Abstract
Most coronaviruses infecting humans cause mild diseases, whereas severe acute respiratory syndrome (SARS)-associated coronavirus is an extremely dangerous pathogen. Here, we report the development of a serologic assay for detection of antibodies to human coronaviruses (HCoVs) based on recombinant nucleocapsid (N) proteins of all known pathogenic strains (229E, NL63, OC43, HKU1, SARS). The novel immunoassay is highly useful for epidemiologic surveys, where use of nucleic acid diagnostics often is limited. Purified recombinant antigens were immobilized on nitrocellulose membranes and applied in a line immunoassay, which allows rapid detection of antibodies to 5 different HCoVs in a single experiment. For assay evaluation, serum samples from persons infected with 229E or OC43 (acute/convalescent), recovered SARS patients and healthy donors were analyzed. Screening for nucleocapsid (N)-specific immunoglobulin G (IgG) in convalescent sera reached 100% sensitivity. With this new technique, we found that recently identified NL63 and HKU1 contribute significantly to the overall spectrum of coronavirus infections. Possibly, cross-reactive antibody responses were observed using 229E and OC43 serum pairs. However, the potential of this assay could clearly be demonstrated employing SARS-positive serum samples, where nonspecific binding to nucleocapsids of other HCoVs was not observed. This coronavirus strain-specific line immunoassay represents a powerful tool for serologic diagnostics.
Keywords: Human coronavirus, Nucleocapsid, Line immunoassay, SARS, Seroprevalence, Cross-reaction
1. Introduction
Coronaviruses (family Coronaviridae, order Nidovirales) are large (120–160 nm), enveloped, roughly spherical particles. They possess a nonsegmented single-stranded RNA genome of up to 31 kb in positive orientation. Based on serologic cross-reactivities and sequence analysis, coronaviruses are classified in 3 distinct groups (Eickmann et al., 2003, Gorbalenya et al., 2004, Kim et al., 2006, Weiss and Navas-Martin, 2005). Coronaviruses are known to infect many domestic animals as well as humans, causing acute and chronic diseases of the respiratory, enteric, and central nervous system (Weiss and Navas-Martin, 2005). Human coronaviruses (HCoVs) 229E and OC43 have 1st been described in the 1960s (Hamre et al., 1967, McIntosh et al., 1967) and are thought to be responsible for 10% to 30% of all common colds (Larson et al., 1980, Myint, 2006). The new infectious disease severe acute respiratory syndrome (SARS) emerged in November 2002 in Guangdong Province, People's Republic of China. Within a few months, SARS had spread to 26 countries, infecting more than 8000 people of whom almost 10% died (Poutanen and Low, 2004). The etiologic agent was recognized to be a novel virus, termed SARS-CoV (Drosten et al., 2003), which is related to, but distinct from other, known coronaviruses. Recent findings suggest a close relationship between SARS-CoV and group II coronaviruses (Kim et al., 2006). In 2004, van der Hoek et al. (2004) reported the isolation of a new group I CoV from a 7-month-old child with bronchiolitis, named HCoV-NL63. Independently described viruses HCoV-NL (Fouchier et al., 2004) and HCoV-NH (Esper et al., 2005b) are closely related to NL63 and likely represent strains of the same species of virus (Kahn, 2006, Van der Hoek and Berkhout, 2005). HCoV-NL63 has been linked to more severe ailments, such as morbus croup (van der Hoek et al., 2005), and a systemic vasculitis in young children, Kawasaki disease, although association with the latter is discussed controversially (Esper et al., 2005a, Shimizu et al., 2005). NL63 infections have been reported throughout the world (Arden et al., 2005, Bastien et al., 2005, Chiu et al., 2005, Garbino et al., 2006), indicating that this HCoV can be regarded as a new major etiologic agent of respiratory disorders. HCoV-HKU1 was 1st isolated 2005 in Hong Kong from a 71-year-old man suffering from pneumonia and was classified as group II coronavirus (Woo et al., 2005a). So far, this strain has been detected in Asia, Europe, Unites States, and Australia in connection with respiratory and enteric tract illness (Esper et al., 2006, Garbino et al., 2006, Lau et al., 2006, Sloots et al., 2006, Vabret et al., 2006).
Coronavirus diagnostics are dominated by nucleic acid technologies (NATs). Because reconvalescence leads to a loss of viremia, NATs are not applicable for coronavirus screening in epidemiologic surveys outside acute pandemics (e.g., SARS). Serology so far is rarely used and not yet developed to distinguish between pathogenic types of HCoVs.
As candidate antigens for immunoassays detecting anticoronavirus antibodies, structural proteins are of special interest. The RNA binding nucleocapsid (N) protein exhibits high immunogenicity and is abundantly produced during infection (Timani et al., 2004). Several methods using nucleocapsids as diagnostic antigens have been designed with special emphasis on SARS N (Chen et al., 2005, Guan et al., 2004, Liang et al., 2005, Tan et al., 2004). Here, we describe the establishment of a line immunoassay for rapid and simultaneous detection of antibodies directed against the 5 known HCoVs (229E, NL63, OC43, HKU1, and SARS) in human sera based on recombinant viral N proteins. We cloned and expressed the corresponding viral genes in Escherichia coli. Purified proteins were subsequently employed in a line immunoassay and tested for suitability as diagnostic antigens.
2. Materials and methods
2.1. Viral strains and viral RNA
HCoVs 229E (ATCC VR-740) and OC43 (ATCC VR-759) were obtained from the American Type Culture Collection (Manassas, VA) and propagated on MRC-5 cells. RNA was isolated from cell culture supernatant using the SV Total RNA Isolation System (Promega, Mannheim, Germany). HCoV-NL63-RNA was kindly provided by L. van der Hoek, Amsterdam, Netherlands. HKU1-RNA (strain Caen) was received from A. Vabret, Caen, France, and SARS-CoV-RNA (strain FRA) was prepared and sent to our laboratory by M. Eickmann, Marburg, Germany.
2.2. Reverse transcriptase polymerase chain reaction, polymerase chain reaction and cloning of coronaviral nucleocapsid genes
cDNA was synthesized by M-MLV reverse transcriptase (Promega) using gene-specific reverse primers derived from full genome sequences (see Table 1 for GenBank accession numbers). Subsequently, cDNA was amplified utilizing PfuTurbo® hotstart DNA polymerase (Stratagene, Heidelberg, Germany) with primer pairs displayed in Table 1. Cloning sites overlapping the start codon and cloning sites downstream of the stop codon were introduced as indicated. All oligonucleotides were synthesized by Metabion, Martinsried, Germany.
