28
-
•
Accurate identification of bloodstream infection associated with fungal infections in the NICU29
-
•
Identification and monitoring the spread of nosocomial outbreak (eg, methicillin-resistant Staphylococcus aureus [MRSA]30 and Candida parapsilosis 31 in the NICU).
Therapeutic management
When appropriate specimens for diagnostic evaluations are collected in clinically stable patients, then empirical antimicrobial therapy should be initiated for neonatal sepsis. It is recommended to discuss complicated cases, such as multidrug resistant organisms and infants not improving while on therapy or those requiring unconventional dosing regimens and antimicrobial agents, with pediatric infectious disease specialists.
Antibiotic Treatment
Ampicillin and gentamicin remains the cornerstone of initial antimicrobial regimen for early-onset neonatal infections. The combination of such broad-spectrum antibiotic regimens cover the most common cause (GBS and E coli in more than 70%)1 of EOS and has synergistic activity (against GBS and Listeria monocytogenes).7, 32 The dosing regimen for ampicillin may change over time based on the chronologic age of the infant and body weight.32 For example, an 8-day-old infant weighing greater than 2000 g may need dosing adjustment of ampicillin from 150 mg/kg/d intravenous (IV) divided every 8 hours to 200 mg/kg/d IV divided every 6 hours.
Once-daily dosing of gentamicin (4 mg/kg IV qd)32 has been used in the term newborn for more than a decade. The pharmacodynamic characteristics of aminoglycosides that allow the use of once-daily dosing include concentration-dependent killing (peak concentration to minimal inhibitory concentration [peak/MIC] ratio),33, 34 postantibiotic effect with leukocyte enhancement,35, 36 and prevention of adaptive resistance.37
Third-generation cephalosporins should be used judiciously. There is significant association between the use of third-generation cephalosporins and invasive candidiasis in preterm infants.9 Cefotaxime has excellent penetration to the cerebrospinal fluid and its therapeutic use should be limited to Gram-negative meningitis.7 Routine use of cefotaxime for EOS may lead to rapid development of drug-resistant organisms.38 Ceftriaxone is contraindicated in neonates for 2 reasons: (1) it is highly protein bound and may displace bilirubin progressing to hyperbilirubinemia7 and (2) concurrent administration with calcium-containing solutions may produce insoluble precipitates (ceftriaxone-calcium salts) leading to cardiorespiratory complications.39
The AAP periodically updates the dosing regimens and recommended therapy for selected neonatal infections through Nelson’s Pediatric Antimicrobial Therapy.32 It provides various antimicrobial regimens (antibiotic, antiviral, and antifungal agents) based on body weight of infants and their chronologic age or gestational and postnatal age. Between new editions, a monthly update of short and interesting reports related to pediatric antimicrobial therapy is posted at www.aap.org/en-us/aap-store/Nelsons/Pages/Whats-New.aspx. Suggested durations of antibiotic therapy for EOS adapted from 2014 Nelson’s Pediatric Antimicrobial Therapy 32 and the AAP Committee on Fetus and Newborn7 are shown in Table 2 .
Table 2.
Duration of antibiotic therapy for early-onset sepsis
| Conditions | Duration & Comments |
|---|---|
| Newborns with early onset pulmonary infiltrates (within 3 d of life) |
|
| Mild or presumed sepsis | 5 or 7 d, no prospective controlled studies; remains controversial |
| Bacteremia without a focus | 10 d |
| Uncomplicated GBS meningitis and other gram-positive bacteria | 14–21 d |
| Gram-negative meningitis | 21 d or 14 d after a negative cerebrospinal fluid culture, whichever is longer |
Data from Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006–15; and American Academy of Pediatrics. Antimicrobial therapy for newborns. In: Bradley JS, editor. 2014 Nelson's pediatric antimicrobial therapy. Elk Grove Village (IL): American Academy of Pediatrics; 2014. p. 17–36.
Antiviral Therapy
There are several antiviral agents that can be used for the treatment of neonatal viral infections. Acyclovir (60 mg/kg/d IV divided every 8 hours) is the treatment of choice for term infants with herpes simplex virus (HSV) and varicella-zoster infections.32 There are several topical agents (0.15% ganciclovir ophthalmic gel, 0.1% iododeoxyuridine, or 1% trifluridine) that may be added to systemic antiviral regimen if there is eye involvement.32 After parenteral therapy with acyclovir, it is recommended to give HSV suppressive regimen (300 mg/m2/dose po tid), which improves neurodevelopmental outcomes of infants with central nervous system involvement.40 There is currently no dosing regimen for valacyclovir in infants younger than 3 months of age.32 The AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn recently published an algorithm-based guideline on the evaluation and treatment of asymptomatic infants born to mothers with active herpes lesions41 (http://www.ncbi.nlm.nih.gov/pubmed/23359576).
