Summary of Suggestions
1. In the event of an incident with mass critical care casualties, we suggest all hospitals within a defined geographic/administrative region (eg, state), health authority, or health-care coalition should implement a uniform triage process and cooperate when critical care resources become scarce.
2. We suggest critical care only be rationed when resources have, or will shortly be, overwhelmed despite all efforts at augmentation and a regional-level authority that holds the legal authority and adequate situational awareness has declared an emergency and activated its mass critical care plan.
3. We suggest health-care systems provide oversight for any triage decisions made under their authority via activation of a mass critical care plan to ensure they comply with the prescribed process and include appropriate documentation.
4. We suggest health-care systems that have instituted a triage policy have a central process to update the triage protocol/system so that information that becomes available during an event informs the process in order to promote the most effective allocation of resources.
5. We suggest health-care systems establish in advance, a formal legal and systematic structure for triage in order to facilitate effective implementation of triage in the event of an overwhelming disaster.
6. We suggest health-care systems that have instituted a triage policy triage patients based on improved incremental survival rather than on a first-come, first-served basis when a substantial incremental survival difference favors the allocation of resources to another patient.
7. Triage officers:
7a. We suggest health-care systems that have instituted a triage policy have clinicians with critical care triage training function as triage officers (tertiary triage) to provide optimum allocation of resources.
7b. We suggest triage officers should have situational awareness at both a regional level and institutional level.
7c. We suggest in trauma or burn disasters, triage be carried out by triage officers who are senior surgeons/physicians with experience in trauma, burns, or critical care and experience in care of the age-group of the patient being triaged.
7d. We suggest in environments where triage is not usual, individual triage officers or teams consisting of a senior intensive care physician and an acute care physician be designated to make mass critical care triage decisions in accordance with previously prepared, publicly vetted, and widely disseminated guidelines.
7e. We suggest in limited resource settings in which there is a limited need for expansion of critical care resources, a continuation of well-established systems is appropriate.
8. We suggest triage protocols (clinical decision support systems), rather than clinical judgment alone, be used in triage whenever possible.
9. We suggest in health-care systems that have instituted a triage policy, technology such as baseline ultrasound, oxygen saturation as measured by pulse oximetry, mobile phone/Internet, and telemedicine be leveraged in triage where appropriate and available to augment clinical assessment in an effort to improve incremental survival and efficiency of resource allocation.
10. We suggest triage decision processes, whenever possible, provide for an appeals mechanism in case of deviation from an approved process (which may be a prospective or retrospective review) or a clinician request for reevaluation in light of novel or updated clinical information (prospective).
11. Triage process:
11a. We suggest tertiary-care triage protocols for use during a disaster that overwhelms or threatens to overwhelm resources be developed with inclusion and exclusion criteria.
11b. We suggest the inclusion criteria for admission to intensive care.
11c. We suggest patients who will have such a low probability of survival that significant benefit is unlikely be excluded from ICUs when resources are overwhelmed.
11d. We suggest consideration be given to excluding patient groups that have a life expectancy < 1 year.
11e. We suggest if a physiologic (nondisease-specific) outcome prediction score can be demonstrated to reliably predict mortality in a specified population upon screening for ICU admission, it is reasonable to use this to exclude admission for patients with a predicted mortality rate > 90%. Similarly if a disease-specific score can be demonstrated to reliably predict mortality when used in the same manner for patients with the disease, we suggest it is reasonable to use this to exclude admissions for patients with a predicted mortality rate of > 90%.
11f. We suggest each patient's condition be reassessed after a suitable time period (eg, 72 h) by the triage officer or triage team. If at that point the patient meets the criteria for exclusion from ICU, consideration should be given to withdrawal of therapy. If in the future a score is demonstrated to reliably predict high mortality when the patient is assessed during ICU stay, this should be used in preference to or as a supplement to clinical judgment.
Introduction
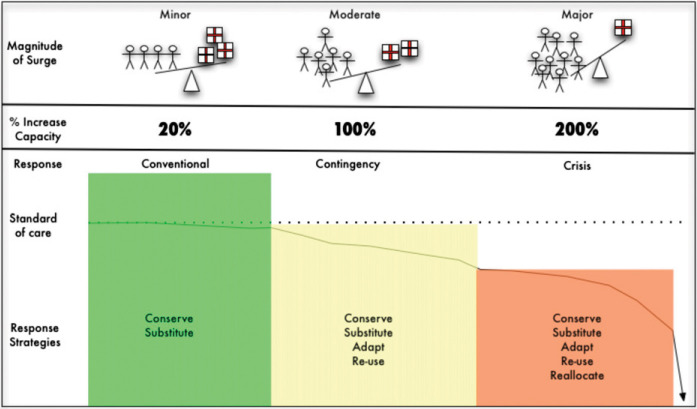
Isolated incidents (static events), such as a natural disaster or terrorism attack, as well as prolonged situations (dynamic events), such as a pandemic, both have the capacity to result in large numbers of critically ill or injured patients within affected health-care systems.1 Depending on the circumstances, the response to these surges may vary (Fig 1 ) from a conventional response where critically ill patients are managed with no significant alterations in standards or processes of care to a crisis response where resource limitations dictate significant alterations in both standards and processes of care to provide minimum basic critical care to the maximum number of patients.2, 3, 4, 5, 6 The prioritization of patients for urgency of treatment occurs at all stages along the continuum; however, this should not be confused with the classic meaning of triage, which includes prioritizing patients for care, rationing scarce resources, and making decisions about who will and will not receive potentially lifesaving therapies.7, 8 In resource-rich settings, rationing of critical care during a disaster should only occur in a crisis response.
Figure 1.
The spectrum of surge from minor to major. The magnitude of surge is illustrated by the alterations in the balance between demand (stick figures) and supply (medication boxes). As surge increases, the demand-supply imbalance worsens. Conventional, contingency, and crisis responses vary with magnitude of surge. Varying response strategies are associated with each level of response. As the magnitude of the surge increases, the response strategies used to cope gradually depart from the usual standard of care (default defining the standards of disaster care) until such point that even with crisis care, delivery of critical care is no longer possible.
Traditionally, triage is considered as occurring at three points along the chain of evacuation, referred to as primary, secondary, and tertiary triage (Fig 2 ), corresponding to decisions regarding evacuation or transport to the hospital (primary triage), during initial treatment (secondary triage), and during definitive care within the hospital (tertiary triage). The following suggestions address tertiary triage only, specifically, decisions regarding the delivery of critical care to any critically ill or injured patient during a pandemic or disaster and admission to ICUs. In resource-rich settings, tertiary triage decisions are only required in the event of a large surge of critically ill or injured casualties. In resource-limited settings, however, clinicians often are required to ration ICU resources under everyday9, 10, 11 circumstances, and hence, when a surge of critically ill patients occurs, the shortfall of resources and the degree of rationing is exacerbated.12 The suggestions offered refer to triage during surge events in both resource-rich and resource-poor settings rather than during long-term shortages of ICU resources.
Figure 2.
