Abstract
Milk contains a variety of substances, which inhibit the infection of pathogens. This is of benefit to the mother, safeguarding the integrity of the lactating mammary gland, but also of huge importance for protection of the suckling offspring. The antimicrobial substances in milk can be classified into two categories. First, nonspecific, broad-spectrum defense substances, which have evolved over long periods of time, and secondly, substances like antibodies, which are specifically directed against particular pathogens and have developed during the mother's lifetime. Substances in both categories may be targets for biological intervention and manipulation with the goal of improving the antimicrobial properties of milk. These alterations of milk composition have applications in human as well as in animal health.
Keywords: Milk, Immunology, Pathogen, Protection, Neutralization
1. Introduction
The major physiological function of milk is the transport of amino acids, carbohydrates, lipids, and minerals (especially calcium) to mammalian offspring. In addition to its nutritional importance, milk also contains a variety of molecules, which provide protection against pathogen infections or modulate immune responses. Antibodies are the most prominent of these molecules, but other nonspecific, broad-spectrum defense substances possess antimicrobial activity as well. Some of these molecules can be altered to improve the antipathogenic properties of milk. These modifications are of particular interest if no suitable vaccines are available and newborn mammals are most severely affected by the infection. This scenario can be the consequence of the low virulence of the pathogen, which does not elicit a major immune response in the adult host [e.g. the human respiratory syncytia virus (RSV)], or the variability of the pathogen, which evades an immune response targeted at variants encountered previously (e.g. influenza virus). Under these circumstances, mothers do not supply protective antibodies to their offspring via placenta and milk and nonspecific defense mechanisms may be too weak to prevent disease. This in turn puts the offspring at an increased risk of infection, as the immune system of newborn mammals is often not sufficiently developed during the early stages of life to cope efficiently with the pathogens. Milk, which has been manipulated to contain large amounts of specific or nonspecific defense substances, may therefore provide a useful tool for disease prevention in these instances.
2. Milk and human health
There is convincing evidence that with regard to infant health, breast-feeding is superior to bottle-feeding Filteau, 2000, Hanson et al., 2000, Hanson, 1998. Protection against infections during lactation has been demonstrated in cases of acute and prolonged diarrhea, respiratory tract infections, otitis media, urinary tract infection, neonatal septicemia, and necrotizing enterocolitis (Hanson, 1998). Breast-feeding provides the newborn baby with a battery of immunologically active substances Table 1, Table 3, Table 4, Table 5 . Antibodies have been demonstrated to efficiently control a variety of pathogen-induced diseases (including malaria, Fried et al., 1998, Kassim et al., 2000), and a wide variety of virus infections (e.g. RSV, Englund, 1994, and rotavirus, Ebina et al., 1992). Lactoferrin, which is present in substantial amounts in human milk, prevents the population of the infant's gut by coliform enterobacteria and acts as an antiinflammatory immune modulator (Nuijens et al., 1996). Other nonspecific defense molecules enhance this protection Newburg, 1996, Labeta et al., 2000. These substances are of particular importance in the newborn prior to acidification of the stomach, which provides an effective barrier to many bacterial infections of the gastrointestinal tract in later life. In addition, there is growing evidence that the transfer of cytokines, growth factors, B-cells, and other lymphocytes from mother to offspring via milk (Zhou et al., 2000) assists the development of the infant's immune system. Babies, who are not breast-fed, are deprived of some of these defense mechanisms. The antibodies in cow's milk will be directed against bovine rather than human pathogens. Bovine lymphocytes even if they would survive the processing of milk to infant formula would be of no benefit to humans. Cow's milk contains very little lactoferrin (Nuijens et al., 1996). In addition, many babies show allergic reactions to cow's milk, with β-lactoglobulin being the major immunogen (Pecquet et al., 2000).
Table 1.
Immunologically active components of milk
| Specific substances | Nonspecific substances |
|---|---|
| Antibodies | Lactoferrin/lactoferricin |
| Antiidiotypes | Casein and casein peptides |
| Lymphocytes | Oligosaccharides |
| Glycolipids | |
| Glycosaminoglycans | |
| Mucin | |
| Growth factors | |
| Cytokines | |
| Complement |
Table 3.
Nonspecific antiinfective components of milk: lactoferrin and lactoferricin
LF=lactoferrin.
| Milk component | Pathogen | Disease | Mode of action | Evidence | Reference |
|---|---|---|---|---|---|
| Lactoferrin | Enterobacteria | Diarrhea | Sequestering of iron and deprivation of pathogenic bacteria | In vitro | Bishop et al. (1976) |
| Haemophilus influenzae | Respiratory tract infections | Inactivation of colonization factors | In vitro | Qiu et al. (1998) | |
| HIV | AIDS | Inhibition of virus–cell fusion via negative charges | In vitro | Harmsen et al. (1995) | |
| hCMV | Necrosis affecting salivary gland, liver, kidney, lung, and brain | Inhibition of virus–cell fusion via negative charges | In vitro | Harmsen et al. (1995) | |
| Hepatitis C virus | Hepatitis, liver cancer | Specific interaction of virus surface proteins with LF N-terminus | In vitro, in vivo | Tanaka et al. (1999) | |
| Candida albicans | Oropharyngeal candidosis | Unknown | In vitro | Kuipers et al. (1999) | |
| Pneumocystis carinii | Pneumonia | Unknown | In vitro | Cirioni et al. (2000) | |
| Lactoferricin |
C. albicans | O. candidosis | Unknown | In vitro, in vivo | Bellamy et al. (1993) |
| Gram-negative and Gram-positive bacteria |
Various |
Effect on membrane function |
In vitro |
Chapple et al. (1998) |
Table 4.
Nonspecific antiinfective components of milk: casein and casein fragments
| Milk component | Pathogen | Disease | Mode of action | Evidence | Reference |
|---|---|---|---|---|---|
| Isracidin (f1-23 bovine αS1 casein) | Staphylococcus aureus, Streptococcus pyogenes, Listeria monocytogenes | Anaphylactic shock, mastitis | Unknown | In vitro, in vivo | Lahov and Regelson (1996) |
| Casocidin I (amino acids 150–188 of bovine αS2 casein) | Escherichia coli | Enteric disease | Unknown | In vitro | Zucht et al. (1995) |
| Fragments of bovine αS2-casein (amino acids 183–207 and 164–179) | E. coli, Bacillus cereus, S. thermophilus | Enteric disease | Unknown | In vitro | Recio and Visser (1999) |
| Human (not bovine) κ-casein | H. pylori | Gastric disease, heart disease (?) | Glycosylated κ-casein inhibits adhesion of H. pylori to gastric cells | In vitro | Stromqvist et al. (1995) |
Table 5.
