Abstract
Background and objective
Chronic obstructive pulmonary disease (COPD) is a chronic progressive lung disease. On the other hand, viral infections of the airway are associated with the acute exacerbations of COPD. A systematic review and meta-analysis were performed to determine the prevalence rate of viral infections in acute exacerbations of COPD patients.
Methods
PubMed database was systematically searched for population-based prevalence studies (1930–2017). Fixed and random effects models were used for estimation of summary effect-sizes. Between-study heterogeneity and publication bias were also calculated. “Viral infections” and “COPD patients with exacerbations” were the two critical inclusion criteria.
Results
Twenty-eight studies were selected out of 26078 articles for the present review. The overall estimation of the prevalence of viral infection was 0.374 (95% C.I: 0.359–0.388). Also, the evident heterogeneity of viral infection was observed among the studies (Cochran Q test, p value < 0.001 and I-squared = 97.5%). The highest and lowest prevalence rate was related to rhinovirus and echovirus, respectively. Also, the results of this study showed that the prevalence of viral infection in exacerbated COPD patients has fluctuation during the years with a slight increase and decrease.
Conclusions
The results of this systematic review demonstrated that respiratory viral infections have an important role in the acute exacerbation of COPD (AECOPD). In addition, determining the exact geographic epidemiology of these viruses is very important to manage the treatment of these infections.
Keywords: COPD, Viral infection, Respiratory virus, Exacerbation, Meta-analysis
Highlights
-
•
Rhinovirus and Echovirus were the most and least common viruses.
-
•
The prevalence of viral infection had a fluctuation by the year of publication.
-
•
PCR resulted in the highest rate of viral detection.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic progressive lung disease, which is the fourth leading cause of death worldwide [1], [2]. About 174.5 million (2.4%) of people worldwide (2015) suffer from COPD [3]. Cough, sputum production, and shortness of breath are the most common symptoms of COPD [1]. COPD Exacerbation is the most complicated status of COPD diagnosed by a sudden worsening of COPD symptoms, including shortness of breath, quantity, and color of phlegm [4]. As many studies imply, COPD acute exacerbations are a multifactorial consequence mainly caused by respiratory infections [5]. Smoking, air pollution, genetics, and viral infections are most important risk factors for the disease [1], [6]. Almost all people experience airway viral infections during their life in which most cases are improved without developing the chronic respiratory disease [6]. Several studies confirmed the association between airway inflammation and the tissue remodeling and destruction [7]. Airway inflammation has a central role in the pathogenesis of COPD, and on the other hand, persistent infections lead to chronic inflammation [7]. In COPD patients, exacerbations are mainly due to frequent infections. According to the role of viruses for inflammation and involvement of inflammation in COPD pathogenesis, studying the presence of viral infections in the airway of high-risk persons is important. The most human respiratory viruses associated with exacerbations of COPD are divided into two categories [1]: Major viruses: human rhinovirus, influenza virus and respiratory syncytial virus (RSV) [2], Minor viruses: parainfluenza virus, coronavirus, human metapneumovirus and adenovirus [8]. The quick and accurate detection of viral infection can be very important in preventing these problems. For this purpose, different techniques were developed, including serology, molecular methods such as PCR and RT-PCR [2]. Respiratory viruses that cause AECOPD are reported in several studies to help with the management of AECOPD patients. We aimed to systematically review the literature and perform a meta-analysis to determine the frequency of viral infection among COPD patients and to evaluate the hypothesis that the viral infection prevalence rate is associated with COPD exacerbation.
2. Methodology
2.1. Search strategy
A systematic search was conducted using PubMed to identify available articles (until March 2017). According to MeSH terms, searches were done by using the following keywords: “chronic obstructive pulmonary disease (COPD)”, “Exacerbation”, “infection”, “microbe”, “bacteria”, “viral” and “colonization” alone or combined together with the Boolean operators “OR”, “AND” and “NOT” in the Keywords/Title/Abstract field. Also, the reference list of selected full-text papers was precisely searched manually to find additional citations not retrieved by the web searching. It should be noted no attempt was made to consider unpublished studies. Furthermore, gray literature, dissertations, and relevant proceedings of international congresses were not explored. Finally, we restricted our search to the original articles or abstracts published which reported the prevalence of viral infection in COPD patients. The literature search was conducted by two independent researchers in two stages. Disagreements among researchers were resolved by discussion or, if necessary, by a third researcher. Journals and authors were not blinded during study selection.
2.2. Inclusion and exclusion criteria
A protocol for inclusion and exclusion criteria was defined for eligible peer-reviewed publications according to the following criteria: 1) PubMed articles published up to 2017; 2) The articles in English language reporting the prevalence of viral infection among COPD patients with exacerbations; 3) All Studies included samples from sputum, nasopharyngeal swab, and nasal lavage; 4) The reported data related to a group of individuals taken from the general population; and 5) Studies that used PCR, Real-Time PCR, RT-PCR, and culture methods. Major exclusion criteria were listed as follows: 1) Studies with unknown sample origins; 2) Studies that failed to present data clearly; 3) Studies were conducted on animal models; 4) Researches that have viral and bacterial co-infections; 5) Studies with overlapping subjects, time, and place of sample collection; 6) Congress abstracts, review articles, case report articles and studies reported in languages other than English, meta-analysis or systematic reviews, and duplicate publication of the same study.
