Abstract
Viral fusion proteins contain a highly hydrophobic segment, named the fusion peptide, which is thought to be responsible for the merging of the cellular and viral membranes. Paramyxoviruses are believed to contain a single fusion peptide at the N terminus of the F1 protein. However, here we identified an additional internal segment in the Sendai virus F1 protein (amino acids 214–226) highly homologous to the fusion peptides of HIV-1 and RSV. A synthetic peptide, which includes this region, was found to induce membrane fusion of large unilamellar vesicles, at concentrations where the known N-terminal fusion peptide is not effective. A scrambled peptide as well as several peptides from other regions of the F1 protein, which strongly bind to membranes, are not fusogenic. The functional and structural characterization of this active segment suggest that the F1 protein has an additional internal fusion peptide that could participate in the actual fusion event. The presence of homologous regions in other members of the same family suggests that the concerted action of two fusion peptides, one N-terminal and the other internal, is a general feature of paramyxoviruses.
Keywords: fusion peptide, membrane fusion, paramyxoviridae, fluorescence, viral entry
Abbreviations: BOC, butyloxycarbonyl; CD, circular dichroism; DMSO, dimethyl sulfoxide; HF, hydrogen fluoride; HIV, human immunodeficiency virus; LUV, large unilamellar vesicles; NBD-F, 4-fluoro-7-nitrobenz-2-oxa-1,3-diazole; NMR, nuclear magnetic resonance; Pam, phenylacetamido-methyl; PBS, phosphate-buffered saline; PC, egg phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; RP-HPLC, reverse phase high-performance liquid chromatography; Rho, tetra-methylrhodamine; RET, resonance energy transfer; RSV, respiratory syncytial virus; SIV, simian immunodeficiency virus; SUV, small unilamellar vesicles; TFA, trifluoroacetic acid
Introduction
Infection of eukaryotic cells by enveloped viruses requires fusion between the viral envelope and the cellular plasma or endosomal membrane Hoekstra and Kok 1989, Stegmann et al 1989. Specific viral envelope glycoproteins help to overcome the energy barriers associated with this process. Most of the known viral fusion proteins are integral membrane proteins that contain a region consisting of about 15–30 mostly apolar amino acids, named the fusion peptide (White, 1990). This region can be located either at the N terminus, as in most orthomyxoviruses, paramyxoviruses, and several retroviruses White et al 1983, Blumberg et al 1985, Gallaher 1987, or in the interior of the fusion protein, as in Rous sarcoma virus (Hunter et al., 1983), vesicular stomatitis virus (Whitt et al., 1990), or Ebola virus (Gallaher, 1996). Various studies have shown the importance of the fusion peptide in membrane fusion. Mutational analysis revealed that substituting critical residues in influenza hemagglutinin (Gething et al., 1986), SIV (Bosch et al., 1989), HIV (Freed et al., 1992), vesicular stomatitis virus (Fredericksen & Whitt, 1995), avian leukosis virus (Hernandez & White, 1998), or murine coronavirus (Luo & Weiss, 1998) fusion peptides is sufficient to abolish membrane fusion. However, mutational studies are sometimes difficult to analyze, since in addition to the local effects, single mutations can also affect the overall folding of the fusion protein. Hydrophobic photoaffinity labeling has been used to show that the fusion peptide of influenza hemagglutinin penetrates deeply into the target membrane during fusion (Harter et al., 1989), and has also helped to identify the fusion domain of the envelope glycoprotein of rabies and vesicular stomatitis viruses (Durrer et al., 1995).
Since the mechanism of membrane fusion is an intricate phenomenon involving the participation of complex proteins, it is difficult to determine the specific role of the fusion peptide in the context of the full-length fusion protein. In an effort to overcome this limitation, the interaction between model membranes and synthetic peptides that mimic the corresponding region in the intact protein has been studied Rafalski et al 1990, Yeagle et al 1991, Martin et al 1994, Nieva et al 1994, Rapaport and Shai 1994, Kliger et al 1997, Ruiz-Arguello et al 1998. The significance of this strategy has been demonstrated by two observations: (i) there is a direct correlation between the effects of mutations in the intact protein and the peptide analogs Freed et al 1992, Horvath and Lamb 1992, Rapaport and Shai 1994, Pereira et al 1995, Martin et al 1996, Kliger et al 1997, Pritsker et al 1999, and (ii) the fusion activity of synthetic peptides, measured in vitro, is sensitive to factors (such as pH or the addition of inhibitory agents) that affect the infectivity of the virus in vivo Wharton et al 1988, Pereira et al 1997.
The family of paramyxoviridae includes well-known respiratory tract pathogens affecting children, which were shown to be a major cause of croup, pneumonia and bronchiolitis (Collins et al., 1996). A prominent feature of paramyxoviridae infection is the fusion of infected cells with their neighbors, leading to the formation of multinucleated cells named syncytia (Lamb & Kolakofsky, 1996), thus highlighting the importance of understanding the mechanism of membrane fusion. The fusion protein of Sendai virus, a member of the paramyxoviridae family, is synthesized as an inactive precursor, F0, which is activated by a host protease, producing two disulfide-linked subunits, F1 and F2 Homma and Ohuchi 1973, Scheid and Choppin 1977. The newly formed N terminus of F1 is known as the fusion peptide (Gething et al., 1978). However, it is unlikely that the whole membrane fusion process is accounted for by a small N-terminal segment of a protein that consists of more than 560 amino acid residues. Moreover, it has been shown that regions other than the fusion peptide in influenza hemagglutinin Yu et al 1994, Epand et al 1999, HIV-1 (Rabenstein & Shin, 1995), and Sendai virus Ghosh et al 1998, Ben-Efraim et al 1999 are able to bind to membranes, thus suggesting that several membrane-interacting segments may have a role in the fusion process. In an attempt to identify regions other than the N-terminal fusion peptide in the Sendai virus F1 protein that could participate in the actual merging of the viral and cellular membranes, we screened its ectodomain for segments with homology to known fusion peptides and found a region, corresponding to amino acids 214–226, highly homologous to the fusion peptide of the HIV-1 and RSV envelope glycoproteins. In order to analyze the fusogenic ability of this specific region, located in the interior of the F1 protein, we synthesized two peptides corresponding to amino acids 208–229 and 201–229, namely SV-208 and SV-201, and compared their ability to induce membrane fusion of large unilamellar vesicles to that of the N-terminal 33-amino acid fusion peptide. We used as controls, a peptide that partially overlaps the N terminus of SV-208 (amino acids 178–210) and one with the same amino acid composition as SV-208, but with a scrambled sequence. We found that SV-208 and SV-201 induce membrane fusion at concentrations at which the N-terminal fusion peptide, the shifted, and the scrambled peptides are inactive. Based on the results presented here, we postulate a revised model for paramyxovirus-induced membrane fusion.
