Abstract
The purpose of this subacute 22-day study was to evaluate methods for canine circulating immunoglobulins (IgM, IgG, and IgE) and select B- and T-lymphocyte populations (CD4-helpers, CD8-suppressors, pan-T and pan-B) for immunotoxicity testing using an organ system (concordance) approach. The challenge substance for immunoglobulin testing was repeated immunization with six-way distemper vaccination (DHLAPP), while the challenge substance for leukocyte subpopulations was treatment with cyclophosphamide. Immunoglobulin measurements were made by capture enzyme-linked immunosorbent assay (ELISA), and leukocyte immunophenotyping by fluorescein isothiocyanate/phycoerythrin conjugation (flow cytometry). A battery of parameters that would be used in a typical regulatory study were taken to aid interpretation of the data generated by these methods. Body weights, food consumption, clinical observations, complete clinical chemistry and urinalysis measurements were taken. Gross pathology and micropathology of sternal bone marrow, spleen, mesenteric and retropharyngeal lymph nodes, thymus, liver and kidney were completed. The ELISA method demonstrated acceptable intra-assay reproducibility for IgM, IgG and IgE, with values in good agreement as reported for radial immunodiffusion. The immunologic challenge demonstrated a biological trend of an increase in IgM that preceded an increase in IgG with no discernible trend in IgE response, and no abnormalities in lymphocyte subpopulations. Principle flow cytometry findings related to cyclophosphamide were that the relative percent of B cells decreased dramatically and progressively after compound administration; being statistically decreased in males on day 22 compared with day −5. The relative percent CD4 and CD8 contribution increased, but the CD4/CD8 ratio remained relatively unchanged as total white blood cells decreased progressively. The increase in relative percent CD4 (males only) was statistically significant according to a two-sample t-test on days 17, 20 and 22 when compared with the pre-treatment day −5. There was a relative percent increase in CD5-panT, but absolute numbers were dramatically decreased. We conclude that an organ system approach to assessment of the immune system which incorporates humoral antibody, enumeration of lymphocyte populations and pathologic evaluation of the lymphoreticular organs assists in the interpretation of an adverse toxicological response. The ELISA method for measurement of Igs detected the expected levels of IgG, IgM and IgE due to repeated vaccinations and to cyclophosphamide treatment. The flow cytometry method was acceptable for measuring select canine lymphocyte populations and detecting the expected decrease in B cells due to cyclophosphamide treatment. Both methods may be added to a testing battery for assessing immunotoxicity in canine regulatory studies.
Keywords: Canine, Immunoglobulins, Flow cytometry, Cyclophosphamide
1. Introduction
The dog is an important immunological model for mechanism work for diseases such as atopy, rheumatoid arthritis, autoimmune hemolytic anemia, autoimmune thrombocytopenia, autoimmune thyroiditis, autoimmune dermal conditions and systemic lupus erythematosis (Pedersen and Pool, 1980). Imunodeficient dwarfism is a relevant model for elucidating the endocrine role of the thymus in its relationships between the neuroendocrine and immune systems in pre-pubertal dogs (Roth et al., 1988). Moreover, the predictivity of pharmaceutical agent human toxicities by pre-clinical animal species is almost 50% from dog or primate studies, but very few toxicities are identified from rat studies alone (Olson et al., 1998). Therefore, the scientific and medical rationale for using the dog as an alternative immunologic model to rodent species extends beyond being the nonrodent alternative in regulatory studies. The use of albino rodent models is often justified on the basis of reduced maintenance costs and ease of reagent development, but recent progress to transgenic models (Burns et al., 1996) seem more conducive to specific hypothesis testing.
In the dog, nonspecific serum immunoglobulin levels are considered one of the most common assessments of immunocompetence in clinical disease (German et al., 1998). Nonspecific immunoglobulin changes have been successfully used in human occupational medicine to investigate possible immunotoxic potential of pesticide mixtures, air pollutants and to screen for possible atopic immunoglobulin (Ig)E-mediated disease (Stiller-Winkler et al., 1999). When the specific antigen exposure is known, it can be injected or exposed to the test subject, to enhance detection of an immunologic response. Some examples of measuring antigen specific response in the dog are vaccination (Barthold et al., 1995), diagnosis of atopic conditions, parasitisms (Hammerberg et al., 1997), autoimmune diseases (Jones et al., 1992) and immunologic challenge from injected sheep red blood cells. The capture enzyme-linked immunosorbent assay (ELISA) method was chosen because it has the advantage of enhanced immunologic sensitivity and accuracy compared with existing radial immunodiffusion (RID) methods (Ginel et al., 1997). Flow cytometry for lymphocyte phenotyping in the dog has been useful in quantifying lymphocyte subpopulations and, coupled with cytochemical methods, the ontogeny and therapeutic sensitivity of myeloproliferative disorders (Modiano et al., 1998).
