Highlights
-
•
+RNA viruses build membranous VRCs.
-
•
+RNA viruses rewire cellular lipid metabolism to build VRCs.
-
•
VRC structure and formation mechanisms differ between disparate viruses.
-
•
Several unrelated viruses usurp membrane contact site machinery to acquire sterols.
Abstract
Positive-strand RNA (+RNA) viruses (e.g. poliovirus, hepatitis C virus, dengue virus, SARS-coronavirus) remodel cellular membranes to form so-called viral replication compartments (VRCs), which are the sites where viral RNA genome replication takes place. To induce VRC formation, these viruses extensively rewire lipid metabolism. Disparate viruses have many commonalities as well as disparities in their interactions with the host lipidome and accumulate specific sets of lipids (sterols, glycerophospholipids, sphingolipids) at their VRCs. Recent years have seen an upsurge in studies investigating the role of lipids in +RNA virus replication, in particular of sterols, and uncovered that membrane contact sites and lipid transfer proteins are hijacked by viruses and play pivotal roles in VRC formation.
Current Opinion in Cell Biology 2017, 47:24–33
This review comes from a themed issue on Cell Organelles
Edited by Bruno Antonny and Catherine Rabouille
For a complete overview see the Issue and the Editorial
Available online 24th February 2017
http://dx.doi.org/10.1016/j.ceb.2017.02.005
0955-0674/© 2017 Elsevier Ltd. All rights reserved.
Introduction
Viruses are obligate intracellular pathogens that depend on host cell metabolism for their replication. Viruses interact with host membranes and lipids at various stages of their life cycle. All positive-strand RNA (+RNA) viruses, which comprise many medically and economically important pathogens of humans, animals, plants and unicellular eukaryotes (Table 1 ), remodel cellular membranes into unique ‘viral replication compartments’ (VRCs) to support replication of the viral genome (for review, see e.g. Ref. [1]). VRCs are generally assumed to support replication by serving as a platform on which proteins involved in genome replication are concentrated and assembled into active replication complexes. Additionally, VRCs are thought to shield the viral RNA from cellular defence systems that patrol the cytoplasm to detect and eliminate intruders (e.g. pattern recognition receptors, RNases). The structure of VRCs and the mechanisms underlying their formation vary greatly between +RNA viruses, but universally depend on cellular lipid and membrane homeostasis. Here we will review how +RNA viruses rewire host lipid metabolism and describe contributions of different lipid categories to VRC formation and functioning.
Table 1.
Examples of well-known pathogenic +RNA viruses
| Family/Genus | Examples | Hosts | Symptoms/disease | VRCsa |
|---|---|---|---|---|
| Bromoviridae | ||||
| Bromovirus | Brome mosaic virus (BMV) | Plants, yeastb | Invag | |
| Coronaviridae | ||||
| Betacoronavirus | MERS coronavirus | Humansc | Severe respiratory disease | Protr |
| SARS coronavirus | Humansc | Severe respiratory disease | Protr | |
| Flaviviridae | ||||
| Hepacivirus | Hepatitis C virus (HCV) | Humans | Hepatitis; liver cancer | Protr |
| Flavivirus | Dengue virus (DENV) | Humansd | Haemorrhagic fever; death | Invag |
| West Nile virus (WNV) | Birds, humansd | Encephalitis; meningitis | Invag | |
| Yellow fever virus | Primates, humansd | Jaundice; liver and kidney damage; bleeding | Invag | |
| Zika virus | Primates, humansd | Microcephaly; Guillain-Barré syndrome | Invag | |
| Nodaviridae | ||||
| Alphanodavirus | Flock House virus | Insects | Aberrant development | Invag |
| Picornaviridae | ||||
| Enterovirus | Coxsackievirus B3 (CVB3) | Humans | Encephalitis; myocarditis | Protr |
| Enterovirus-A71 | Humans | Hand, foot and mouth disease; paralysis | Protr | |
| Enterovirus-D68 | Humans | Severe respiratory disease; paralysis | Protr | |
| Poliovirus | Humans | Poliomyelitis (paralysis) | Protr | |
| Rhinovirus | Humans | Common cold; exacerbations of chronic pulmonary diseases like asthma | Protr | |
| Cardiovirus | Encephalomyocarditis virus (EMCV) | Rodents, pigs, elephantse | Encephalitis; premature abortions; death | Protr |
| Saffold virus | Humans | Respiratory symptoms; gastrointestinal disease | ||
| Aphthovirus | Foot-and-mouth disease virus | Cloven-hoofed ruminants | Foot-and-mouth disease | Protr |
| Hepatovirus | Hepatitis A virus | Humans | Hepatitis; jaundice; acute liver failure | Protr |
| Kobuvirus | Aichivirus | Humans | Diarrhoea; vomiting | |
| Parechovirus | Human parechovirus 1 | Humans | Meningitis; sepsis | Protr |
| Tombusviridae | ||||
| Tombusvirus | Tomato bushy stunt virus (TBSV) | Tomato, yeastb | Stunting of growth; deformed or absent fruit | Invag |
| Carnation Italian ringspot virus | Dianthus, yeastb | Stunting of growth; spots on leaves | Invag | |
Viruses are grouped by family and genus to indicate evolutionary relationships. Some of the relevant hosts, and a selection of symptoms or diseases associated with infection by the virus are listed.
Type of VRC generated by the virus (Protr = protrusion-type; Invag = invagination-type).
The natural hosts for bromoviruses and tombusviruses are plants, but many of those viruses can also infect yeast as a surrogate host.
Viruses having a zoonotic origin, likely originating from bats and being transmitted to humans through camels (MERS) or civet cats (SARS).
Viruses are transmitted via mosquito bites.
The natural hosts for encephalomyocarditis virus are rodents, but the virus can cause zoonotic infections in many other animal species with devastating results.
