Abstract
The Cadi ThermoSENSOR skin-contact thermometer measures body temperature continuously and transmits readings wirelessly to a central server. This study evaluated the ThermoSENSOR against ear temperatures (ETs) measured by a Braun ThermoScan ear thermometer and axillary temperatures (ATs) measured by a Terumo digital clinical thermometer. The test participants consisted of 109 children aged 6 months to 16 years from a pediatric ward. The sensor was attached to the lower abdomen at least 15 minutes before the first measurement. ET, AT, and ThermoSENSOR temperatures (TTs) were recorded up to three times at the usual measurement times. The TTs differed from ETs by −0.23°C ± 0.47°C (mean ± standard deviation, n = 271) and from ATs by +0.21°C ± 0.46°C (n = 270). The ETs differed from ATs by +0.43°C ± 0.42°C (n = 315). These results suggest that the TTs were comparable to the ETs and ATs.
Key words: Axillary temperature, Contact tracing, Continuous monitoring, Ear temperature, Radio frequency identification, Skin-contact thermometer, ThermoSENSOR, Tympanic temperature, Wireless monitoring, Wireless sensor network
BODY TEMPERATURE IS an important physiological parameter used routinely in the clinical management of critically ill patients. For both children and adults, it is usually measured manually using rectal, ear, oral, or axillary methods (Asher and Northington, 2008, National Institute for Health and Clinical Excellence (NICE), 2007, O'Grady et al., 2008). In patients with fever, body temperature is assessed frequently, causing constant disturbance to the patients and increasing nursing workload. Automated wireless monitoring of temperature enables nurses and clinicians to monitor a patient's temperature continuously without disturbing the patient, enhancing patient comfort and mobility. It also enables readings to be automatically stored, retrieved, and analyzed for trends, saving time and minimizing errors associated with manual recording and analysis.
In view of the worldwide shortage of nurses (Oulton, 2006, World Health Organization (WHO), 2006) and the ongoing need to improve patient care, KK Women's and Children's Hospital in Singapore explored the use of an automated wireless system for monitoring body temperature. Developed by Cadi Scientific in Singapore as part of an integrated wireless system for temperature monitoring and location tracking, this system uses a reusable skin-contact thermometer or sensor called the ThermoSENSOR. This thermometer takes the form of a small disc that can be easily adhered to the patient's skin, and each disc is assigned a unique radio frequency identification (RFID) number (Figure 1 ). The thermometer measures body temperature continuously and transmits a temperature reading and the RFID number approximately every 30 seconds to a computer or server through one or more signal receivers (nodes) installed in the vicinity of the patient (Figure 2 ).
Figure 1.
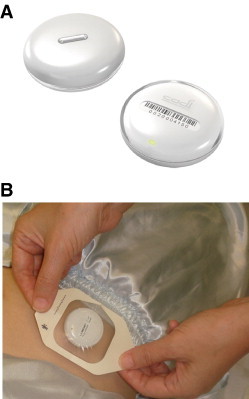
(A) The ThermoSENSOR wireless thermometer. The disc has an elliptical cross-section, and the sensing element consists of a metal strip located at the center of the skin-contact side. (B) A ThermoSENSOR, having been placed over the first piece of hypoallergenic adhesive film dressing on the lower abdomen, about to be secured to the lower abdomen by a second piece of the same dressing.
Figure 2.
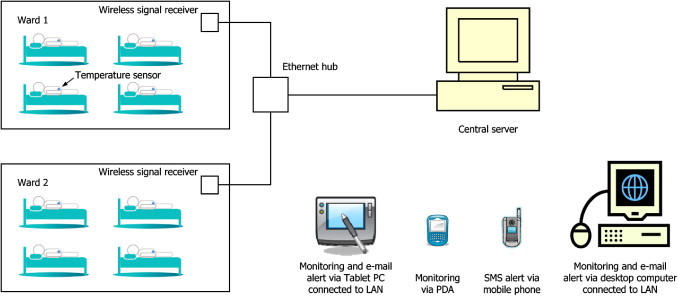
A setup of the ThermoSENSOR wireless temperature monitoring system. Each sensor transmits data wirelessly to a signal receiver (node) that is within the prescribed transmission range. The signal receiver uploads the data to a central server through the LAN, through which the data can be accessed from computers and other devices that are connected, wirelessly or by wired means, to the LAN. The server can be configured to send out e-mail and short message service (SMS) alerts.
A search of the published literature revealed the use of two types of continuous wireless thermometry systems—one type that measures core temperature using a disposable ingestible capsule (Byrne & Lim, 2007) and another type that measures skin temperature using a disposable dermal patch (Racinais, Gaoua, & Grantham, 2008). In these systems, temperature readings are transmitted wirelessly at regular intervals to a patient monitor or data recorder. Ingestible thermometers have been used to study the thermoregulation of sports persons and soldiers during physical exercise (Gant et al., 2006, Lim et al., 2008) and investigated for use in monitoring body temperature during cardiac surgery (Markides, Omorphos, Kotoulas, & Prendergast, 2007), but they are not suitable for routine monitoring of temperature in young children. The published literature also revealed the use of a skin-contact thermometer that automatically measures skin temperature at predetermined intervals, but its temperature readings are not transmitted wirelessly, are stored in the thermometer, and have to be downloaded to a computer by wired means (Sarabia et al., 2008, van Marken Lichtenbelt et al., 2006). In comparison, although the ThermoSENSOR is also a skin-contact thermometer, its readings are intended to reflect core temperature.
As with any physiological parameter, accurate temperature measurement is critical to accurate patient assessment. For this reason, a study was conducted to evaluate the accuracy of the ThermoSENSOR against ear temperature (ET) and axillary temperature (AT) in a pediatric population. This article reports on this evaluation.
Methods
Test Subjects
The test subjects consisted of inpatient children from a pediatric ward at KK Women's and Children's Hospital, Singapore. Children with an implanted pacemaker, those in isolation rooms, and psychiatric patients were excluded. The study protocol was approved by the hospital's institutional review board. Consent was obtained from each patient's guardian or parents before the study.
The Environment
KK Women's and Children's Hospital is the largest hospital in Singapore dedicated to providing care for women, babies, and children. The ward within which the study was conducted is one of the hospital's general pediatric medical wards catering to children from birth to 16 years. It has six five-bedded rooms, two isolation rooms, a play area, and a nursing station. Children admitted to the ward represent mainly cases of fever arising from respiratory and gastroenterological infections. An average of 12 new patients are admitted to the ward per day, and their length of stay ranges from 1 to 5 days. On any given day, six to seven nurses provide care to up to 32 patients.