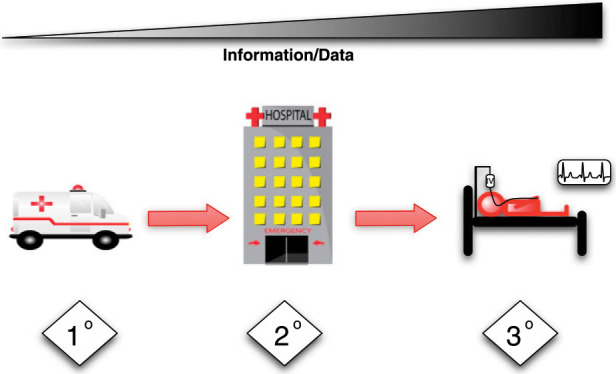
Primary triage (1°) involves decisions in the prehospital setting about the priority for treatment on the scene and evacuation to the hospital. Secondary triage (2°) involves decisions regarding the priority for assessment and initial treatment of patients when they first arrive at the hospital (usually the ED). Tertiary triage (3°) involves decisions regarding the priority for definitive care of patients in the ICU or operating room. The degree to which the decisions at each stage involve resource allocation (rationing) in addition to prioritization depends on the degree of imbalance between the demand for and supply of resources. As one progresses from primary through secondary and tertiary triage, the information and data available on which to base the triage decisions increase but so too does the complexity of the decisions. (Ambulance and hospital images courtesy of pamsclipart.com.)
The suggestions for resource allocation provide general principles supplemented by descriptive guidance for the application of the principles. This approach acknowledges the variability required in conducting tertiary triage, depending on the context of the disaster (resource rich vs poor), the magnitude of the surge, the ethical perspective of the region, and the affected age-groups. Although most of the general principles apply to both adult and pediatric populations, some, such as excluding from critical care patients with a very low survival probability, will have a very different impact on the amount of resources required for an adult vs a child.13 The suggestions in this article are important for all involved in a pandemic or disaster with multiple critically ill patients, including front-line clinicians, hospital administrators, and public health or government officials. Although it is important for all providers to be familiar with all aspects of critical care triage, Table 1 provides an overview of the suggestions of most interest to each group.
TABLE 1.
Primary Target Audiences for Suggestions
| Suggestion Number | Primary Target Audience | ||
|---|---|---|---|
| Clinicians | Hospital Administrators | Public Health/Government | |
| 1 | ✓ | ✓ | |
| 2 | ✓ | ||
| 3 | ✓ | ||
| 4 | ✓ | ||
| 5 | ✓ | ||
| 6 | ✓ | ||
| 7 | ✓ | ✓ | |
| 8 | ✓ | ✓ | |
| 9 | ✓ | ||
| 10 | ✓ | ✓ | ✓ |
| 11 | ✓ | ✓ | |
Materials and Methods
The methods used by the Triage topic panel in developing the suggestions in this article were consistent with the policies of the American College of Chest Physicians (CHEST) Guidelines Oversight Committee. The panel reviewed previous task force suggestions14 and identified 17 key questions for which literature searches were conducted to identify studies upon which evidence-based recommendations could be made (e-Appendix 1). No studies of sufficient quality were identified upon which to make evidence-based recommendations. Therefore, the panel developed expert opinion-based suggestions using a modified Delphi process.15 Suggestions from the previous task force that were not being updated were also included for validation by the expert panel.
Results
1. In the event of an incident with mass critical care casualties, we suggest all hospitals within a defined geographic/administrative region (eg, state), health authority, or health-care coalition implement a uniform triage process and cooperate should critical care resources become scarce.
It is essential that hospitals within the affected region have a consistent and coordinated approach between and among pediatric and adult critical care communities to ensure equitable and ethical allocation of resources within that region. Failure to do so may lead to situations that are ethically compromising and undermine public trust, such as when access to critical care resources is denied based on crisis standards by one institution without referral to a nearby institution capable of providing critical care. Regions should institute an Incident Management System (IMS) at the facility, local, regional or state, and national level to exercise command and control over scarce resources.16 Furthermore, decision-makers should communicate with community emergency services and local hospitals to ensure a coordinated approach to patient transfers, standards of care, and resource allocation.16, 17
2. We suggest critical care only be rationed when resources have, or will shortly be, overwhelmed despite all efforts at augmentation and a regional-level authority that holds the legal authority and adequate situational awareness has declared an emergency and activated its mass critical care plan.
A basic tenet of ethically conducted triage is that the degree of rationing through triage is proportionate to the anticipated or realized shortfall in resources.18 Therefore, the rationing of critical care should be held as a last resort and only implemented when all efforts have been made to optimize the use of resources. The task force considers all efforts at augmentation to include all attempts to acquire scarce critical resources or to transfer patients to other health-care facilities that are able to provide care (state, national, and even international partner institutions).19 In rapidly evolving disasters, critical care may need to be rationed before the appropriate authority has declared an emergency or activated its mass critical care plan, but this should only be done in exceptional circumstances. Similarly, in some jurisdictions, the relevant authority will be responsible for declaring an emergency and activating a mass critical care plan but may not have statutory powers. Nevertheless, a decision to ration critical care should not be made unilaterally at an institutional level because individual institutions do not possess the situational awareness in isolation to operationally or ethically justify such a decision. Similarly, the decision to cease triage should occur in a graduated and coordinated manner by altering the prioritization criteria and exclusion thresholds as resources become available.19
3. We suggest health-care systems provide oversight for any triage decisions made under their authority via activation of a mass critical care plan to ensure they comply with the prescribed process and include appropriate documentation.
Any authority (government, public health agency, health-care coalition, hospital) that either authorizes or participates in critical care triage should ensure that it provides oversight of this process and the ensuing decisions.14, 20, 21, 22, 23, 24, 25, 26, 27 Oversight may include, but is not limited to, logging triage decisions, tracking patient outcomes, benchmarking outcomes between facilities, and reviewing situations with outliers. During a prolonged event, such as a pandemic, it may be possible to undertake this oversight in real time, such as daily or weekly comparisons between decisions and outcomes at various facilities. In a sudden, brief event, however, oversight will most likely have to be conducted retrospectively but should still be undertaken to ensure accountability.
4. We suggest health-care systems that have instituted a triage policy have a central process to update the triage protocol/system so that further information that becomes available during an event is built into the process in order to promote the most effective allocation of resources.
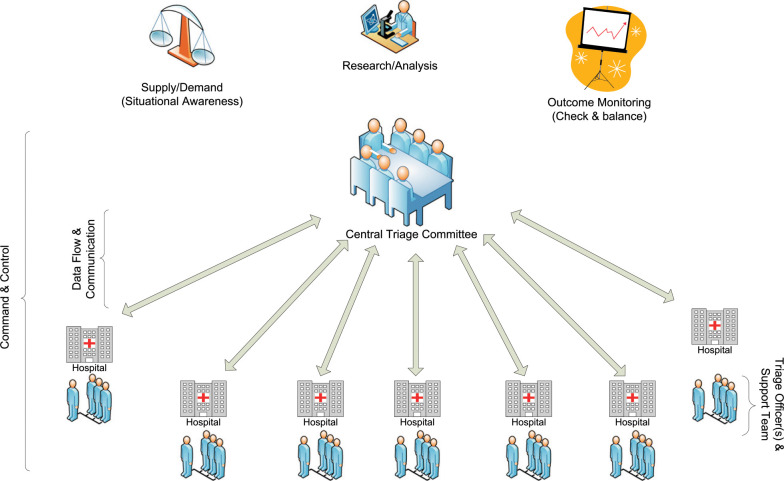
Tertiary triage decisions are dynamic and based on several factors, including supply and demand of resources and variables predicting patient outcome. In circumstances of a newly emerging infection, such as Severe Acute Respiratory Syndrome,28 or a novel strain of a known virus, such as 2009 influenza A(H1N1),29 key information about the precipitating factor inciting the surge event may not be known in the early days of the event. However, this information is crucial in effective and ethical triage decision-making. Therefore, as information becomes available, flexible systems and processes must be in place to modify existing protocols (Fig 3 ) and guide oversight and research.