Results
An internal region in the ectodomain of Sendai virus F1 protein is homologous to known fusion peptides
In order to identify new regions, apart from the N-terminal fusion peptide, which could be involved in the actual fusion process, we screened the extracellular domain of the Sendai virus F1 protein for segments with homology to known fusion peptides. We found a 13-residue segment (amino acids 214–226) highly homologous to the HIV-1 gp41 (amino acids 8–20) and RSV F1 (amino acids 1–13) fusion peptides (Figure 1(a)). In order to determine the role of the fusion peptide-like segment in the process of membrane fusion, we synthesized a peptide corresponding to amino acids 208–229 of the Sendai virus F1 protein, namely SV-208, (Figure 1(b) and (c)) and compared its fusogenic ability to that of the N-terminal fusion peptide from the same protein (amino acids 117–149). Since the fusion peptide-like sequence is located in the interior of the F1 protein, a peptide elongated seven amino acids to the N terminus, SV-201, was also synthesized. A peptide with the same amino acid composition as SV-208, but with the fusion peptide-like sequence scrambled, namely Mu-SV-208, and one that partially overlaps its N terminus (amino acids 178–210), namely SV-178, were used as controls.
Figure 1.
(a) Sequence alignment of the fusion peptide-like sequence of SV-208 (amino acids 214–226), with homologous regions in the fusion peptide of HIV-1 gp41 (amino acids 8–20) and RSV (amino acids 1–13); (b) representation of the F1 subunit of the fusion protein of Sendai virus; (c) designations and sequences of the peptides used. Mutated amino acids are underlined.
The internal fusion peptide induces lipid mixing to a higher extent than the N-terminal fusion peptide
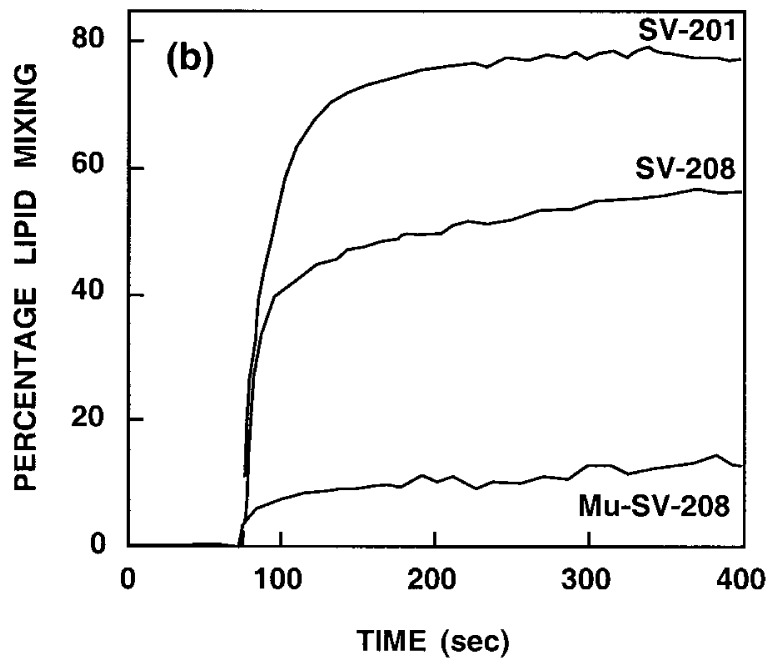
The fusogenic activity of the peptides was determined by their ability to cause lipid mixing of large unilamellar vesicles (LUV) composed of PC/PG (1:1) (100 nm diameter) as revealed by the probe dilution assay (Struck et al., 1981). Briefly, a population of LUV labeled both with NBD-PE and Rho-PE (22 μM) is mixed with a population of unlabeled LUV (88 μM) and different concentrations of peptides are added. Fusion between the labeled and unlabeled vesicles caused by the peptide results in dilution of the labeled lipids and therefore reduced energy transfer between NBD-PE and Rho-PE. This change is visualized as an increase of NBD fluorescence. The dependence of both the extent and the kinetics of lipid mixing on the lipid to peptide molar ratio were analyzed. In separate experiments, increasing amounts of SV-208, SV-201, Mu-SV-208, SV-178, and the N-terminal fusion peptide, were added to a fixed amount of vesicles. In order to compare the activity of the different peptides, the percentage of lipid mixing as a function of the lipid to peptide molar ratio is shown in Figure 2(a). Clearly, SV-208 is more active than the N-terminal fusion peptide in inducing lipid mixing of model membranes. Elongation of SV-208 does not affect its activity, suggesting that the ability of SV-208 to induce membrane fusion is not related to the N-terminal proximity of the fusion peptide-like sequence in the synthetic peptide. On the contrary, the scrambled peptide and SV-178 are substantially less active, indicating that the fusogenic activity depends on the peptide sequence. Furthermore, a peptide corresponding to the homologous region from measles virus was also active (unpublished results), suggesting a similar role for the homologous region in different paramyxoviruses. Similar results were observed with PC/PS (1:1) LUV and therefore are not shown. Time profiles depicted in Figure 2(b) show that maximal activities were reached at about five minutes.
Figure 2.
(a) Dose dependence of lipid mixing of PC/PG (1:1) LUV induced by different peptides. Peptide aliquots were added to mixtures of LUV (22 μM) containing 0.6% each of NBD-PE and Rho-PE, and unlabeled LUV (88 μM) in PBS. The increase in the fluorescence was measured 15 minutes after the addition of the peptide. The fluorescence intensity upon the addition of reduced Triton X-100 (0.25%, v/v) was referred to as 100%. Symbols: SV-208, filled squares; SV-201, open squares; N-terminal fusion peptide, open triangles; Mu-SV-208, filled triangles; SV-178, open circles. (b) Time-course of lipid mixing induced by SV-201, SV-208, and Mu-SV-208, under the same conditions described in (a) at 0.12 lipid/peptide molar ratio. Time-course traces corresponding to the N-terminal fusion peptide and SV-178 were similar to that of Mu-SV-208, and therefore are not shown.
Intervesicular lipid mixing is a result of membrane fusion
In order to confirm that the observed intervesicular lipid mixing was indeed the result of membrane fusion, suspensions of LUV were directly visualized under an electron microscope, before and after the treatment with the peptides. Briefly, PC/PG (1:1) LUV of 100 nm diameter (200 μM) were incubated for 15 minutes alone, with SV-208, or Mu-SV-208 (peptide/lipid molar ratio of 0.12) in PBS, before visualization. Figure 3 shows representative micrographs of the LUV (a) without any peptide, (b) with SV-208, and (c) with Mu-SV-208. It is evident from the micrographs that the lipid mixing observed with SV-208 appears concurrently with an increase in size of the vesicles, confirming that the ability of SV-208 to induce lipid mixing is the result of membrane fusion. Such a size increase was not observed with the N-terminal fusion peptide (Ghosh & Shai, 1999).
Figure 3.

Electron micrographs of negatively stained vesicles. (a) PC/PG (1:1) LUV alone; (b) incubated with SV-208 for 15 minutes; (c) incubated with Mu-SV-208 for 15 minutes. The bar represents 200 nm.
SV-208 and Mu-SV-208 have similar membrane-binding affinities
In order to determine whether the difference between the fusogenic activity of SV-208 and Mu-SV-208 was due to a difference in their membrane-binding ability, the increases in the fluorescence intensities of NBD-labeled peptides, because of membrane binding, were recorded as a function of the lipid to peptide molar ratios. The binding curves obtained for both peptides are very similar (Figure 4(a)). Since both SV-208 and Mu-SV-208 are monomeric in aqueous solution, as revealed by rhodamine dequenching experiments (data not shown), we were able to analyze the binding isotherms as partition equilibria (Rapaport & Shai, 1991). The surface partition coefficients were estimated from the initial slopes of the curves shown in Figure 4(b) (Beschiaschvili & Seelig, 1990), and were found to be 6.7(±0.4) × 104 M−1, and 7.3(±0.2) × 104 M−1, for SV-208 and Mu-SV-208, respectively. Since these values are similar, we have concluded that the peptides have similar membrane-binding affinities. However, as the concentration of peptide increases, the bound fraction, corresponding to SV-208, increases faster than that of Mu-SV-208, suggesting positive cooperativity for the binding of the wild-type peptide. Since this indicates that SV-208 may self-associate in the membrane, the oligomerization state of the membrane-bound peptides was further analyzed (see below).