Oral valganciclovir (16 mg/kg/dose po bid) is the drug of choice for infants with symptomatic congenital cytomegalovirus (CMV) disease with or without central nervous system involvement.32, 42 The treatment of congenital CMV should be initiated in the first month of life. Kimberlin and colleagues42 concluded from the phase III randomized double-blind placebo-controlled multinational study that 6 months of valganciclovir regimen for symptomatic congenital CMV disease significantly improves hearing and neurodevelopmental outcomes. There is significant improvement in language and receptive communication at 2 years of age. There was less grade 3 to 4 neutropenia at 6 weeks oral valganciclovir (∼19%) compared with 6 weeks of IV ganciclovir (63%) reported previously.
IV ganciclovir (6 mg/kg/dose bid) can be used initially for infants with symptomatic congenital CMV disease if oral valganciclovir is contraindicated due to extreme prematurity or NEC.32 The same dosing regimen is the treatment of choice for perinatally or postnatally acquired CMV disease associated encephalitis, hepatitis, pneumonitis, or persistent thrombocytopenia.
Oral oseltamivir (3 mg/kg/dose bid) remains the treatment of choice for term infants with influenza infections.32, 43 Oral suspension formulation is available (6 mg/mL) and should be offered to young infants with suspected or confirmed influenza infection regardless of severity because they are at higher risk for complications.43 Limited data are available for the weight-based dosing regimen for preterm infants using postmenstrual age (ie, gestational age plus chronologic age):
-
•
Less than 38 weeks postmenstrual age, 1 mg/kg/dose po bid
-
•
38 to 40 weeks, 1.5 mg/kg/dose po bid
-
•
Greater than 40 weeks, 3 mg/kg/dose po bid.
There is currently no dosing regimen for inhalational zanamivir for young infants.
Suggested durations of antiviral therapy, prophylaxis, and suppressive regimen for congenital and perinatal or postnatally acquired viral infections adapted from 2014 Nelson’s Pediatric Antimicrobial Therapy 32 and the AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn41 are shown in Table 3 .
Table 3.
Duration of antiviral therapy and suppressive regimen for congenital and perinatal or postnatally acquired viral infections
| Conditions | Duration & Comments |
|---|---|
| HSV: central nervous system or disseminated disease | Acyclovir IV for 21 d, continue treatment until repeat cerebrospinal fluid HSV PCR negative |
| HSV: skin, eye, or mouth disease | Acyclovir IV for 14 d |
| HSV suppressive regimen after IV therapy | Acyclovir po for 6 mo, monitor for neutropenia |
| Congenital CMV disease | Valganciclovir po for 6 mo |
| Perinatally or postnatally acquired CMV disease associated encephalitis, hepatitis, pneumonitis, or persistent thrombocytopenia | Ganciclovir IV for 14–21 d, monitor for relapse after treatment completion |
| Influenza A and B viruses therapy | Oseltamivir po for 5 d |
| Influenza A and B viruses prophylaxis | Generally not recommended because of limited safety and efficacy data, discuss with pediatric infectious disease specialist |
Antifungal Treatment and Prophylaxis
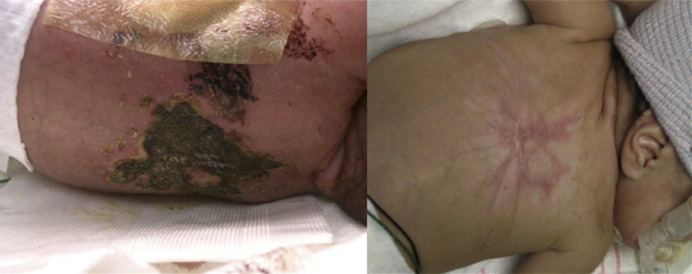
For candidiasis, IV amphotericin B deoxycholate (1 mg/kg/d) and IV fluconazole (loading dose 25 mg/d on day 1, then 12 mg/kg/d on day 2) may be used for susceptible isolates.32 Lipid-based amphotericin (3–5 mg/kg/d) can be used if there is no renal involvement because of inadequate kidney penetration. For aspergillosis, voriconazole (loading dose 18 mg/kg/d divided every 12 hours on day 1, then 16 mg/kg/d on day 2) is the drug of choice. Local debridement may be needed for cutaneous aspergillosis. However, if there is clinical deterioration and if the infant is unstable for surgical intervention, consultation with pediatric infectious diseases specialists is recommended.18 Fig. 2 shows an extensive cutaneous aspergillosis on a preterm infant who was a poor surgical risk successfully treated with combination antifungal agents.
Fig. 2.
4 × 6 cm necrotic black eschar on the back of a preterm infant due to Aspergillus fumigatus and the residual scarring after several weeks of combination antifungal agents.