The structure of VRCs
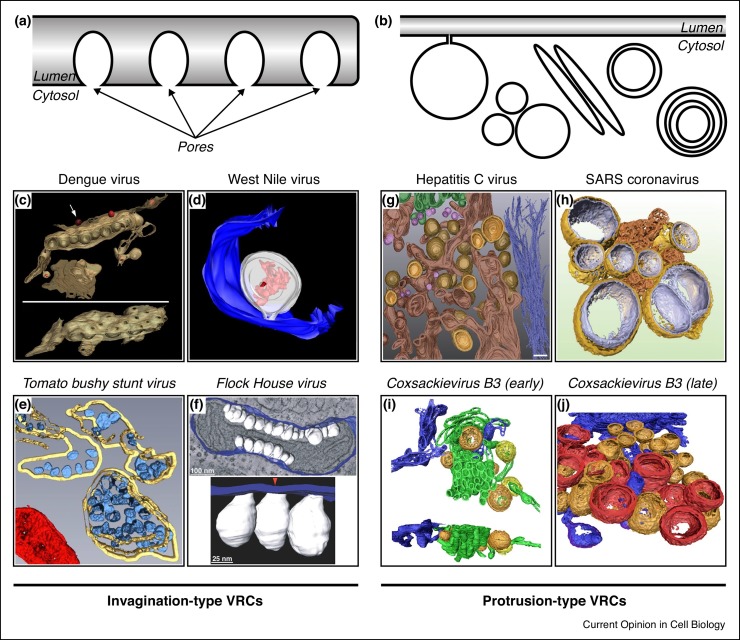
Recent advances in 3D electron microscopy have revolutionised our understanding of the structure of VRCs. VRCs can be subdivided into two morphological classes (Figure 1 ) (reviewed in Ref. [2]). Invagination-type VRCs are formed by bending of the donor membrane away from the cytoplasm (i.e. by induction of negative membrane curvature), resulting in a secluded environment that contains all factors required for genome replication. These VRCs are usually connected to the cytoplasm by a narrow pore that allows passing of small molecules such as nucleotides but restricts access of the antiviral defence machinery. Viruses with invagination-type VRCs include flaviviruses, tombusviruses and nodaviruses (Table 1 ).
Figure 1.
Overview of different VRC morphologies.
(a–b) Schematic overview of (a) invagination-type VRCs and (b) protrusion-type VRCs ([networks of] single- and double-membrane vesicles, multilamellar vesicles, tubules). (c–f) Three-dimensional reconstructions of invagination-type VRCs from various viruses. (c) VRCs of dengue virus in ER membranes. The tilted bottom panel is rotated by 90° to highlight the pores in the ER membrane that connect the VRC interior to the cytoplasm. (d) VRC (white) of West Nile virus in the lumen of the ER (blue). Viral RNA in the VRC lumen is displayed in red. (e) VRCs (blue) of tomato bushy stunt virus in the lumen of the peroxisome (yellow). A mitochondrion is shown in red. (f) VRCs (white) of Flock House virus in the intermembrane space of a mitochondrion connected to the outer mitochondrial membrane (blue). (g–j) Three-dimensional reconstructions of protrusion-type VRCs from various viruses. (g) Early stage of hepatitis C virus VRCs showing single-membrane (pink) and double-membrane (yellow inner membrane, light brown outer membrane) structures interspersed with ER membranes (dark brown). Golgi apparatus is shown in green, intermediate filaments are coloured dark blue. (h) Double-membrane vesicle VRCs of SARS-coronavirus (outer membrane in gold, inner membrane in silver) connected to so-called convoluted membranes (bronze). (i) Early stage tubular (green) and vesicular (orange, yellow) VRCs of the enterovirus coxsackievirus B3. ER is depicted in blue. (j) Late-stage VRCs of coxsackievirus B3 showing double-membrane vesicles (orange) and multilamellar vesicles (red). C has been reprinted from Ref. [79] with permission from Elsevier, D has been reproduced from Ref. [80] with permission from American Society for Microbiology, and E has been reproduced with permission from Journal of Cell Science from Ref. [81]. f–j are reproduced from open access publications [82] (f), [7] (g), [83] (h) and [5] (i, j).
Protrusion-type VRCs are generated by bending of the donor membrane into the cytoplasm (i.e. through induction of positive membrane curvature), resulting in single-membrane and double-membrane structures. Viruses that generate protrusion-type VRCs include hepatitis C virus (HCV), coronaviruses and enteroviruses (e.g. poliovirus, coxsackievirus, and rhinovirus) (Table 1 ). Protrusion-type VRCs often form a network of tightly packed membranes, which has been proposed to protect and hide the viral RNA [3, 4, 5, 6, 7, 8]. Biochemical support for this idea comes from work with poliovirus. In isolated poliovirus VRC networks, the viral RNA is protected from RNase digestion, but it becomes RNase-sensitive upon reversible disruption of the network (without the use of detergents, thus retaining membrane integrity) [4], indicating that the RNA does not reside inside the VRCs. In line with this, pores allowing access to the VRC lumen appear to be absent from single/double-membrane protrusion-type VRCs [5, 6, 7]. Collectively, these data indicate that RNA replication takes place on the cytoplasmic face of protrusion-type VRCs. Importantly, for enteroviruses and HCV it was shown that VRCs are dynamic structures that gradually transform from single-membrane structures into double-membrane and multilamellar structures, reminiscent of autophagic structures [5, 6, 7]. The single/double-membrane structures likely primarily support genome replication. The role of the multilamellar structures is less clear. In enterovirus-infected cells, they have been implicated in the en bloc release of virions in extracellular vesicles [9•]. In HCV-infected cells, they may be the result of a cellular stress response [7].
Sterols
Intracellular sterol distribution
Sterols are important components of eukaryotic membranes, which affect membrane properties like thickness and lipid packing, and are enriched particularly in late Golgi, endocytic compartments and the plasma membrane [10]. While mammalian cells incorporate cholesterol in membranes, plants and fungi use other sterols like ergosterol. Sterols are synthesised in the endoplasmic reticulum (ER) and from there redistributed to other organelles, keeping ER cholesterol levels low. Sterol biosynthesis is tightly regulated depending on extracellular supplies, which are taken up and redistributed intracellularly through the endosomal system. A significant portion of sterols is redistributed through non-vesicular transport by lipid transfer proteins, which often operate at membrane contact sites (MCSs), where membranes of two distinct organelles approximate closely (typically <30 nm). Sterol transporters include oxysterol-binding protein (OSBP) and OSBP-related proteins (ORPs) [11].
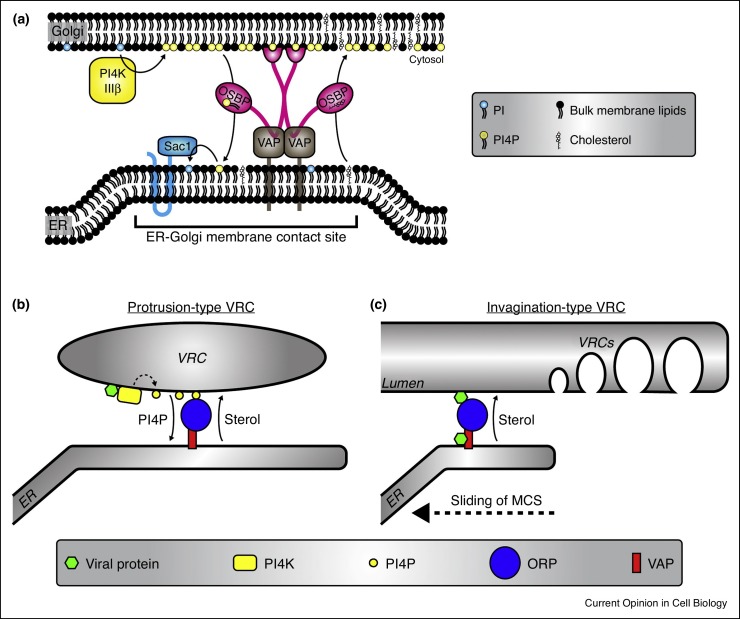
OSBP is a mammalian protein that operates at ER-Golgi MCSs. OSBP docks to trans-Golgi membranes through the small GTPase Arf1 and phosphatidylinositol 4-phosphate (PI4P) lipids, primarily produced by phosphatidylinositol 4-kinase type IIIβ (PI4KIIIβ). Simultaneously, OSBP binds to the ER via the transmembrane proteins VAP-A/B. OSBP shuttles cholesterol from the ER to the Golgi against the concentration gradient, which is powered by a counterflux of PI4P along the concentration gradient from the Golgi to the ER, where Sac1 removes the phosphate [12••] (Figure 2 a).