2.3. Quality assessment and data extraction
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines were used to assess the quality of the included studies. The PRISMA Statement consists of a 27-item checklist and a four-phase flow diagram [9]. A complete information list was extracted from the articles into a Microsoft Excel worksheet. These data were including the first author's name, publication date, sample size, the prevalence of viral infection, detection method, research location, and references. Extracted data were qualified by two other researchers, independently. Furthermore, unclear data were consulted and achieved consensus before recording an entry in the dataset. Cohen's kappa as the agreement coefficient between the researchers was acceptable and was equal to 0.85.
2.4. Statistical methods
Pooled relative frequency (RF) and its corresponding 95% CI was used to evaluate the prevalence of viral infection in COPD disease. The inverse of the Freeman-Tukey Double arcsine transformation of relative frequencies to calculate a pooled RF [10]. The heterogeneity and the variation in the pooled estimations were assessed by using Cochran's Q test and I2, respectively, and significance was considered at P < 0.05 level [11]. The pooled RF was made in a random effect model while heterogeneity existed between the individual studies and otherwise this pooled effect sizes were derived from a fixed effect model. However, sensitivity analysis was done by successively removing a particular study or group of studies (if any) that had the highest impact on the heterogeneity test. A funnel plot was established for checking the existence of publication bias. The funnel plot asymmetry was measured by Egger's linear regression test and Begg's test (P < 0.05 levels were considered statistically significance for publication bias) [12]. Finally, the sub-group analysis was used on the detection method, kind of sample, geographic continents, year of publication, and type of virus. All statistical analyses were conducted by using data analysis and statistical software (STATA) (version 11.0; Stata Corporation, College Station, TX).
3. Results
3.1. Search results
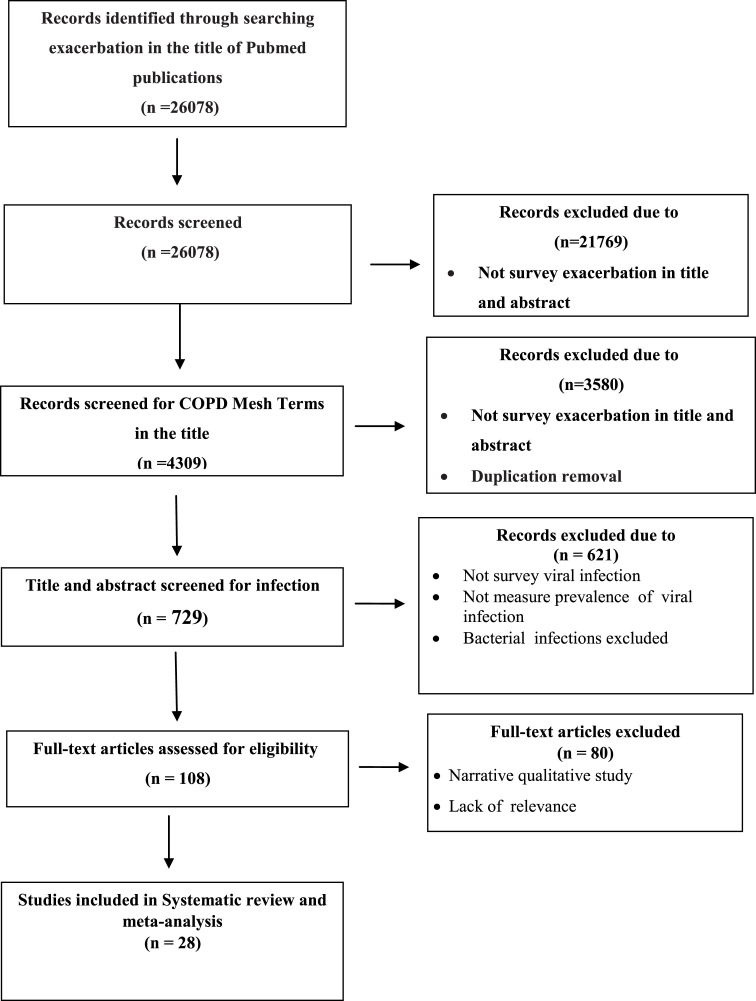
A total of 26078 articles were retrieved from PubMed. In a primary screening process, 21769 of the publications were excluded according to COPD Mesh terms in the title or abstract (COPD, chronic obstructive pulmonary disease). The retained 3580 publications were screened according to “Infection” Mesh terms, including “Infect*” OR “Microb*” OR “Virus” OR “Viral” OR “Bacteri*” OR “Probiotic” OR “Influenza” OR “Colonization” in the title and abstract which resulted in 724 publications. Then, all 724 publications were manually assessed for viral infections in the title and abstract, resulting in 108 papers. After eligibility evaluation, finally, 23 papers were retained for full-text evaluation. The study selection process and flowchart of the literature search is shown in Fig. 1 .
