Highlights
► We compared the amounts of otitis media episodes in OPV vs. IPV vaccinated children. ► OPV provides some protection against otitis media. ► The protective effect is strongest at the age of 6–18 months.
Abbreviations: OPV, oral polio vaccine; IPV, inactivated polio vaccine; DIPP, Diabetes Prediction and Prevention study; OM, otitis media
Keywords: OPV, Otitis media, Daycare, Children, Vaccine
Abstract
Background
The goal of this study was to evaluate whether a live attenuated poliovirus vaccine (OPV) has clinically relevant interfering effect with non-polio infections causing otitis media in young children.
Methods
Open trial in which the intervention group (64 children) received OPV at the age of 2, 3, 6 and 12 months. The control group (250 children) received IPV (inactivated polio vaccine) at the age of 6 and 12 months. Clinical symptoms were recorded by a questionnaire at the age of 3, 6, 12, 18 and 24 months.
Results
Otitis media episodes were less frequent in the OPV than in the control group. A significant difference was seen at the age of 6–18 months (IRR = 0.76 [95% CI 0.59–0.94], P = 0.011) and was particularly clear among children, who attended daycare (IRR 0.37 [95% CI 0.19–0.71], P = 0.003).
Conclusions
OPV provides some protection against otitis media. This effect may be mediated by viral interference with non-polio viruses.
1. Introduction
Acute upper respiratory infections (URIs), like common cold, are prominent acute diseases in children and picornaviruses are the causal agent in the majority of the cases [1]. URIs and acute otitis media (AOM) are closely linked; 29–61% of all cases of URI are complicated by AOM [2]. Otitis media can be caused by viruses and bacteria, and mixed infections are common. The most common viruses found in AOM are rhino- and enteroviruses, which both belong to the enterovirus genus [3].
Vaccines against the most well-known enteroviruses, polioviruses, are routinely used in almost all countries, while vaccines are not available for other enteroviruses. In Finland, as well as in an increasing number of other countries, children are routinely vaccinated against poliomyelitis with the inactivated poliovirus vaccine (IPV, Salk vaccine). The other type of polio vaccine, oral live vaccine (OPV, Sabin vaccine), is also used widely. In contrast to IPV, OPV induces a strong local immune response in the gastrointestinal tract and a strong systemic cell-mediated immune response [4]. Previous studies have shown that cell-mediated immune responses to polioviruses cross-react with non-polio enteroviruses due to their structural similarities [5], [6], [7]. However, it is not clear if this cross-reactivity can provide cross-protection against non-polio enteroviruses [8]. In addition, the replication of OPV strains for prolonged periods leads to long-lasting type 1 interferon response and an antiviral state, which can further improve the resistance to other viruses. In contrast to OPV, IPV lacks these properties. IPV induces mainly antibodies, and the antibody-mediated protection is poliovirus specific. In addition, IPV is considered to induce much weaker and shorter type 1 interferon secretion.
Viral interference has been reported in a number of studies. Recently Wang et al. noticed a negative association between the presence of rhinovirus (HRV) and adenovirus (HAdV) in military recruits, suggesting viral interference between HRV and HAdV [9]. In addition, the start of the influenza pandemic was delayed at the beginning of the autumn in 2009 in France, possibly due to viral interference between rhinoviruses and influenza A (H1N1) viruses [10]. Such interference may result from the non-specific innate immune response. The interferon response caused by HRV induces a refractory state in the respiratory tract preventing infections by other respiratory viruses. A study assessing the factors affecting immunogenicity of the first two doses of OPV among unimmunized Mayan infants revealed viral interference also between Sabin type 2 and Sabin type 3 polioviruses. In addition, concurrent enteric non-polio enterovirus infections seemed to lead to reduced antibody responses to Sabin types 1 and 2 [11].
The objective of this study was to assess whether the above-mentioned properties of live attenuated poliovirus vaccine (OPV) can lead to clinically relevant interference with non-polio viruses. Therefore, we carried out a vaccine trial to study the effect of OPV on the occurrence otitis media in children younger than 2 years of age. As enteroviruses, including also rhinoviruses, are a common cause of otitis media, we hypothesized that OPV would reduce the incidence of otitis media.