Figure 2.
Schematic depiction of cholesterol shuttling at MCSs.
(a) Schematic depiction of OSBP-mediated cholesterol shuttling at ER-Golgi MCSs (based upon the model presented in [12••]). PI4KIIIβ produces PI4P lipids at the Golgi. PI4P serves as a docking site for OSBP dimers. VAP-A/B transmembrane proteins link OSBP to the ER. OSBP transports cholesterol against the concentration gradient from ER to Golgi. A counterflux of PI4P along the concentration gradient provides the driving force for cholesterol transport. In the ER, Sac1 hydrolyses PI4P into PI to keep the PI4P gradient intact. (b) Model of cholesterol shuttling at ER-VRC MCSs as proposed for enteroviruses, cardioviruses and HCV (recently reviewed in [84]). Viral proteins (i.e. enterovirus 3A, cardiovirus 3A or HCV NS5A) recruit a PI4K (i.e. PI4KIIIβ for enteroviruses, PI4KIIIα for HCV and cardioviruses) to enrich the VRC membranes in PI4P lipids (dotted arrow). Reminiscent of the physiological situation at the Golgi, the PI4P lipids anchor ORPs (in this case OSBP) to the VRCs and drive and OSBP-mediated cholesterol accumulation. (c) Model of cholesterol transport to invagination-type VRCs as proposed for TBSV [38••]. The viral protein p33 recruits ORPs (in this case Osh3, Osh5, Osh6 and Osh7) to the MCS between ER and the peroxisomes, while p33 also binds VAP at the ER. The ORPs mediate cholesterol accumulation at the peroxisome. Of note, Osh6 and Osh7 were shown to exchange PS instead of cholesterol for PI4P [85•], suggesting that also PS may be shuttled to peroxisomes through the ER-peroxisome MCS. It has been hypothesised that the MCS slides along the surface of the peroxisome and that cholesterol accumulation primes the membrane for VRC formation in the wake of the sliding MCS [39].
Roles of sterols in VRC formation and function
Several evolutionary unrelated viruses, both with protrusion-type and invagination-type VRCs accumulate sterols at their VRCs. Several picornaviruses and HCV all employ a similar mechanism depending on PI4Ks, PI4P and OSBP (Figure 2b). Enteroviruses, through their 3A protein, recruit PI4KIIIβ to VRCs to enrich them in PI4P [13••]. The PI4P lipids serve as an anchor for OSBP and drive OSBP-mediated cholesterol accumulation at VRCs [14•, 15•, 16•]. Another picornavirus, Aichi virus, also recruits PI4KIIIβ and enriches VRCs in PI4P, whereas encephalomyocarditis virus (EMCV) hijacks PI4KIIIα instead of PI4KIIIβ to enrich PI4P at VRCs to mediate OSBP docking and cholesterol shuttling to VRCs [17, 18, 19•]. HCV has evolved a similar mechanism as EMCV to acquire cholesterol, involving PI4KIIIα recruitment by viral protein NS5A (although some genotypes also use PI4KIIIβ) to enrich PI4P at VRCs (reviewed in Ref. [20]) and drive OSBP-dependent cholesterol accumulation [21••].
In line with the essential role of the PI4K-PI4P-OSBP axis in recruiting cholesterol, enteroviruses, EMCV, human Saffold virus (which like EMCV belongs to the cardiovirus genus), and HCV are all sensitive to pharmacological inhibitors of the respective PI4K and OSBP [13••, 15•, 16•, 19•, 21••, 22, 23, 24, 25, 26, 27, 28]. Importantly, not all viruses with protrusion-type VRCs depend on the PI4K-PI4P-OSBP axis. Picornaviruses from several other genera (e.g. foot-and-mouth disease virus, hepatitis A virus and human parechovirus) are insensitive to inhibitors of PI4KIIIα/β and/or OSBP [16•, 19•, 26, 29, 30, 31], and also mouse hepatitis virus, a coronavirus, is insensitive to OSBP inhibition [29].
The involvement of OSBP in cholesterol accumulation at VRCs suggests that cholesterol, at least in part, originates from the ER. Since cholesterol is synthesised in the ER, it would be logical to assume that VRC cholesterol is newly synthesised. Alternatively, cholesterol from other organelles may be distributed through the ER to the VRCs. Enteroviruses and cardioviruses likely do not rely on newly synthesised cholesterol, but instead depend on redistribution of pre-existing cholesterol pools [32]. Enteroviruses may mobilise cholesterol stores from lipid droplets [15•]. Furthermore, they enhance endocytosis to increase uptake of cholesterol, which is delivered to VRCs via recycling endosomes [33••, 34]. This latter pathway may also involve a role of OSBP, since OSBP was recently shown to operate at ER-endosome MCSs as well [35•].
For HCV, inhibition of PI4K-PI4P-OSBP-mediated cholesterol shuttling disrupts the lipid environment of the viral proteins and alters VRC ultrastructure [21••]. The PI4K-PI4P-OSBP-cholesterol system is likely also involved in picornavirus VRC formation, since inhibition of PI4KIIIα or OSBP alters global EMCV VRC organisation [19•]. Viral rewiring of sterols may support multiple aspects of virus replication, as inhibition of the PI4K-PI4P-OSBP axis and treatments that reduce VRC cholesterol availability also alter proteolytic processing of the enteroviral polyprotein – which is a membrane-dependent process [36] – by viral proteinases [33••, 37].
A number of unrelated viruses that generate invagination-type VRCs also rely on sterols, while there is little if any evidence that they use PI4Ks or PI4P. This process is best understood for the tombusviruses tomato bushy stunt virus (TBSV) (Figure 2c), a plant virus that in experimental settings can also replicate in yeast. TBSV forms VRCs in peroxisomes and recruits several ORPs to ER-peroxisome MCSs through its viral protein p33 [38••]. Moreover, TBSV interacts with the ER-localised VAP homologue Scs2. Observations pointing to direct recruitment of ORPs were also made for the tombusvirus carnation Italian ringspot virus, which forms its VRCs in mitochondria [38••]. Thus, tombusviruses shuttle sterols to peroxisomes apparently independent of PI4P. How they fuel sterol accumulation is unknown. Unlike protrusion-type VRCs, invagination-type VRCs are formed in a pre-existing organelle. Therefore, viruses that build invagination-type VRCs may rely on – and perhaps reinforce – MCSs and lipid transport mechanisms that already exist in uninfected cells.
