1. Introduction
All marine organisms have been proven to be a veritable cornucopia of unusual steroid metabolites, but some believe that marine sponges may provide the most diverse and biogenetically unprecedented array of unconventional steroids in the entire animal kingdom [1]. The steroids isolated from sponges are sometimes very complex mixtures of highly functionalized compounds, many of which have no terrestrial counterpart. Commonly encountered features in these compounds include additional oxygenation of both the nucleus and the side-chain, side-chains extensively modified by alkylation and degradation, and structural modification of the basic carbon skeleton. The occurrence of sulfate esters of polyoxygenated sterols in sponges has also been well documented [2], [3].
Unconventional steroids often co-occur with con-ventional ones and are sometimes present in small amounts; however, many exceptions are reported for some sponges that are found with unusual structures as the predominant steroids rather than cholesterol or the conventional 3β-hydroxy sterols [4], [5], [6]. It is, therefore, particularly interesting when a sponge contains unusual steroids in large quantities, as these very likely play a functional (rather than metabolic) role in maintaining the integrity of membranous structures. It has been hypothesized and, to some extent, documented that the uniqueness of sterols in cell membranes of sponges is related to the other membrane components, particularly the phospholipids. These latter compounds seem to have head groups and fatty acids very different from those of higher animals; therefore, the structural modifications exhibited by the sponge sterols may be a sort of structural adjustments for a better fit with other membrane components [7], [8], [9], [10].
The highly functionalized steroids have recently attracted considerable attention because of their biological and pharmacological activities. Remarkable examples are herbasterol [11], which is ichthyotoxic, xestobergsterols [12], [13], and contignasterol [14], potent inhibitors of histamine release from rat mast cells induced by anti-IgE, and halistanol disulfate B [15], an endothelin-converting enzyme inhibitor. Most of the oxygenated cholesterol derivatives have been shown to exert a cytotoxic activity on human cancer cells lines in vitro. Also, enzymatic transformations leading to secosterols, i.e. ring cleavage products of cholesterol, may result in the formation of products with cell division-inhibitory properties [16]. Finally, the recent discovery of the antiviral properties of sulfated polyhydroxysterols has increased interest in these compounds [17].
Up to the present, several excellent reviews have been published on the structure and distribution of the steroids of marine invertebrates, including the sponges, the last one dating back to 1991 [2]. A study dedicated exclusively to polyoxygenated sterols was also published in 1993 [3]. Furthermore, recent literature in this area demonstrates that marine sponges continue to be a rich source of interesting new steroids.
This review was intended to be a survey on the new spongal steroids recently reported in the literature (1991–1997). The material is organized on the basis of the level of deviation respect to a common steroid compound (e.g. cholesterol); this arrangement led to the following five classes of compounds: (1) polyoxygenated steroids, (2) steroid sulfates, (3) steroids with unconventional side-chain, (4) unconventional nuclei, and (5) miscellaneous. Of course, some compounds may belong to more than one of the above classes; so, the following criteria have been used for their classification. Section 2 includes polyoxygenated steroids with conventional side-chain and nucleus; Section 3 contains all the steroids possessing sulfate functions; Section 4 includes steroids with unconventional side-chain and conventional nucleus; and Section 5 reports all the steroids possessing a modified nucleus. Occasionally, a few marine sponges were found to contain atypical steroids, which cannot be included in the above classes; they are reported in Section 6 (miscellaneous) of the present review.
2. Polyoxygenated steroids
Six new 3β,5α,6β-trihydroxysterols (1–6) with a saturated nucleus have been isolated from two different populations of the sponge Cliona copiosa, collected from two different sites of the bay of Naples [18]. Partial synthesis of compounds 1, 5, and 6 confirmed the structures of these compounds. Successively, several new Δ5-3β-hydroxy-7-ketosteroids and the related Δ5-3β,7β-, and Δ5-3β,7α-dihydroxysterols (7–15) have been described as constituent of the same species [19].