Table 1.
Accession numbers and oligonucleotides used for reverse transcription and amplification of cDNA
| HCoV | Accession number, position | Primer (5′→3′) | Cloning site |
|---|---|---|---|
| 229E | DQ243939a | GAACGAACATATGGCTACAGTCAAATGGG | NdeI |
| (VR-740) | GTGGATCCTTTAGTTTAC | BamHI | |
| OC43 | AY585228b | AAATTTTACATATGTCTTTTACTCCTGG | NdeI |
| (VR-759) | 29079–30425c | GGTGAATTCTCTTATATTTCT | EcoRI |
| NL63 | AY567487b | TAAACTAAACCATATGGCTAGTG | NdeI |
| 26133–27266c | TGGAATTCCAAAACAATTTAATGC | EcoRI | |
| SARS (FRA) | AY310120b | ACAAATTCATATGTCTGATAATGG | NdeI |
| 28120–29388c | GGGGATCCTGAGTGTTTATGCC | BamHI | |
| HKU1 (Caen) | DQ778921a | ATCTACCCGCTTAGTATGTCTTATAC | FauI |
| ATTAGAATTCATTCTCAATTAAG | EcoRI |
Boldface letters in primer sequences indicate restriction endonuclease cleavage sites.
Accession numbers for nucleocapsid genes.
Accession numbers for full genome sequences.
Nucleotide positions of corresponding nucleocapsid genes.
The resulting polymerase chain reaction (PCR) products were cloned into expression vector pCS04 (Lindner, unpublished), a derivative of pET22b (Novagen, Schwalbach, Germany), encoding 6 N-terminal histidine residues for purification. Sequence identity of expression constructs pCS04/229E-N, pCS04/OC43-N, pCS04/NL63-N, pCS04/HKU1-N, and pCS04/SARS-N was confirmed by DNA sequencing (Geneart, Regensburg, Germany). E. coli BL21 competent cells CodonPlus® (DE3)-RP (Stratagene) were transformed with the constructed expression plasmids.
2.3. Expression of N genes and purification of recombinant N proteins
Transformed cells were grown in Luria–Bertani medium (supplemented with 100 μg/mL ampicillin and 34 μg/mL chloramphenicol) at 37 °C under vigorous shaking until optical density at 600 nm reached approximately 0.6. Nucleocapsid protein synthesis was induced by addition of isopropylthio-β-d-galactoside (final concentration 1 mmol/L). After 3 h, cells were harvested by centrifugation (10 800 × g, 10 min, 4 °C) and stored at −80 °C.
Nucleocapsid genes of 229E, NL63, and SARS were expressed as highly soluble proteins, whereas OC43 N and HKU1 N had to be purified from inclusion bodies. Therefore, buffers used for purification of OC43 N and HKU1 N were supplemented with 1 or 3 mol/L urea, respectively. Frozen cells (6–8 g wet weight) were thawed and suspended in loading buffer containing 50 mmol/L sodium phosphate (pH 8.0), 500 mmol/L NaCl, and 20 mmol/L imidazole at 10 mL per gram wet weight. Protease inhibitor was added before disruption of cells (2 mmol/L Pefabloc SC®; BIOMOL, Hamburg, Germany). Cells were lysed by 2 passages through the high pressure system Basic-Z (IUL Instruments, Königswinter, Germany) at 1500 bar. To avoid DNA contamination, we 1st incubated the crude extract with 2 U/mL Benzonase® (Merck, Darmstadt, Germany) for 20 min and then precipitated with 0.7% (w/v) protamine sulfate (Sigma-Aldrich, Steinheim, Germany). The lysate was then cleared by centrifugation (45 000 × g, 10 min, 4 °C). Soluble histidine-tagged nucleocapsid proteins (229E N, NL63 N, and SARS N) present in the supernatant were purified by affinity chromatography using a Ni-Sepharose column (bed volume, 17 mL; GE Healthcare, Munich, Germany). Bound proteins were eluted with a linear gradient of imidazole (20–500 mmol/L) in loading buffer. The nucleocapsid-containing fractions (350–450 mmol/L imidazole) were pooled, dialyzed (50 mmol/L sodium phosphate, pH 8.0, 50 mmol/L NaCl), and applied on an SP-Poros ion-exchange column (bed volume, 1.6 mL; Applied Biosystems, Weiterstadt, Germany). Elution was conducted in loading buffer (50 mmol/L sodium phosphate, pH 8.0) with a linear gradient from 50 mmol/L NaCl to 1 mol/L NaCl. Nucleocapsid fractions (500–900 mmol/L NaCl) were pooled and loaded on a preparative Superdex 75 gel filtration column (bed volume, 120 mL; 16/60 Superdex™ 75 prep grade; GE Healthcare) and equilibrated with loading buffer (150 mmol/L NaCl). The nucleocapsid-containing fractions were pooled, aliquoted, and stored at −80 °C.
Insoluble proteins OC43 N and HKU1 N were purified as described above for soluble nucleocapsids, whereas all buffers used for purification of OC43 N and HKU1 N were supplemented with 1 or 3 mol/L urea, respectively.
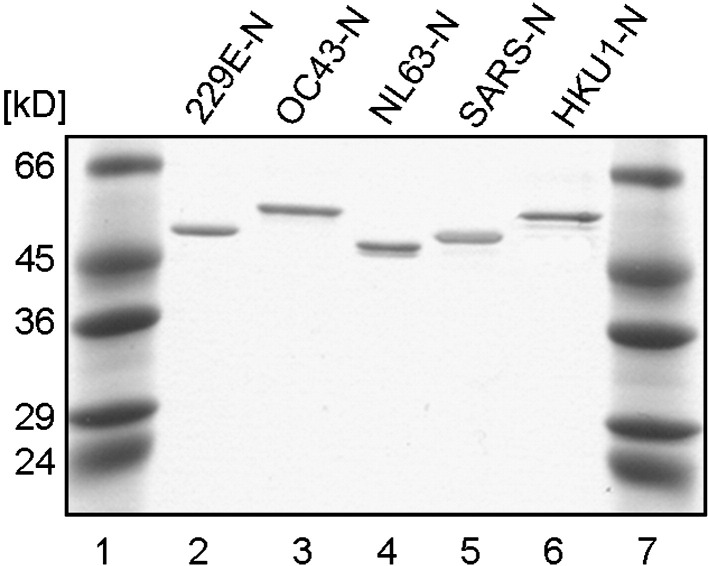
High protein purity was confirmed by analysis on a Coomassie-stained polyacrylamide gel (12.5%, Fig. 1 ). Nucleocapsid protein concentration was determined by the bicinchoninic acid (BCA) method (Sigma-Aldrich) or spectrophotometrically.