Figure 4.
(a) Increase in the fluorescence of NBD-labeled peptides (0.2 μM) upon titration with PC/PG (1:1) SUV. Titrations were performed at room temperature in PBS. The excitation wavelength was set at 467 nm (8 nm slit) and emission recorded at 530 nm (8 nm slit). Symbols: SV-208, open circles; Mu-SV-208, filled squares; (b) binding isotherms derived from (a) by plotting Xb∗ (the molar ratio of bound peptide/60% of the total lipid) versus Cf (equilibrium concentration of free peptide in solution). Symbols: SV-208, open circles; Mu-SV-208, filled squares.
The amino terminus of SV-208 and Mu-SV-208 is relocated to a more hydrophobic environment upon addition of vesicles
The amino terminus of several fusion peptides has been shown to be inserted in the membrane Rapaport and Shai 1994, Kliger et al 1997. In order to determine whether this was also true for SV-208, the fluorescence of NBD-labeled peptides was analyzed upon binding to the membrane. The sensitivity of the NBD moiety to the dielectric constant of its surroundings allows us to determine the environment of the NBD-labeled peptides in their membrane-bound state. It has already been shown that the fluorescence emission of NBD shifts to lower wavelengths upon relocation of the NBD moiety to a more hydrophobic environment (Rajarathnam et al., 1989). The fluorescence emission spectra of NBD-labeled SV-208 and Mu-SV-208 were measured in aqueous solution and in the presence of PC/PG (1:1) SUV. In both cases a shift toward lower wavelengths, with a concomitant increase in the fluorescence intensity, was observed after addition of the vesicles (data not shown), indicating that the NBD-labeled peptides interact with the lipidic membranes. The maximal fluorescence emission was 524 nm and 525 nm, for SV-208 and Mu-SV-208, respectively. A similar value was obtained for the HIV-1 fusion peptide (524 nm) (Kliger et al., 1997), which is known to be inserted into the membrane (Chang & Cheng, 1998). Furthermore, Rajarathnam and colleagues have shown that the emission maximum of NBD when inserted into membranes is close to 526 nm, whereas a maximum around 535 nm was observed when the NBD was linked to the polar head of phosphatidylethanolamine, located on the membrane’s surface (Rajarathnam et al., 1989). Therefore we can deduce that the N terminus of both SV-208 and the mutant peptide is located close to the hydrophobic core of the membrane.
SV-208 and Mu-SV-208 are accessible to proteolytic degradation when bound to membranes
The susceptibility of a membrane-bound peptide to proteolytic digestion can be used to determine the location of the backbone with respect to the plane of the membrane. When a peptide is located inside the bilayer, it is protected from the protease, whereas location on the surface renders the peptide susceptible to proteolytic digestion (Gazit & Shai, 1993). As depicted in Figure 5(a), adding proteinase K to a mixture of vesicles and NBD-labeled SV-208, results in a rapid decrease in the NBD fluorescence, demonstrating its release from the hydrophobic environment of the membrane. Similar results were obtained with NBD-Mu-SV-208 (Figure 5(c)). In control experiments, the final level of fluorescence intensity was the same when the protease was added before adding the vesicles (Figure 5(b) and (d)). These results demonstrate that at least part of the peptide backbones are located on the surface of the vesicles. A similar behavior was observed for the HIV-1 fusion peptide (Kliger et al., 1997).
Figure 5.
Susceptibility to enzymatic digestion of membrane-bound peptides. The fluorescence emission of NBD-labeled peptides was monitored at 530 nm with the excitation set at 467 nm: (a) SUV (200 μM) was added to NBD-SV-208 (0.1 μM), followed by the addition of 10 μl of Proteinase K (0.5 mg/ml); (b) as a control experiment, 10 μl of Proteinase K (0.5 mg/ml) was added to NBD-SV-208 (0.1 μM) before the addition of vesicles (200 μM); (c) and (d), same as indicated in (a) and (b), respectively, but with NBD-Mu-SV-208.
SV-208 but not Mu-SV-208 self-associates in its membrane-bound state
Several models of viral-induced membrane fusion postulate that the fusion proteins oligomerize during the actual fusion step Bentz et al 1993, Stegmann and Helenius 1993, Zimmerberg et al 1993, Stegmann 1994, Blumenthal et al 1996. Moreover, several known fusion peptides have been shown to oligomerize in the membrane Rapaport and Shai 1994, Kliger et al 1997. To determine whether the fusogenic activity of SV-208 is correlated with its ability to oligomerize in the membrane, the aggregation state of SV-208 and Mu-SV-208 was determined by resonance energy transfer measurements. Briefly, peptides labeled at their N terminus either with NBD, serving as energy donors, or with rhodamine, serving as energy acceptors, were used. In a typical experiment, a donor peptide (final concentration of 0.06 μM) was added to a dispersion of PC/PG (1:1) SUV (200 μM) in PBS, followed by the addition of an acceptor peptide in several sequential doses. As depicted in Figure 6, when NBD- and Rho-labeled SV-208 were used as a donor and an acceptor, respectively, dose-dependent quenching of the donor’s emission was observed. However, when NBD and Rho-labeled Mu-SV-208 were used, the observed energy transfer was similar to that expected from randomly distributed donors and acceptors (Fung & Stryer, 1987). The lipid to peptide ratio was kept high (>3000:1) to create a low surface density of donors and acceptors to reduce the energy transfer between unassociated peptides, and to ensure that essentially all the peptide is bound to membranes (as deduced from Figure 4(a)). Note that the acceptor-peptide was added only after the donor-peptide was already bound to the membrane, thus preventing any association in solution. These results indicate that SV-208 but not Mu-SV-208 is able to oligomerize in its membrane-bound state.
Figure 6.
Detection of oligomerization of membrane-bound peptides by fluorescence resonance energy transfer. Transfer efficiencies between donor and acceptor-SV-208 (filled squares), and donor and acceptor- Mu-SV-208 (open circles), are plotted as a function of [bound acceptor]/[lipid] molar ratio. A theoretical plot showing energy transfer efficiency as a function of the surface density of the acceptors, assuming a random distribution of donors and acceptors, and R0 = 51 Å, is given for comparison (broken line). SUV were used.