(From Santos RP, Sanchez PJ, Mejias A, et al. Successful medical treatment of cutaneous aspergillosis in a premature infant using liposomal amphotericin B, voriconazole and micafungin. Pediatr Infect Dis J 2007;26(4):364–6; with permission.)
Fluconazole prophylaxis (6 mg/kg/d twice a week) may be indicated in high-risk infants with birth weight of less than 1000 g from institutions with high incidence of candidiasis (ie, above 10%).32 Fluconazole prophylaxis (25 mg/kg once weekly) may be offered to young infants younger than 4 months old on extracorporeal membrane oxygenation.
Suggested durations of antifungal treatment of candidiasis and aspergillosis adapted from 2014 Nelson’s Pediatric Antimicrobial Therapy 32 are shown in Table 4 .
Table 4.
Duration of antifungal treatment regimen for candidiasis and aspergillosis
| Conditions | Duration & Comments |
|---|---|
| Candidiasis |
|
| Aspergillosis | Depends on disease and local debridement |
Data from American Academy of Pediatrics. Antimicrobial therapy for newborns. In: Bradley JS, editor. 2014 Nelson's pediatric antimicrobial therapy. Elk Grove Village (IL): American Academy of Pediatrics; 2014. p. 17–36.
Surgical treatment options
Surgical interventions may be indicated for the source control of neonatal infections. In a single-center 20-year retrospective study, NEC-associated blood stream infection (BSI) occurred within 3 days of NEC diagnosis and was noted in approximately 43% (69 out of 158 infants with one episode of BSI). Infants with NEC-associated BSI had higher odds (adjusted odds ratio 3.51; 95% CI 1.98–6.24) of having surgical interventions compared with those without BSI.44 It is of utmost importance to correspond with pediatric surgery regarding source control of infection if clinically indicated because NEC-associated BSI had higher odds of death (adjusted odds ratio 2.88; 95% CI 1.39–5.97).44
The following includes disease-specific conditions that may require surgical interventions for adequate source control of infections if the infant is clinically stable. Pediatric providers are encouraged to discuss with their surgical colleagues the following surgical treatment options32:
-
•
Early debridement of cutaneous lesions with disseminated aspergillosis
-
•
Surgical drainage of peritonitis with bowel rupture
-
•
Wound cleaning and debridement rapidly spreading cellulitis (S aureus), necrotizing fasciitis (group A or B streptococci), tetanus neonatorum
-
•
Surgical drainage of pus in osteomyelitis and suppurative arthritis
-
•
Thoracostomy drainage of empyema
-
•
Surgical drainage of breast abscess may be needed to minimize damage to breast tissue.
Surgical interventions for primary diseases in infants may also increase the risk for neonatal infections. Higher rates of surgical site infection defined as superficial, deep, and organ infections within 30 days of surgical procedures were noted among infants following closure of gastroschisis.45 It is important to closely monitor infants with surgical site infection because they require significantly longer hospital stay.45
Preventive strategies
There are various measures that can be used, depending on the availability of local resources, to prevent neonatal infections. These include but are not limited to GBS prophylaxis, hand hygiene, immunization and immunoprophylaxis, ASP, probiotics and prebiotics, and care bundles.
Group B Streptococcal Prophylaxis
IAP is the only preventive strategy that substantially reduces the incidence of early-onset GBS.7, 46 The following are indications for IAP:
-
•
Previous infant with invasive GBS disease
-
•
GBS bacteriuria during the current pregnancy
-
•
Positive GBS vaginal-rectal screening (at 35–37 week gestation) except for cesarean delivery without labor or ruptured membrane
-
•
Unknown maternal GBS status with delivery at less than 37 weeks, rupture of membrane at or before 18 hours, or fever equal to or greater than 100.4°F (≥38°C).
Adequate IAP means receiving penicillin, ampicillin, or cefazolin for at least 4 hours before delivery. Cefazolin may be used if with nonserious β-lactam allergy. If there is history of serious β-lactam allergy (anaphylaxis, angioedema, respiratory insufficiency, or urticarial rash) and if GBS isolate is susceptible, clindamycin may be used. Otherwise, vancomycin is an alternative. Because of high resistance rates, erythromycin is not recommended.
The Center for Disease Control and Prevention has an extensive online resource on GBS for clinicians, including the algorithm-based guidance on secondary prevention of EOS in newborns.47 The Web page also provides an application, Prevent Group B Strep, which includes guidance on various patient scenarios in collaboration with different medical societies, such as the AAP and the American College of Obstetricians and Gynecologists (http://www.cdc.gov/groupBstrep/guidelines/index.html).
