Abstract
Ethnopharmacological relevance
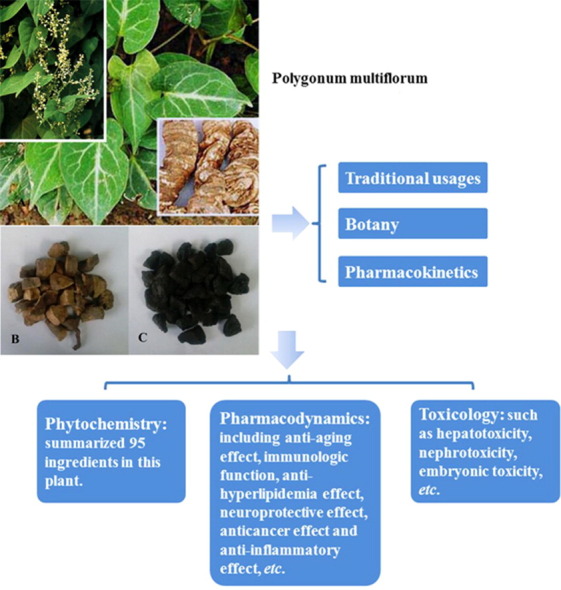
Polygonum multiflorum Thunb., which is known as Heshouwu (何首乌 in Chinese) in China. It is traditionally valued and reported for hair-blacking, liver and kidney-tonifying and anti-aging effects as well as low toxicity. The aim of this review is to provide comprehensive information on the botany, traditional uses, phytochemistry, pharmacological research and toxicology of Polygonum multiflorum, based on the scientific literature. Moreover, trends and perspectives for future investigation of this plant are discussed. It will build up a new foundation for further study on Polygonum multiflorum.
Materials and methods
A systematic review of the literature on Polygonum multiflorum was performed using several resources, including classic books on Chinese herbal medicine and various scientific databases, such as PubMed, SciFinder, the Web of Science, Science Direct, China Knowledge Resource Integrated (CNKI).
Results
Polygonum multiflorum is widely distributed throughout the world and has been used as a traditional medicine for centuries in China. The ethnomedical uses of Polygonum multiflorum have been recorded in many provinces of China and Japan for nine species of adulterants in six families. More than 100 chemical compounds have been isolated from this plant, and the major components have been determined to be stilbenes, quinones, flavonoids and others. Crude extracts and pure compounds of this plant are used as effective agents in pre-clinical and clinical practice due to their anti-aging, anti-hyperlipidaemia, anti-cancer and anti-inflammatory effects and to promote immunomodulation, neuroprotection, and the curing of other diseases. However, these extracts can also lead to hepatotoxicity, nephrotoxicity and embryonic toxicity. Pharmacokinetic studies have demonstrated that the main components of Polygonum multiflorum, such as 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranoside and emodin are distributed among many organs and tissues.
Conclusion
Therapeutic potential of Polygonum multiflorum has been demonstrated in the conditions like Alzheimer׳s disease, Parkinson׳s disease, hyperlipidaemia, inflammation and cancer, which is attributed to the presence of various stilbenes, quinones, flavonoids, phospholipids and other compounds in the drug. On the other hand, the adverse effects (hepatotoxicity, nephrotoxicity, and embryonic toxicity) of this plant were caused by the quinones, such as emodin and rhein. Thus more pharmacological and toxicological mechanisms on main active compounds are necessary to be explored, especially the combined anthraquinones (Emodin-8-O-β-d-glucopyranoside, Physcion-8-O-β-d-glucopyranoside, etc.) and the variety of stilbenes.
Keywords: Polygonum multiflorum Thunb, Traditional usages, Botany, Phytochemistry, Pharmacology and toxicology
Graphical abstract
1. Introduction
Polygonum multiflorum ( Fig. 1) is one of the most popular traditional Chinese medicines and is an ingredient in many medicines and prescriptions. It has been widely used to treat various diseases that have been commonly associated with aging for many centuries in China. A recent study proved that it can exhibit antioxidative activity (Lv et al., 2007, Wang, 2005a, Wang et al., 2008, Wang et al., 2009a), mainly due to its flavonoid and phenolic acid constituents. The pharmacological effects of stilbene in Polygonum multiflorum have been reported to promote anti-aging effects (Long and Dougherty, 2003, Lin et al., 2008, Chen et al., 2001a, Chan et al., 2002, Um et al., 2006, Cheung et al., 2014) and hepatoprotective activities (Huang et al., 2007, Liu et al., 1992). Anthraquinones, another main component of this plant, also have many biological activities, such as effects against cancer (Ma et al., 2012, Wang et al., 2011b, Way et al., 2014, Yu et al., 2013, Liu et al., 2009a, Lin et al., 2006, Tabolacci et al., 2010), developmental anomalies (Yon et al., 2013), and tonic tension (Lim et al., 2014). However, an increasing number of recently published studies have demonstrated the adverse effects of Polygonum multiflorum. Some researchers have found that Polygonum multiflorum shows not only hepatotoxicity but also a possible drug interaction with warfarin to result in bone marrow suppression. Specifically, the long-term use of Polygonum multiflorum may lead to liver and kidney toxicity. The toxicity of emodin has been detailed by the U.S. National Toxicology Program (NTP technical report, 2001). In the current review, we provide a comprehensive overview of the existing knowledge and traditional uses of Polygonum multiflorum, including its botany, phytochemistry, pharmacodynamics and potential applications, toxicology and pharmacokinetics.
Fig. 1.
Whole Polygonum multiflorum plant (A); The roots of Polygonum multiflorum (B); the roots of Polygonum multiflorum Praeparata (C).
2. Traditional usages
With a wide spectrum of biological and pharmacological effects, Polygonum multiflorum has been used as a traditional medicine for many centuries in China. The Compendium of Materia Medica (Ben cao Gang mu) reported that it exerts liver-tonic and hair-blacking effects. In Diannan Bencao, a famous monograph of traditional Chinese medicine, this plant was described to be useful for the treatment of sore scabies, ringworm and pruritus (Lan, 1959). The toxicity of this plant was first described in Bencao Huiyan (another famous monograph of traditional Chinese medicine written in China during the Ming dynasty), which described this plant to be of minimal toxicity to humans. In other monographs of Materia Medica, such as Kaibao Bencao, Bencao Mengquan, and Xinbian Bencao, this plant was described to be used for the treatment of scrofula, carbuncles, and postpartum and morbid leucorrhea and to reinforce the kidney and promote anti-aging effects.
The root of this plant is used as the effective agent after its common processing by steaming with black bean (Zhou et al., 2010), rehmannia juice, or a wine- or ginger-black bean mixture (Committee on the Programming of Teaching Material for Higher TCM Education, 1996).
The black bean method (black beans are the dry and mature seeds of Glycine max (L.) Merr.) (Yang et al., 2008) is the most commonly used processing method, as described in “Xian Shou Li Shang Xu Duan Mi Fang” (Secret Formulary for Traumatology and Fracture Taught by Immortal), which was written during the Tang Dynasty. This work first proposed that the plant be prepared as follows: “10 lb, cooked with half a catty of black bean”. The book “Sue Shen Liang fang” of the Song Dynasty states that “After a one-day-long immersion, cut the Polygonum multiflorum into half-inch-thick sections and then mix well with the water of the black bean by placing fleece-flower root layer upon layer with the black bean. This should be followed by steaming until the beans become rotten, removal of the beans, and drying of the root in the shade”. The Qing Dynasty “Cheng Fang Qie Yong” proposed that the plant be “mixed seven times with black bean juice” (Yu, 2014). In modern times, the 2010 edition of “Chinese Pharmacopoeia” described the following detailed rules for the Polygonum multiflorum processing method: Mix the slices or pieces of Polygonum multiflorum thoroughly with black bean juice (Preparation of black bean juice: Boil 10 kg of black bean in a sufficient quantity of water for 4 h and stew to get about 15 kg of juice. Boil the bean residue again in water for about 3 h and stew to get about 10 kg of juice. Combine to get about 25 kg of the black bean juice). Carry out the stewing method in a suitable non-ferrous container until the juice is exhausted or carry out the steaming method, steam it alone or steam it after being mixed with black bean juice to a brown color on all sides, dry in the sun to partial dryness, then cut into slice and dry. For each 100 kg of slice (piece) of Polygonum multiflorum use 10 kg of black bean (Editorial Committee of Chinese Pharmacopoeia., 2010).
Steaming is another common preparation method for Polygonum multiflorum. In this method, slightly moist Polygonum multiflorum is enclosed in boiler and steamed until the drugs both inside and outside are brown, at which point it is removed and desiccated (Zhou et al., 2010, Tian et al., 2007, Li et al., 2012g). In addition, there are other processing methods, including steaming with wine, rehmannia juice and boiling with a black bean-ginger mixture. There are also novel and improved processing methods using modern science and technology, such as fermentation and autoclave steaming. The pressure processing parameters are as follows: the pressure ranges from 0.08 to 0.25 MPa, the heating time ranges from 4 to 10 h, and the temperature is 120 °C (Li et al., 2012f, Li et al., 2012g, Du et al., 2012, Sun, 1996c, Liu et al., 2013, Xu et al., 2011, Qiu and Zeng, 2006b).
Currently in China, Polygonum multiflorum is a well-known traditional herb that is used as the main component of powders, decoctions or infusions for the treatments of leptotrichia (Wang et al., 2001), hyperlipidaemia (Yang et al., 2005), inflammation (Lv et al., 2001), learning and memory obstructions (Liu et al., 2004) and hypoimmunity (Ma and Du, 2001) and as an antioxidant and anti-aging compound (Xiao et al., 1993). It has been widely used in clinical and traditional practice. An analysis of dozens of traditional Chinese medicine prescription books, such as “Beijing traditional Chinese medicine prescription anthology”, “Ji Shan Tang Fang”, “Bi Hua Yi Jing”, “Bianque Xin Shu-Shen Fang”, “Bu Ju Ji”, “Chuai Mo You De Ji”, “Dan Xi Xin Fa”, and “Gu Fang Hui Jing” revealed 242 prescriptions containing Polygonum multiflorum, and the compatible herbs that are most frequently found include Angelica sinensis, Radix rehmanniae, Glycyrrhiza, Rhizoma Chuanxiong, and Radix sileris. The 2010 edition of the Chinese Pharmacopoeia lists 46 Chinese patent medicines containing Polygonum multiflorum, and the compatible herbs that are more frequently described include Radix rehmanniae, astragalus, Angelica sinensis, Salviae miltiorrhizae, and Radix ophiopogonis. Table 1 lists subsets of the Chinese patent drugs and decoctions containing Polygonum multiflorum.
Table 1.
The traditional and clinical uses of Polygonum multiflorum in China.
| Preparation name | Compositions | Used | References |
|---|---|---|---|
| Ren Shen Zai Zao pill | Scrophulariae Radix, Ephedrae Herba, Cyperi Rhizome, Angelicae Dahuricae Radix, Polygonum multiflorum, Rehmannia Radix Preparata, Asari Radix et Rhizome and others. | Curing apoplexia, facial paralysis and hemiplegia. | “Beijing traditional Chinese medicine prescription anthology”, page 10. |
| Chan Ling pellet | Angelica Sinensis, Atractylodes Rhizome, Radix Auckladiae, Polygonum multiflorum, Radix Auckladiae, Monkshood Root, Rhizome Ligustici, Radix Saposhnikoviae, Angelicae Dahuricae Radix and others. | Curing woman postpartum lochiometra, thoracic and abdominal distension, stabbing hypochondrium pain. | “Beijing traditional Chinese medicine prescription anthology”, page 194. |
| Duo Zi ingots | Lanceolata, Eucommia, Herba Cistanche, Common Anemarrhena Rhizome, Phellodendron Bark, Radix Polygala Tenuifolia, Alisma Orientale, Chinese Yam Root, Glycyrrhiza Uralensis Fisch, Polygonum multiflorum and others. | Curing deficiency of kidney qi, listlessmess and ache of waist. | “Beijing traditional Chinese medicine prescription anthology”, page 114. |
| Anti-asthma pill | Seeds of Brassica Alba, Folium Perillae, Lilium Brownie, Apricot Kernel, Radish Seed, Polygonum multiflorum, Radix Asparagi, Fritillaria Cirrhosa, Roots of Common Anemarrhena, Pinellia Ternate, Angelica Sinensis and others. | Curing deficiency syndrome of the lung, cough, dyspnea with cough and Phlegm | “Beijing traditional Chinese medicine prescription anthology”, page 139. |
| Qi Bao Mei Ran pellet | Red Polygonum multiflorum, White Polygonum multiflorum, Red Poria Cocos, White Poria Cocos, Achyranthes Root, Angelica Sinensis, Barbary Wolfberry, Cuscuta Chinensis Lam and Fructus Psoralea. | Curing leukotrichia, lipsotrichia, dysgenesis, metrorrhagia and leukorrhagia, teeth shake, liver and kidney deficiencies | “Compendium of Materia Medica”, qing dynasty, vol. 18. |
| Li Yin He Zhong decotion | Rehmanniae Radix, Radix Glehniae, Cortex Lycii Radicis, Concha Ostreae, Polygonum multiflorum, White Peony Root, Magnolia Officinalis, Cortex Moutan, Artemisia Annua, Fructus Setariae Germinates and Fructus Hordei Germinatus. | Curing children with rickets. | “Bi Hua Yi Jing”, qing dynasty, vol. 3. |
| Pei Tu Yang Yin decotion | Prepared Polygonum multiflorum, Salvia Militiorrhiza, Hyacinth Bean, Fructus Setariae Germinates, White Peony Root, Plantain Herb, Lotusty and Porcine Kidney. | Curing consumptive disease, apocleisis, phlegmatic, spontaneous perspiration, night sweat and spermatorrhea. | “Bu Ju Ji”, qing dynasty, batch 1, vol. 10. |
| Li Pi Yi Ying decotion | Prepared Polygonum multiflorum, Sea Cucumber, Lotusty, Black Soybean, Rhizoma Dioscorea and Hyacinth Bean. | Curing insufficiency of the spleen, insufficiency of blood, fever due to yin deficiency. | “Bu Ju Ji”, qing dynasty, batch 1, vol. 10. |
| Qing Ji decotion | Pinellia Ternate, Grassleaved Sweetflag Rhizome, Sophora Flavescens Ait, Linseed, Radix Saposhnikoviae, Atractylodes Rhizome, Radix Angelica Sinensis, Polygonum multiflorum, Rehmanniae Radix, Dried Ginger, Sixpetal Clematis Root and Carthamus Tinctorius. | Curing scabies and familial benign pemphigus. | “Chuang Yang Jing Yan Quan Shu”, song dynasty, vol. 6. |
| Ji Yin Hui Chun decotion | Fresh Polygonum multiflorum, Radix Angelica Sinensis, White Peony Roots, Glycyrrhiza Uralemis Fisch, Raphanus Seed, Plantain Seed, Citrus Aurtantium, Tangerine Peel and Simmer Radix Aucklandiae. | Curing dysentery. | “Ci Hang Ji”, qing dynasty, vol. 4. |
| Ju Sheng Zi pill | Prepared Radix Rehmanniae, Radix Rehmanniae, Polygonum multiflorum, Achyranthes Root, Cistanche Deserticola, Fructus Dipsaci, Poria Cocos, Seed of Oriental Arborvitae, Morinda Officinalis How, Rhizoma Dioscorea, Radix Dipsaci and others. | Curing feeble pulse, asynodia. | “Dan Xi Xin Fa”, yuan dynasty, vol. 3. |
| Red bean powder | Polygala Tenuifolium, Polygonum multiflorum Peel, Red bean, Carthamus Tinctorius and Schizonepeta Tenuifolia Briq. | Curing ulcer. | “Gu Fang Jing Hui”, qing dynasty, vol. 2. |
| Zheng Qi decotion | Schizonepeta Tenuifolia Briq, Rhizome of Chuanxiong, Angelica Sinensis, Xia Qu, Radix Paeoniae Rubra, Atractylodes Rhizome, White Atractylodes Rhizome, Tangerine Peel, Red Polygonum multiflorum, Citrus Aurtantium, Wrinkled Giant Hyssop and Cassia Twig. | Curing Pregnancy malaria | “Gu Fang Jing Hui”, qing dynasty, vol. 3. |
| San Xian pill | Polygonum multiflorum, Atractylodes Rhizome, Foeniculum Vulgare, Cyperus Rotundus, Fructus Toosendan, Concha Ostreae and White Ginger. | Curing epicophosis, dim vision and woman splenic blood disease. | “Pu Ji Fang” qing dynasty, vol. 219. |
| Gou Pi plaster | Citrus Aurtantium, Muscardine Silkworm, Alisma Orientale, Aconite Root, Phellodendron Bark, Polygonum multiflorum, Pinellia Ternate, Myrrha, Cortex Acanthopanacis Radices, Achyranthes Root, Platycodon Grandiflorum, Rhizoma Gastrodiae and others. | Curing osphyalgia, skelalgia and brachialgia. | Zhao et al. (2011a) |
| Huo Luo pellet | Tiger bone, Garter Snake, Deinagkistrodon, Radix Clematidis, Polygonum multiflorum, Rhizoma Coptidis, Rhizoma Gastrodiae, Boswellia Carterii, Myrrha, Rhizoma Typhonii, Scutellaria Baicalensis Root, Eucommia Ulmoides, Rhizome of Chuanxiong and others. | Curing rheumatic paralysis, acroanesthesia, low back and leg pain, arthralgia and myalgia, Phlegm heat syndrome. | Li et al. (2012c) |
| San Shen pill | Prepared Rhizome of Rehmannia, Cortex Moutan, Cynomorium Songaricum, Herba Cistanches, Poria Cocos, Barbary Wolfberry Fruit, White Atractylodes Rhizome, Radix Aconiti Carmichaeli, Rhizome of Chuanxiong, Rhizoma Dioscoreae, Eucommia Ulmoides, Angelicae Sinensis, Polygonum multiflorum and others. | Curing kidney asthenia, aversion to cold, overwork asthma, limb fatigue, lower energizer asthenia cold, hypofunction reproduct. | Zhou. (2010) |
| Die Da Sun Shang wine | Musk, Boswellia carterii, Native Copper, Carthamus Tinctorious, Pseudo-ginseng, Rhizoma Cyperi, Rhizoma Curcumae, Aristolochiae Lignum, Radix Clematidis, Cynomorium Songaricum, Evodia Rutaecarpa, Polygonum multiflorum, Radix Liquiritiae, Radix Angelicae and others. | Curing traumatic injury. | Yuan et al. (2004) |
| Wu Mei Shang Yu decotion | Schisandra Chinensis, Fructus Mume, Fructus Corni, Radix Liquiritiae, Polygonum multiflorum, Herbaceous Peony, Fossil Fragments and Concha Ostreae. | Curing corectasis. | “Si Sheng Xin Yuan”, qing dynasty, vol. 8. |
| Gui Zhi Wu Ling decotion | Cassia Twig, Herbaceous Peony, Radix Liquiritiae, Polygonum multiflorum, Poria Cocos and Fructus Amomi. | Curing apoplexia and the left half hemiplegia. | “Si Sheng Xin Yuan”, qing dynasty, vol. 7. |
| Yu Feng pellet | Rhubarb, Mirabilite, Schizonepeta Tenuifolia Briq, Ephedra Intermedia, Gardenia Jasminoides Ellis, Radix Paeoniae Rubra, Fructus Forsythiae, Radix Liquiritiae, Polygonum multiflorum, Field Mint, Scutellaria Baicalensis Georgi, Rhizoma Gastrodiae and others. | Curing apoplexia. | “Zheng Zhi Bao Jian”, mngo, vol. 1. |
| Yi Gan Ning granule | Herba Hedyotis, Polygonum Filiforme, Mongolian Dandelion Herb, Cortex Moutan Radices, Poria Cocos, White Atractylodes Rhizome, Astragalus Mongholicus, Artemisia Capillaris Thumb, Codonopsis Pilosula, Polygonum multiflorum, Radix Salviae Miltiorrhizae, White Peony Root and Fructus Toosendan | Curing chronic hepatitis, blood stasis blocking collaterals and damp heat toxin accumulation syndrome. | Yang and Zhang (1999) and Wu et al. (2001) |
| He Shou Wu granule | Polygonum multiflorum | Treatment of pregnancy constipation. | Zhu (2011) |
| Qi Shu decotion | Astragalus, Radix Codonopsis, Lycium, Donkeyhide Glue, Angelica Sinensis, Polygonum Multiflorum, Cauliss Patholobi, Glycyrrhiza Uralensis. | Treatment of NSCLC patients with leukopenia after Chemotherapy | Xiang and Han, (2013) |
| Zi Shen Bu Gan Fang | Polygonum Multiflorum, Polygonatum Canaliculatum, Fructus Ligustri Lucidi, Fructus Lycii. | Anti-aging | Wang (2012b) |
| Pei Bu Gan Shen Fomula | Fructus broussonetiae, Semen Astragali complanati, Prepared Rhizome of Rehmannia, Prepared Polygonum multiflorum, Glue of Tortoise Plastron, Fructus Lycii, Morinda Officinalis, Radix Achyranthis Bidentatae, Herba Cistanches and others. | Treatment Parkinson׳s disease and intestinal dysfunction | Chen (2013) |
| Ren Shen Shou Wu capsule | Radix Ginseng Rubra and Prepared Polygonum multiflorum. | Curing premature graying hair, insomnia, loss of appetite and body weakness due to qi-blood deficiency. | “Chinese Pharmacopoeia”, vol. 1. |
| Tian Ma Shou Wu tablets | Rhizoma Gastrodiae, Polygonum multiflorum, Radix Angelicae, Radix Rehmanniae Praeparata, Radix Salviae Miltiorrhizae, Rhizome of Chuanxiong, Radix Angelicae Sinensis, Stir-baked Fructus Tribuli, Folium Mori, Herba Ecliptae, Fructus Ligustri Lucidi, Radix Paeoniae Alba, Polygonatum Canaliculatum and Radix Liquiritiae. | Curing dizzy, headache, tinnitus, bitter mouth, dry pharynx, soreness and weakness of waist and knees due to the deficiency of liver-yin and kidney-yin. Curing cerebral arteriosclerosis, cluster headache syndrome and alopecia seborrheica. | “Chinese Pharmacopoeia”, vol. 1. |
| Xin Nao Kang capsule | Radix Salviae Miltiorrhizae, Prepared Polygonum multiflorum, Red Paeony Root, Radix Puerariae, Carthamus Tinctorious, Root of Bidentate Achyranthes, Radix Curcumae, Barbary Wolfberry Fruit, Rhizome of Chuanxiong, Rhizoma Alismatis, Polygala Tenuifolia and others. | Curing thoracic obstruction, circumgyration, coronary heart disease, angina pectoris and cerebral arteriosclerosis. | “Chinese Pharmacopoeia”, vol. 1. |
| Xue Zhi Ning Pill | Rhizoma Alismatis, Cassia Seed, Hawkthorn and Prepared Polygonum multiflorum. | Curing hyperlipoidemia, dizziness, chest tightness and constipated. | “Chinese Pharmacopoeia”, vol. 1. |
| Jiang Zhi Ling tablets | Prepared Polygonum multiflorum, Barbary Wolfberry Fruit, Polygonatum Canaliculatum, Hawkthorn and Cassia Seed. | Curing hyperlipoidemia, dizziness, premature graying hair. | “Chinese Pharmacopoeia”, vol. 1. |
3. Botany
Polygonum multiflorum, an herbaceous perennial plant, was originally called Caulis Polygoni Multiflori in the book “He Shou Wu Zhuan” written during the Tang dynasty (Li et al., 2003). Its root is tuberous, hypertrophic, oblong and dark brown in color. Its stems are approximately 2–4 m in length with a twine-like appearance, many branches, longitudinal ribs, glabrous and micro-rough skin and exhibit lignification in the lower parts. The leaves are ovate or broadly elliptic and 3–7×2–5 cm2 in size with an acuminate apex and a cordate or subcordate base. Both sides of the leaf are coarse and have entire margins; the petioles are 1.5–3 cm in length; and the ochreas are membranous, oblique, glabrous and 3–5 mm in length. The inflorescences are paniculate, terminal or axillary and approximately 10–20 cm in length; the branches are expansive, with longitudinal ridges and small dense protrusions along the ridge. The bracts are triangular and ovate with small protrusions, and the apex is acute. In addition, each inflorescence contains three to four flowers. The pedicels are 2–3 mm in length and slender, and the perianths are white or greenish and five-parted. The perianth segments are oblong and of non-standard size. The male flowers have eight stamens, and the lower parts of the filaments are wide and very short, exhibit three styles, and have stigmas with a capitulum. The achenes are ovate with three ribs, black-brown, shiny, and approximately 2.5–3 mm in length (Editorial Board of Flora of China, 1998).