Fig. 1.
Flowchart of literature search and study selection.
3.2. Study characteristics
Eleven of the studies had been published before 2010 and the others were 2010 and later. The most and the lowest studies were conducted on the European continent (18/28; 64%), and in England and the America continent (1/23; 3.5%), respectively. In addition, 9 studies were conducted in Asia (9/28 (32%)). Only in one study was used nasal lavage and oropharyngeal swab samples, 6 and 17 studies were used nasopharyngeal swab and sputum samples respectively. The laboratory techniques used in the studies were PCR, Real-Time PCR, RT-PCR, and culture, which were used in 10, 12, 5, and 2 studies, respectively. More information was presented in Table 1 .
Table 1.
Characteristics of studies included in the systematic review and meta-analysis.
| First Author | Year of Study | City/Country/Province | Total of sample size | Number of cases | Technique | Most common virus |
Ref |
|---|---|---|---|---|---|---|---|
| Raquel Almansa | 2012 | Valladolid/Spain/Europe | 57 | 20 | RT-PCR | influenza A/H1N1 Virus | [13] |
| Ramon Boixeda | 2012 | Barcelona/Spain/Europe | 132 | 14 | RT-PCR | RSV | [5] |
| Seemunga | 2000 | London/UK/Europe | 43 | 29 | PCR | Rhinovirus | [14] |
| M Roland | 2000 | London/UK/Europe | 22 | 10 | RT-PCR | Rhinovirus | [15] |
| Terense Seemubgal | 2001 | London/UK/Europe | 168 | 53 | PCR, Culture | Rhinovirus | [16] |
| G. Rohde | 2002 | Bochum/Germany/Europe | 85 | 48 | RT-PCR | Picornavirus | [17] |
| Jadwiga A. Wedzicha | 2003 | London/United Kingdom/Europe | 87 | 24 | PCR | Rhinovirus | [4] |
| M.E. Hamelin | 2005 | Quebec/Canada/America | 64 | 15 | RT-PCR | RSV | [18] |
| Tom M. A. Wilkinson | 2005 | London/UK/Europe | 56 | 18 | PCR | Rhinovirus | [19] |
| Anastasia F. Hutchinson | 2007 | Victoria 3050/Australia/Asia | 148 | 33 | PCR | Rhinovirus | [20] |
| T.E. McManus | 2007 | Belfast/UK/Europe | 136 | 65 | Real-time PCR, PCR | EBV | [21] |
| Felix C Ringshausen | 2009 | Bochum/Germany/Europe | 123 | 9 | PCR | … | [22] |
| Omar Kherad | 2010 | Geneva/Switzerland/Europe | 86 | 44 | RT-PCR | Picornavirus | [23] |
| Jennifer K. Quint | 2009 | London/England/Europe | 72 | 68 | Real-time PCR | Rhinovirus | [24] |
| Jeanne-Marie Perotin | 2013 | Reims/France/Europe | 45 | 24 | PCR | Rhinovirus | [25] |
| Lucas Boeck | 2014 | Basel/Switzerland/Europe | 208 | 86 | Serologic method | Adenovirus | [26] |
| Sîobhán N. George | 2014 | London/UK/Europe | 107 | 64 | Real-time PCR | Rhinovirus | [27] |
| Tristan W. Clark | 2014 | Cambridge/UK/Europe | 264 | 100 | Real-time PCR, RT-PCR | influenza A, B Virus | [28] |