Hand Hygiene
There is no doubt that hand hygiene remains the cornerstone in decreasing health care–associated infections in different hospital settings, including the NICU. In fact, there are various educational programs, multidisciplinary quality-improvement teams, and guidelines on the proven effectiveness of hand hygiene in decreasing infection; however, this is significantly affected by compliance.48, 49 The Center for Disease Control and Prevention has a Web site (http://www.cdc.gov/handhygiene/) containing resources for hand hygiene in health care settings including an application, iScrub, for monitoring hand hygiene compliance using an iPhone or iPod Touch.50 Thus, hand hygiene guidelines are effective in reducing infections only if we use it.
Soap and water is recommended for decontaminating visibly soiled hands by rubbing hands together vigorously for 15 seconds.48, 49 Alcohol-based gel or foam or an antiseptic soap may be used for routine hand hygiene if not grossly contaminated.48, 49 Hand hygiene compliance is improved if with available alcohol-based products at the infant’s bedside.48 Antimicrobial-impregnated towelettes or wipes are considered alternatives but not substitutes for washing with soap and water or alcohol-based gel or foam.49
Immunization and Immunoprophylaxis
The development of a safe and effective vaccine is arguably one of the greatest medical interventions in the last century.51 Hepatitis B vaccine is the only agent in the United States recommended to be administered at birth. The various brands available in the United States have an efficacy of 90% to 95% in preventing hepatitis B virus infection and disease.52 Additional information regarding recommended dosages of hepatitis B vaccines are available in the most recent edition of the Red Book 52 and in the annual publication from the Advisory Committee on Immunization Practices.53 The first dose in the primary series of the subsequent vaccines (diphtheria and tetanus toxoids and acellular pertussis vaccine [DTaP], Haemophilus influenza type B, inactivated polio, pneumococcal, or rotavirus vaccine) can be administered at a minimum age of 6 weeks.53
Care givers at home should be advised on the importance of immunizing family members to protect infants who are too young to be vaccinated. This is called cocooning54 and prevents vaccine-preventable diseases, such as pertussis and influenza, in young infants. Educational materials on cocooning for parents and clinicians are available at the AAP Web site http://www2.aap.org/immunization/families/cocooning.html.
In October 2012, the use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) during every pregnancy was recommended because of increasing cases of pertussis in young infants in the recent years.55 The mother’s protective antibodies against pertussis are short-lived and a dose of Tdap in a previous pregnancy may not be protective to the infants of subsequent pregnancies.56
Young preterm infants (<1 year of age born at <29 weeks and 0-day gestation) should receive palivizumab during the RSV season for immunoprophylaxis.57 Five monthly doses of palivizumab at 15 mg/kg given intramuscularly will provide adequate protection for 6 months. Other indications during the RSV season include preterm infants (<1 year of age born at <32 weeks and 0-day gestation) with chronic lung disease of prematurity who required greater than 21% oxygen for at least the first 28 days of life and young infants (<1 year of age) with certain hemodynamically significant heart disease.57 Clinicians should consult the most current guidelines or policy statement from the AAP regarding palivizumab prophylaxis among young children at increased risk for hospitalization for RSV infection.
Antimicrobial Stewardships
Injudicious use of antibiotics can alter the neonates’ microflora that increases exposure and pressure that leads to antimicrobial resistance. The NICU milieu and interventions are permissive for the development of antibiotic-resistant organisms.48, 58 The AAP Committee on Fetus and Newborn48 have listed ASP strategies that may be useful in the NICU setting based on the guideline from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America (Box 1 ).59
Box 1. Antimicrobial stewardship strategies in the nursery intensive care unit.
-
•
Audit antimicrobial use and provide feedback to providers
-
•
Formulary restriction and preauthorization requirements for selected antibiotics
-
•
Education of care providers regarding antibiotic use or misuse and the development of resistance
-
•
Development of clinical guidelines for selected medical conditions
-
•
Antimicrobial order forms
-
•
Specific plans for streamlining (broad-to-narrow spectrum antibiotic agents) and de-escalation (elimination of redundant or unnecessary) antibiotic agents
-
•
Optimize dosing regimen based on individual patient characteristics such as weight, renal status, or drug-drug interactions
-
•
Change from parenteral to oral antibiotic agents when appropriate and feasible
Data from Polin RA, Denson S, Brady MT, Committee on Fetus and Newborn, Committee on Infectious Diseases. Strategies for prevention of health care-associated infections in the NICU. Pediatrics 2012;129(4):e1085–93; and Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44(2):159–77.
Probiotics and Prebiotics
There is some medical evidence supporting the use of probiotics in the prevention of NEC in preterm infants. Probiotic is an oral supplement containing sufficient amount of viable microorganisms that alters the host microflora with potential for health benefits.60 A meta-analysis based on 9 randomized control trials involving approximately 1400 infants born before or at 37 weeks gestation and/or weighing less than or equal to 2500 g at birth showed that enteral use of probiotic significantly decreased the incidence of severe NEC and mortality.61 There were no severe adverse events or systemic infections directly related to the probiotics used were reported.