This plant is widely cultivated in many provinces of China, including Gansu, Shanxi, Sichuan, Yunnan, Guizhou, and Henan, and other countries, such as Japan. It grows in valley shrubs, hillside forests, gutter rock crevices and other locations with altitudes of 200–3000 m (Editorial Board of Flora of China, 1998, Zhou, 1993).
As a widely used traditional Chinese medicine, there are some adulterants of this plant, involving nine species belonging to six families. The original plants are Pteroxygonum giraldii Damm et Diels, P. cillinerve (Nakai) Ohwi, P. subertii L. Henry, Cynanchum auriculatum Royle ex Wight, C. wilfordi (Maxim) Hemsl., Stephania cepharantha Hayata, Musa basjoo Sieb. et Zucc, Dioscorea bulbifera L. and Rodgersia aesculifolia Batal. (Chen et al., 1999a, Cheng and Zhou, 2005a, Zhao et al., 1998, Xia and Li, 2003). Until now, several methods have been developed to identify and distinguish them, including experiential identification, morphological identification, ultraviolet spectrophotometry, the TLC method, HPLC, gel electrophoresis, HPLC-ESI/MS and ITS2 rDNA sequencing (Chen et al., 1998, Li et al., 1995, Ge et al., 2011; Sun et al., 1996a; Zhang and Shi, 2007a, Zheng et al., 2009, Zheng, 2010).
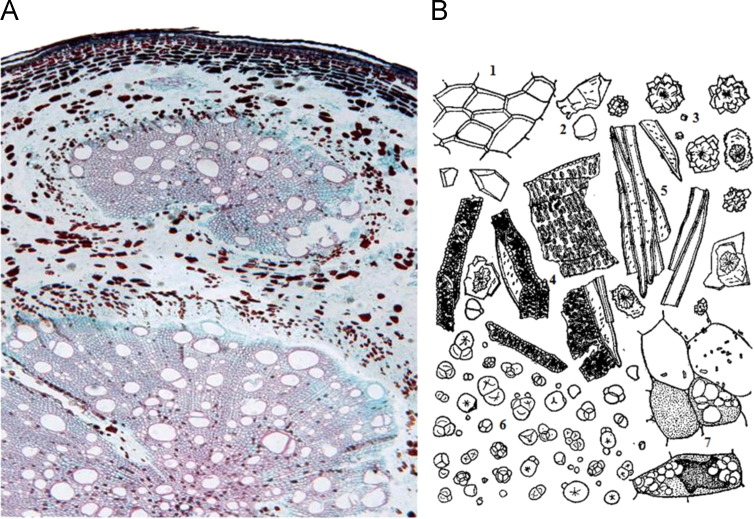
The morphological identification method is regarded as the most popular method for identifying Polygonum multiflorum. The fracture plane of Polygonum multiflorum is yellowish-brown or reddish-brown and starchy and exhibits a brocaded pattern (abnormal vascular bundle). The cork layer of the root transverse sections shows 2–3 columns of rectangular cells. The phelloderm is a sequence of tangential prolonged cells, the surface view of the cork cells is subpolygonal, and there are decadent epidermal cells on the outside. The phloem is broad and mainly composed of parenchymatous cells, with the brown cells distributed in the parenchyma, and the phloem ray is not obvious. There are several uniquely shaped compound vascular bundles and scattered fiber bundles, with subpolygonal or irregular fibers that are 5–30 μm in diameter with a wall thickness of 2.5 μm. Clusters of calcium oxalate are scattered in the cambium ring. The xylem are more developed, and the vessels are subrounded, primarily bordered, pitted, and 13–101 μm in diameter. The fibers are square and 5–18 μm in diameter with a wall thickness of 2.5 μm. The sequence of wood ray cells is wide but not very obvious, and the majority of the cell walls are thick. The starch granules are fairly abundant with simple spherical granules that are 4–39 μm in diameter. The hilum are stellate or forked, and compound starch granules are common, whereas brown blocks are ubiquitously scattered ( Fig. 2). Of the counterfeits of Polygonum multiflorum, Musa basjoo Sieb. et Zucc has no cork tissue but does have needle-like calcium oxalate crystals. In Pteroxygonum giraldii Damm et Diels, the cork cells of are yellowish-brown, there are fewer vessels in the xylem, and the vessels are primarily reticulate. In this same species, there is a single starch granule that is circular, triangular, oblong or subovate. The hilum is pointed, cleft or cruciate. In the counterfeit species Dioscorea bulbifera L, the cork cells are approximately square, and the walls are pale brown. The mucilage cells are oblong, and there are calcium oxalate raphides that are approximately 50 μm in length. The species P. cillinerve (Nakai) Ohwi has khaki-coloured sections, and the cork layer of the transverse root sections is composed of a sequence of rectangular cells. The vessels are subrounded and mostly singularly arranged, and these are larger on the outside and become smaller gradually toward the interior. The wood ray is obvious, and there are clusters of calcium oxalate in the wood ray cells. Moreover, none of the counterfeits of Polygonum multiflorum have a “brocaded pattern” (Ge et al., 2011, Cheng and Zhou, 2005b, He and Sun, 2010, Guo et al., 2007a, Lu et al., 2012, Sun and Bai, 1996a, Sun and Bai, 1996b, Xu, 1986).
Fig. 2.
Cross section (A) and microscopic structure (B, 1-Cork cell; 2-Brown block; 3-Clusters of calcium oxalate; 4-Vessel; 5-Cork fiber; 6-Starch granules; 7-Brown cell) of Polygonum multiflorum.
HPLC is the most widely used method for evaluating the quality and authenticity of Polygonum multiflorum. 2,3,5,4′-Tetrahydroxystilbene-2-O-glc in combination with anthraquinone are used as the indicator compounds to characterize the quality of this plant with minimum contents of 1.0% and 0.10%, respectively. The content of the combined anthraquinones is calculated using the amounts of emodin and physcion, and the process is described in the Pharmacopoeia of the People׳s Republic of China.
4. Phytochemistry
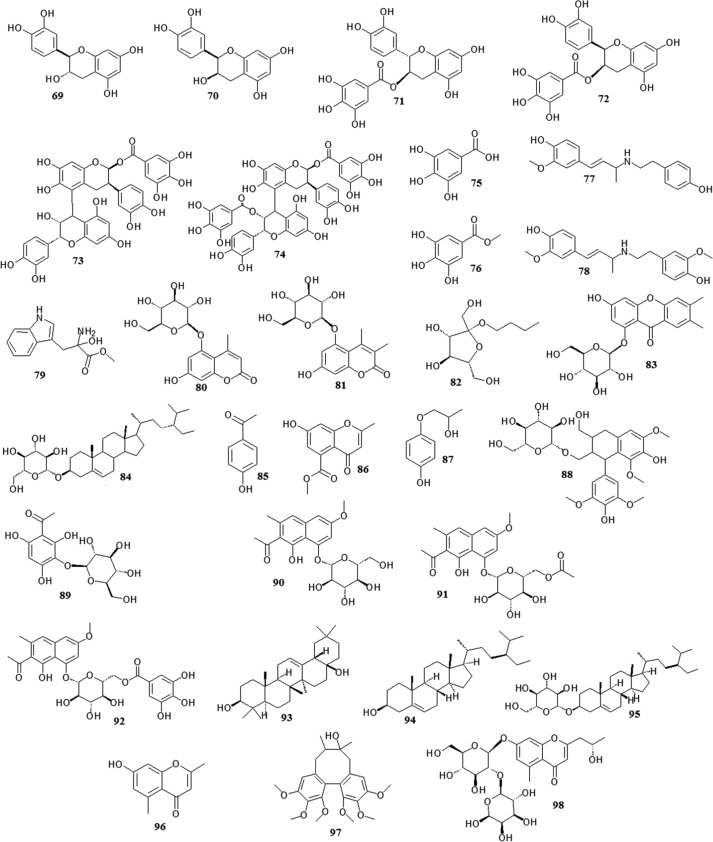
There are many chemical constituents in Polygonum multiflorum, including flavones, quinones, and stilbenes. In this section, we describe the major chemical constituents of this plant and their structures ( Table 2) ( Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8).
Table 2.
Chemical compounds isolated from Polygonum multiflorum.
| Classification | NO | Chemical component | Reference |
|---|---|---|---|
| Stilbenes | 1 | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d- glucopyranoside | Yang. (1976) |
| 2 | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-(2″-O-monogalloyl esters)-glucopyranoside | Nonaka et al. (1982) | |
| 3 | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-(3″-O-monogalloyl esters)-glucopyranoside | Nonaka et al. (1982) | |
| 4 | 2,3,5,4′-tetrahydroxystilbene-2,3-di-O-β-d- glucopyranoside | Zhou et al. (1994) | |
| 5 | 2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-α- d-glucopyranosyl)-β-d-glucopyranoside | Chen et al. (2000a) | |
| 6 | 2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-acetyl)-β-d-glucopyranoside | Chen et al. (2000a) | |
| 7 | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-xyloside | Sun et al. (2013) | |
| 8 | 2,3,5,4′-tetrahydroxystilbene-2-O-(4″-O-α-d-glucopyranosyl)-β-d-glucopyranoside | Li et al. (2013b) | |
| 9 | 2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-β-d-glucopyranosyl)-β-d-glucopyranoside | Li et al. (2013b) | |
| 10 | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-4′-O-α-d-glucopyranoside | Li et al. (2013b) | |
| 11 | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-5-O-α-d-glucopyranoside | Li et al. (2013b) | |
| 12 | 2,3,5,4′-tetrahydroxystilbene-2-O-(2″-O-β-d-fructofuranosyl)-β-d-glucopyranoside | Li et al. (2013b) | |
| 13 | Resveratrol | Xu et al. (2009) | |
| 14 | Polydatin | Xu et al. (2009) | |
| 15 | Rhaponticoside | Yi et al. (2007) | |
| 16 | Cis-2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoyranoside | Sun et al. (2009) | |
| 17 | Cis-2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-α- d-glucopyranosyl)-β-d-glucopyranoside | Xiao et al. (2002) | |
| 18 | Polygonumosides A | Yan et al. (2014) | |
| 19 | Polygonumosides B | Yan et al. (2014) | |
| 20 | Polygonumosides C | Yan et al. (2014) | |
| 21 | Polygonumosides D | Yan et al. (2014) | |
| Quinones | 22 | Emodin | Sun et al. (2013); Li et al. (2013); Li and Lin (1993); Kato and Morita (1987); Wang et al. (2005b); Chen et al. (1999b); Zuo et al. (2008); Zhang et al. (2006a) |
| 23 | Aloe-emodin | ||
| 24 | Chrysophanol | ||
| 25 | Physcion | ||
| 26 | Rhein | ||
| 27 | 1,6-dimethyl ether-emodin | ||
| 28 | Emodin-8-methyl ether | ||
| 29 | Citreorosein | ||
| 30 | Citreorosein-8-methyl ether | ||
| 31 | Emodin-3- methyl ether | ||
| 32 | Fallacinol | ||
| 33 | Emodin-6,8-dimethylether | ||
| 34 | 2-acetylemodin | ||
| 35 | Emodin-8-O-β-d-glucopyranoside | Qiu et al. (2013); Li et al. (2013) | |
| 36 | Physcion-8-O-β-d-glucopyranoside | Qiu et al. (2013); Li et al. (2013) | |
| 37 | Emodin-3- methyl ether-8-O-β-d-glucopyranoside | Li et al. (2006) | |
| 38 | Physcion-8-O-(6′-O-acetyl)-β-d-glucopyranoside | Sun et al. (2009) | |
| 39 | Emodin-8-O-( 6′-O-acetyl)- β-d-glucopyranoside | Zhang et al. (2006a) | |
| 40 | Chrysophanol-8-O-β-d-glucopyranoside | Yang et al. (1998) | |
| 41 | 6-methoxyl-2-acetyl-3-methyl-1,4-naphthoquinone-8-O-β-d-glucopyranoside | Chen et al. (2000c) | |
| 42 | 2-Methoxy-6-acethyl-7-methyliuglone | Li and Lin (1993) | |
| Flavonoids | 43 | Tricin | Xu et al. (2006); Li and Lin (1993); Chen et al. (2001c); Chen et al. (2000b) |
| 44 | Rutin | ||
| 45 | Luteolin | ||
| 46 | Quercetin | ||
| 47 | Kaempferol | ||
| 48 | Isoorientin | ||
| 49 | Apigenin | ||
| 50 | Hyperoside | ||
| 51 | Vitexin | ||
| 52 | Quercetin-3-O-arabinoside | ||
| 53 | Polygonflavanol A | Chen et al. (2012a) | |
| Phospholipids | 54 | Phosphatidyl ethanolamine | Chen et al. (2001c) |
| 55 | Copaene | Chen et al. (2001c) | |
| 56 | Eicosane | Chen et al. (2001c) | |
| 57 | Hexanoic acid | Chen et al. (2001c) | |
| 58 | Hexadecanoic acid methyl ester | Chen et al. (2001c) | |
| 59 | Hexadecanoic acid ethyl ester | Chen et al. (2001c) | |
| 60 | Octadecanoic acid methyl ester | Chen et al. (2001c) | |
| 61 | Octadecanoic acid ethyl ester | Chen et al. (2001c) | |
| 62 | Ethyl oleate | Chen et al. (2001c) | |
| 63 | Docosanoic acid methyl lester | Chen et al. (2001c) | |
| 64 | Tetradecanoic acid ethyl ester | Chen et al. (2001c) | |
| 65 | Squalene | Chen et al. (2001c) | |
| 66 | 1, 2- dihydroxynonadecone- 3 | Chen et al. (2000b) | |
| 67 | 1-O-stearoyl-2-O-Δ4′,7′-dodecenoyl-3-O-phosphatidic acid-O-β-d-glucoside | Chen et al. (2001b) | |
| 68 | 1-O-stearoyl-2-O-Δ4′,7′-dodecenoyl-3-O-phosphatidic acid-O-(6″-O-α-d-glucoside)-β-d-glucoside | Chen et al. (2001b) | |
| Other compounds | 69 | Catechin | Chen et al. (1999c) |
| 70 | Epicatechin | Chen et al. (1999c) | |
| 71 | 3-O-galloyl-(-)-catechin | Nonaka et al. (1982) | |
| 72 | 3-O-galloyl-(-)-epicatechin | Nonaka et al. (1982) | |
| 73 | 3-O-galloyl-procyanidin B2 | Nonaka et al. (1982) | |
| 74 | 3,3′-di-O-galloyl-procyanidin B2 | Nonaka et al. (1982) | |
| 75 | Gallic acid | Li and Lin (1993) | |
| 76 | Methyl gallate | Yang et al. (1998) | |
| 77 | N-trans-Feruloyl tyramine | Li and Lin (1993) | |
| 78 | N-trans-feruloyl-3-methyldopamine | Li and Lin (1993) | |
| 79 | Indole-3-(L-α-amino-α-hydroxypropionicacid) methyl ester | Yang et al. (1998) | |
| 80 | 7-hydroxy-4-methylcoumarin-5-O-β-d-glucopyranoside | Yu et al. (2008) | |
| 81 | 7-hydroxy-3,4-dimethylcoumarin-5-O-β-d-glucopyranoside | Yu et al. (2008) | |
| 82 | n-butyl-β-d-fructopyranoside | Zhang, et al. (2006b) | |
| 83 | 1,3-dihydroxy-6,7-dimethylxanthone -1-O-β-d-glueopyranoside | Zhou et al. (1994) | |
| 84 | Daucosterol | Li and Lin (1993); Rao et al. (2009) | |
| 85 | 4-hydroxybenzaldehyde | Li et al. (2006) | |
| 86 | 5-carboxymethyl-7-hydroxy-2-methylchromone | Li et al. (2006) | |
| 87 | 1, 2-propanediol-1-(4-hydroxy-phenyl) | Yamaguchi et al. (1969) | |
| 88 | (+)-lyoniresinol-3α-O-β-d-glucopyranoside | Achenbach et al. (1992) | |
| 89 | 2,3,4,6-tetrahy-droxyacetophenone-3-O-β-d-glucoside | Yoshizaki et al. (1987),Yoshizaki et al. (1987) | |
| 90 | Torachrysone-8-O-β-d-glucopyranoside | Li et al. (2006) | |
| 91 | Torachrysone-8-O-(6′-O-acetyl)-β-d-glucopyranoside | Sun et al. (2009) | |
| 92 | Torachrysone-8-O-(6′-O- galloyl)-β-d-glucoside | Li and Lin (1993) | |
| 93 | β-amyrin | Rao et al. (2009) | |
| 94 | β-sitosterol | Rao et al. (2009) | |
| 95 | β-Sitosterol-3-O-β-d-glucoside | Xu et al. (2006) | |
| 96 | 2,5-dimethyl-7-hydroxychromone | Liang et al. (2009) | |
| 97 | Schizandrin | Chen et al. (1999b) | |
| 98 | (S)-2-(2′-hydroxypropyl)-5-methyl-7-hydroxyl chromone-7-O-α-l-fucosel(1→2)-β-d-glycoside | Zhao et al. (2014) | |
| New compounds after processed | 99 | 2,3-di-hydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one | Liu et al. (2007b); Liu et al. (2009c); Liu et al. (2008b). |
| 100 | Hydroxymaltol | ||
| 101 | 5-Hydroxy methyl-furfuran | ||
| 102 | Butanedioic acid | ||
| 103 | 5-dihydroxy-6-methyl-4(H)-pyran-4-one | ||
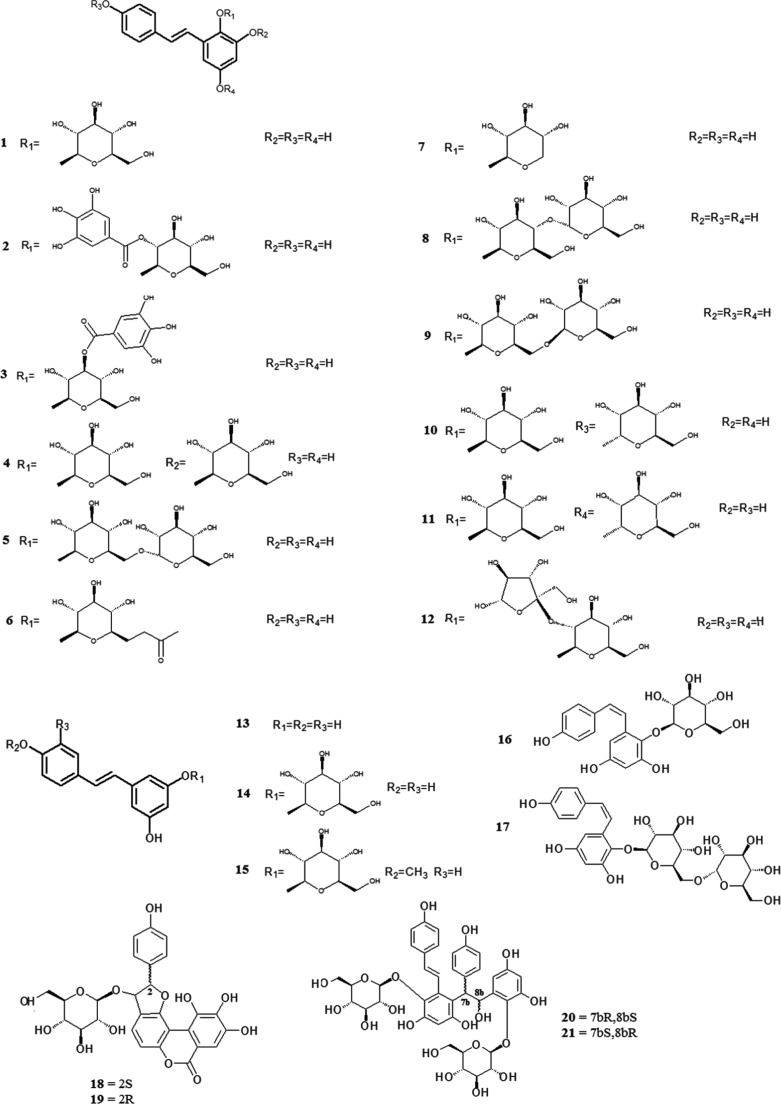
Fig. 3.