Figure 3.
Triage infrastructure: the optimal relationship between the state or regional central triage committee and the triage officers at individual hospitals. The central triage committee must have situational awareness (knowledge of the resources supply and demand) and the capacity to conduct research in order to modify triage protocols and monitor triage outcomes. A bidirectional communication network between the central triage committee and hospitals is required to achieve situational awareness, monitor outcomes, and communicate modifications to the triage protocols. At the individual hospitals, the triage officers are supported by a staff or team.
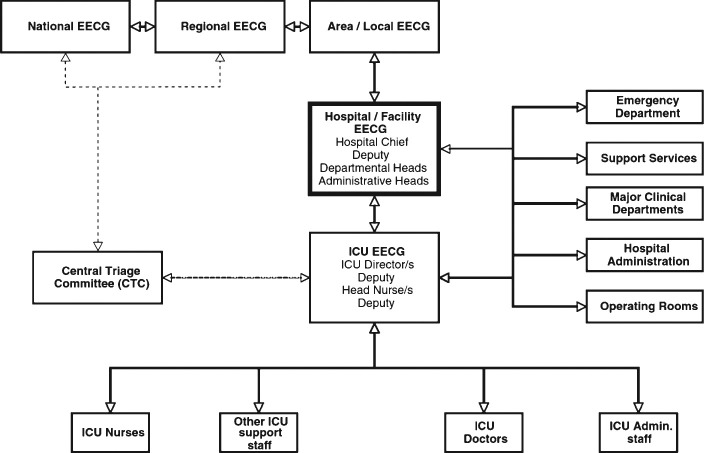
ICUs should interface with their hospital's coordinating (IMS) structure and detailed standard operating procedures for coordination, and collaboration should be developed and tested through simulated exercises before a crisis occurs.16 Effective triage will depend on the existence of a well-functioning communication and ICM among the ICU, administration, key departments, and hospital services (Fig 4 ).16
Figure 4.
Schematic showing key lines of authority (command chain) and information flow (bidirectional) required for an effective response in a disaster, including performing triage. EECG = executive emergency control group. (Reprinted with permission from Joynt et al.16)
5. We suggest health-care systems establish in advance, a formal legal and systematic structure for triage in order to facilitate effective implementation of triage in the event of an overwhelming disaster.
A more complete discussion and suggestions of the legal and policy aspects of mass critical care are provided the “Legal Preparedness” article by Courtney et al30 in this consensus statement. The task force believes that authorities should ensure that there is a legislative framework and structure to support critical care triage. The legislative and legal frameworks to address issues, especially rationing, during a disaster or public health emergency are highly complex and in many jurisdictions, are unclear or nonexistent.22, 25, 31, 32, 33, 34, 35, 36, 37 Clarity regarding the legal environment, and ideally safeguards for clinicians practicing mass critical care, is likely to enhance clinicians' ability to perform effective triage.
6. We suggest health-care systems that have instituted a triage policy triage patients based on improved incremental survival rather than on a first-come, first-served basis when a substantial incremental survival difference favors the allocation of resources to another patient.
Admission to an ICU should result in an increased chance of a better outcome compared with not being admitted.38, 39 However, survival is not the only outcome of interest, and although the outcome data are easily accessible and obvious, additional measures such as quality of life and resource utilization warrant consideration.39 If there is considerable uncertainty when predicting the outcome of individual patients and when incremental survival differences are believed to be minimal, after applying inclusion and exclusion criteria, it may be appropriate to allocate resources on a first-come, first-served basis or following an alternative process for prioritization on the basis of a lottery.
7. Triage officers:
7a. We suggest health-care systems that have instituted a triage policy have clinicians with critical care triage training function as triage officers to provide optimum allocation of resources (tertiary triage).
7b. We suggest triage officers should have situational awareness at both a regional level and institutional level.
7c. We suggest in trauma or burn disasters, triage be carried out by triage officers who are senior surgeons/physicians with experience in trauma, burns, or critical care and experience in care of the age-group of the patient being triaged.
7d. We suggest in environments where triage is not usual, individual triage officers or teams consisting of a senior intensive care physician and an acute care physician be designated to make triage decisions in accordance with previously prepared, publicly vetted, and widely disseminated guidelines for mass critical care triage.
7e. We suggest in situations in limited resource settings in which there is a limited need for expansion of critical care resources, a continuation of well-established systems is appropriate.
Triage officers or teams should have training expertise in mass casualty triage.7, 40, 41, 42, 43 They should receive training in ethics, communication, incident management, crisis resource management, and the importance and need for situational awareness. Experienced physicians should serve as triage officers whenever possible to improve efficiency and efficacy of decisions. The use of senior physicians and surgeons as triage officers is based on their training and decision-making capacity, which makes them ideal candidates.40, 41, 42, 43, 44, 45 Specifically for trauma, burns, and critically ill or injured patients, senior trauma surgeons, burn surgeons, and intensivists are best qualified to fulfill the role in each of the respective situations. Ideally, to enable timely decisions and avoid duty-to-care conflicts, triage officers should not be involved in triage and direct care of patients requiring triage simultaneously.42, 43, 46
The decision to have a single triage officer vs a triage team depends on the context of the situation and the philosophic approach adopted. Using a sole triage officer model allows for efficiency of decision-making, minimizes the impact on staff resources, and avoids conflicting opinions. However, a sole officer carries a significant intellectual and emotional burden. Furthermore, in situations with diverse groups of patients requiring critical care (eg, during a pandemic as opposed to a sudden static event), it is less likely that a very-well-defined set of criteria will exist to guide triage officers, and predicting patient outcomes will be more challenging. Therefore, in such settings, using a triage team comprising a senior intensivist and another senior physician from an acute care background may have advantages. This model ensures that at least two perspectives are provided on each case. Given the aim is to maximize the incremental benefit of critical care, the combination of an intensivist and a nonintensivist physician may be advantageous as one estimates the probability of good outcome with intensive care and the other without intensive care. Furthermore, a team approach may mitigate some of the emotional burden associated with sole decision-making. A team model, however, increases physician resources and requires processes to deal with disagreements within the team. Hospitals that choose to have a triage team must ensure that this approach does not compromise the ability to make effective and efficient decisions. Finally, it will likely be necessary for support staff (clinical and clerical) to be provided for the triage officers and teams.
This suggestion applies specifically to the role of the triage officer and team in the execution of triage during a mass casualty event. This is in contradistinction to the development and oversight of the triage process and the crafting of guidelines and protocols that relies on multidisciplinary teams representing a variety of stakeholders. Furthermore, this process should adhere to the suggestions made by the task force regarding public engagement and transparency.47
In limited resource settings, particularly in middle- and low-income countries, triage and refusal of ICU admission are everyday occurrences. In these settings, well-established triage systems may exist; therefore, we suggest that it is most appropriate to continue using the existing model for triage decision-making rather than implementing a new process during a disaster or pandemic.
