Abstract
The Sofia™ Influenza A + B FIA demonstrated 74.0% sensitivity and 95.4% specificity for influenza A in patients with influenza-like illness in 2012–2013 season. It yielded higher sensitivity than SD Bioline Influenza Ag A/B/A(H1N1/2009) (54.1%) for influenza A (P < 0.01). The Sofia™ Influenza A + B FIA might be useful for rapid diagnosis of influenza.
Keywords: Influenza A virus, Point-of-care systems
Highlights
-
•
Sofia™ Influenza A + B FIA is a fluorescent immunoassay of “walk-away” mode for diagnosis of influenza using analyzer.
-
•
Sofia™ Influenza A + B FIA showed relatively high sensitivity for influenza A viruses during 2012–2013 season in Northern Hemisphere.
-
•
Sofia™ Influenza A + B FIA might be useful for rapid diagnosis of influenza.
A rapid diagnosis of influenza allows for early decision making and timely intervention in patients with influenza-like illness (ILI). It is also advantageous in the selection of an appropriate antiviral treatment, the reduction of antibiotics use, and the avoidance of unnecessary diagnostic examination, which, in turn, reduces medical costs (Bonner et al., 2003, Nitsch-Osuch et al., 2013). Rapid influenza diagnostic tests (RIDTs) have been widely used in clinical practice to diagnose influenza because they are easy to use and provide results within 10–15 minutes (Centers fro Disease Control and Prevention (CDC), 2013, Cho et al., 2013). However, the results of conventional RIDTs are limited in reliability due to various and unsatisfactory performances (Centers fro Disease Control and Prevention (CDC), 2013, Cho et al., 2013).
The sensitivity of an RIDT varies depending on the type of test and has been shown to be affected by multiple factors: study population, elapsed time from symptom onset, viral titer, sample type and status, circulating influenza virus, and epidemic size. The clinical performances of RIDTs have either been evaluated in a clinical field or by using frozen samples, with reported sensitivities ranging from 40.3% to 73.3% compared to PCR-based detection (Choi et al., 2011, Leonardi et al., 2013, Stripeli et al., 2010, Sutter et al., 2012).
The Sofia™ Influenza A + B FIA (Quidel Corporation, CA, USA) is a novel fluorescent immunoassay used to detect influenza A and B within 15 minutes using the Sofia Analyzer (Lewandrowski et al., 2013). In this study, the clinical performance of the Sofia™ Influenza A + B FIA was prospectively evaluated and compared with the performance of the SD Bioline Influenza Ag A/B/A(H1N1/2009) (Standard Diagnostics, Yongin, South Korea) in cases of ILI in adult patients.
From December 23, 2012, to April 11, 2013, a prospective study was conducted in 3 teaching hospitals in South Korea. Two nasopharyngeal swabs were obtained from adult patients (≥18 years) with ILI who visited an emergency department or outpatient clinic. ILI was defined as an acute respiratory infection with measured fever of ≥38 °C and a cough that occurred within 7 days.
Among 2 nasopharyngeal swabs, 1 flocked swab was randomly placed in 900 μL of viral transport medium (VTM) (BD, NJ, USA) and was used to perform promptly RIDTs at patients' bedsides. After agitating the flocked swab thoroughly in a vial, samples were tested using both Sofia™ Influenza A + B FIA and SD Bioline Influenza Ag A/B/A(H1N1/2009) simultaneously. All procedures were conducted according to the manufacturers' protocols (Quidel, n.d, Standard Diagnostics, n.d).
The other flocked swab was immediately placed in 3 mL of VTM (BD), and samples were kept at −70 °C until use. Total RNA was extracted automatically by Nimbus (Hamilton Robotics, Reno, NV, USA) as per the manufacturer's protocol. Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using Anyplex™ II RV16 Detection (Seegene, Seoul, South Korea) to detect influenza A and B viruses. Among the samples tested, those positive for influenza A virus were selected and tested to differentiate subtype using Seeplex® Influenza A/B Onestep Typing (Seegene) and the PowerChek™ Influenza SIH1/H3/H5 Real-time RT PCR kit (Kogenebiotech, Seoul, South Korea).
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each RIDT were calculated using the results of RT-PCR as a gold standard. A McNemar test was performed to compare the sensitivities of each test. To calculate the sensitivity of a test according to age and time from symptom onset to hospital visit, a chi-square test or Fisher's exact test was used. P < 0.05 was considered to be statistically significant.
The study protocol was approved by the institutional review board in each hospital: Korea University Guro Hospital (MD12024-001), Korea University Ansan Hospital (ASMD12-002), and Hallym University Kangnam Sacred Heart Hospital (2012-11-102).