| Meng-Yuan Dai | 2013 | Anhui/China/Asia | 81 | 58 | PCR | influenza virus | [29] |
| G. Dimopoulos | 2012 | Athens/Greece/Europe | 247 | 133 | PCR | RSV | [30] |
| Seyedeh Somayeh Hosseini | 2015 | Tehran/Iran/Asia | 170 | 81 | PCR | Influenza A virus | [31] |
| Nurdan Kokturk | 2015 | Ankara/Turkey/Asia | 27 | 20 | PCR | RSV | [32] |
| Kenichiro Shimizu | 2015 | Tokyo/Japan/Asia | 50 | 17 | Real-time PCR | Influenza A virus | [33] |
| E. Biancardi | 2016 | Sydney/Australia/Asia | 153 | 59 | PCR | Influenza A Rhinovirus RSV A/B |
[34] |
| Tiping Yin | 2017 | Shanghai/China/Asia | 264 | 72 | RT-PCR | Influenza A | [35] |
| Miguel Gallego1 | 2016 | Galdakao/Spain/Europe | 380 | 96 | RT-PCR | Rhinovirus | [36] |
| Hyun Jung Kwak | 2017 | Bejjing/China/Asia | 213 | 62 | PCR | Rhinovirus | [37] |
| Parvaiz A Koul | 2017 | Maharashtra/India/Asia | 233 | 46 | Real-Time PCR | Influenza A/H3N2 rhinoviruse | [38] |
3.3. Overall prevalence
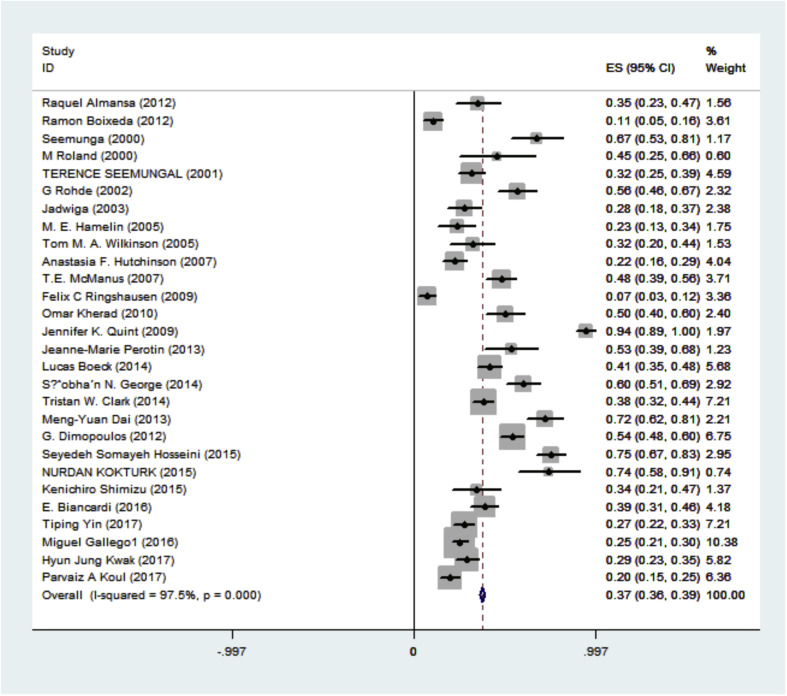
It was shown that the pooled estimation of the prevalence of viral infections in COPD patients was 0.374 (95% C.I: 0.359–0.388). Among all patients, 1368 cases (36.7%) were viral infections positive. Also, the heterogeneity in estimating the pooled prevalence among the studies was statistically significant; Cochran Q test, P < 0.001, I2 = 97.7% (Fig. 2 & Table 2 ).
Fig. 2.
Forest Plot indicates an estimation for the prevalence of viral infection in the COPD patients.
Table 2.
Subgroing Analysis for year of publication, detection methods, kind of sample, geographic continent, and type of virus.
| Characteristics | Categories | No. of Studies | Pooled Prevalence (95% C.I) |
Heterogeneity test () |
Model |
|---|---|---|---|---|---|
| All Studies | – | 28 | 0.374 (0.359 0.388) | (97.5%; P < 0.001) | Random |
| Year of Publication | <2010 | 11 | 0.371 (0.344, 0.397) | (98.5%; P < 0.001) | Random |
| ≥2010 | 17 | 0.375 (0.358, 0.392) | (96%; P < 0.001) | Random | |
| Detection Method | PCR | 10 | 0.397 (0.374, 0.421) | (97.1%; P < 0.001) | Random |
| Real-Time PCR | 12 | 0.316 (0.294, 0.338) | (98.6%; P < 0.001) | Random | |
| RT-PCR | 5 | 0.279 (0.252, 0.306) | (90%; P < 0.001) | Random | |
| Culture | 2 | 0.215 (0.161, 0.269) | (97.1%; P < 0.001) | Random | |
| Kind of Sample | Sputum sample | 17 | 0.378 (0.359, 0.397) | (97%; P < 0.001) | Random |
| Nasopharyngeal swab | 6 | 0.258 (0.232, 0.284) | (90.2%; P < 0.001) | Random | |
| Nasal lavage | 1 | 0.565 (0.459, 0.670) | NA | NA | |
| Oropharyngeal swab | 1 | 0.197 (0.0146, 0.249) | NA | NA | |
| Geographic continent | Asia | 9 | 0.351 (0.327, 0.375) | (96.5%; P < 0.001) | Random |
| Europe | 18 | 0.390 (0.372, 0.409) | (98.0%; P < 0.001) | Random | |