Nonspecific antiinfective components of milk: other molecules
| Milk component | Pathogen | Disease | Mode of action | Evidence | Reference |
|---|---|---|---|---|---|
| Mucin | Rotavirus | Diarrhea (mainly in infants) | Glycosylation-dependent inhibition of virus infection | In vitro, in vivo | Yolken et al. (1992) |
| Mannosylated glycopeptide | Enterohaemorrhagic E. coli (EHEC) | Haemorrhagic colitis | Binding of bacteria to cell surface receptor inhibited | In vitro | Ashkenazi et al. (1991) |
| CD14 | E. coli | Enteric disease | Binding of bacterial LPS and whole bacteria | In vitro | Labeta et al. (2000) |
| Complement | General cytotoxicity | In vitro | Ogundele (1999a) | ||
| Glycolipid (ganglioside fraction of human milk) | Vibrio cholerae, E. coli, Campylobacter jejuni | Diarrhea | Inhibition of toxin action by competition of milk fat globule-bound glycolipids with cell surface receptors | In vitro, in vivo | Laegreid and Kolsto-Otnaess (1987) |
| Glycosaminoglycan | HIV | AIDS | GAGa binds/competes with HIV cell surface receptor CD4 | In vitro | Newburg et al. (1995) |
| Lactadherin | Rotavirus | Diarrhea | Inhibits virus binding | In vivo | Newburg et al. (1998) |
| Lipids and fatty acids | Enveloped viruses, S. epidermidis, E. coli | Various | Free fatty acids impair membrane function | In vitro, in vivo | Isaacs et al. (1990) |
| Giardia lamblia trophozoites | Diarrhea | Free fatty acids impair membrane | In vitro | Rohrer et al. (1986) | |
| α-lactalbumin fragments | Gram-positive bacteria | Unknown | In vitro | Pelligrini et al. (1999) |
GAG=Glycosaminoglycan.
However, even breast-feeding fails to supply adequate protection against a variety of pathogens. This is exemplified by the frequent hospitalization of children (below the age of 6 months) infected with RSV. Although the levels of maternal antibody are inversely correlated with the severity of RSV-induced disease (broncholitis and pneumonia; Lamprecht et al., 1976), most mothers transfer negligible amounts of virus-neutralizing antibodies to their children. This is a consequence of the minor immune reaction that RSV elicits in adults with most infections being subacute. Available vaccines do not lead to a long-lasting immune response. The first RSV vaccine (using formalin-inactivated virus) even resulted in an increased incidence and severity of disease in vaccinated children (Englund, 1999). Therefore, although RSV-specific antibodies in milk are able to control RSV-induced disease, they are usually scarce. In a scenario like this, it is obvious that the manipulation of antimicrobial substances in milk would have beneficial health effects.
3. Potential targets for manipulation
Of the plethora of immunologically active substances in milk, only a few are realistic targets for biological or genetic modification (Table 2) , as discussed next.
Table 2.
Potential targets for the manipulation of immunologically active milk-components
| Immunization of mothers | Immunization of ruminants | Transgenic expression |
|---|---|---|
| Antigen | Antigen | Neutralizing antibodies |
| Neutralizing antibody | Neutralizing antibody | Antiidiotypes |
| Nonspecific defense proteins | ||
| Overexpression of IgA transporter | ||
| Insertion of human immunoglobulin gene loci |
3.1. Antibodies and antiidiotypes
Antibodies are the most prominent and most specific defense molecules in milk. Depending on the species, immunoglobulin A or G (IgA or IgG) is the predominant isotype in colostrum and milk. They are able to efficiently control a variety of bacterial and viral diseases, which affect newborn mammals.
3.1.1. Immunization of mothers
Milk contains antibodies directed against a variety of pathogens that the mother has encountered during her lifetime. Some pathogens elicit a major immune response and a long-lasting immunity. Other pathogens only lead to a transient immune response. In this case, the antibody content of human milk can be manipulated by immunizing mothers during pregnancy. This can be done as a passive immunization by infusing preparations of pathogen-specific antisera or, more frequently, as active immunization by vaccination. Vaccination in pregnancy leads to a normal immune response and no pregnancy-specific side effects have been reported Englund et al., 1998, Munoz and Englund, 2000. This approach is particularly useful in cases (like RSV), where vaccination only leads to a short-term immune response and infants can be seriously affected by the infection. Immunization of mothers during pregnancy with RSV antigen leads to the transfer of antibodies to the offspring via placenta and colostrum and helps to prevent RSV-induced disease Englund, 1994, Englund et al., 1998. A potential drawback to this approach may relate to the finding that maternal antibodies can suppress the infant's own immune response to certain pathogens (Englund et al., 1998). Therefore, careful analysis of the host–pathogen interactions is required before embarking on vaccination during pregnancy. Nevertheless, immunization during pregnancy may help to prevent a wide spectrum of diseases.
Milk can also be used as a medium for the active immunization of the neonate with antiidiotypes. This can be achieved by immunizing mothers with pathogen-neutralizing antibodies. The mother will develop an immune response against the variable region of the neutralizing antibody and transmit secondary antibodies (i.e. antiidiotypes) to the offspring via placenta and milk. The offspring's immune system will respond by generating tertiary antibodies, which recognize the same epitope as the primary neutralizing antibody. The viability of such an approach has been demonstrated in animal experiments (Okamoto et al., 1989). The transfer of antiidiotypes via placenta and milk may also be a mechanism of natural immunization in humans (Hanson et al., 2000).
3.1.2. Immunization of ruminants
Immunization during pregnancy requires a substantial financial input in terms of vaccine development and clinical testing (Englund et al., 1998), as the outcome of the vaccines is not always predictable. As an alternative, the manipulations of antimicrobial properties of milk can be carried out in ruminants. Cattle can be successfully immunized against a battery of human pathogens (Jenkins et al., 1999). Pathogen-specific antibodies can be found in substantial concentrations in bovine colostrum and milk and can be processed to provide a marketable product (Stephan et al., 1990). Cow's milk containing antibodies directed against rotavirus has been successfully used to prevent rotavirus-induced diarrhea in children (Ebina, 1996). In another experiment, disease symptoms caused by enterotoxigenic E. coli (ETEC) were efficiently suppressed in adult volunteers by cow's milk containing ETEC-specific immunoglobulin (Freedman et al., 1998). These examples demonstrate that bovine antibodies in milk are able to control pathogen-induced diseases in humans. Cattle may also be immunized with pathogen-neutralizing antibodies to generate milk containing antiidiotypes, thereby providing a recombinant vaccine.
3.1.3. Transgenesis
Alternatively, ruminants expressing pathogen-neutralizing antibodies can be generated by transgenesis. This has a variety of advantages. Transgenesis circumvents the problem of differences in isotype usage in milk. Cows mainly produce IgG, whereas human milk mainly contains IgA (Larson, 1992). IgG can activate complement via its Fc portion and is very potent against bacteria, whereas IgA (due to its dimeric nature) has a strongly neutralizing effect against viruses. Humanized IgA molecules can theoretically be produced in the milk of transgenic ruminants. The concentration of the recombinant antibody present in milk would exceed the levels of antibodies generated by vaccination by some orders of magnitude. Antibody concentrations of 10 mg/mL have been obtained in transgenic goats (Pollock et al., 1999), while the total immunoglobulin content of ruminant milk is about 1 mg/ml. In addition, the most potent antibodies directed against a given pathogen can be selected in vitro.