SV-208 has a higher α-helical content than Mu-SV-208 in membranes
Since the structural conformation has been shown to be important for the fusogenic activity of fusion peptides Lee et al 1992, Rapaport et al 1993, Nieva et al 1994, the secondary structure of the peptides in aqueous solution and in membranes was analyzed from their CD spectra, as measured in PBS, 1% SDS, and in the presence of PC/PG (1:1) SUV. As shown in Figure 7, the α-helical content of the peptides in PBS is low, but it increases in 1% SDS, a membrane-mimetic environment. In the presence of PC/PG (1:1) SUV, the spectra were not reliable below 212 nm due to light-scattering, and therefore are not shown. The fractional helicities, calculated according to the elipticity at 222 nm (Wu et al., 1981), are 17% in PBS, 25% in PC/PG (1:1) SUV, and 35% in 1% SDS for SV-208, and 8 % in PBS, 18% in PC/PG (1:1) SUV, and 24% in 1% SDS, for Mu-SV-208. Note that the lipid/peptide molar ratio used was about 500:1, and according to the binding curve (see Figure 4(a)) at this ratio only about 75% of the peptides are bound to the vesicles. It was not possible to increase the lipid/peptide ratio due to light-scattering caused by the vesicles.
Figure 7.
CD spectra of SV-208 and Mu-SV-208 in PBS and 1% SDS. Spectra were taken at peptide concentrations of 5 μM. SV-208 in PBS, dotted line; SV-208 in 1% SDS, continuous line; Mu-SV-208 in PBS, dotted-broken line, Mu-SV-208 in 1% SDS, broken line.
Discussion
A second fusion peptide in paramixovirus F1 protein
Here we have demonstrated that a peptide corresponding to amino acids 208–229 of the Sendai virus fusion protein, with a 13-residue segment homologous to the fusion peptides of the HIV-1 envelope glycoprotein and the RSV F1 protein (Figure 1), is able to induce rapid membrane fusion. Moreover, SV-208 induces fusion more efficiently than the 33-amino acid N-terminal fusion peptide (Figure 2). Furthermore, a peptide elongated to the N terminus, SV-201, is also active, indicating that SV-208 can act as an internal fusion peptide, suggesting that our findings could also be relevant in the context of the full-length protein. On the other hand, the scrambled and the shifted peptides are not active, although both peptides bind to membranes. Similarly, two peptides corresponding to the N-terminal and the C-terminal heptad repeats of the Sendai virus F1 protein, which were shown to bind to membranes (Ben-Efraim et al., 1999), were also tested and found not to induce lipid mixing (data not shown). This confirms that membrane-binding ability itself is not enough to achieve fusion. Furthermore, in spite of the low sequence homology, a peptide modeled after the homologous region of the measles virus F1 protein is also active (unpublished results), suggesting that the presence of an internal fusion peptide is a general feature of paramyxoviruses. To our knowledge, this is the first time that the specific location of a second fusion active segment has been determined in a viral fusion protein. It has been shown very recently that a 127 amino acid segment corresponding to the full-length ectodomain of influenza hemagglutinin promotes rapid pH-dependent membrane fusion (Epand et al., 1999). The 20 amino acid fusion peptide and a 95 amino acid construct, lacking the fusion peptide, were also able to induce some lipid mixing, although substantially less efficiently than the full-length ectodomain. This suggests that regions from the ectodomain of influenza hemagglutinin, other than the fusion peptide, might be able to induce lipid mixing. However, the specific location of such regions remains unknown. Moreover, it should be noted that the ectodomain of influenza hemagglutinin is much shorter than those of paramyxoviruses, and its coiled coil is formed by a C-terminal heptad repeat (Bullough et al., 1994), whereas paramixoviruses contain two heptad repeats, one following the N-terminal fusion peptide and the other preceding the transmembrane domain, which are separated by more than 280 amino acids (Lamb & Kolakofsky, 1996).
SV-208 has characteristics similar to other known fusion peptides
The orientation of fusion peptides with respect to the plane of the membrane is thought to be important for their activity. An oblique orientation has been found for the fusion peptides of influenza (Tatulian et al., 1995), SIV (Martin et al., 1994), bovine leukemia virus (Voneche et al., 1992), Sendai virus (Rapaport & Shai, 1994), Newcastle disease virus (Brasseur, 1991), measles virus (Brasseur et al., 1990), and HIV-1 (Pritsker et al., 1999), and is believed to facilitate membrane destabilization (reviewed by Epand, 1998). Here we analyzed the extent of membrane penetration by fluorescence spectroscopy and enzymatic digestion experiments. While the NBD fluorescence emission indicates that the N terminus of the peptides is located close to the hydrophobic core of the bilayer, the susceptibility of the membrane-bound peptides to enzymatic digestion shows that at least part of the peptide backbones lie on the membrane surface (Figure 5), thus suggesting an oblique orientation.
There is much evidence indicating that fusion proteins are oligomers in their active conformation Freed et al 1992, Wahlberg et al 1992. Furthermore, the ability of fusion peptides to oligomerize in their membrane-bound state may be a determining factor in their fusion activity Zimmerberg et al 1993, Kliger et al 1997. In the present study, resonance energy transfer experiments have demonstrated that SV-208 oligomerizes in its membrane-bound state, even at very low concentrations (Figure 6). In contrast, the inactive scrambled peptide does not oligomerize in the membrane. Similarly, the inability of a V2E mutant of the HIV-1 fusion peptide to form high-order oligomers was correlated with its loss of fusion activity (Kliger et al., 1997).
Secondary structure of fusion peptides seems to be important for their activity. Disruption of the α-helical structure of fusogenic peptides either by the addition of proline residues (Lee et al., 1992) or by the substitution of two l-amino acids with their d-enantiomers (Rapaport et al., 1993) abolished the fusogenic activity of the peptides. Here, we found a correlation between the higher α-helical content of SV-208 and its fusogenic ability, when compared with those of Mu-SV-208. However, the role of other secondary structure elements in the process of membrane fusion cannot be ruled out Epand 1998, Pritsker et al 1999.