| America | 1 | 0.234 (0.131, 0.338) | NA | NA | |
| Type of virus | Rhinovirus | 18 | 0.320 (0.300, 0.340) | (96.5%; P < 0.001) | Random |
| Metapneumovirus | 11 | 0.229 (0.207, 0.252) | (88.9%; P < 0.001) | Random | |
| RSV | 14 | 0.247 (0.222, 0.271) | (94.3%; P < 0.001) | Random | |
| RSV A | 4 | 0.272 (0.234, 0.309) | (93.7%; P < 0.001) | Random | |
| RSV B | 3 | 0.267 (0.215, 0.318) | (96.5%; P < 0.001) | Random | |
| Influenza A | 16 | 0.196 (0.175, 0.216) | (98%; P < 0.001) | Random | |
| Influenza B | 11 | 0.191 (0.169, 0.214) | (95.4%; P < 0.001) | Random | |
| Influenza C | 2 | 0.051 (0.022, 0.081) | (66.2%; P = 0.085) | Fixed | |
| Influenza virus | 3 | 0.243 (0.211 0.275) | (70.6%; P = 0.033) | Random | |
| parainfluenza viruses | 9 | 0.241 (0.217, 0.266) | (96.9%; P < 0.001) | Random | |
| Parainfluenza 3 virus | 8 | 0.180 (0.150, 0.210) | (95.6%; P < 0.001) | Random | |
| Parainfluenza 1 virus | 5 | 0.208 (0.168, 0.247) | (96.2%; P < 0.001) | Random | |
| Parainfluenza 2 virus | 3 | 0.262 (0.211, 0.314) | (97.7%; P < 0.001) | Random | |
| Coronavirus | 10 | 0.238 (0.216, 0.260) | (93.7%; P < 0.001) | Random | |
| Adenovirus | 12 | 0.218 (0.199, 0.238) | (98.4%; P < 0.001) | Random | |
| Echovirus | 1 | 0.008 (0.000, 0.022) | NA | NA | |
| Bocavirus | 4 | 0.20 (0.169, 0.230) | (95.9%; P < 0.001) | Random | |
| Picornavirus | 2 | 0.467 (0.366, 0.569) | (0.0%; P = 0.548) | Fixed | |
| Enterovirus | 1 | 0.030 (0.001, 0.063) | NA | NA | |
| WU polyomavirus | 1 | 0.125 (0.000, 0.354) | NA | NA |
3.4. Subgroup analysis
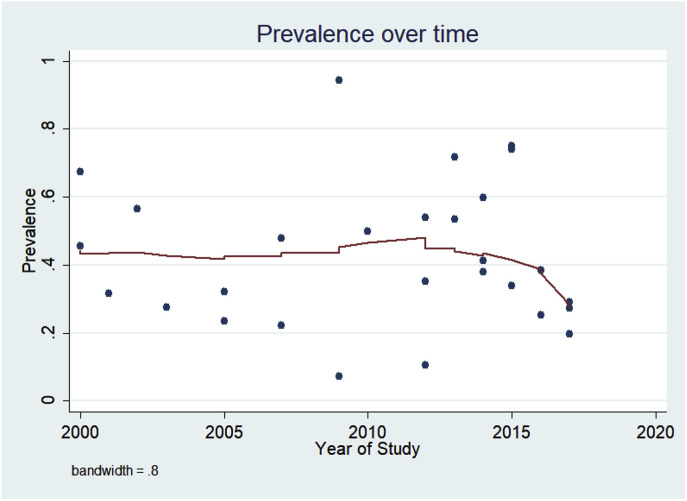
According to the sub-group analysis, the highest prevalence of viral infections in COPD patients was related to rhinovirus in the first sub-group (0.320; 95% C.I: 0.300 to 0.340), studies that published after 2010 in the second sub-group (0.375; 95% C.I: 0.358 to 0.392), studies that used PCR for detection in the third sub-group (0.397; 95% C.I: 0.374 to 0.421), studies that used the nasal lavage in the next sub-group (0.565; 95% C.I: 0.459, 0.670), and studies that were conducted in Europe in the last sub-group (0.390; 95% C.I: 0.372 to 0.409). The lowest prevalence of viral infection was related to Echovirus (0.008; 95% C.I: 0.000 to 0.022), and Enterovirus (0.030; 95% C.I: 0.001 to 0.063), respectively (Table 2). According to Lowess smoothing analysis, the prevalence of viral infection in exacerbated COPD patients has a fluctuation during the years with a non-significant slight increase and decrease. (Fig. 3 ).
Fig. 3.
Prevalence of viral infection in COPD patients over time. As indicated here, there is a fluctuation prevalence over the time.