Cyclophosphamide (CY) is a drug that interferes with cell proliferation by cross-linking DNA (Calabresi and Chabner, 1990), useful as a standard challenge substance in immunotoxicology investigations (Burns et al., 1996, Dean et al., 1998), and as anti-neoplastic therapy in the dog and humans (Medleau et al., 1983, Calabresi and Chabner, 1990). In general, CY is known to suppress both humoral and cell-mediated immune responses in rodents and in the rat model, and causes reduced CD4, B-cell numbers and SRBC-specific serum IgM levels (Ladics et al., 1995, Burns et al., 1996, Dean et al., 1998). The preferential inhibition of B-cell response may possibly be due to decreased production and surface expression of immunoglobulins (Burns et al., 1996). In the dog, Medleau et al. (1983) reported that CY reduces leukocytes, but that antibody response and lymphocyte blastogenesis were not affected.
The objective of an organ system approach to immunotoxicology assessment is detecting adverse effects of suppression, hypersensitivity, endocrine disruption (pre-pubertal thymus) that are directly or indirectly related to exposure of a drug or chemical directed toward components of the immune system (Burns et al., 1996, US-EPA-TSCA, 1997). The purpose of this study was to evaluate immunoglobulins (IgM, IgG, IgE) by ELISA and lymphocytes (CD4-helpers, CD8-suppressors, pan-T CD5 and pan-B CD21) by flow cytometry for detecting adverse effects in dogs administered positive control challenges to the immune system.
2. Materials and methods
2.1. Materials and reagents
The reagents used for ELISA consisted of: capture antibody-sheep anti-dog IgG-affinity purified, goat anti-dog IgE-affinity purified, goat anti-dog IgM, affinity purified (Bethyl Laboratories, Montgomery, TX), standard canine immunoglobulin reference serum (Bethyl Laboratories), secondary antibody-sheep anti-dog IgG horeseradish peroxidase (HRP), goat anti-dog IgE HRP, and goat anti-dog IgM HRP Conjugate (Bethyl Laboratories). The materials and chemicals used were: Immulon® 2 Flat-Bottom Plates (Dynatech Labs, Burlington, MA), blocking buffer of 10 mM phosphate-buffered saline (pH 7.4) with 0.05% Tween 20, wash buffer of 2 ml Tween 20 and 4 l high-performance liquid chromatography (HPLC) water, phosphate-citrate buffer with urea hydrogen peroxide tablets, 2,2′-azino-bis(3-ethylbenzthiazoline 6-sulfonic acid) diammonium (ABTS), all supplied by Sigma Chemical (St. Louis, MO), and U-bottom 96-well microplates.
Flow cytometry was performed using a FACSCalibur Flow cytometer (Becton Dickinson, San Jose, CA). The reagents used were: phycoerythrin (PE) anti-human CD21, fluorescein isothiocyanate (FITC) rat IgG1, FITC rat IgG2a; PE rat IgG1 (PharMingen, San Diego, CA), and rat anti-canine CD4:FITC, rat anti-canine CD8:PE; rat anti-canine CD5:FITC (Serotec, Raleigh, NC), Dulbecco’s phosphate-buffered saline (Pierce, Rockford, IL), and fetal calf serum (Sigma). The materials included: HPLC grade water, ammonium chloride, sodium azide, potassium bicarbonate, ethylenediamine tetraacetic acid (EDTA)-tetrasodium (Sigma), Falcon 2052 test tubes, and 30 ml centrifuge tubes with caps.
Positive control substances for testing biological specificity were Duramune DA2PP+CvK/LCI: Canine Distemper, Adenovirus, Parainfluenza, Parvovirus, Leptospira and Coronavirus (Fort Dodge Laboratories, Fort Dodge, IA) and cyclophosphamide (1-bis(2-chloroethyl)amino-1-oxo-2-aza-5-oxaphosphoridin) from (Sigma), considered as challenge substances A and B, respectively. The animal ration fed was Purina Mills Lab Canine Diet 5006-3, analyzed for contaminants by PMI Feeds, Inc. (St. Louis, MO).
2.2. Immunoglobulin ELISA
Immunoglobulins in dog serum were measured by an antibody capture ELISA method (Immunotox, 1997). Serum samples were taken from control animals to conduct the method testing. Serum test samples taken were aliquotted and stored at −50°C or below, then thawed and brought to room temperature before being analyzed. Prior to running samples, optimal concentrations of antigen coating (usually 1 μg/ml) and conjugate were determined. Optimal dilutions for standards and test sera were also determined.
In this procedure, Immulon 2 Flat Bottom Plates were prepared by adding 100 μl of the optimal concentration of capture antibody in 10 mM phosphate-buffered saline (pH 7.4) to all test wells in columns 2–12 of the microplate. Column 1 of the plate was used to determine any nonspecific binding and orientation of the plate, and column 2 served as the blank. After the addition of capture antibody, plates were sealed to prevent evaporation and stored overnight at 2–8°C.
The capture antibody binding step was followed by a wash step using a Bio-Tek Microplate Washer with the following settings: 200 μl wash buffer fill volume, 5 s soak time, and three cycles. This step was used between each incubation of the procedure. Test wells were then blocked with the addition of 175 μl of 10 mM phosphate-buffered saline (pH 7.4) with 0.05% Tween 20 for 1 h at room temperature. After incubation, plates were washed as previously described, then 100 μl blocking buffer was added to each well in columns 2–12. Next, appropriate test samples and standard were diluted in blocking buffer, and 150 μl each were added to a U-bottom plate. All samples and standard were performed in duplicate on each plate. The addition of 100 μl standard and test sera from the U-bottom plate to column 3 of the Immulon plate was performed using a multichannel pipette. Serial dilutions were prepared from columns 3 to 12. Plates were incubated for 1 h at room temperature.