The AAP Committee on Nutrition,60 however, cannot recommend the use of all probiotics in young infants until further studies are done to resolve problematic issues. They noted the large heterogeneity of the studies included in the review, the different mixture of probiotics used, and that the combinations of probiotics used in the studies are not available in the United States. Further, there remains some gap in knowledge on which probiotic bacteria species to use, the microbial dose, as well as the duration of administration.
In 2014, an updated review of the aforementioned meta-analysis of randomized controlled trials continues to support a change in practice of supplementing preterm infants with probiotics. The review provided similar results involving more than 5000 infants in whom probiotics significantly reduced severe NEC and mortality.62 However, the previously mentioned gap in knowledge remains, as well as the need for comparative studies.
There is scarcity of medical evidence to recommend the addition of prebiotics such as oligosaccharides in infant formula. Prebiotics are nondigestible food ingredients that occur naturally or as dietary supplements that enhance growth of probiotic bacteria such as Bifidobacterium spp.60 Several studies had reported that the addition of prebiotics in infant formula significantly increased the bifidobacteria counts in their stool without adverse effects noted. However, clinical efficacy as well as cost-benefit analyses regarding the addition of oligosaccharides to infant formulas is lacking.60
For infants, human milk remains the best source of naturally occurring prebiotics and probiotics, and immunoprotective compounds known to decrease the incidence of respiratory and gastrointestinal infections.48, 60
Nursery Intensive Care Unit Care Bundles
There are invasive procedures that may increase the infant’s risk of health care–associated infections in the NICU setting. These infections include central line–associated BSIs (CLABSIs), pneumonia, skin, and soft tissue infections; and, occasionally, vaccine-preventable diseases and outbreak of respiratory viral infections. Care bundles are sets of interventions aimed at reducing health care–associated infections in the NICU.48
The most common cause of CLABSI48 and LOS8 are coagulase-negative staphylococci. Several randomized clinical trials on the use of low-dose vancomycin in parenteral solutions in preterm infants did not show significant decrease in the length of stay and mortality.48 There is an antibiotic-lock therapy done in neonates that significantly decrease CLABSI however it was not powered to answer whether vancomycin resistance occurred. Both are currently not recommended because of the lack of long-term efficacy evidence as well as concern for development of drug-resistant organisms.
Infection control intended to decrease CLABSI in the NICU should include measures to decrease extraluminal and intravascular catheter–related infections. Various techniques and guidelines in the prevention of CLABSI in infants adapted from the AAP Committee on Fetus and Newborn are shown in Box 2 .48
Box 2. Techniques and guidelines in the prevention of central line–associated bloodstream infections in the nursery intensive care unit.
Techniques in the prevention of extraluminal catheter contamination
-
•
Hand hygiene
-
•
Aseptic catheter insertion and the use of maximal sterile barrier for catheter insertion and care
-
•
Use of topical antiseptic
-
•
Use of sterile dressing
Guidelines in the prevention of intravascular catheter infection
-
•
Remove and do not replace umbilical artery catheters if signs of CLABSI, thrombosis, or vascular insufficiency in the lower extremities are present.
-
•
Remove and do not replace umbilical venous catheters if signs of CLABSI or thrombosis are present.
-
•
Clean the umbilical insertion site using an antiseptic such as povidone-iodine before catheter insertion.
-
•
Avoid using topical antibiotic ointment or creams on insertion sites to prevent fungal infections and antimicrobial resistance.
-
•
Use low doses of heparin (0.25–1.0 U/mL) to the fluid infused through umbilical arterial catheter.
-
•
Remove umbilical catheters as soon as no longer needed or if signs of vascular insufficiency to the lower extremities (for umbilical artery access) are present; they may be replaced if malfunctioning. Umbilical artery catheters should not be left in place for more than 5 days. Umbilical venous catheters may be used up to 14 days if managed aseptically.
Data from Polin RA, Denson S, Brady MT, Committee on Fetus and Newborn, Committee on Infectious Diseases. Strategies for prevention of health care-associated infections in the NICU. Pediatrics 2012;129(4):e1085–93.