2. Materials and methods
2.1. Subjects
In this open trial the intervention group (64 children; 34 boys and 30 girls) was vaccinated with the oral polio vaccine (OPV) at the age of 2, 3, 6, and 12 months during the years 1999–2001 (Fig. 1 ). The control group comprising 250 children (122 boys and 128 girls) received inactivated poliovirus vaccine (IPV) at the age of 6, 12, and 24 months according to the standard immunization protocol in Finland at that time. The intervention and control groups were matched for gender, date of birth (±2 months) and HLA type, thus controlling for possible confounding effect of the season on the number of infections and that of HLA on infection immunity.
Fig. 1.
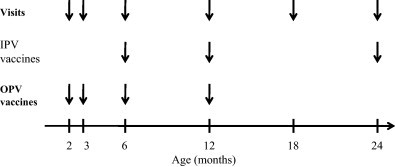
Vaccination schedule in the intervention (OPV) and control groups (IPV).
All children were healthy newborns who were recruited to the prospective Type 1 Diabetes Prediction and Prevention study (DIPP) at Tampere University Hospital in Finland. In the DIPP study all newborn infants whose families gave informed consent to the study were first screened using cord blood for HLA class II alleles conferring susceptibility to type 1 diabetes, and those with increased risk were recruited to prospective follow-up [12]. This set-up made it possible to match the intervention and control groups for their HLA alleles; they all carried the DQB1*02/0302 or the DQB1*0302/x genotype, where x refers to alleles other than *02, *0301, or *0602-03 [13].
OPV was given to all DIPP children whose parents consented to OPV vaccination during the recruitment period and who did not have immunodeficiency, malignant disease or immunosuppressive treatment. Maximally five control children were then selected for each OPV-vaccinated child from the large cohort of all DIPP children (randomly from all children who fulfilled matching criteria described above). Allocation ratio of 1:2–5 was chosen to maximize the number of participants (IPV group followed the normal Finnish vaccination schedule which made it possible to have more IPV vaccinated children). Table 1 compares some of the demographic features of the groups. The only significant difference was in the proportion of children who were breastfed at the age of 6 months, which was higher in the OPV group.
Table 1.
Demographic characteristics of the study subjects.
| Oral polio vaccine | Inactivated polio vaccine | P | |
|---|---|---|---|
| No. of children vaccinated | 64 | 250 | |
| Males (%) | 34 (53.1) | 122 (48.8) | NS |
| No. of subjects in daycare at the age of 12 months (%) | 11 (17.2) | 56 (22.6) | NS |
| No. of siblings (mean) | 1.09 | 0.88 | NS |
| No. of subjects with pets (%) | 17 (26.6) | 75 (30.5) | NS |
| No. of subjects breastfed at the age of 6 months (%) | 45 (72.6) | 137 (56.1) | 0.014 |
| No. of mothers who smoked during pregnancy | 9 (14.3) | 22 (9.0) | NS |
NS, not significant.
The local Ethics committee and the National Agency for Medicine in Finland approved the study. A written informed consent was obtained from the parents of all study children.
2.2. Recording of infections
The subjects visited the study center at the age of 2, 3, 6, 12, 18, and 24 months. The parents were interviewed by a study nurse using a standard questionnaire during each visit. The number of AOM episodes which the child had encountered since their last visit to the clinic were recorded and stored in the study database. All AOM episodes which were reported by parents and diagnosed by a physician were counted. Thus, all episodes were diagnosed by a doctor. Prolonged otitis media such as secretory otitis media was counted only once when first diagnosed in the acute phase. Information on the diet and the type of daycare (such as care at home, family care, daycare center, other) was also documented in the context of each visit. The number of siblings and pets were recorded as well.
2.3. Statistical methods
Poisson regression model was used as a multivariate analysis comparing the effects of vaccination type, gender, starting age of daycare, duration of breastfeeding, number of siblings and pets to the number of infections recorded in the 1:2–5 matched case–control groups in this cohort study. Results are presented as incidence rate ratios (IRR). The χ 2-test was used to analyze the statistical significance of differences observed between the smoking habits during pregnancy of the mothers of OPV and IPV vaccinated children. The software packages used were SPSS, version 15.0 (SPSS, Chicago, IL, USA) and Stata, version 8.2 (StataCorp, 4905 College Station, TX, USA). Differences were considered significant at P < 0.05.
3. Results
All the participants received polio vaccines as planned. The total duration of follow-up was 614 person-years and the median duration 24.3 months. Altogether 89.8% of the children (92.2% of the OPV vaccinated and 89.2% of the IPV vaccinated) completed the entire follow-up. In the OPV group 3.1% and 4.7% of the subjects dropped out at the ages of 12 and 18 months, and in the IPV group 0.4%, 6.0% and 4.4% dropped out at the ages of 6, 12 and 18 months, respectively. A total number of 854 OM episodes were recorded with an average of 2.72 per child during the follow-up.