Equipment
Before the study, a ThermoSENSOR wireless temperature monitoring system was installed in the ward. A wireless signal receiver (node) was installed on the ceiling of each of the six five-bedded rooms, one on the ceiling between the two isolation rooms (which were smaller), and one on the ceiling at the play area. These receivers were connected to the hospital's local area network (LAN). The system worked in such a way that temperature readings and RFID numbers transmitted by a sensor were received by one or more wireless receivers in the vicinity of the sensor and transferred through the LAN to a personal computer that was also connected, by wired means, to the LAN. This computer was located at the nursing station. Web-based application software designed for use with the wireless system and installed on the computer was used to configure the computer to receive, store, and display the temperature and RFID data. A total of 32 sensors were used for the study.
The ThermoSENSOR uses a thermistor as the sensing element. When in use, the sensor is attached to the patient using a two-layer dressing system that prevents the sensor from coming in direct contact with the skin (Figure 1). The sensor is water resistant and can be cleaned by immersing it in a cleaning or disinfectant solution. The manufacturer provided the following specifications for the sensor: operating ambient temperature range, 10°C to 50°C; thermistor accuracy, ±0.2°C for temperature range of 32.0°C to 42.0°C; data transmission rate, every 30 seconds on average; radio frequency, 868.4 MHz; typical transmission range, 10 m (unblocked); power source, internal 3-V lithium coin-cell battery; battery life, 12 months (continuous operation); dimensions, diameter of 36 mm, height of 11.6 mm; weight, 10 g without battery; applicable radio equipment standards, ETSI EN 300 220, ETSI EN 301 489.
A Braun ThermoScan ear thermometer, type 6021 (model PRO 4000, Braun, Kronberg, Germany), was used to measure ET. Two units were used for the study. This thermometer model was used throughout the hospital for routine temperature measurement. The manual indicated the following specifications: displayed temperature range, 20°C to 42.2°C (68°F–108°F); operating ambient temperature range, 10°C to 40°C (50°F–104°F); display resolution, 0.1°C or 0.1°F; accuracy for displayed temperature range, ±0.2°C (±0.4°F) for the range 35.5°C to 42°C (95.9°F–107.6°F) and ±0.25°C (±0.5°F) outside this range; measurement time, 3 to 7 seconds; power source, 2 × MN 1500 or 1.5-V AA (LR6) batteries; battery life, 6 months or 1,000 measurements; dimensions, 152 × 44 × 33 mm; weight, 100 g without batteries; applicable accuracy standards, ASTM E1965-98, EN 12470-5.
A Terumo digital clinical thermometer, model C202 (Terumo, Tokyo, Japan), was used to measure AT. Two new units were used for the study. This thermometer is designed for use at the axilla and uses a thermistor as the sensing element. It has two modes of operation: the predictive measurement mode and the direct measurement mode. In the predictive mode, the microprocessor in the thermometer analyzes the rising curve of the temperature detected by the sensor and predicts the body temperature after about 90 seconds, giving a beep upon completion of the measurement. If the thermometer remains in the axilla after this beep, it will switch to the direct mode, in which the thermometer will measure the temperature directly like a standard mercury thermometer and give the temperature reading along with a second beep after about 10 minutes from the start of measurement. The following specifications were obtained from the manufacturer and the instructions for use: measurement range, 32°C to 42°C; operating ambient temperature range, 10°C to 40°C; accuracy, ±0.2°C (95% confidence interval) for predictive mode, ±0.1°C for direct mode, both with respect to a standard mercury thermometer; display resolution, 0.1°C; measurement time, about 90 seconds in predictive measurement mode, about 10 minutes in direct measurement mode; power source, 3-V lithium coin-cell battery; battery life, approximately 10,000 measurements; dimensions, 126 × 16 × 10 mm; weight, 10 g with battery; applicable accuracy standard, EN 12470-3.
Procedure
The evaluation procedure was designed to obtain up to three sets of readings for each patient, each set consisting of an ET reading, an AT reading, and a ThermoSENSOR temperature (TT) reading.
Before performing the first measurement, a ThermoSENSOR was attached to the middle of the left or right quadrant of the lower abdomen, which, depending on the physical size of the child, is about 2 to 6 cm below the navel. This was accomplished by applying a piece of hypoallergenic adhesive film dressing (Tegaderm transparent film dressing, 3M Health Care, St. Paul, MN) to the skin, placing the sensor over the dressing, and overlaying a second piece of the same dressing over the sensor and surrounding area to hold the sensor securely against the skin (Figure 1). The sensor was then covered with whatever diaper or clothing the patient was wearing. This was done at least 15 minutes before the first measurement to give time for the sensor to warm up to body temperature and for the sensor reading to stabilize.
For each patient, temperature measurements were taken up to three times at the usual times at which the patient's temperature was taken. Before each measurement, the Web-based application software on the computer was set to receive, store, and display temperature readings and RFID numbers received through the LAN. Immediately after the measurement start time was recorded on the case report form (CRF), the ET reading was first taken from the left or right ear. This was immediately followed by the predictive-mode AT reading from the left or right axilla. These readings were taken in accordance with the hospital's policy and procedure guidelines and recorded on the CRF. Each set of readings for a measurement was taken by only one nurse, and to minimize variability in the procedure, only two nurses were assigned to take temperature readings. The corresponding TT reading was retrieved from the computer after all measurements for the patient were completed.
For any patient who experienced discomfort with the sensor, the sensor was removed and the study discontinued for the patient. This was to ensure patient safety and well-being.
Analysis
The analysis was designed to compare the following paired readings: TT and ET, TT and AT, and ET and AT. Readings were used for analysis as long as any of these paired readings could be formed.
For each measurement, the pattern of TT readings that appeared after the recorded measurement start time was examined. The first available stabilized TT reading that occurred within 5 minutes after the recorded measurement start time was taken as the corresponding TT reading. There were three situations in which there would be no corresponding TT reading. The first situation was when the TT readings were near room temperature, which would indicate that the sensor was not attached to the patient at the time the ET and AT readings were taken. The second situation was when the TT reading was falling toward room temperature, indicating that the sensor had just been removed. The third situation was when the TT reading had not stabilized and was still rising, which would indicate that the sensor had not been given sufficient time to warm up to body temperature before the ET and AT readings were taken.
To provide a quantitative picture of the distribution of the reference readings, the percentage of ET readings in each of the following three ranges was determined: low, <36.8°C; medium, ≥36.8°C, <38.0°C; high, ≥38.0°C. These ranges were based on the normal ET range of 35.8°C to 38.0°C established by Chamberlain et al. (1995). A width of 1.2°C, about 54% of the normal range, was arbitrarily used for the medium range. Similarly, the percentage of AT readings in each of the following three ranges was also determined: low, <35.9°C; medium, ≥35.9°C, <37.3°C; high, ≥37.3°C. These ranges were based partly on the normal AT range of 34.7°C to 37.3°C established by Chamberlain & Terndrup (1994). A width of 1.4°C, also about 54% of the normal range, was arbitrarily used for the medium range.