Fig. 1.
Purified recombinant coronaviral N proteins were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Lanes 1 and 7, molecular weight markers; lanes 2 to 6, recombinant protein, 250 ng of each recombinant protein was loaded per lane as indicated.
2.4. Instrumentation
Affinity chromatography and gel filtration were performed by use of the High Load™-System (Pharmacia, Freiburg, Germany). Ion-exchange chromatography was run on a BioCad 700E workstation (Applied Biosystems).
2.5. Production of immunoassay strips
Nucleocapsid antigen solutions (NL63 N, OC43 N, and SARS N: 50 ng/μL; 229E N: 25 ng/μL; HKU1 N: 15 ng/μL) and appropriate controls (strong positive control: human immunoglobulin G (IgG) 100 ng/μL; weak positive control: human IgG 3.5 ng/μL) were immobilized as parallel lines on nitrocellulose transfer membranes (Protran, 0.45 μm; Schleicher and Schuell, Dassel, Germany) using the Accutran ACC100/0 cross-blot system (Schleicher and Schuell). Recombinant antigens were incubated on the membrane for 2.5 h at room temperature under soft shaking. Nonspecific binding sites were blocked with 0.2% I-Block™ (w/v) (Tropix, Bedford, MA) supplemented with 1% (v/v) Tween 20 for 2 h at 37 °C. Finally, membranes were air-dried, cut into strips (3 × 57 mm), and stored at −20 °C.
2.6. Human sera
Acute and convalescent phase sera from 6 patients with recent HCoV-OC43 infection and from 6 patients with recent HCoV-229E infection were obtained from the serum bank of the Respiratory Pathogen Research Unit of the Baylor College of Medicine, Houston, TX (R.B. Couch). These samples were tested by enzyme-linked immunosorbent assay (ELISA) as well as microneutralization experiments. Convalescent phase sera were shown to have high OC43- and 229E-specific antibody titers. Samples from convalescent SARS patients were provided by the Chinese Center for Disease Control (China CDC), Beijing, PR China. These sera were collected from hospitalized patients during the SARS outbreak 2003 in Inner Mongolia, PR China. SARS-CoV infection was confirmed previously by ELISA, Western blot analysis, and immunofluorescence (IF). Further serum samples were derived from healthy donors and stored at the serum bank of the University of Regensburg, Germany.
2.7. Antibodies and antisera
To screen for contamination during purification steps, we used polyclonal rabbit anti-E. coli antibodies (1.3 μg/mL; Dako, Hamburg, Germany). For detection of His-tagged recombinant protein, we utilized mouse Penta His™ antibodies (dilution, 1:1000; QIAGEN, Hilden, Germany). We further raised anti-SARS nucleocapsid antibodies by injection of recombinant nucleocapsid protein into rabbits (Davids Biotechnologie, Regensburg, Germany). Western blot analysis was carried out with rabbit SARS N antiserum at a dilution of 1:10 000. Rabbit NL63 N antiserum was provided by L. van der Hoek, Amsterdam, Netherlands, and used at a dilution of 1:1000. All primary antibodies and sera were diluted in Tris-buffered saline (TBS), pH 7.5. For secondary detection, horseradish peroxidase (HRP)-conjugated polyclonal rabbit anti-human IgGs (Dako) were used for detection of anti-N antibodies in human sera. HRP-conjugated polyclonal swine anti-rabbit Ig and rabbit anti-mouse Ig (1.3 μg/mL, Dako) were used for detection of corresponding primary antibodies.
2.8. Line immunoassay arrangement
Immunoassay strips were incubated in human sera (dilution 1:100 in TBS, pH 7.5; total incubation volume, 1.5 mL) for 1 h under shaking and washed twice for 5 min in TBS supplemented with 0.05% (v/v) Tween 20 (TTBS). The strips were then incubated with secondary antibody solution for 45 min under shaking. To reduce background and matrix effects, we diluted 1:1000 the secondary antibodies in a buffer containing 70% LowCross™-Buffer (Candor Bioscience, Münster, Germany) and 30% TBS. After washing 3 times for 15 min in TTBS, bound antibodies were detected colorimetrically by addition of NiCl2/diaminobenzidine solution containing H2O2 for 3 min. All steps were performed at room temperature.
2.9. Nucleotide sequence accession numbers
The nucleotide sequence of the N gene of HCoV-HKU1 strain Caen was deposited in the GenBank sequence database under accession number DQ778921. GenBank accession numbers are listed in Table 1.
3. Results
3.1. Sequence analysis of cloned genes
Cloned nucleotide sequences of 229E N, OC43 N, NL63 N, and SARS N match completely to published sequences, whereas the nucleocapsid gene of coronavirus HKU1 differed slightly from already published HKU1 sequences. Viral RNA isolated from a nasal aspirate sampled from an infant with respiratory infection in Caen, France (Vabret, personal communication), was used for reverse transcriptase PCR (RT-PCR) amplification of the HKU1 N gene. The newly identified protein sequence (corresponding to GenBank accession number DQ778921) exhibits 97.3% amino acid identity and 98.6% amino acid similarity to the N protein of HKU1, strain N23 (accession number DQ437631). Genetic variability among HCoV-HKU1 isolates has been studied previously (Sloots et al., 2006, Woo et al., 2005b, Woo et al., 2006). For further classification of HCoV-HKU1, strain Caen, sequencing of the S and pol genes will be necessary.
3.2. Production of recombinant proteins
Nucleocapsid genes of all 5 known HCoVs were heterologously expressed in E. coli and purified to homogeneity. Highly purified recombinant antigens are shown in Fig. 1. Lack of any interfering protein contamination originating from the expression host was demonstrated by Western blotting. In purified nucleocapsid fractions, no bacterial proteins were detected using mouse anti-E. coli antibodies. Recombinant proteins could be identified during the purification process by use of antibodies recognizing the N-terminal histidine tag (data not shown).