Following the wash step, 100 μl secondary antibody was added to each test well and incubated for 1 h at room temperature. Preparation of the citrate buffer (one tablet per 100 ml distilled water) was performed approximately 30 min prior to use. The peroxidase substrate was prepared by adding one ABTS tablet for every 50 ml citrate buffer. After the wash step, 100 μl substrate was added to all wells of the plate. Plates were incubated until the standard on the first plate reached an optimal optical density at 405 nm on the Bio-Tek Ceres 900 Microplate Reader. Subsequent plates were read using this timing as the read point.
Data were collected from the microplate reader and entered into a Corel Quattro Pro worksheet. The mean absorbance values were calculated for each dilution of standard and sample. The linear portion of the standard curve was identified, using a log–log curve fit. The standard curve concentration and absorbance values were converted to log scale for calculation of the regression equation. Once the curve was established, absorbance values of the unknown were compared with the standard to determine gravimetric units of the unknown sample. Gravimetric units close together were averaged and used to obtain the final gravimetric unit. Log values were converted to base 10, and the mean, standard deviation and coefficient of variation were obtained for the gravimetric units of the samples.
2.3. Lymphocyte flow cytometry
Fresh whole blood samples were collected from control animals by cephalic venipuncture to conduct the method testing. The flow cytometer was used to analyze FITC- or PE-conjugated monoclonal antibodies to cell antigens. Optimal antibody titers had been previously determined. Antibodies were diluted in working buffer with protein carrier (0.5 ml fetal calf serum in 24.5 ml working buffer), prepared fresh daily and protected from light. The lysing buffer 10× stock solution (pH 7.3) was prepared by dissolving 22.5 g ammonium chloride, 2.5 g potassium bicarbonate, and 0.093 g EDTA-tetrasodium in 250 ml HPLC grade water. A 1× working solution of lysing buffer was prepared and maintained at room temperature. A working buffer was prepared from Dulbecco’s phosphate-buffered saline (DPBS) containing no calcium or magnesium, with the addition of 0.5 g sodium azide to 500 ml DPBS. The cold buffer was used for diluting and washing cells.
The procedure was as follows: 1 ml whole blood was added to 14 ml of 1× Lysing Buffer at room temperature, the tube capped and mixed by inversion, then left to stand at room temperature for 3–5 min. The mixture was centrifuge at 300×g for 5 min at room temperature. The supernatant was aspirated, the remaining contents mixed gently and 5 ml cold working buffer was added, mixed and centrifuged again at 300×g for 5 min at 2–8°C. The supernatant was aspirated and the pellet re-suspended in 0.5–1.0 ml working buffer with protein carrier. Confirmation of cell viability may be performed at this point. It was our experience that the optimal concentration should be approximately (3.0–6.0)−107/ml, but lower concentrations were found to be acceptable. For single color staining, 20 μl FITC- or PE-labeled antibody were added to the appropriately labeled Falcon test tube, then 50 μl cell preparation pipetted into each tube, and the mixture incubated in a dark ice bath for at least 30 min. An auto and isotype control were analyzed with each sample. After incubation, approximately 1 ml working buffer was added to each tube, and if unable to analyze immediately, were stored in a dark ice bath to be analyzed within 4 h. Results obtained from the FACSCalibur were entered into a spread sheet and appropriate statistics generated.
2.4. Criteria for method acceptance
The limited number of published canine values by capture ELISA and flow cytometry, caused a robust set of acceptance criteria to be applied prior to use in Good Laboratory/Good Automated Laboratory Practices regulatory studies (Taylor, 1987). The acceptance criteria for ELISA included: linearity, intra-assay reproducibility (system precision), inter-assay reproducibility (method precision), accuracy (% observed/expected), and immunological specificity. The acceptance criteria for flow cytometry was intra-assay reproducibility (system precision), while biological specificity for both assays was determined by use of positive control challenge substances in the target species.
2.5. Experimental animals
Purebred male and female (nulliparous and nonpregnant) beagle dogs, Canis familiaris, were obtained from White Eagle Laboratories (Doylestown, PA). The-study design required eight animals (four males and four females) who were approximately 5 months of age that had been immunized with a similar modified-live vaccine 3 weeks previous to the start of the study. The beagle was selected as the test species because of its acceptance as the nonrodent species of choice for regulatory testing, the availability of a large historical database on the strain, and because the dog is an important mechanistic model for several autoimmune diseases. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee. Upon receipt, animals were given complete physical examinations by a veterinarian, placed into individual pens, then acclimated and quarantined prior to pre-treatment measurements. The animals were housed in an accredited facility, where the environment was regulated and continuously monitored to maintain a room temperature range of 18–29°C, a relative humidity range of 30–70% and a daily photoperiod of approximately 12 h light and 12 h darkness.