The range, mean, and standard deviation of each of the three main groups of readings (AT, ET, and TT) were determined, and the following differences were computed: TT minus ET, TT minus AT, and ET minus AT. Agreement between the paired readings was assessed using mean difference statistics (Bland and Altman, 1986, Bland and Altman, 1995) and a nonparametric method that uses the percentages of differences within certain limits (O'Brien et al., 1993, O'Brien et al., 2002). The range, mean, standard deviation, p value (two-tailed), and distribution of differences for these three groups of data were then determined. In this analysis, the repeated measurements for each subject were treated as independent measurements. Although this might underestimate the true standard deviations of the differences, the effect was expected to be clinically insignificant because the number of repeated measurements (maximum of two) was considerably lower than the number of subjects (Bland & Altman, 2007). To assess the validity of this approach, the standard deviations of differences were also computed using a modified Bland–Altman method that takes into account repeated measurements (Bland & Altman, 2007, “Method where the true value varies” section, pp. 575–578). Bland–Altman plots were used to graphically illustrate agreement between the paired readings for the main groups. In addition, a post hoc statistical power analysis based on the computed mean difference statistics for the main groups of readings was performed to assess the adequacy of the sample sizes.
For each of the three main groups of differences, the mean, standard deviation, p value, and distribution of readings for each subrange (low, medium, or high) were also determined if there were at least 30 readings in the subrange, the number 30 being to ensure a statistically reasonable estimate of the mean difference. This analysis was intended to assess how the agreement between the paired readings might vary with temperature.
Results
Temperature readings used for analysis came from 109 children aged 6 months to 16 years, distributed as follows: paired TT–ET and paired TT–AT readings, 43 boys, 62 girls, 6.1 ± 4.7 years; paired ET–AT readings, 46 boys, 63 girls, 6.1 ± 4.6 years. The range, mean, standard deviation, and distribution of the temperature readings are summarized in Table 1 . The range, mean, standard deviation, p value, and distribution of the differences for the various groups of differences are summarized in Table 2 . Bland–Altman plots for the three main groups of readings are given in Figure 3, Figure 4, Figure 5 . All these readings were derived from 316 measurements, of which only 270 had complete sets of readings. Most of the patients had 3 measurements, but for various reasons as summarized in Table 3 , some patients had only one or two measurements, and some of the readings for a measurement were incomplete. The average ambient temperature in the ward was 23°C.
Table 1.
Range, Mean (M), Standard Deviation (SD), and Distribution of Temperature Readings
| Parameter | Quantity |
|---|---|
| ET | |
| n | 316 |
| Range | 35.3°C–40.4°C |
| M ± SD | 37.35°C ± 0.65°C |
| No. of readings | |
| Low range: <36.8°C | 42 (13.3%) |
| Medium range: ≥36.8°C, <38.0°C | 233 (73.7%) |
| High range: ≥38.0°C | 41 (13.0%) |
| AT | |
| n | 315 |
| Range | 34.9°C–39.8°C |
| M ± SD | 36.91°C ± 0.63°C |
| No. of readings | |
| Low range: <35.9°C | 6 (1.9%) |
| Medium range: ≥35.9°C, <37.3°C | 244 (77.5%) |
| High range: ≥37.3°C | 65 (20.6%) |
| TT | |
| n | 271 |
| Range | 36.1°C–39.5°C |
| M ± SD | 37.12°C ± 0.54°C |
Table 2.
Range, Mean, Standard Deviation, p Value (Two-Tailed), and Distribution of Differences Between Readings
| Difference | n | Range (°C) | M ± SD (°C) | p | ≤0.5°C (%) | ≤1.0°C (%) | ≤1.5°C (%) |
|---|---|---|---|---|---|---|---|
| TT minus ET | |||||||
| All readings | 271 | −2.6 to +1.3 | −0.23 ± 0.47 | <.001 | 75.3 | 96.3 | 99.3 |
| Low range: ET <36.8°C | 38 | −0.5 to +1.3 | +0.31 ± 0.42 | <.001 | 73.7 | 94.7 | 100.0 |
| Medium range: ET ≥36.8°C, <38.0°C | 196 | −1.2 to +0.8 | −0.26 ± 0.36 | <.001 | 80.6 | 98.5 | 100.0 |
| High range: ET ≥38.0°C | 37 | −2.6 to +0.3 | −0.62 ± 0.54 | <.001 | 48.6 | 86.5 | 94.6 |
| TT minus AT | |||||||
| All readings | 270 | −1.8 to +1.4 | +0.21 ± 0.46 | <.001 | 72.2 | 96.3 | 99.6 |
| Low range: AT <35.9°C | 5 | 0.5 to 1.1 | No further computation because n <30 | ||||
| Medium range: AT ≥35.9°C, <37.3°C | 212 | −0.8 to +1.4 | +0.30 ± 0.41 | <.001 | 70.8 | 96.7 | 100.0 |
| High range: AT ≥37.3°C | 53 | −1.8 to +0.8 | −0.17 ± 0.42 | .0082 | 83.0 | 96.2 | 98.1 |
| ET minus AT | |||||||
| All readings | 315 | −0.9 to +2.0 | +0.43 ± 0.42 | <.001 | 61.3 | 93.3 | 98.7 |
| Low range: ET <36.8°C | 42 | −0.7 to +0.7 | +0.11 ± 0.33 | .0356 | 88.1 | 100.0 | 100.0 |
| Medium range: ET ≥36.8°C, <38.0°C | 232 | −0.9 to +2.0 | +0.46 ± 0.38 | <.001 | 59.9 | 94.8 | 99.6 |
| High range: ET ≥38.0°C | 41 | −0.4 to +1.6 | +0.65 ± 0.49 | <.001 | 41.5 | 78.0 | 92.7 |
Figure 3.
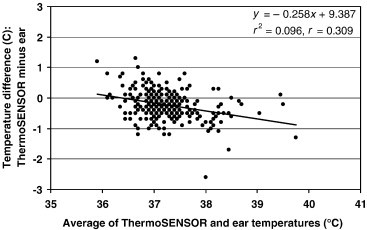
Bland–Altman plot for differences between ThermoSENSOR and ETs (n = 271). The limits of agreement are −0.23°C ± 0.47°C (mean ± standard deviation). The lowest point pertains to the third measurement for Patient 88. The continuous line is the linear regression line.