3.3. Line immunoassay development and evaluation
Recombinant proteins were immobilized as horizontal lines on membrane strips. The optimal concentration was individually determined for each antigen. Best signal-to-noise ratios were achieved by using protein concentrations of 15 to 50 ng/μL (data not shown). Reliability of the testing system was improved by including a strong positive (human IgG; 100 ng/μL) and a weak positive control (human IgG; 3.5 ng/μL) on each strip. The weak positive control represents a cutoff control. Only signals of the same intensity as or stronger than the defined weak positive control were considered positive.
Performance of the immunoassay was tested with 3 different sample pools: serum pairs of patients who suffered from infections with HCoV-OC43 (BCM-1A/B to BCM-6A/B, n = 12) or HCoV-229E (BCM-7A/B to BCM-12A/B, n = 12), sera gathered from convalescent SARS patients (n = 49), and randomly selected specimen from healthy donors (n = 25; 18 adults, 7 children). Unfortunately, no human sera collected after confirmed HCoV-NL63 or HCoV-HKU1 infection were available. Additionally, rabbit NL63 N antiserum and rabbit SARS N antiserum were utilized, showing specific reactions with their respective antigens (Fig. 3, anti-N, and Fig. 4, 23N). All experiments were carried out at least in triplicate.
Fig. 3.
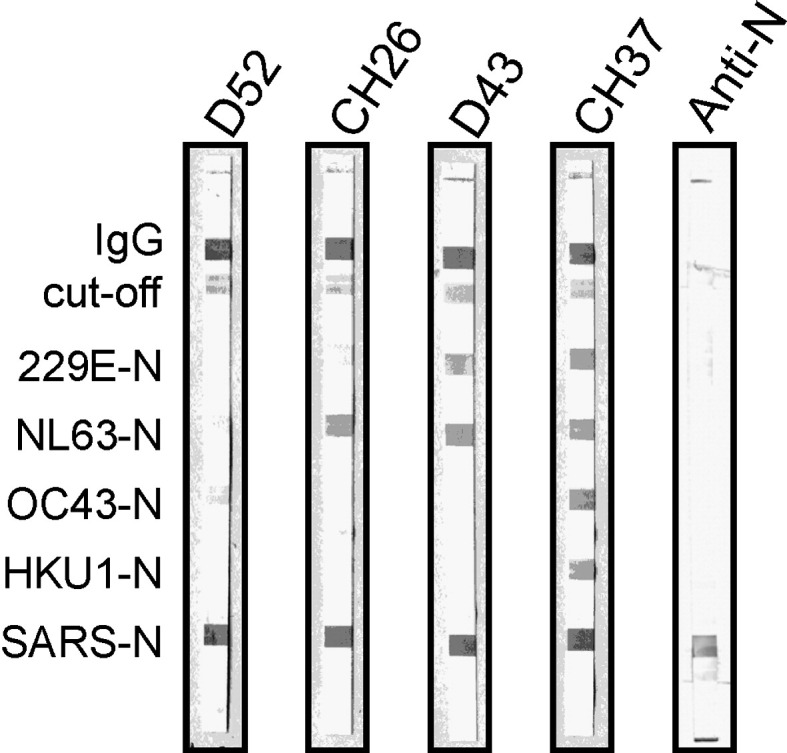
Line immunoassay evaluation probing sera sampled from convalescent SARS patients. A representative selection of immunoassay strips out of 49 samples is shown. Without exception, all samples clearly display strong signals to SARS nucleocapsid as expected. In addition, various combinations of reactions with other HCoV strains are shown. Anti-N, serum from rabbits immunized with recombinant SARS-CoV nucleocapsid was used in the assay. The immunoassay demonstrates a distinct and specific reaction with SARS N.
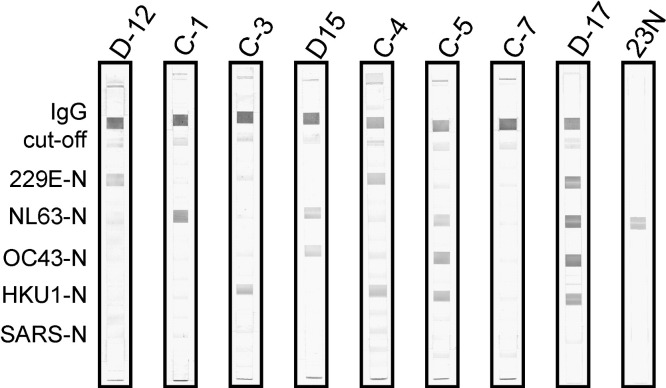
Fig. 4.
Arbitrarily selected sera from healthy donors were applied in our line immunoassay. Single, double, and multiple anticoronaviral immune responses in various combinations could be verified as shown. In addition, blood samples without seroconversion were identified (C-7). 23N, serum from rabbits immunized with truncated HCoV-NL63 nucleocapsid was used.
3.3.1. HCoV-OC43 and HCoV-229E
First, serum pairs (acute/convalescent) from patients suffering from HCoV-OC43 or HCoV-229E infection were screened regarding 2 aspects: specific and sensitive reaction with immobilized antigens; comparison of signal intensities (fraction of bound antibodies to relevant antigens) between acute (A) and convalescent (B) phase sera (Table 2 , Fig. 2 ). All sera obtained from convalescent patients (HCoV-OC43 or HCoV-229E infection) reacted with the respective antigens as expected. A strong induction of humoral responses was observed in 5 out of 6 (OC43) and 3 out of 6 (229E) cases (Table 2). Yet, amplified signal intensities in postinfection OC43 sera (BCM-1B to BCM-6B) were accompanied by sturdy reactions with the HKU1 antigen, except for BCM-3B, where, overall, only weak signals were detected (Table 2). Reaction intensities of sera derived from HCoV-229E–infected patients increased from acute to convalescent phase in 3 serum pairs (BCM-9A/B, BCM-11A/B, and BCM-12A/B), whereas in the remaining 3 sample pairs (BCM-7A/B, BCM-8A/B, BCM-10A/B), no clear boost of antibodies could be detected (Table 2). However, serum BCM-10B, responding only weakly to 229E N, exhibited distinct reactions to OC43 and to HKU1 N antigens. This observation raises the possibility of a collateral acute infection with HCoV-OC43. In addition, defined sera from HCoV-229E–infected patients (BCM-7 to BCM-12) uniformly showed presence of a basal level of NL63-specific antibodies. Stronger antibody response in case of samples BCM-9A/B does not necessarily imply nonspecific cross-reactions between 229E and NL63. Remarkably, none of the tested coronavirus positive sera reacted with the SARS antigen (Fig. 2, Table 2).