2.6. Experimental design of challenge study in dogs
A treatment-by-study design (Bruning and Kintz, 1968) was chosen to reduce the number of animals needed by using each animal as its own control to minimize inter-subject variability and for similar statistical power as used in a regulatory study. The rationale was to utilize an organ system (concordance) approach to assess the immune system, meaning an incorporation of humoral antibody, enumeration of lymphocyte populations and pathologic evaluation of the lymphoreticular organs to aid in interpretation of a potential adverse toxicological response.
2.6.1. Repeated immunization
Clinical chemistries, urinalysis, complete blood count (including differentials) and flow cytometry phenotyping were performed on all animals twice prior to administration of challenge substance A. Serum samples were retained for measurement of immunoglobulins. The combination vaccine was given on day 0, 2 and 7. The next day following each immunization, serum samples were taken for analysis of IgG, IgM and IgE, along with clinical chemistry, hematology, urinalysis, and flow cytometry on day 8, to evaluate possible influences of repeated immunization.
2.6.2. Cyclophosphamide
On day 14, CY was administered as challenge substance B. Prior to dosing, a single pre-treatment clinical chemistry, urinalysis, complete blood count (including differentials), and flow cytometry phenotyping with serum retained for immunoglobulins was done on each dog. The B- and T-lymphocyte subpopulations measured were CD4-helpers, CD8-suppressors, pan-T (CD5) and pan-B (CD21). Each dog was given a single 20 mg/kg intraperitoneal injection of CY daily for 3 days (day 14, 15 and 16). A clinical chemistry, hematology, urinalysis, flow cytometry phenotyping and retained serum profile was done on each animal on days 17, 20 and 22.
2.7. Statistical analysis
Statistical analysis was performed using number cruncher Statistical Software (NCSS, Kaysville, UT). The continuous data were analyzed by the Mann–Whitney U-test or paired t-test. A probability value of P<0.05 was accepted as significant. The data were visually inspected for trends or with the aid of orthogonal polynomial linear trend analysis.
3. Results
3.1. Immunoglobulin by ELISA
The acceptance criteria results for the ELISA IgG, IgM and IgE method are given in Table 1 . All three immunoglobulin assays had acceptable linearity. The intra-assay coefficient of variation was acceptable with 5, 2 and 6% for IgG, IgM and IgE, respectively. As expected when conducted on different days, the method precision (inter-assay) coefficient of variation was greater, but considered acceptable (Table 1). The accuracy, defined as percent observed/expected, averaged 130, 101 and 129% for IgG, IgM and IgE, respectively.
Table 1.
Results of acceptance criteria for the capture ELISA immunoglobulin assaysa
| Acceptance criteria | IgG | IgM | IgE |
|---|---|---|---|
| Linearity (r2) | 0.993 | 0.999 | 0.999 |
| Precision | |||
| System (%CV) | 5 | 2 | 6 |
| Method (%CV) | 17, 21, 23 | 4, 5, 11, 6, 7 | 17, 16, 4 |
| Accuracy (%O/E) | 140, 120 | 100, 102 | 136, 123 |
| Specificity | |||
| Immunological | Curves parallel | Curves parallel | Curves parallel |
| Biological | Acceptable | Acceptable | Acceptable |
| Detection limit (ng/ml) | 5 | 7 | 39 |
| Range (ng/ml) | 5–86 | 27–219 | 39–265 |
CV, Coefficient of variation; O/E, observed/experimental.
The immunological specificity for canine was parallel with respect to the standard curve for the rat for all three immunoglobulins. The detection limit was 5, 7 and 39 ng/ml for IgG, IgM and IgE, respectively (Table 1). The linear range for IgG (5–86 ng/ml), IgM (27–219 ng/ml) and IgE (39–625 ng/ml) was considered acceptable based on the subsequent biological specificity testing (days −4 and 5 in Fig. 1 A,B and Fig. 2 ).
Fig. 1.
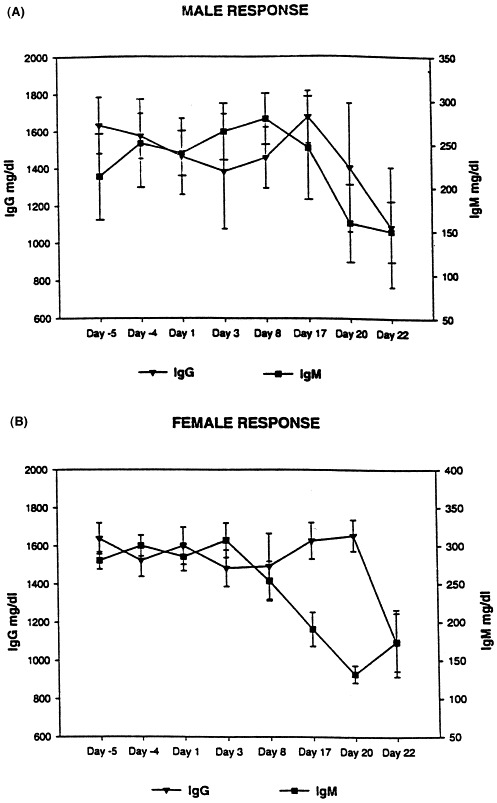
The IgG and IgM antibody levels at pre-treatment (days −4 and −5), during repeated immunization (days 1, 3 and 8) and following CY treatment (days 17, 20 and 22), determined by capture ELISA: (A) males and (B) females.