A role for the internal fusion peptide in the process of membrane fusion induced by paramixovirus F protein
Synthetic peptides can only partly mimic the complex fusogenic properties of a viral protein. Nevertheless, properties that were found to be crucial in the present study, such as fusogenic activity, oligomerization in the membrane and α-helical secondary structure, were proposed to be also requirements of viral-induced membrane fusion (White, 1990). Recently, it has been shown that both the N-terminal and the C-terminal heptad repeats of the Sendai virus fusion protein, which form a strong complex in solution (Ghosh et al., 1998), dissociate into α-helical monomers upon binding to membranes (Ben-Efraim et al., 1999). In agreement with these observations, NMR studies showed that the N-terminal heptad repeat of the homologous Newcastle disease virus fusion protein has an α-helical structure in SDS, consistent with the notion that it binds parallel with the bilayer, as a monomer, with its hydrophobic face buried in the membrane (Young et al., 1999). Furthermore, the N-terminal heptad repeat of the Sendai virus F1 protein, located between the N-terminal fusion peptide and SV-208, has been shown to assist the N-terminal fusion peptide in inducing membrane fusion (Ghosh & Shai, 1999). These results suggest that the region of the F1 protein that corresponds to the internal fusion peptide SV-208 could be in proximity to the membrane during the fusion process, thus supporting our observations. The previous model for Sendai virus-induced membrane fusion is shown in Figure 8(a), (b) and (c) (Ben-Efraim et al., 1999). Our results led us to extend this model, suggesting a specific role for the internal fusion peptide in relation to the full-length F1 protein (Figure 8(a), (d), (e) and (f)). According to the extended model, binding of the viral surface glycoprotein to cell receptors (Figure 8(a)) results in a conformational change of the fusion protein, which leads to the formation of a trimeric coiled-coil, analogous to the conformation observed in the 3-D structures of other fusion proteins Bullough et al 1994, Fass et al 1996, Chan et al 1997, Weissenhorn et al 1997, Weissenhorn et al 1998, Malashkevich et al 1998, Malashkevich et al 1999, Baker et al 1999, Kobe et al 1999, Yang et al 1999. It should be noted that a leucine zipper-like sequence, SV-269, is located at a position following the internal fusion peptide. This region has been shown to interact with the C-terminal heptad repeat (Ghosh et al., 1997). However, this interaction was found to be thermally unstable, suggesting that this region is not a component of the final, most stable core of the F protein (Dutch et al., 1999). There is no experimental evidence yet to determine whether the N-terminal fusion peptide or the internal fusion peptide inserts into the target membrane first. Although we cannot rule out the possibility that the N-terminal fusion peptide inserts into the target membrane first (Figure 8(d)), the existence of a second fusion peptide consecutive to the N-terminal heptad repeat opens the possibility that the initial interaction with the target membrane is achieved by means of the internal fusion peptide, which in this conformation is located on top of the coiled-coil, as suggested in Figure 8(e). According to CD spectroscopy (Figure 7) and resonance energy transfer (Figure 6), the internal fusion peptide oligomerizes and adopts an α-helical structure upon membrane binding. In this conformation, the N-terminal fusion peptide might be embedded in the viral membrane, in agreement with the models suggested by Kozlov and Chernomordik 1998, Bentz 2000. It has been shown by Ben-Efraim et al. (1999) that the affinity of both the N-terminal and the C-terminal heptad repeats to the membrane causes the “umbrella”-like opening of the coiled-coil (Figure 8(f)). This membrane-induced conformational change consequently causes the cellular and viral membranes to approach each other. Subsequently, both the N-terminal and the internal fusion peptides induce the actual merging of the membranes. Further experiments are needed to determine whether both fusion-active segments destabilize the same membrane. The location of the internal and the N-terminal fusion peptides, both in the same membrane (Figure 8(f)) is therefore speculative.
Figure 8.
The umbrella mechanism of Sendai virus-induced membrane fusion. A previous model is presented in (a)-(c) (Ben-Efraim et al., 1999). Our extended model is shown in (a) and (d)-(f). According to the revised model, binding of the viral surface glycoprotein (a) to cell receptors results in a conformational change of the fusion protein, leading to the formation of a trimeric coiled-coil. Two alternative pathways are possible: the N-terminal fusion peptide inserts into the target membrane first (d); or the initial interaction with the target membrane is achieved by means of the internal fusion peptide, which in this conformation is located on top of the coiled-coil (e). After the initial binding, the affinity of both the N-terminal and the C-terminal heptad repeats to the membrane causes the opening of the coiled-coil (f). This membrane-induced conformational change causes the cellular and viral membranes to approach each other. Subsequently, the internal and the N-terminal fusion peptides induce the merging of the membranes.
Materials and methods
Materials
BOC-amino acids were purchased from Novabiochem AG (Läufelfingen, Switzerland), and BOC-amino acid phenylacetamidomethyl (PAM)-resin was obtained from Applied Biosystems (Foster City, CA). NBD-fluoride and other reagents for peptide synthesis were obtained from Sigma. Egg phosphatidylcholine (PC), and phosphatidylglycerol (PG) were purchased from Lipid Products (South Nutfield, U.K). N-[Lissamine-rhodamine B-sulfonyl]- dioleoylphosphatidylethanolamine (Rho-PE), N-[7-nitrobenz-2-oxa-1,3-diazole-4-yl]-dioleoylphosphatidylethanolamine (NBD-PE), N-[7-nitrobenz-2-oxa-1,3-diazole-fluoride] (NBD-fluoride), and 5-(and 6)-carboxytetramethyl-rhodamine (Rho), succinimidyl ester were purchased from Molecular Probes (Eugene, OR). All other reagents were of analytical grade. Buffers were prepared using double glass-distilled water. Phosphate-buffered saline (PBS) is composed of NaCl (8 g/l), KCl (0.2 g/l), KH2PO4 (0.2 g/l), and Na2HPO4 (1.09 g/l), pH 7.3.
Peptide synthesis and fluorescent labeling
The peptides were synthesized by a standard solid phase method on PAM-resin as described Merrifield et al 1982, Shai et al 1990. NBD and Rho-labeling of the N terminus of the resin-bound peptides was achieved as previously described (Rapaport & Shai, 1992). The peptides were cleaved from the resin by HF treatment and purified by RP-HPLC. Purity (∼99%) was confirmed by analytical HPLC. The peptide compositions were determined by amino acid analysis.
Preparation of lipid vesicles
Small unilamellar vesicles (SUV) were prepared by sonication of PC/PG (1:1) as described earlier (Shai et al., 1991). Large unilamellar vesicles (LUV) were also prepared from PC/PG (1:1), and when necessary with different amounts of Rho-PE and NBD-PE, as follows: dry lipids were suspended in PBS by vortexing to produce large multilamellar vesicles. The lipid suspension was freeze-thawed six times and then extruded 20 times through polycarbonate membranes with 0.1 μm diameter pores (Nuclepore Corp., Pleasanton, CA).
Peptide-induced lipid mixing
Lipid mixing of large unilamellar vesicles was measured using a fluorescence probe dilution assay (Struck et al., 1981). Lipid vesicles containing 0.6 mol% each of NBD-PE (energy donor) and Rho-PE (energy acceptor) were prepared in PBS as described before. A 1:4 mixture of labeled and unlabeled vesicles (110 μM total phospholipid concentration) was suspended in 400 μl of PBS, and a small volume of peptide in DMSO was added. The increase in NBD fluorescence at 530 nm was monitored with the excitation set at 467 nm. The fluorescence intensity before the addition of the peptide was referred to as 0% lipid mixing, and the fluorescence intensity upon the addition of Triton X-100 (0.25%, v/v) was referred to as 100% lipid mixing. All the fluorescence measurements in the present study were done on a Perkin-Elmer LS-50B spectrofluorometer.
Electron microscopy
The effects of the peptides on liposomal suspensions were examined by negative-staining electron microscopy. A drop containing PC/PG (1:1) LUV alone or a mixture of LUV and peptide was deposited onto a carbon-coated grid and negatively stained with 2% uranyl acetate. The grids were examined using a JEOL JEM 100B electron microscope (Japan Electron Optics Laboratory Co., Tokyo, Japan).
Membrane binding experiments
The degree of peptide association with PC/PG (1:1) SUV was measured by adding increasing amounts of vesicles to 0.2 μM NBD-labeled peptides dissolved in PBS. The fluorescence intensity was measured as a function of the lipid/peptide molar ratio, with excitation set at 467 nm (8 nm slit), and emission set at 530 nm (8 nm slit). The fluorescence values were corrected by substracting the corresponding blank (PBS with the same amount of vesicles). The binding isotherms were analyzed as partitium equilibriums (Beschiaschvili & Seelig, 1990), as described previously (Rapaport & Shai, 1991).
NBD fluorescence measurements
Changes in the fluorescence of NBD-labeled peptides were measured upon their binding to vesicles. NBD-labeled peptide (0.1 μM) was added to 2 ml of PBS, containing PC/PG (1:1) SUV (200 μM). Emission spectra were recorded (8 nm slit), with excitation set at 467 nm (8 nm slit), and compared with the emission spectra of the NBD-labeled peptide in iposome-free PBS. The peptide/lipid molar ratio was kept at a level such that the majority of the peptides were bound to the vesicles and the contribution of the free peptide to fluorescence could be neglected.