The average number of OM episodes was lower among girls than boys (2.33 vs. 3.12 per child, IRR = 0.71 [95% CI 0.55–0.92], P = 0.010). Being in daycare outside home at the age of 12 months significantly increased the average number of OM episodes during the first 24 months of life (3.64 vs. 2.49 per child, IRR = 1.61 [95% CI 1.36–1.92], P < 0.001). Having one or more siblings increased also the number of OM episodes (2.97 vs. 2.48 per child, IRR = 1.19 [95% CI 1.09–1.30], P < 0.001). The average number of OM was lower among subjects with one or more pets compared to subjects with no pets (2.45 vs. 2.86, IRR = 0.78 [95% CI 0.66–0.94], P = 0.007). The duration of breastfeeding for more than 6 months did not have a significant effect on the rate of OM or other recorded infections.
The OPV vaccinated children had slightly less OM episodes during the first 2 years of life than the IPV vaccinated controls (average number 2.59 vs. 2.75 per child, IRR = 0.87 [95% CI 0.72–1.04]), but the difference was not statistically significant (P = 0.121). Since the protecting effect of OPV probably occurred at the age when vaccine viruses were actively replicating in the gut mucosa (at the age of 2–18 months) and when the incidence of OM peaks (at the age of 6–18 months), the numbers of OM episodes were further analyzed between the age of 6 and 18 months. A significant difference was seen between the OPV and IPV groups in the number of OM episodes during this age period (1.58 vs. 1.88 episodes per child, IRR = 0.76 [95% CI 0.59–0.94], P = 0.011). The protective effect of OPV was even stronger among subjects who had started daycare already at the age of 12 months or earlier (P = 0.003; Fig. 2 ). All these analyses were adjusted for the effect of possible confounding factors, i.e. for daycare, pets, breastfeeding and number of siblings.
Fig. 2.
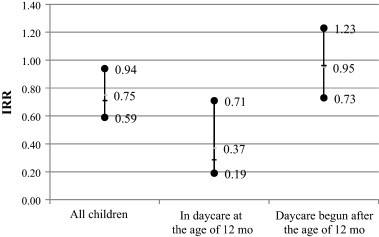
The incidence rate ratio (IRR; mean and 95% CI) for otitis media at the age of 6–18 months in OPV vs. IPV vaccinated children according to the type of daycare (home/daycare center). Statistical significance for all children: P = 0.011; in daycare at the age of 12 months: P = 0.003; daycare begun after the age of 12 months: P = NS. Possible confounding factors, i.e. daycare, pets, breastfeeding and number of siblings, have been taken in to account in statistical analyses.
4. Discussion
Acute OM episodes are one of the most common reasons for a child to visit a doctor under the age of 3 years being the most frequent indication for the prescription of antibiotics. Furthermore, OM causes significant economical losses to the families and to the society [14], [15]. Viruses play an important role in the etiology of OM and predispose to bacterial invasion to the middle ear leading to purulent infection. It has also been estimated that 20–50% of acute OM cases are due to virus infections without bacterial co-infection [1], [16]. Picornaviruses (including rhino- and enteroviruses) are the most common viruses in acute OM in children followed closely by other viruses such as influenza virus, parainfluenza virus, RSV, adenovirus, human bocavirus, human metapneumovirus and coronavirus [17], [18]. Moreover, rhinoviruses have been shown to be the most prevalent pathogens in lower respiratory infections in infants [19], [20]. Recent studies have shown that particularly the C-type rhinoviruses are able to cause severe infections in young children [21].
As a virus infection often initiates bacterial OM, it would be highly beneficial to prevent the initial viral infection. In fact, the influenza A vaccine has been observed to decrease OM episodes by 32–71% during the influenza seasons [22], [23]. The efficacy is so better than that of the pneumococcal conjugate vaccine, but the type of vaccine may also be important for this protection [24]. Conjugated polysaccharide vaccines, like the heptavalent pneumococcal conjugate vaccine, are serotype-specific and do not protect from infections caused by serotypes not included in the vaccine [25]. In contrast, live attenuated virus vaccines can often induce viral-interference and immunological cross-reactivity against other genetically related agents.