Chemical structures of stilbenes.
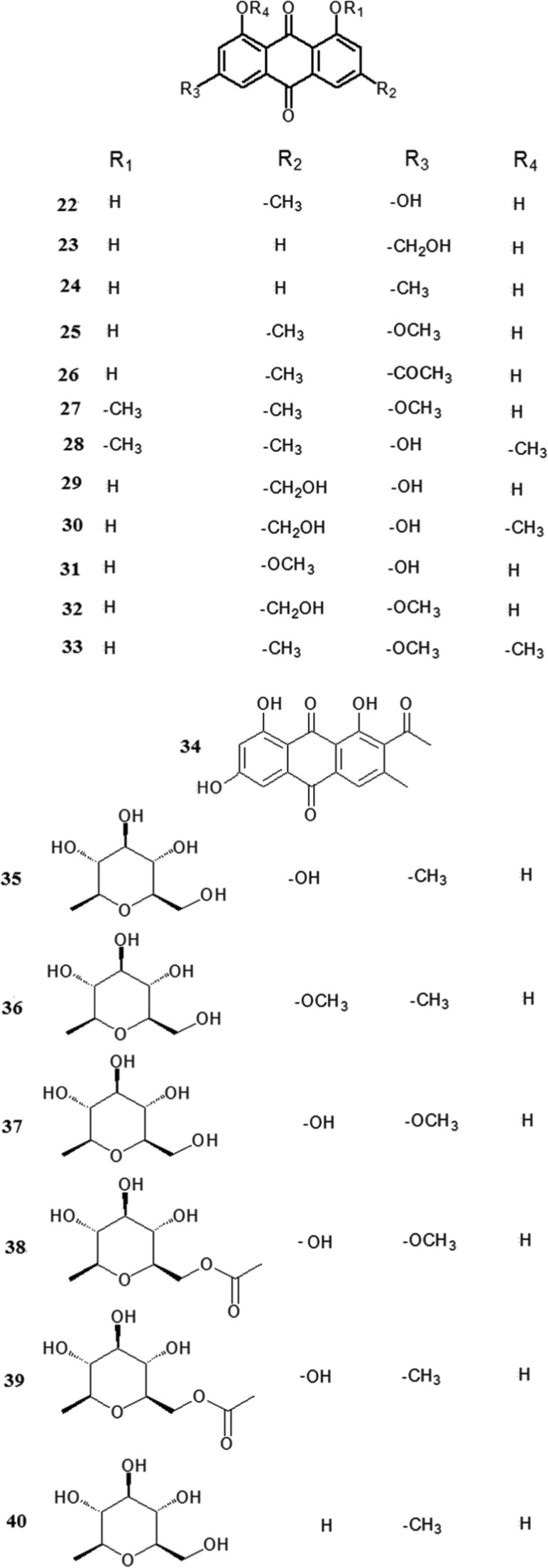
Fig. 4.
Chemical structures of quinones.
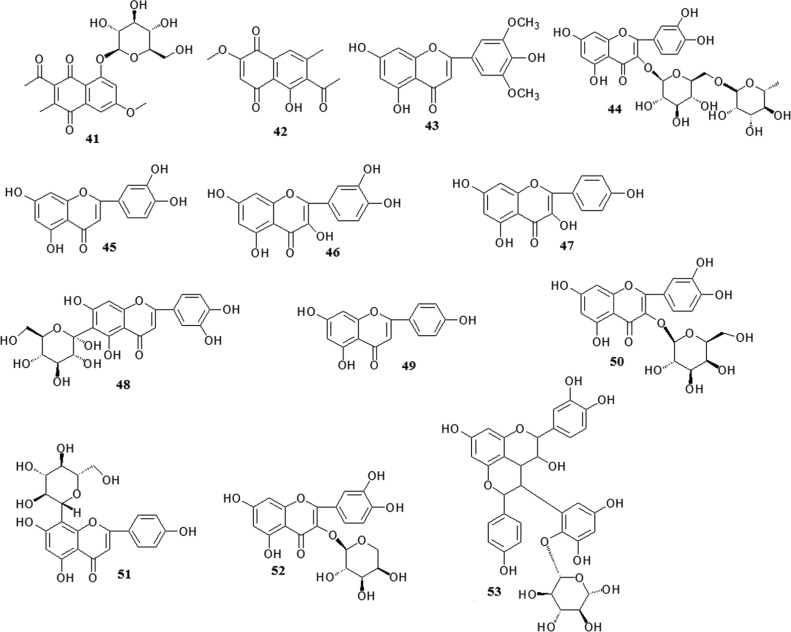
Fig. 5.
Chemical structures of flavonoids.
Fig. 6.
Chemical structures of phospholipids.
Fig. 7.
Chemical structures of other compounds.
Fig. 8.
Chemical structures of new compounds after processed.
4.1. Stilbenes
Stilbenes are the main characteristic components in Polygonum multiflorum. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside (1) was first isolated and identified from this plant in 1976 (Yang, 1976), and another two stilbenes, which were identified as 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-(2″-O-monogalloyl esters)-glucopyranoside (2) and 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-(3″-O-monogalloyl esters)-glucopyranoside (3), were then isolated (Nonaka et al., 1982). 2,3,5,4′-Tetrahydroxystilbene-2,3-di-O-β-d-glucopyranoside (4) was isolated from the ethyl acetate insoluble fraction of an ethanol extract of the plant (Zhou et al., 1994). Two tetrahydroxystilbenes were isolated from this plant in 2000 and identified as 2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-α- d-glucopyranosyl)-β-d-glucopyranoside (5) (Chen et al., 2000a) and 2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-acetyl)-β-d-glucopyranoside (6) (Chen et al., 2000c). Another stilbene, which was identified as 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-xyloside (7) (Sun et al., 2013), was isolated from a 70% ethanol extract of Polygonum multiflorum. Five stilbene glycosides, which were identified as 2,3,5,4′-tetrahydroxystilbene-2-O-(4″-O-α-d-glucopyranosyl)-β-d-glucopyranoside (8), 2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-β-d-glucopyranosyl)-β-d-glucopyranoside (9), 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-4′-O-α-d-glucopyranoside (10), 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-5-O-α-d-glucopyranoside (11) and 2,3,5,4′-tetrahydroxystilbene-2-O-(2″-O-β-d-fructofuranosyl)-β-d-glucopyranoside (12), were isolated from Polygonum multiflorum in 2013 (Li, et al., 2013b). Moreover, resveratrol (13), polydatin (14) (Xu et al., 2009), rhaponticoside (15) (Yi et al., 2007), cis-2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoyranoside (16) (Sun et al., 2009) and cis-2,3,5,4′-tetrahydroxystilbene-2-O-(6″-O-α-d-glucopyranosyl)-β-d-glucopyranoside (17) (Xiao et al., 2002) were also isolated from Polygonum multiflorum. Four stilbene derivatives, denoted polygonumosides A-D (18–21), were isolated and identified from this plant in 2014 (Yan et al., 2014). Other stilbenes, such as tetrahydroxystilbene-O-(malonyl)-hex, tetrahydroxystilbene-O-deoxyhex and tetrahydroxystilbene-O-(caffeoyl)-hex, were also found in Polygonum multiflorum, but their structures have not yet been identified (Qiu et al., 2013).
4.2. Quinones
Quinones are the other characteristic components in Polygonum multiflorum. Quinones and their derivatives have been isolated and identified, and most of them are anthraquinones. The predominant anthraquinones are emodin-type anthraquinones, including emodin (22), aloe-emodin (23), chrysophanol (24), physcion (25), rhein (26), 1,6-dimethyl ether-emodin (27), emodin-8-methyl ether (28), citreorosein (29), citreorosein-8-methyl ether (30), emodin-3- methyl ether (31) fallacinol (32), emodin-6,8-dimethylether (33) and 2-acetylemodin (34) (Sun et al., 2013, Li et al., 2013c, Li and Lin, 1993, Kato and Morita, 1987, Wang et al., 2005b, Chen et al., 1999b, Zuo et al., 2008, Zhang et al., 2006a). There are also many combined anthraquinones in Polygonum multiflorum, such as emodin-8-O-β-d-glucopyranoside (35), physcion-8-O-β-d-glucopyranoside (36) (Qiu et al., 2013, Li et al., 2013c), emodin-3-methyl ether-8-O-β-d-glucopyranoside (37) (Li et al., 2006), physcion-8-O-(6′-O-acetyl)-β-d-glucopyranoside (38) (Sun et al., 2009), emodin-8-O-(6′-O-acety1)-β-d-glucopyranoside (39) (Zhang et al., 2006a) and chrysophanol-8-O-β-d-glucopyranoside (40) (Yang et al., 1998). Two naphthoquinones, namely 6-methoxyl-2-acetyl-3-methyl-1,4-naphthoquinone-8-O-β-d-glucopyranoside (41) (Chen et al., 2000c) and 2-methoxy-6-acethyl-7-methyliuglone (42) (Li et al., 1993), were also isolated from Polygonum multiflorum.
4.3. Flavonoids
Flavonols exist in numerous plants, including Polygonum multiflorum. These compounds have antioxidant and free radical scavenging activities (Li et al., 2012e). The flavonols in Polygonum multiflorum include tricin (43), rutin (44), luteolin (45), quercetin (46), kaempferol (47), isoorientin (48), apigenin (49), hyperoside (50), vitexin (51) and quercetin-3-O-arabinoside (52) (Xu et al., 2006, Li and Lin, 1993, Chen et al., 2000b, Chen et al., 2001c). In addition, a novel flavonostilbene glycoside was isolated from Polygonum multiflorum and identified as polygonflavanol A (53) (Chen et al., 2012a).
4.4. Phospholipids
Polygonum multiflorum is rich in phospholipids and may be associated with the tonic effect of Polygonum multiflorum. These phospholipids include phosphatidyl ethanolamine (54), copaene (55), eicosane (56), hexanoic acid (57), hexadecanoic acid methyl ester (58), hexadecanoic acid ethyl ester (59), octadecanoic acid methyl ester (60), octadecanoic acid ethyl ester (61), ethyl oleate (62), docosanoic acid methyl ester (63), tetradecanoic acid ethyl ester (64), squalene (65) (Chen et al., 2001c), 1,2-dihydroxynonadecone-3 (66) (Chen et al., 2000b), 1-O-stearoyl-2-O-Δ4′,7′-dodecenoyl-3-O-phosphatidic acid-O-β-d-glucoside (67) and 1-O-stearoyl-2-O-Δ4′,7′-dodecenoyl-3-O-phosphatidic acid-O-(6″-O-α-d-glucoside)-β-d-glucoside (68) (Chen et al., 2001b).
4.5. Other compounds
There are also other components in Polygonum multiflorum, and these include the following polyphenolic compounds: catechin (69), epicatechin (70) (Chen et al., 1999c), 3-O-galloyl-(-)-catechin (71), 3-O-galloyl-(-)-epicatechin (72), 3-O-galloyl-procyanidin B2 (73), 3,3′-di-O-galloyl-procyanidin B2 (74) (Nonaka et al., 1982), gallic acid (75) (Li and Lin, 1993) and methyl gallate (76) (Yang et al., 1998). Three nitrogenous compounds, namely N-trans-feruloyltyramine (77), N-trans-feruloyl-3-methyldopamine (78) (Li and Lin, 1993) and indole-3-(l-α-amino-α-hydroxypropionic acid) methyl ester (79) (Yang et al., 1998), were also isolated and identified from this plant. Two coumarin glucosides, which were identified as 7-hydroxy-4-methylcoumarin-5-O-β-d-glucopyranoside (80) and 7-hydroxy-3,4-dimethylcoumarin-5-O-β-d-glucopyranoside (81) (Yu et al., 2008), were also isolated. Furthermore, the following compounds were also isolated from Polygonum multiflorum: n-butyl-β-d-fructopyranoside (82) (Zhang, et al., 2006b), 1,3-dihydroxy-6,7-dimethylxanthone-1-O-β-d-glueopyranoside (83) (Zhou et al., 1994), daucosterol (84) (Li and Lin, 1993, Rao et al., 2009), 4-hydroxybenzaldehyde (85), 5-carboxymethyl-7-hydroxy-2-methylchromone (86) (Li et al., 2006), 1,2-propanediol-1-(4-hydroxy-phenyl) (87) (Yamaguchi et al., 1969), (+)-lyoniresinol-3α-O-β-d-glucopyranoside (88) (Achenbach et al., 1992), 2,3,4,6-tetrahy-droxyacetophenone-3-O-β-d-glucoside (89) (Yoshizaki et al., 1987), torachrysone-8-O-β-d-glucopyranoside (90) (Li et al., 2006), torachrysone-8-O-(6′-O-acetyl)-β-d-glucopyranoside (91) (Sun et al., 2009), torachrysone-8-O-(6′-O- galloyl)-β-d-glucoside (92) (Li and Lin, 1993), β-amyrin (93), β-sitosterol (94) (Rao et al., 2009), β-sitosterol-3-O-β-d-glucoside (95) (Xu et al., 2006), 2,5-dimethyl-7-hydroxychromone (96) (Liang et al., 2009), schisandrin (97) (Chen et al., 1999b), (S)-2-(2′-hydroxypropyl)-5-methyl-7-hydroxyl, and chromone-7-O-α-l-fucosel(1→2)-β-d-glycoside (98) (Zhao et al., 2014).
4.6. Changes in the chemical constitution after processing
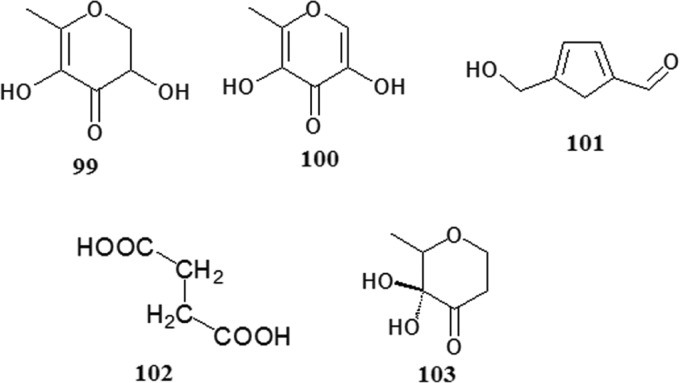
The chemical constituents in Polygonum multiflorum changes after processing, and novel components can be created. The combined anthraquinone content decreases with increased processing. The content of free anthraquinones, such as emodin and physcion, increases with prolonged processing time. The content of d-glucose increases gradually, whereas the d-fructose and sucrose contents decrease gradually, and the content of polysaccharide slightly increases. The content of stilbene glucoside (index component of Polygonum multiflorum) is reduced with an increase in the processed time, and the content exhibited the following order: rice wine mixed with steamed>steamed>rice wine and fried>black bean juice products. After processing, the content of tannin decreased, and the antioxidant activity of the gallic acid content increased; however, catechin was almost undetectable after processing for 16 h. Other studies have found that the content of trace elements in fleece-flower root before and after processing changed only slightly (Zhang et al., 2009, Chen et al., 2012d, Fu, 2013, Liu et al., 2008b, Qiu and Huang, 2006a, Qiu and Zeng, 2006b, Wang et al., 2004, Shi, 2003, Liu et al., 2005c, Liu et al., 2009b, Nie and Liu, 2002).
According to a previous study, Polygonum multiflorum processing resulted in the production of five ingredients: 2,3-di-hydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one (99), hydroxymaltol (100), 5-hydroxym ethyl-furfural (101), butanedioic acid (102), and 5-dihydroxy-6-methyl-4(H)-pyran-4-one (Liu et al., 2007b, Liu et al., 2008c, Liu et al., 2009c). In conclusion, processing has a marked influence on the chemical constituents of Polygonum multiflorum, and the fact that the toxicity of Polygonum multiflorum preparata is lower than that of the crude drugs may be associated with the decreased levels of some of the components after processing.
5. Pharmacodynamics and potential applications
5.1. Anti-aging effect
Age is the leading risk factor for many of the most prevalent and devastating diseases, including Alzheimer׳s disease (AD) and Parkinson׳s disease (PD). AD is a neurodegenerative disease characterized by progressive memory loss and cognitive impairment and is the most common type of dementia in the aging population (Long and Dougherty., 2003). PD is a common progressive neurodegenerative disorder characterized by the loss of specific populations of neurons and the accumulation of protein aggregates in the brain. The disease affects more than 1% of the population over the age of 60, and as the population ages, this frequency is likely to increase (Fahad et al., 2014, Lang and Lozano, 1998, Obeso et al., 2010). The main effect of Polygonum multiflorum is anti-aging given that it can be used to treat AD and PD. Specifically, 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside (TSG), one of the effective components of this plant, may be able to treat AD and PD (Li et al., 2010b, Zhang et al., 2010, Su et al., 2014). Polygonum multiflorum and TSG may potentially treat AD and PD through the following mechanisms: inhibition of acetylcholinesterase (AChE), neuroprotection, antioxidant activity and cognitive enhancement.
5.1.1. AChE inhibition
AChE inhibitors are commonly used to treat AD. TSG can decrease AChE activity and increase the expression of protein phosphatase-2A (PP-2A) and microtubule associated protein-2 (MAP-2) in the hippocampus of model rats following the i.g. administration of 30, 60 and 120 mg/kg/day for 11 weeks (Liu et al., 2008a). Emodin-8-O-β-d-glucopyranoside (EG), another effective component of Polygonum multiflorum, was tested in vitro against AChE I zymogen (extracted from the mice cerebral cortex) at doses ranging from 23.1 to 92.6 μmol/mL, and the results indicate that EG can inhibit AChE I activity. In addition, in vivo AChE I activity could also be inhibited by EG (0.5–80 mg/kg/day, p.o., once or every day for a period of 15 days), and this inhibition was reversible (Chen et al., 2001a). Previous studies have also demonstrated that Polygonum multiflorum extracts can treat insomnia and brain function disorders by inhibiting the activity of AChE. These experiments were conducted on a 96-well plate assay using Ellman׳s method. The AChE activity inhibition percentages of aqueous and ethanol Polygonum multiflorum extracts at doses of 20 μg/mL were 59.88 and 47.24, respectively (Lin et al., 2008). Polygonum multiflorum aqueous extract, which was administered p.o. to mice for 28 days, obviously improve the cognitive deficits induced by Aβ25-35 on mice. And, the acetylcholinesterase and GPx activity were increased, the TBARS level was decreased in mice brain after administration for 4 weeks. These effects might be related to the antioxidant activity of Polygonum multiflorum (Um et al., 2006).
5.1.2. Neuroprotective effect
Many studies suggested that JNK played an important role in the mediation of MPP+-induced neurotoxicity, and a research demonstrated the TSG could protect against MPP+-induced apoptosis in a dose-dependent manner (1, 5 and 10 μM) in PC12 cells. The results showed that TSG pretreatment attenuated the expression of the p-JNK, this effect related to the antioxidantive ability (blocked the ROS increase) of TSG (Li et al., 2010c). Another study verified that the neuroprotective effects of TSG against MPP+-induced damage and apoptosis in PC12 cells (0.1, 1 and 10 μM) are likely to be mediated by the activating the PI3K signaling pathway, the stimulation of PI3K activity leads to downstream target Akt phosphorylation and subsequent inhibition of cell apoptosis (Qin et al., 2011). TSG also protected human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity (3.125, 6.25, 12.5, 25 and 50 μM). The mechanisms underlying this effect may be mediated through (i) its protection of mitochondrial function, (ii) attenuating the accumulation of intracellular reactive oxygen species, (iii) and inhibiting the MPP+-induced mitochondrial apoptotic pathway by decreasing the ratio of Bax to Bcl-2 and preventing caspase-3 activation (Sun et al., 2011). These mechanisms were also demonstrated in a study on staurosporine (STS)-induced toxicity in cultured rat hippocampal neurons. The results of the study showed that the administration of 200 μM TSG can significantly protect against STS-induced apoptosis in cultured rat hippocampal neurons (Yang et al., 2014). In addition, TSG (5–400 μmol/L) significantly promoted cell viability and reduced cell membrane damage in β-amyloid 25-35- and H2O2-treated human neuroblastoma SK-N-SH cells. TSG may initially antagonize the cell damage induced by hydrogen peroxide and prevent the subsequent toxicity of amyloid beta protein in nerve cells (Zhang et al., 2004, Xu and Yi, 2013).
Polygonum multiflorum ethyl acetate extracts (0.1, 0.5, 1, 3, 5, and 10 μg/mL) may exert neuroprotective effects through activation of extracellular regulated kinase (ERK) and p38 via MAPK and the calpain-STEP signaling pathways, and ERK and p38 were major contributing factors to its protection. These extracts may also be used as therapeutic interventions for the treatment of oxidative neuronal death. A previous study showing this effect was conducted by evaluating glutamate-induced oxidative cell death in HT22 hippocampal cells (Kim et al., 2013). An 80% ethanol extract of Polygonum multiflorum protected U373 human astrocytes from hydrogen peroxide-induced cell death (LC50: 35.2±1.2 μg/mL, EC50:<0.2 μg/mL for hydrogen peroxide). The protective mechanism was mediated by its free radical scavenging and antioxidant capacities (Steele et al., 2013). The hexane extract of Polygonum multiflorum also exerts a protective effect against glutamate-induced neurotoxicity in primary cultured cortical neurons. This neuroprotection may be mediated through both DR- and mitochondrial-mediated apoptotic pathways involving DR4, Bcl-2, XIAP, and cIAP-1(Jang et al., 2013).