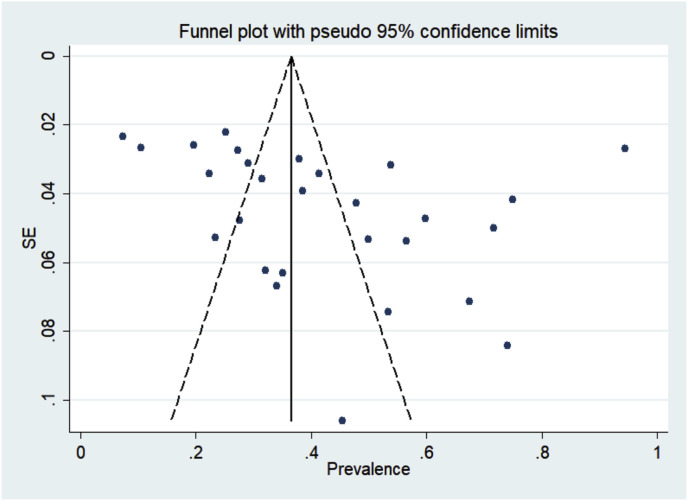
3.5. Publication bias and sensitivity analysis
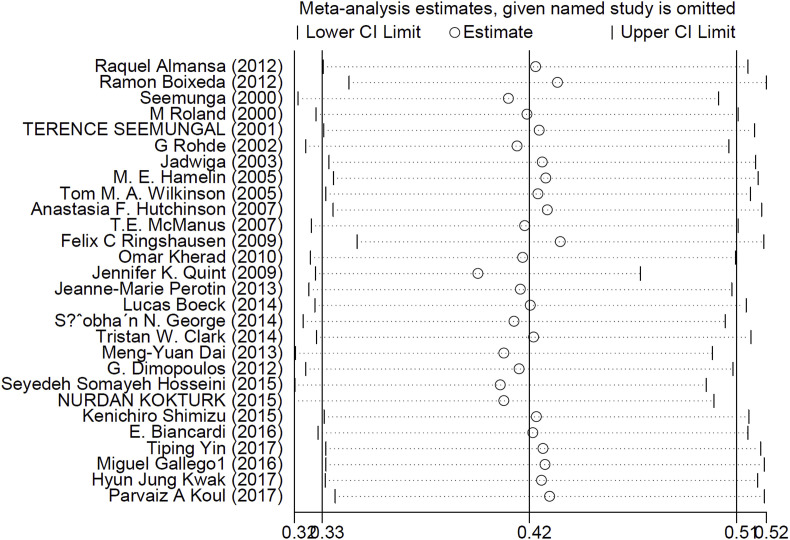
Based on Egger's regression test, in most of the cases, the publication bias was not statistically significant. In addition, no publication bias was detected according to the Begg's adjusted rank correlation test (Table 2 & Fig. 4 ). Sensitivity analysis was performed by sequential omission of individual studies. The pooled prevalence from sequential omission was not significantly changed (0.420; 95% C.I: 0.320 to 0.510), indicating that the results were statistically robust (Fig. 5 ).
Fig. 4.
Publication Bias assessment plot (Funnel plot) indicating the no publication bias according to the Begg's adjusted rank correlation test.
Fig. 5.
Influence plot for sensitivity analysis.
4. Discussion
This meta-analysis is performed to estimate the geographical distribution of viral infections in COPD patients worldwide. According to the sensitivity analysis, the results of this meta-analysis is robust against any included study. But, the funnel plot indicated non-significant right bias, which assumes in general a symmetry assumption. Previous studies demonstrated that during the exacerbation of COPD, some viruses are frequently detected and the immune system responses to these viruses may be involved in the severity of exacerbations (for example, increased levels of IL-8 due to rhinovirus infection) [8]. Another effective factor is the increased oxidant stress response to viral infections in COPD exacerbation [4]. During exacerbations, levels of IL-6 and plasma fibrinogen increase significantly, suggesting that respiratory viral infections increase systemic inflammation and thus increase the frequency of AECOPD [4]. In most recent studies, PCR based methods have been used extensively for detection, which resulted in the increased sensitivity of viral detection 2 to 3 times more than other methods [2], [39]. In addition, in our meta-analysis study, PCR based methods were the most common detection methods. It has been confirmed in many studies that respiratory viruses are involved in 30% of AECOPD patients [39]. Through more sensitive molecular methods, the role of viruses in the COPD pathogenesis is better understandable [40]. Seemungal's group (The United Kingdom, 2001) demonstrated that 40% of AECOPD were associated with respiratory viral infections [16]. In AECOPD patients, the etiology of respiratory infections depends vastly on the specific geographical area [2]. Wilkinson et al. (The United Kingdom, 2006) reported the prevalence of RSV in 32.8% of sputum samples. Also, they demonstrated that the persistent infection of RSV in COPD patients was associated with airway inflammation [41]. In another investigation, Almansa et al. (Spain, 2011) detected some viral infections, including 1, 3, 1, 6, and 1 cases of rhinovirus (1 case), RSV (3 cases), metapneumovirus (1 case), influenza A/H1N1 ns (6 cases), and influenza B (1 case) in COPD patients. In addition, they demonstrated that COPD patients with viral infections had higher inflammatory cytokine levels compared to COPD patients with negative viral infections and healthy controls. They showed the direct association between viral infections and COPD exacerbations [13]. Among all the viruses found in COPD patients, the rhinovirus is the most common virus (58%), maybe due to the fact that this virus is the major cause of common cold and also is widespread in the community [16]. Rohde et al. (Germany, 2003) performed a case-control study on AECOPD patients and detected 56% viral infections in the AECOPD group compared to 19% in controls, suggesting a possible association between respiratory viral infections and COPD exacerbations. Also, they demonstrated a higher rate of viral detection in the sputum sample than nasal lavage [17]. In another study, Hutchinson et al. (Australia, 2007) demonstrated that some viruses such as parainfluenza, influenza, and picornavirus are strongly associated with the development of AECOPD [20]. Previous studies showed that picornavirus was the most common virus associated with AECOPD, followed by influenza and RSV [2]. In our study, the overall prevalence of viral infections in COPD patients was 42.7%, the most detected viruses were rhinovirus, influenza A, and RSV, and the last ones were echovirus, enterovirus, and WU polyoma virus. The difference in the prevalence of viral infections in these patients could have many reasons such as geographical region, sample type, sample size, sample time, sample transport system, and type of the technique). The current study demonstrated that the prevalence of viral infection in COPD patients was slightly increased by the year of publication and then was again decreased. The possible reasons may be increased individual and social health levels, vaccination for some viruses, prophylaxis of patients, etc. Before developing the laboratory molecular detection methods, the role of viral infections in COPD patients was underestimated, but after the development of sensitive and specific molecular methods, it became clear. In the first line of defense in AECOPD patients, they are treated with antibiotics based on two factors, including the severity of the COPD and the severity of exacerbation [42]. It is obvious that overused or unnecessary antibiotic therapies for treatment of viral infections may simultaneously lead to the advent of multidrug resistant bacteria that are not the real causal agent of the current infection. Therefore, applying more sensitive and rapid methods for a definitive diagnosis of the real cause of the disease can help to make a precise and more exact treatment of AECOPD patients with viral infections and avoid the unnecessary use of antibiotics. On the other hand, these rapid methods can reduce patients' costs and prevent antibiotic resistance to bacteria. In addition, according to the association between COPD exacerbations and respiratory viral infections, more effective control, prevention, and therapy for these viral infections are needed.