This is the first study to assess the effect of a live attenuated enterovirus vaccine (OPV) on non-polio infections in childhood. Picornaviruses comprise approximately 300 different serotypes capable of infecting humans and causing symptoms. As polioviruses belong to picornaviruses, which are the main causative group of viruses causing acute OM, our finding that OPV can indeed reduce acute OM episodes suggests that OPV may interfere with other picornaviruses which are causing OM. Our finding is also indirectly supported by some earlier studies. According to reports from the 1960s, concurrent infection with other enteroviruses demonstrated interference with the OPV vaccination, being infavourable for the vaccine [26], [27]. Epidemiological observations from South America suggest that non-specific intestinal interference generated by OPV strains reduced mortality from infantile diarrhea [28]. This knowledge was utilized in Bulgaria in 1975, when universal administration of OPV was applied to help terminate a non-poliovirus epidemic [29]. Thus, this kind of viral interference seems to have a clear biological effect. Such interference can be caused by at least two different mechanisms: (1) cross-protection against genetically related non-polio enteroviruses mediated by cross-reactive cellular immune responses or (2) induction of type 1 interferons and other antiviral innate immune system responses which can protect against many different viruses. Compared to many other viruses the interfering effect of OPV may be particularly strong, since the replication of vaccine virus strains lasts for longer periods than that of many other viruses replicating for weeks and sometimes even for months in vaccinated individuals and leading to long-lasting and strong activation in both the innate and the adaptive immune system. As enteroviruses (including also rhinoviruses) constitute the most important virus group causing OM, even a modest protective effect may have an important clinical impact.
It is known that approximately 70% of children have experienced at least one episode of OM by the age of 24 months, with a peak incidence between 6 and 18 months [14], [30]. Several risk factors for OM have been identified. Our findings that daycare, number of siblings and male gender increase the incidence of OM are in line with previous observations [31]. The fact that the protective effect of OPV was seen particularly among those children who started daycare at a young age is probably due to their strong exposure to viruses causing OM. In addition, the protective effect was strongest at the age of 6–18 months when acute OM peaks. This fits also into the time period when the interfering effect of OPV is expected to occur, if the vaccine is given four times before age of 12 months. However, these results should be interpreted with caution, since the number of the study subjects was relatively small and certain possible confounding factors, e.g. antibiotic treatments during the follow-up could not be taken in to account. The possible confounding effect of other vaccines is unlikely, since the coverage of the national vaccination program was very high (93.3–95.2%) and additional vaccines were rarely given (data not shown).
Interestingly, pets at home had an independent protective effect against OM, a phenomenon which has also been reported previously [32]. The reason to this is not known. It may be related to the avoidance of pets by atopic families (atopy is a risk factor for OM) or to possible booster effect of pets on immune protection against infections. A recent study suggests that furry pets can also reduce the risk of recurrent acute respiratory illnesses [33].
The identification of OM episodes was based on information given by the parents during the interview. Both intervention and control groups were interviewed prospectively in a structured manner using a standard questionnaire, which minimized possible bias in recordings between the groups. In addition, acute OM leads usually to a quite clear episode, and its diagnosis was always made by a doctor. Our study has also the important advantage that the symptoms were recorded during consecutive visits at the study clinic in a prospective follow-up where the maximum time period from the previous visit was only 6 months. Thus, it is very likely that the parents were able to recall OM episodes reliably over such a short period. In fact, the mean number of acute OM episodes parents reported was 1.39 per child-year, similar to that found in previous studies, further suggesting that the reporting of these episodes was quite reliable [3], [34].
5. Conclusions
This study suggests that a live attenuated enterovirus vaccine may play a role in modulating immunity against other microbial infections. The protective effect of OPV against OM suggests that such interference may provide important clinical benefit. Even though the safety profile of OPV may not be optimal for the prevention of OM in general, this study suggests that live virus vaccines may have beneficial effects which are much wider than their original indication. Therefore, the mechanisms of viral interference induced by live virus vaccines should be studied further. It is possible that such an interfering effect could be applied in the development of new vaccines against picornavirus-related diseases such as OM, meningitis, myocarditis and type 1 diabetes.
Acknowledgements
The study was financially supported by the Academy of Finland, the Juvenile Diabetes Research Foundation and the Competitive Research Funding of the Tampere University Hospital.
Conflict of interest statement: H. Hyöty and M. Knip are minor shareholders in Vactech LtD, which develops vaccines against picornaviruses. All other authors declare that there is no duality of interest associated with this manuscript. Funding: OPV vaccines were received as a gift from GSK (former SmithKlineBeecham). IPV vaccines were given in primary health care as a part of the national vaccination program.