Whereas protrusion-type VRCs can directly form MCSs with the ER, this is topologically impossible for invagination-type VRCs. Instead, in TBSV-infected cells MCSs form between ER and peroxisomes close to the VRCs. It has been proposed that these MCSs slide along the peroxisome surface, possibly mediated by actin, to locally enrich sterols and prime the membrane for VRC formation [39, 40]. Likely, TBSV requires sterols for the assembly, stability and functioning of the replication machinery [38••, 41], although it is not known whether this is related to VRC formation.
The closely related flaviviruses West Nile virus (WNV) and dengue virus (DENV) also replicate independently of PI4Ks and PI4P, but are still sensitive to disruptions of cholesterol homeostasis [42, 43, 44, 45••, 46, 47]. Unfortunately, the role of cholesterol in flavivirus replication has not been comprehensively studied. WNV accumulates cholesterol at its VRCs [42], but similar studies are missing for DENV. DENV replication is insensitive to the OSBP inhibitor OSW-1 [21••], but there are no data for WNV. Assuming similar replication mechanisms, these findings suggest that flaviviruses accumulate cholesterol at VRCs independent of the PI4K-PI4P-OSBP axis. It remains to be studied whether flaviviruses build ER-VRC MCSs at all and usurp other cholesterol transfer proteins to accumulate cholesterol at VRCs, or whether they depend on disparate mechanisms like vesicular cholesterol delivery.
Glycerophospholipids
Glycerophospholipids (e.g. phosphatidylcholine [PC], phosphatidylethanolamine [PE], phosphatidylserine [PS], phosphatidylinositol [PI]) are major constituents of most membranes. While the different head groups are prime determinants of lipid properties like charge, shape and interactions with peripheral membrane proteins, FAs varying in length and saturation affect membrane properties like lipid packing and fluidity [48].
PC
Both protrusion- and invagination-type VRC-generating viruses (e.g. poliovirus, EMCV, Flock House virus, HCV, DENV and brome mosaic virus [BMV]) increase cellular levels of PC, the most prevalent glycerophospholipid [49, 50, 51, 52, 53, 54••]. In fact, poliovirus, EMCV, HCV and BMV accumulate PC at VRCs, while BMV has even been shown to recruit PC biosynthetic machinery to VRCs for local PC production [54••]. Other viruses likely also modulate local lipid synthesis at or near VRCs. For example, DENV and WNV recruit fatty acid synthase (FASN), the key enzyme in FA biogenesis, to VRCs [44, 55, 56]. In line with a requirement for de novo FA biosynthesis, several viruses, including enteroviruses, DENV, WNV and HCV, are sensitive to pharmacological inhibition of FASN and in some cases upregulate FASN [44, 53, 55, 56, 57, 58, 59, 60].
As an alternative strategy to support increased membrane lipid biosynthesis, several enteroviruses and EMCV increase uptake of FAs [61••]. The underlying mechanism has been investigated in detail in poliovirus-infected cells, which revealed specific increases in PC species with FAs with 16 or 18 carbons, likely matching VRC membrane properties. To increase uptake and activation of those FAs in particular, poliovirus harnesses the long chain acyl-CoA synthase Acsl3. Furthermore, while in non-infected cells most of the absorbed FAs are stored in lipid droplets, poliovirus reroutes them to membrane lipids at VRCs [61••].
PC is synthesised via two routes catalysed by ER-localised enzymes, either by addition of the choline head group to a diacylglycerol backbone (Kennedy pathway), or from PE by three subsequent methylation events of the head group (reviewed in Ref. [62]). In yeast, PE methylation is the major route for PC production, which may (partly) occur at MCSs, at least at ER-plasma membrane MCSs [63]. In contrast, the Kennedy pathway is the major pathway for PC biosynthesis in most mammalian cell types. Nevertheless, it is tempting to speculate that viruses alter host lipid metabolism to locally produce PC via in trans PE methylation at ER-VRC MCSs, which would allow in situ PC production at VRCs without the need for relocalisation of the enzymes from ER to VRCs. Which biosynthetic pathway mediates PC biosynthesis at VRCs and whether this involves virus-induced MCSs, remains to be established.
PE
+RNA viruses use a variety of other glycerophospholipids during replication, although their role in the viral life cycle and the involvement of MCSs in their homeostasis is often poorly understood. For example, TBSV accumulates PE at VRCs, possibly as a docking station for the viral replication machinery [64••]. Correspondingly, replication of TBSV is sensitive to disruptions of PE biosynthesis. The requirement for PE is likely more widespread among tombusviruses, including some that form VRCs in other organelles. It has been speculated that, apart from serving a role in the recruitment of viral replication proteins, PE through its small head group and resulting conical shape may contribute to the induction of the negative membrane curvature that is important for the invagination-type VRCs [65], but proof for this is lacking.
PS
Enteroviruses accumulate PS at VRC subdomains [9•]. Later in infection, PS is found at autophagosome-like double-membrane structures, which have been proposed to mediate non-lytic virus release. The resulting virus-containing extracellular vesicles are enriched in PS, which facilitates PS-receptor dependent uptake and infection in other cells [9•]. Numerous other viruses employ PS-dependent uptake in a process termed ‘apoptotic mimicry’ [66]. How PS accumulates at VRCs is unknown. Recently, the MCS proteins ORP5 and ORP8 were shown to facilitate a PS/PI4P exchange at ER-plasma membrane MCSs [67•]. Possibly, ORP5/8 are hijacked to facilitate PS accumulation at VRCs. It remains to be determined whether PS also has a role in enterovirus VRC formation and/or function.
PI(4,5)P2
Besides having functions of its own, PI4P is also a direct precursor for the PI-bisphosphates PI(3,4)P2 and PI(4,5)P2 [68•]. PI(4,5)P2 plays a role in cellular lipid transport by anchoring MCS proteins at multiple MCSs (e.g. ER-plasma membrane, lysosome-peroxisome) [69, 70]. HCV accumulates PI(4,5)P2 at its VRCs and for this it requires PIP5KIα [71••], suggesting that PI(4,5)P2 is locally produced from PI4P. PI(4,5)P2 interacts with a novel amphipathic helical motif in viral NS5A and enhances the interaction between NS5A and the cellular ER-localised Rab1 GTPase activating protein TBC1D20, which is an important host factor for HCV replication [71••, 72, 73]. Interestingly, the enteroviral protein 2C harbours a similar motif. Whether the presence of this motif is indicative of the accumulation of PI(4,5)P2 at enterovirus VRCs, and what the role of PI(4,5)P2 in enterovirus replication would be, remains to be investigated. Whether other PI-phosphates are in any way involved in +RNA virus replication is yet also unknown.