Figure 4.
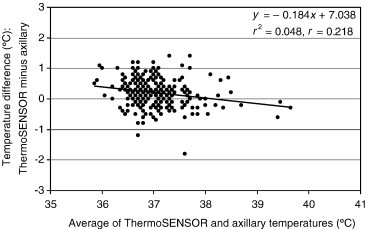
Bland–Altman plot for differences between ThermoSENSOR and ATs (n = 270). The limits of agreement are +0.21°C ± 0.46°C (mean ± standard deviation). The lowest point pertains to the third measurement for Patient 88. The continuous line is the linear regression line.
Figure 5.
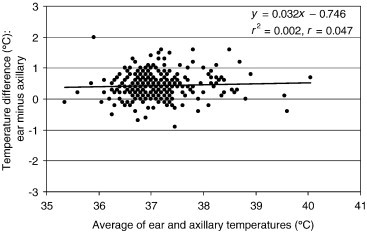
Bland–Altman plot for differences between ET and AT (n = 315). The limits of agreement are +0.43°C ± 0.42°C (mean ± standard deviation). The highest point pertains to the first measurement for Patient 26. The continuous line is the linear regression line.
Table 3.
Observations Related to Incomplete Data Sets
| Type of Observation | No. of Cases | Description |
|---|---|---|
| Procedure related | 25 | No TT reading was available at the time the ET and ET readings were taken because the ThermoSENSOR either was inadvertently not attached to the patient or was already removed from the patient. Most of these cases happened after the second measurement but before the third measurement. |
| Procedure related | 9 | No TT reading was available because the sensor was not given sufficient time to warm up to body temperature. Most of these cases occurred during the first measurement. |
| Patient related | 2 | Only one complete set of readings was available because the patient was discharged or transferred to another ward after the first measurement. |
| Patient related | 1 | Only two measurements were made because the patient was discharged before the third measurement was performed. |
| Patient related | 1 | Only two measurements were made because the patient removed the sensor after the second measurement and refused to have it put back. |
| Patient related | 1 | The AT reading for the third measurement was not available because the patient refused to have it taken after the ET reading was taken. |
| Patient related | 1 | Only one set of ET and AT readings was available because the patient refused to have the sensor attached to his abdomen. |
| Patient related | 2 | The sensor was removed after the patient complained of itchiness. For one patient, one set of ET and AT readings was taken. For the other patient, no readings were taken. |
| Signal reception | 4 | No TT reading was available because the receiver failed to receive readings from the sensor. |
| Signal reception | 1 | The TT reading for a measurement was excluded from analysis because the first stabilized reading occurred 5.2 minutes after the measurement start time; the analysis included only TT readings that occurred within 5 minutes of the measurement start time. |
| Signal reception | 1 | No TT reading was available for the first measurement because of a technical problem with the signal receiver. |
| Computer related | 3 | The TT reading was not available because the data file, for some unknown reason, became corrupted during analysis and no backup of the file was available. |
The time intervals at which measurements were taken ranged from 5 minutes to 5 hours (163 ± 45 minutes). For four patients, the interval between the first and second measurements was relatively short, ranging from 5 to 17 minutes, for one of two reasons. First, the first measurement was taken just before a shift change and the second measurement just after. Second, the second measurement was taken shortly after the first for verification purposes. For one patient, the interval between the second and third measurements was 5 hours. A total of 271 TT readings were used for analysis. They occurred within 4.7 minutes (0.4 ± 0.5) of the recorded measurement start time, and 93.4% of them (253 readings) occurred within the first minute.
For all measurements considered (Table 2), the TT readings were on average 0.23°C lower than the ET readings and 0.21°C higher than the AT readings, and the ET readings were on average 0.43°C higher than the AT readings. The standard deviations of differences for these readings were identical to those computed using the modified Bland–Altman method, which takes into account repeated measurements. This confirms the validity of treating all the repeated measurements in this study as independent measurements. The mean differences for the various subranges ranged from −0.62°C to +0.31°C for the TT–ET readings and from −0.17°C to +0.30°C for the TT–AT readings. For all groups of TT-ET and TT-AT readings, the standard deviations ranged from 0.36°C to 0.54°C and the proportions of differences within ±0.5°C, ±1.0°C, and ±1.5°C ranged from 48.6% to 100%. The mean ET–AT differences for the various subranges ranged from 0.11°C to 0.65°C. For all groups of ET–AT readings, the standard deviations ranged from 0.33°C to 0.49°C, and the proportions of differences within ±0.5°C, ±1.0°C, and ±1.5°C ranged from 41.5% to 100%. All the p values were less than .05 (Table 3), indicating that the mean differences were statistically significant at the 5% level.
A post hoc power analysis for the three main groups of readings gave the following minimum sample sizes required to achieve a power of 80%, 90%, and 98% at a significance level of 5%: TT–ET readings, 35, 46, and 70, respectively; TT–AT readings, 40, 53, and 80, respectively; ET–AT readings, 10, 13, and 18, respectively. On the basis of these results, we consider the sample sizes of 105 subjects for the TT–ET and TT–AT readings and 109 subjects for the ET–AT readings to be more than adequate.
The linear regression lines of the Bland–Altman plots of Figure 3, Figure 4, Figure 5 exhibit gentle slopes of −0.258, −0.184, and +0.032°C/°C, respectively, and low correlation coefficients (r) of 0.309, 0.218, and 0.047, respectively. For Patient 88, the TT reading (36.7°C) for the third measurement was considerably lower than the corresponding AT (38.5°C) and ET (39.3°C) readings. We were unable to ascertain the cause of this outlier, which is evident in the Bland–Altman plots (Figure 3, Figure 4). For Patient 26, the AT reading (34.9°C) for the first measurement was significantly lower than the corresponding ET reading (36.9°C); this measurement had no corresponding TT reading because the ThermoSENSOR was not given sufficient time to warm up to body temperature (Table 3). We were also unable to ascertain the cause of this outlier (Figure 5).
The mean difference statistics and the proportion of differences for the TT–ET and TT–AT readings suggest that the temperatures recorded by the ThermoSENSOR were comparable to the ETs recorded by the Braun ThermoScan and the ATs recorded by the Terumo digital thermometer. We consider the mean differences of −0.23°C for the TT-ET readings and +0.21°C for the TT–AT readings to be clinically significant, so assessment of a patient's condition using ThermoSENSOR readings must take into account these differences.