A total of 394 patients with ILI were enrolled in this study, and the median age of patients was 40 years (interquartile range [IQR], 30–59). Influenza vaccination (for the 2012–2013 season) rate was 44.2% (174/394). At least 1 of chronic underlying disease was detected in 111 patients (28.2%). Median time from symptom onset to sample collection was 1 day (IQR, 0–2) (Table 1 ).
Table 1.
Demographic characteristics of patients with influenza-like illness.
| Parameter | Influenza positive (n = 199) | Influenza negative (n = 195) | P |
|---|---|---|---|
| Sex (male), n (%) | 61 (30.7) | 87 (44.6) | <0.01 |
| Age (years), mean ± SD | 43.4 ± 17.8 | 46.7 ± 19.2 | 0.14 |
| Any comorbidity | 39 (19.6) | 72 (36.9) | <0.01 |
| Influenza vaccination (2012–2013) | 85 (42.7) | 89 (45.6) | 0.6 |
| Time to hospital visit from symptom onset (days), mean ± SD | 1.5 ± 1.4 | 1.5 ± 1.7 | 0.40 |
| Detected virus | |||
| Influenza A virus | 196 (98.5) | ||
| H3N2 | 149 (74.9) | ||
| A(H1N1)pdm09 | 36 (18.1) | ||
| Subtype not determined | 11 (5.5) | ||
| Influenza B virus | 3 (1.5) | ||
| Other respiratory viruses | 62 (31.8) | ||
| Negative | 133 (68.2) |
Among 394 specimens, 196 (49.7%) tested positive for influenza A virus by real-time RT-PCR. H3N2 influenza virus was the dominant strain (149/196, 76.0%) and A(H1N1)pdm09 virus was detected from 18.4% patients (36/196). Among 196 influenza A viruses, subtypes of 11 samples were not determined. Samples in which other respiratory viruses were detected accounted for 62. Sixty-eight respiratory viruses were detected in 62 samples: human metapneumovirus, 18; coronavirus, 18; human rhinovirus, 16; respiratory syncytial virus, 9; adenovirus, 5; parainfluenza virus, 1; bocavirus, 1.
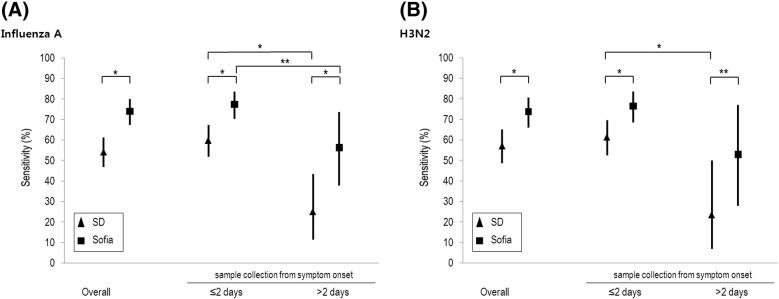
The performance of the Sofia™ Influenza A + B FIA for the influenza A virus was estimated as follows: sensitivity 74.0% (95% confidence interval [CI], 67.3–80.0), specificity 95.4% (95% CI, 91.4–97.9), PPV 94.2% (95% CI, 89.2–97.3), and NPV 78.5% (95% CI, 72.7–83.5) (Table 2 ). The SD Bioline Influenza Ag A/B/A(H1N1/2009) showed sensitivity of 54.1% (95% CI, 46.8–61.2), specificity of 95.9% (95% CI, 92.1–98.2), PPV of 93.0% (95% CI, 86.6–96.9), and NPV of 67.5% (95% CI, 61.7–73.0). The Sofia™ Influenza A + B FIA yielded higher sensitivity than SD Bioline Influenza Ag A/B/A(H1N1/2009) for influenza A virus significantly (P < 0.01) (Fig. 1 ).
Table 2.
Performance of rapid influenza diagnostic tests compared to PCR-based detection of influenza A virus.