However, there are also serious disadvantages of the transgenic approach. Transgenesis will lead to milk containing only one monospecific antibody directed against a single epitope (in contrast to vaccination, which leads to a whole battery of pathogen-specific antibodies). Pathogens are often able to evade the neutralizing effect of an antibody by mutating the recognized protein domains. Therefore, antibodies expressed in the milk of transgenic animals will have to be selected carefully, so that they are directed against essential epitopes, which cannot be mutated without impairing infectivity (Sola et al., 1998). Transgenesis in ruminants is also expensive and time consuming. The market for milk as a nutraceutical containing a recombinant, pathogen-neutralizing antibody may be therefore only profitable enough for some exceptional human pathogens with worldwide significance. The biggest disadvantage of transgenic technology will be the current adverse public perception. However, the transgenic approach may find public approval in the area of animal health where it could partly obviate the need for antibiotics.
The porcine coronavirus transmissible gastroenteritis virus (TGEV) illustrates the possible application of transgene-mediated modification of milk as a means to prevent disease. TGEV causes gastroenteritis and diarrhea in pigs (Enjuanes and van der Zeijst, 1995). Newborn animals are most severely affected by the infections with a mortality of up to 100%. Coronaviruses in general elicit an immune response that is highly strain-specific and often short-lived. Although a variety of routes were investigated to obtain useful vaccines against TGEV, no marketable vaccine has been developed yet. Oral administration of neutralizing antibody has been shown to efficiently prevent TGEV infection (Torres et al., 1995). Milk containing this neutralizing antibody may therefore provide a route to protecting piglets against TGEV infections Sola et al., 1998, Castilla et al., 1998. To provide a proof of principle, a mouse model for this approach was established (Fig. 1) . Transgenic mice, which express a highly neutralizing antibody directed against a murine coronavirus (the mouse hepatitis virus MHV-JHM) in the lactating mammary gland, were generated. Newborn mice suckling the milk of transgenic dams were fully protected against a challenge with a lethal dose (7000×LD50) of MHV-JHM. Cross-fostering experiments demonstrated that the milk-borne recombinant antibody was sufficient for this protection (Kolb et al., 2001). Although there are differences in the disease pattern elicited by MHV and TGEV and the immunoglobulin isotypes typically found in the milk of mice and pigs, these experiments demonstrate that modification of milk via a transgenic route can have a very beneficial impact on animal health.
Fig. 1.
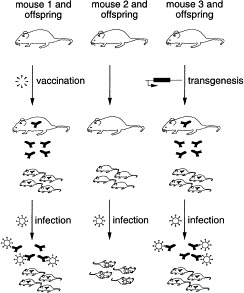
A transgenic approach to lactogenic immunity: schematic representation of the outcome of a coronavirus infection in naive mice (Mouse 2 and offspring), immunized mice (Mouse 1 and offspring), and transgenic mice expressing a coronavirus-neutralizing antibody (Mouse 3 and offspring). Immunizations against coronaviruses are often short-lived. Therefore, Mouse 1 would only supply virus-neutralizing antibodies to their offspring for a limited time-span. Mouse 3, however, will consistently supply their offspring with large quantities of the recombinant virus-neutralizing antibody throughout every lactation period.
Combinations of transgenesis and vaccination may provide other promising approaches for the generation of milk containing pathogen-specific antibodies. Cattle mainly express antibodies of the IgG isotype, whereas IgA is the predominant isotype in humans (Larson, 1992). In mice, IgG and IgA are present at roughly equal levels (Larson, 1992). By overexpressing the polymeric immunoglobulin transporter in the lactating mammary gland of transgenic mice, the IgA content of murine milk could be increased twofold (De Groot et al., 2000). A similar approach may also be used to generate cattle that are able to secrete increased amounts of IgA into milk. Immunization of these transgenic cows would then lead to milk rich in pathogen-specific IgA.
By using homologous recombination, researchers at Abigenix (〈www.abigenix.com〉) have replaced the murine immunoglobulin loci by their human counterparts. The mice will therefore respond to immunization by producing human antibodies. Monoclonal antibodies derived from these mice are human monoclonal antibodies and can be used therapeutically with minimal side effects. A similar approach could be taken in ruminants by using nuclear transfer techniques. Ruminants thus modified could be immunized against pathogens of interest and produce milk containing human antibodies.
3.2. Lymphocytes
There is growing evidence that lymphocytes are transferred from mother to offspring via milk. In mouse experiments, B-cells carrying a reporter gene could be transferred to suckling mice via milk Zhou et al., 2000, Arvola et al., 2000. Breast-feeding mothers transfer lymphocytes to their babies, which induce an immune tolerance of the baby against the mother. One well-documented consequence of this is the fact that breast-feeding mothers are often suitable organ donors for their children in later life (Hanson, 2000). Immunization during pregnancy may also affect the properties of these transferred immune cells. However, too little is known about lymphocyte transfer from mother to offspring to warrant direct manipulations of this process.
3.3. Lactoferrin and lactoferricin
Lactoferrin is a glycosylated milk protein, which is also found in other mucosal secretions and is expressed in neutrophils. It possesses a wide variety of antimicrobial functions (Table 3) . Its actions against viruses, bacteria, and fungi can be classified into several different modes of action. (1) By sequestering free iron, lactoferrin can restrict the growth of Gram-positive and Gram-negative bacteria and a variety of fungi Kuipers et al., 1999, Cirioni et al., 2000, Andersson et al., 2000. The bacteriostatic effects can be counteracted in vitro by addition of excess iron. (2) Lactoferrin and (even more potently) its pepsin-breakdown products lactoferricin B and H (from bovine and human lactoferrin, respectively), which are derived from the N-terminus of the protein, have a bacteriocidal effect by interfering with bacterial membrane function (Hwang et al., 1998). Lactoferrin's activity against fungal infection in vitro and in vivo Kuipers et al., 1999, Bellamy et al., 1993, Wakabayashi et al., 2000 may have the same biochemical basis. (3) Lactoferrin can also bind bacterial lipopolysaccharide (LPS), thereby impairing bacterial cell wall/membrane function Nuijens et al., 1996, Odell et al., 1996. By binding free LPS or LPS bound to other proteins, it can also act as an antiinflammatory immune modulator Baveye et al., 1999, Baveye et al., 2000. (4) Lactoferrin purified from milk and colostrum is able to block human cytomegalovirus (hCMV) replication as well as inhibit HIV-1-induced cytopathic effects. Negatively charged lactoferrin derivatives obtained by acylation of the amino function of lysine residues even had a fourfold stronger antiviral activity against HIV-1 but lost their anti-hCMV activity. Native and acylated lactoferrin strongly binds to the V3 domain of the HIV envelope protein gp120, suggesting that the shielding of this domain results in the inhibition of virus–cell fusion and virus entry. Addition of positive charges to lactoferrin by amination resulted in an increased anti-hCMV activity and a loss of anti-HIV activity (Swart et al., 1998). (5) Lactoferrin also inhibits the entry of hepatitis virus C (HCV) into susceptible cells by direct interaction with the viral surface glycoproteins (Ikeda et al., 1998). Pilot studies in HCV-infected patients, however, only demonstrated a moderate antiviral activity in vivo (Tanaka et al., 1999). Whereas the antibacterial effects of lactoferrin probably reflect a natural function, the antiviral activities may be fortuitous. However, lactoferrin, α-lactalbumin, and β-lactoglobulin, which can also be derivatized to yield compounds with strong anti-HIV activity (Swart et al., 1996), may provide lead structures for the development of antiviral drugs (Swart et al., 1999).