Table 2.
Reactions of clinically defined sera with HCoV nucleocapsids
| Serum no. | 229E-N | NL63-N | OC43-N | HKU1-N | SARS-N | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCoV-OC43a | BCM-1A | BCM-1B | ++ | ++ | ++ | ++ | − | +++ | − | +++ | − | − |
| BCM-2A | BCM-2B | − | − | + | + | − | +++ | − | ++ | − | − | |
| BCM-3A | BCM-3B | + | + | − | − | + | + | − | − | − | − | |
| BCM-4A | BCM-4B | − | − | + | + | + | +++ | − | +++ | − | − | |
| BCM-5A | BCM-5B | ++ | +++ | ++ | +++ | + | +++ | + | +++ | − | − | |
| BCM-6A | BCM-6B | ++ | ++ | +++ | ++ | + | ++ | + | ++ | − | − | |
| HCoV-229Eb | BCM-7A | BCM-7B | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | − | − |
| BCM-8A | BCM-8B | ++ | ++ | ++ | ++ | + | + | + | − | − | − | |
| BCM-9A | BCM-9B | − | +++ | ++ | +++ | + | + | − | − | − | − | |
| BCM-10A | BCM-10B | + | + | + | + | − | ++ | − | + | − | − | |
| BCM-11A | BCM-11B | ++ | +++ | ++ | ++ | +++ | +++ | ++ | +++ | − | − | |
| BCM-12A | BCM-12B | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | − | |
BCM-1 to BCM-6: serum samples of HCoV-OC43–infected patients.
BCM-7 to BCM-12: serum samples of HCoV-229E–infected patients; A: acute phase; B: convalescent phase; positive reactions are indicated with + (moderate), ++ (strong), and +++ (very strong); negative/no reactions are indicated with −.
Fig. 2.
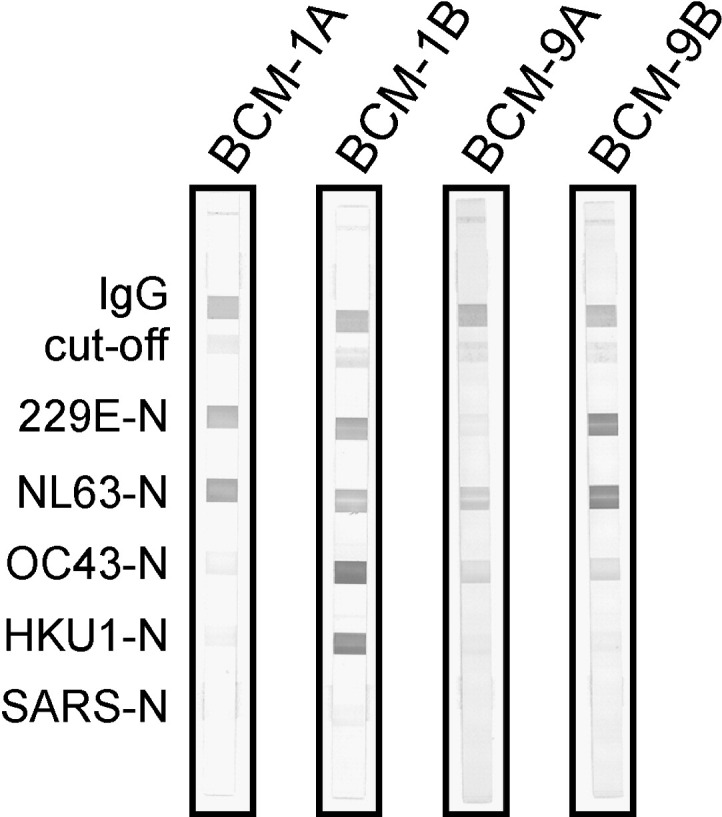
Evaluation of the line immunoassay with clinically defined serum pairs from HCoV-OC43 (BCM-1A/B)– or HCoV-229E (BCM-9A/B)–infected patients. Membrane strips were coated with recombinant N proteins and controls as described in Materials and methods. Representative immunoassay strips are shown. BCM-1A, serum from acute phase of HCoV-OC43 infection; BCM-1B, serum from convalescent phase of HCoV-OC43 infection, collected 55 days after BCM-1A. BCM-9A, serum from acute phase of HCoV-229E infection; BCM-9B, serum from convalescent phase of HCoV-229E infection, collected 116 days after BCM-9A. In sera taken during acute phase of HCoV infection, no OC43- or 229E-specific antibodies could be detected, whereas samples collected after convalescence strongly reacted with the corresponding antigens. Coreaction with HKU1 nucleocapsid (BCM-1) or NL63 nucleocapsid (BCM-9) might be due to cross-reactions.
3.3.2. SARS-CoV
We analyzed 49 sera obtained from convalescent SARS patients. All samples showed strong distinct signals with SARS nucleocapsids.
Moreover, 42 (85.7%) specimen responded to HCoV-229E N, 46 (93.9%) to HCoV-NL63 N, 40 (81.6%) to HCoV-OC43 N, and 26 (53.1%) to HCoV-HKU1 N. A representative set of assay strips indicating the variety of obtained antibody reactions is displayed in Fig. 3 .
3.3.3. Healthy donors
Twenty-five sera, randomly selected from healthy blood donors, were tested to investigate natural coronavirus prevalence. Applying these samples in the line immunoassay, we found evidence of single, double, and multiple coronavirus infections in various combinations (Fig. 4 ). We were also able to identify 3 blood samples showing no seroconversion, 2 of which were derived from children (Fig. 4, C-7). In summary, sera collected from healthy donors reacted with HCoV nucleocapsids as follows: 229E, 14 (56%); NL63, 15 (60%); OC43, 13 (52%); and HKU1, 12 (48%). As expected, no responses to SARS N were detected (Fig. 4).