Fig. 2.
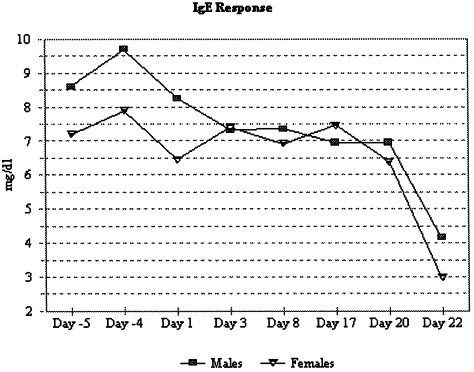
The IgE antibody levels at pre-treatment (days −4 and −5), during repeated immunization (days 1, 3 and 8) and following CY treatment (days 17, 20 and 22), determined by capture ELISA.
3.2. Lymphocyte flow cytometry
The intra-assay reproducibility for measurement of lymphocyte subpopulations was found to be acceptable (Table 2 ). The percent coefficients of variation for CD5, CD4, CD8 and CD21 were 2, 2, 3 and 5%, respectively. The biological specificity was based on treatment with CY as the positive control challenge substances in the target species (Fig. 3 A,B).
Table 2.
Intra-assay reproducibility of lymphocyte flow cytometry assay
| Animal number | T-cells CD5 | T-helper CD4 | T-Suppressor CD8 | CD4/CD8 Ratio | B-cells CD21 |
|---|---|---|---|---|---|
| 1 | 78.60 | 59.70 | 20.57 | 2.90 | 0.67 |
| 2 | 82.87 | 57.27 | 20.87 | 2.74 | 11.33 |
| 3 | 82.47 | 58.77 | 20.63 | 2.85 | 11.10 |
| 4 | 81.73 | 56.30 | 20.30 | 2.77 | 11.07 |
| 5 | 81.20 | 58.53 | 20.97 | 2.79 | 10.17 |
| 6 | 83.77 | 57.13 | 19.53 | 2.93 | 12.10 |
| 7 | 83.93 | 59.63 | 21.40 | 2.79 | 11.87 |
| 8 | 81.67 | 57.07 | 20.20 | 2.83 | 11.17 |
| 9 | 82.77 | 57.60 | 20.17 | 2.86 | 11.00 |
| 10 | 81.93 | 57.40 | 19.90 | 2.88 | 11.33 |
| Mean | 82.09 | 57.94 | 20.45 | 2.83 | 11.18 |
| ISD | 1.52 | 1.15 | 0.55 | 0.06 | 0.55 |
| %CV | 2 | 2 | 3 | 2 | 5 |
Fig. 3.
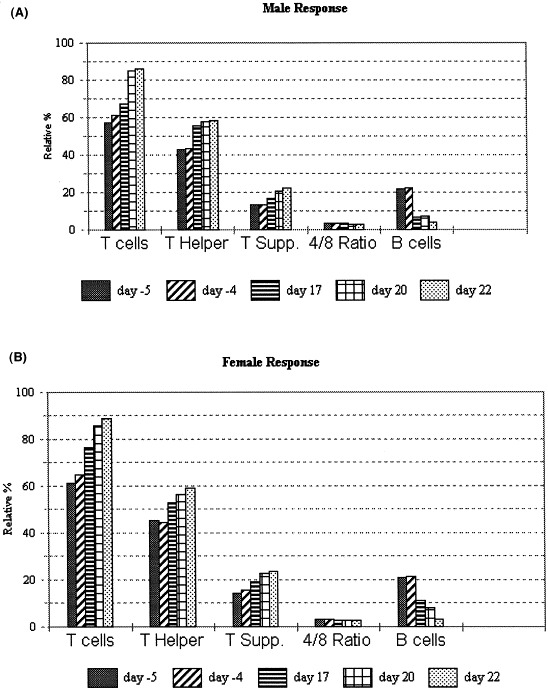
Mean relative% lymphocyte subpopulations at pre-treatment (days −4 and −5) and following CY treatment (days 17, 20 and 22) as determined by flow cytometry: (A) males and (B) females. Relative% B cells were decreased in males on day 22 compared with day −5 (P<0.05). Relative% T-helper cells (males only) were increased (P<0.05) compared with day −5 according to a two-sample t-test on days 17, 20 and 22; however, absolute numbers were dramatically decreased.
3.3. Challenge study in dogs
3.3.1. Repeated immunization
The predominant basal immunoglobulin at pre-treatment was IgG (1385–1851 mg/dl), followed by IgM (166–337 mg/dl), and the lowest basal concentration was IgE (0.7–14.7 mg/dl) according to the capture ELISA method (Fig. 1A,B and Fig. 2). Serum samples taken after the previous day’s immunologic challenge on days 1, 3 and 8 demonstrated a biological trend (nonstatistical, physiologically relevant) in the humoral response of an increase in IgM that preceded an increase in IgG in both sexes. There was no discernible trend in IgE response due to immunization (Fig. 2), which was consistent since IgE functions primarily in type I hypersensitivity and release of vasoactive agents. Statistically, there were no differences on day 8 due to multiple vaccination when compared with pre-treatment day −5.