Enzymatic digestion of membrane-bound peptides
The susceptibility of the peptides to proteolytic degradation in their membrane-bound state was determined as described previously (Rapaport et al., 1995). Briefly, PC/PG (1:1) SUVs (200 μM) were added to 0.1 μM NBD-labeled peptide, followed by the addition of 10 μl of Proteinase-K (0.5 mg/ml). Fluorescence intensity at 530 nm (8 nm slit) was recorded as a function of time before and after the addition of the enzyme, excitation was set at 467 nm (8 nm slit). In a control experiment, the enzyme was added before the addition of the vesicles.
Resonance energy transfer measurements
Fluorescence resonance energy transfer was measured using NBD-labeled peptides serving as energy donors and Rho-labeled peptides serving as energy acceptors (Gazit & Shai, 1993). Fluorescence spectra (8 nm slit) were obtained at room temperature, with excitation set at 467 nm (8 nm slit). In a typical experiment, donor peptide (final concentration of 0.06 μM) was added to a dispersion of PC/PG (1:1) SUV (200 μM) in PBS, followed by the addition of acceptor peptide in several sequential doses. Fluorescence spectra were obtained before and after the addition of the acceptor. The efficiency of energy transfer (E) was determined by measuring the decrease in the quantum yield of the donor as a result of the presence of the acceptor. E was determined experimentally from the ratio of the fluorescence intensities of the donor with (I da) and without (I d) the acceptor, at the donor’s maximal emission wavelength. The percentage of transfer efficiency (E), is given by:E=(1-Ida/Id)×100% Correction for the contribution of acceptor emission as a result of direct excitation was made by subtracting the signal produced by the acceptor-labeled peptide added to the non-labeled donor. The contribution of buffer and vesicles was subtracted from all measurements.
Circular dichroism (CD) spectroscopy
CD spectra were obtained using an Aviv 202 spectropolarimeter. The spectra were scanned with a thermostated quartz optical cell with a path length of 1 mm, at 25°C. Each spectrum was recorded with an averaging time of ten seconds, at a wavelength range of 260 to 195 nm. Fractional helicities (Wu et al., 1981) were calculated as follows:fh=[θ]222−[θ]2220[θ]222100−[θ]2220 where [θ]222 is the experimentally observed mean residue elipticity at 222 nm, and values for [θ]222 0 and [θ]222 100, corresponding to 0% and 100% helix content at 222 nm, were estimated at −2000 and −32,000 deg cm2/dmol, respectively. Each experiment was repeated twice and found to be in good agreement.
Footnotes
Edited by A. R.Fersht
References
- Baker K.A, Dutch R.E, Lamb R.A, Jardetzky T.S. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim I, Kliger Y, Hermesh C, Shai Y. Membrane-induced step in the activation of Sendai virus fusion protein. J. Mol. Biol. 1999;285:609–625. doi: 10.1006/jmbi.1998.2370. [DOI] [PubMed] [Google Scholar]
- Bentz J. Minimal aggregate size and minimal fusion unit for the first fusion pore of influenza hemagglutinin-mediated membrane fusion. Biophys. J. 2000;78:227–245. doi: 10.1016/S0006-3495(00)76587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz J, Ellens H, Alford D. Architecture of the influenza hemagglutinin membrane fusion site. In: Bentz J, editor. Viral Fusion Mechanisms. CRC Press; Boca Raton: 1993. pp. 163–199. [Google Scholar]
- Beschiaschvili G, Seelig J. Melittin binding to mixed phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry. 1990;29:52–58. doi: 10.1021/bi00453a007. [DOI] [PubMed] [Google Scholar]
- Blumberg B.M, Giorgi C, Rose K, Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J. Gen. Virol. 1985;66:317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Blumenthal R, Sarkar D.P, Durell S, Howard D.E, Morris S.J. Dilation of the influenza hemagglutinin fusion pore revealed by the kinetics of individual cell-cell fusion events. J. Cell. Biol. 1996;135:63–71. doi: 10.1083/jcb.135.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M.L, Earl P.L, Fargnoli K, Picciafuoco S, Giombini F, Wong-Staal F, Franchini G. Identification of the fusion peptide of primate immunodeficiency viruses. Science. 1989;244:694–697. doi: 10.1126/science.2541505. [DOI] [PubMed] [Google Scholar]
- Brasseur R. Differentiation of lipid-associating helices by use of three-dimensional molecular hydrophobicity potential calculations. J. Biol. Chem. 1991;266:16120–16127. [PubMed] [Google Scholar]
- Brasseur R, Vandenbranden M, Cornet B, Burny A, Ruysschaert J.M. Orientation into the lipid bilayer of an asymmetric amphipathic helical peptide located at the N terminus of viral fusion proteins. Biochim. Biophys. Acta. 1990;1029:267–273. doi: 10.1016/0005-2736(90)90163-i. [DOI] [PubMed] [Google Scholar]
- Bullough P.A, Hughson F.M, Skehel J.J, Wiley D.C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Chan D.C, Fass D, Berger J.M, Kim P.S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Chang D.K, Cheng S.F. Determination of the equilibrium micelle-inserting position of the fusion peptide of gp41 of human immunodeficiency virus type 1 at amino acid resolution by exchange broadening of amide proton resonances. J. Biomol. NMR. 1998;12:549–552. doi: 10.1023/a:1008399304450. [DOI] [PubMed] [Google Scholar]
- Collins P.L, Chanock R.M, McIntosh K. Parainfluenza Viruses. In: Fields B.N, Knipe D.M, Howley P.M, editors. Virology. Lippincott-Raven; Philadelphia: 1996. pp. 1205–1241. [Google Scholar]
- Durrer P, Gaudin Y, Ruigrok R.W, Graf R, Brunner J. Photolabeling identifies a putative fusion domain in the envelope glycoprotein of rabies and vesicular stomatitis viruses. J. Biol. Chem. 1995;270:17575–17581. doi: 10.1074/jbc.270.29.17575. [DOI] [PubMed] [Google Scholar]
- Dutch R.E, Leser G.P, Lamb R.A. Paramyxovirus fusion protein: characterization of the core trimer, a rod-shaped complex with helices in anti-parallel orientation. Virology. 1999;254:147–159. doi: 10.1006/viro.1998.9532. [DOI] [PubMed] [Google Scholar]
- Epand R.M. Lipid polymorphism and protein-lipid interactions. Biochim. Biophy. Acta. 1998;1376:353–368. doi: 10.1016/s0304-4157(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Epand R.F, Macosko J.C, Russell C.J, Shin Y.K, Epand R.M. The ectodomain of HA2 of influenza virus promotes rapid pH dependent membrane fusion. J. Mol. Biol. 1999;286:489–503. doi: 10.1006/jmbi.1998.2500. [DOI] [PubMed] [Google Scholar]