| Test | Influenza | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|---|
| Sofia Influenza A + B FIA | Influenza A | 145/196, 74.0 (67.3–80.0) | 186/195, 95.4 (91.4–97.9) | 145/154, 94.2 (89.2–97.3) | 186/237, 78.5 (72.7–83.5) |
| 18–49 yearsa | 101/132, 76.5 (68.4–83.5) | 103/109, 94.5 (88.4–97.9) | 101/107, 94.4 (88.2–97.9) | 103/134, 76.9 (68.8–83.7) | |
| ≥50 yearsa | 44/64, 68.8 (55.9–79.8) | 83/86, 96.5 (90.1–99.2) | 44/47, 93.6 (82.4–98.6) | 83/103, 80.6 (71.6–87.7) | |
| ≤2 daysb | 127/164, 77.4 (70.3–83.6) | 144/151, 95.4 (90.7–98.1) | 127/134, 94.8 (89.5–97.9) | 144/181, 79.6 (72.9–85.2) | |
| >2 daysb | 18/32, 56.3 (37.7–73.6) | 42/44, 95.5 (84.5–99.3) | 18/20, 90.0 (68.3–98.5) | 42/56, 75.0 (61.6–85.6) | |
| H3N2 | 110/149, 73.8 (66.0–80.7) | 186/195, 95.4 (91.4–97.9) | 110/119, 92.4 (86.1–96.5) | 186/225, 82.7 (77.1–87.4) | |
| 18–49 yearsa | 71/94, 75.5 (65.6–83.8) | 103/109, 94.5 (88.4–97.9) | 71/77, 92.2 (83.8–97.1) | 103/126, 81.8 (73.9–88.1) | |
| ≥50 yearsa | 39/55, 70.9 (57.1–82.4) | 83/86, 96.5 (90.1–99.2) | 39/42, 92.9 (80.5–98.4) | 83/99, 83.8 (75.1–90.5) | |
| ≤2 daysb | 101/132, 76.5 (68.4–83.5) | 144/151, 95.4 (90.7–98.1) | 101/108, 93.5 (87.1–97.3) | 144/175, 82.3 (75.8–87.6) | |
| >2 daysb | 9/17, 52.9 (27.9–77.0) | 42/44, 95.5 (84.5–99.3) | 9/11, 81.8 (48.2–97.2) | 42/50, 84.0 (70.9–92.8) | |
| SD Bioline Influenza Ag A/B/A(H1N1/2009) | Influenza A | 106/196, 54.1 (46.8–61.2) | 187/195, 95.9 (92.1–98.2) | 106/114, 93.0 (86.6–96.9) | 187/277, 67.5 (61.7–73.0) |
| 18–49 yearsa | 73/132, 55.3 (46.4–64.0) | 105/109, 96.3 (90.9–99.0) | 73/77, 94.8 (87.2–98.5) | 105/164, 64.0 (56.2–71.4) | |
| ≥50 yearsa | 33/64, 51.6 (38.7–64.3) | 82/86, 95.4 (88.5–98.7) | 33/37, 89.2 (74.6–96.9) | 82/113, 72.6 (63.4–80.5) | |
| ≤2 daysb | 98/164, 59.8 (51.8–67.3) | 144/151, 95.4 (90.7–98.1) | 98/105, 93.3 (86.7–97.3) | 144/210, 68.6 (61.8–74.8) | |
| >2 daysb | 8/32, 25.0 (11.5–43.4) | 43/44, 97.7 (87.9–99.6) | 8/9, 88.9 (51.7–98.2) | 43/67, 64.2 (51.5–75.5) | |
| H3N2 | 85/149, 57.1 (48.7–65.1) | 187/195, 95.9 (92.1–98.2) | 85/93, 91.4 (83.8–96.2) | 187/251, 74.5 (68.6–79.8) | |
| 18–49 yearsa | 54/94, 57.5 (46.8–67.6) | 105/109, 96.3 (90.9–99.0) | 54/58, 93.1 (83.3–98.1) | 105/145, 72.4 (64.4–79.5) | |
| ≥50 yearsa | 31/55, 56.4 (42.3–69.7) | 82/86, 95.4 (88.5–98.7) | 31/35, 88.6 (73.2–96.7) | 82/106, 77.4 (68.2–84.9) | |
| ≤2 daysb | 81/132, 61.4 (52.5–69.7) | 144/151, 95.4 (90.7–98.1) | 81/88, 92.1 (84.3–96.7) | 144/195, 73.9 (67.1–79.9) | |
| >2 daysb | 4/17, 23.5 (7.0–49.9) | 43/44, 97.7 (87.9–99.6) | 4/5, 80.0 (28.8–96.7) | 43/56, 76.8 (63.6–87.0) |
Patient's age.
Time to hospital visit from symptom onset.
Fig. 1.
Test sensitivities were compared between the Sofia™ Influenza A + B FIA and the SD Bioline Influenza Ag A/B/A(H1N1/2009) (A) influenza A virus (B) A/H3N2 influenza virus. The black bars represent a 95% confidence interval. ⁎P < 0.01, ⁎⁎P < 0.05.