As expected, food consumption and body weight were not affected by immunization, nor were there clinical signs attributed to this immunological challenge. There was no evidence of clinical chemistry, hematology or urinalysis alterations, nor was there discernible influence on select lymphocyte parameters attributed to the immunization challenge.
3.3.2. Cyclophosphamide
Cyclophosphamide treatment on days 14–16, contributed to a trend of decreased IgM that preceded an increase in IgG in both sexes, possibly influenced by the normal decline of the anamnestic response to hyper-immunization (Fig. 1A,B). The Ig response occurred despite a more than 50% decreased in relative percent of (relative%) B cells and total leukocyte count. Immunoglobulin levels appeared little changed by CY treatment until day 22, when all three immunoglobulins decreased dramatically as a nonstatistical biological trend.
Principle flow cytometry findings related to CY were that the relative% B-cell decreased dramatically and progressively after compound administration; being statistically decreased in males on day 22 compared with day −5 (Fig. 3A,B). The relative% CD4 and CD8 contribution increased, but the CD4/CD8 ratio remained relatively unchanged as total white blood cells (WBC) decreased progressively. The increase in relative% CD4 (males only) was statistically significant according to a two-sample t-test on days 17, 20 and 22 when compared with pre-treatment day −5. There was a relative% increase in CD5-panT, but absolute numbers were dramatically decreased.
As expected (data not presented), the principle hematologic findings noted were that the relative% segmented neutrophils were increased and relative% lymphocytes were decreased 3 days after CY, suggesting a stress leukogram. However, beginning 6 days after CY, relative segmented neutrophils were markedly decreased and relative lymphocytes increased, along with a reduced M/E ratio that suggested granulopoietic hypoplasia. The relative% monocyte and eosinophil decreased progressively, and there was evidence of a moderate nonregenerative anemia and marked thrombocytopenia.
The principle gross pathology observations considered due to CY treatment included: inflammation of the urogenital tract, gray discolored tonsil, and thymic atrophy. Principle micropathology findings consisted of lymphoid depletion of the lymph nodes (mesenteric and retropharyngeal), thymus, spleen and tonsil, along with erythrophagocytosis in both lymph nodes. In addition, there was diffuse, marked hypocellularity of the sternal bone marrow in all animals. Food consumption and body weight were dramatically reduced. Clinical signs and observations attributed to CY treatment included: abdominal ascites, dark/mucoid feces, decreased activity, red penis (males) and vaginal opening (females), hematuria, hematochezia, vomiting, dehydration, and thin body condition. Additional systemic effects of CY included decreased TSH and T4, increased ALP and bile acids, along with proteinuria and hematuria 6 and 8 days after initiation of CY challenge.
4. Discussion
The dog represents animal welfare challenges with regard to numbers of subjects that can be used in a regulatory study, as well as scientific challenge, as the common nonrodent species for extrapolation to man for risk assessment. Therefore, this study design approached scientific and clinical validation from the limitations of a regulatory study, and evaluated the immune system based on a concordance or holistic (Keil et al., 1999) assessment of humoral antibody response, enumeration of lymphocyte populations and functionality of lymphoreticular organs (Burns et al., 1996).
The ELISA and flow cytometry assessments met current industry objectives for Tier I and II immunologic testing designed for rodents (Dean et al., 1998, Pallardy et al., 1998). However, the application of these methods to toxicology testing in dog studies conducted under Good Laboratory Practices has been minimal. The ELISA method was chosen because it has the advantage of enhanced immunologic sensitivity and accuracy compared with existing RID methods (Tizard, 1987). Nonspecific immunoglobulin changes have been successfully used in human occupational medicine to detect possible immunotoxic potential when the antigen exposure is a pesticide mixture or pollutant, and cannot be injected or exposed directly to the test subject of interest. (Stiller-Winkler et al., 1999). This study found that when multiple vaccinations were administered, a nonadverse humoral antibody response was detectable. The general immunoglobulin response pattern due to administration of a vaccine challenge was typical of an initial IgM response that preceded IgG then, upon subsequent challenge, an enhanced anamnestic response of both immunoglobulins was observed (Tizard, 1987). Immunoglobulin E in normal dogs was found in extremely low concentrations, but is of major importance in type I hypersensitivity reactions, atopy, and parastisms (Hammerberg et al., 1997, Ginel et al., 1998). The coefficient of variation was largest for IgG but all were of similar magnitude as previously reported (German et al., 1998). This vaccination challenge demonstrated a normal functional response of increased IgG and IgM, and a lack of IgE response that did not translate into an adverse toxicological response.