Sphingolipids
Sphingolipids represent a third major category of membrane lipids. This category of lipids is enriched in the plasma membrane, late Golgi and endolysosomal compartments [48]. MCSs and PI4P play crucial roles in the biosynthesis of sphingolipids (reviewed in Ref. [74]). The ceramide transporter (CERT) docks to the Golgi through PI4P and delivers ceramide, a substrate for sphingomyelin (SM) synthesis, from ER to Golgi at MCSs. Emerging evidence points to a role of sphingolipids in +RNA virus replication. This is best studied for HCV. Replication of HCV is sensitive to inhibitors of SM biosynthesis. SM binds the viral polymerase NS5B in vitro, supposedly to recruit it to SM-enriched detergent-resistant membranes, and activates the polymerase, although this may differ between genotypes [75]. However, there is no evidence yet that SM accumulates at VRCs and recruits and activates NS5B in HCV-infected cells.
The four-phosphate adaptor protein 2 (FAPP2) docks to the Golgi through PI4P and shuttles the glycosphingolipid glucosylceramide (GlcCer), a substrate for the synthesis of lactosylceramide (LacCer) and a subset of complex glycosphingolipids. FAPP2 localises to HCV VRCs and mediates LacCer accumulation [76]. FAPP2 depletion impairs HCV replication and disrupts VRC formation [76]. The adverse effect of replication of FAPP2 depletion can be efficiently rescued by addition of different (complex) glycosphingolipids, but not all, implying that HCV has specific requirements for sphingolipids at its VRC.
Also some viruses with invagination-type VRCs may use sphingolipids. SM localises to WNV VRCs and WNV replication is sensitive to inhibition of SM biosynthesis, albeit moderately [77]. Correspondingly, ceramide is redistributed to VRCs and inhibition of ceramide biosynthesis impairs WNV replication [78]. For DENV, two studies report contrasting results on the accumulation and importance of sphingolipids [53, 78], which may represent differences in biology or experimental approach. This leaves the matter whether these closely related flaviviruses have similar or different interactions with sphingolipids currently unresolved.
Outlook
Although lipids are essential components of membranes and thus indispensible for the replication +RNA viruses, their diverse roles are quite poorly studied. Recent years have seen an upsurge of the field, spurred by emerging insights in basic lipid and membrane biology. In the near future, novel technologies (e.g. lipidomics, fluorescent probes to detect lipids) will spur further studies into how +RNA viruses rewire host lipid metabolism to optimally support genome replication. Such fundamental insights into virus replication may provide a basis for the development of novel antiviral drugs.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We apologise to all colleagues whose (primary) work could not be cited due to space restrictions. Research in the authors’ lab is supported by grants from the European Union (Horizon2020 Marie Sklodowska Curie European Training Network “ANTIVIRALS”, grant agreement number 642434 to FJMvK) and from the Netherlands Organisation for Scientific Research (NWO) (VENI-722.012.066 to JRPMS and VICI-91812628 to FJMvK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Miller S., Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 2008;6:363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero-Brey I., Bartenschlager R. Membranous replication factories induced by plus-strand RNA viruses. Viruses. 2014;6:2826–2857. doi: 10.3390/v6072826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bienz K., Egger D., Pfister T., Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egger D., Pasamontes L., Bolten R., Boyko V., Bienz K. Reversible dissociation of the poliovirus replication complex: functions and interactions of its components in viral RNA synthesis. J Virol. 1996;70:8675–8683. doi: 10.1128/jvi.70.12.8675-8683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limpens R.W.A.L., van der Schaar H.M., Kumar D., Koster A.J., Snijder E.J., van Kuppeveld F.J.M., Bárcena M. The transformation of enterovirus replication structures: a three-dimensional study of single- and double-membrane compartments. mBio. 2011;2:e00111–e00166. doi: 10.1128/mBio.00166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belov G.A., Nair V., Hansen B.T., Hoyt F.H., Fischer E.R., Ehrenfeld E. Complex dynamic development of poliovirus membranous replication complexes. J Virol. 2012;86:302–312. doi: 10.1128/JVI.05937-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero-Brey I., Merz A., Chiramel A., Lee J.Y., Chlanda P., Haselman U., Santarella-Mellwig R., Habermann A., Hoppe S., Kallis S. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012;8:e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neufeldt C.J., Joyce M.A., Van Buuren N., Levin A., Kirkegaard K., Gale M., Jr., Tyrrell D.L., Wozniak R.W. The hepatitis C virus-induced membranous web and associated nuclear transport machinery limit sccess of pattern recognition receptors to viral replication sites. PLoS Pathog. 2016;12:e1005428. doi: 10.1371/journal.ppat.1005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Chen Y.H., Du W., Hagemeijer M.C., Takvorian P.M., Pau C., Cali A., Brantner C.A., Stempinski E.S., Connelly P.S., Ma H.C. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates for the first time that enteroviruses accumulate PS at their VRCs and provides evidence that PS ends up at the exposed face of extracellular vesicles containing multiple virions, thus helping PS-dependent en bloc uptake of viruses. This paper provides a functional and mechanistic explanation for previous observations that enteroviruses are transmitted via extracellular vesicles.
- 10.Holthuis J.C., Menon A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature. 2014;510:48–57. doi: 10.1038/nature13474. [DOI] [PubMed] [Google Scholar]
- 11.Moser von Filseck J., Drin G. Running up that hill: how to create cellular lipid gradients by lipid counter-flows. Biochimie. 2016;130:115–121. doi: 10.1016/j.biochi.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 12••.Mesmin B., Bigay J., Moser von Filseck J., Lacas-Gervais S., Drin G., Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]; This manuscript shows for the first time that OSBP employs a counterflux with PI4P to shuttle cholesterol. It has set a mechanistic framework for other lipid transfer proteins, some of which have now also been shown to perform lipid counterfluxes (see e.g. Refs [67] & [85]).
- 13••.Hsu N.-Y., Ilnytska O., Belov G., Santiana M., Chen Y.-H., Takvorian P.M., Pau C., van der Schaar H., Kaushik-Basu N., Balla T. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using enteroviruses as a model system, this is the first publication showing that a virus recruits a PI4K to its VRCs and enriches them in PI4P lipids.
- 14•.Arita M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol Immunol. 2014;58:239–256. doi: 10.1111/1348-0421.12144. [DOI] [PubMed] [Google Scholar]; This reference demonstrates that the PI4P lipids at enterovirus VRCs serve to recruit OSBP and drive cholesterol accumulation at the VRCs.
- 15•.Roulin P.S., Lötzerich M., Torta F., Tanner L.B., van Kuppeveld F.J.M., Wenk M.R., Greber U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe. 2014;16:677–690. doi: 10.1016/j.chom.2014.10.003. [DOI] [PubMed] [Google Scholar]; This reference demonstrates that the PI4P lipids at enterovirus VRCs serve to recruit OSBP and drive cholesterol accumulation at the VRCs.