The neuroprotective effects of Polygonum multiflorum and TSG were also investigated in vivo. Treatment with TSG (20 and 40 mg/kg/day, p.o., for 14 days) protected dopaminergic neurons by preventing MPTP-induced decreases in substantia nigra tyrosine hydroxylase (TH)-positive cells and striatal dopaminergic transporter (DAT) protein levels in mice (Zhang et al., 2013). Another study also demonstrated this neuroprotective effect. This report investigated the degeneration of nigrostriatal dopaminergic neurons induced by a combination of paraquat and maneb (PQMB) in male C57BL/6 mice after the administration of 75% Polygonum multiflorum ethanol extract (400 and 800 mg/kg/day, p.o., for 47 days) and the ethanol-soluble (250 mg/kg/day) and ethanol-insoluble (500 mg/kg/day) fractions. The results suggested that the neuroprotective effect of the 75% Polygonum multiflorum ethanol extract is attributable to a substance(s) in the ethanol-soluble fraction (Li et al., 2005).
5.1.3. Antioxidant effect
Antioxidant and free radical scavenging can also be used to treat AD and PD. A recent study evaluated the protective effect of TSG against d-galactose-induced aging in mice (42, 84 and 168 mg/kg, p.o., for 8 weeks). The results suggested that TSG is able to improve the memory ability and regulated the body weight of d-galactose induced aging mice through reducing the levels of ROS, NO and IGF-1 and increasing the levels of superoxide dismutase (SOD), Ca2+ and Klotho protein in the serum (Zhou et al., 2013). Another study also proved that TSG (20 and 40 mg/kg/day, p.o., for 42 days) can increase SOD and glutathione peroxidase (GSH-Px) activities in the serum and organs and decrease the content of 2-thiobarbituric acid-reactive substances (TBARS) in a d-galactose-induced mouse model of dementia (Lv et al., 2007). However, TSG also has anti-melanogenic activity at doses ranging from 60 μM to 240 μM through a mechanism that is likely mediated through the non-competitive inhibition of tyrosinase, the down-regulation of the expression of melanogenic proteins, and a reduction of tyrosinase/tyrosinase-related protein 1 complex formation (Cheung et al., 2014).
In addition, extracts of Polygonum multiflorum also have antioxidant capacities. Polygonum multiflorum extract (70% ethanol extract, 0.32 g crude drugs/kg/day, p.o., 5 days/week, for 4 weeks) has anti-aging effects in a mouse model of d-galactose-induced subacute aging. The mechanism for this effect may be mediated by decreases in the levels of LPF in the brain and kidney and enhancements in the activities of Na+/K+-ATPase in the heart and SOD in the liver (Song et al., 2003). Another study showed that mice exhibit a better active shuttle avoidance response, fewer vacuoles, less lipofuscin in the hippocampus, and lower MDA concentrations in the brain after being fed Polygonum multiflorum extracts (2.5 g/kg/day, p.o., for 18 weeks; 50% ethanol, 95% ethanol, or aqueous). Consequently, these effects promote improved learning and memory abilities (Chan et al., 2002), which may be due to the antioxidant phytochemicals of the extracts (Chan et al., 2003).
5.1.4. Enhanced cognition
TSG can treat AD by enhancing learning and memory abilities. A previous study has shown that TSG (1 and 5 μM) promotes an ERK-dependent differentiation of PC12 cells, directly enhances ERK1/2 activation and intracellular calcium level in hippocampal neurons. TSG-mediated enhancement of ERK1/2 phosphorylation is involved in the facilitation of hippocampal long-term potentiation (LTP) by TSG, the processes may be involved in the effect of TSG on hippocampal LTP including the ERK–CREB pathway and modulation of the response to the NMDA receptor by calcium/calmodulin-dependent protein kinase II (CaMKII) activity. This effect may contribute to the enhancement of learning and memory observed in animal models (Wang et al., 2011a). This effect was verified in AD-like APP transgenic mice of different ages treated with TSG (120 and 240 μmol/kg/day, p.o., for 4, 10 and 16 months). The results showed that TSG can significantly improve the learning-memory abilities (Zhang et al., 2006c).
5.2. Immunomodulating effect
The immunomodulating effect of Polygonum multiflorum is mainly due to its polysaccharides and anthraquinone glycosides. A polysaccharide fraction was purified from Polygonum multiflorum Praeparata, which is composed of rhamnose, arabinose, xylose and glucose at a molar ratio of 1.64:1.00:1.34:6.06. This fraction increased the levels of serum IL-2 and hematological parameters, enhanced the antioxidant profiles, and promoted the haematopoiesis of splenocytes by up-regulating the expression of EPOR and GATA-1 proteins in a cyclophosphamide (Cy)-induced mouse anemia model (20, 40 and 80 mg/kg/day, for 7 days, intraperitoneal). This result indicated that the polysaccharide fraction is a potential immunomodulatory agent (Chen et al., 2012b). Another polysaccharide extracted from Polygonum multiflorum was administered orally at doses of 0.4, 0.8 and 1.6 g/kg/day to mice for 10 days. Moreover, the results reflected that this fraction inhibited the Cy-induced weight loss of immune organs, decreased the number of blood cells, increased the phagocytic percentage and phagocytic index in peritoneal macrophages, increased the contents of serum hemolysin and the esterase-positive rate of T-lymphocytes, and enhanced the ConA-induced proliferation of splenic T-lymphocytes (Ge and Liu, 2007). A previous investigation also showed that the polysaccharide extracted from Polygonum multiflorum Praeparata exerts immunomodulatory effects (mice, 100, 200 and 400 mg/kg/day, p.o., for 7 days) (Zhang et al., 2008). These findings demonstrate that polysaccharides from Polygonum multiflorum and Polygonum multiflorum Praeparata have immunomodulatory effects; however, the structure and composition of these immunomodulatory polysaccharides have not been clarified. Similar findings were also observed for anthraquinone glycoside extracted from Polygonum multiflorum. This compound, when used in vitro at doses ranging from 31.25 to 125 μg/mL, (i) significantly accelerated T and B lymphocyte proliferation and mixed lymphocyte reaction, (ii) improved macrophage phagocytosis, (iii) increased tumour necrosis factor (TNF) secretion and the activity of natural killer (NK) cells, (iv) and antagonized the restraining effect of the lymphocyte helper/suppressor ratio induced by mitomycin (MitC) (Sun et al., 2006).
The water extract of Polygonum multiflorum and Polygonum multiflorum Praeparata significantly increased the serum IgM level in rats after oral administration at a dose of 30 g/kg for 90 days (Hu et al., 2009). After treatment with the water extract of Polygonum multiflorum at doses of 2 and 4 mg/mice/day (i.p. injection, 7 days), the ConA- and LPS-induced lymphocyte proliferation in mouse splenocytes and the function of antibody-secreting cells were enhanced (Qin et al., 1990). The enhancement effects of ConA and LPS were also observed in mouse spleen lymphocytes after treatment with n-butanol, ethyl acetate and chloroform extracts at dosages of 50, 100 and 200 μg/mL (Deng et al., 2008).
5.3. Anti-hyperlipidaemia effect
Dyslipidemia is characterized by increased total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) levels and by decreased high-density lipoprotein cholesterol (HDL-C) levels (Toth, 2010). Recently, the effects of TCM have been increasingly demonstrated to be helpful for hyperlipidemic patients. More than 50 TCM formulas have been used to treat hyperlipidaemia. Polygonum multiflorum and Polygonum multiflorum Praeparata are the commonly used herbs in these formulas (Xie et al., 2012). The anti-hyperlipidaemia effect of Polygonum multiflorum was mainly due to the antioxidant function of certain ingredients, such as TSG, polysaccharides and anthraquinones.
TSG (30 and 60 mg/kg/day, p.o., for 28 days) can remarkably decrease the levels of serum TC, TG, LDL-C, and malondialdehyde (MDA). TSG was also shown to decrease the TC/high-density lipoprotein cholesterol (HDL-C) ratio and to markedly increase the levels of serum HDL-C, nitric oxide (NO) and SOD (Wang, 2005a, Wang et al., 2009a). These results were verified by another study through an experimental hyperlipidemic rat model treated with TSG (90 and 180 mg/kg/day, p.o., for 7 days) (Gao et al., 2007). Calreticulin, vimentin, HSP 70, lipocortin 1, and Apo A-I are proteins that may be molecular targets responsible for the TSG-induced atherogenesis suppression (Yao et al., 2013). The anti-hyperlipidaemia effect of Polygonum multiflorum polysaccharides in mice were investigated through its administration at doses of 50 and 200 mg/kg/day for 28 days. The results showed that the serum levels of TC, TG and AI were significantly decreased, whereas the HDL-C, LPL, HL and LA levels were significantly increased (Zhai et al., 2010). In addition, a previous study investigated the mechanism underlying the lipid regulation activity of the major chemical components of Polygonum multiflorum in L02 cells. The results showed that TSG increased the content of cholesterol 7α-hydroxylase (CYP7A) and subsequently promoted the lipolysis of cholesterol. TSG also showed the best LDL-reducing effect. Moreover, emodin inhibited 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase and diacylglycerol acyltransferase 1 (DGAT1), which are key enzymes in the synthesis of TC and TG. Physcion increased the content of hepatic triglyceride lipase (HTGL), subsequently boosting the lipolysis of triglycerides. At the same time, physcion showed the best VLDL-reducing effect (Wang et al., 2014). Another study investigated the anti-hyperlipidemic effect in the combined hyperlipidaemia rat model by treatment with emodin alone (75, 150 and 300 mg/kg/day, p.o., for 4 weeks). The result showed that emodin may be effective for the prevention and treatment of combined hyperlipidaemia model rats. The mechanisms may be mediated by increases in the anti-oxidative effects of blood SOD and GSH-PX and reductions in the blood MDA levels (Wu, 2008).
The extracts of Polygonum multiflorum also exert anti-hyperlipidaemia effects. A previous study using mice to evaluate the reductions in the blood lipid contents induced by different Polygonum multiflorum extracts obtained through different methods, including supercritical fluid extraction (SFE), systematic solvents (petroleum benzene, ethyl acetate, n-butanol, 75% ethanol and water) and ethanol precipitation after water extraction, showed that the SFE extract was active (0.5 to 1.25 g/kg, p.o., one administration) (Li et al., 2008). Water extracts of both Polygonum multiflorum and Polygonum multiflorum Praeparata reduced the total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) contents in the rat blood. Moreover, the two extracts showed dose-dependent TC- and triglyceride (TG)-decreasing effects in liver tissue samples (0.405, 0.81, and 1.62 mg/kg/day for Polygonum multiflorum; 0.81, 1.62, and 3.24 mg/kg/day for Polygonum multiflorum Praeparata, p.o., for 24 days) (Li et al., 2012b). Another study used hepatic steatosis L02 cells to compare the relative activities of Polygonum multiflorum and Polygonum multiflorum Praeparata extracts (extracted with water or 50% ethanol; all extracts at doses of 10, 20, 40, 80 and 100 μg/mL). The results showed that the intracellular contents of TG and TC were increased from 16.50±1.29 mmol/L to 34.40±1.36 mmol/L and from 5.07±1.80 mmol/L to 11.79±0.54 mmol/L, respectively, in steatosis L02 cells. The water extract of Polygonum multiflorum showed markedly higher TG- and TC-regulation effects than the Polygonum multiflorum Praeparata water extract (Wang et al., 2012a).
5.4. Hepatoprotective effect
The compounds and extracts of Polygonum multiflorum exert hepatoprotective effects via the actions of anthraquinones and polysaccharides, among others. More research studies have investigated the hepatoprotective effects of anthraquinones compared with those of polysaccharides.
Emodin (30 and 50 mg/kg, p.o., one administration) was isolated and exhibited hepatoprotective effects on carbon tetrachloride (CCl4)- as well as d-galactosamine (d-GalN)-induced liver damage in rats. The histopathological examination also clearly showed that emodin reduced lymphocyte cells, Kupffer cells, ballooning degeneration, cell necrosis and hyaline degeneration following CCl4- and d-galactosamine-induced damage (Lin et al., 1996). Treatment with emodin (20, 30 and 40 mg/kg, p.o., one administration) significantly lessened the observed toxicity by protecting the acetaminophen-induced alterations in various blood and tissue biochemical variables in rats 24 h after administration, and the protective effect was dose-dependent (Bhadauria, 2010). The protective effect of emodin may be related to anti-inflammatory and anti-oxidant activities. In turn, these mechanisms were mediated by blockade of TLR4/MD2 complex expression on the cell surface of macrophages, which led to the deactivation of MAPKs and NF-κB signaling pathways, and the inhibition of TNF-α production (Yin et al., 2014). Rhein (10, 20 and 40 mg/kg, p.o., one administration) can reduce the levels of glutamate-pyruvate transaminase (GPT), glutamate-oxaloacetic transaminase (GOT), creatinine (CREA), urea nitrogen (UREA) and reactive oxygen species (ROS) in acetaminophen-induced hepatotoxicity and nephrotoxicity rats. The histopathological damage of the liver and kidney were also significantly ameliorated by rhein treatment (Zhao et al., 2011b).
Antioxidant activity tests have been performed, and a homogeneous polysaccharide with a molecular weight of 6.1×102 kDa was identified at concentrations of 0.1–1.5 mg/mL. The free radical scavenging activity of this polysaccharide was exhibited in the order: superoxide anion (IC50 0.47 mg/mL)>hydrogen peroxide (IC50 0.60 mg/mL)>hydroxyl radical (IC50 0.93 mg/mL), and this polysaccharide also displayed better effects on inhibiting the formation of advanced glycation end products (AGEs) (Lv et al., 2014).
Many studies have investigated the hepatoprotective effect of Polygonum multiflorum extracts. The methanol extract of this plant (1–1000 μg/mL) promoted the expression of hepatocyte growth factor (HGF) for hepatic non-parenchymal cells. Consequently, the proliferation of stellate cells was inhibited, and the proliferation of primary liver cells was increased. Moreover, the phagocytic activity of Kupffer cells was enhanced, as determined using fluorescein-labelled Escherichia coli as the target. Using dimethylnitrosamine-induced liver cirrhosis mice, the methanol extract of Polygonum multiflorum at 20, 23, 30, 37, and 44 mg/kg/day (p.o., for 25 days) was to promote the expression of HGF that stimulates the proliferation of hepatocyte enhances the regenerative potential of liver to reverse dimethylnitrosamine-induced liver damage (Huang et al., 2007). Both Polygonum multiflorum and Polygonum multiflorum Praeparata extracts (water-extracted, 15 g/kg/day, p.o., for 8 days) can be used to treat the hepatic lipid accumulation caused by prednisone acetate-, carbon tetrachloride- or thioacetamide-induced liver damage in mice (Liu et al., 1992). Moreover, these extracts attenuated liver damage by reducing lipid peroxidation as well as by positively modulating inflammation (Lee et al., 2012).
5.5. Anticancer effect
The compounds of Polygonum multiflorum also possess anti-cancer effects. The main effective substances are thought to be anthraquinones, such as emodin and aloe-emodin. The anti-cancer effects of anthraquinones have been studied in different tumour cell lines and in pre-clinical animal models. The main identified mechanisms underlying the anti-cancer effects involve the induction of apoptosis and the activation of the PI3K/AKT/mTOR pathways.
5.5.1. Effects on apoptosis
Apoptosis is generally triggered through two major pathways. One is the death receptor-induced extrinsic pathway, which includes ligands and their receptors, such as FAS, TNF, TRAIL, caspases and Bcl2. The other pathway is the mitochondria-apoptosome-mediated intrinsic pathway, which includes key effector caspases-8, -9 and -10. Emodin is characterized as a strong apoptotic agent. Emodin has been shown to significantly inhibit the growth of HepG2 cells, as evidenced by an IC50 of 36±2.6 μg/mL (Liu et al., 2003). Emodin at a concentration of 50 μM arrests liver cancer Huh7, Hep3B, and HepG2 cells in the G2/M phase, this effect was accompanied by induced cyclin A, cyclin B, Chk2, Cdk2 and P27 expression and down regulated Cdc25c and P21 expression in time-dependent fashion. This result demonstrated that emodin can regulate common gene expression or suppression in different liver cancer cells and the potential biological pathways activated by emodin (Hsu et al., 2010). Aloe-emodin, another anti-apoptotic agent found in this plant, inhibited the growth of human cervical cancer cells (HeLa cells) at the G2/M phase in a dose-dependent manner at concentrations between 2.5 and 40 µmol/L. This effect was associated with decreased cyclin A and cyclin-dependent kinases 2 (CDK2) expression and increased cyclin B1 and CDK1 expression. Aloe-emodin had a down regulatory effect on the expression of c-myc in human cervical cancer cells through the PKCα pathway (Guo et al., 2007a). Aloe-emodin also inhibited T24 human bladder cancer cell viability, inducing G2/M arrest and apoptosis at doses of 5, 10, 25 and 50 μM. Furthermore, aloe-emodin could increase the accumulation of p53, enhancing the expression of p21, Fas, Bax and cytochrome c, and decreasing Bcl-2 before leading to apoptosis (Lin et al., 2006). Moreover, this treatment (10, 20, 30, 40 or 50 μM) induced cell death through S-phase arrest and caspase-dependent pathways in human tongue squamous cancer SCC-4 cells (Chiu et al., 2009) and exhibited cytotoxic effects against neuroblastoma cells (SJ-N-KP) (Pecere et al., 2000) and B16-F10 melanoma cells (Tabolacci et al., 2010).
In vivo, emodin (40 mg/kg/every 3 days, i.p. injection, 39 days) significantly reduced the colon tumour volume (46%) and tumour weight (42%). These effects were mediated by inducing cell morphological changes and G2/M phase arrest, decreasing the percentage of viability, increasing ROS and Ca2+ production, and inducing the loss of the mitochondrial membrane potential in human colon cancer cells (LS1034). Apoptosis was also confirmed by DAPI staining, and these effects were concentration-dependent (Ma et al., 2012). In another mouse experiment examining gallbladder tumors induced by an injection of human gallbladder cancer SGC996 cells, emodin (50 mg/kg/day, i.p. injection, 18 days) exerted significant antitumor effects by enhancing the apoptosis of gallbladder cancer cells (Wang et al., 2011b).
5.5.2. Effect on the PI3K/AKT/mTOR pathway
Improper regulation of PI3K/AKT pathways has been reported in many human cancers (Fresno Vara et al., 2004). A previous study demonstrated that aloe-emodin (2.5, 5, 10, 20, 40 and 80 μM) can reduce the cytotoxicity of the pro-inflammatory cytokine tumour necrosis factor (TNF) in L929 mouse fibrosarcoma and U251 human glioma cell line through a mechanism involving the induction of autophagy and he blockade of ERK activation. Emodin (1, 5, 10, 20 and 40 μM in the HeLa cell line) induces apoptosis, and the anti-apoptotic effect of CK2 is partially mediated by targeting the phosphorylation and up-regulation of AKT. This is the mechanism of action associated with the PI3K/AKT pathway, the deregulation of which has been linked numerous times to malignant transformation (Birgitte et al., 2007).
In vivo, an i.p. injection of emodin (25 and 50 mg/kg /day, 5 days per week, for 4 weeks) has also shown significant antitumor effects in head and neck squamous cell carcinomas induced by FaDu-pFLAG-CMV or FaDu-pFLAG-TWIST1 cells in mice. The anti-cancer mechanism of emodin is hypothesized to be an inhibition of TWIST1 expression by inhibiting the β-catenin and Akt pathways, up regulating E-cadherin mRNA and protein expression and down regulating vimentin mRNA and protein expression (Way et al., 2014).
Emodin has also been found to exhibit cytotoxic effects against HepG2 cells (Yu, et al., 2013), the prostate cancer cell lines LNCaP and PC-3, the lung cancer cell line A549, the colon cancer cell line HCT-15, the bone cancer cell line MG-63 (Masaldan and Iyer, 2014) and the esophageal cancer cell lines EC-109 (Liu et al., 2009a).
5.6. Anti-inflammatory effects
Polygonum multiflorum also exerts anti-inflammatory effects. The main effective substances associated with this effect are believed to be TSG and emodin. The anti-inflammatory effects of TSG and emodin have been associated with antioxidant activity and an inhibition of the pro-inflammatory transcription factor NF-κB. TSG (60 and 120 mg/kg/day, p.o., for 7 days) significantly ameliorated colon damage (induced by acetic acid), inhibited the increase in myeloperoxidase (MPO) activity induced by acetic acid, depressed MDA and NO levels, and enhanced SOD activity in mice (Wang et al., 2008). TSG (50 μM) attenuated the LPS-mediated induction of pro-inflammatory factors in microglia by reducing iNOS protein expression and the TNF-α, IL-6, and NO levels. TSG also increased apoptosis, caspase-3 cleavage, and the lactate dehydrogenase (LDH) levels. Another effect of TSG was found to be a reduction in the binding of NF-κB to its DNA element in LPS-stimulated BV-2 cells (Huang et al., 2013).
In another experiment, TSG inhibited mouse ear and paw edema with a percentage of inhibition of 87% after oral administration at a dose of 9.2 mg/kg. This percentage was 56% after oral administration at a dose of 12.8 mg/kg. The mechanism underlying these effects is likely related to the suppressed cyclooxygenase-2 (COX-2) gene expression or directly inhibited COX-2 enzyme activity in RAW264.7 macrophage cells (Zhang et al., 2007b). TSG also showed beneficial effects on acetic acid-induced experimental colitis in mice following intragastric administration at 10, 30, or 60 mg/kg for 7 days and exerted beneficial effects on acetic acid-induced experimental colitis by upregulating the PPAR-γ mRNA and protein levels and inhibiting the NF-κB pathway. These effects in turn decreased the expressions of the downstream inflammatory mediators TNF-α, IL-6 and COX-2 and MDA content (Zeng et al., 2011). A study showed that administrations of emodin (1, 2, and 4 mg/kg, i.p., one administration) could lessen the LPS-induced mammary gland injury and inflammatory cell infiltration, decrease the myeloperoxidase (MPO) activation in the mammary gland, down-regulate the expression and production of TNF-a, IL-6, and interleukin-1 beta (IL-1b) in a dose dependent manner. The anti-inflammatory mechanism was inhibited by the activation of NF-kB and MAPKs signal pathways (Li et al., 2013a). The Polygonum multiflorum extract (70% ethanol extract, p.o., 0.58, 1.15, 2.30, 4.60 and 9.20 g/kg, for 3 days) exhibited strong anti-inflammatory activity based on edema severity and vasopermeability in a mouse model of inflammation and in the response of mice to pain induced by acetate acid. The mechanism was proposed to be related to its immunosuppressive effects (Lv et al., 2001).