Cholest-4-ene-3,6-diones (16 and 17) were isolated from Cinachyra tarentina [20]. Compound 16 had been previously synthesized starting from cholesterol; structure 17 has been confirmed by its synthesis starting from sitosterol through a Jones oxidation that allowed also to assign the R chirality at C-24.
The five novel 5α-hydroxy-6-keto-Δ7-sterols (18–22) isolated from Oscarella lobularis [21] may be considered the ‘missing links’ in the hypothesized biosynthetic pathway leading to the incisterols, a class of C18 compounds deriving from the biodegradation of the steroidal nucleus, isolated from the sponge Dyctionella incisa [22].
Seven new Δ8(14)-3β,7α-dihydroxysterols (23–29) were isolated from Pellina semitubulosa. Compound 23 has been synthesized starting from 5α-cholest-7-en-3β-ol, following a previously reported synthetic procedure [23].
Two clionasterol derivatives, (24S)-24-ethylcholesta-3β,5α,6α-triol (30) and (24S)-24-ethylcholesta-3β,5α-diol-6-one (31), have been isolated from Spirastrella incostans [24], [25]. The structure 31 has been confirmed by semisynthesis starting from clionasterol.
Extensive investigations performed on Dysidea species showed that highly oxygenated sterols are widespread in this genus. All the isolated sterols are characterized by oxygenated functions exclusively on rings A and B, as well as at C-11. Significant variations in the sterol profile have been observed within the genus and even for specimens of the same species coming from different habitats. Sterols 32 through 34, all bearing a 24-methylene side-chain, have been isolated as their triacetates from D. herbacea [26]. The sterol acetates were found to possess antitumor activity against P388 cell line. A novel polyhydroxylated sterol with Δ8(14),24 unsaturations (35) has been isolated from a Dysidea species [27]. Incrustasterols A and B (36 and 37) have been isolated from a sample of D. incrustans collected in the Mediterranean sea along the coasts of Tunisia [28]. They contain the rare Δ8(9)-11-keto functionality and were shown to be cytotoxic against human non-small-cell lung, renal carcinoma, and melanoma cell lines. Because very limited amounts of the incrustasterols had been isolated from the natural source, their synthesis has been performed starting from the easy available Δ7-cholesterol [29]. A variable sterol pattern has been described for the same species, D. fragilis, coming from different geographical areas. Two highly hydroxylated sterols (38 and 39) were isolated from the Black Sea sponge D. fragilis [30]. Biosynthetic experiments performed on the sponge fed with [4-14C]cholesterol showed that dietary cholesterol suffers biological oxidation leading to 38. 5α-Cholesta-8(14),24-diene-3β,6α-diol (40) has been isolated from a D. fragilis collected along the South China coasts [31]. Finally, the steroid composition of D. fragilis collected in the lagoon of Venice differed remarkably from the aforementioned specimens of the same species. The lagoonal sample contained large amounts of polar steroids, comprising 13 polyhydroxysterols; eight of them (36, 88–90, and 174–177) were novel compounds; as reported above, compound 36 was independently found as constituent of D. incrustans [28]. Compounds 88 through 90 and 174 and 175 are described in 3, 5, respectively; 3β,5α,6β,7α-tetrahydroxy-cholest-8(9)-en-11-one (36), was proved to be cytotoxic on two different cell lines in vitro [6]. Fig 1 , Fig. 2 , Fig. 3 , Fig. 4 , Fig. 5 , Fig. 6 , Fig. 7 , Fig. 8 , Fig. 9 Fig. 10 Fig. 11 , Fig. 12 , and Fig. 13
Figure 1.