- 16•.Strating J.R., van der Linden L., Albulescu L., Bigay J., Arita M., Delang L., Leyssen P., van der Schaar H.M., Lanke K.H., Thibaut H.J. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep. 2015;10:600–615. doi: 10.1016/j.celrep.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference demonstrates that the PI4P lipids at enterovirus VRCs serve to recruit OSBP and drive cholesterol accumulation at the VRCs.
- 17.Greninger A.L., Knudsen G.M., Betegon M., Burlingame A.L., DeRisi J.L. The 3A protein from multiple picornaviruses utilizes the Golgi adaptor protein ACBD3 to recruit PI4KIIIβ. J Virol. 2012;86:3605–3616. doi: 10.1128/JVI.06778-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki J., Ishikawa K., Arita M., Taniguchi K. ACBD3-mediated recruitment of PI4KB to picornavirus RNA replication sites. EMBO J. 2012;31:754–766. doi: 10.1038/emboj.2011.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Dorobantu C.M., Albulescu L., Harak C., Feng Q., van Kampen M., Strating J.R., Gorbalenya A.E., Lohmann V., van der Schaar H.M., van Kuppeveld F.J. Modulation of the host lipid landscape to promote RNA virus replication: the picornavirus encephalomyocarditis virus converges on the pathway used by hepatitis C virus. PLoS Pathog. 2015;11:e1005185. doi: 10.1371/journal.ppat.1005185. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that cardioviruses hijack PI4KIIIα to recruit OSBP and drive cholesterol accumulation at VRCs. It is the first time that a picornavirus is shown to use PI4KIIIα for this purpose, which is different from the use of PI4KIIIβ by enteroviruses and kobuviruses.
- 20.Bishé B., Syed G., Siddiqui A. Phosphoinositides in the hepatitis C virus life cycle. Viruses. 2012;4:2340–2358. doi: 10.3390/v4102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Wang H., Perry J.W., Lauring A.S., Neddermann P., De Francesco R., Tai A.W. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology. 2014;146:1373–1385. doi: 10.1053/j.gastro.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using HCV, this manuscript shows the recruitment of OSBP to VRCs and the importance of OSBP-mediated accumulation of cholesterol in VRC formation. This is the first report of the involvement of any ORP in virus replication.
- 22.Arita M., Kojima H., Nagano T., Okabe T., Wakita T., Shimizu H. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J Virol. 2011;85:2364–2372. doi: 10.1128/JVI.02249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianco A., Reghellin V., Donnici L., Fenu S., Alvarez R., Baruffa C., Peri F., Pagani M., Abrignani S., Neddermann P. Metabolism of phosphatidylinositol 4-kinase IIIalpha-dependent PI4P Is subverted by HCV and is targeted by a 4-anilino quinazoline with antiviral activity. PLoS Pathog. 2012;8:e1002576. doi: 10.1371/journal.ppat.1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaillancourt F.H., Brault M., Pilote L., Uyttersprot N., Gaillard E.T., Stoltz J.H., Knight B.L., Pantages L., McFarland M., Breitfelder S. Evaluation of phosphatidylinositol-4-kinase IIIalpha as a hepatitis C virus drug target. J Virol. 2012;86:11595–11607. doi: 10.1128/JVI.01320-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arita M., Kojima H., Nagano T., Okabe T., Wakita T., Shimizu H. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. J Virol. 2013;87:4252–4260. doi: 10.1128/JVI.03546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Schaar H.M., Leyssen P., Thibaut H.J., de Palma A., van der Linden L., Lanke K.H., Lacroix C., Verbeken E., Conrath K., Macleod A.M. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIbeta. Antimicrob Agents Chemother. 2013;57:4971–4981. doi: 10.1128/AAC.01175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bojjireddy N., Botyanszki J., Hammond G., Creech D., Peterson R., Kemp D.C., Snead M., Brown R., Morrison A., Wilson S. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J Biol Chem. 2014;289:6120–6132. doi: 10.1074/jbc.M113.531426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorobantu C.M., Harak C., Klein R., van der Linden L., Strating J.R., van der Schaar H.M., Lohmann V., van Kuppeveld F.J. Tyrphostin AG1478 inhibits encephalomyocarditis virus and hepatitis C virus by targeting phosphatidylinositol 4-kinase IIIalpha. Antimicrob Agents Chemother. 2016;60:6402–6406. doi: 10.1128/AAC.01331-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albulescu L., Strating J.R., Thibaut H.J., van der Linden L., Shair M.D., Neyts J., van kuppeveld F.J. Broad-range inhibition of enterovirus replication by OSW-1, a natural compound targeting OSBP. Antiviral Res. 2015;117:110–114. doi: 10.1016/j.antiviral.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Esser-Nobis K., Harak C., Schult P., Kusov Y., Lohmann V. Novel perspectives for hepatitis A virus therapy revealed by comparative analysis of hepatitis C virus and hepatitis A virus RNA replication. Hepatology. 2015;62:397–408. doi: 10.1002/hep.27847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berryman S., Moffat K., Harak C., Lohmann V., Jackson T. Foot-and-mouth disease virus replicates independently of phosphatidylinositol 4-phosphate and type III phosphatidylinositol 4-kinases. J Gen Virol. 2016;97:1841–1852. doi: 10.1099/jgv.0.000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albulescu L., Wubbolts R., van Kuppeveld F.J., Strating J.R. Cholesterol shuttling is important for RNA replication of coxsackievirus B3 and encephalomyocarditis virus. Cell Microbiol. 2015;17:1144–1156. doi: 10.1111/cmi.12425. [DOI] [PubMed] [Google Scholar]
- 33••.Ilnytska O., Santiana M., Hsu N.Y., Du W.L., Chen Y.H., Viktorova E.G., Belov G., Brinker A., Storch J., Moore C. Enteroviruses harness the cellular endocytic machinery to remodel the host cell cholesterol landscape for effective viral replication. Cell Host Microbe. 2013;14:281–293. doi: 10.1016/j.chom.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ilnytska et al. show that enteroviruses upregulate endocytosis to increase cholesterol uptake and deliver cholesterol to VRCs via recycling endosomes, thus revealing a new route via which viruses can acquire cholesterol.
- 34.Cornell C.T., Kiosses W.B., Harkins S., Whitton J.L. Coxsackievirus B3 proteins directionally complement each other to downregulate surface major histocompatibility complex class I. J Virol. 2007;81:6785–6797. doi: 10.1128/JVI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Dong R., Saheki Y., Swarup S., Lucast L., Harper J.W., De Camilli P. Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell. 2016;166:408–423. doi: 10.1016/j.cell.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this hallmark paper, the authors show that OSBP also operates at endosome-ER MCSs and regulates endosomal lipid homeostasis and protein sorting.