References
- 1.Nokso-Koivisto J., Hovi T., Pitkäranta A. Viral upper respiratory tract infections in young children with emphasis on acute otitis media. Int J Pediatr Otorhinolaryngol. 2006;70(8):1333–1342. doi: 10.1016/j.ijporl.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chonmaitree T., Revai K., Grady J.J., Clos A., Patel J.A., Nair S. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46(6):815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nokso-Koivisto J., Räty R., Blomqvist S., Kleemola M., Syrjänen R., Pitkäranta A. Presence of specific viruses in the middle ear fluids and respiratory secretions of young children with acute otitis media. J Med Virol. 2004;72:241–248. doi: 10.1002/jmv.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jesus N.H. Epidemics to eradication: the modern history of poliomyelitis. Virol J. 2007;4:70. doi: 10.1186/1743-422X-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cello J., Strannegård O., Svennerholm B. A study of the cellular immune response to enteroviruses in humans: identification of cross-reactive T cell epitopes on the structural proteins of enteroviruses. J Gen Virol. 1996;77(Pt. 9):2097–2108. doi: 10.1099/0022-1317-77-9-2097. [DOI] [PubMed] [Google Scholar]
- 6.Juhela S., Hyöty H., Lönnrot M., Roivainen M., Simell O., Ilonen J. Enterovirus infections and enterovirus specific T-cell responses in infancy. J Gen Virol. 1998;54(3):226–232. doi: 10.1002/(sici)1096-9071(199803)54:3<226::aid-jmv14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Marttila J., Hyöty H., Vilja P., Härkönen T., Alho A., Roivainen M. T cell epitopes in coxsackievirus B4 structural proteins concentrate in regions conserved between enteroviruses. Virology. 2002;293(2):217–224. doi: 10.1006/viro.2001.1259. [DOI] [PubMed] [Google Scholar]
- 8.Juhela S., Hyöty H., Uibo R., Meriste S.H., Uibo O., Lönnrot M. Comparison of enterovirus-specific cellular immunity in two populations of young children vaccinated with inactivated or live poliovirus vaccines. Clin Exp Immunol. 1999;117(1):100–105. doi: 10.1046/j.1365-2249.1999.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., Malanoski A.P., Lin B., Long N.C., Leski T.A., Blaney K.M. Broad spectrum respiratory pathogen analysis of throat swabs from military recruits reveals interference between rhinoviruses and adenoviruses. Microb Ecol. 2010;59(4):623–634. doi: 10.1007/s00248-010-9636-3. [DOI] [PubMed] [Google Scholar]
- 10.Casalegno J.S., Ottmann M., Duchamp M.B., Escuret V., Billaud G., Frobert E. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect. 2010;16(4):326–329. doi: 10.1111/j.1469-0691.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 11.Maldonado Y.A., Peña-Cruz V., de la Luz Sanchez M., Logan L., Blandon S., Cantwell M.F. Host and viral factors affecting the decreased immunogenicity of Sabin type 3 vaccine after administration of trivalent oral polio vaccine to rural Mayan children. J Infect Dis. 1997;175(3):545–553. doi: 10.1093/infdis/175.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Näntö-Salonen K., Kupila A., Simell S., Siljander H., Salonsaari T., Hekkala A. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 13.Kupila A., Muona P., Simell T., Arvilommi P., Savolainen H., Hamalainen A.M. Juvenile Diabetes Research Foundation Centre for the Prevention of Type I Diabetes in Finland Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia. 2001;44:290–297. doi: 10.1007/s001250051616. [DOI] [PubMed] [Google Scholar]
- 14.Cripps A.W., Otczyk D.C., Kyd J.M. Bacterial otitis media: a vaccine preventable disease? Vaccine. 2005;23(17–18):2304–2310. doi: 10.1016/j.vaccine.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Nurmi T., Salminen E., Pönkä A. Infections and other illnesses of children in day-care centers in Helsinki II: the economic losses. Infection. 1991;19(5):331–335. doi: 10.1007/BF01645358. [DOI] [PubMed] [Google Scholar]