5.7. Other pharmacological effects
In addition to the pharmacological effects described above, Polygonum multiflorum and its ingredients have other pharmacological effects, such as increased hair-fiber length, anti-brain infarct activity, and anti-thromboembolic disorders. Some of these effects are discussed briefly below.
Torachrysone-8-O-β-d-glucoside, a recently discovered compound in Polygonum multiflorum, induced a strong increase in the proliferation of dermal papilla cells and increased the hair-fiber length significantly at doses of 10 and 20 μM (Sun et al., 2013). TSG (10 and 20 mg/kg/day, p.o., for 56 days) ameliorated diabetic nephropathy in rats. The therapeutic mechanisms of TSG on diabetic nephropathy involved in inhibiting oxidative stress, inflammation, and the expression of TGF-β1, which partly mediated by activation of SIRT1. These effects occur in part via the activation of SIRT1 (Li et al., 2010a). TSG has been shown to attenuate reactive oxygen/nitrogen species (ROS/RNS) formation and to protect against ischemia/reperfusion injury. The neuroprotective effect of TSG is caused by multifunctional cytoprotective pathways (Wang et al., 2009b). TSG (30, 60, 120 mg/kg/day) was administered to rats for 30 days in a cardiac remodeling study. In this context, TSG prevented cardiac remodeling induced by pressure overload in rats. The underlying mechanisms may be related to a decrease in the angiotensin II level, an antioxidant effect of the tested compound, the suppression of transforming growth factor-β1 expression, and the inhibition of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase activation (Xu et al., 2014). Another study indicated that TSG exerted potent anti-platelet activity against collagen-induced aggregation. TSG is likely to exert protective effects in platelet-associated thromboembolic disorders by modulating human platelets (Xiang et al., 2014).
Importantly, emodin (IC50 was 200 μM) seriously blocks both the binding of SARS-CoV S protein to angiotensin-converting enzyme 2 (ACE2) and the infectivity of S protein-pseudotyped retrovirus to Vero E6 cells. Therefore, emodin may be considered a potential lead therapeutic to treat SARS (Ho et al., 2007). Another study revealed that co-treatment with emodin (1×10−5 and 1×10−4 μg/ml) significantly prevented ethanol-induced developmental anomalies in cultured mouse fetuses by modulating hypoxia and antioxidant enzymes and attenuating the enhanced levels of TNF-α and caspase 3 in cultured embryos (Yon et al., 2013). Emodin (1 μM to 10 μM) also inhibited tonic tension in a concentration- and time-dependent manner by suppressing the PKCδ-mediated inhibition of myosin phosphatase in isolated rat thoracic aortas (Lim et al., 2014).
A study that investigated the cerebrovascular protective effects of Polygonum multiflorum against ischemic brain injury used an in vivo photothrombotic mouse model. Hexane, ethyl acetate and methanol extracts of Polygonum multiflorum (100 mg/kg) were administered i.p. 30 min prior to ischemic insult. The result showed that the hexane extracts induced a significant reduction in infarct volume and subsequent neurological deficits compared with the other extracts. This cerebroprotective effect is primarily mediated via an eNOS-dependent mechanism (Lee et al., 2014).
5.8. Clinical application
Polygonum multiflorum is not usually used as a single herb for clinical application but shows better therapeutic effects when used in combination with other herbs. A previous study investigated the effect of Buyanghuanw Pill Jiajian Fang (BPJF, composed of Radix astragali, Angelica sinensis, Ligusticum wallichii, Radix Paeoniae alba, peach kernel, safflower, Lumbricus, Fructus Psoraleae, and Cistanche salsa) on patients suffering from PD. Sixty patients with PD were randomly divided into the treatment group (BPJF+Madopar group, n=30) or the control group (Madopar group, n=30) and treated for 3 months. The total effective rate of the treatment group was 87.5%, whereas that of the control group was 77.5%, and this difference was significant (Zhang, 2008). Another study observed the effect of Qishu decoction (contains Astragalus, prepared Rhizome of rehmannia, Radix codonopsis, lyceum, donkey-hide glue, Angelica sinensis, Polygonum multiflorum, Caulis Spatholobi, and Glycyrrhiza uralensis) on the treatment of NSCLC patients with leukopenia after chemotherapy. Two-hundred-forty patients with NSCLC were randomly divided into the treatment group (Qishu decoction group n=120) or control group (Leucogen and Batilol tablet group, both n=120). Both groups were treated for 10 days. The total effective rate in the treatment group was 91.5%, whereas that for the control group was 72.8%. This result suggested that the Qishu decoction can greatly improve bone marrow depression, promote blood-producing functions and recover leukocytes, thereby benefiting NSCLC patients (Xiang and Han, 2013). The He shou wu and Rou cong rong decoction (composed of Polygonum multiflorum, Cistanche salsa, Radix Astragali preparata, Ligusticum wallichii, Angelica sinensis, and Salviae miltiorrhizae) was demonstrated to enhance immunoregulation and improve the clinical symptoms in a study of 34-year-old inpatients with kidney deficiency and blood stasis KDBS (Chen et al., 2006). The Shou wu ji li formula decoction (composed of Polygoni multiflori preparata, stir-baked Fructus tribuli, Angelica sinensis, Fructus ligustri lucidi, Ecliptae herba, Rehmannia glutinosa libosch, duckweed, Fructus Xanthii, Clematis chinensis, Radix sileris, Semen Astragali Complanati, and Radix Glycyrrhizae preparata) combined with the Kaliziran tincture formula was effective for treating blood deficiency and wind sheng-type vitiligo, and there were fewer adverse reactions. The mechanism of action may be through an increase in tyrosinase activity and modulation of the immune system. This combination was found to change the white spots in vitiligo to become darker (Bai, 2013). Polygonum multiflorum is used widely in clinical practice, and there are many studies similar to those mentioned above; we therefore do not list all of the studies in this manuscript.
5.9. Summary of pharmacological effects
Polygonum multiflorum exerts a wide spectrum of pharmacological effects, including anti-aging, immunologic, anti-hyperlipidaemia, neuroprotective, anti-cancer and anti-inflammatory effects ( Table 3). Based on these pharmacological effects, we can conclude that the extracts and the compounds from this plant can prevent or treat certain diseases, such as hyperlipidaemia, inflammation/infection, cancer and AD. However, there are insufficient data for these chemical compounds and their pharmacological effects. Therefore, it is necessary and important to investigate the pharmacological effects and molecular mechanisms of these chemical compounds based on our modern understanding of these diseases׳ pathophysiologies.
Table 3.
Pharmacological effects of Polygonum multiflorum.
| Pharmacological effects | Detail | Active extract/fraction/compound | Effective concentration/dose/pattern | Study model | Reference |
|---|---|---|---|---|---|
| Anti-aging effect | Increase the activities of SOD and GSH-Px | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 20 and 40 mg/kg/day, po., for 42 days | in vivo | Liu et al. (2008a) |
| Inhibit AChE I activity | Emodin-8-O-β-d-glucopyranoside | 0.5–80 mg/kg/day, po., once or 15 days | in vivo | Chen et al. (2001a) | |
| Decrease the AChE activity | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 30,60 and 120 mg/kg/day, ig., for 11 weeks | in vivo | Lin et al. (2008) | |
| Preventive effect against cognitive deficits | Water extract | The experimental diet was based on the RAIN 76 formula, and comprised either 0.5% or 1% Polygonum multiflorum water extract, po., for 28 days | in vivo | Um et al. (2006) | |
| Protect against MPP+-induced apoptosis | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 1, 5 and 10 μM | in vitro | Li et al. (2010c) | |
| Protect against MPP+-induced apoptosis | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 0.1, 1 and 10 μM | in vitro | Qin et al. (2011) | |
| Protected human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 3.125, 6.25, 12.5, 25 and 50 μM | in vitro | Sun et al. (2011) | |
| promoted cell viability and reduced cell membrane damage | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 5–400 μmol/L | in vitro | Zhang et al. (2004); Xu and Yi (2013) | |
| Neuroprotective effect | Ethyl acetate extract | 0.1, 0.5, 1, 3, 5, 10 μg/mL | in vivo | Kim et al. (2013) | |
| Protect U373 human astrocytes | 80% ethanol extract | LC50 was 35.2±1.2 μg/mL, EC50 was <0.2 μg/mL for hydrogen peroxide | in vitro | Steele et al. (2013) | |
| Inhibition of apoptosis of primary cortical neurons | Hexane extract | 0.1, 0.5, 1, 5 and 10 μg/mL | in vitro | Jang et al. (2013) | |
| Neuroprotective effects | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 20 and 40 mg/kg/day, po., for 14 days | in vivo | Zhang et al. (2013) | |
| The neuroprotective effect was attributable to some substance(s) Included in the ethanol-soluble fraction. | 75% ethanol extract | 400 and 800 mg/kg/day, po., for 47 days. | in vivo | Li et al. (2005) | |
| Against d-galactose-induced aging | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 42, 84 and 168 mg/kg, po., for 8 weeks | in vivo | Zhou et al. (2013) | |
| Antioxidant effect | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 20 and 40 mg/kg/day, po., for 42 days | in vivo | Lv et al. (2007) | |
| Antimelanogenic activity | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 60 μM to 240 μM | in vitro | Cheung et al. (2014) | |
| decreased the level of LPF in brain and kidney, enhanced the activities of Na+/K+-ATPase in heart and SOD in liver | Ethanol extract | 0.32 g crude drugs/kg/day, po., 5 days/week, for 4 weeks | in vivo | Song et al. (2003) | |
| Promote learning and memory ability | 50% ethanol, 95% ethanol, or water extracts | 2.5 g/kg/day, po., for 18 weeks | in vivo | Chan et al. (2002) | |
| Enhancement of learning and memory | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 1 and 5 μM | in vitro | Wang et al. (2011a) | |
| Enhancement of learning and memory | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 120, 240 μmol/kg/day, po., for 4, 10 and 16 months | in vivo | Zhang et al. (2006c) | |
| Immunomodulating effect | Immunomodulatory effects | Polysaccharide fraction | 20, 40 and 80 mg/kg/day, intraperitoneal, for 7 days | in vivo | Chen et al. (2012b) |
| Immunomodulatory effects | Polysaccharide fraction | 0.4, 0.8 or 1.6 g/kg/day. po., for 10 days | in vivo | Ge et al. (2007) | |
| Immunomodulatory effects | Polysaccharide fraction | 100, 200 and 400 mg/kg/day, po., for 7 days | in vivo | Zhang et al. (2008) | |
| Accelerate T and B lymphocytes proliferation | Anthraquinone glycoside | 31.25–125 μg/mL | in vitro | Sun et al. (2006) | |
| Increased of serum IgM | Water extract of Polygonum multiflorum and Polygonum multiflorum Praeparata | 30 g/kg, po., for 90 days | in vivo | Hu et al. (2009) | |
| Enhance antibody Secreting cells׳ function | Water extract | 2 mg or 4 mg /mice/day, intraperitoneal injection, for 7 days. | in vivo | Qin et al. (1990) | |
| ConA and LPS enhancement | n-butanol, ethyl acetate and chloroform extracts | 50, 100 and 200 μg/mL | in vitro | Deng et al. (2008) | |
| Anti-hyperlipidaemia effect | Decrease the levels of TC, TG, LDL-C, MDA and TC/HDL-C, HDL-C, NO and SOD. | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 30, 60 mg/kg/day, po., for 28 days | in vivo | Wang (2005a); Wang et al. (2009a) |
| Decrease the levels of TC, TG, LDL-C, MDA and TC/HDL-C, HDL-C, NO and SOD. | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 90, 180 mg/kg/day, po., for 7 days | in vivo | Gao et al. (2007) | |
| Decrease the TC, TG and AI levels. | Polygonum multiflorum polysaccharides | 50 and 200 mg/kg/day, po., for 28 days | in vivo | Zhai et al. (2010) | |
| Lipid regulation activity | major chemical components from Polygonum multiflorum (Emodin, Physcion and 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside) | 50 μM to 300 μM | in vitro | Wang et al. (2014) | |
| Anti-hyperlipidemic effect | Emodin | 75, 150 and 300 mg/kg/day, po., for 4 weeks | in vivo | Wu et al. (2012) | |
| Reduce blood lipid effect | SFE, systematic solvents (petroleum benzin, ethylacetate, n-butanol, 75 % ethanol and water) and ethanol-precipitation after water-extraction extracts. | 0.5 to 1.25 g/kg, po., for once | in vivo | Li et al. (2008) | |
| TC-lowing effects, reduce LDL-C | Water extracts | 0.405, 0.81, 1.62 mg/kg/day for Polygonum multiflorum, 0.81, 1.62, 3.24 mg/kg/day for Polygonum multiflorum Praeparata, po., for 24 days | in vivo | Li et al. (2012b) | |
| Increase the intracellular contents of TG and TC | Water and 50% ethanol extracts | 10, 20, 40, 80 and 100 μg/mL | in vitro | Wang et al. (2012a) | |
| Hepatoprotective effect | Hepatoprotective effects | Emodin | 30 and 50 mg/kg, po. | in vivo | Lin et al. (1996) |
| Hepatoprotective effects | Emodin | 20, 30 and 40 mg/kg, po., one administration | in vivo | Bhadauria et al. (2010) | |
| Hepatoprotective and renal protection | Rhein | 10, 20 and 40 mg/kg, po., for once | in vivo | Zhao et al. (2011b) | |
| Antioxidant activity | Homogeneous polysaccharide | IC50 values were 0.47, 0.6 and 0.93 mg/mL for superoxide anion scavenging, hydroxyl radical scavenging, and hydroxyl peroxide scavenging | in vitro | Lv et al. (2014) | |
| Promote the expression of HGF | Methanol extract | 1–1000 μg/mL | in vitro | Huang et al. (2007) | |
| Increases the expression of HGF messenger RNA in liver tissue | Methanol extract | 20, 23, 30, 37, and 44 mg/kg/day, po., for 25 days. | in vivo | Huang et al. (2007) | |
| Treatment the hepatic lipid accumulation | Water extracted | 15 g/kg/day, po., for 8 days | in vivo | Liu et al. (1992) | |
| Anticancer effect | Inhibit the growth of HepG2 cells | Emodin | IC50 was 36±2.6 μg/ml | in vitro | Liu et al. (2003) |
| Arrests liver cancer Huh7, Hep3B, and HepG2 cells in G2/M phase, | Emodin | 50 μM | in vitro | Hsu et al. (2010) | |
| Anti-cervical cancer | Aloe-emodin | 2.5 and 40 µmol/L | in vitro | Guo et al. (2007b) | |
| Anti-bladder cancer | Aloe-emodin | 5, 10, 25 and 50 μM | in vitro | Lin et al. (2006) | |
| Anti-tongue squamous cancer | Aloe-emodin | 10, 20, 30, 40 and 50 μM | in vitro | Chiu et al. (2009) | |
| Exhibited cytotoxic effects against neuroblastoma cells | Aloe-emodin | ED50 of 7 μM | in vitro | Pecere et al. (2000) | |
| Exhibited cytotoxic effects against B16–F10 melanoma cells | Aloe-emodin | IC50 values of 60 μM | in vitro | Tabolacci et al. (2010) | |
| Anti-colon cancer | Emodin | 40 mg/kg/every 3 days, intraperitoneal injection, for 39 days | in vivo | Ma et al. (2012) | |
| Anti-gallbladder cancer | Emodin | 50 mg/kg/day, intraperitoneal injection, for 18 days | in vivo | Wang et al. (2011) | |
| Reduce the cytotoxicity of fibrosarcoma and U251 human glioma cell lines. | Aloe-emodin | 2.5, 5, 10, 20, 40 and 80 μM | in vitro | Birgitte et al. (2007) | |
| Anti-apoptotic effect | Emodin | 1, 5, 10, 20 and 40 μM | in vitro | Birgitte et al. (2007) | |
| Anti-head and neck squamous cell carcinoma | Emodin | 25 and 50 mg/kg/day, 5 days per week, intraperitoneal injection, for 4 weeks | in vivo | Way et al. (2014) | |
| Exhibited cytotoxic effects against HepG2 cells | Emodin | 30–120 μM | in vitro | Yu, et al. (2013) | |
| Anti-prostate cancer, lung cancer, colon cancer, bone cancer | Emodin | GL50(μM)—LNCaP(10), PC-3(36.4), A549(21.7), HCT-15(33.1), MG-63(50) | in vitro | Masaldan and Iyer (2014) | |
| Anti-esophageal cancer | Emodin | 2.5,5.0,10.0,20.0 μg/mL | in vitro | Liu et al. (2009a) | |
| Anti-inflammatory effects | Ameliorated colon damage | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 60 and 120 mg/kg/day, po., for 7 days | in vivo | Wang et al. (2008) |
| Attenuates proinflammatory factors in microglia | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 50 μM | in vitro | Huang et al. (2013) | |
| Inhibited mouse ear edema and rat paw edema | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 9.2 mg/kg for inhibited mouse ear edema, 12.8 mg/kg for inhibited rat paw edema | in vivo | Zhang et al. (2007b) | |
| Beneficial effects on colitis | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | intragastric administered 10, 30, 60 mg/kg for 7 days | in vivo | Zeng et al. (2011) | |
| Increased in LPS-induced mouse mastitis | Emodin | 1, 2, and 4 mg/kg, i.p., one administration | in vivo | Li et al. (2013a) | |
| Exhibited intensive anti-inflammation effect | Ethanol extract | po., 0.58 g/kg, 1.15 g/kg, 2.30 g/kg, 4.60 g/kg and 9.20 g/kg, for 3 days | in vivo | Lv et al. (2001) | |
| Promote hair growth | Increase in the proliferation of dermal papilla cells and increased hair-fiber length | Torachrysone-8-O-β-d-glucoside | 10 and 20 μM | in vitro | Sun et al. (2013) |
| Ameliorates diabetic Nephropathy | Alleviation of oxidative stress injury and overexpression of COX-2 and TGF-β1, partially via activation of SIRT1 | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 10 and 20 mg/kg/day, po., for 56 days | in vivo | Wang et al. (2009b) |
| Treatment SARS | Blocked the S protein and ACE2 | Emodin | IC50 was 200 μM | in vitro | Ho et al. (2007) |
| Prevent cardiac remodeling | Prevent cardiac remodeling | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 30, 60, 120 mg/kg/day | in vivo | Xu et al. (2014) |
| Anti-platelet activity | Anti-platelet activity | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside | 10 and 50 μM | in vitro | Xiang et al. (2014) |
| Prevented developmental anomalies | Prevented developmental anomalies in cultured mouse fetus induced by ethanol | Emodin | 1×10−5 and 1×10−4 μg/ml | in vitro | Yon et al. (2013) |
| Inhibit tonic tension | Inhibit tonic tension through suppressing PKCδ-mediated inhibition of myosin phosphatase | Emodin | 1 μM to 10 μM | in vitro | Lim et al. (2014) |
| Cerebrovascular protective effects | Against ischemic brain injury | Hexane extracts, ethyl acetate extracts and methanol extracts of Polygonum multiflorum | 100 mg/kg | in vivo | Lee et al. (2014) |
6. Toxicology
The number of reports on the adverse effects of Polygonum multiflorum has recently increased. Some researchers have found that Polygonum multiflorum shows not only hepatotoxicity and kidney toxicity, particularly in long-term use, but also a possible drug interaction with warfarin to result in bone marrow suppression.
6.1. Hepatotoxicity
The adverse hepatic effects of Polygonum multiflorum have been reported commonly in China and other countries (Niu, 1996, Ye, 1996, Dong et al., 2014), and acute toxic hepatitis was the most frequently reported (Park et al., 2001, Min et al., 2008, Jung et al., 2011). A study that evaluated the hepatotoxicity of the aqueous and acetone extracts of Polygonum multiflorum and Polygonum multiflorum Praeparata on mice (5, 10, and 20 g/kg/day, p.o., for 28 days) indicated that the hepatotoxicity of the aqueous decoction was much higher than that of the acetone extract. Moreover, the hepatotoxicity of the acetone extract of Polygonum multiflorum was considerably higher than that of Polygonum multiflorum Praeparata (Wu et al., 2012). Another study established the “dose-time-toxicity” relationship of the hepatotoxicity caused by administration of a single dose of the water-extracted and ethanol-extracted components of Polygonum multiflorum to mice. The water-extracted components (from 5.5 to 30.75 g/kg) and the ethanol-extracted components (from 8.5 to 24.5 g/kg) caused obvious damage to the liver organization, resulting in significantly increased ALT and AST serum levels, and this effect was dose-dependent (Huang, et al., 2011). Other studies have demonstrated that Polygonum multiflorum Praeparata also exerts side effects on the rat liver; however, the toxicity was less than that obtained with Polygonum multiflorum (Li et al., 2012d, Hu et al., 2006a, Hu et al., 2006b, Hu et al., 2007).
Emodin and rhein, the major components of Polygonum multiflorum, have been demonstrated to exert concentration- and time-dependent toxic effects on human liver (L-02) cells. Emodin at a concentration of 30 μM led to significant apoptosis in a time-dependent manner, as determined by morphological changes in the drug-treated cells (Li et al., 2012a). An in vitro study suggested the occurrence of time- and dosage-dependent (in the range of 6.25–50 μmol/L) toxic effects of rhein on L-02 cells (Sun et al., 2010).
6.2. Nephrotoxicity
Emodin at a concentration of 40 μM displayed apoptotic/necrotic effects in a dose- and time-dependent manner on human proximal tubular epithelial (HK-2) cells. Cell cycle analysis revealed that the HK-2 cells were locked in G1 phase, and the observed apoptosis was mediated through the induction of a caspase 3-dependent pathway (Wang et al., 2007a). Rhein also induced the apoptosis of HK-2 cells at a dose of 40 μM. However, the toxicity of rhein was weaker than that of emodin (Da et al., 2009). Another study demonstrated that emodin, rhein and physcion can significantly inhibit the proliferation of HK-2 cells with IC50 values of 130.65, 82.97 and 76.02 μmol/L, respectively (Wang et al., 2007c). The suppression of the phosphorylation of ERK through the MAPK/ERK signal pathway and alterations in the proportions of lipid moieties may lead to the impairment of the outer mitochondrial membrane in renal tubular epithelial cells. This effect can in turn cause the release of cytochrome c and lead to apoptosis, inducing a decline in the reabsorption of low-molecular-weight compounds in the renal tubule, aminoaciduria and glucosuria (Wang, 2007b).