- 36.Molla A., Paul A.V., Wimmer E. Effects of temperature and lipophilic agents on poliovirus formation and RNA synthesis in a cell-free system. J Virol. 1993;67:5932–5938. doi: 10.1128/jvi.67.10.5932-5938.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford Siltz L.A., Viktorova E.G., Zhang B., Kouiavskaia D., Dragunsky E., Chumakov K., Isaacs L., Belov G.A. New small molecule inhibitors effectively blocking picornavirus replication. J Virol. 2014;88:11091–11107. doi: 10.1128/JVI.01877-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Barajas D., Xu K., de Castro Martin I.F., Sasvari Z., Brandizzi F., Risco C., Nagy P.D. Co-opted oxysterol-binding ORP and VAP proteins channel sterols to RNA virus replication sites via membrane contact sites. PLoS Pathog. 2014;10:e1004388. doi: 10.1371/journal.ppat.1004388. [DOI] [PMC free article] [PubMed] [Google Scholar]; This publication provides the first example of a virus with an invagination-type VRC that hijacks ORPs and VAP to facilitate cholesterol accumulation at VRCs.
- 39.Nagy P.D., Strating J.R., van Kuppeveld F.J. Building viral replication organelles: close encounters of the membrane types. PLoS Pathog. 2016;12:e1005912. doi: 10.1371/journal.ppat.1005912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nawaz-ul-Rehman M.S., Prasanth K.R., Xu K., Sasvari Z., Kovalev N., de Castro Martin I.F., Barajas D., Risco C., Nagy P.D. Viral replication protein inhibits cellular cofilin actin depolymerization factor to regulate the actin network and promote viral replicase assembly. PLoS Pathog. 2016;12:e1005440. doi: 10.1371/journal.ppat.1005440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma M., Sasvari Z., Nagy P.D. Inhibition of sterol biosynthesis reduces tombusvirus replication in yeast and plants. J Virol. 2010;84:2270–2281. doi: 10.1128/JVI.02003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackenzie J.M., Khromykh A.A., Parton R.G. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe. 2007;2:229–239. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Rothwell C., Lebreton A., Young Ng C., Lim J.Y., Liu W., Vasudevan S., Labow M., Gu F., Gaither L.A. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389:8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 44.Martín-Acebes M.A., Blázquez A.-B., Jiménez de Oya N., Escribano-Romero E., Saiz J.-C. West Nile Virus replication requires fatty acid synthesis but is independent on phosphatidylinositol-4-phosphate lipids. PLoS One. 2011;6:e24970. doi: 10.1371/journal.pone.0024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Reiss S., Rebhan I., Backes P., Romero-Brey I., Erfle H., Matula P., Kaderali L., Poenisch M., Blankenburg H., Hiet M.-S. Recruitment and activation of a lipid kinase by hepatitis C Virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper from the Bartenschlager lab shows for the first time that PI4KIIIα is recruited to HCV ROs to accumulate PI4P and that PI4KIIIα is important for proper VRC formation.
- 46.Soto-Acosta R., Mosso C., Cervantes-Salazar M., Puerta-Guardo H., Medina F., Favari L., Ludert J.E., del Angel R.M. The increase in cholesterol levels at early stages after dengue virus infection correlates with an augment in LDL particle uptake and HMG-CoA reductase activity. Virology. 2013;442:132–147. doi: 10.1016/j.virol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Poh M.K., Shui G., Xie X., Shi P.-Y., Wenk M.R., Gu F. U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral Res. 2012;93:191–198. doi: 10.1016/j.antiviral.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 48.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amako K., Dales S. Cytopathology of mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967;32:201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- 50.Vance D.E., Trip E.M., Paddon H.B. Poliovirus increases phosphatidylcholine biosynthesis in HeLa cells by stimulation of the rate-limiting reaction catalyzed by CTP:phosphocholine cytidylyltransferase. J Biol Chem. 1980;255:1064–1069. [PubMed] [Google Scholar]
- 51.Castorena K.M., Stapleford K.A., Miller D.J. Complementary transcriptomic, lipidomic, and targeted functional genetic analyses in cultured Drosophila cells highlight the role of glycerophospholipid metabolism in Flock House virus RNA replication. BMC Genomics. 2010;11:183. doi: 10.1186/1471-2164-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roe B., Kensicki E., Mohney R., Hall W.W. Metabolomic profile of hepatitis C virus-infected hepatocytes. PLoS One. 2011;6:e23641. doi: 10.1371/journal.pone.0023641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perera R., Riley C., Isaac G., Hopf-Jannasch A.S., Moore R.J., Weitz K.W., Pasa-Tolic L., Metz T.O., Adamec J., Kuhn R.J. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012;8:e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54••.Zhang J., Zhang Z., Chukkapalli V., Nchoutmboube J.A., Li J., Randall G., Belov G.A., Wang X. Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites. Proc Natl Acad Sci U S A. 2016;113:E1064–E1073. doi: 10.1073/pnas.1519730113. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this publication, the authors show for the first time the concept that a virus (brome mosaic virus) locally synthesises PC at VRCs by recruiting PC biosynthetic machinery to VRCs. They also elegantly show that HCV and poliovirus accumulate newly synthesised PC at their VRCs.
- 55.Heaton N.S., Perera R., Berger K.L., Khadka S., Lacount D.J., Kuhn R.J., Randall G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci U S A. 2010;107:17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang W.C., Lin R.J., Liao C.L., Lin Y.L. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol. 2014;88:6793–6804. doi: 10.1128/JVI.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guinea R., Carrasco L. Phospholipid biosynthesis and poliovirus genome replication, two coupled phenomena. EMBO J. 1990;9:2011–2016. doi: 10.1002/j.1460-2075.1990.tb08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rassmann A., Henke A., Jarasch N., Lottspeich F., Saluz H.P., Munder T. The human fatty acid synthase: a new therapeutic target for coxsackievirus B3-induced diseases? Antiviral Res. 2007;76:150–158. doi: 10.1016/j.antiviral.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Yang W., Hood B.L., Chadwick S.L., Liu S., Watkins S.C., Luo G., Conrads T.P., Wang T. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48:1396–1403. doi: 10.1002/hep.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilsky S., Sobotta K., Wiesener N., Pilas J., Althof N., Munder T., Wutzler P., Henke A. Inhibition of fatty acid synthase by amentoflavone reduces coxsackievirus B3 replication. Arch Virol. 2012;157:259–269. doi: 10.1007/s00705-011-1164-z. [DOI] [PubMed] [Google Scholar]
- 61••.Nchoutmboube J.A., Viktorova E.G., Scott A.J., Ford L.A., Pei Z., Watkins P.A., Ernst R.K., Belov G.A. Increased long chain acyl-CoA synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles. PLoS Pathog. 2013;9:e1003401. doi: 10.1371/journal.ppat.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nchoutmboube et al. show that enteroviruses and cardioviruses upregulate the uptake of fatty acids to provide building blocks for PC synthesis for VRC formation and to support virus replication. Importantly, they show that poliovirus induces an specific uptake of a subset of FAs and provide a mechanistic explanation of how poliovirus achieves this.