- Fass D, Harrison S.C, Kim P.S. Retrovirus envelope domain at 1.7 angstrom resolution. Nature Struct. Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- Fredericksen B.L, Whitt M.A. Vesicular stomatitis virus glycoprotein mutations that affect membrane fusion activity and abolish virus infectivity. J. Virol. 1995;69:1435–1443. doi: 10.1128/jvi.69.3.1435-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E.O, Delwart E.L, Buchschacher G.L, Jr, Panganiban A.T. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc. Natl Acad. Sci. USA. 1992;89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B.K, Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1987;17:5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- Gallaher W.R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987;50:327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Gallaher W.R. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell. 1996;85:477–478. doi: 10.1016/s0092-8674(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Gazit E, Shai Y. Structural and functional characterization of the alpha 5 segment of Bacillus thuringiensis delta-endotoxin. Biochemistry. 1993;32:3429–3436. doi: 10.1021/bi00064a029. [DOI] [PubMed] [Google Scholar]
- Gething M.J, White J.M, Waterfield M.D. Purification of the fusion protein of Sendai virus: analysis of the NH2-terminal sequence generated during precursor activation. Proc. Natl Acad. Sci. USA. 1978;75:2737–2740. doi: 10.1073/pnas.75.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M.J, Doms R.W, York D, White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J. Cell. Biol. 1986;102:11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J.K, Shai Y. Direct evidence that the N-terminal heptad repeat of Sendai virus fusion protein participates in membrane fusion. J. Mol. Biol. 1999;292:531–546. doi: 10.1006/jmbi.1999.3097. [DOI] [PubMed] [Google Scholar]
- Ghosh J.K, Ovadia M, Shai Y. A leucine zipper motif in the ectodomain of Sendai virus fusion protein assembles in solution and in membranes and specifically binds biologically-active peptides and the virus. Biochemistry. 1997;36:15451–15462. doi: 10.1021/bi971152i. [DOI] [PubMed] [Google Scholar]
- Ghosh J.K, Peisajovich S.G, Ovadia M, Shai Y. Structure-function study of a heptad repeat positioned near the transmembrane domain of Sendai virus fusion protein which blocks virus-cell fusion. J. Biol. Chem. 1998;273:27182–27190. doi: 10.1074/jbc.273.42.27182. [DOI] [PubMed] [Google Scholar]
- Harter C, James P, Bachi T, Semenza G, Brunner J. Hydrophobic binding of the ectodomain of influenza hemagglutinin to membranes occurs through the “fusion peptide”. J. Biol. Chem. 1989;264:6459–6464. [PubMed] [Google Scholar]
- Hernandez L.D, White J.M. Mutational analysis of the candidate internal fusion peptide of the avian leukosis and sarcoma virus subgroup A envelope glycoprotein. J. Virol. 1998;72:3259–3267. doi: 10.1128/jvi.72.4.3259-3267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D, Kok J.W. Entry mechanisms of enveloped viruses. Implications for fusion of intracellular membranes. Biosci. Rep. 1989;9:273–305. doi: 10.1007/BF01114682. [DOI] [PubMed] [Google Scholar]
- Homma M, Ohuchi M. Trypsin action on the growth of sendai virus in tissue culture cells. J. Virol. 1973;12:1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C.M, Lamb R.A. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J. Virol. 1992;66:2443–2455. doi: 10.1128/jvi.66.4.2443-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E, Hill E, Hardwick M, Bhown A, Schwartz D.E, Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J. Virol. 1983;46:920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliger Y, Aharoni A, Rapaport D, Jones P, Blumenthal R, Shai Y. Fusion peptides derived from the HIV type 1 glycoprotein 41 associate within phospholipid membranes and inhibit cell-cell fusion. Structure-function study. J. Biol. Chem. 1997;272:13496–13505. doi: 10.1074/jbc.272.21.13496. [DOI] [PubMed] [Google Scholar]
- Kobe B, Center R.J, Kemp B.E, Poumbourios P. Crystal structure of human T cell leukemia virus type 1 gp21 ectodomain crystallized as a maltose-binding protein chimera reveals structural evolution of retroviral transmembrane proteins. Proc. Natl Acad. Sci. USA. 1999;96:4319–4324. doi: 10.1073/pnas.96.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M.M, Chernomordik L.V. A mechanism of protein-mediated fusion: coupling between refolding of the influenza hemagglutinin and lipid rearrangements. Biophys. J. 1998;75:1384–1396. doi: 10.1016/S0006-3495(98)74056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R.A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B.N, Knipe D.M, Howley P.M, editors. Virology. Lippincott-Raven; Philadelphia: 1996. pp. 1177–1204. [Google Scholar]
- Lee S, Aoki R, Oishi O, Aoyagi H, Yamasaki N. Effect of amphipathic peptides with different alpha-helical contents on liposome-fusion. Biochim. Biophys. Acta. 1992;1103:157–162. doi: 10.1016/0005-2736(92)90069-x. [DOI] [PubMed] [Google Scholar]
- Luo Z, Weiss S.R. Roles in cell-to-cell fusion of two conserved hydrophobic regions in the murine coronavirus spike protein. Virology. 1998;244:483–494. doi: 10.1006/viro.1998.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashkevich V.N, Chan D.C, Chutkowski C.T, Kim P.S. Crystal structure of the simian immunodeficiency virus (SIV) gp41 core: conserved helical interactions underlie the broad inhibitory activity of gp41 peptides. Proc. Natl Acad. Sci. USA. 1998;95:9134–9139. doi: 10.1073/pnas.95.16.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashkevich V.N, Schneider B.J, McNally M.L, Milhollen M.A, Pang J.X, Kim P.S. Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9 Å resolution. Proc. Natl Acad. Sci. USA. 1999;96:2662–2667. doi: 10.1073/pnas.96.6.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Dubois M.C, Defrise-Quertain F, Saermark T, Burny A, Brasseur R, Ruysschaert J.M. Correlation between fusogenicity of synthetic modified peptides corresponding to the NH2-terminal extremity of simian immunodeficiency virus gp32 and their mode of insertion into the lipid bilayer: an infrared spectroscopy study. J. Virol. 1994;68:1139–1148. doi: 10.1128/jvi.68.2.1139-1148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Schaal H, Scheid A, Ruysschaert J.M. Lipid membrane fusion induced by the human immunodeficiency virus type 1 gp41 N-terminal extremity is determined by its orientation in the lipid bilayer. J. Virol. 1996;70:298–304. doi: 10.1128/jvi.70.1.298-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R.B, Vizioli L.D, Boman H.G. Synthesis of the antibacterial peptide cecropin A (1–33) Biochemistry. 1982;21:5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Nieva J.L, Nir S, Muga A, Goni F.M, Wilschut J. Interaction of the HIV-1 fusion peptide with phospholipid vesicles: different structural requirements for fusion and leakage. Biochemistry. 1994;33:3201–3209. doi: 10.1021/bi00177a009. [DOI] [PubMed] [Google Scholar]
- Pereira F.B, Goni F.M, Nieva J.L. Liposome destabilization induced by the HIV-1 fusion peptide effect of a single amino acid substitution. FEBS Letters. 1995;362:243–246. doi: 10.1016/0014-5793(95)00257-a. [DOI] [PubMed] [Google Scholar]