The sensitivities of both RIDTs for influenza A virus were higher for patients who had visited the hospital within 2 days of symptom onset than for patients who visited hospital more than 2 days after symptom onset (77.4% versus 56.3% using the Sofia™ Influenza A + B FIA, P = 0.01; 59.8% versus 25.0% by the SD Bioline Influenza Ag A/B/A(H1N1/2009), P < 0.01). The Sofia™ Influenza A + B FIA showed sensitivity of 76.5% in patients aged 18–49 years and 68.8% in patients ≥50 years (P = 0.25). The sensitivity of SD Bioline Influenza Ag A/B/A(H1N1/2009) did not significantly differ according to age group (55.3% in 18–49 years and 51.6% in patients ≥50 years, P = 0.62).
For A/H3N2 influenza virus, sensitivity, specificity, PPV, and NPV of the Sofia™ Influenza A + B FIA were 73.8% (95% CI, 66.0–80.7), 95.4% (95% CI, 91.4–97.9), 92.4% (95% CI, 86.1–96.5), and 82.7% (95% CI, 77.1–87.4), respectively. The Sofia™ Influenza A + B FIA yielded higher sensitivity than the SD Bioline Influenza Ag A/B/A(H1N1/2009) for H3N2 influenza (73.8% versus 57.1%, P < 0.01). For the A(H1N1)pdm09 virus, the Sofia™ Influenza A + B FIA showed a sensitivity of 91.7% (33/36; 95% CI, 77.5–98.2), a specificity of 95.4% (186/195; 95% CI. 91.4–97.9), a PPV of 78.6% (33/42; 95% CI, 63.2–89.7), and an NPV of 98.4% (186/189; 95% CI, 95.4–99.7). In contrast, the SD Bioline Influenza Ag A/B/A(H1N1/2009) detected A(H1N1)pdm09 virus in 6 patients (sensitivity, 16.7%; 95% CI, 6.4-32.8).
In a previous study, the sensitivity of the Sofia™ Influenza A + B FIA was reported to be 78.1% during the 2011–2012 influenza season, when implemented at the bedsides of infants and children (Rath et al., 2012). Using stored samples, it displayed a sensitivity of 82.2% for influenza A, compared to real-time RT-PCR; however, the mean age of the study population was younger (21.3 years old) than ours (Lee et al., 2012). In another prospective study, the sensitivity of Sofia™ Influenza A + B FIA for the influenza A virus, compared to real-time RT-PCR, was 85% and 69% using nasal or nasopharyngeal swabs, respectively (Lewandrowski et al., 2013). Although more than 90% of those patients were younger than 22 years old, this study showed a lower sensitivity for detection of influenza A using nasopharyngeal swab than our results (Lewandrowski et al., 2013). However, direct comparison of the performance of RIDTs between these studies should be limited in interpretation because the clinical performance of RIDTs can be affected by multiple factors, some of which are uncontrollable.
In our study, the sensitivities of both RIDTs for influenza A virus were higher in patients who visited the hospital within 2 days of symptom onset, compared to those who visited hospital 2 days after the symptom onset. This might be due to high viral titer during the early infection phase, and this finding is consistent with a previous study (Choi et al., 2011). However, in a previous report, the sensitivity of RIDT was low during the very early period (within 3 hours of symptom onset) and the time interval from onset to consultation was shorter in RIDT false-negative group (5.5 hours) than in true-positive group (11.5 hours) (Harada et al., 2012). Time from symptom onset to hospital visit was recorded in the unit of day in our study; thus, analysis based on hours was not available.
This study has some limitations. First, we used limited commercial RT-PCR kits as a gold standard. The sensitivities to detect influenza viruses vary with each commercial kit. Second, samples were used promptly for Sofia™ Influenza A + B FIA after specimen collection. However, samples for PCR were eluted in 3 mL of VTM and were frozen at −70 °C and used after thawing. There is a chance that influenza virus RNA was diluted in VTM. Freezing and thawing can reduce viral titer in clinical samples. These limitations can produce false negatives in PCR, causing false positives of the Sofia™ Influenza A + B FIA in this study. Also, there is a chance that uneven distribution of influenza virus between 2 nasopharyngeal swabs could affect the results. Only adult patients were included in this study and relatively lower viral titer in adult than children could affect low sensitivity of RIDTs. However, viral titer was not determined. During the study period, influenza B virus rarely circulated; thus, we did not evaluate the performance of RIDTs for influenza B. In addition, direct comparison of sensitivity against influenza A(H1N1)pdm09 between Sofia™ Influenza A + B FIA and SD Bioline Influenza Ag A/B/A(H1N1/2009) is limited in interpretation. Because the Sofia™ Influenza A + B FIA distinguishes influenza A and B, whereas the SD Bioline Influenza Ag A/B/A(H1N1/2009) has 3 lines to differentiate influenza A, B, and influenza A(H1N1)pdm09.