4. Discussion
The SARS epidemic of 2002 and 2003 led to a boost in coronavirus research. Two, so far, unknown viruses, NL63 and HKU1, have since been identified, and detection of further HCoVs in the future is likely. The major technique to diagnose HCoV infections today is RT-PCR, whereas serologic assays distinguishing between all types of HCoVs are, to our knowledge, almost completely missing. Yet, epidemiologic studies regarding coronavirus prevalence are of great interest. However, techniques such as nucleic acid diagnostics are not suitable because coronaviral RNA can only be detected during the acute phase of infection or shortly thereafter. In addition, if clinical symptoms are mild or missing altogether, serology opens the possibility to identify silent converters, which, in the case of SARS, represents highly valuable information on the actual virus distribution among the population.
Based on previous reports (Tan et al., 2004, Timani et al., 2004, Zhu et al., 2006) and protein sequence comparisons, which revealed relatively low sequence identities and similarities (Table 3 ), we considered nucleocapsid proteins as appropriate antigenic markers for retrospective diagnosis of coronavirus infections in humans. The highest amino acid sequence identities are present between HKU1- and OC43-encoded nucleocapsids (68.2% identity, 74.5% similarity). N protein sequences of all other coronaviruses considered as antigens in our immunoassay exhibit much lower amino acid identities when compared with each other (31.9–49.3% identity, 38.5–57.8% similarity; Table 3). Therefore, we established a line immunoassay using recombinant N proteins as antigens to specifically detect coronavirus-directed antibodies in human sera. Validation of the newly developed test was carried out by assaying well-defined sera of patients who suffered from various coronaviral infections. Consistently, all available human sera sampled from convalescent patients (tested positive for HCoVs 229E, OC43, or SARS-CoV by microneutralization experiments, Western blotting, ELISA, or IF) reacted with their respective antigens as expected, indicating an excellent sensitivity of our line immunoassay. Although antigenic cross-reactivity to SARS nucleocapsid has been described earlier (Chan et al., 2005, Sun and Meng, 2004, Woo et al., 2004, Yip et al., 2007), we could not detect any serologic cross-reactivity with SARS N, even in those cases where donors strongly responded to the other 4 nucleocapsids (Fig. 4, D-17). Accordingly, only sera obtained from convalescent SARS patients reacted with the SARS antigen. Moreover, antibody binding reactions with SARS N were consistently strong, as demonstrated in Fig. 3. We identified 6 samples showing a single distinct band proving presence of anti–SARS-CoV antibodies. Five specimens reacted only with 1 additional HCoV antigen (e.g., Fig. 3, D52 and CH26), deriving from preexisting antibodies of anterior HCoV infections. Based on these findings, we conclude that there is no evidence of any nonspecific binding of SARS-specific antibodies to antigens of other HCoVs in convalescent SARS sera. Thus, SARS N protein provides a powerful tool to differentiate between SARS infection and infections with other HCoVs in serologic studies. However, cross-reactions among other HCoVs might occur. N proteins of OC43 and HKU1 (group I HCoVs) display the highest amino acid identities among all coronaviruses (68.2%, Table 3), and nonspecific binding of antibodies directed against OC43 N to HKU1 N is possibly observed (Fig. 2, BCM-1A/B, Table 2). The donor of serum sample BCM-1A/B was not tested for HKU1 infection (samples were characterized before primary detection of HKU1). However, double infection with OC43 and HKU1 seems unlikely because simultaneous detection of HKU1- and OC43-directed antibodies occurred in all but 1 serum pair (BCM-3A/B). Nonetheless, we also tested sera comprising IgGs that recognized OC43 N without binding to HKU1 N (Fig. 4, D-15), contradicting a general cross-reactivity. Nonspecific cross-reactions between 229E and NL63 (group I HCoVs) antigens appear rather unlikely because amino acid identities between both nucleocapsids are relatively low (49.3%, Table 3). Nevertheless, in a single 229E-positive serum sample, NL63 antibody response seemed to be coinduced (BCM-9A/B; Table 2, Fig. 2). The other defined sera sampled from 229E-infected patients (BCM-7 to BCM-12) consistently indicated antibody binding signals to NL63 N. These sera were initially not characterized for the presence of NL63 viral agent because NL63 was actually not discovered at sampling time. Thus, proof of NL63-specific antibodies in these sera is not necessarily evidence for cross-reactivity. Coinfections of 229E and NL63, or previous contact with NL63 earlier in the lives of these patients, resulting in basal antibody levels, cannot be excluded. Furthermore, positive reactions with OC43 N and HKU1 N in sample BCM-10B (Fig. 2, Table 2) could be due to a true HCoV-OC43 infection.
Table 3.
HCoV nucleocapsid protein analysis: pairwise comparison of coronavirus nucleocapsid proteins (GCG Wisconsin Package, bestfit, scoring matrix: blosum62)
| SARS-N | 100a | ||||
|---|---|---|---|---|---|
| 229E-N | 31.9 (38.5) | 100 | |||
| OC43-N | 42.8 (49.1) | 38.3 (44.9) | 100 | ||
| NL63-N | 35.8 (44.2) | 49.3 (57.8) | 35.4 (41.2) | 100 | |
| HKU1-N | 41.8 (49.4) | 42.0 (48.7) | 68.2 (74.5) | 36.4 (44.1) | 100 |
| SARS-N | 229E-N | OC43-N | NL63-N | HKU1-N |
Values indicate % amino acid identity (% amino acid similarity).
Antibodies specific to HCoVs 229E and OC43 can be identified in about 90% of adults throughout the world (Modrow et al., 2007). Although immunologic data concerning seroprevalence of anti–HCoV-229E and anti–HCoV-OC43 antibodies are available (Hruskova et al., 1990, Mourez et al., 2006), information about humoral responses concerning recently discovered HCoV-NL63 and HCoV-HKU1 is rather scarce, despite their widespread association with respiratory tract infections. The global SARS epidemic of 2002/2003 was controlled by a highly successful public health effort, and there have been no reported cases since April 2004. Thus, it appears that SARS-CoV is currently not circulating in humans. However, studies on the actual distribution of SARS-CoV are lacking, and surveys screening healthy donors for antibodies specific for SARS-CoV are rare (Yu et al., 2005). Contact leading to seroconversion may result in asymptomatic or subclinical manifestations (Che et al., 2006, Wilder-Smith et al., 2005) and are therefore not recognized as SARS infections. Hence, serology remains an important tool in SARS diagnosis.