- Pereira F.B, Goni F.M, Nieva J.L. Membrane fusion induced by the HIV type 1 fusion peptide: modulation by factors affecting glycoprotein 41 activity and potential anti-HIV compounds. AIDS Res. Hum. Retroviruses. 1997;13:1203–1211. doi: 10.1089/aid.1997.13.1203. [DOI] [PubMed] [Google Scholar]
- Pritsker M, Rucker J, Hoffman T.L, Doms R.W, Shai Y. Effect of nonpolar substitutions of the conserved Phe11 in the fusion peptide of HIV-1 gp41 on its function, structure, and organization in membranes. Biochemistry. 1999;38:11359–11371. doi: 10.1021/bi990232e. [DOI] [PubMed] [Google Scholar]
- Rabenstein M, Shin Y.K. A peptide from the heptad repeat of human immunodeficiency virus gp41 shows both membrane binding and coiled-coil formation. Biochemistry. 1995;34:13390–13397. doi: 10.1021/bi00041a016. [DOI] [PubMed] [Google Scholar]
- Rafalski M, Lear J.D, DeGrado W.F. Phospholipid interactions of synthetic peptides representing the N terminus of HIV gp41. Biochemistry. 1990;29:7917–7922. doi: 10.1021/bi00486a020. [DOI] [PubMed] [Google Scholar]
- Rajarathnam K, Hochman J, Schindler M, Ferguson-Miller S. Synthesis, location, and lateral mobility of fluorescently labeled ubiquinone 10 in mitochondrial and artificial membranes. Biochemistry. 1989;28:3168–3176. doi: 10.1021/bi00434a009. [DOI] [PubMed] [Google Scholar]
- Rapaport D, Shai Y. Interaction of fluorescently labeled pardaxin and its analogues with lipid bilayers. J. Biol. Chem. 1991;266:23769–23775. [PubMed] [Google Scholar]
- Rapaport D, Shai Y. Aggregation and organization of pardaxin in phospholipid membranes. A fluorescence energy transfer study. J. Biol. Chem. 1992;267:6502–6509. [PubMed] [Google Scholar]
- Rapaport D, Shai Y. Interaction of fluorescently labeled analogues of the amino-terminal fusion peptide of Sendai virus with phospholipid membranes. J. Biol. Chem. 1994;269:15124–15131. [PubMed] [Google Scholar]
- Rapaport D, Hague G.R, Pouny Y, Shai Y. pH- and ionic strength-dependent fusion of phospholipid vesicles induced by pardaxin analogues or by mixtures of charge-reversed peptides. Biochemistry. 1993;32:3291–3297. doi: 10.1021/bi00064a011. [DOI] [PubMed] [Google Scholar]
- Rapaport D, Ovadia M, Shai Y. A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 1995;14:5524–5531. doi: 10.1002/j.1460-2075.1995.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Arguello M.B, Goni F.M, Pereira F.B, Nieva J.L. Phosphatidylinositol-dependent membrane fusion induced by a putative fusogenic sequence of Ebola virus. J. Virol. 1998;72 doi: 10.1128/jvi.72.3.1775-1781.1998. 177517–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A, Choppin P.W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977;80:54–60. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Shai Y, Bach D, Yanovsky A. Channel formation properties of synthetic pardaxin and analogues. J. Biol. Chem. 1990;265:20202–20209. [PubMed] [Google Scholar]
- Shai Y, Hadari Y.R, Finkels A. pH-dependent pore formation properties of pardaxin analogues. J. Biol. Chem. 1991;266:22346–22354. [PubMed] [Google Scholar]
- Stegmann T. Membrane fusion. Anchors aweigh. Curr. Biol. 1994;4 doi: 10.1016/s0960-9822(00)00123-8. 551–4. [DOI] [PubMed] [Google Scholar]
- Stegmann T, Helenius A. Influenza Virus fusion: from models toward a mechanism. In: Bentz J, editor. Viral Fusion Mechanisms. CRC Press; Boca Raton: 1993. pp. 89–111. [Google Scholar]
- Stegmann T, Doms R.W, Helenius A. Protein-mediated membrane fusion. Annu. Rev. Biophys. Biophys. Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Struck D.K, Hoekstra D, Pagano R.E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981;20:4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Tatulian S.A, Hinterdorfer P, Baber G, Tamm L.K. Influenza hemagglutinin assumes a tilted conformation during membrane fusion as determined by attenuated total reflection FTIR spectroscopy. EMBO J. 1995;14:5514–5523. doi: 10.1002/j.1460-2075.1995.tb00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voneche V, Portetelle D, Kettmann R, Willems L, Limbach K, Paoletti E, Ruysschaert J.M, Burny A, Brasseur R. Fusogenic segments of bovine leukemia virus and simian immunodeficiency virus are interchangeable and mediate fusion by means of oblique insertion in the lipid bilayer of their target cells. Proc. Natl Acad. Sci. USA. 1992;89:3810–3814. doi: 10.1073/pnas.89.9.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J.M, Bron R, Wilschut J, Garoff H. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 1992;66:7309–7318. doi: 10.1128/jvi.66.12.7309-7318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Harrison S.C, Skehel J.J, Wiley D.C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W, Carfi A, Lee K.H, Skehel J.J, Wiley D.C. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol. Cell. 1998;2:605–616. doi: 10.1016/s1097-2765(00)80159-8. [DOI] [PubMed] [Google Scholar]
- Wharton S.A, Martin S.R, Ruigrok R.W, Skehel J.J, Wiley D.C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J. Gen. Virol. 1988;69:1847–1857. doi: 10.1099/0022-1317-69-8-1847. [DOI] [PubMed] [Google Scholar]
- White J, Kielian M, Helenius A. Membrane fusion proteins of enveloped animal viruses. Quart. Rev. Biophys. 1983;16:151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- White J.M. Viral and cellular membrane fusion proteins. Annu. Rev. Physiol. 1990;52:75–97. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- Whitt M.A, Zagouras P, Crise B, Rose J.K. A fusion-defective mutant of the vesicular stomatitis virus glycoprotein. J. Virol. 1990;64:4907–4913. doi: 10.1128/jvi.64.10.4907-4913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.S.C, Ikeda K, Yang J.T. Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry. 1981;20:566–570. doi: 10.1021/bi00506a019. [DOI] [PubMed] [Google Scholar]
- Yang Z.N, Mueser T.C, Kaufman J, Stahl S.J, Wingfield P.T, Hyde C.C. The crystal structure of the SIV gp41 ectodomain at 1.47 Å resolution. J. Struct. Biol. 1999;126:131–144. doi: 10.1006/jsbi.1999.4116. [DOI] [PubMed] [Google Scholar]
- Yeagle P.L, Epand R.M, Richardson C.D, Flanagan T.D. Effects of the ‘fusion peptide’ from measles virus on the structure of N-methyl dioleoylphosphatidylethanolamine membranes and their fusion with Sendai virus. Biochim. Biophys. Acta. 1991;1065:49–53. doi: 10.1016/0005-2736(91)90009-w. [DOI] [PubMed] [Google Scholar]
- Young J.K, Li D, Abramowitz M.C, Morrison T.G. Interaction of peptides with sequences from the Newcastle disease virus fusion protein heptad repeat regions. J. Virol. 1999;73:5945–5956. doi: 10.1128/jvi.73.7.5945-5956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.G, King D.S, Shin Y.K. Insertion of a coiled-coil peptide from influenza virus hemagglutinin into membranes. Science. 1994;266:274–276. doi: 10.1126/science.7939662. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Vogel S.S, Chernomordik L.V. Mechanisms of membrane fusion. Annu. Rev. Biophys. Biomol. Struct. 1993;22:433–466. doi: 10.1146/annurev.bb.22.060193.002245. [DOI] [PubMed] [Google Scholar]