Despite limitations, this study is valuable as a prospective study on the performance of the Sofia™ Influenza A + B FIA in the clinical field during the 2012–2013 influenza season in Northern Hemisphere. Due to its ability to provide relatively high sensitivity in the detection of the influenza A virus, it will be one of viable tools for the rapid diagnosis of influenza in clinical practice.
Funding
This work was supported by a grant of the TEPIK (Transgovernmental Enterprise for Pandemic Influenza in Korea), which is part of the Korea Healthcare Technology R&D Project by Ministry of Health & Welfare, Republic of Korea (grant number A103001) and Dow Biomedica (Seoul, South Korea). The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
This study was supported in part by Dow Biomedica (Seoul, Korea).
References
- Bonner A.B. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2):363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- Centers fro Disease Control and Prevention (CDC) Evaluation of 11 commercially available rapid influenza diagnostic tests--United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2012;61(43):873–876. [PubMed] [Google Scholar]
- Cho C.H., Woo M.K., Kim J.Y., Cheong S., Lee C.K., An S.A. Evaluation of five rapid diagnostic kits for influenza A/B virus. J Virol Methods. 2013;187(1):51–56. doi: 10.1016/j.jviromet.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Choi W.S., Noh J.Y., Huh J.Y., Kee S.Y., Jeong H.W., Lee J. The clinical usefulness of the SD Bioline Influenza Antigen Test(R) for detecting the 2009 influenza A (H1N1) virus. Yonsei Med J. 2011;52(4):683–685. doi: 10.3349/ymj.2011.52.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada D., Nishiuchi R., Iwasaki Y., Watanabe H., Tokorodani C., Kanazawa A. Reliability of a rapid test for the clinical diagnosis of influenza A/H1N1 2009. Scand J Infect Dis. 2012;44(10):776–781. doi: 10.3109/00365548.2012.686670. [DOI] [PubMed] [Google Scholar]
- Lee C.K. Evaluation of Sofia fluorescent immunoassay analyzer for influenza A/B virus. J Clin Virol. 2012;55(3):239–243. doi: 10.1016/j.jcv.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Leonardi G.P., Wilson A.M., Zuretti A.R. Comparison of conventional lateral-flow assays and a new fluorescent immunoassay to detect influenza viruses. J Virol Methods. 2013;189(2):379–382. doi: 10.1016/j.jviromet.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Lewandrowski K. Detection of influenza a and B viruses with the sofia analyzer: a novel, rapid immunofluorescence-based in vitro diagnostic device. Am J Clin Pathol. 2013;139(5):684–689. doi: 10.1309/AJCP7ZTLJCP3LLMA. [DOI] [PubMed] [Google Scholar]
- Nitsch-Osuch A., Stefanska I., Kuchar E., Brydak L.B., Pirogowicz I., Zycinska K. Influence of rapid influenza test on clinical management of children younger than five with febrile respiratory tract infections. Adv Exp Med Biol. 2013;755:237–241. doi: 10.1007/978-94-007-4546-9_30. [DOI] [PubMed] [Google Scholar]
- Quidel Sofia® Influenza A+B FIA. https://www.quidel.com/sites/default/files/product/documents/sofia_influenza_pi.pdf Available at: [Accessed April 18, 2015]
- Rath B., Tief F., Obermeier P., Tuerk E., Karsch K., Muehlhans S. Early detection of influenza A and B infection in infants and children using conventional and fluorescence-based rapid testing. J Clin Virol. 2012;55(4):329–333. doi: 10.1016/j.jcv.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Standard Diagnostics SD BIOLINE Influenza Ag A/B/A(H1N1) pandemic. http://www.standardia.com/en/home/product/rapid/infectious-disease/Influenza_Ag_A-B-A-H1N1.html Available at: [Accessed April 18, 2015]
- Stripeli F., Sakkou Z., Papadopoulos N., Georgiou V., Gratsia P., Christodoulou I. Performance of rapid influenza testing in hospitalized children. Eur J Clin Microbiol Infect Dis. 2010;29(6):683–688. doi: 10.1007/s10096-010-0914-2. [DOI] [PubMed] [Google Scholar]
- Sutter D.E., Worthy S.A., Hensley D.M., Maranich A.M., Dolan D.M., Fischer G.W. Performance of five FDA-approved rapid antigen tests in the detection of 2009 H1N1 influenza A virus. J Med Virol. 2012;84(11):1699–1702. doi: 10.1002/jmv.23374. [DOI] [PubMed] [Google Scholar]