Neither the immunization or CY challenges revealed a clear immunoglobulin response difference attributed to sex of the animal in this small study. However, given the thymus involution and pubertal influences that occur in dogs used in regulatory testing (age range, 4–18 months), it seems appropriate to account for variation in age and sex when measuring immunoglobulins, as has been reported for IgE (Racine et al., 1999), IgG, IgM and IgA (Schreiber et al., 1992, German et al., 1998). The decline in IgM and IgG after CY testing may have reflected the half-life of circulating immunoglobulins, considered to be approximately 7 days (Burns et al., 1996). Additionally, immunoglobulin levels during CY challenge may have been influenced by secondary systemic effects on protein homeostasis and albumin concentrations, known to correlate with IgG (German et al., 1998). Previous work with CY in the dog (single dose, 10 mg/kg, intravenously) found decreased lymphocyte numbers but no effect on antibody response or lymphocyte blastogenesis (Medleau et al., 1983).
Flow cytometry is becoming a critical cell biology tool for quantifying and sorting specific phenotypes, and characterizing structure and functional attributes to meet the increased demands for mechanistic work in regulatory studies (Gossett et al., 1999). The use of flow cytometry demonstrated that a relative% B-cell decrease was attributed to CY treatment, but the increased CD4, CD8 and CD5% contributions, when considered with the dramatic decreased in total WBC and unchanged CD4/CD8 ratio, indicates absolute values were dramatically decreased. The preferential inhibition of B-cell response may possibly be due to decreased production and surface expression of immunoglobulins (Burns et al., 1996). Adverse reactions in humans associated with clinical use of cyclophosphamide include vomiting, anorexia, hemorrhagic colitis, leukopenia, hemorrhagic ureteritis and cystitis (Calabresi and Chabner, 1990), most of which were observed clinically or at necropsy in this study.
It has previously been demonstrated that neurological and ophthalmological investigations are appropriate to conduct within the framework of existing guideline studies as a nested hypothesis, benefiting from the battery of tests for an organ system assessment of drugs and chemicals toxicodynamic profiles (Jones et al., 1999). Multivariant statistical analysis of data gathered from assessment of the immune system as a whole, as described by Keil et al. (1999), would seem a useful tool toward attempting to characterize the complexity of immunotoxicities. We conclude that a concordance approach to assessment of the immune system that incorporates humoral antibody, enumeration of lymphocyte populations and pathologic evaluation of the lymphoreticular organs assists in the interpretation of an adverse toxicological response. The ELISA method for measurement of Igs detected a nonspecific biological response following immunization and after CY treatment. The flow cytometry method detected adverse changes in lymphocyte subpopulations after CY treatment. Both methods are acceptable and may be added to a screening battery, if needed for assessing Tier II immunotoxicity, within the framework of existing guidelines for chronic nonrodent regulatory studies.
References
- Barthold S.W., Levy S.A., Fikrig E., Bochenstedt L.K., Smith A.L. Serologic responses of dogs naturally exposed to or vaccinated against Borrelia burgdorferi infection. J. Am. Vet. Med. Assoc. 1995;207:1435–1440. [PubMed] [Google Scholar]
- Bruning, J.L., Kintz, B.L. (Eds.), 1968. Computational Handbook of Statistics. Scott, Foresman and Company.
- Burns L.A., Meade B.J., Munson A.E. Toxic responses of the immune system. In: Klaassen C.D., editor. Casarett & Doull’s Toxicology — The Basic Science of Poisons. McGraw-Hill; New York: 1996. pp. 355–402. [Google Scholar]
- Calabresi P., Chabner B.A. Chemotherapy of neoplastic disease. In: Gilman A.F., Rall T.W., Nies A.S., Taylor P., editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Pergamon Press; New York: 1990. pp. 1215–1219. [Google Scholar]
- Dean J.H., Hincks J.R., Remandet B. Immunotoxicity assessment in the pharmaceutical industry. Toxicol. Lett. 1998;102–103:247–255. doi: 10.1016/s0378-4274(98)00314-2. [DOI] [PubMed] [Google Scholar]
- German A.J., Hall E.J., Day M.J. Measurement of IgG, IgM and IgA concentrations in canine serum, saliva, tears and bile. Vet. Immunol. Immunopathol. 1998;64:107–121. doi: 10.1016/s0165-2427(98)00132-9. [DOI] [PubMed] [Google Scholar]
- Ginel P.J., Margario J.M., Lucena R., Molleda J.M. Concentrations of plasma immunoglobulins in the dog as determined by laser nephelometry. Comparison with radial immunodiffusion and enzyme-linked immunosorbent assay. Eur. J. Clin. Chem. Clin. Biochem. 1997;35:223–228. doi: 10.1515/cclm.1997.35.3.223. [DOI] [PubMed] [Google Scholar]
- Ginel P.J., Riano C., Lucena R. Evaluation of a commercial ELISA test for the detection of allergen-specific IgE antibodies in atopic dogs. J. Vet. Med. B. 1998;45:421–425. doi: 10.1111/j.1439-0450.1998.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Gossett K.A., Narayanan P.K., Williams D.M., Gore E.R., Herzyk D.J., Hart T.K., Sellers T.S. Flow cytometry in the preclinical development of biopharmaceuticals. Toxicol. Pathol. 1999;27:32–37. doi: 10.1177/019262339902700107. [DOI] [PubMed] [Google Scholar]
- Hammerberg B., Bevier D., BeBoer D.J., Olivry T. Auto IgG, anti-IgE and IgG times IgE immune complex presence and effects on ELISA-based quantitation of IgE in canine atopic dermatitis, demodectic acariasis and helminthiasis. Vet. Immunol. Immunopathol. 1997;60:33–46. doi: 10.1016/s0165-2427(97)00119-0. [DOI] [PubMed] [Google Scholar]
- Immunotox, 1997. Capture ELISA for Total IgG levels in rat serum.