8. We suggest triage protocols (clinical decision support systems), rather than clinical judgment alone, be used in triage whenever possible.
Clinical decision support systems (CDSSs), “a tool to aid the physician in patient care, in data acquisition, and in decision making,”48 can improve clinician performance and patient outcomes.49, 50, 51, 52, 53 A triage protocol7, 19, 54, 55 (manual or electronic) can serve as a CDSS, and therefore, we suggest that triage guidelines or protocols be used to support triage officer decision-making. As a CDSS, a triage protocol should support the decision-making by the clinician but should not be used as a checklist that substitutes for clinical experience. The aim of using a triage protocol is to improve incremental survival in patients, to allocate resources efficiently, and to minimize moral distress among care providers. Studies and experience suggest that clear guidelines developed by a group may help to mitigate54 some of the psychologic and moral pressures27, 35, 42 experienced by triage officers.
9. We suggest in health-care systems that have instituted a triage policy, technology such as baseline ultrasound, oxygen saturation as measured by pulse oximetry, mobile phone/Internet, and telemedicine be leveraged in triage where appropriate and available to augment clinical assessment in an effort to improve incremental survival and efficiency of resource allocation.
Technology that allows rapid acquisition of data on which to base assessments and rapid access to prognostic information or expert opinion may facilitate the accuracy of estimates of benefit without undue delay in decision-making. Examples of such technology include the use of oxygen saturation as a measure pulse oximetry to guide triage decisions regarding pediatric patients in resource-poor countries where oxygen and critical care resources are scarce56, 57 or the potential use of focused assessment with sonography in trauma ultrasound examination in trauma triage.
10. We suggest triage decision processes, whenever possible, provide for an appeals mechanism in case of deviation from an approved process (which may be a prospective or retrospective review) or a clinician request for reevaluation in light of novel or updated clinical information (prospective).
The appeals mechanism is distinct from institutional oversight. Three different scenarios are envisioned. The first is a request to change the approved triage process due to, for example, new knowledge regarding the outcomes or prognostic factors of a new disease epidemic. In this case, the appeal mechanism should be sufficiently rapid to allow a meaningful change in the process during the disaster. The second is a retrospective appeal by clinicians, patients, or relatives on the basis of deviation from the approved triage process by the triage officer or team. The third is a clinician request for reevaluation of a patient because the initial evaluation was based on incorrect or incomplete information, the patient's clinical state has changed, or new clinical information is available. In these scenarios, the process should be sufficiently rapid to allow a change in decision to be clinically meaningful.
11. Triage process:
11a. We suggest tertiary-care triage protocols for use during a disaster that overwhelms or threatens to overwhelm resources be developed with inclusion and exclusion criteria.
11b. We suggest the inclusion criteria for admission to intensive care as described in this article.
11c. We suggest patients who will have such a low probability of survival that significant benefit is unlikely be excluded from ICU when resources are overwhelmed.
11d. We suggest consideration be given to excluding patient groups that have a life expectancy < 1 year.
11e. We suggest if a physiologic (nondisease-specific) outcome prediction score can be demonstrated to reliably predict mortality in a specified population upon screening for ICU admission, it is reasonable to use this to exclude admissions for patients with a predicted mortality rate > 90%. Similarly, if a disease-specific score can be demonstrated to reliably predict mortality when used in the same manner for patients with the disease, we suggest it is reasonable to use this to exclude admissions for patients with a predicted mortality rate of > 90%.
11f. We suggest each patient's condition be reassessed after a suitable time period (eg, 72 h) by the triage officer or triage team. If at that point the patient meets the criteria for exclusion from ICU, consideration should be given to withdrawal of therapy. If in the future a score is demonstrated to reliably predict high mortality when the patient is assessed during ICU stay, this should be used in preference to or as a supplement to clinical judgment.
The goal of intensive care is generally considered to be more than survival to hospital discharge, and most would consider that survival for some reasonable length of time in the community is required for a good outcome. Clearly, this duration will be affected by premorbid conditions that severely limit life expectancy. To achieve the greatest benefits from the resources used, it is important to consider a patient's incremental increase in survival in the context of his or her predicted critical care resource consumption.
Although a few studies have assessed the impact of inclusion and exclusion criteria for tertiary triage,58, 59, 60 the evidence was considered too weak and inconclusive to support evidence-based recommendations. The inclusion criteria are based on forms of organ support that are not commonly available outside ICUs. The goal of inclusion criteria is to identify patients who may benefit from ICU admission. The inclusion criteria are not absolute and will need to be altered depending on the scale of the incident (TABLE 2, TABLE 3 ). Exclusion criteria aim to identify patients who are not candidates for ICU admission, including those (1) with a poor prognosis despite ICU care, (2) requiring resources that cannot be provided, and (3) whose underlying illness has a poor prognosis with a high likelihood of death.
TABLE 2.
Inclusion Criteria for Critical Care Admission
| Variable | Inclusion Criteria for Critical Care Admission |
|---|---|
| Requirement for invasive ventilatory support | Refractory hypoxemia (Spo2 < 90% on nonrebreather mask Fio2 > 0.85) Respiratory acidosis with pH < 7.2 Clinical evidence of respiratory failure Inability to protect or maintain airway |
| Hypotension | SBP < 90 mm Hg for adults (see BP parameters for all age-groups in Table 3) or relative hypotension with clinical evidence of shock for all ages (altered level of consciousness, decreased urine output, other end-organ failure) refractory to volume resuscitation requiring vasopressor/inotrope support that cannot be managed on the ward |
SBP = systolic BP; Spo2 = oxygen saturation as measured by pulse oximetry.
TABLE 3.
Age-Based BP Parameters for Defining Hypotension
| Group | Age | BP Parameter | Value |
|---|---|---|---|
| Adult | > 10 y | SBP | < 90 |
| Child | 1-10 y | SBP | < [70 + (2 × age in y)] |
| Infant | 1 mo-1 y | SBP | < 70 |
| Neonate | Term newborn-1 mo | SBP | < 60 |
| Premature neonate | Preterm newborn | MAP | < Gestational age in wk |
MAP = mean arterial pressure. See Table 2 legend for expansion of other abbreviation.
We suggest triage be based on incremental probability of survival. When the absolute probability of survival is low, then the incremental probability of survival (probability of survival with intensive care minus probability of survival without intensive care) will inevitably be as low or lower. The need for triage and, therefore, the threshold for excluding patients will vary depending on the scale of the incident (eg, bombing vs a pandemic) and the severity of disease. This does not mean that triage officers and teams are at liberty to unilaterally change exclusion criteria. The exclusion criteria being used should be determined by the central triage committee based on the supply-demand imbalance and then disseminated to the triage officers to incorporate in their triage protocol (decision support system) (TABLE 4, TABLE 5 ).
TABLE 4.
Low Probability of Survival Exclusion Criteria
| Criterion | Explanation |
|---|---|
| Cardiac arrest | Unwitnessed cardiac arrest |
| Witnessed adult cardiac arrest, not responsive to electrical therapy (defibrillation or pacing) | |
| Witnessed pediatric cardiac arrest, not responsive to initial 15 min of effective PALS or NRP | |
| Recurrent cardiac arrest | |
| Severe trauma | Trauma Injury Severity Score with predicted mortality > 90% |
| Severe burns | Predicted mortality > 90% |
| Severe and irreversible neurologic event or condition | Severe anoxic brain injury postcardiac arrest, massive stroke |
| Severe prematurity | < 24 wk estimated gestational age |
NRP = neonatal resuscitation program; PALS = pediatric advanced life support.