- 62.Vance J.E. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 63.Tavassoli S., Chao J.T., Young B.P., Cox R.C., Prinz W.A., de Kroon A.I., Loewen C.J. Plasma membrane-endoplasmic reticulum contact sites regulate phosphatidylcholine synthesis. EMBO Rep. 2013;14:434–440. doi: 10.1038/embor.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Xu K., Nagy P.D. RNA virus replication depends on enrichment of phosphatidylethanolamine at replication sites in subcellular membranes. Proc Natl Acad Sci U S A. 2015;112:E1782–E1791. doi: 10.1073/pnas.1418971112. [DOI] [PMC free article] [PubMed] [Google Scholar]; In studying tombusviruses, this manuscript demonstrates for the first time the enrichment of PE at VRCs and the importance of this for replication.
- 65.Altan-Bonnet N. Lipid tales of viral replication and transmission. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazzon M., Mercer J. Lipid interactions during virus entry and infection. Cell Microbiol. 2014;16:1493–1502. doi: 10.1111/cmi.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Chung J., Torta F., Masai K., Lucast L., Czapla H., Tanner L.B., Narayanaswamy P., Wenk M.R., Nakatsu F., De Camilli P. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this publication, the authors report the groundbreaking finding that other ORPs than OSBP also employ a lipid counterflux. Specifically, they show that ORP5 and ORP8 perform a PS/PI4P counterflux at ER-plasma membrane MCSs to shuttle PS to the plasma membrane. It appeared back-to-back with publication 85, which shows a similar mechanism for the yeast ORPs Osh6 and Osh7.
- 68•.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this encyclopedic review, Tamas Balla provides a comprehensive overview of our current knowledge on phosphoinositides, their metabolism and their roles in health and disease.
- 69.Chu B.B., Liao Y.C., Qi W., Xie C., Du X., Wang J., Yang H., Miao H.H., Li B.L., Song B.L. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell. 2015;161:291–306. doi: 10.1016/j.cell.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 70.Perez-Lara A., Jahn R. Extended synaptotagmins (E-Syts): architecture and dynamics of membrane contact sites revealed. Proc Natl Acad Sci U S A. 2015;112:4837–4838. doi: 10.1073/pnas.1504487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71••.Cho N.J., Lee C., Pang P.S., Pham E.A., Fram B., Nguyen K., Xiong A., Sklan E.H., Elazar M., Koytak E.S. Phosphatidylinositol 4,5-bisphosphate is an HCV NS5A ligand and mediates replication of the viral genome. Gastroenterology. 2015;148:616–625. doi: 10.1053/j.gastro.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]; This publication reports that HCV accumulates PI(4,5)P2 at VRCs and unravels a role in regulating the interaction between viral NS5A and host TBC1D20. The authors uncover a novel PI(4,5)P2-binding amphipatic helical motif that also occurs in various other cellular and viral proteins. It is the first report of the involvement of bisphosphorylated phosphoinositides in virus replication.
- 72.Sklan E.H., Serrano R.L., Einav S., Pfeffer S.R., Lambright D.G., Glenn J.S. TBC1D20 is a Rab1 GTPase-activating protein that mediates hepatitis C virus replication. J Biol Chem. 2007;282:36354–36361. doi: 10.1074/jbc.M705221200. [DOI] [PubMed] [Google Scholar]
- 73.Sklan E.H., Staschke K., Oakes T.M., Elazar M., Winters M., Aroeti B., Danieli T., Glenn J.S. A Rab-GAP TBC domain protein binds hepatitis C virus NS5A and mediates viral replication. J Virol. 2007;81:11096–11105. doi: 10.1128/JVI.01249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaji T., Hanada K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic. 2015;16:101–122. doi: 10.1111/tra.12239. [DOI] [PubMed] [Google Scholar]
- 75.Weng L., Hirata Y., Arai M., Kohara M., Wakita T., Watashi K., Shimotohno K., He Y., Zhong J., Toyoda T. Sphingomyelin activates hepatitis C virus RNA polymerase in a genotype-specific manner. J Virol. 2010;84:11761–11770. doi: 10.1128/JVI.00638-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan I., Katikaneni D.S., Han Q., Sanchez-Felipe L., Hanada K., Ambrose R.L., Mackenzie J.M., Konan K.V. Modulation of hepatitis C virus genome replication by glycosphingolipids and four-phosphate adaptor protein 2. J Virol. 2014;88:12276–12295. doi: 10.1128/JVI.00970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin-Acebes M.A., Gabande-Rodriguez E., Garcia-Cabrero A.M., Sanchez M.P., Ledesma M.D., Sobrino F., Saiz J.C. Host sphingomyelin increases West Nile virus infection in vivo. J Lipid Res. 2016;57:422–432. doi: 10.1194/jlr.M064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aktepe T.E., Pham H., Mackenzie J.M. Differential utilisation of ceramide during replication of the flaviviruses West Nile and dengue virus. Virology. 2015;484:241–250. doi: 10.1016/j.virol.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 79.Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C.K., Walther P., Fuller S.D., Antony C., Krijnse-Locker J., Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gillespie L.K., Hoenen A., Morgan G., Mackenzie J.M. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J Virol. 2010;84:10438–10447. doi: 10.1128/JVI.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernández de Castro I., Fernández J.J., Barajas D., Nagy P.D., Risco C. Three-dimensional imaging of the intracellular assembly of a functional viral RNA replicase complex. J Cell Sci. 2017;130:260–268. doi: 10.1242/jcs.181586. [DOI] [PubMed] [Google Scholar]
- 82.Kopek B.G., Perkins G., Miller D.J., Ellisman M.H., Ahlquist P. Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle. PLoS Biol. 2007;5:e220. doi: 10.1371/journal.pbio.0050220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knoops K., Kikkert M., Worm S.H., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Schaar H.M., Dorobantu C.M., Albulescu L., Strating J.R., van Kuppeveld F.J. Fat(al) attraction: picornaviruses usurp lipid transfer at membrane contact sites to create replication organelles. Trends Microbiol. 2016;24:535–546. doi: 10.1016/j.tim.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85•.Moser von Filseck J., Copic A., Delfosse V., Vanni S., Jackson C.L., Bourguet W., Drin G. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349:432–436. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]; In this paper, the authors report the groundbreaking finding that the yeast ORPs Osh6 and Osh7 mediate and PS/PI4P exchange, reminiscent of the cholesterol/PI4P exchange that OSBP performs. The manuscript appeared back-to-back with publication 67, which shows a similar mechanism for the mammalian proteins ORP5 and ORP8.