- Jones D.R.E., Jones-Gruffydd T.J., Stokes C.R., Bourne F.J. Use of a direct enzyme-linked antiglobulin test for laboratory diagnosis of immune-mediated hemolytic anemia in dogs. Am J. Vet. Res. 1992;53:457–465. [PubMed] [Google Scholar]
- Jones R.D., Hastings T.F., Lanes A.M. Absence of neurovisual effects due to tissue and blood cholinesterase depression in a chronic disulfoton feeding study in dogs. Toxicol. Lett. 1999;106:181–190. doi: 10.1016/s0378-4274(99)00064-8. [DOI] [PubMed] [Google Scholar]
- Keil D., Luebke R.W., Ensley M., Gerard P.D., Pruett S.B. Evaluation of multivariate statistical methods for analysis and modeling of immunotoxicology data. Toxicol. Sci. 1999;51:245–258. doi: 10.1093/toxsci/51.2.245. [DOI] [PubMed] [Google Scholar]
- Ladics G.S., Smith C., Heaps K., Elliott G.S., Slone T.W., Loveless S.E. Possible incorporation of an immunotoxicogical functional assay for assessing humoral immunity for hazard identification purposes in rats on standard toxicology study. Toxicology. 1995;96:225–238. doi: 10.1016/0300-483x(94)02967-y. [DOI] [PubMed] [Google Scholar]
- Medleau L., Dawe D.L., Calvert C.A. Immunosuppressive effects of cyclophosphamide, vincristine, and l-asparaginase in dogs. Am. J. Vet. Res. 1983;44:176–180. [PubMed] [Google Scholar]
- Modiano J.F., Smith R., Wojcieszyn J., Thomas J.S., Rosenbaum B.A., Ball C., Nicholds E.A., Anthony M.A., Barton C.L. The use of cytochemistry, immunophenotyping, flow cytometry, and in vitro differentiation to determine the ontogeny of a canine monoblastic leukemia. Vet. Clin. Pathol. 1998;27:40–49. doi: 10.1111/j.1939-165x.1998.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Olson H., Betton G., Stritar J., Robinson D. The predictivity of the toxicity of pharmaceuticals in humans from animal data — an interim assessment. Toxicol. Lett. 1998;102–103:535–538. doi: 10.1016/s0378-4274(98)00261-6. [DOI] [PubMed] [Google Scholar]
- Pallardy M., Kerdine S., Lebrec H. Testing strategies in immunotoxicology. Toxicol. Lett. 1998;102–103:257–260. doi: 10.1016/s0378-4274(98)00315-4. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Pool R. Immune-mediated arthropathies of the canine. In: Shifrine M., Wilson F.D., editors. The Canine as a Biomedical Research Model: Immunological, Hematological, and Oncological Aspects. Department of Energy; Washington DC: 1980. pp. 263–277. [Google Scholar]
- Racine B.P., Marti E., Busato A., Weilenmann R., Lazary S., Griot-Wenk M.E. Influence of sex and age on serum total immunoglobulin E concentration in Beagles. Am. J. Vet. Res. 1999;60:93–97. [PubMed] [Google Scholar]
- Roth J.A., Goff L., Monroe W.E. Immunodeficient dwarfism in dogs: a model for neuroimmunomodulation. Int. J. Neurosci. 1988;38:443–454. doi: 10.3109/00207458808990705. [DOI] [PubMed] [Google Scholar]
- Schreiber M., Kantimm D., Kirchhoff D., Heimann G., Bhargava A.S. Concentrations in serum of IgG, IgM and IgA and their age-dependence in Beagle dogs as determined by a newly developed enzyme-linked-immuno-sorbent-assay (ELISA) Eur. J. Clin. Chem. Clin. Biochem. 1992;30:775–778. doi: 10.1515/cclm.1992.30.11.775. [DOI] [PubMed] [Google Scholar]
- Stiller-Winkler R., Hadnagy W., Leng G., Straube E., Idel H. Immunological parameters in humans exposed to pesticides in the agricultural environment. Toxicol. Lett. 1999;107:219–224. doi: 10.1016/s0378-4274(99)00050-8. [DOI] [PubMed] [Google Scholar]
- Taylor, J.K. (Ed.), 1987. Principles of measurement. In: Quality Assurance of Chemical Measurements. Lewis Publishers, USA, pp. 75–92.
- Tizard I.R. Veterinary Immunology. WB Saunders; Philadelphia, PA: 1987. pp. 1–176. [Google Scholar]
- US-EPA-TSCA, 1997. Immunotoxicity Testing Guidelines, 40 CFR Section 799.9780, August.