Discussion
The mean differences and standard deviations in this study are within ranges reported in other studies comparing readings at different sites (Craig et al., 2002, Craig et al., 2000, Devrim et al., 2007, Lawson et al., 2007). In one review using data from 20 studies, the rectal temperatures were higher than ATs by 0.17°C ± 0.16°C for neonates and by 0.92°C ± 0.54°C for older children and young people (Craig et al., 2000). In another review using data from 44 studies, ETs (measured in actual mode, as opposed to calibration mode) differed from rectal temperatures by +0.70°C ± 0.45°C for children (Craig et al., 2002). In a study on 60 adults using invasive pulmonary artery temperature as the reference (Lawson et al., 2007), ETs differed by +0.36°C ± 0.56°C and ATs (measured using a digital thermometer) by −0.23°C ± 0.44°C, suggesting that the ETs differed from ATs by about +0.59°C ± 0.71°C (Taylor & Kuyatt, 1994). In a study on 102 children, ETs (measured using a clinical ear thermometer) differed from ATs (measured using a mercury-in-glass thermometer) by +0.74°C ± 0.52°C (Devrim et al., 2007).
The results of this study and those of other studies (Craig et al., 2000, Craig et al., 2002, Devrim et al., 2007, El-Radhi and Barry, 2006, Heusch and McCarthy, 2005, Lawson et al., 2007, Smith, 2003, Smith, 2004) affirm the phenomenon that body temperature varies with measurement site and measurement method. Because of this, the range of normal temperatures and that for fever thresholds must depend on the same. The definition of fever appears to be arbitrary, depending on the clinical setting and patient condition (American College of Emergency Physicians Clinical Policies Committee, & American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever, 2003, Chamberlain and Terndrup, 1994, Chamberlain et al., 1995, Hughes et al., 2002, Kaul et al., 2006, O'Grady et al., 2008, Ryan and Levy, 2003). The American College of Emergency Physicians (2003) defines fever as a rectal temperature higher than 38°C for children aged 1 day to 3 years presenting to the emergency department. The American College of Critical Care Medicine and the Infectious Diseases Society of America suggested that a core temperature of 38.3°C or higher should be considered a fever (O'Grady et al., 2008) but noted at the same time that a variety of arbitrary definitions are acceptable, depending on the desired level of sensitivity in fever detection. The Infectious Diseases Society of America defines fever for neutropenic patients with cancer as a single oral temperature of 38.3°C or higher or a temperature 38°C or higher for 1 hour or longer (Hughes et al., 2002). At KK Women's and Children's Hospital, the fever threshold is 37.8°C for ET and 37.5°C for AT. Comprehensive ranges for normal body temperatures by site have been established (Chamberlain and Terndrup, 1994, Chamberlain et al., 1995): axillary, 34.7°C to 37.3°C; oral, 35.5°C to 37.5°C; rectal, 36.6°C to 38.0°C; ear, 35.8°C to 38.0°C. Although the average ThermoSENSOR readings in this study lie between the corresponding average ear and axillary readings (Table 2), separate studies using a wider range of subjects and temperatures are required to establish the range of normal temperatures based on the sensor.
The negative slopes of the regression lines in the Bland–Altman plots (Figure 3, Figure 4) for the TT–ET and TT–AT readings (−0.258 and −0.184°C/°C, respectively) suggest that both the TT–ET and TT–AT differences might decrease with increasing temperature. This suggestion is also evident in the increasingly negative mean differences at higher reference temperatures (Table 2). However, this relationship is not conclusive because the correlation coefficients for the regression lines were rather low (0.309 for TT–ET readings and 0.218 for TT–AT readings). Further studies using more data in the higher and lower temperature ranges are required to characterize the relationship between difference and magnitude. For the TT–AT readings (Figure 5), the gentle slope (+0.032°C/°C) and low correlation (0.047) suggest that the ET–AT difference might be independent of magnitude, but this relationship needs to be confirmed by further studies.
For the outlier (low TT reading) for Patient 88 (Figure 3, Figure 4), we suspect that the attachment between the ThermoSENSOR and the skin was slightly loose at the time the ThermoSENSOR reading was taken. To minimize erroneous TT readings, it is important to keep the sensor in close contact against the skin at all times and provide sufficient time for the sensor to warm up to body temperature. For the outlier (low AT reading) for Patient 26 (Figure 5), we suspect that the Terumo thermometer might not have been properly placed in the axilla. In both cases, we did verify, however, that the inclusion of these outliers in the analysis did not affect the mean difference statistics to any significant extent.
This study was carried out in an air-conditioned environment in which the ambient temperature varied little (average of 23°C). As with most thermometers, ambient temperature can affect temperature measurement. We have, however, observed from laboratory tests that the sensor will give stable readings as long as it is properly attached to the skin, it is given sufficient time to warm up, and both the sensor and its surrounding region are not subjected to any localized heat or cold. When the ThermoSENSOR disc is in close contact with the skin, its sensing element, being at the center of the disc (Figure 1), is not directly exposed to ambient temperature but lies at the center of a relatively large region that is exposed to skin temperature. We believe this design helps to minimize the effect of ambient temperature. We recommend using the ThermoSENSOR within the specified ambient temperature range of 10°C to 50°C, which is easily met in a hospital environment. Although we have not specifically tested the effect of clothing covering the sensor on the accuracy of TT readings, we anticipate that any effect will be minimal. Further studies are, however, needed to characterize the effects of ambient temperature on ThermoSENSOR readings.
Several improvements can be considered for future studies. First, the protocol can be modified to allow for simultaneous or almost simultaneous measurement of all the temperatures, so as to minimize the effect of physiological variations. Because the ear thermometer takes only 3 to 7 seconds to complete a measurement whereas the axillary thermometer requires a much longer time of about 90 seconds, the AT could be taken first followed by the ET, and the time of completion of the AT measurement could be recorded and used later to identify the next ThermoSENSOR reading that is closest in time to this recorded time. We recognize that this revised procedure may not work out if the child refuses to have the ET taken immediately after the AT is taken, in which case the axillary measurement may have to be repeated. Second, the study can be improved by exercising better coordination to reduce the number of cases in which the sensor is not given sufficient time to warm up to body temperature, is inadvertently not attached to the patient, or is already removed before reference temperatures are taken (Table 3). Third, the study can be extended by evaluating the thermometer against other temperatures such as oral and rectal temperatures. Last but not least, the base of test subjects should be expanded to include more temperatures in the lower and higher ranges to better assess the ability of the ThermoSENSOR to measure a wide range of temperatures (Table 1). In addition, standardized protocols for evaluating clinical thermometers should be developed that stipulate, among other things, a minimum sample size based on sound statistical considerations, age groups, temperature ranges, the minimum percentage of readings in each range, the method of analysis, and accuracy criteria for clinical use. One such protocol is being developed by the International Organization for Standardization (ISO) as part of a comprehensive standard on the performance of clinical thermometers (ISO, 2008). Standardized protocols, which have long been used for evaluation of blood pressure monitors (Association for the Advancement of Medical Instrumentation (AAMI), 2003, European Committee for Standardization (CEN), 2004, O'Brien et al., 1993, O'Brien et al., 2002), not only help ensure that devices meet minimum performance standards but also facilitate comparison of one device with another.