TABLE 5.
Short Life Expectancy Exclusion Criteria
| Patient Condition | Age Group |
|---|---|
| Metastatic malignancies | Adult and pediatric |
| Hematologic malignancies with poor prognosis | Adult and pediatric |
| End-stage organ failure with expected survival < 1 y, such as end-stage cardiac failure (NYHA class IV), severe chronic lung disease, advanced hepatic failure (MELD score > 20) | Adult and pediatric |
| Very advanced age | Adult |
| Advanced and irreversibly immunocompromised, such as drug-resistant AIDS | Adult and pediatric |
| Congenital anomalies with expected survival < 1 y | Pediatric |
MELD = Model for End-Stage Liver Disease; NYHA = New York Heart Association.
Triage decision-making is believed to be more objective if it is guided by a decision support system that includes a prognostic scoring system, such as that discussed in the “Ethical Considerations” article by Daugherty Biddison et al47 in this consensus statement. A number of prognostic scores have been developed for use in the critical care setting. We believe that for a prognostic score to be useful, it must reliably predict mortality, with an initial cutoff of > 90% mortality being reasonable to consider excluding a patient from admission to the ICU during a mass casualty event. Currently, there are no scores that meet these criteria. Previously, an admission Sequential Organ Failure Assessment (SOFA) score of > 11 had been recommended as an exclusion criterion predicting significant mortality.7, 54, 55, 61, 62 However, some studies showed conflicting evidence regarding mortality rates associated with this threshold.60, 63, 64 Therefore, predictive ability of the SOFA score varies across populations and, hence, a specific SOFA score threshold cannot be recommended for use in all adult populations. The SOFA score has not been validated in children.
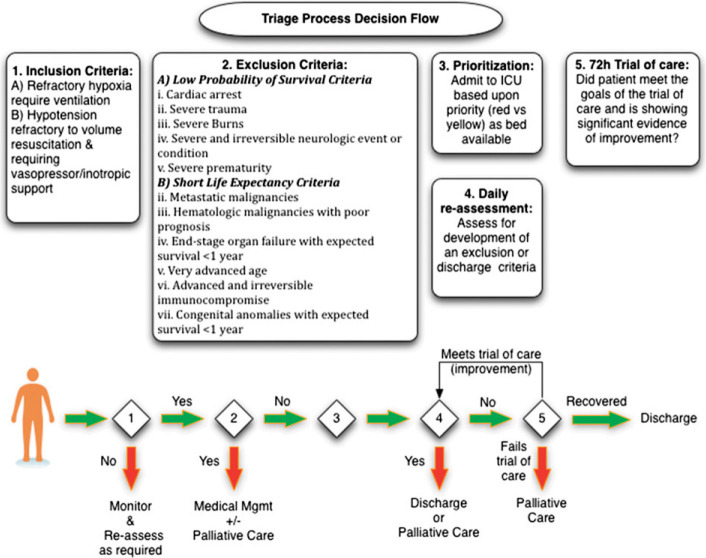
In view of the limitations of scores to predict outcome, to offer resources to as many as possible, it is useful to consider thresholds for offering resources. As discussed, the threshold of 90% may need to be adjusted up or down to include or exclude patients, depending on available resources. Similarly, the duration of the time period for reassessments (currently suggested as 72 h but as 96 h in prior publications) may need to be varied, depending on the natural history of the specific disease. In addition, instead of mortality prediction, a score may be more valuable if it predicts critical care resource utilization with a goal of maximizing the number of lives saved per available resource (Fig 5 ). This would be particularly useful in populations with low mortality rates, such as pediatrics.
Figure 5.
A conceptualized framework for how the critical care (tertiary) triage process and decisions would flow in a disaster or pandemic.
This discussion of inclusion and exclusion criteria has focused on the decision to admit or deny ICU care based on a crisis response where emergency mass critical care has already been fully implemented. In altering standards from conventional to contingency and, subsequently, to crisis care, decisions will already have been made to limit the use of therapies that require extraordinarily expensive equipment or consume extensive staff or hospital resources. Examples of such therapies include advanced ventilatory support and rescue therapies, such as inhaled nitric oxide, high-frequency ventilation, prone positioning ventilation, and extracorporeal membrane oxygenation.65
Areas for Future Research
During a disaster, there is an obligation to provide clinical care as well as research to guide effective and efficient care. Studies and trial designs should be preplanned and modified quickly when specific events occur. During the 2009 influenza A(H1N1) pandemic, observational study case report forms, registry, clinical trial design, and cooperation of international critical care research organizations occurred.66 Rigorous, relevant, timely, and ethical clinical and health services research is sorely lacking but crucial to improve care and outcomes of patients during disasters. Recommendations have been made to clinical investigators and research ethics committees for critical care research during a disaster that proposed strategies for expedited and centralized research ethics committee reviews and alternate consent models.67
The literature search presented in this article reveals an almost complete absence of data on which to base recommendations. Conducting research on triage during a static event is likely to be challenging. However, even simple observational data on the feasibility of the suggestions given in this article would be useful. More comprehensive research is more feasible during a dynamic event, such as an epidemic. Preliminary research on outcomes, including patients admitted and not admitted to the ICU in the interepidemic period, will facilitate predictions of benefit for those with nonepidemic diseases and provide clues about the data to collect during the epidemic. This may allow for the rapid development of appropriate CDSSs early in the epidemic.
Conclusions
Critical care triage is a complex process that requires significant planning, preparation, and infrastructure for it to be conducted ethically and efficiently. At present, the prognostic tools required to produce an effective decision support system (triage protocol) are lacking along with most of the infrastructure, processes, legal protections, and training for critical care triage. Therefore, critical care triage is best avoided, if at all possible, through the use of mass critical care strategies. When critical care triage is required, the suggestions from this Task Force outline the key principles upon which it should be based as well as suggest a path for the development of plans, processes, and infrastructure.
Acknowledgments
Author contributions: M. D. C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. D. C., C. L. S., M. A. K., J. R. D., N. K., A. V. D., and C. D. G. contributed to the development of PICO questions; M. D. C., C. L. S., and C. D. G. conducted the literature review; M. D. C., C. L. S,. M. A. K., J. R. D., N. K., A. V. D., and C. D. G. contributed to development of expert opinion suggestion; M. D. C., C. L. S., M. A. K., J. R. D., N. K., A. V. D., and C. D. G. contributed to the conception and design, or acquisition of data, or analysis and interpretation of data from the Delphi process; M. D. C., C. L. S., M. A. K., and C. D. G. developed and drafted the manuscript; and M. D. C., C. L. S., J. R. D., N. K., A. V. D., and C. D. G. revised the manuscript critically for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Endorsements: This consensus statement is endorsed by the American Association of Critical-Care Nurses, American Association for Respiratory Care, American College of Surgeons Committee on Trauma, International Society of Nephrology, Society for Academic Emergency Medicine, Society of Critical Care Medicine, Society of Hospital Medicine, World Federation of Pediatric Intensive and Critical Care Societies, World Federation of Societies of Intensive and Critical Care Medicine.