Because we decided to test 25 arbitrarily selected sera, we assumed that most samples collected from adults would exhibit positive IgG reactions to at least 1, but possibly several coronavirus nucleocapsids, because sustained coronavirus infections become more likely with increasing age. Consistent with our assumptions, we found sera recognizing all antigens (except SARS) as well as samples showing evidence of infection with a single type of virus. Furthermore, we identified 3 HCoV-negative sera (2 children, 1 adult). Although the tested children most likely never had contact to coronaviruses, the seronegative adult might have had coronavirus infections earlier in his life, which became nondetectable because antinucleocapsid IgG titers decrease over time.
In conclusion, we consider our line immunoassay approach to be highly favorable for large-scale epidemiologic studies on coronaviruses. Our assay system allows rapid and easy detection of antibodies against all known HCoVs in a single experiment with 1 antigen-coated strip, requiring only very low serum volumes (15 μL). By replacing secondary anti-human IgG with anti-human IgM, the immunoassay could easily be adjusted to screen for human IgM, which might flank RT-PCR–based diagnosis of acute HCoV infections in clinical settings. In this context, it would be advisable to test sequential (acute and convalescent) serum samples to track seroconversion and to define the immunoassay's role in the diagnosis of acute infections. Assay setup and test strip production are straightforward and cost-effective. N proteins are appropriate antigens because they are extremely immunogenic and provide a sensitive and reliable tool for antibody detection. Yet, beyond acute infections, our data also indicate that cross-reactive epitopes among HCoVs within group I or group II might be involved in IgG response to HCoV nucleocapsids. In addition, the line immunoassay may detect all remote infections experienced in the past. To gain even better specificity and to exclude possible false-positive results, it would be beneficial to extend the antigen portfolio on the strips. Therefore, future experiments will aim at additional studies focusing on potential cross-reactivities and fine-tuning of our assay by inclusion of other antigens or specific immunoreactive peptides in addition to full-length N proteins.
Acknowledgments
The authors thank Lia van der Hoek, Markus Eickmann, and Astrid Vabret for providing viral RNA, and Robert B. Couch for providing human sera. Christian Gerdes provided valuable support in terms of protein purification. The authors thank Michael Nevels for critical reading of the manuscript. This work was funded by the Boehringer Ingelheim Fonds, Germany.
References
- Arden K.E., Nissen M.D., Sloots T.P., Sloots T.P., Mackay I.M. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J. Med. Virol. 2005;75:455–462. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N., Anderson K., Hart L., Van Caeseele P., Brandt K., Milley D., Hatchette T., Weiss E.C., Li Y. Human coronavirus NL63 infection in Canada. J. Infect. Dis. 2005;191:503–506. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Cheng V.C., Woo P.C., Lau S.K., Poon L.L., Guan Y., Seto W.H., Yuen K.Y., Peiris J.S. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E OC43, and NL63. Clin. Vaccine Immunol. 2005;12:1317–1321. doi: 10.1128/CDLI.12.11.1317-1321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X.Y., Di B., Zhao G.P., Wang Y.D., Qui L.W., Hao W., Wang M., Qin P.Z., Liu Y.F., Chan K.H., Cheng V.C., Yuen K.Y. A patient with asymptomatic severe acute respiratory syndrome (SARS) and antigenemia from the 2003–2004 community outbreak of SARS in Guangzhou, China. Clin. Infect. Dis. 2006;43:e1–e5. doi: 10.1086/504943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lu D., Zhang M., Che J., Yin Z., Zhang S., Zhang W., Bo X., Ding Y., Wang S. Double-antigen sandwich ELISA for detection of antibodies to SARS-associated coronavirus in human serum. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:549–553. doi: 10.1007/s10096-005-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S.S., Chan K.H., Chu K.W., Kwan S.M., Guan Y., Poon L.L., Peiris J.S. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong China. Clin. Infect. Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günter S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Eickmann M., Becker S., Klenk H.D., Doerr H.W., Stadler K., Censini S., Guidotti S., Misagnani V., Scarselli M., Mora M., Donati C., Han J.H., Song H.C., Abrignani S., Covacci A., Rappuoli R. Phylogeny of the SARS coronavirus. Science. 2003;302:1504–1505. doi: 10.1126/science.302.5650.1504b. [DOI] [PubMed] [Google Scholar]
- Esper F., Shapiro E.D., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Association between a novel human coronavirus and Kawasaki disease. J. Infect. Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J. Infect. Dis. 2005;191:492–498. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Coronavirus HKU1 infection in the United States. Emerg. Infect. Dis. 2006;12:775–779. doi: 10.3201/eid1205.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H., Osterhaus A.D. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbino J., Crespo S., Aubert J.D., Rochat T., Ninet B., Deffernez C., Wunderli W., Pache J.C., Soccal P.M., Kaiser L. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (Non-SARS)–related human coronavirus infection. Clin. Infect. Dis. 2006;43:1009–1015. doi: 10.1086/507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Snijder E.J., Spaan W.J. Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 2004;78:7863–7866. doi: 10.1128/JVI.78.15.7863-7866.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan M., Chen H.Y., Foo S.Y., Tan Y.J., Goh P.Y., Wee S.H. Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients. Clin. Vac Immunol. 2004;11:287–291. doi: 10.1128/CDLI.11.2.287-291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Kinding D.A., Mann J. Growth and intracellular development of a new respiratory virus. J. Virol. 1967;1:810–816. doi: 10.1128/jvi.1.4.810-816.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrusková J., Heinz F., Svandová E., Penningerová S. Antibodies to human coronaviruses 229E and OC43 in the population of C.R. Acta Virol. 1990;34:346–352. [PubMed] [Google Scholar]