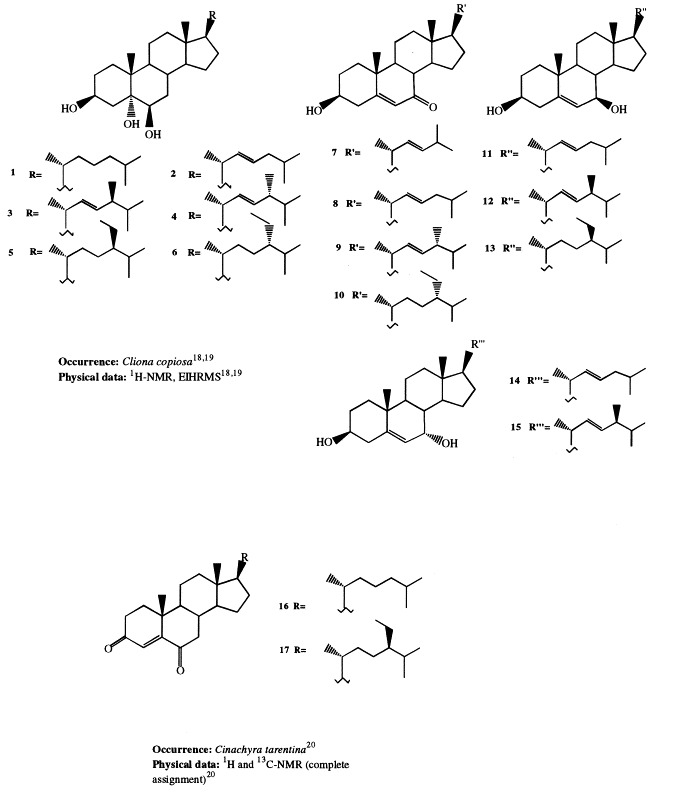
Figure 2.
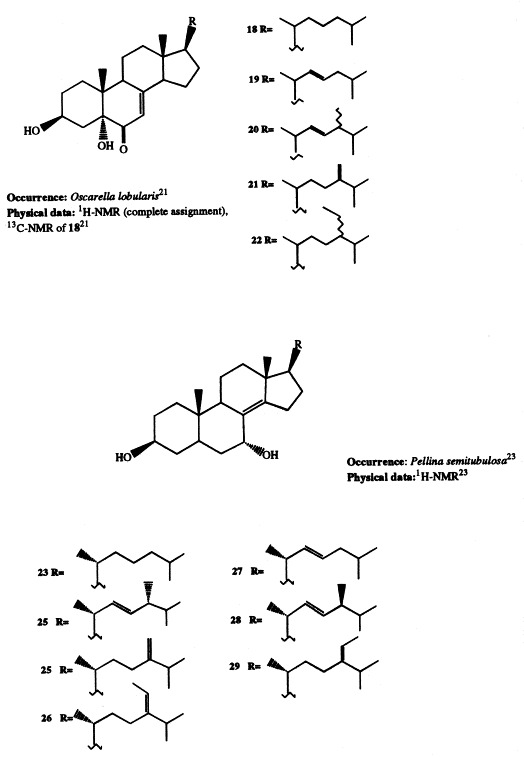
Figure 3.
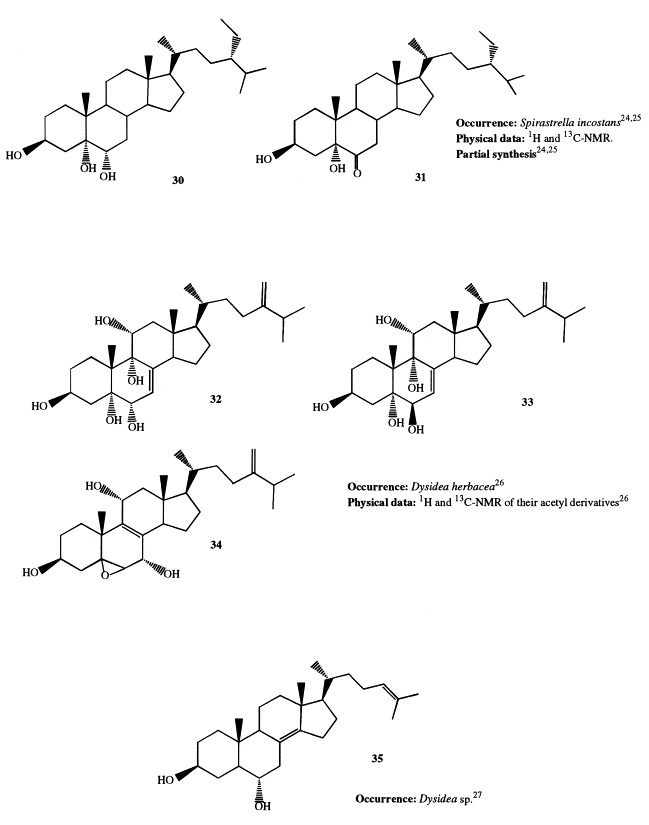
Figure 4.