In addition to its direct effects on pathogens, lactoferrin fulfills a variety of other functions. Lactoferrin binds to a cell surface receptor (McAbee et al., 1998). It is taken up into cells and transported to the nucleus, where it binds to DNA Nuijens et al., 1996, Kanyshkova et al., 1999. It inhibits growth of tumor cells by modulating the expression and the activity of key G1 regulatory proteins (Damiens et al., 1999) and metastasis of tumors (Iigo et al., 1999). It also influences cellular responses to Listeria infections Moriishi et al., 1999, Valenti et al., 1999. Lactoferrin is also reported to significantly decrease the cell number of Helicobacter pylori (a suspected cause of stomach cancer) in the stomach of experimental animals (Wada et al., 1999).
Due to the wide variety of applications, lactoferrin has long been a prominent target for recombinant production in a variety of host organisms Krimpenfort et al., 1991, Salmon et al., 1998, Sun et al., 1999, Chong and Langridge, 2000. Milk containing high amounts of lactoferrin may be used directly as a nutraceutical to prevent or treat bacterial infections of the digestive tract. Lactoferrin purified from milk could serve a variety of other purposes. Transgenic cattle expressing human lactoferrin have been generated (Krimpenfort et al., 1991) and the product is in clinical trials (〈www.pharming.com/products〉).
Lactoferricin B and H are derived from lactoferrin by pepsin cleavage in the digestive tract. The 25 amino acid lactoferricin peptide folds into a distorted antiparallel β-sheet, whereas the N-terminal 13 amino acids in the native lactoferrin adopt a α-helical structure (Hwang et al., 1998). The bacteriocidal effect of lactoferricin is far greater than that of native lactoferrin (Bellamy et al., 1992). Two tryptophan residues in the bovine lactoferricin peptide are indispensable for this activity (Strom et al., 2000). Although lactoferricin has been shown to depolarize bacterial membranes Aguilera et al., 1999, Ulvatne et al., 2001, the precise basis of its antimicrobial action remains unclear. In order to induce the activity of lactoferricin in milk, the pepsin cleavage site may be engineered to be recognized by a protease, which is resident in the lactating mammary gland. This may boost the antibacterial potency of milk and reduce the number of bacteria (especially enterobacteria, which are particularly susceptible to lactoferricin action) in milk. These manipulations, however, would probably alter the processing abilities of milk. In addition, the modified milk may also have an unwanted immunogenic/allergenic effect.
3.4. Casein and casein fragments
Caseins and casein fragments also display antimicrobial activities, which, however, are not very well defined (Table 4) . Isracidin is a chymosin cleavage product of bovine αS1 casein encompassing amino acids 1–23 (Fig. 2) . It possesses strong antibacterial activity (at concentrations similar to that of penicillin) and was found to be effective against a variety of Gram-negative and Gram-positive bacteria (Lahov and Regelson, 1996). Surprisingly, isracidin is most potent when injected intramuscularly in vivo but only displays a modest antibacterial activity in vitro. This suggests that the effect of isracidin may be indirect. Therefore, it is uncertain whether transgenic overexpression of an isracidin-peptide in the lactating mammary gland would increase the antibacterial potency of milk. The chymosin cleavage sites in αS1 casein are conserved in ruminant proteins but not in other mammals, indicating that the formation of isracidin may not be essential for the well-being of mammals in vivo (Fig. 3) . Although isracidin may not augment the antibacterial properties of milk, transgenic expression may nevertheless be useful, as large-scale production of isracidin was mainly hampered by the lack of suitable production systems (the peptide had to be isolated from milk after chymosin treatment). Production of larger amounts of isracidin in the milk of transgenic animals could be achieved either by expressing it as a peptide or by introduction of a cleavage site for a mammary-resident protease.
Fig. 2.
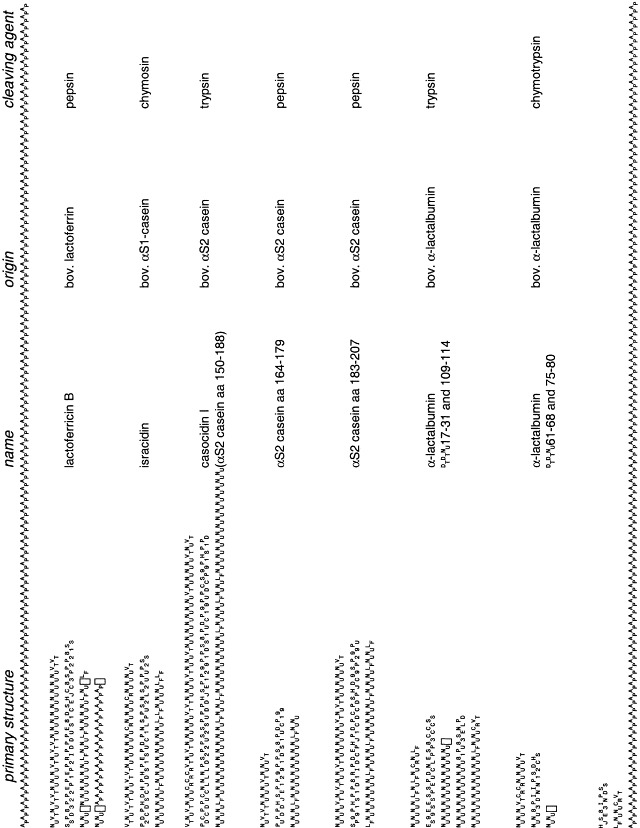
Primary structure of bactericidal peptides derived from milk proteins. Charged amino acids are marked (+) or (−); hydrophobic amino acids are designated with (*). Disulfide bridges between cysteine residues are indicated as lines. The distribution of charged and hydrophobic residues in lactoferricin B allows the peptide to fold into antiparallel amphipathic β-sheets (Hwang et al., 1998), which can interact with membranes. Similar structures could be formed by the other casein-derived peptides whose structures have not been identified yet. Bov.: bovine, aa: amino acids.
Fig. 3.

Alignment of the N-terminal regions of ruminant (cow, buffalo, sheep, and goat) and nonruminant (pig, mouse, and guinea pig) αS1 casein proteins. Dark and light background shading indicate the sequences, which correspond to the signal peptide and isracidin, respectively. Whereas there is a high degree of sequence conservation between the ruminant and nonruminant signal peptides, there is no significant sequence conservation between the N-terminal segments of the mature proteins, which correspond to isracidin.