Conflict of interest
None to declare.
Contributor Information
Sadegh Azimzadeh Jamalkandi, Email: azimzadeh.jam.sadegh@gmail.com, azimzadeh@nigeb.ac.ir.
Ali Ahmadi, Email: ahmadi1919@gmail.com.
References
- 1.Vestbo J., Hurd S.S., Agustí A.G., Jones P.W., Vogelmeier C., Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Mohan A., Chandra S., Agarwal D., Guleria R., Broor S., Gaur B. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15(3):536–542. doi: 10.1111/j.1440-1843.2010.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay S.I., Jayaraman S.P., Truelsen T., Sorensen R.J., Millear A., Giussani G. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015 (vol. 388, pg 1545, 2016) Lancet. 2017;389(10064):E1. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedzicha J.A. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2004;1(2):115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 5.Boixeda R., Rabella N., Sauca G., Delgado M., Martínez-Costa X., Mauri M. Microbiological study of patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) and the usefulness of analytical and clinical parameters in its identification (VIRAE study) Int. J. chronic Obstr. Pulm. Dis. 2012;7:327. doi: 10.2147/COPD.S30568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto K., Inoue H. Viral infections in asthma and COPD. Respir. Investig. 2014;52(2):92–100. doi: 10.1016/j.resinv.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 7.O’donnell R., Breen D., Wilson S., Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61(5):448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurai D., Saraya T., Ishii H., Takizawa H. Virus-induced exacerbations in asthma and COPD. Front. Microbiol. 2013:4. doi: 10.3389/fmicb.2013.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller J.J. The inverse of the Freeman–Tukey double arcsine transformation. Am. Statistician. 1978;32(4):138. [Google Scholar]
- 11.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. methods. 2006;11(2):193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 12.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almansa R., Socias L., Andaluz-Ojeda D., Martín-Loeches I., Bobillo F., Blanco J. Viral infection is associated with an increased proinflammatory response in chronic obstructive pulmonary disease. Viral Immunol. 2012;25(4):249–253. doi: 10.1089/vim.2011.0095. [DOI] [PubMed] [Google Scholar]
- 14.Seemungal T., Harper-Owen R., Bhowmik A., Jeffries D., Wedzicha J. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 2000;16(4):677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roland M., Bhowmik A., Sapsford R., Seemungal T., Jeffries D., Warner T. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax. 2001;56(1):30–35. doi: 10.1136/thorax.56.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seemungal T., Harper-Owen R., Bhowmik A., Moric I., Sanderson G., Message S. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 17.Rohde G., Wiethege A., Borg I., Kauth M., Bauer T., Gillissen A. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamelin M., Côtù S., Laforge J., Lampron N., Bourbeau J., Weiss K. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin. Infect. Dis. 2005;41(4):498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson T.M., Hurst J.R., Perera W.R., Wilks M., Donaldson G.C., Wedzicha J.A. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest J. 2006;129(2):317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson A.F., Ghimire A.K., Thompson M.A., Black J.F., Brand C.A., Lowe A.J. A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir. Med. 2007;101(12):2472–2481. doi: 10.1016/j.rmed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 21.McManus T.E., Marley A.-M., Baxter N., Christie S.N., Elborn J.S., O'Neill H.J. High levels of epstein–barr virus in COPD. Eur. Respir. J. 2008;31(6):1221–1226. doi: 10.1183/09031936.00107507. [DOI] [PubMed] [Google Scholar]
- 22.Ringshausen F.C., Heckmann M., Weissbrich B., Neske F., Borg I., Knoop U. No evidence for WU polyomavirus infection in chronic obstructive pulmonary disease. Infect. agents cancer. 2009;4(1):12. doi: 10.1186/1750-9378-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kherad O., Kaiser L., Bridevaux P.-O., Sarasin F., Thomas Y., Janssens J.-P. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. CHEST J. 2010;138(4):896–904. doi: 10.1378/chest.09-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quint J.K., Donaldson G.C., Goldring J.J., Baghai-Ravary R., Hurst J.R., Wedzicha J.A. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. CHEST J. 2010;137(4):812–822. doi: 10.1378/chest.09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perotin J.M., Dury S., Renois F., Deslee G., Wolak A., Duval V. Detection of multiple viral and bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a pilot prospective study. J. Med. virology. 2013;85(5):866–873. doi: 10.1002/jmv.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeck L., Gencay M., Roth M., Hirsch H.H., Christ-Crain M., Mueller B. Adenovirus-specific IgG maturation as a surrogate marker in acute exacerbations of COPD. CHEST J. 2014;146(2):339–347. doi: 10.1378/chest.13-2307. [DOI] [PubMed] [Google Scholar]