There are specific practices that may be adapted in the local setting for preventing vaccine-preventable diseases and outbreaks of respiratory viral infections. These include but are not limited to vaccination of health care providers against influenza and pertussis (Tdap), visitation guidelines to screen ill or symptomatic visitors, and cohorting in cases of clustering of infections or in outbreak situations.48 Cohorting may only be possible if early screening procedures, such as the use of PCR-based assays, are in place if available in cases of clustering of respiratory viral infections.14, 15, 16 Further, appropriate isolation (eg, contact precautions for MRSA, droplet precautions for influenza, and airborne precautions for measles) should be observed if the infant is colonized or infected with a pathogen requiring additional protection beyond standard precautions.63
Summary
Neonatal infections continue to cause morbidity and mortality in infants. GBS and E coli are the most common agents of EOS, whereas coagulase-negative Staphylococcus is the predominant cause for LOS. There is increasing recognition of respiratory viral infections contributing to ruling out sepsis in very young infants whose presentations are similar to bacterial infections. Blood culture at birth and white blood cell with or without CRP has been used in the algorithm-based guideline for the evaluation of asymptomatic term and preterm infants with risk factors for sepsis. Ampicillin and gentamicin remains the cornerstone of initial antimicrobial regimen for neonatal infections. Third-generation cephalosporins should be used judiciously. The use of antiviral (acyclovir, ganciclovir, valganciclovir, and oseltamivir) and antifungal (fluconazole, amphotericin B, and voriconazole) treatment and prophylactic regimens may reduce mortality and morbidity to specific viral and fungal disease in infants. There are various strategies, such as GBS prophylaxis, hand hygiene, immunization, and immunoprophylaxis, ASP, probiotics, and prebiotics, and NICU care bundles, which may be used in preventing infections in infants.
Footnotes
Disclosures: None.
References
- 1.Stoll B.J., Hansen N.I., Sanchez P.J. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho-Gonzalez A., Spearman P.W., Stoll B.J. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013;60(2):367–389. doi: 10.1016/j.pcl.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schelonka R.L., Maheshwari A. The many faces of B cells: from generation of antibodies to immune regulation. NeoReviews. 2013;14:e438–e447. [Google Scholar]
- 4.Kollmann T.R., Crabtree J., Rein-Weston A. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 6.Randolph D. The neonatal adaptive immune system. NeoReviews. 2005;6:e454–e462. [Google Scholar]
- 7.Polin R.A., Committee on Fetus and Newborn Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(5):1006–1015. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi N., Cotten C.M., Smith P.B. Antibiotic use and misuse in the neonatal intensive care unit. Clin Perinatol. 2012;39(1):61–68. doi: 10.1016/j.clp.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotten C.M., McDonald S., Stoll B. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118(2):717–722. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 10.Cotten C.M., Taylor S., Stoll B. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuppala V.S., Meinzen-Derr J., Morrow A.L. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordero L., Ayers L.W. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol. 2003;24(9):662–666. doi: 10.1086/502270. [DOI] [PubMed] [Google Scholar]
- 13.Rhedin S., Lindstrand A., Rotzen-Ostlund M. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133(3):e538–e545. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 14.Ronchi A., Michelow I.C., Chapin K.C. Viral respiratory tract infections in the neonatal intensive care unit: the VIRIoN-I study. J Pediatr. 2014;165(4):690–696. doi: 10.1016/j.jpeds.2014.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett N.J., Tabarani C.M., Bartholoma N.M. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr. 2012;161(5):814–818. doi: 10.1016/j.jpeds.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner M., Strassl R., Straub J. Nosocomial rhinovirus infection in preterm infants. Pediatr Infect Dis J. 2012;31(12):1302–1304. doi: 10.1097/INF.0b013e31826ff939. [DOI] [PubMed] [Google Scholar]
- 17.Smith P.B., Benjamin D.K. Clinical approach to the infected neonate. In: Long S.S., Pickering L.K., Prober C.G., editors. Principles and practice of pediatric infectious diseases. 4th edition. Elsevier Saunders; Philadelphia: 2012. pp. 536–538. [Google Scholar]
- 18.Santos R.P., Sanchez P.J., Mejias A. Successful medical treatment of cutaneous aspergillosis in a premature infant using liposomal amphotericin B, voriconazole and micafungin. Pediatr Infect Dis J. 2007;26(4):364–366. doi: 10.1097/01.inf.0000258698.98370.89. [DOI] [PubMed] [Google Scholar]
- 19.Bizzarro M.J., Raskind C., Baltimore R.S. Seventy-five years of neonatal sepsis at Yale: 1928-2003. Pediatrics. 2005;116(3):595–602. doi: 10.1542/peds.2005-0552. [DOI] [PubMed] [Google Scholar]
- 20.Escobar G.J., Li D.K., Armstrong M.A. Neonatal sepsis workups in infants >/=2000 grams at birth: a population-based study. Pediatrics. 2000;106(2 Pt 1):256–263. doi: 10.1542/peds.106.2.256. [DOI] [PubMed] [Google Scholar]