Role of sponsors: The American College of Chest Physicians was solely responsible for the development of these guidelines. The remaining supporters played no role in the development process. External supporting organizations cannot recommend panelists or topics, nor are they allowed prepublication access to the manuscripts and recommendations. Further details on the Conflict of Interest Policy are available online at http://chestnet.org.
Other contributions: The opinions expressed within this manuscript are solely those of the author (M. D. C.) and do not represent the official position or the policy of the Royal Canadian Medical Service, Canadian Armed Forces, or Department of National Defence.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Collaborators: Executive Committee: Michael D. Christian, MD, FRCPC, FCCP; Asha V. Devereaux, MD, MPH, FCCP, co-chair; Jeffrey R. Dichter, MD, co-chair; Niranjan Kissoon, MBBS, FRCPC; Lewis Rubinson, MD, PhD; Panelists: Dennis Amundson, DO, FCCP; Michael R. Anderson, MD; Robert Balk, MD, FCCP; Wanda D. Barfield, MD, MPH; Martha Bartz, MSN, RN, CCRN; Josh Benditt, MD; William Beninati, MD; Kenneth A. Berkowitz, MD, FCCP; Lee Daugherty Biddison, MD, MPH; Dana Braner, MD; Richard D Branson, MSc, RRT; Frederick M. Burkle Jr, MD, MPH, DTM; Bruce A. Cairns, MD; Brendan G. Carr, MD; Brooke Courtney, JD, MPH; Lisa D. DeDecker, RN, MS; COL Marla J. De Jong, PhD, RN [USAF]; Guillermo Dominguez-Cherit, MD; David Dries, MD; Sharon Einav, MD; Brian L. Erstad, PharmD; Mill Etienne, MD; Daniel B. Fagbuyi, MD; Ray Fang, MD; Henry Feldman, MD; Hernando Garzon, MD; James Geiling, MD, MPH, FCCP; Charles D. Gomersall, MBBS; Colin K. Grissom, MD, FCCP; Dan Hanfling, MD; John L. Hick, MD; James G. Hodge Jr, JD, LLM; Nathaniel Hupert, MD; David Ingbar, MD, FCCP; Robert K. Kanter, MD; Mary A. King, MD, MPH, FCCP; Robert N. Kuhnley, RRT; James Lawler, MD; Sharon Leung, MD; Deborah A. Levy, PhD, MPH; Matthew L. Lim, MD; Alicia Livinski, MA, MPH; Valerie Luyckx, MD; David Marcozzi, MD; Justine Medina, RN, MS; David A. Miramontes, MD; Ryan Mutter, PhD; Alexander S. Niven, MD, FCCP; Matthew S. Penn, JD, MLIS; Paul E. Pepe, MD, MPH; Tia Powell, MD; David Prezant, MD, FCCP; Mary Jane Reed, MD, FCCP; Preston Rich, MD; Dario Rodriquez, Jr, MSc, RRT; Beth E. Roxland, JD, MBioethics; Babak Sarani, MD; Umair A. Shah, MD, MPH; Peter Skippen, MBBS; Charles L. Sprung, MD; Italo Subbarao, DO, MBA; Daniel Talmor, MD; Eric S. Toner, MD; Pritish K. Tosh, MD; Jeffrey S. Upperman, MD; Timothy M. Uyeki, MD, MPH, MPP; Leonard J. Weireter Jr, MD; T. Eoin West, MD, MPH, FCCP; John Wilgis, RRT, MBA; ACCP Staff: Joe Ornelas, MS; Deborah McBride; David Reid; Content Experts: Amado Baez, MD; Marie Baldisseri, MD; James S. Blumenstock, MA; Art Cooper, MD; Tim Ellender, MD; Clare Helminiak, MD, MPH; Edgar Jimenez, MD; Steve Krug, MD; Joe Lamana, MD; Henry Masur, MD; L. Rudo Mathivha, MBChB; Michael T. Osterholm, PhD, MPH; H. Neal Reynolds, MD; Christian Sandrock, MD, FCCP; Armand Sprecher, MD, MPH; Andrew Tillyard, MD; Douglas White, MD; Robert Wise, MD; Kevin Yeskey, MD.
Footnotes
FUNDING/SUPPORT: This publication was supported by the Cooperative Agreement Number 1U90TP00591-01 from the Centers of Disease Control and Prevention, and through a research sub award agreement through the Department of Health and Human Services [Grant 1 - HFPEP070013-01-00] from the Office of Preparedness of Emergency Operations. In addition, this publication was supported by a grant from the University of California–Davis.
COI grids reflecting the conflicts of interest that were current as of the date of the conference and voting are posted in the online supplementary materials.
DISCLAIMER: American College of Chest Physicians guidelines and consensus statements are intended for general information only, are not medical advice, and do not replace professional care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this consensus statement can be accessed at http://dx.doi.org/10.1378/chest.1464S1.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Supplementary Material
References
- 1.Christian MD, Devereaux AV, Dichter JR, Geiling JA, Rubinson L. Definitive care for the critically ill during a disaster: current capabilities and limitations: from a Task Force for Mass Critical Care summit meeting, January 26-27, 2007, Chicago, IL. Chest. 2008;133(5_suppl):8S–17S. doi: 10.1378/chest.07-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(suppl 1):S59–S67. doi: 10.1097/DMP.0b013e31819f1ae2. [DOI] [PubMed] [Google Scholar]
- 3.Hick JL, Christian MD, Sprung CL. European Society of Intensive Care Medicine's Task Force for Intensive Care Unit Triage During an Influenza Epidemic or Mass Disaster. Chapter 2. Surge capacity and infrastructure considerations for mass critical care. Recommendations and standard operating procedures for intensive care unit and hospital preparations for an influenza epidemic or mass disaster. Intensive Care Med. 2010;36(suppl 1):S11–S20. doi: 10.1007/s00134-010-1761-4. [DOI] [PubMed] [Google Scholar]
- 4.Hick JL, Hanfling D, Burstein JL. Health care facility and community strategies for patient care surge capacity. Ann Emerg Med. 2004;44(3):253–261. doi: 10.1016/j.annemergmed.2004.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereaux A, Christian MD, Dichter JR, Geiling JA, Rubinson L. Task Force for Mass Critical Care. Summary of suggestions from the Task Force for Mass Critical Care summit, January 26-27, 2007. Chest. 2008;133(5_suppl):1S–7S. doi: 10.1378/chest.08-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine; Committee on Guidance for Establishing Standards of Care for Use in Disaster Situations Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations: A Letter Report. Washington, DC: National Academies Press. 2009 [PubMed] [Google Scholar]
- 7.Christian MD, Hawryluck L, Wax RS. Development of a triage protocol for critical care during an influenza pandemic. CMAJ. 2006;175(11):1377–1381. doi: 10.1503/cmaj.060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian MD, Stewart TE, Lapinsky SE, Cashman J, Grounds M. Critical care and biological disasters: lessons learned from SARS and pandemic influenza planning. In: Recent Advances in Anaesthesia and Intensive Care. Cambridge, England: Cambridge University Press. 2007:264. [Google Scholar]
- 9.Sprung CL, Geber D, Eidelman LA. Evaluation of triage decisions for intensive care admission. Crit Care Med. 1999;27(6):1073–1079. doi: 10.1097/00003246-199906000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Joynt GM, Gomersall CD, Tan P, Lee A, Cheng CA, Wong EL. Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome. Intensive Care Med. 2001;27(9):1459–1465. doi: 10.1007/s001340101041. [DOI] [PubMed] [Google Scholar]