Improvements can also be considered for the ThermoSENSOR and its application method. First, for babies aged between 6 months and 1 year, we found the sensor somewhat bulky because it covered a relatively large area of their small abdomen. This problem can be mitigated by using a sensor that is smaller and thinner. Second, for these babies, we also found that wet diapers tended to loosen the adhesive film dressing. This problem can perhaps be mitigated by using an adhesive dressing that provides stronger adhesion to the skin or by changing the adhesive dressing periodically. Third, we encountered toddlers who simply refused to have a foreign object pasted on their body. We anticipate that this kind of refusal is likely to happen in practice, especially for young children, but think that a smaller and thinner sensor that is also easier to attach to the skin may make it easier for a child to agree to using it. The manufacturer has been requested to consider such a design for young children.
Readings provided by the ThermoSENSOR are not pure skin temperature readings but are readings that have been calibrated to reflect core or body temperature. Studies have shown that skin temperature itself in general neither reflects nor correlates with body temperature (Thomas, 2003, Thomas et al., 2004). Theoretically, any of several noninvasive methods of measuring body temperature could be used for wireless temperature monitoring, including the regular infrared ear thermometry (Chamberlain and Terndrup, 1994, Chamberlain et al., 1995), infrared ear thermometry using the arterial heat balance method (Loveys et al., 1999, Powell et al., 2001), infrared skin-contact thermometry at the temporal artery using the arterial heat balance method (Hebbar et al., 2005, Kistemaker et al., 2006, Kimberger et al., 2007, Roy et al., 2003), other types of skin-contact thermometry (Thomas, 2003, Thomas et al., 2004), and non-skin-contact infrared thermometry (De Curtis et al., 2008, Osio and Carnelli, 2007). However, skin-based thermometry appears to be more suited for wireless monitoring because the human body has a large skin surface, and more options are available for selecting an area that would make the sensor easier to install, less obtrusive, and more aesthetically appealing.
Advances in wireless technologies have made possible a wide range of applications in physiological monitoring (Budinger, 2003, Curtis et al., 2008, Gao et al., 2006). The ThermoSENSOR, when used with the associated application software, enables nurses and clinicians to view patients' readings and receive alerts for abnormal readings not only from computers at nursing stations but also from portable wireless devices that are connected to the hospital's LAN, as well as from mobile telecommunication devices if the server is configured to communicate with such devices (Figure 2). Because of its use of RFID technology, the ThermoSENSOR also functions as a location sensor, which can be used for contact tracing during outbreaks of contagious diseases such as severe acute respiratory syndrome and influenza (Eames and Keeling, 2003, Klinkenberg et al., 2006). In addition, the ThermoSENSOR can potentially be used in nonclinical settings that require a person's temperature and location to be tracked in an ambulatory manner, such as at home, in a nursing home, or in the workplace.
Notwithstanding its advantages, wireless physiological monitoring requires several technical considerations. First, wireless devices are subject to loss of signals caused by distance and physical obstructions between the sensor and the receiver (Molisch, 2005). In this study, the sensor was transmitting a reading every 30 seconds on average, so the first TT reading after the recorded measurement start time should have always occurred within the first minute. As the results show (Results section and Table 3), this did not always happen. This signal reception problem can be mitigated by installing more receivers distributed in the vicinity of the patients. Second, wireless devices are also subject to loss of signals caused by electromagnetic interference from electromagnetic signals emitted by other devices in the neighborhood if the emitted signals include the radio frequencies used by the wireless devices (Ashar and Ferriter, 2007, Bit-Babik et al., 2007). Last but not least, signals emitted by wireless devices can potentially interfere with the normal functions of other devices in the neighborhood if these other devices share the same frequencies as those of the wireless devices (Bit-Babik et al., 2007, van der Togt et al., 2008). For this reason, the ThermoSENSOR is not recommended for use on patients with an implanted cardiac device such as a pacemaker or an internal cardioverter-defibrillator (Dyrda and Khairy, 2008, Sweesy et al., 2004, Yerra and Reddy, 2007). Because of all these technical characteristics, installation of the ThermoSENSOR system or any other wireless physiological monitoring system in any location must take into consideration the physical structures of the location and the electromagnetic compatibility characteristics of other devices—wireless and nonwireless—that are used or will be used in and around the location. With proper site survey, planning, and system design and installation, the undesirable effects of these technical characteristics can be mitigated, and the benefits of a wireless monitoring system will outweigh the costs of setting up and running the system.
Acknowledgments
Cadi Scientific provided the wireless temperature monitoring system for the study. KK Women's and Children's Hospital provided the manpower for collecting the temperature data. We thank Milagrosa Pumaren and Yingying Qu of KK Women's and Children's Hospital for collecting the temperature data and May-Yin Ng of the hospital for supervising the data collection. We also thank Grace Too of Cadi Scientific for training and supporting the team at the hospital on the use of the ThermoSENSOR and associated application software. We further thank the reviewers for their helpful comments.
Footnotes
Potential conflict of interest: Dr. Zenton Goh is CEO and a director of Cadi Scientific, and Dr. Soh-Min Lim is the chief marketing officer and also a director of the company. Dr. Kim-Gau Ng participated in the study out of educational interest, on a voluntary basis, and on his personal time; he received no financial rewards and no in-kind benefits for the participation. In addition, at the time of the study and at the writing of this article, Dr. Ng was not involved in the development or marketing of temperature monitoring systems and was not affiliated with any other organization that develops or markets such systems, so he had no conflict of interest.
Contributions: S.T.W. is the principal investigator and coordinated the study. S.T.W., S.M.L., and Z.G. jointly developed the protocol. K.G.N. wrote the article, analyzed the data, and interpreted the results. All the authors reviewed and approved the article.