Two C-terminal pepsin fragments of bovine αS2 casein have also been shown to possess antibacterial activity (Zucht et al., 1995, Recio and Visser, 1999; Fig. 2). The precise mechanism by which these peptides exert their bacteriocidal effect is unclear. However, the αS2 casein-derived peptides share some similarities with other antibacterial peptides (Boman, 1995) and may be a component of an innate defense system of the mammary gland.
Human κ-casein strongly inhibits binding of H. pylori to susceptible intestinal cells (Stromqvist et al., 1995). Glycosylation of κ-casein was found to be crucial for this activity. Casein proteins, in general, may be more difficult to manipulate than whey proteins as caseins also contribute to the generation of casein micelles. Manipulations, which disturb the micelle formation, may therefore impair the animal's ability to lactate. This would influence efficiency of production as well as animal welfare.
3.5. Complement
The cytotoxic activity of milk is inactivated by incubation at 56°C, suggesting that complement is a major cytotoxic factor Ogundele, 1999a, Ogundele, 1999b. Complement activity, however, needs to be regulated to maintain the integrity of the mammary epithelium of the mother and the epithelium of the digestive tract in the offspring. Lactoferrin, complement protein C1, and the complement regulator CD59, which are expressed in the mammary epithelium, have been implicated in that control (Bjorge et al., 1996). Manipulation of complement proteins in milk without endangering the integrity of the mammary epithelium may be difficult.
3.6. Other milk-borne molecules
Other milk-borne molecules also have antimicrobial potency (Table 5) . Milk mucin inhibits rotavirus infections in vitro and in vivo (Yolken et al., 1992), presumably by its sialic acid residues competing with natural virus receptors (Guerrero et al., 2000). Tryptic fragments of bovine α-lactalbumin (Fig. 2) efficiently restrict the growth of Gram-positive bacteria (Pellegrini et al., 1999). Other milk proteins have been shown to have neutralizing effects against specific pathogens (Table 5) but the protective mechanisms are not clear Labeta et al., 2000, Newburg, 1999. Milk is also rich in oligosaccharides and glycoconjugates (Newburg, 1996). Many of which have been demonstrated to possess antiviral, antibacterial, or antifungal effects (see Table 2). Lipids in milk have also shown antimicrobial effects, which are not well defined at present (Goldman et al., 1996). These molecules may represent components of an innate defense system of the mammary gland. Synthesis of these substances, however, depends on the interplay of a number of enzymes. Manipulation of these pathways would require major efforts and may therefore not be cost-effective.
Various milk proteins, which are chemically modified, are also powerful antagonists of virus infections Neurath et al., 1996, Berkhout et al., 1997. Although some of these activities are fortuitous, milk may provide a cheap source for the production for these agents. In addition, a wide variety of immunomodulatory substances are found in milk (Goldman, 2000; Table 5). Examples include interleukins, transforming growth factors α and β, or the complement regulatory protein CD59 (Bjorge et al., 1996). Some of the abovementioned molecules (e.g. lactoferrin and isracidin) also possess immune modulatory activity. Little is known about the precise role of these proteins in the mother or in the offspring (Goldman et al., 1996). However, the importance of these factors is exemplified by the fact that TGF-β knockout mice, which die shortly after birth, can be rescued by TGF-β supplied by maternal sources (via placenta and milk; Letterio et al., 1994). As the precise significance of these factors is not established, they are not likely candidates for targeted manipulation.
3.7. Nonmilk proteins and peptides
Other molecules with antimicrobial properties, e.g. porins, can theoretically be expressed in the milk of transgenic animals. However, these molecules may also have a deleterious effect against the mammary epithelium of the dam or the gut epithelium of the offspring. Therefore, these proteins or peptides would have to be expressed as preproteins, which have to be cleaved proteolytically (ideally by a pathogen-specific protease) to yield a functional toxin.
4. Conclusions
There are two routes for the manipulation of immunologically active, protective components of milk: vaccination and transgenesis (Table 2). Vaccination of mothers during pregnancy requires a careful consideration of potential risks and benefits for each pathogen in question. Vaccination of ruminants can lead to the production of milk highly enriched in antibodies directed against particular pathogens Ebina, 1996, Freedman et al., 1998. Since antibodies in milk are relatively stable (Schmidt, 1982), formula containing antibodies directed against a variety of pathogens can be generated and used for the prevention and treatment of infectious diseases of the digestive tract. The modified milk will have its major application in infectious diseases, which are acute but not life-threatening and do not lead to hospitalization. However, the economic value of this strategy may be considerable. In addition, the time span between the occurrence of a new pathogen (or a new variant) and the development of a vaccine may be shortened significantly by DNA vaccination. DNA vaccines have been effective in many instances (Turnes et al., 1999) and lead to the production of pathogen-specific antibodies in colostrum and milk (Jenkins et al., 1999). Manipulation of milk by transgenesis will be useful in the veterinary field. Milk containing either a defined, highly neutralizing antibody directed against a pathogen or a high concentration of a nonspecific defense molecule may be applied for the prevention of infectious diseases, when suitable vaccines are unavailable. This approach, however, will be subject to a stringent cost-benefit analysis. The transgenes encoding the antipathogenic molecules will not be integrated into the conventional breeding programs but used for the generation of a herd of animals, which provides modified milk as a product (Whitelaw, 1999).
The modification of the immunological properties of milk by vaccination, transgenesis, or a combination of both may be a promising approach, which leads to products with a clear health benefit in the medical and veterinary field. Different mammalian species employ different strategies to provide immune protection to their offspring. Also, pathogens use different strategies to overcome the host's immune defenses. Therefore, there will not be a single approach for the prevention of infectious diseases via immunologically modified milk, but a variety of approaches tailored to specific classes of pathogens will have to be used to turn milk into an effective disease-preventing nutraceutical.