- 11.Simchen E, Sprung CL, Galai N. Survival of critically ill patients hospitalized in and out of intensive care units under paucity of intensive care unit beds. Crit Care Med. 2004;32(8):1654–1661. doi: 10.1097/01.ccm.0000133021.22188.35. [DOI] [PubMed] [Google Scholar]
- 12.Aschkenasy-Steuer G, Shamir M, Rivkind A. Clinical review: the Israeli experience: conventional terrorism and critical care. Crit Care. 2005;9(5):490–499. doi: 10.1186/cc3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christian MD, Toltzis P, Kanter RK, Burkle FM, Jr, Vernon DD, Kissoon N. Task Force for Pediatric Emergency Mass Critical Care. Treatment and triage recommendations for pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6):S109–S119. doi: 10.1097/PCC.0b013e318234a656. [DOI] [PubMed] [Google Scholar]
- 14.Devereaux AV, Dichter JR, Christian MD. Task Force for Mass Critical Care. Definitive care for the critically ill during a disaster: a framework for allocation of scarce resources in mass critical care: from a Task Force for Mass Critical Care summit meeting, January 26-27, 2007, Chicago, IL. Chest. 2008;133(5_suppl):51S–66S. doi: 10.1378/chest.07-2693. [DOI] [PubMed] [Google Scholar]
- 15.Pill J. The Delphi method: substance, context a critique and an annotated bibliography. Socio-Econ Plan Sci. 1971;5:57–71. [Google Scholar]
- 16.Joynt GM, Loo S, Taylor BL. European Society of Intensive Care Medicine's Task Force for intensive care unit triage during an influenza epidemic or mass disaster. Chapter 3. Coordination and collaboration with interface units. Recommendations and standard operating procedures for intensive care unit and hospital preparations for an influenza epidemic or mass disaster. Intensive Care Med. 2010;36(suppl 1):S21–S31. doi: 10.1007/s00134-010-1762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbera JA, Macintyre AG. Medical Surge Capacity and Capability: A Management System for Integrating Medical and Health Resources During Large-Scale Emergencies. Washington, DC: US Department of Health and Human Services. 2004 [Google Scholar]
- 18.Barnett DJ, Taylor HA, Hodge JG, Jr, Links JM. Resource allocation on the frontlines of public health preparedness and response: report of a summit on legal and ethical issues. Public Health Rep. 2009;124(2):295–303. doi: 10.1177/003335490912400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christian MD, Joynt GM, Hick JL, Colvin J, Danis M, Sprung CL. European Society of Intensive Care Medicine's Task Force for Intensive Care Unit Triage During an Influenza Epidemic or Mass Disaster. Chapter 7. Critical care triage. Recommendations and standard operating procedures for intensive care unit and hospital preparations for an influenza epidemic or mass disaster. Intensive Care Med. 2010;36(suppl 1):S55–S64. doi: 10.1007/s00134-010-1765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabery J, Mackett CW., III University of Pittsburgh Medical Center Pandemic Influenza Task Force's Triage Review Board. Ethics of triage in the event of an influenza pandemic. Disaster Med Public Health Prep. 2008;2(2):114–118. doi: 10.1097/DMP.0b013e31816c408b. [DOI] [PubMed] [Google Scholar]
- 21.Ventilator Document Workgroup; Ethics Subcommittee of the Advisory Committee to the Director, Centers for Disease Control and Prevention Ethical Considerations for Decision Making Regarding Allocation of Mechanical Ventilators during a Severe Influenza Pandemic or Other Public Health Emergency. Atlanta, GA: Centers for Disease Control and Prevention. 2010 [Google Scholar]
- 22.Barnett DJ, Taylor HA, Hodge JG, Jr, Links JM. Resource allocation on the frontlines of public health preparedness and response: report of a summit on legal and ethical issues. Public Health Rep. 2009;124(2):295–303. doi: 10.1177/003335490912400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White DB, Katz MH, Luce JM, Lo B. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150(2):132–138. doi: 10.7326/0003-4819-150-2-200901200-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuschner WG, Pollard JB, Ezeji-Okoye SC. Ethical triage and scarce resource allocation during public health emergencies: tenets and procedures. Hosp Top. 2007;85(3):16–25. doi: 10.3200/HTPS.85.3.16-25. [DOI] [PubMed] [Google Scholar]
- 25.Lemon SM, Hamburg MA, Sparling PF, Choffnes ER, Mack A. Forum on Microbial Threats. Ethical and Legal Considerations in Mitigating Pandemic Disease. Washington, DC: The National Academies Press. 2007 [PubMed] [Google Scholar]
- 26.O'Laughlin DT, Hick JL. Ethical issues in resource triage. Respir Care. 2008;53(2):190–197. [PubMed] [Google Scholar]
- 27.Klein KR, Pepe PE, Burkle FM, Jr, Nagel NE, Swienton RE. Evolving need for alternative triage management in public health emergencies: a Hurricane Katrina case study. Disaster Med Public Health Prep. 2008;2(suppl 1):S40–S44. doi: 10.1097/DMP.0b013e3181734eb6. [DOI] [PubMed] [Google Scholar]
- 28.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38(10):1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawood FS, Jain S, Finelli L. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 30.Courtney B, Hodge JG, Jr, Toner ES. on behalf of the Task Force for Mass Critical Care. Legal preparedness: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4_suppl):e134S–e144S. doi: 10.1378/chest.14-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gostin LO, Sapsin JW, Teret SP. The Model State Emergency Health Powers Act: planning for and response to bioterrorism and naturally occurring infectious diseases. JAMA. 2002;288(5):622–628. doi: 10.1001/jama.288.5.622. [DOI] [PubMed] [Google Scholar]
- 32.Hodge JG., Jr Protecting the public's health in an era of bioterrorism: the Model State Emergency Health Powers Act. Account Res. 2003;10(2):91–107. doi: 10.1080/08989620300507. [DOI] [PubMed] [Google Scholar]
- 33.Hodge JG, Jr, Anderson ED, Kirsch TD, Kelen GD. Facilitating hospital emergency preparedness: introduction of a model memorandum of understanding. Disaster Med Public Health Prep. 2011;5(1):54–61. doi: 10.1001/10-v4n2-hsf10003. [DOI] [PubMed] [Google Scholar]
- 34.Hodge JG, Jr, O'Connell JP. The legal environment underlying influenza vaccine allocation and distribution strategies. J Public Health Manag Pract. 2006;12(4):340–348. doi: 10.1097/00124784-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Eastman N, Philips B, Rhodes A. Triaging for adult critical care in the event of overwhelming need. Intensive Care Med. 2010;36(6):1076–1082. doi: 10.1007/s00134-010-1862-0. [DOI] [PubMed] [Google Scholar]
- 36.Courtney B, Hodge JG., Jr Task Force for Pediatric Emergency Mass Critical Care. Legal considerations during pediatric emergency mass critical care events. Pediatr Crit Care Med. 2011;12(6):S152–S156. doi: 10.1097/PCC.0b013e318234a7e1. [DOI] [PubMed] [Google Scholar]