References
- American College of Emergency Physicians Clinical Policies Committee, & American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever Clinical policy for children younger than three years presenting to the emergency department with fever. Annals of Emergency Medicine. 2003;42:530–545. doi: 10.1067/s0196-0644(03)00628-0. [comments in 42, 546–549] [DOI] [PubMed] [Google Scholar]
- Ashar B.S., Ferriter A. Radiofrequency identification technology in health care: Benefits and potential risks. Journal of the American Medical Association. 2007;298:2305–2307. doi: 10.1001/jama.298.19.2305. [DOI] [PubMed] [Google Scholar]
- Asher C., Northington L.K. Position statement for measurement of temperature/fever in children. Journal of Pediatric Nursing. 2008;23:234–246. [Google Scholar]
- Association for the Advancement of Medical Instrumentation (AAMI) Author [amendments appeared as ANSI/AAMI SP10:2002/A1:2003 and ANSI/AAMI SP10:2002/A2:2006]; Arlington, Virginia: 2003. American national standard ANSI/AAMI SP10:2002. Manual, electronic, or automated sphygmomanometers. [Google Scholar]
- Bit-Babik G., Morrissey J.J., Faraone A., Balzano Q. Electromagnetic compatibility management of wireless transceivers in electromagnetic-interference-sensitive medical environments. Annali dell'Istituto Superiore di Sanità. 2007;43:218–224. [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Agreement between methods of measurement with multiple observations per individual. Journal of Biopharmaceutical Statistics. 2007;17:571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- Budinger T.F. Biomonitoring with wireless communications. Annual Review of Biomedical Engineering. 2003;5:383–412. doi: 10.1146/annurev.bioeng.5.040202.121653. [DOI] [PubMed] [Google Scholar]
- Byrne C., Lim C.L. The ingestible telemetric body core temperature sensor: A review of validity and exercise applications. British Journal of Sports Medicine. 2007;41:126–133. doi: 10.1136/bjsm.2006.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J.M., Terndrup T.E., Alexander D.T., Silverstone F.A., Wolf-Klein G., O'Donnell R. Determination of normal ear temperature with an infrared emission detection thermometer. Annals of Emergency Medicine. 1995;25:15–20. doi: 10.1016/s0196-0644(95)70349-7. [comments in 25, 97–99] [DOI] [PubMed] [Google Scholar]
- Chamberlain J.M., Terndrup T.E. New light on ear thermometer readings. Contemporary Pediatrics. 1994;11:66–76. [PubMed] [Google Scholar]
- Craig J.V., Lancaster G.A., Taylor S., Williamson P.R., Smyth R.L. Infrared ear thermometry compared with rectal thermometry in children: A systematic review. Lancet. 2002;360:603–609. doi: 10.1016/S0140-6736(02)09783-0. [comments in 360, 584 & 1881–1883] [DOI] [PubMed] [Google Scholar]
- Craig J.V., Lancaster G.A., Williamson P.R., Smyth R.L. Temperature measured at the axilla compared with rectum in children and young people: Systematic review. BMJ. 2000;320:1174–1178. doi: 10.1136/bmj.320.7243.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D.W., Pino E.J., Bailey J.M., Shih E.I., Waterman J., Vinterbo S.A. SMART—An integrated wireless system for monitoring unattended patients. Journal of the American Medical Informatics Association. 2008;15:44–53. doi: 10.1197/jamia.M2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Curtis M., Calzolari F., Marciano A., Cardilli V., Barba G. Comparison between rectal and infrared skin temperature in the newborn. Archives of Disease in Childhood, Fetal and Neonatal Edition. 2008;93:F55–F57. doi: 10.1136/adc.2006.114314. [DOI] [PubMed] [Google Scholar]
- Devrim I., Kara A., Ceyhan M., Tezer H., Uludağ A.K., Cengiz A.B. Measurement accuracy of fever by tympanic and axillary thermometry. Pediatric Emergency Care. 2007;23:16–19. doi: 10.1097/PEC.0b013e31802c61e6. [DOI] [PubMed] [Google Scholar]
- Dyrda K., Khairy P. Implantable rhythm devices and electromagnetic interference: Myth or reality? Expert Review of Cardiovascular Therapy. 2008;6:823–832. doi: 10.1586/14779072.6.6.823. [DOI] [PubMed] [Google Scholar]
- Eames K.T.D., Keeling M.J. Contact tracing and disease control. Proceedings of the Royal Society, B, Biological Sciences. 2003;270:2565–2571. doi: 10.1098/rspb.2003.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Radhi A.S., Barry W. Thermometry in paediatric practice. Archives of Disease in Childhood. 2006;91:351–356. doi: 10.1136/adc.2005.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Committee for Standardization (CEN) Author; Brussels: 2004. European standard EN 1060-4:2004. Non-invasive sphygmomanometers—Part 4: Test procedures to determine the overall system accuracy of automated non-invasive sphygmomanometers. [Google Scholar]
- Gant N., Atkinson G., Williams C. The validity and reliability of intestinal temperature during intermittent running. Medicine & Science in Sports & Exercise. 2006;38:1926–1931. doi: 10.1249/01.mss.0000233800.69776.ef. [DOI] [PubMed] [Google Scholar]
- Gao T., Hauenstein L.K., Alm A., Crawford D., Sims C.K., Husain A. Vital signs monitoring and patient tracking over a wireless network. Johns Hopkins APL Technical Digest. 2006;27:66–74. [Google Scholar]
- Hebbar K., Fortenberry J.D., Rogers K., Merritt R., Easley K. Comparison of temporal artery thermometer to standard temperature measurements in pediatric intensive care unit patients. Pediatric Critical Care Medicine. 2005;6:557–561. doi: 10.1097/01.pcc.0000163671.69197.16. [DOI] [PubMed] [Google Scholar]
- Heusch A.I., McCarthy P.W. The patient: A novel source of error in clinical temperature measurement using infrared aural thermometry. Journal of Alternative and Complementary Medicine. 2005;11:473–476. doi: 10.1089/acm.2005.11.473. [DOI] [PubMed] [Google Scholar]
- Hughes W.T., Armstrong D., Bodey G.P., Bow E.J., Brown A.E., Calandra T. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clinical Infectious Diseases. 2002;34:730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization (ISO) Author; Geneva: 2008. Draft international standard ISO/DIS 80601-2-56. Medical electrical equipment—Part 2-56: Particular requirements for basic safety and essential performance of clinical thermometers for body temperature measurement. [Google Scholar]
- Kaul D.R., Flanders S.A., Beck J.M., Saint S. Brief report: Incidence, etiology, risk factors, and outcome of hospital-acquired fever: A systematic, evidence-based review. Journal of General Internal Medicine. 2006;21:1184–1187. doi: 10.1111/j.1525-1497.2006.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberger O., Cohen D., Illievich U., Lenhardt R. Temporal artery versus bladder thermometry during perioperative and intensive care unit monitoring. Anesthesia & Analgesia. 2007;105:1042–1047. doi: 10.1213/01.ane.0000281927.88935.e0. [DOI] [PubMed] [Google Scholar]