References
- Aguilera O, Ostolaza H, Quiros LM, Fierro JF. Permeabilizing action of an antimicrobial lactoferricin-derived peptide on bacterial and artificial membranes. FEBS Lett. 1999;462:273–277. doi: 10.1016/s0014-5793(99)01545-8. [DOI] [PubMed] [Google Scholar]
- Andersson Y, Lindquist S, Lagerqvist C, Hernell O. Lactoferrin is responsible for the fungistatic effect of human milk. Early Hum Dev. 2000;59:95–105. doi: 10.1016/s0378-3782(00)00086-4. [DOI] [PubMed] [Google Scholar]
- Arvola M, Gustafsson E, Svensson L, Jansson L, Holmdahl R, Heyman B, Okabe M, Mattsson R. Immunoglobulin-secreting cells of maternal origin can be detected in B cell-deficient mice. Biol Reprod. 2000;63:1817–1824. doi: 10.1095/biolreprod63.6.1817. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S, Newburg DS, Cleary TG. The effect of human milk on the adherence of enterohemorrhagic E. coli to rabbit intestinal cells. Adv Exp Med Biol. 1991;310:173–177. doi: 10.1007/978-1-4615-3838-7_21. [DOI] [PubMed] [Google Scholar]
- Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- Baveye S, Elass E, Fernig DG, Blanquart C, Mazurier J, Legrand D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect Immun. 2000;68:6519–6525. doi: 10.1128/iai.68.12.6519-6525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol. 1992;73:472–479. doi: 10.1111/j.1365-2672.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- Bellamy W, Wakabayashi H, Takase M, Kawase K, Shimamura S, Tomita M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med Microbiol Immunol (Berlin) 1993;182:97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- Berkhout B, Derksen GC, Back NK, Klaver B, de KC, Visser S. Structural and functional analysis of negatively charged milk proteins with anti-HIV activity. AIDS Res Hum Retroviruses. 1997;13:1101–1107. doi: 10.1089/aid.1997.13.1101. [DOI] [PubMed] [Google Scholar]
- Bishop JG, Schanbacher FL, Ferguson LC, Smith KL. In vitro growth inhibition of mastitis-causing coliform bacteria by bovine apo-lactoferrin and reversal of inhibition by citrate and high concentrations of apo-lactoferrin. Infect Immun. 1976;14:911–918. doi: 10.1128/iai.14.4.911-918.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorge L, Jensen TS, Kristoffersen EK, Ulstein M, Matre R. Identification of the complement regulatory protein CD59 in human colostrum and milk. Am J Reprod Immunol. 1996;35:43–50. doi: 10.1111/j.1600-0897.1996.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Castilla J, Pintado B, Sola I, Sanchez-Morgado JM, Enjuanes L. Engineering passive immunity in transgenic mice secreting virus-neutralizing antibodies in milk. Nat Biotechnol. 1998;16:349–354. doi: 10.1038/nbt0498-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple DS, Mason DJ, Joannou CL, Odell EW, Gant V, Evans RW. Structure–function relationship of antibacterial synthetic peptides homologous to a helical surface region on human lactoferrin against Escherichia coli serotype O111. Infect Immun. 1998;66:2434–2440. doi: 10.1128/iai.66.6.2434-2440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DK, Langridge WH. Expression of full-length bioactive antimicrobial human lactoferrin in potato plants. Transgenic Res. 2000;9:71–78. doi: 10.1023/a:1008977630179. [DOI] [PubMed] [Google Scholar]
- Cirioni O, Giacometti A, Barchiesi F, Scalise G. Inhibition of growth of Pneumocystis carinii by lactoferrins alone and in combination with pyrimethamine, clarithromycin and minocycline. J Antimicrob Chemother. 2000;46:577–582. doi: 10.1093/jac/46.4.577. [DOI] [PubMed] [Google Scholar]
- Damiens E, El Yazidi I, Mazurier J, Duthille I, Spik G, Boilly-Marer Y. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J Cell Biochem. 1999;74:486–498. [PubMed] [Google Scholar]
- de Groot N, van Kuik-Romeijn P, Lee SH, de Boer HA. Increased immunoglobulin A levels in milk by over-expressing the murine polymeric immunoglobulin receptor gene in the mammary gland epithelial cells of transgenic mice. Immunology. 2000;101:218–224. doi: 10.1046/j.1365-2567.2000.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina T. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Arch Virol Suppl. 1996;12:217–223. doi: 10.1007/978-3-7091-6553-9_23. [DOI] [PubMed] [Google Scholar]
- Ebina T, Ohta M, Kanamaru Y, Yamamoto Osumi Y, Baba K. Passive immunizations of suckling mice and infants with bovine colostrum containing antibodies to human rotavirus. J Med Virol. 1992;38:117–123. doi: 10.1002/jmv.1890380209. [DOI] [PubMed] [Google Scholar]
- Englund JA. Passive protection against respiratory syncytial virus disease in infants: the role of maternal antibody. Pediatr Infect Dis J. 1994;13:449–453. doi: 10.1097/00006454-199405000-00037. [DOI] [PubMed] [Google Scholar]
- Englund JA. Prevention strategies for respiratory syncytial virus: passive and active immunization. J Pediatr. 1999;135:38–44. [PubMed] [Google Scholar]
- Englund J, Glezen WP, Piedra PA. Maternal immunization against viral disease. Vaccine. 1998;16:1456–1463. doi: 10.1016/s0264-410x(98)00108-x. [DOI] [PubMed] [Google Scholar]
- Enjuanes L, van der Zeijst BAM. Molecular basis of transmissible gastroenteritis virus epidemiology. In: Siddell SG, Siddells SG, editors. The coronaviridae. Plenum; New York: 1995. pp. 337–376. [Google Scholar]
- Filteau SM. Role of breast-feeding in managing malnutrition and infectious disease. Proc Nutr Soc. 2000;59:565–572. doi: 10.1017/s002966510000080x. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Tacket CO, Delehanty A, Maneval DR, Nataro J, Crabb JH. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis. 1998;177:662–667. doi: 10.1086/514227. [DOI] [PubMed] [Google Scholar]
- Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr. 2000;130:426S–431S. doi: 10.1093/jn/130.2.426S. [DOI] [PubMed] [Google Scholar]
- Goldman AS, Chheda S, Garofalo R, Schmalstieg FC. Cytokines in human milk: properties and potential effects upon the mammary gland and the neonate. J Mammary Gland Biol Neoplasia. 1996;1:251–258. doi: 10.1007/BF02018078. [DOI] [PubMed] [Google Scholar]
- Guerrero CA, Mendez E, Zarate S, Isa P, Lopez S, Arias CF. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc Natl Acad Sci USA. 2000;97:14644–14649. doi: 10.1073/pnas.250299897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol. 1998;81:523–533. doi: 10.1016/S1081-1206(10)62704-4. [DOI] [PubMed] [Google Scholar]
- Hanson LA. The mother–offspring dyad and the immune system. Acta Paediatr. 2000;89:252–258. [PubMed] [Google Scholar]
- Hanson LA, Ceafalau L, Mattsby-Baltzer I, Lagerberg M, Hjalmarsson A, Ashraf R, Zaman S, Jalil F. The mammary gland–infant intestine immunologic dyad. Adv Exp Med Biol. 2000;478:65–76. doi: 10.1007/0-306-46830-1_6. [DOI] [PubMed] [Google Scholar]