- 16.Bulut Y., Güven M., Otlu B., Yenisehirli G., Aladag I., Eyibilen A. Acute otitis media and respiratory viruses. Eur J Pediatr. 2007;166:223–228. doi: 10.1007/s00431-006-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruohola A., Meurman O., Nikkari S., Skottman T., Salmi A., Waris M. Microbiology of acute otitis media in children with tympanostomy tubes: prevalences of bacteria and viruses. Clin Infect Dis. 2006;43(11):1417–1422. doi: 10.1086/509332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezes S., Söderlund-Venermo M., Roivainen M., Kemppainen K., Szabo Z., Sziklai I. Human bocavirus and rhino-enteroviruses in childhood otitis media with effusion. J Clin Virol. 2009;46(3):234–237. doi: 10.1016/j.jcv.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusel M., de Klerk N.H., Holt P.G., Kebadze T., Johnston S.L., Sly P.D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life. Pediatr Infect Dis J. 2006;25(8):680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 20.Van der Zalm M.M., Uiterwaal S.P.M., Wilbrink B., de Jong B.M., Verheij T.J.M., Kimpen J.L.L. Respiratory pathogens in respiratory tract illness during the first year of life: a birth cohort study. Pediatr Infect Dis J. 2009;28(6):472–476. doi: 10.1097/inf.0b013e318195e26e. [DOI] [PubMed] [Google Scholar]
- 21.Gern J.E. The ABCs of rhinoviruses, wheezing, and asthma. J Virol. 2010;84(15):7418–7426. doi: 10.1128/JVI.02290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clements D.A., Langdon L., Bland C., Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med. 1995;149(10):1113–1117. doi: 10.1001/archpedi.1995.02170230067009. [DOI] [PubMed] [Google Scholar]
- 23.Jansen A.G., Sanders E.A., Hoes A.W., van Loon A.M., Hak E. Effects of influenza plus pneumococcal conjugate vaccination versus influenza vaccination alone in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2008;153(6):764–770. doi: 10.1016/j.jpeds.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskola J., Kilpi T., Palmu A., Jokinen J., Haapakoski J., Herva E. Finnish Otitis Media Study Group Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien K.L., Santosham M. Potential impact of conjugate pneumococcal vaccines on pediatric pneumococcal diseases. Am J Epidemiol. 2004;159(7):634–644. doi: 10.1093/aje/kwh082. [DOI] [PubMed] [Google Scholar]
- 26.Ingram V., Lepow M., Warren R., Robbins F. Behavior of Sabin type 1 attenuated poliovirus in infant population infected with ECHO 14 virus. Pediatrics. 1962;29:174–180. [Google Scholar]
- 27.Feldman R.A., Holguin A.H., Gelfand H.M. Oral poliovirus vaccination in children: a study suggesting enterovirus interference. Pediatrics. 1964;33:526–533. [PubMed] [Google Scholar]
- 28.Contreras G. Effect of the administration of the oral poliovirus vaccine on infantile diarrhea mortality. Vaccine. 1989;7:211–212. doi: 10.1016/0264-410x(89)90230-2. [DOI] [PubMed] [Google Scholar]
- 29.Shindarov L.M., Chumakov M.P., Voroshilova M.K., Bojinov S., Vasilenko S.M., Iordanov I. Epidemiological, clinical, pathomorphological characteristics of epidemic poliomyelitis-like disease caused by enterovirus 71. J Hyg Epidemiol Microbiol Immunol. 1979;3:284–295. [PubMed] [Google Scholar]
- 30.Alho O.P., Koivu M., Sorri M., Rantakallio P. The occurrence of acute otitis media in infants. A life-table analysis. Int J Pediatr Otorhinolaryngol. 1991;21(1):7–14. doi: 10.1016/0165-5876(91)90054-f. [DOI] [PubMed] [Google Scholar]
- 31.Alho O.P. Incidence and risk factors in acute otitis media in early childhood. Duodecim. 1992;108(5):475–479. [PubMed] [Google Scholar]
- 32.Sipilä M., Karma P., Pukander J., Timonen M., Kataja M. The Bayesian approach to the evaluation of risk factors in acute and recurrent otitis media. Acta Otolaryngol. 1988;106:94–101. doi: 10.3109/00016488809107375. [DOI] [PubMed] [Google Scholar]
- 33.Hatakka K., Piirainen L., Pohjavuori S., Poussa T., Savilahti E., Korpela R. Factors associated with acute respiratory illness in day care children. Scand J Infect Dis. 2010;42:704–711. doi: 10.3109/00365548.2010.483476. [DOI] [PubMed] [Google Scholar]
- 34.Teele D.W., Klein J.O., Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]