- Kistemaker J.A., Den Hartog E.A., Daanen H.A. Reliability of an infrared forehead skin thermometer for core temperature measurements. Journal of Medical Engineering & Technology. 2006;30:252–261. doi: 10.1080/03091900600711381. [DOI] [PubMed] [Google Scholar]
- Klinkenberg D., Fraser C., Heesterbeek H. The effectiveness of contact tracing in emerging epidemics. PLoS ONE. 2006;1:e12. doi: 10.1371/journal.pone.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson L., Bridges E.J., Ballou I., Eraker R., Greco S., Shively J. Accuracy and precision of noninvasive temperature measurement in adult intensive care patients. American Journal of Critical Care. 2007;16:485–496. [PubMed] [Google Scholar]
- Lim C.L., Byrne C., Lee J.K. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Annals of the Academy of Medicine, Singapore. 2008;37:347–353. [PubMed] [Google Scholar]
- Loveys A.A., Dutko-Fioravanti I., Eberly S.W., Powell K.R. Comparison of ear to rectal temperature measurements in infants and toddlers. Clinical Pediatrics (Philadelphia) 1999;38:463–466. doi: 10.1177/000992289903800804. [DOI] [PubMed] [Google Scholar]
- Markides G.A., Omorphos S., Kotoulas C., Prendergast B. Evaluation of a wireless ingestible temperature probe in cardiac surgery. Thoracic and Cardiovascular Surgeon. 2007;55:442–446. doi: 10.1055/s-2007-965371. [DOI] [PubMed] [Google Scholar]
- Molisch A.F. Wiley-IEEE; Chichester: 2005. Wireless communications. [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) Author; London: 2007. Feverish illness in children: Assessment and initial management in children younger than 5 years. Clinical guideline.http://www.nice.org.uk/Guidance/CG47 Retrieved September 20, 2008, from. [Google Scholar]
- O'Brien E., Petrie J., Littler W., de Swiet M., Padfield P.L., Altman D.G. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. Journal of Hypertension. 1993;11(Suppl. 2):S43–S62. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- O'Brien E., Pickering T., Asmar R., Myers M., Parati G., Staessen J. Working group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Pressure Monitoring. 2002;7:3–17. doi: 10.1097/00126097-200202000-00002. [comments in 7, 1–2, 289–291] [DOI] [PubMed] [Google Scholar]
- O'Grady N.P., Barie P.S., Bartlett J.G., Bleck T., Carroll K., Kalil A.C. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Critical Care Medicine. 2008;36:1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [erratum in 36, 1992] [DOI] [PubMed] [Google Scholar]
- Osio C.E., Carnelli V. Comparative study of body temperature measured with a non-contact infrared thermometer versus conventional devices: The first Italian study on 90 pediatric patients. Minerva Pediatrica. 2007;59:327–336. [PubMed] [Google Scholar]
- Oulton J.A. The global nursing shortage: An overview of issues and actions. Policy, Politics, & Nursing Practice. 2006;7(3 Suppl):34S–39S. doi: 10.1177/1527154406293968. [DOI] [PubMed] [Google Scholar]
- Powell K.R., Smith K., Eberly S.W. Ear temperature measurements in healthy children using the arterial heat balance method. Clinical Pediatrics (Philadelphia) 2001;40:333–336. doi: 10.1177/000992280104000605. [DOI] [PubMed] [Google Scholar]
- Racinais S., Gaoua N., Grantham J. Hyperthermia impairs short term memory and peripheral motor drive transmission. Journal of Physiology. 2008;19:4751–4762. doi: 10.1113/jphysiol.2008.157420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Powell K., Gerson L.W. Temporal artery temperature measurements in healthy infants, children, and adolescents. Clinical Pediatrics (Philadelphia) 2003;42:433–447. doi: 10.1177/000992280304200508. [DOI] [PubMed] [Google Scholar]
- Ryan M., Levy M.M. Clinical review: Fever in intensive care unit patients. Critical Care. 2003;7:221–225. doi: 10.1186/cc1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabia J.A., Rol M.A., Mendiola P., Madrid J.A. Circadian rhythm of wrist temperature in normal-living subjects: A candidate of new index of the circadian system. Physiology & Behavior. 2008;95:570–580. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Smith L.S. Reexamining age, race, site, and thermometer type as variables affecting temperature measurement in adults—A comparison study. BMC Nursing. 2003;2:1. doi: 10.1186/1472-6955-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.S. Temperature measurement in critical care adults: A comparison of thermometry and measurement routes. Biological Research for Nursing. 2004;6:117–125. doi: 10.1177/1099800404268917. [DOI] [PubMed] [Google Scholar]
- Sweesy M.W., Holland J.L., Smith K.W. Electromagnetic interference in cardiac rhythm management devices. AACN Clinical Issues. 2004;15:391–403. doi: 10.1097/00044067-200407000-00007. [DOI] [PubMed] [Google Scholar]
- Taylor B.N., Kuyatt C.E. National Institute of Standards and Technology; Gaithersburg, Maryland: 1994. NIST technical note 1297. Guidelines for evaluating and expressing the uncertainty of NIST measurement results. [Google Scholar]
- Thomas K.A. Comparability of infant abdominal skin and axillary temperatures. Newborn & Infant Nursing Reviews. 2003;3:173–178. [Google Scholar]
- Thomas K.A., Burr R., Wang S.Y., Lentz M.J., Shaver J. Axillary and thoracic skin temperatures poorly comparable to core body temperature circadian rhythm: Results from 2 adult populations. Biological Research for Nursing. 2004;5:187–194. doi: 10.1177/1099800403260620. [DOI] [PubMed] [Google Scholar]
- van der Togt R., van Lieshout E.J., Hensbroek R., Beinat E., Binnekade J.M., Bakker P.J. Electromagnetic interference from radio frequency identification inducing potentially hazardous incidents in critical care medical equipment. Journal of the American Medical Association. 2008;299:2884–2890. doi: 10.1001/jama.299.24.2884. [comments in 299, 2898–2899] [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W.D., Daanen H.A., Wouters L., Fronczek R., Raymann R.J., Severens N.M. Evaluation of wireless determination of skin temperature using iButtons. Physiology & Behavior. 2006;88:489–497. doi: 10.1016/j.physbeh.2006.04.026. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Author; Geneva: 2006. The world health report 2006: Working together for health. [Google Scholar]
- Yerra L., Reddy P.C. Effects of electromagnetic interference on implanted cardiac devices and their management. Cardiology in Review. 2007;15:304–309. doi: 10.1097/CRD.0b013e31813e0ba9. [DOI] [PubMed] [Google Scholar]