- 21.Edwards M.S., Baker C.J. Bacterial infections in neonate. In: Long S.S., Pickering L., Prober C.G., editors. Principles and practice of pediatric infectious diseases. 4th edition. Elsevier Saunders; Philadelphia: 2012. pp. 538–543. [Google Scholar]
- 22.Piantino J.H., Schreiber M.D., Alexander K. Culture negative sepsis and systemic inflammatory response syndrome in neonates. NeoReviews. 2013;14:e294–e305. [Google Scholar]
- 23.Ottolini M.C., Lundgren K., Mirkinson L.J. Utility of complete blood count and blood culture screening to diagnose neonatal sepsis in the asymptomatic at risk newborn. Pediatr Infect Dis J. 2003;22(5):430–434. doi: 10.1097/01.inf.0000068206.11303.dd. [DOI] [PubMed] [Google Scholar]
- 24.Effective biomarkers for diagnosis of neonatal sepsis. J Ped Infect Dis. 2014;3(3):234–245. doi: 10.1093/jpids/piu063. [DOI] [PubMed] [Google Scholar]
- 25.Baron E.J., Miller J.M., Weinstein M.P. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a) Clin Infect Dis. 2013;57(4):e22–e121. doi: 10.1093/cid/cit278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mussap M. Laboratory medicine in neonatal sepsis and inflammation. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):32–34. doi: 10.3109/14767058.2012.715000. [DOI] [PubMed] [Google Scholar]
- 27.Lartigue M.F., Kostrzewa M., Salloum M. Rapid detection of “highly virulent” Group B Streptococcus ST-17 and emerging ST-1 clones by MALDI-TOF mass spectrometry. J Microbiol Methods. 2011;86(2):262–265. doi: 10.1016/j.mimet.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Mekouar H., Voortman G., Bernard P. Capnocytophaga species and perinatal infections: case report and review of the literature. Acta Clin Belg. 2012;67(1):42–45. doi: 10.2143/ACB.67.1.2062626. [DOI] [PubMed] [Google Scholar]
- 29.Iatta R., Cafarchia C., Cuna T. Bloodstream infections by Malassezia and Candida species in critical care patients. Med Mycol. 2014;52(3):264–269. doi: 10.1093/mmy/myt004. [DOI] [PubMed] [Google Scholar]
- 30.Schlebusch S., Price G.R., Hinds S. First outbreak of PVL-positive nonmultiresistant MRSA in a neonatal ICU in Australia: comparison of MALDI-TOF and SNP-plus-binary gene typing. Eur J Clin Microbiol Infect Dis. 2010;29(10):1311–1314. doi: 10.1007/s10096-010-0995-y. [DOI] [PubMed] [Google Scholar]
- 31.Pulcrano G., Roscetto E., Iula V.D. MALDI-TOF mass spectrometry and microsatellite markers to evaluate Candida parapsilosis transmission in neonatal intensive care units. Eur J Clin Microbiol Infect Dis. 2012;31(11):2919–2928. doi: 10.1007/s10096-012-1642-6. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Pediatrics . Antimicrobial therapy for newborns. In: Bradley J.S., editor. 2014 Nelson's pediatric antimicrobial therapy. American Academy of Pediatrics; Elk Grove Village (IL): 2014. pp. 17–36. [Google Scholar]
- 33.de Hoog M., Mouton J.W., van den Anker J.N. The use of aminoglycosides in newborn infants. In: Choonara I., Nun A.J., Kearns G., editors. Introduction to paediatric and perinatal drug therapy. Nottingham University Press; Notthingham (England): 2003. pp. 117–140. [Google Scholar]
- 34.Begg E.J., Barclay M.L., Kirkpatrick C.J. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 1999;47(1):23–30. doi: 10.1046/j.1365-2125.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novelli A., Mazzei T., Fallani S. In vitro postantibiotic effect and postantibiotic leukocyte enhancement of tobramycin. J Chemother. 1995;7(4):355–362. doi: 10.1179/joc.1995.7.4.355. [DOI] [PubMed] [Google Scholar]
- 36.Craig W.A. Once-daily versus multiple-daily dosing of aminoglycosides. J Chemother. 1995;7(Suppl 2):47–52. [PubMed] [Google Scholar]
- 37.Rotschafer J.C., Zabinski R.A., Walker K.J. Pharmacodynamic factors of antibiotic efficacy. Pharmacotherapy. 1992;12(6 Pt 2):64S–70S. [PubMed] [Google Scholar]
- 38.Bryan C.S., John J.F., Jr., Pai M.S. Gentamicin vs cefotaxime for therapy of neonatal sepsis. Relationship to drug resistance. Am J Dis Child. 1985;139(11):1086–1089. doi: 10.1001/archpedi.1985.02140130024022. [DOI] [PubMed] [Google Scholar]
- 39.Bradley J.S., Wassel R.T., Lee L. Intravenous ceftriaxone and calcium in the neonate: assessing the risk for cardiopulmonary adverse events. Pediatrics. 2009;123(4):e609–e613. doi: 10.1542/peds.2008-3080. [DOI] [PubMed] [Google Scholar]
- 40.Kimberlin D.W., Whitley R.J., Wan W. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med. 2011;365(14):1284–1292. doi: 10.1056/NEJMoa1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimberlin D.W., Baley J., Committee on Infectious Diseases Guidance on management of asymptomatic neonates born to women with active genital herpes lesions. Pediatrics. 2013;131(2):e635–e646. doi: 10.1542/peds.2012-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimberlin DW, Jester P, Sanchez PJ, et al. Six months versus six weeks of oral valganciclovir for infants with symptomatic congenital cytomegalovirus (CMV) disease with and without central nervous system (CNS) involvement: Results of a Phase III, randomized, double-blind, placebo-controlled, multinational study. 2013 ID Week. Infectious Disease Society of America. San Francisco (CA), October 5, 2013. Available at: https://idsa.confex.com/idsa/2013/webprogram/Paper43178.html. Accessed October 7, 2014.