6.3. Other toxicities
Emodin induces embryonic toxicity in mouse blastocysts at doses of 25, 50 and 75 μM. The injury in mouse blastocysts occurs via the intrinsic apoptotic signaling processes, impairing subsequent embryonic development (Chang et al., 2012). Moreover, the adverse effects of Polygonum multiflorum can be observed not only in the liver and kidney but also in the lung. An ethanol extract of Polygonum multiflorum was found to exert toxic effects in the rat lung after repeated intragastric administration for 28 days at a dosage of 40 g/kg (Li et al., 2013b). Emodin and rhein also showed mutagenicity in TK gene mutation analysis at the concentrations of 80 and 120 μg/mL (Zhu et al., 2011). In addition, a previous study compared the acute toxicity of different components of Polygonum multiflorum on mice. The acute toxicity of dual extraction components was stronger than that of the ethanol- and/or water-extracted components, and the maximum tolerated doses (MTD) were 20.0, 98.4 and 78.0 g/kg, respectively (Huang et al., 2010).
7. Pharmacokinetics
In pharmacokinetic experiments, rats were administered Polygonum multiflorum extracts. After the tissues were extracted with methanol, the metabolites were identified by LC-MS/MS. TSG, the main component of Polygonum multiflorum, was rapidly absorbed into the body fluids, widely distributed throughout the body and quickly eliminated. TSG was mainly distributed in the liver and lung but hardly penetrated the blood-brain and blood-testicle barriers (Lv et al., 2011). Emodin was found predominantly in the liver and brain (Lin et al., 2012). Another study evaluated the pharmacokinetic behaviors of typical constituents of different types of processed Polygonum multiflorum after oral administration in rats by LC-MS/MS. The results showed that the bioavailability of gallic acid improved, and the absorption of TSG, polydatin and emodin from the processed product in rats was reduced compared with that obtained with the raw product (Zhang et al., 2013). Some studies have found that stilbene glucoside and emodin were rapidly converted to glucuronide conjugates in rats after oral administration (Sun, 2004, Liu et al., 2011). Further pharmacokinetic studies indicated that the AUC, C max and T 1/2 of emodin were increased after stilbene glucoside treatment, and the glucuronidation of the emodin levels was significantly inhibited. This phenomenon may be attributed to the inhibition of UGT1A8 mRNA expression by stilbene glucoside (Ma et al., 2013).
8. Future perspectives and conclusions
Polygonum multiflorum is one of the most important and frequently used traditional Chinese herbal medicines because it exhibits anti-aging, immunologic, anti-hyperlipidaemia, neuroprotective, anti-cancer, and anti-inflammatory effects, among others. Quinones and stilbenes are considered to be the major constituents with pharmacological effects. However, some aspects need to be further investigated.
Many recent studies have investigated the effective constituents of Polygonum multiflorum from different geographical areas. HPLC-fingerprint chromatography is usually used to compare the differences in Polygonum multiflorum from different geographical areas (Wu et al., 2006, Luo et al., 2008, Liu et al., 2007a, Cai et al., 2011, Xue et al., 2004, Zhang et al., 2003). These studies have only focused on the differences in the contents of common components but have ignored differences in the more minor components in these plants, which is crucially important for Polygonum multiflorum not only in terms of quality but also clinical use. The combined anthraquinones are used as indicator compounds to characterize the quality of this plant, which should have a minimum content of 0.10%, as delineated in the Pharmacopoeia of the People׳s Republic of China; this percentage is calculated based on the amounts of emodin and physcion. However, chrysophanol-8-O-β-d-glucopyranoside and other combined anthraquinones (Yang et al., 1998) are also found in Polygonum multiflorum. Therefore, the development of a method to accurately measure the amount of the combined anthraquinones requires further investigation. Although we summarized 102 ingredients in this plant, this number is quite limited at present, and future research on the chemical composition of the plant should be more precise.
In traditional Chinese medicine, Polygonum multiflorum is commonly used with other herbs. However, modern experiments have validated that this plant alone exhibits significant pharmacological effects. Therefore, it is important to investigate the molecular mechanisms of Polygonum multiflorum combined with other herbs based on traditional uses. Second, the pharmacological effects of some traditional uses of Polygonum multiflorum have been validated in recent studies, but these studies were primarily conducted in vitro. Thus, the effects of these compounds need to be verified in vivo. Third, to date, approximately 100 chemical compounds have been isolated from this plant; however, the pharmacological effects of only a few of the ingredients, such as 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranoside, resveratrol, polydatin, emodin, and physcion, have been studied. Some stilbenes and quinones, the major components of Polygonum multiflorum, have not been sufficiently researched in terms of their pharmacological effects, and this is also true for emodin glycosides, physcion glycosides, and some stilbenes, among others.
The number of reports of the adverse effects of Polygonum multiflorum, such as hepatotoxicity, nephrotoxicity, and embryonic toxicity, is increasing. The primary toxicity among these is hepatotoxicity, which is of wide concern worldwide. However, the identity if the component(s) that causes hepatotoxicity remains unclear. Some studies have shown that free anthraquinones, such as emodin and physcion, were the main hepatotoxicants (Li et al., 2012a, Sun et al., 2010). Other studies have demonstrated that the hepatotoxicity of the water extract was stronger than that of the ethanol and acetone extracts of Polygonum multiflorum (Wu et al., 2012, Huang et al., 2011). Moreover, the hepatotoxicity of Polygonum multiflorum was also stronger than that of Polygonum multiflorum Praeparata. The emodin and physcion contents increased after processing of this herb (Zhang et al., 2003, Chen et al., 2012c, Liu et al., 2005a, Zhou, 2005, Liu et al., 2005b), and the contents of these components in the ethanol and acetone extracts of this herb were higher than those of the water extract. Thus, the above-described results appear to be contradictory.
Another issue is that Polygonum multiflorum exhibits both hepatoprotection and hepatotoxicity, which appears to be contradictory. Based on an examination of the literature, we speculate that the possible reasons are as follows. The first is the size of the administered dose. High doses are more likely to result in liver toxicity, and low doses may show liver protection. The second is the length of the delivery time: long-term drug delivery can easily show liver toxicity, whereas short-term drug delivery may result in liver protection. Third, a study revealed that TSG and physcion likely attenuated the cytotoxic effect of emodin (Yu et al., 2011); therefore, the different experimental results may be due to the different TSG, physcion and emodin ratios in the herbs. Fourth, TSG and emodin are also hepatoprotective due to their antioxidant activity. Thus, this issue requires further study.
In conclusion, Polygonum multiflorum is one of the most popular traditional Chinese medicines, and it is an ingredient of many patent medicines or prescriptions. It has been widely used to treat various diseases commonly associated with aging in China for many centuries. Modern research demonstrated that Polygonum multiflorum have therapeutic potential in the conditions like Alzheimer׳s disease, Parkinson׳s disease, hyperlipidaemia, inflammation and cancer attributed to the presence of various stilbenes, quinones, flavonoids, phospholipids and other compounds present in the drug. The adverse effects (hepatotoxicity, nephrotoxicity, and embryonic toxicity) of this plant were due to the quinones, such as emodin and rhein. Thus more pharmacological and toxicological mechanisms on main active compounds are necessary to be explored, especially the combined anthraquinones (such as emodin-8-O-β-d-glucopyranoside, Physcion-8-O-β-d-glucopyranoside, etc.) and the variety of stilbenes.
References
- Achenbach H., Lowell M., Waibel R., Gupta M., Solis P. New lignin glucosides from Stemmadenia minima. Planta Medica. 1992;58:270–272. doi: 10.1055/s-2006-961451. [DOI] [PubMed] [Google Scholar]
- Bai P. The Clinical Efficacy of Compound Radix Tribulus Decoction for Treatment on Vitiligo and it׳s Effect of Tyrosinase Activity. Shandong Traditional Chinese Medicine University; Jinan: 2013. [Google Scholar]
- Bhadauria M. Dose-dependent hepatoprotective effect of emodin against acetaminophen-induced acute damage in rats. Experimental and Toxicologic Pathology. 2010;62:627–635. doi: 10.1016/j.etp.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Birgitte B.O., Marina B.P., Barbara G. Emodin negatively affects the phosphoinositide 3-kinase/AKT signalling pathway: a study on its mechanism of action. The International Journal of Biochemistry & Cell Biology. 2007;39:227–237. doi: 10.1016/j.biocel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Cai L.F., Zhong G,Y., Zhang Q., Qu X.Y. Quality evaluation of raw and prepared radix polygonui multiflori from different producing areas and markets. Traditional Chinese Drug Research & Clinical Pharmacology. 2011;22:204–208. [Google Scholar]
- Chan Y.C, Cheng F.C., Wang M.F. Beneficial effects of different Polygonum multiflorum Thunb. extracts on memory and hippocampus morphology. Journal of Nutritional Science and Vitaminology. 2002;48:491–497. doi: 10.3177/jnsv.48.491. [DOI] [PubMed] [Google Scholar]
- Chan Y.C., Wang M.F., Chang H.C. Polygonum multiflorum extracts improve cognitive performance in senescence accelerated mice. American Journal of Chinese Medicine. 2003;31:171–179. doi: 10.1142/S0192415X03000862. [DOI] [PubMed] [Google Scholar]
- Chang M.H., Huang F.J., Chan W.H. Emodin induces embryonic toxicity in mouse blastocysts through apoptosis. Toxicology. 2012;299:25–32. doi: 10.1016/j.tox.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Chen G.H., Pan G.H., Shen S.X., Meng X.F., Zhou R.H. Effect of heshouwu and roucongrong compound on immunological function of elderly patients with kidney deficiency and blood stasis. Chinese Journal of Clinical Rehabilitation. 2006;10:13–15. [Google Scholar]
- Chen J.Y., Chen L., Chen S.X., An Z.B., Tong Y.X. Identification index of Polygonum multiflorum thunb and its Adulterants (I.Plant, Macrostructure) Lishizhen Medicine and Materia Medica Research. 1999;10:44–45. [Google Scholar]
- Chen J.Y., Tong Y.X., An Z.B., Chen L. Gel electrophoresis identification of radix polygoni multiflori and its adulterants. Journal of Chinese Medicinal Materials. 1998;21:71–72. [Google Scholar]
- Chen L. Study on the Curative Effect of Peibu Ganshen Compound in Treating Parkinson׳s Disease and Intestinal Dysfunction. Beijing University of Chinese Medicine; Beijing: 2013. [Google Scholar]
- Chen L.L., Huang X.J., Li M.M., Ou G.M., Zhao B.X., Chen M.F., Zhang Q.W., Wang Y., Ye W.C. Polygonflavanol A, a novel flavonostilbene glycoside from the roots of Polygonum multiflorum. Phytochemistry Letters. 2012;5:756–760. [Google Scholar]
- Chen Q., Zhang S.Z., Ying H.Z., Daic X.Y., Li X.X., Yu C.H., Ye H.C. Chemical characterization and immunostimulatory effects of a polysaccharide from Polygoni Multiflori Radix Praeparata in cyclophosphamide-induced anemic mice. Carbohydrate Polymers. 2012;88:1476–1482. [Google Scholar]
- Chen Q.T., Zhou L.H., Xu W., Huang Z.H., Qiu X.H. Content changes of 5 components in Polygohum muhiflorum during processing. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18:66–71. [Google Scholar]
- Chen Q.T., Zhuo L.H., Xu W., Huang Z.H., Qiu X.H. The content change of 5 kinds of chemical components in Polygonum multiflorum Thunb during the processing. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18:66–71. [Google Scholar]
- Chen W.S., Fan W., Yang G.J., Qiao C.Z., Chen H.S., Yuan Y. Studies on the chemical constituents of radix Polygoni multiflori preparata. Academic Journal of Second Military Medical University. 1999;20:438–440. [Google Scholar]
- Chen W.S., Liu W.Y., Yang G.J., Zhang W.D., Chu Z.Y., Chen H.S., Qiao C.Z. Structural elucidation of a new tetrahydroxystilbene of radix polygoni multiflori preparata and study on its cardiovascular activity. Acta Pharmaceutica Sinica. 2000;35:906–908. [PubMed] [Google Scholar]
- Chen W.S., Xu J.P., Li L., Qiao C.Z. Studies on nootropic activity and mechanism of emodin-8-O-β-d-glucopyranoside. Chinese Traditional and Herbal Drugs. 2001;32:39–41. [Google Scholar]
- Chen W.S., Yang G.J., Zhang W.D., Chen H.S., Qiao C.Z. A new fatty ketone of radix Polygoni multiflori preparata. China Journal of Chinese Materia Medica. 2000;25:476–477. [PubMed] [Google Scholar]
- Chen W.S., Yang G.J., Zhang W.D., Chen H.S., Qiao C.Z. Studies on two new phospholipids of radix Polygoni multiflori preparata. Chinese Pharmaceutical Journal. 2001;36:155–157. [Google Scholar]
- Chen W.S., Yang G.J., Zhang W.D., Qiao C.Z., Chen H.S. Two new compounds of radix polygoni multiflori preparata. Acta Pharmaceutica Sinica. 2000;35:273–276. [PubMed] [Google Scholar]
- Chen W.S., Zhang W.D., Qiao C.Z. Analysis of the constituents of essential oil from radix Polygoni multiflori preparata. Journal of Chinese Medicinal Materials. 2001;23:684–685. [PubMed] [Google Scholar]
- Chen Y., Wang M., Rosen R.T., HO C.T. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. Journal of Agricultural and Food Chemistry. 1999;47:2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- Cheng R.Y., Zhou J.L. Identification of fleece-flower root cut crude drug and spurious breed airpotato yam in crude drug. Journal of Anhui Traditional Chinese Medical College. 2005;24:24. [Google Scholar]
- Cheng R.Y., Zhou J.L. Identification of fleece-flower root cut crude drug and spurious breed airpotato yam in crude drug. Journal of Anhui TCM College. 2005;24:52–53. [Google Scholar]
- Cheung F.W., Leung A.W., Liu W.K., Che C.T. Tyrosinase inhibitory activity of a glucosylated hydroxystilbene in mouse melan-a melanocytes. Journal of Natural Products. 2014;77:1270–1274. doi: 10.1021/np4008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T.H., Lai W.W., Hsia T.C., Yang J.S., Lai T.Y., Wu P.P., Ma C.Y., Yeh C.C., Ho C.C., Lu H.F., Wood W.G., Chung J.G. Aloe-emodin induces cell death through S-phase arrest and caspase-dependent pathways in human tongue squamous cancer SCC-4 cells. Anticancer Research. 2009;29:4503–4512. [PubMed] [Google Scholar]
- Committee on the programming teaching material for higher TCM education . Science of Processing Chinese Materia Medica. Shanghai Science and Technology Press; Shanghai: 1996. p. 235. (1996) [Google Scholar]
- Da H.Y., Jiang Z.Z., Wang C.F., Zhang L.Y., Liu G.Q. The toxic effects of rhein and emodin on human renal tubular epithelial cells in vitro. Chinese Traditional and Herbal Drugs. 2009;40:102–105. [Google Scholar]
- Deng X.C., Huang J.N., Lian Z.W., Sun G.B., Song W. Proliferative effects of different extract of Polygonum multiflorum thunberg on spleen lymphocytes in mice. Chinese Journal of Modern Drug Application. 2008;2:1–3. [Google Scholar]
- Dong H.H., Slain D., Cheng J., Ma W.H., Liang W.F. Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complementary Therapies in Medicine. 2014;22:70–74. doi: 10.1016/j.ctim.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Du C.H., Hai Q.S., Yan Y., Yuan H.X., Zhao R.H., Zhao S.L., Wang X.H. Preliminary study on the processing mechanism of Polygonum multiflorum Thunb by microbial fermentation. Natural Product Research & Development. 2012;24:212–215. [Google Scholar]
- Editorial Board of Flora of China . Vol. 25. Science Publishing House; Beijing: 1998. pp. 102–103. (Flora of China). [Google Scholar]
- Editorial Committee of Chinese Pharmacopoeia . 2010 ed. Medical Science and Technology Press; Beijing, China: 2010. Chinese Pharmacopoeia; pp. 164–165. [Google Scholar]
- Fahad A., Simon R.W., Stott Roger, Barker A. Stem cells and the treatment of Parkinson׳s disease. Experimental Neurology. 2014;260:3–11. doi: 10.1016/j.expneurol.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Fresno Vara J.A., Casado E., de Castro J., Cejas P., Belda-Iniesta C., González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treatment. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Fu W.H. HPLC determination of the main chemical ingredients content in polygonum multiflorum during the processing. Asia-Pacific Traditional Medicine. 2013;9:50–51. [Google Scholar]
- Gao X., Hu Y.J., Fu L.C. Blood lipid-regulation of stilbene glycoside from Polygonum multiflorum. China Journal of Chinese Materia Medica. 2007;32:323–326. [PubMed] [Google Scholar]
- Ge C.L., Liu Y. Polysaccharide from Polygonum multiflorum Thunb potentiates the immunological function in immunosuppressed mice. Chinese Journal of New Drugs. 2007;16:2040–2042. [Google Scholar]
- Ge L.W., Wu Z.C., Zhang Z.F., Zhang M. Identification of fallopia multiflora and its adulterants. Chinese Wild Plant Resources. 2011;30:64–68. [Google Scholar]
- Guo J.M., Xiao B.X., Liu Q., Zhang S., Liu D.H., Gong Z.H. Anticancer effect of aloe-emodin on cervical cancer cells involves G2/M arrest and induction of differentiation. Acta Pharmacologica Sinica. 2007;28:1991–1995. doi: 10.1111/j.1745-7254.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- Guo Y.Q., Chen D.X., Guo M. Quality identification on the authenticity of fleece-flower root. Journal of Traditional Chinese Medicine University of Hunan. 2007;27:71–73. [Google Scholar]
- He E.H., Sun W.J. Physical and chemical identification of radix polygoni multiflori and its adulterants. Aerospace Medicine. 2010;21:380–381. [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Research. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.M., Hsu Y.A., Tsai Y., Shieh F.K., Huang S.H., Wan L., Tsai F.J. Emodin inhibits the growth of hepatoma cells: finding the common anti-cancer pathway using Huh7, Hep3B, and HepG2 cells. Biochemical and Biophysical Research Communications. 2010;392:473–478. doi: 10.1016/j.bbrc.2009.10.153. [DOI] [PubMed] [Google Scholar]
- Hu X.Q., Fang H.L., Quan Z.B., Geng Z.Y. Experimental study of Radix Plygoni Multiflori peparata on liver׳s biochemical matters. Journal of Shaanxi College of Traditional Chinese Medicine. 2007;30:63–64. [Google Scholar]
- Hu X.Q., Li Y.L., Lin F. Effect of polygonum multiflorum and polygonum multiflorum preparation on immunoglobulin in rats. Tianjin Journal of Traditional Chinese Medicine. 2009;26:139–141. [Google Scholar]
- Hu X.Q., Yang H.L., Zhang X.Q., Miao Y.X., Geng Z.Y. Experimental study on the toxicology of prepared Radix Polygoni Multiflori to the rats׳ liver. Journal of Shaanxi College of Traditional Chinese Medicine. 2006;29:40–42. [Google Scholar]
- Hu X.Q., Yang X.Q., Xing Y.R., Miao Y.X., Fang H.L. Study on liver injury induced by prepared Radix Polygoni Multiflori. Shaanxi Journal of Traditional Chinese Medicine. 2006;27:625–626. [Google Scholar]
- Huang C., Wang Y.Z., Wang J., Yao W.J., Chen X.F., Zhang W. TSG (2,3,4′,5-tetrahydroxystilbene 2-O-β-d-glucoside) suppresses induction of pro-inflammatory factors by attenuating the binding activity of nuclear factor-κB in microglia. Journal of Neuroinflammation. 2013;10:129–141. doi: 10.1186/1742-2094-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.H., Homg L.Y., Chen C.F., Wu R.T. Chinese herb Radix Polygoni Multiflori as a therapeutic drug for liver cirrhosis in mice. Journal of Ethnopharmacology. 2007;114:199–206. doi: 10.1016/j.jep.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Huang W., Zhang Y.N., Sun R. Comparative study on acute toxicity of different components of Polygonum multiflorum in mice. Chinese Journal of Pharmacovigilance. 2010;7:705–707. [Google Scholar]