- Harmsen MC, Swart PJ, de Bethune MP, Pauwels R, de Clercq E, The TH, Meijer DK. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172:380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- Hwang PM, Zhou N, Shan X, Arrowsmith CH, Vogel HJ. Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry. 1998;37:4288–4298. doi: 10.1021/bi972323m. [DOI] [PubMed] [Google Scholar]
- Iigo M, Kuhara T, Ushida Y, Sekine K, Moore MA, Tsuda H. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin Exp Metastasis. 1999;17:35–40. doi: 10.1023/a:1026452110786. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama K, Tanaka T, Tanaka K, Sckihara H, Shimotohno K, Kato N. Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes. Biochem Biophys Res Commun. 1998;245:549–553. doi: 10.1006/bbrc.1998.8481. [DOI] [PubMed] [Google Scholar]
- Isaacs CE, Kashyap S, Heird WC, Thormar H. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch Dis Child. 1990;65:861–864. doi: 10.1136/adc.65.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MC, O'Brien C, Trout J, Guidry A, Fayer R. Hyperimmune bovine colostrum specific for recombinant Cryptosporidium parvum antigen confers partial protection against cryptosporidiosis in immunosuppressed adult mice. Vaccine. 1999;17:2453–2460. doi: 10.1016/s0264-410x(98)00369-7. [DOI] [PubMed] [Google Scholar]
- Kanyshkova TG, Semenov DV, Buneva VN, Nevinsky GA. Human milk lactoferrin binds two DNA molecules with different affinities. FEBS Lett. 1999;451:235–237. doi: 10.1016/s0014-5793(99)00579-7. [DOI] [PubMed] [Google Scholar]
- Kassim OO, Ako-Anai KA, Torimiro SE, Hollowell GP, Okoye VC, Martin SK. Inhibitory factors in breastmilk, maternal and infant sera against in vitro growth of Plasmodium falciparum malaria parasite. J Trop Pediatr. 2000;46:92–96. doi: 10.1093/tropej/46.2.92. [DOI] [PubMed] [Google Scholar]
- Kolb AF, Pewe L, Webster J, Perlman S, Whitelaw CB, Siddell SG. Virus-neutralizing monoclonal antibody expressed in milk of transgenic mice provides full protection against virus-induced encephalitis. J Virol. 2001;75:2803–2809. doi: 10.1128/JVI.75.6.2803-2809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimpenfort P, Rademakers A, Eyestone W, van der Schans A, van den Broek S, Kooiman P, Kootwijk E, Platenburg G, Pieper F, Strijker R, de Boer HA. Generation of transgenic dairy cattle using ‘in vitro’ embryo production. Biotechnology (NY) 1991;9:844–847. doi: 10.1038/nbt0991-844. [DOI] [PubMed] [Google Scholar]
- Kuipers ME, de VH, Eikelboom MC, Meijer DK, Swart PJ. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob Agents Chemother. 1999;43:2635–2641. doi: 10.1128/aac.43.11.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeta MO, Vidal K, Nores JE, Arias M, Vita N, Morgan BP, Guillemot JC, Loyaux D, Ferrara P, Schmid D, Affolter M, Borysiewicz LK, Donnet-Hughes A, Schiffrin EJ. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J Exp Med. 2000;191:1807–1812. doi: 10.1084/jem.191.10.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laegreid A, Kolsto-Otnaess AB. Trace amounts of ganglioside GM1 in human milk inhibit enterotoxins from Vibrio cholerae and Escherichia coli. Life Sci. 1987;40:55–62. doi: 10.1016/0024-3205(87)90252-9. [DOI] [PubMed] [Google Scholar]
- Lahov E, Regelson W. Antibacterial and immunostimulating casein-derived substances from milk: casecidin, isracidin peptides. Food Chem Toxicol. 1996;34:131–145. doi: 10.1016/0278-6915(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Lamprecht CL, Krause HE, Mufson MA. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis. 1976;134:211–217. doi: 10.1093/infdis/134.3.211. [DOI] [PubMed] [Google Scholar]
- Larson BL. Immunoglobulins of the mammary secretions. In: F PF, F PFs, editors. Advanced dairy chemistry vol. 1 — proteins. Elsevier; London: 1992. pp. 231–254. [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science. 1994;264:1936–1938. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- McAbee DD, Bennatt DJ, Ling YY. Identification and analysis of a Ca(2+)-dependent lactoferrin receptor in rat liver. Lactoferrin binds to the asialoglycoprotein receptor in a galactose-independent manner. Adv Exp Med Biol. 1998;443:113–121. [PubMed] [Google Scholar]
- Moriishi K, Inoue S, Koura M, Amano F. Inhibition of listeriolysin O-induced hemolysis by bovine lactoferrin. Biol Pharm Bull. 1999;22:1167–1172. doi: 10.1248/bpb.22.1167. [DOI] [PubMed] [Google Scholar]
- Munoz FM, Englund JA. A step ahead Infant protection through maternal immunization. Pediatr Clin North Am. 2000;47:449–463. doi: 10.1016/s0031-3955(05)70217-0. [DOI] [PubMed] [Google Scholar]
- Neurath AR, Jiang S, Strick N, Lin K, Li YY, Debnath AK. Bovine beta-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV. Nat Med. 1996;2:230–234. doi: 10.1038/nm0296-230. [DOI] [PubMed] [Google Scholar]
- Newburg DS. Oligosaccharides and glycoconjugates in human milk: their role in host defense. J Mammary Gland Biol Neoplasia. 1996;1:271–283. doi: 10.1007/BF02018080. [DOI] [PubMed] [Google Scholar]
- Newburg DS. Human milk glycoconjugates that inhibit pathogens. Curr Med Chem. 1999;6:117–127. [PubMed] [Google Scholar]
- Newburg DS, Linhardt RJ, Ampofo SA, Yolken RH. Human milk glycosaminoglycans inhibit HIV glycoprotein gp120 binding to its host cell CD4 receptor. J Nutr. 1995;125:419–424. doi: 10.1093/jn/125.3.419. [DOI] [PubMed] [Google Scholar]
- Newburg DS, Peterson JA, Ruiz-Palacios GM, Matson DO, Morrow AL, Shults J, Guerrero ML, Chaturvedi P, Newburg SO, Scallan CD, Taylor MR, Ceriani RL, Pickering LK. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet. 1998;351:1160–1164. doi: 10.1016/s0140-6736(97)10322-1. [DOI] [PubMed] [Google Scholar]
- Nuijens JH, van Berkel PH, Schanbacher FL. Structure and biological actions of lactoferrin. J Mammary Gland Biol Neoplasia. 1996;1:285–295. doi: 10.1007/BF02018081. [DOI] [PubMed] [Google Scholar]
- Odell EW, Sarra R, Foxworthy M, Chapple DS, Evans RW. Antibacterial activity of peptides homologous to a loop region in human lactoferrin. FEBS Lett. 1996;382:175–178. doi: 10.1016/0014-5793(96)00168-8. [DOI] [PubMed] [Google Scholar]
- Ogundele MO. Cytotoxicity by stored human breast-milk: possible contribution of complement system. Cell Biol Int. 1999;23:585–588. doi: 10.1006/cbir.1999.0425. [DOI] [PubMed] [Google Scholar]
- Ogundele MO. Complement-mediated bactericidal activity of human milk to a serum-susceptible strain of E. coli 0111. J Appl Microbiol. 1999;87:689–696. doi: 10.1046/j.1365-2672.1999.00911.x. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Tsutsumi H, Kumar NS, Ogra PL. Effect of breast feeding on the development of anti-idiotype antibody response to F glycoprotein of respiratory syncytial virus in infant mice after post-partum maternal immunization. J Immunol. 1989;142:2507–2512. [PubMed] [Google Scholar]