- Kahn J.S. The widening scope of coronaviruses. Curr. Opin. Pediatr. 2006;18:42–47. doi: 10.1097/01.mop.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- Kim O.J., Lee D.H., Lee C.H. Close relationship between SARS-coronavirus and group 2 coronavirus. J. Microbiol. 2006;44:83–91. [PubMed] [Google Scholar]
- Larson H.E., Reed S.E., Tyrrell D.A. Isolation of rhinoviruses and coronaviruses from 38 colds in adults. J. Med. Virol. 1980;5:221–229. doi: 10.1002/jmv.1890050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Woo P.Y., Yip C.C., Tse H., Tsoi H., Cheng V.C., Lee P., Tang B., Cheung C., Lee R.A., So L., Lau Y., Chan K., Yuen K. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J. Clin. Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Wan Y., Qui L.W., Zhou J., Ni B., Guo B., Zuo Q., Zou L., Zhou W., Jia Z., Che X.Y., Wu Y. Comprehensive antibody epitope mapping of the nucleocapsid protein of severe acute respiratory syndrome (SARS) coronavirus: insight into the humoral immunity of SARS. Clin. Chem. 2005;51:1382–1396. doi: 10.1373/clinchem.2005.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Becker W.B., Chanock R.M. Growth in suckling-mouse brain of “IBV-like” viruses from patients with upper respiratory tract disease. Proc. Natl. Acad. Sci. U. S. A. 1967;58:2268–2273. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow S., Falke D., Truyen U. Molekulare Virologie. 2nd ed. Spektrum Akademischer Verlag; Heidelberg: 2003. Humanpathogene Coronaviren; pp. 221–223. [Google Scholar]
- Mourez T., Vabret A., Han Y., Dina J., Legrand L., Corbert S., Freymuth F. Baculovirus expression of HCoV-OC43 nucleocapsid protein and development of a Western blot assay for detection of human antibodies against HCoV-OC43. J. Virol. Methods. 2006;139:175–180. doi: 10.1016/j.jviromet.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint S.H. Human coronaviruses: a brief review. Rev. Med. Virol. 2006;4:35–46. [Google Scholar]
- Poutanen S.M., Low D.E. Severe acute respiratory syndrome: an update. Curr. Opin. Infect. Dis. 2004;17:287–294. doi: 10.1097/01.qco.0000136924.45049.7e. [DOI] [PubMed] [Google Scholar]
- Shimizu C., Shike H., Baker S.C., Garcia F., van der Hoek L., Kujipers T.W., Reed S.L., Rowley A.H., Shulman S.T., Talbot H.K., Williams J.V., Burns J.C. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J. Clin. Infect. Dis. 2005;192:1767–1771. doi: 10.1086/497170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloots T.P., McEarlean P., Speicher D.J., Arden K.E., Nissen M.D., Mackay I.M. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J. Clin. Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.F., Meng X.J. Antigenic cross-reactivity between the nucleocapsid protein of severe acute respiratory syndrome (SARS) coronavirus and polyclonal antisera of antigenic group I animal coronaviruses: implication for SARS diagnosis. J. Clin. Microbiol. 2004;42:2351–2352. doi: 10.1128/JCM.42.5.2351-2352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.J., Goh P.Y., Fielding B.C., Shen S., Chou C.F., Fu J.L., leong H.N., Leo Y.S., Ooi E.E., Ling A.E., Lim S.G., Hong W. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Vaccine Immunol. 2004;11:362–371. doi: 10.1128/CDLI.11.2.362-371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timani K.A., Ye L., Zhu Y., Wu Z., Gong Z. Cloning, sequencing, expression, and purification of SARS-associated coronavirus nucleocapsid protein for serodiagnosis of SARS. J. Clin. Virol. 2004;30:309–312. doi: 10.1016/j.jcv.2004.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A., Dina J., Gouarin S., Patitjean J., Cohert S., Freymuth F. Detection of the new human coronavirus HKU1: a report of 6 cases. Clin. Infect. Dis. 2006;42:634–639. doi: 10.1086/500136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Berkhout B. Questions concerning the New Haven coronavirus. J. .Infect. Dis. 2005;192:350–351. doi: 10.1086/430795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-van Dillen P.M., Kaandorp J., Spaargagen J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Sure K., Ihorst G., Stang A., Pyrc K., Jebbink M.F., Petersen G., Foster J., Berkhout B., Uberla K. Croup is associated with the novel coronavirus NL63. PLoS. Med. 2005;2:e240. doi: 10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Teleman M.D., Heng B.H., Earnest A., Ling A.E., Leo Y.S. Asymptomatic SARS coronavirus infection among healthcare workers Singapore. Emerg. Infect. Dis. 2005;11:1142–1145. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.M., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L., Wong S.S., Guan Y., Peiris J.S., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Tsoi H.W., Huang Y., Poon R.W., Chu C.M., Lee R.A., Luk W.K., Wong G.K., Wong B.H., Cheng V.C., Tang B.S., Wu A.K., Yung R.W., Chen H., Guan Y., Chan K.H., Yuen K.Y. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J. Infect. Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Wong B.H., Chan K.H., Hui W.T., Kwan G.S., Peiris J.S., Couch R.B., Yuen K.Y. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide. J. Clin. Microbiol. 2004;42:5885–5888. doi: 10.1128/JCM.42.12.5885-5888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Woo P.C., Lau S.K., Yip C.C., Huang Y., Tsoi H.W., Chan K.H., Yuen K.Y. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J. Virol. 2006;80:7136–7145. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip Y.P., Hon C.C., Zeng F., Chow K.Y., Chan K.H., Peiris J.S., Leung F.C. Naturally occurring anti-Escherichia coli protein in the sera of healthy humans cause analytical interference in a recombinant nucleocapsid protein-based enzyme-linked immunosorbent essay for serodiagnosis of severe acute respiratory syndrome. Clin Vaccine Immunol. 2007;14:99–107. doi: 10.1128/CVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Qui M., Chen Z., Ye X., Gao Y., Wei A., Wang X., Yang L., Wang J., Wen J., Song Y., Pei D., Dai E., Guo Z., Cao C., Wang J., Yang R. Retrospective serological investigation of severe acute respiratory syndrome coronavirus antibodies in recruits from mainland China. Clin. Vac. Immunol. 2005;12:552–554. doi: 10.1128/CDLI.12.4.552-554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Hu S., Jona G., Zhu X., Kreiswirth N., Willey B.M., Mazzulli T., Liu G., Song Q., Chen P., Cameron M., Tyler A., Wang J., Wen J., Chen W., Compton S., Snyder M. Severe acute respiratory syndrome diagnostics using a coronavirus protein microarray. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4011–4016. doi: 10.1073/pnas.0510921103. [DOI] [PMC free article] [PubMed] [Google Scholar]