- Huang W., Zhang Y.N., Sun R. Experimental study on the “dose- time- toxicity” relationship of acute hepatotoxicity induced by different components from Polygonum multiflorum in mice. Chinese Journal of Pharmacovigilance. 2011;8:193–197. [Google Scholar]
- Jang J.Y., Kim H.N., Kim Y.R., Choi Y.W., Choi Y.H., Lee J.H., Shin H.K., Choi B.T. Hexane extract from Polygonum multiflorum attenuates glutamate-induced apoptosis in primary cultured cortical neurons. Journal of Ethnopharmacology. 2013;145:261–268. doi: 10.1016/j.jep.2012.10.061. [DOI] [PubMed] [Google Scholar]
- Jung K.A., Min H.J., Yoo S.S., Kim H.J., Choi S.N., Ha C.Y., Kim H.J., Kim T.H., Jung W.T., Lee O.J., Lee J.S., Shim S.G. Drug-induced liver injury: twenty five cases of acute hepatitis following ingestion of polygonum multiflorum thunb. Gut Liver. 2011;5:493–499. doi: 10.5009/gnl.2011.5.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Morita Y. Anthraquinones components in Rumex acetosa L. Shoy akugaku Zasshi. 1987;41:67–74. [Google Scholar]
- Kim H.N., Kim Y.R., Jang J.Y., Choi Y.W., Baek J.U., Hong J.W., Choi Y.H., Shin H.K., Choi B.T. Neuroprotective effects of Polygonum multiflorum extract against glutamate-induced oxidative toxicity in HT22 hippocampal cells. Journal of Ethnopharmacology. 2013;150:108–115. doi: 10.1016/j.jep.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Lan M. Diannan Bencao. ed. People׳s Medical Publishing House; Beijing: 1959. p. 111. (1959) [Google Scholar]
- Lang A.E., Lozano A.M. Parkinson׳s disease. First of two parts. New England Journal of Medicine. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Huang Y.Y., Duh P.D., Wu S.C. Hepatoprotection of emodin and Polygonum multiflorum against CCl(4)-induced liver injury. Pharmaceutical Biology. 2012;50:351–359. doi: 10.3109/13880209.2011.604335. [DOI] [PubMed] [Google Scholar]
- Lee S.V., Choi K.H., Choi Y.W., Hong J.W., Baek J.U., Choi B.T., Shin H.K. Hexane extracts of Polygonum multiflorum improve tissue and functional outcome following focal cerebral ischemia in mice. Molecular Medicine Reports. 2014;9:1415–1421. doi: 10.3892/mmr.2014.1943. [DOI] [PubMed] [Google Scholar]
- Li C.L., Ma J., Zheng L., Li H.J., Li P. Determination of emodin in L-02 cells and cell culture media with liquid chromatography-mass spectrometry: application to a cellular toxicokinetic study. Journal of Pharmaceutical and Biomedical Analysis. 2012;71:71–78. doi: 10.1016/j.jpba.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Li C.R., Cai F., Yang Y.Q., Zhao X.Y., Wang C., Li J., Jia Y.L., Tang J., Liu Q. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: involvement of SIRT1 and TGF-β1 pathway. European Journal of Pharmacology. 2010;649:382–389. doi: 10.1016/j.ejphar.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Li D.P., Zhang N.S., Cao Y.G., Zhang W., Su G.L., Sun Y., Liu Z.C., Li F.Y., Liang D.J., Liu B., Guo M.Y., Fu Y.H., Zhang X.C., Yang Z.T. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-kB and MAPKs signal pathways. European Journal of Pharmacology. 2013;705:79–85. doi: 10.1016/j.ejphar.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Li J., Xu G.J., Xu L.S., Jin R.L. Studies on the traditional Chinese medicine Shouwu II. botanical origins and applied drugs. Chinese Traditional and Herbal Drugs. 1995;26:33–35. [Google Scholar]
- Li J.B., Lin M. Study on the chemical constituents of polygonum multiflorum Thunb. Chinese Traditional and Herbal Drugs. 1993;3:115–118. [Google Scholar]
- Li J.K., Jiang Z.T., Li R., Tan J. Investigation of antioxidant activities and free radical scavenging of flavonoids in leaves of polygonum multiflorum thumb. China Food Additives. 2012;2:69–74. [Google Scholar]
- Li L., Zhang L., Zhang R.Y. Effects of tetrahydroxystilbene glucoside on multiple targets of Alzheimer׳s disease in seven Alzheimerlike animal models. Alzheimer׳s & Dementia. 2010;6:S579–S580. (Poster Presentations) [Google Scholar]
- Li N., Chen Z., Mao X., Yu J., Zhao R. Effects of lipid regulation using raw and processed radix Polygoni multiflori in rats fed a high-Fat diet. Evidence-Based Complementary and Alternative Medicine. 2012 doi: 10.1155/2012/329171. (Article ID 329171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhao K.J., Zhao Y.L., Wang J.B., Fang F., Lv Y., Ma Z.J., Jiang B.Q., Pu S.B., Zou Z.S., Teng G.J., Gong M., Xiao X.H. High dosage administration of Polygonum multiflorum alcohol extract caused the multi-organ injury in rats. Global Traditional Chinese Medicine. 2013;6:1–7. [Google Scholar]
- Li S.G., Chen L.L., Huang X.J., Zhao B.X., Wang Y., Ye W.C. Five new stilbene glycosides from the roots of Polygonum multiflorum. Journal of Asian Natural Products Research. 2013;15:1145–1151. doi: 10.1080/10286020.2013.837454. [DOI] [PubMed] [Google Scholar]
- Li T.H., Luo S.F., Yang S.Y. 58 cases of Xiaohuoluo pill in treating knee osteoarthritis. Henan Traditional Chinese Medicine. 2012;32:489–490. [Google Scholar]
- Li W.X., Zhang Q., Wang G.R., Lin X.L., Peng Y.T. Study on liver injury induced by different processing of prepared Radix Polygoni multiflori. Guangming Journal of Chinese Medicine. 2012;27:595–597. [Google Scholar]
- Li W.X., Zhang Q., Wang G.R., Lin X.L., Peng Y.T. Quality standard of Polygonum by different processing methods. China Medical Herald. 2012;9:41–47. [Google Scholar]
- Li W.X., Zhang Q., Wang G.R., Lin X.L., Peng Y.T. Study on the quality standard of high-pressure steamed radix polygoni multiflori at different time. Guide of China Medicine. 2012;10:396–397. [Google Scholar]
- Li X., Matsumoto K., Murakami Y., Tezuka Y., Wu Y., Kadota S. Neuroprotective effects of Polygonum multiflorumon nigrostriatal dopaminergic degeneration induced by paraquat and maneb in mice. Pharmacology, Biochemistry and Behavior. 2005;82:345–352. doi: 10.1016/j.pbb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Li X.B., Li Y., Chen J.Z., Sum J., Li X.F., Sun X., Kang X.G. Tetrahydroxystilbene glucoside attenuates MPP+-induced apoptosis in PC12 cells by inhibiting ROS generation and modulating JNK activation. Neuroscience Letters. 2010;483:1–5. doi: 10.1016/j.neulet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Li X.E., Liu J.Z., Liao S.T., Xu L.X., Wei X.Y. Chemical constituents from tubers of Polygonum multiflorum Thunb. Journal of Tropical and Subtropical Botany. 2006;17:617–620. [Google Scholar]
- Li Z., Hang Y.Y., Zhou Y.F. Germplasm appraisements of Plolygonum Multiflorum from main producing areas in China by content of tetrahydroxystilbene. Chemistry and Industry of Forest Products. 2003;23:37–41. [Google Scholar]
- Li Z.C., Jin Z.J., Cui S., Hong Y.J. Experimental study on the active situs of radix polygoni multiflori to reduce blood lipid and their dose-effect relation. China Practical Medicine. 2008;3:3–5. [Google Scholar]
- Liang Y., Tian W.X., Ma X.F. Chemical cnstituents of Caulis Polygoni Multiflori(the stem of Polygonum multiflorum Thunb.) Journal of Shenyang Pharmaceutical University. 2009;26:536–538. [Google Scholar]
- Lim K.M., Kwon J.H., Kim K., Noh J.Y., Kang S., Park J.M., Lee M.Y., Bae O.N., Chung J.H. Emodin inhibits tonic tension through suppressing PKCδ-mediated inhibition of myosin phosphatase in isolated rat thoracic aorta. British Journal of Pharmacology. 2014;171:4300–4310. doi: 10.1111/bph.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.C., Chang C.H., Yang J.J., Namba T., Hattori M. Hepatoprotective effects of emodin from Ventilago leiocarpa. Journal of Ethnopharmacoiogy. 1996;52:107–111. doi: 10.1016/0378-8741(96)01397-9. [DOI] [PubMed] [Google Scholar]
- Lin H.Q., Ho M.T., Lau L.S., Wong K.K., Shaw P.C., Wan D.C. Anti-acetylcholinesterase activities of traditional Chinese medicine for treating Alzheimer׳s disease. Chemico-Biological Interactions. 2008;175:352–354. doi: 10.1016/j.cbi.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Lin J.G., Chen G.W., Li T.M., Chouh S.T., Tan T.W., Chung J.G. Aloe-emodin induces apoptosis in T24 human bladder cancer cells through the p53 dependent apoptotic pathway. The Jouranl of Urology. 2006;175:343–347. doi: 10.1016/S0022-5347(05)00005-4. [DOI] [PubMed] [Google Scholar]
- Lin S.P., Chu P.M., Tsai S.Y., Wu M.H., Hou Y.C. Pharmacokinetics and tissue distribution of resveratrol, emodin and their metabolites after intake of Polygonum cuspidatum in rats. Journal of Ethnopharmacology. 2012;144:671–676. doi: 10.1016/j.jep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Liu Z.L., Li F.L., Song Z.Q., Lv A.P. Changes before and after processing of Radix Polygoni Multiflori HPLC fingerprints. Chinese Traditional and Herbal Drugs. 2005;36:1644–1646. [Google Scholar]
- Liu Z.L., Song Z.Q., Zhang L., Li S.L. Influence of process methods on contents of chemical component Radix Polygoni Multiflori. China Journal of Chinese Materia Medica. 2005;30:336–340. [PubMed] [Google Scholar]
- Liu C.J., Zhang Q.H., Lin J. Effect of the root of Polygonum multiflorum Thunb and its processed products on fat accumulation in the liver of mice. China Journal of Chinese Materia Medica. 1992;17:595–596. [PubMed] [Google Scholar]
- Liu J.B., Gao X.G., Lian T., Zhao A.Z., Li K.Z. Apoptosis of human hepatoma HepG2 cells induced by emodin in vitro. Chinese Journal of Cancer. 2003;22:1280–1283. [PubMed] [Google Scholar]
- Liu L., Li L., Zhao L., Zhang L., Li Y.L., Ye C.F. Effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside on learning and memory abilities of rats with chronic cerebral ischemia. Chinese Journal of Pharmacology and Toxicology. 2008;22:108–115. [Google Scholar]
- Liu M.H., Jing X.B., Cai X.B., Chen S.Z., Cai J.Y. The mechanism about the inhibition effects of emodin on the proliferation of EC-109 cell in esophageal cancer. Journal of Shantou University Medical College. 2009;22:12–14. [Google Scholar]
- Liu S.Q., Wang L., Yue l. Influence of high pressure processing on contents of active ingredients from Polygoni multiflori radix. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19:37–40. [Google Scholar]
- Liu W., Zheng Z.J., Liu X., Gao S., Ye L., Yang Z., Hu M., Liu Z.Q. Sensitive and robust UPLC-MS/MS method to determine the gender-dependent pharmacokinetics in rats of emodin and its glucuronide. Journal of Pharmaceutical and Biomedical Analysis. 2011;54:1157–1162. doi: 10.1016/j.jpba.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.J., Li L., Ye C.F., Wang Y.Q. The effects of tetrahydroxylstilbene on learning and memory ability and NMDA-receptor binding to [3H]MK801 in forebrain of ischemia-reperfusion gerbils. Chinese New Drugs Journal. 2004;13:223–226. [Google Scholar]
- Liu Z.L., Chao Z.M., Li L.F., Song Z.Q., Wang C., Lv S. HPLC-ELSD determination of the content change of monosaccharides and disaccharides in polygonum multiflorum during the processing. Chinese Journal of Experimental Traditional Medical Formulae. 2008;14:6–8. [Google Scholar]
- Liu Z.L., Li F.L., Song Z.Q., Zhang L. Quality Evaluation of Radix Polygoni Multiflori. Journal of Chinese Medicinal Materials. 2007;30:278–281. [PubMed] [Google Scholar]
- Liu Z.L., Li L.F., Chao Z.M., Lv S.Y., Wang C., Li L.F. Content analysis of the newly generated component in radix polygoni multiflori after processing. China Journal of Chinese Materia Medica. 2008;33:2326–2329. [PubMed] [Google Scholar]
- Liu Z.L., Li L.F., Chao Z.M., Song Z.Q., Wang C., Zhang L. Study on chemical constituents of radix polygoni multiflori after processing. Natural Product Research & Development. 2009;21:239–241. (248) [Google Scholar]
- Liu Z.L., Li L.F., Song Z.Q., Wang C., Zhang L., Chao Z.M. New chemical constituents from radix polygoni multiflori after processing. Journal of Chinese Medicinal Materials. 2007;30:1505–1507. [PubMed] [Google Scholar]
- Liu Z.L., Song Z.Q., Chao Z.M., Wang C., Lv S.Y., Wang C., Li L.F. HPLC analysis of the content change of gallic acid and catechin which antioxidant constituents in radix polygoni multiflori before and after processing. Chinese Traditional Patent Medicine. 2009;31:1392–1394. [Google Scholar]
- Liu Z.L., Song Z.Q., Zhang L., Li S.L. The impact of different processing methods on the component content of polygonum multiflorum. China Journal of Chinese Materia Medica. 2005;30:336–340. [PubMed] [Google Scholar]
- Long C.O., Dougherty J. What׳s new in Alzheimer׳s disease? Home Health Nurse. 2003;21:8–14. doi: 10.1097/00004045-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Lu X.L., Li J.S., Zhang T., Wu T. Identification of unknown unqualified polygonum mulitflorum system. Chinese Archives of Traditional Chinese Medicine. 2012;30:1087–1088. [Google Scholar]
- Luo W., Liu B., Wang W., Shi R.B. HPLC fingerprint chromatogram of Polygonum multiflorum Thunb. Journal of Beijing University of Traditional Chinese Medicine. 2008;31:557–560. [Google Scholar]
- Lv G.Y., Lou Z.H., Chen S.H., Gu H., Shan L.T. Pharmacokinetics and tissue distribution of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside from traditional Chinese medicine Polygonum multiflorum following oral administration to rats. Journal of Ethnopharmacology. 2011;137:449–456. doi: 10.1016/j.jep.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Lv J.S., Meng D.S., Xiang M.F., Feng Y.Y. Preliminary study on the Anti-inflammatory effect of Polygonum multiflorum. China Pharmacy. 2001;12:712–714. [Google Scholar]
- Lv L.H., Cheng Y.H., Zheng T.S., Li X.M., Zhai R. Purification, antioxidant activity and antiglycation of polysaccharides from Polygonum multiflorum Thunb. Carbohydrate Polymers. 2014;99:765–773. doi: 10.1016/j.carbpol.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Lv L.S., Gu X.H., Tang J., Ho C.T. Antioxidant activity of stilbene glycoside from Polygonum multiflorum Thunb in vivo. Food Chemistry. 2007;104:1678–1681. [Google Scholar]
- Ma J., Zheng L., Deng T., Cui L.L., He Y.S., Li H.J., Li P. Stilbene glucoside inhibits the glucuronidation of emodin in rats through the down-regulation of UDP-glucuronosyltransferases 1A8: application to a drug-drug interaction study in Radix Polygoni Multiflori. Journal of Ethnopharmacology. 2013;147:335–340. doi: 10.1016/j.jep.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Ma Y.M., Du H.Q. Effect of Shouwushen (SWS) on Ability of learning and memory in mice. Pharmacology and Clinics of Chinese Materia Medica. 2001;17:35–37. [Google Scholar]
- Ma Y.S., Weng S.W., Lin M.W., Lu C.C., Chiang J.H., Yang J.S., Lai K.C., Lin J.P., Tang N.Y., Lin J.G., Chung J.G. Antitumor effects of emodin on LS1034 human colon cancer cellsin vitro and in vivo: roles of apoptotic cell death and LS1034 tumor xenografts model. Food and Chemical Toxicology. 2012;50:1271–1278. doi: 10.1016/j.fct.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Masaldan S., Iyer V.V. Exploration of effects of emodin in selected cancer cell lines: enhanced growth inhibition by ascorbic acid and regulation of LRP1 and AR under hypoxia-like conditions. Journal of Applied Toxicology. 2014;34:95–104. doi: 10.1002/jat.2838. [DOI] [PubMed] [Google Scholar]
- Min H.J., Jung K.A., Kim H.J., Kim T.H., Jung W.T., Lee O.K. Twelve cases of toxic hepatitis related to the root of Polygonum multiflorum Thunb. Journal of Hepatology. 2008;48:S356. (Posters) [Google Scholar]
- National Toxiclogy Program. NTP technical report . NIH Publication; 2001. The Toxicology and Carcinogenesis Studies of Emodin in F344/N Rats and B6C3F1 Mice. (Report No. 01-3952) (PP1–278) [PubMed] [Google Scholar]
- Nie X.P., Liu Z.G. Study overview of the processing of polygonum multiflorum thumb. Shangdong Pharmaceutical Industry. 2002;21:24. [Google Scholar]
- Niu J.H. A severe liver damage case induced by Shou Wu Pian though oral administration. Chinese Journal of New Drugs and Clinical Remedies. 1996;15:382. [Google Scholar]
- Nonaka G.I., Miwa N., Nishioka I. Stilbene glycoside gallates and proanthocyanidins from Polygonum multiflorum. Phytochemistry. 1982;21:429–432. [Google Scholar]
- Obeso J.A., Rodriguez-Oroz M.C., Goetz C.G., Marin C., Kordower J.H., Rodriguez M., Hirsch E.C., Farrer M., Schapira A.H., Halliday G. Missing pieces in the Parkinson׳s disease puzzle. Nature Medicine. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Park G.J., Mann S.P., Ngu M.C. Acute hepatitis induced by Shou-Wu-Pian, a herbal product derived from Polygonum multiflorum. Journal of Gastroenterology and Hepatology. 2001;16:115–117. doi: 10.1046/j.1440-1746.2001.02309.x. [DOI] [PubMed] [Google Scholar]
- Pecere T., Gazzola M.V., Mucignat C., Parolin C., Vecchia F.D., Cavaggioni A., Basso G., Diaspro A., Salvato B., Carli M., Palu G. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Research. 2000;60:2800–2804. [PubMed] [Google Scholar]
- Qin F.H., Xie S.S., Zhang W.R., Long Z.Z., Liu F.J. Study on the immune function influence by Polygonum multiflorum in mice. Immunological Journal. 1990;6:252–254. [Google Scholar]
- Qin R., Li X.B., Li G., Tao L.Z., Li Y., Sun J., Kang X.G., Chen J.Z. Protection by tetrahydroxystilbene glucoside against neurotoxicity induced by MPP+: The involvement of PI3K/Akt pathway activation. Toxicology Letters. 2011;202:1–7. doi: 10.1016/j.toxlet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Qiu X.H., Huang Z.H. Content comparative analysis of water soluble saccharides in radix polygoni multiflori before and after its processing. China Pharmacy. 2006;17:954–956. [Google Scholar]
- Qiu X.H., Zeng W.H. On Processing Technique of Radix Polygoni Multiflori. China Pharmacy. 2006;17:1270–1273. [Google Scholar]
- Qiu X.H., Zhang J., Huang Z.H., Zhu D.Y., Xu W. Profiling of phenolic constituents in Polygonum multiflorum Thunb. by combination of ultra-high-pressure liquid chromatography with linear ion trap-Orbitrap mass spectrometry. Journal of Chromatography A. 2013;1292:121–131. doi: 10.1016/j.chroma.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Rao G.X., Xue Y.M., Hui T.T., Wang W.J., Zhang Q.L. Studies on the chemical constituents of the leaves of Polygonum multiflorum. Journal of Chinese Medicinal Materials. 2009;32:891–893. [PubMed] [Google Scholar]
- Shi G.B. Study on the influence of concoction over active ingredients in Polygonum multiflorum. Chinese Hospital Pharmacy Journal. 2003;23:95–97. [Google Scholar]
- Song S.J., Li F.F., Yue H., Yin Z.W. Study on the anti-aging effects of radix Polygonum multiflorum. Journal of Hebei Medical University. 2003;24:90–91. [Google Scholar]
- Steele M.L., Truong J., Govindaraghavan S., Ooi L., Sucher N.J., Münch G. Cytoprotective properties of traditional Chinese medicinal herbal extracts in hydrogen peroxide challenged human U373 astroglia cells. Neurochemistry International. 2013;62:522–529. doi: 10.1016/j.neuint.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Su Y., Wang Q.H., Wang C.F., Chan K., Sun Y.P., Kuang H.X. The treatment of Alzheimer׳s disease using Chinese Medicinal Plants: from disease models to potential clinical applications. Journal of Ethnopharmacology. 2014;152:403–423. doi: 10.1016/j.jep.2013.12.053. [DOI] [PubMed] [Google Scholar]
- Sun F.L., Zhang L., Zhang R.Y., Li L. Tetrahydroxystilbene glucoside protects human neuroblastoma SH-SY5Y cellsagainst MPP+-induced cytotoxicity. European Journal of Pharmacology. 2011;660:283–290. doi: 10.1016/j.ejphar.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Sun G.B., Guo B.J., Li X.E., Huang J.N., Xue H.B., Sun X.B. The effect of anthraquinone glycoside from Polygonum multiflorum Thunb on cellular immunological function in mice. Pharmacology and Clinics of Chinese Materia Medica. 2006;22:30–32. [Google Scholar]
- Sun J.H. Studies on the Pharmacokinetics and Metaboliteof Stilbene Glycoside. Heibei Medical University; ShijiaZhuang: 2004. [Google Scholar]
- Sun J.L., Huang X.L., Wu H.Q., Huang F. HPLC/IT-MS analysis of glycosides in Radix polygoni multiflori. Natural Product Research & Development. 2009;21:806–812. [Google Scholar]
- Sun Q.H., Bai Q. The differentiation on Polygonum multiflorum thumb and its forgery human-Like Polygonum multiflorum thunb (Musa basjoo Sieb. et Zucc) Journal of North Sichuan Medical College. 1996;11:54–56. [Google Scholar]
- Sun Q.H., Bai Q. The differentiation on polygonum mulitflorum thunb and its forgery. Journal of North Sichuan Medical College. 1996;11:44–46. [Google Scholar]
- Sun S.F. The processing method of Polygonum multiflorum Thunb. China Journal of Chinese Materia Medica. 1996;21:360. [Google Scholar]