- Pecquet S, Bovetto L, Maynard F, Fritsche R. Peptides obtained by tryptic hydrolysis of bovine beta-lactoglobulin induce specific oral tolerance in mice. J Allergy Clin Immunol. 2000;105:514–521. doi: 10.1067/mai.2000.103049. [DOI] [PubMed] [Google Scholar]
- Pellegrini A, Thomas U, Bramaz N, Hunziker P, von Fellenberg R. Isolation and identification of three bactericidal domains in the bovine alpha-lactalbumin molecule. Biochim Biophys Acta. 1999;1426:439–448. doi: 10.1016/s0304-4165(98)00165-2. [DOI] [PubMed] [Google Scholar]
- Pollock DP, Kutzko JP, Birck WE, Williams JL, Echelard Y, Meade HM. Transgenic milk as a method for the production of recombinant antibodies. J Immunol Methods. 1999;231:147–157. doi: 10.1016/S0022-1759(99)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Hendrixson DR, Baker EN, Murphy TF, St. Geme JW, Plaut AG. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc Natl Acad Sci USA. 1998;95:12641–12646. doi: 10.1073/pnas.95.21.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio I, Visser S. Identification of two distinct antibacterial domains within the sequence of bovine alpha(s2)-casein. Biochim Biophys Acta. 1999;1428:314–326. doi: 10.1016/s0304-4165(99)00079-3. [DOI] [PubMed] [Google Scholar]
- Rohrer L, Winterhalter KH, Eckert J, Kohler P. Killing of Giardia lamblia by human milk is mediated by unsaturated fatty acids. Antimicrob Agents Chemother. 1986;30:254–257. doi: 10.1128/aac.30.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon V, Legrand D, Slomianny MC, el Yazidi I, Spik G, Gruber V, Bournat P, Olagnier B, Mison D, Theisen M, Merot B. Production of human lactoferrin in transgenic tobacco plants. Protein Expression Purif. 1998;13:127–135. doi: 10.1006/prep.1998.0886. [DOI] [PubMed] [Google Scholar]
- Schmidt E. Effects of varying degrees of heat treatment on milk protein and its nutritional consequences. Acta Paediatr Scand Suppl. 1982;296:41–43. doi: 10.1111/j.1651-2227.1982.tb09593.x. [DOI] [PubMed] [Google Scholar]
- Sola I, Castilla J, Pintado B, Sanchez-Morgado JM, Whitelaw CB, Clark AJ, Enjuanes L. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J Virol. 1998;72:3762–3772. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan W, Dichtelmuller H, Lissner R. Antibodies from colostrum in oral immunotherapy. J Clin Chem Clin Biochem. 1990;28:19–23. [PubMed] [Google Scholar]
- Strom MB, Rekdal O, Svendsen JS. Antibacterial activity of 15-residue lactoferricin derivatives. J Pept Res. 2000;56:265–274. doi: 10.1034/j.1399-3011.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- Stromqvist M, Falk P, Bergstrom S, Hansson L, Lonnerdal B, Normark S, Hernell O. Human milk kappa-casein and inhibition of Helicobacter pylori adhesion to human gastric mucosa. J Pediatr Gastroenterol Nutr. 1995;21:288–296. doi: 10.1097/00005176-199510000-00006. [DOI] [PubMed] [Google Scholar]
- Sun XL, Baker HM, Shewry SC, Jameson GB, Baker EN. Structure of recombinant human lactoferrin expressed in Aspergillus awamori. Acta Crystallogr, Sect D: Biol Crystallogr. 1999;55:403–407. doi: 10.1107/s0907444998011226. [DOI] [PubMed] [Google Scholar]
- Swart PJ, Kuipers ME, Smit C, Pauwels R, de Bethune MP, de Clercq E, Meijer DK, Huisman JG. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vitro. AIDS Res Hum Retroviruses. 1996;12:769–775. doi: 10.1089/aid.1996.12.769. [DOI] [PubMed] [Google Scholar]
- Swart PJ, Kuipers EM, Smit C, van der Strate BW, Harmsen MC, Meijer DK. Lactoferrin. Antiviral activity of lactoferrin. Adv Exp Med Biol. 1998;443:205–213. [PubMed] [Google Scholar]
- Swart PJ, Harmsen MC, Kuipers ME, Van Dijk AA, van der Strate BW, van Berkel PH, Nuijens JH, Smit C, Witvrouw M, de Clercq E, de Bethune MP, Pauwels R, Meijer DK. Charge modification of plasma and milk proteins results in antiviral active compounds. J Pept Sci. 1999;5:563–576. doi: 10.1002/(SICI)1099-1387(199912)5:12<563::AID-PSC226>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ikeda M, Nozaki A, Kato N, Tsuda H, Saito S, Sekihara H. Lactoferrin inhibits hepatitis C virus viremia in patients with chronic hepatitis C: a pilot study. Jpn J Cancer Res. 1999;90:367–371. doi: 10.1111/j.1349-7006.1999.tb00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Sanchez C, Sune C, Smerdou C, Prevec L, Graham F, Enjuanes L. Induction of antibodies protecting against transmissible gastroenteritis coronavirus (TGEV) by recombinant adenovirus expressing TGEV spike protein. Virology. 1995;213:503–516. doi: 10.1006/viro.1995.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnes CG, Aleixo JA, Monteiro AV, Dellagostin OA. DNA inoculation with a plasmid vector carrying the faeG adhesin gene of Escherichia coli K88ab induced immune responses in mice and pigs. Vaccine. 1999;17:2089–2095. doi: 10.1016/s0264-410x(98)00384-3. [DOI] [PubMed] [Google Scholar]
- Ulvatne H, Haukland HH, Olsvik O, Vorland LH. Lactoferricin B causes depolarization of the cytoplasmic membrane of Escherichia coli ATCC 25922 and fusion of negatively charged liposomes. FEBS Lett. 2001;492:62–65. doi: 10.1016/s0014-5793(01)02233-5. [DOI] [PubMed] [Google Scholar]
- Valenti P, Greco R, Pitari G, Rossi P, Ajello M, Melino G, Antonini G. Apoptosis of Caco-2 intestinal cells invaded by Listeria monocytogenes: protective effect of lactoferrin. Exp Cell Res. 1999;250:197–202. doi: 10.1006/excr.1999.4500. [DOI] [PubMed] [Google Scholar]
- Wada T, Aiba Y, Shimizu K, Takagi A, Miwa T, Koga Y. The therapeutic effect of bovine lactoferrin in the host infected with Helicobacter pylori. Scand J Gastroenterol. 1999;34:238–243. doi: 10.1080/00365529950173627. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H, Uchida K, Yamauchi K, Teraguchi S, Hayasawa H, Yamaguchi H. Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J Antimicrob Chemother. 2000;46:595–602. doi: 10.1093/jac/46.4.595. [DOI] [PubMed] [Google Scholar]
- Whitelaw B. Toward designer milk. Nat Biotechnol. 1999;17:135–136. doi: 10.1038/6134. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Peterson JA, Vonderfecht SL, Fouts ET, Midthun K, Newburg DS. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Yoshimura Y, Huang YY, Suzuki R, Yokoyama M, Okabe M, Shimamura M. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101:570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucht HD, Raida M, Adermann K, Magert HJ, Forssmann WG. Casocidin-I: a casein-alpha s2 derived peptide exhibits antibacterial activity. FEBS Lett. 1995;372:185–188. doi: 10.1016/0014-5793(95)00974-e. [DOI] [PubMed] [Google Scholar]