- 43.Committee On Infectious Diseases Recommendations for prevention and control of influenza in children, 2014-2015. Pediatrics. 2014;134(5):e1503–e1519. doi: 10.1542/peds.2014-2413. [DOI] [PubMed] [Google Scholar]
- 44.Bizzarro M.J., Ehrenkranz R.A., Gallagher P.G. Concurrent bloodstream infections in infants with necrotizing enterocolitis. J Pediatr. 2014;164(1):61–66. doi: 10.1016/j.jpeds.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Segal I., Kang C., Albersheim S.G. Surgical site infections in infants admitted to the neonatal intensive care unit. J Pediatr Surg. 2014;49(3):381–384. doi: 10.1016/j.jpedsurg.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verani J.R., McGee L., Schrag S.J. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR–10):1–36. [PubMed] [Google Scholar]
- 47.Prevention CfDCa. Group B Strep (GBS). 2014. Available at: http://www.cdc.gov/groupBstrep/guidelines/index.html. Accessed October 8, 2014.
- 48.Polin R.A., Denson S., Brady M.T. Strategies for prevention of health care-associated infections in the NICU. Pediatrics. 2012;129(4):e1085–e1093. doi: 10.1542/peds.2012-0145. [DOI] [PubMed] [Google Scholar]
- 49.Boyce J.M., Pittet D., Healthcare Infection Control Practices Advisory Committee Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23(12 Suppl):S3–S40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 50.CDC. Hand Hygiene in Healthcare Settings. 2014. Available at: http://www.cdc.gov/handhygiene/. Accessed September 30, 2014.
- 51.Healy C.M., Pickering L.K. How to communicate with vaccine-hesitant parents. Pediatrics. 2011;127(Suppl 1):S127–S133. doi: 10.1542/peds.2010-1722S. [DOI] [PubMed] [Google Scholar]
- 52.American Academy of Pediatrics . Hepatitis B. In: Pickering L.K., editor. Red Book. American Academy of Pediatrics; Elk Grove Village (IL): 2012. pp. 369–390. [Google Scholar]
- 53.Committee on Infectious Diseases, American Academy of Pediatrics Recommended childhood and adolescent immunization schedule—United States, 2014. Pediatrics. 2014;133(2):357–363. doi: 10.1542/peds.2013-3965. [DOI] [PubMed] [Google Scholar]
- 54.Lessin H.R., Edwards K.M., Committee On Practice And Ambulatory Medicine Immunizing parents and other close family contacts in the pediatric office setting. Pediatrics. 2012;129(1):e247–e253. doi: 10.1542/peds.2011-2937. [DOI] [PubMed] [Google Scholar]
- 55.Sawyer M., Liang J.L., Messonnier N. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Recomm Rep. 2013;62(7):131–135. [PMC free article] [PubMed] [Google Scholar]
- 56.Healy C.M., Rench M.A., Baker C.J. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013;56(4):539–544. doi: 10.1093/cid/cis923. [DOI] [PubMed] [Google Scholar]
- 57.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 58.Basu S. Neonatal sepsis: the gut connection. Eur J Clin Microbiol Infect Dis. 2014 doi: 10.1007/s10096-014-2232-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Dellit T.H., Owens R.C., McGowan J.E., Jr. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 60.Thomas D.W., Greer F.R., American Academy of Pediatrics Committee on Nutrition Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126(6):1217–1231. doi: 10.1542/peds.2010-2548. [DOI] [PubMed] [Google Scholar]
- 61.Alfaleh K., Anabrees J., Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011;(3) doi: 10.1002/14651858.CD005496.pub3. CD005496. [DOI] [PubMed] [Google Scholar]
- 62.AlFaleh K., Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4) doi: 10.1002/14651858.CD005496.pub4. CD005496. [DOI] [PubMed] [Google Scholar]
- 63.American Academy of Pediatrics . Transmission-based precautions. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red ook: 2012 report of the committee on infectious diseases. 29th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2012. pp. 164–167. [Google Scholar]