- Sun X.H., Sun Y.W., Li H., Sun W. Influence of main component of Heshouwu such as emodin, rhein and toluylene glycoside onhepatic cells and hepatomacarcinoma cell. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2010;19:1315–1319. [Google Scholar]
- Sun Y.N., Cui L., Li W., Yan X.T., Yang S.Y., Kang J.I., Kang H.K., Kim Y.H. Promotion effect of constituents from the root of Polygonum multiflorumon on hair growth. Bioorganic & Medicinal Chemistry Letters. 2013;23:4801–4805. doi: 10.1016/j.bmcl.2013.06.098. [DOI] [PubMed] [Google Scholar]
- Tabolacci C., Lentini A., Mattioli P., Provenzano B., Oliverio S., Carlomosti F., Beninati S. Antitumor properties of aloe-emodin and induction of transglutaminase 2 activity in B16-F10 melanoma cells. Life Sciences. 2010;87:316–324. doi: 10.1016/j.lfs.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Tian Y.H., Zhang L.Y., Yang Y.Q., Li J., Li M., Yu Q.L. Optimization of processing technologies for steamed Polygonum multiflorum by colligation score. Journal of Guiyang College of Traditional Chinese Medicine. 2007;29:15–17. [Google Scholar]
- Toth P.P. Drug treatment of hyperlipidaemia: a guide to the rational use of lipid-lowering drugs. Drugs. 2010;70:1363–1379. doi: 10.2165/10898610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Um M.Y., Choi W.H., Aan J.Y., Kim S.R., Ha T.Y. Protective effect of Polygonum multiflorumThunb on amyloid β-peptide 25-35 induced cognitive deficits in mice. Journal of Ethnopharmacology. 2006;104:144–148. doi: 10.1016/j.jep.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Wang C.F., Wu X.D., Chen M., Duan W.G., Sun L.X., Yan M., Zhang L.Y. Emodin induces apoptosis through caspase 3-dependent pathway in HK-2 cells. Toxicology. 2007;231:120–128. doi: 10.1016/j.tox.2006.11.064. [DOI] [PubMed] [Google Scholar]
- Wang C.Y. Studies on Antihyperlipidemic Effects and Pharmacokinetics of Stilbene Glycoside from Radix Polygoni Multiflori. Hebei Medical University; Shijiazhuang: 2005. [Google Scholar]
- Wang C.Y., Gu J.M., Liu W.N., Yang W., Zhang L.T. Studies on pharmacokinetics and tissue distribution of stilbene glycoside in the hyperlipide miamodel rats. Chinese Journal of Pharmaceutical Analysis. 2009;29:1073–1078. [Google Scholar]
- Wang H.Y., Li S.X., Ma Y.Z. Clinical observation of tuber fleeceflower root injection in the treatment of hair loss. Journal of Traditional Chinese Medicine and Chinese Materia Medica of Jilin. 2001;1:35. [Google Scholar]
- Wang J.K., Gao Y.M., Chen H.L. The determination of stilbene glucoside in Fleece-flower root raw product and four kinds of processed products. Studies of Trace Elements and Health. 2004;21:27–28. [Google Scholar]
- Wang M.J., Zhao R.H., Wang W.G., Mao X.J., Yu J. Lipid regulation effects of Polygoni Multiflori Radix, its processed products and its major substances on steatosis human liver cell line L02. Journal of Ethnopharmacology. 2012;139:287–293. doi: 10.1016/j.jep.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Wang Q.X. Study on the Toxicity and its Mechanism Sofrhubarbandits Major Constituents. Academy of Military Medical Sciences; Beijing: 2007. [Google Scholar]
- Wang Q.X., Wu C.Q., Yang H.L., Jing S.F., Jin C., Xiao X.H., Liao M.Y. Cytotoxicity of free anthraquinone from Radix et Rhizoma Rhei to HK-2 Cells. Chinese Journal of New Drugs. 2007;16:189–199. [Google Scholar]
- Wang T., Gu J., Wu P.F., Wang F., Xiong Z., Yang Y.J., Wu W.N., Dong L.D., Chen J.G. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-κB pathways and inhibition of intracellular ROS/RNS generation. Free Radical Biology and Medicine. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Wang T., Yang Y.J., Wu P.F., Wang W., Hu Z.L., Long L.H., Xie N., Fu H., Wang F., Chen J.G. Tetrahydroxystilbene glucoside, a plant-derived cognitive enhancer, promotes hippocampal synaptic plasticity. European Journal of Pharmacology. 2011;650:206–214. doi: 10.1016/j.ejphar.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Wang W., He Y., Lin P., Li Y., Sun R., Gu W., Yu J., Zhao R. In vitro effects of active components of Polygonum Multiflorum Radix on enzymes involved in the lipid metabolism. Journal of Ethnopharmacology. 2014;153:763–770. doi: 10.1016/j.jep.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Wang W., Sun Y., Li X., Li H., Chen Y., Tian Y., Yi J., Wang J. Emodin potentiates the anticancer effect of cisplatin on gallbladder cancer cells through the generation of reactive oxygen species and the inhibition of survivin expression. Oncology Reports. 2011;26:1143–1148. doi: 10.3892/or.2011.1390. [DOI] [PubMed] [Google Scholar]
- Wang W.J., Zhang W.M., Dong X.L., Zhao R.H., Rao G.X. Studies on chemical constituents of Polygonum multiflorun from Yunnan. Journal of Yunnan College of Traditional Chinese Medicine. 2005;28:10–12. [Google Scholar]
- Wang X.L. The Study of Anti-aging Mechanism of Zishen Yigan Fang in vivo and vitro. Hubei University of Chinese Medicine; Wuhan: 2012. [Google Scholar]
- Wang X.M., Zhao L.B., Han T.Z., Chen S.F., Wang J.L. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. European Journal of Pharmacology. 2008;578:339–348. doi: 10.1016/j.ejphar.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Way T.D., Huang J.T., Chou C.H., Huang C.H., Yang M.H., Ho C.T. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the β-catenin and Akt pathways. European Journal of Cancer. 2014;50:366–378. doi: 10.1016/j.ejca.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Wu X.M. Effect of emodin on combined hyperlipidemia familial in rats. Anhui Medical and Pharmaceutical Journal. 2008;12:1026–1028. [Google Scholar]
- Wu X.Q., Chen X.Z., Huang Q.C., Fang D.M., Li G.Y., Zhang G.L. Toxicity of raw and processed roots of Polygonum multiflorum. Fitoterapia. 2012;83:469–475. doi: 10.1016/j.fitote.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Wu Y.W., Zhang D.K., Yu C.Y. Yiganning granule on liver injury animal model research of protecting liver and reducing enzyme and immune function. Journal of Hunan College of Traditional Chinese Medicine. 2001;21:14–15. [Google Scholar]
- Wu Z., Zhang M., Zhang C.F., Wang Z.T. Study on Quality Evaluation for 36 Samples of Radix Polygonui Multiflori by HPLC-Fingerprints. Chinese Pharmaceutical Journal. 2006;41:257–260. [Google Scholar]
- Xia C.L., Li L.X. Identification of radix polygoni multiflori and silvervine fleeceflower root. LiShiZhen Medicine and Materia Medica Research. 2003;14:540. [Google Scholar]
- Xiang J.H., Han P.K. Efficacy of Qishu decoction on 120 NSCLC patients with leucopenia after-Chemotherapy experienced prescription. Gansu Medical Journal. 2013;32:171–173. [Google Scholar]
- Xiang K., Liu G., Zhou Y.J., Hao H.Z., Yin Z., He A.D., Da X.W., Xiang J.Z., Wang J.L., Ming Z.Y. 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside (THSG) attenuates human platelet aggregation, secretion and spreading in vitro. Thrombosis Research. 2014;133:211–217. doi: 10.1016/j.thromres.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Xiao K., Xuan L.J., Xu Y.M., Bai D.L. Novel stilbene glycosides from Polygonum multiflorum. Acta Botanica Sinica. 2002;44:1491–1494. [Google Scholar]
- Xiao P.G., Xing S.T., Wang L.W. Immunological aspects of Chinese medicinal plants as anti-ageing drugs. Journal of Ethnopharmacology. 1993;38:167–175. doi: 10.1016/0378-8741(93)90012-t. [DOI] [PubMed] [Google Scholar]
- Xie W.D., Zhao Y.N., Du L.J. Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. Journal of Ethnopharmacology. 2012;140:345–367. doi: 10.1016/j.jep.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Xu D.J., Tao Y., Wang S.X., Cheng W.S., Zhong X.F., You B.H. Effect of high pressure processing onstilbene glycoside and phosphatidylcholine in Polygoni Multiflori Radix. Chinese Traditional and Herbal Drugs. 2011;42:78–80. [Google Scholar]
- Xu G.J. ed. People׳s Health Publishing House; Beijing, China: 1986. Microscopic identification of Chinese medicinal materials powder; pp. 102–103. (1986) [Google Scholar]
- Xu M.L., Zheng M.S., Lee Y.K., Moon D.C., Lee C.S., Woo M.H., Jeong B.S., Lee E.S., Jahng Y., Chang H.W., Lee S.H., Son J.K. A new stilbene glucoside from the roots of Polygonum multiflorum Thunb. Archives of Pharmacal Research. 2006;29:946–951. doi: 10.1007/BF02969276. [DOI] [PubMed] [Google Scholar]
- Xu X.L., Zhu Q.Y., Zhao C., Wang F., Zhou Z.Y., Hu Y.E., Zhang W. The effect of 2,3,4′,5-tetrahydroxystilbene-2-O-β-d-glucoside on pressure overload-induced cardiac remodeling in rats and its possible mechanism. Planta Medica. 2014;80:130–138. doi: 10.1055/s-0033-1360198. [DOI] [PubMed] [Google Scholar]
- Xu Y.L., Dong Q., Hu F.Z. Simultaneous quantitative determination of eight active components in Polygonum multiflorum thunb by RPHPLC. Journal of Chinese Pharmaceutical Sciences. 2009;18:358–361. [Google Scholar]
- Xu Z.H., Yi J.J. Mechanism of the protection of stilbene glycoside which is the effective component of tuber fleece flower root on nerve cells. Asia-Pacific Traditional Medicine. 2013;9:61–62. [Google Scholar]
- Xue Y.M., Ma S., Lang X.F., Zhang T., Zhao S.L., Zhao R.H., Rao G.X. Determination of Anthraquinone In Polygonum Multiflorum From Different Regions of Yunnan. Journal of Yunnan College of Traditional Chinese Medicine. 2004;27:42–45. [Google Scholar]
- Yamaguchi A., Hiroi T., Miyazaki M. Synthesis of some new phydroxyphenylpropane monomer. Mokuzai Gakkaishi. 1969;15:256–261. [Google Scholar]
- Yan S.L., Su Y.F., Chen L., Que M., Gao X.M., Chang J.B. Polygonumosides A-D, stilbene derivatives from processed roots of Polygonum multiflorum. Journal of Natural Products. 2014;77:397–401. doi: 10.1021/np400720y. [DOI] [PubMed] [Google Scholar]
- Yang J., Song J.F., Li P.Y. Study on the quality standard of Chinese traditional medicine processing excipient of black bean juice. China Association of Chinese Medicine – Processing Branch. 2008:281–283. [Google Scholar]
- Yang P.Y., Almofti M.R., Lu L., Kang H., Zhang J., Li T.J., Rui Y.C., Sun L.N., Chen W.S. Reduction of atherosclerosisin cholesterol-fed rabbits and decrease of expressions of intracellular adhesion molecule-1 and vascular endothelial growth factor in foam cells by a water-soluble fraction of Polygonum multiflorum. Journal of Pharmacological Sciences. 2005;99:294–300. doi: 10.1254/jphs.fp0050333. [DOI] [PubMed] [Google Scholar]
- Yang X.P., Liu T.Y., Qin X.Y., Yu L.C. Potential protection of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside against staurosporine-induced toxicity on cultured rat hippocampus neurons. Neuroscience Letters. 2014;576:79–83. doi: 10.1016/j.neulet.2014.05.045. [DOI] [PubMed] [Google Scholar]
- Yang X.W., Gu Z.M., Ma C.M., Hattori M., Namba T. A New indole derivative isolated from the root of tuber fleeceflower (Polygonum multiflorum) Chinese Traditional and Herbal Drugs. 1998;1:5–11. [Google Scholar]
- Yang Y.Z. The new ingredients of Polygonum multiflorum thunb: hydroxyl on stilbene glucoside. Foreign Medical Reference. SCI. 1976;3:247. [Google Scholar]
- Yang Z.X., Zhang G.C. 48 cases of Yiganning granules in treating chronic hepatitis B. Hunan Journal of Traditional Chinese Medicine. 1999;15:13. [Google Scholar]
- Yao W.J., Fan W.J., Huang C., Zhong H., Chen X.F., Zhang W. Proteomic analysis for anti-atherosclerotic effect of tetrahydroxystilbene glucoside in rats. Biomedicine & Pharmacotherapy. 2013;67:140–145. doi: 10.1016/j.biopha.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Ye Q.H. An acute toxic hepatopathy case induced by Radix Polygoni Multiflori. Chinese Journal of Integrative Medicine. 1996;16:732. [Google Scholar]
- Yi T., Leung K.S., Lu G.H., Zhang H., Chan K. Identification and determination of the major constituents in traditional Chinese medicinal plant Polygonum multiflorum thunb by HPLC coupled with PAD and ESI/MS. Phytochemical Analysis. 2007;18:181–187. doi: 10.1002/pca.963. [DOI] [PubMed] [Google Scholar]
- Yin X.R., Gong X., Jiang R., Kuang G., Wang B., Zhang L., Xu G., Wan J.Y. Emodin ameliorated lipopolysaccharide-induced fulminant hepatic failure by blockade of TLR4/MD2 complex expression in d-galactosamine-sensitized mice. International Immunopharmacology. 2014;23:66–72. doi: 10.1016/j.intimp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Yon J.M., Lin C., Oh K.W., Baek H.S., Lee B.J., Yun Y.W., Nam S.Y. Emodin prevents ethanol-induced developmental anomalies in cultured mouse fetus through multiple activities. Birth Defects Research B: Developmental and Reproductive Toxicology. 2013;98:268–275. doi: 10.1002/bdrb.21061. [DOI] [PubMed] [Google Scholar]
- Yoshizaki M., Fujino H., Arise A., Ohmura K., Arrisawa M., Morita N. Polygoacetophenoside, a new acetophenone glucoside from Polygonum multiflorum 1. Planta Medica. 1987;53:273–275. doi: 10.1055/s-2006-962703. [DOI] [PubMed] [Google Scholar]
- Yu G.Q. The processing history of Polygonum multiflorum. Journal of North Pharmacy. 2014;11:63–64. [Google Scholar]
- Yu J., Xie J., Mao X.J., Wang M.J., Li N., Wang J., Zhaori G.T., Zhao R.H. Hepatoxicity of major constituents and extractions of Radix Polygoni Multiflori and Radix Polygoni Multiflori Praeparata. Journal of Ethnopharmacology. 2011;137:1291–1299. doi: 10.1016/j.jep.2011.07.055. [DOI] [PubMed] [Google Scholar]
- Yu J.Q., Bao W., Lei J.C. Emodin regulates apoptotic pathway in human liver cancer cells. Phytotherapy Research. 2013;27:251–257. doi: 10.1002/ptr.4703. [DOI] [PubMed] [Google Scholar]
- Yu R.M., Zhou L.B., Yan C.Y., Duan G.Y., Zhao Y. Two new coumarin glucosides biosynthesized by transgenic hairy roots of Polygonum multiflorum. Chinese Chemical Letters. 2008;19:76–78. [Google Scholar]
- Yuan X.W., Deng Z.H., Ye L.X., Luo Y.C. Preparation and clinical curative effect observation of Zhentongdieda wine. Journal of Chinese Medicinal Materials. 2004;27:544–545. [Google Scholar]
- Zeng C., Xiao J.H., Chang M.J., Wang J.L. Beneficial effects of THSG on acetic acid-induced experimental colitis: involvement of upregulation of PPAR-γ and inhibition of the Nf-Κb inflammatory pathway. Molecules. 2011;16:8552–8568. doi: 10.3390/molecules16108552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R., Lv L.S., Jin B.Q. Hypolipidemic effect of the polysaccharide from polygonum multiflorum. Food and Machinery. 2010;26:87–101. [Google Scholar]
- Zhang C.M. The Study of Effects of Buyanghuanw Pill Jiajian Fang and Madopar on Parkinson׳s Disease. Shandong Traditional Chinese Medicine University; Jinan: 2008. [Google Scholar]
- Zhang H.Y., Shi X.G. Analysis of rDNA ITS sequences in root tuber of Polygonum multiflorum from various habitats. Chinese Traditional and Herbal Drugs. 2007;38:911–914. [Google Scholar]
- Zhang L., Li L., Li Y.L. Mechanism of the protection of stilbene glycoside which is the effective component of tuber fleece flower root on nerve cells. Chinese Journal of Clinical Rehabilitation. 2004;8:118–120. [Google Scholar]
- Zhang L., Lin L., Xing Y. Effects of tetrahydroxystilbene glucoside on mapks signaltransduction pathway in APP transgenic model of Alzheimer׳s disease. Alzheimers & Dementia. 2010;7:S668–S669. (Poster presentations) [Google Scholar]
- Zhang L., Ma W.F., Li J., He J., Zhang P., Zheng F., Zhang B.L., Gao X.M., Chang Y.X. Influence of processing on pharmacokinetic of typical constituents in radix polygoni multiflori after oral administration by LC-ESI-MS/MS. Journal of Ethnopharmacology. 2013;148:246–253. doi: 10.1016/j.jep.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Zhang L., Xing Y., Ye C.F., Ai H.X., Wei H.F., Li L. Learning-memory deficit with aging in APP transgenic mice of Alzheimer׳s disease and intervention by using tetrahydroxystilbene glucoside. Behavioural Brain Research. 2006;173:246–254. doi: 10.1016/j.bbr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Zhang L.L., Huang L.H., Chen L.W., Hao D.J., Chen J.Z. Neuroprotection by tetrahydroxystilbene glucoside in the MPTP mouse model of Parkinson׳s disease. Toxicology Letters. 2013;222:155–163. doi: 10.1016/j.toxlet.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Zhang T., Lv G.Y., Chen S.H., Yu X.J. Comparative study of pre and post processed Polygonum multiforum anthraquinones content. Journal of Zhejiang Chinese Medical University. 2009;33:872–873. [Google Scholar]
- Zhang Y.L., Yang Y.Q., Gao Y.M. Comparison the content of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside of wild and cultivated Radix Polygoni Multiflori from different areas of Guizhou. China Journal of Chinese Materia Medica. 2003;28:786–787. [Google Scholar]
- Zhang Y.Z., Shen J.F., Xu J.Y., Xiao J.H., Wang J.L. Inhibitory effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside on experimental inflammation and cyclooxygenase 2 activity. Journal of Asian Natural Products Research. 2007;9:355–363. doi: 10.1080/10286020600727772. [DOI] [PubMed] [Google Scholar]
- Zhang Z.G., Lv T.S., Yao Q.Q. Studies on the anthraquinone chemical constituents of radix Polygoni multiflori. Chinese Traditional and Herbal Drugs. 2006;37:1311–1313. [Google Scholar]
- Zhang Z.G., Lv T.S., Yao Q.Q. Studies on the non-anthraquinone chemical constituents of radix Polygoni multiflori. China Journal of Chinese Materia Mediea. 2006;31:1027–1029. [Google Scholar]
- Zhang Z.Y., Miao M.S., Gu L.Y. Study on the immune function influence by polysaccharide from Polygonum multiflorum Peparata in mice. Traditional Chinese Medicinal Research. 2008;21:18–19. [Google Scholar]
- Zhao G.Q., Li F.F., Li C.G., Zhang Y., Zeng Y., Meng X.L. Experimental studies on the anti-inflammatory and analgesic effects of Gou-pi plaster. Pharmacy and Clinics of Chinese Materia Medica. 2011;2:27–29. [Google Scholar]
- Zhao H.N., Chen L.L., Huang X.J., Wang Y., Li Y.L., Ye W.C. A new chromone glycoside from roots of Polygonum multiflorum. China Journal of Chinese Materia Medica. 2014;39:1441–1444. [PubMed] [Google Scholar]
- Zhao H.Y., Xu X.R., Chen Y.L., Chen H.N., Cheng J.Y., Liu Y. Study on the identification of radix polygoni multiflori and its adulterants of rodgersia aesculifolia. ShiZhen Journal of Traditional Chinese Medicine Research. 1998;9:147–148. [Google Scholar]
- Zhao Y.L., Zhou G.D., Yang H.B., Wang J.B., Shan L.M., Li R.S., Xiao X.H. Rhein protects against acetaminophen-induced hepatic and renal toxicity. Food and Chemical Toxicology. 2011;49:1705–1710. doi: 10.1016/j.fct.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Zheng C.J., Zhao S.J., Zhao Z.H., Ge J. Molecular identification of fallopia multiflora by PCR-RFLP based on rDNA ITS sequence. Journal of South China University of Technology. 2009;37:74–78. [Google Scholar]
- Zheng G.H. Study on the Radix Polygoni Multiflori Pieces Quality Standards and Fingerprint. Hubei University of Chinese Medicine; Wuhan: 2010. [Google Scholar]
- Zhou C.H. Effects of processing on the content of effective components in Radix Polygoni Multiflori. TCM Research. 2005;18:18–19. [Google Scholar]
- Zhou L.X., Lin M., Li J.B., Li S.Z. Chemical studies on the ethyl acetate insoluble fraction of the roots of Polygonum mutifloum thunb. Acta Pharmaceutica Sinica. 1994;29:107–110. [Google Scholar]
- Zhou R.H. Science of Chinese Medicinal Material Resources. Chinese Medical Science and Technology Press; Beijing: 1993. pp. 265–269. [Google Scholar]
- Zhou W.Q. 82 cases of Sanshen pill in treating male erectile dysfunction. Chinese Medicine Modern Distance Education of China. 2010;8:176. [Google Scholar]
- Zhou X.X., Yang Q., Xie Y.H., Sun J.Y., Qiu P.C., Cao W., Wang S.W. Protective effect of tetrahydroxystilbene glucoside against d-galactose induced aging process in mice. Phytochemistry Letters. 2013;6:372–378. [Google Scholar]
- Zhou Y., Luo C.J., Deng Z.J. The processing history research of Radix Polygoni multiflori. China Medical Herald. 2010;7:9–10. [Google Scholar]
- Zhu Q.Z., Chen W., Zhang L.S. Evaluation of in vitro genotoxicity of emodinanrhein. Carcinogenesis,Teratogenesis, and Mutagenesis. 2011;23:65–67. [Google Scholar]
- Zhu S.M. Clinical observation of treating pregnancy constipation with Polygonum multiflorum granule therapy in 52 cases. Zhejiang Journal of Traditional Chinese Medicine. 2011;46:129–130. [Google Scholar]
- Zuo Y.G., Wang C.J., Lin Y.J., Guo J.W., Deng Y.W. Simultaneous determination of anthraquinones in radix Polygoni multiflori by capillary gas chromatography coupled with flame ionization and mass spectrometric detection. Journal of Chromatography A. 2008;1200:43–48. doi: 10.1016/j.chroma.2008.01.058. [DOI] [PubMed] [Google Scholar]