- 37.Ransom MM, Goodman RA, Moulton AD. Addressing gaps in health care sector legal preparedness for public health emergencies. Disaster Med Public Health Prep. 2008;2(1):50–56. doi: 10.1097/DMP.0b013e3181587cff. [DOI] [PubMed] [Google Scholar]
- 38.Gomersall CD, Joynt GM. What is the benefit in triage? Crit Care Med. 2011;39(4):911–912. doi: 10.1097/CCM.0b013e31820b7415. [DOI] [PubMed] [Google Scholar]
- 39.Christian MD, Fowler R, Muller MP. PREEDICCT Study Group. Critical care resource allocation: trying to PREEDICCT outcomes without a crystal ball. Crit Care. 2013;17(1):107. doi: 10.1186/cc11842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frykberg ER. Medical management of disasters and mass casualties from terrorist bombings: how can we cope? J Trauma. 2002;53(2):201–212. doi: 10.1097/00005373-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Iserson KV, Moskop JC. Triage in medicine, part I: concept, history, and types. Ann Emerg Med. 2007;49(3):275–281. doi: 10.1016/j.annemergmed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Moskop JC, Iserson KV. Triage in medicine, part II: underlying values and principles. Ann Emerg Med. 2007;49(3):282–287. doi: 10.1016/j.annemergmed.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy K, Aghababian RV, Gans L, Lewis CP. Triage: techniques and applications in decision making. Ann Emerg Med. 1996;28(2):136–144. doi: 10.1016/s0196-0644(96)70053-7. [DOI] [PubMed] [Google Scholar]
- 44.Kirschenbaum L, Keene A, O'Neill P, Westfal R, Astiz ME. The experience at St. Vincent's Hospital, Manhattan, on September 11, 2001: preparedness, response, and lessons learned. Crit Care Med. 2005;33:S48–S52. doi: 10.1097/01.ccm.0000151067.76074.21. [DOI] [PubMed] [Google Scholar]
- 45.Burkle FM, Sanner PH, Wolcott BW. Disaster Medicine: Application for the Immediate Management and Triage of Civilian and Military Disaster Victims. New Hyde Park, NY: Medical Examination Publishing. 1984 [Google Scholar]
- 46.Baker MS. Creating order from chaos: part I: triage, initial care, and tactical considerations in mass casualty and disaster response. Mil Med. 2007;172(3):232–236. doi: 10.7205/milmed.172.3.232. [DOI] [PubMed] [Google Scholar]
- 47.Daugherty Biddison L, Berkowitz KA, Courtney B. on behalf of the Task Force for Mass Critical Care. Ethical considerations: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4_suppl):e145S–e155S. doi: 10.1378/chest.14-0742. [DOI] [PubMed] [Google Scholar]
- 48.Richardson JE, Ash JS, Sittig DF. Multiple perspectives on the meaning of clinical decision support. AMIA Annu Symp Proc. 2010;2010:1427–1431. [PMC free article] [PubMed] [Google Scholar]
- 49.Bright TJ, Wong A, Dhurjati R. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157(1):29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. [DOI] [PubMed] [Google Scholar]
- 50.Garg AX, Adhikari NK, McDonald H. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 51.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipton J, Hazelzet JA. Clinical decision support systems: important tools when appropriately used. Pediatr Crit Care Med. 2009;10(1):128–129. doi: 10.1097/PCC.0b013e31819838f9. [DOI] [PubMed] [Google Scholar]
- 53.Mack EH, Wheeler DS, Embi PJ. Clinical decision support systems in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10(1):23–28. doi: 10.1097/PCC.0b013e3181936b23. [DOI] [PubMed] [Google Scholar]
- 54.Powell T, Christ KC, Birkhead GS. Allocation of ventilators in a public health disaster. Disaster Med Public Health Prep. 2008;2(1):20–26. doi: 10.1097/DMP.0b013e3181620794. [DOI] [PubMed] [Google Scholar]
- 55.Hick JL, O'Laughlin DT. Concept of operations for triage of mechanical ventilation in an epidemic. Acad Emerg Med. 2006;13(2):223–229. doi: 10.1197/j.aem.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 56.Duke T, Subhi R, Peel D, Frey B. Pulse oximetry: technology to reduce child mortality in developing countries. Ann Trop Paediatr. 2009;29(3):165–175. doi: 10.1179/027249309X12467994190011. [DOI] [PubMed] [Google Scholar]
- 57.Mwaniki MK, Nokes DJ, Ignas J. Emergency triage assessment for hypoxaemia in neonates and young children in a Kenyan hospital: an observational study. Bull World Health Organ. 2009;87(4):263–270. doi: 10.2471/BLT.07.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bailey A, Leditschke I, Ranse J, Grove K. Impact of a pandemic triage tool on intensive care admission. Crit Care. 2008;12(suppl 2):349. [Google Scholar]
- 59.Christian MD, Hamielec C, Lazar NM. A retrospective cohort pilot study to evaluate a triage tool for use in a pandemic. Crit Care. 2009;13(5):R170. doi: 10.1186/cc8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guest T, Tantam G, Donlin N, Tantam K, McMillan H, Tillyard A. An observational cohort study of triage for critical care provision during pandemic influenza: ‘clipboard physicians’ or ‘evidenced based medicine’? Anaesthesia. 2009;64(11):1199–1206. doi: 10.1111/j.1365-2044.2009.06084.x. [DOI] [PubMed] [Google Scholar]
- 61.Ashton-Cleary D, Tillyard A, Freeman N. Intensive care admission triage during a pandemic: a survey of the acceptability of triage tools. J Intensive Care Soc. 2011;12(3):180–186. [Google Scholar]
- 62.Hick JL, Rubinson L, O'Laughlin DT, Farmer JC. Clinical review: allocating ventilators during large-scale disasters—problems, planning, and process. Crit Care. 2007;11(3):217. doi: 10.1186/cc5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan Z, Hulme J, Sherwood N. An assessment of the validity of SOFA score based triage in H1N1 critically ill patients during an influenza pandemic. Anaesthesia. 2009;64(12):1283–1288. doi: 10.1111/j.1365-2044.2009.06135.x. [DOI] [PubMed] [Google Scholar]
- 64.Shahpori R, Stelfox HT, Doig CJ, Boiteau PJE, Zygun DA. Sequential Organ Failure Assessment in H1N1 pandemic planning. Crit Care Med. 2011;39(4):827–832. doi: 10.1097/CCM.0b013e318206d548. [DOI] [PubMed] [Google Scholar]
- 65.Sprung CL, Kesecioglu J. European Society of Intensive Care Medicine's Task Force for Intensive Care Unit Triage During an Influenza Epidemic or Mass Disaster. Chapter 5. Essential equipment, pharmaceuticals and supplies. Recommendations and standard operating procedures for intensive care unit and hospital preparations for an influenza epidemic or mass disaster. Intensive Care Med. 2010;36(suppl 1):S38–S44. doi: 10.1007/s00134-010-1763-2. [DOI] [PubMed] [Google Scholar]
- 66.Fowler RA, Webb SA, Rowan KM. Early observational research and registries during the 2009-2010 influenza A pandemic. Crit Care Med. 2010;38(4 suppl):e120–e132. doi: 10.1097/CCM.0b013e3181d20c77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook D, Burns K, Finfer S. Clinical research ethics for critically ill patients: a pandemic proposal. Crit Care Med. 2010;38(4 suppl):e138–e142. doi: 10.1097/CCM.0b013e3181cbaff4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.