- 27.George S.N., Garcha D.S., Mackay A.J., Patel A.R., Singh R., Sapsford R.J. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur. Respir. J. 2014;44(1):87–96. doi: 10.1183/09031936.00223113. [DOI] [PubMed] [Google Scholar]
- 28.Clark T.W., Medina M.-J., Batham S., Curran M.D., Parmar S., Nicholson K.G. C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation of COPD. Eur. Respir. J. 2015;45(1):76–86. doi: 10.1183/09031936.00092214. [DOI] [PubMed] [Google Scholar]
- 29.Dai M.-Y., Qiao J.-P., Xu Y-h, Fei G.-H. Respiratory infectious phenotypes in acute exacerbation of COPD: an aid to length of stay and COPD assessment test. Int. J. chronic Obstr. Pulm. Dis. 2015;10:2257. doi: 10.2147/COPD.S92160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimopoulos G., Tsiodras S., Lerikou M., Chranioti A., Perros E., Anagnostopoulou U. Viral profile of COPD exacerbations according to patients. open Respir. Med. J. 2015;9:1. doi: 10.2174/1874306401509010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosseini S.S., Ghasemian E., Jamaati H., Tabaraie B., Amini Z., Cox K. Association between respiratory viruses and exacerbation of COPD: a case-control study. Infect. Dis. 2015;47(8):523–529. doi: 10.3109/23744235.2015.1022873. [DOI] [PubMed] [Google Scholar]
- 32.Kokturk N., Bozdayi G., Yilmaz S., Doğan B., Gulbahar O., Rota S. Detection of adenovirus and respiratory syncytial virus in patients with chronic obstructive pulmonary disease: exacerbation versus stable condition. Mol. Med. Rep. 2015;12(2):3039–3046. doi: 10.3892/mmr.2015.3681. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu K., Yoshii Y., Morozumi M., Chiba N., Ubukata K., Uruga H. Pathogens in COPD exacerbations identified by comprehensive real-time PCR plus older methods. Int. J. chronic Obstr. Pulm. Dis. 2015;10:2009. doi: 10.2147/COPD.S82752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biancardi E., Fennell M., Rawlinson W., Thomas P.S. Viruses are frequently present as the infecting agent in acute exacerbations of chronic obstructive pulmonary disease in patients presenting to hospital. Intern. Med. J. 2016;46(10):1160–1165. doi: 10.1111/imj.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin T., Zhu Z., Mei Z., Feng J., Zhang W., He Y. Analysis of viral infection and biomarkers in patients with acute exacerbation of chronic obstructive pulmonary disease. Clin. Respir. J. 2017;00:1–12. doi: 10.1111/crj.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallego M., Pomares X., Capilla S., Marcos M.A., Suárez D., Monsó E. C-reactive protein in outpatients with acute exacerbation of COPD: its relationship with microbial etiology and severity. Int. J. chronic Obstr. Pulm. Dis. 2016;11:2633. doi: 10.2147/COPD.S117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du X., Ma X., Gao Y., Wen L., Li J., Wang Z. Prevalence and risk factors of respiratory viral infection in acute exacerbation of chronic obstructive pulmonary disease. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chin. J. Tuberc. Respir. Dis. 2017;40(4):263. doi: 10.3760/cma.j.issn.1001-0939.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Koul P.A., Mir H., Akram S., Potdar V., Chadha M.S. Respiratory viruses in acute exacerbations of chronic obstructive pulmonary disease. Lung India official organ Indian Chest Soc. 2017;34(1):29. doi: 10.4103/0970-2113.197099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kherad O., Kaiser L., Bridevaux P.-O., Sarasin F., Thomas Y., Janssens J.-P. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest. 2010;138(4):896–904. doi: 10.1378/chest.09-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. CHEST J. 2000;117(5_suppl_2) doi: 10.1378/chest.117.5_suppl_2.380s. 380S-5S. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson T.M., Donaldson G.C., Johnston S.L., Openshaw P.J., Wedzicha J.A. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. care Med. 2006;173(8):871–876. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]
- 42.Peces-Barba G., Albert Barbera J., Agusti A., Casanova C., Casas A., Luis Izquierdo J. Diagnosis and management of chronic obstructive pulmonary disease: joint guidelines of the Spanish society of pulmonology and thoracic surgery (SEPAR) and the Latin American thoracic society (ALAT) Arch. De. Bronconeumologia. 2008;44(5):271–281. [PubMed] [Google Scholar]