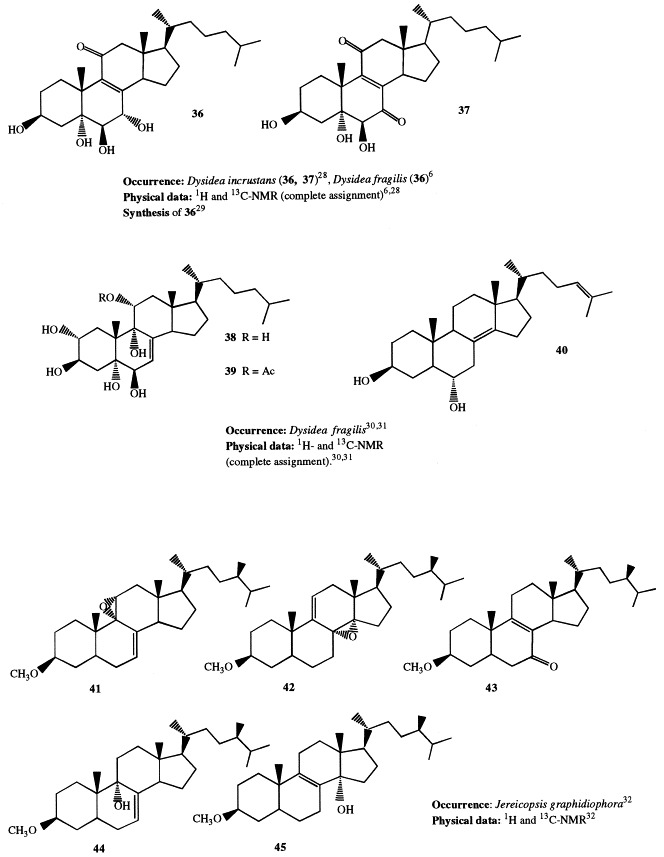
Figure 5.
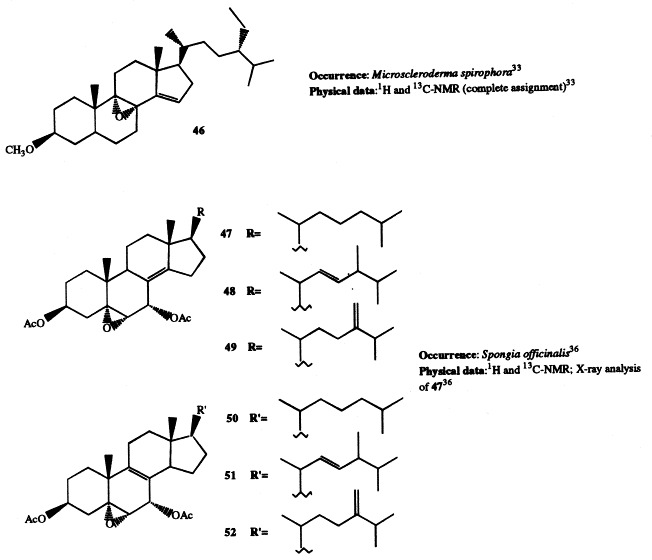
Figure 6.
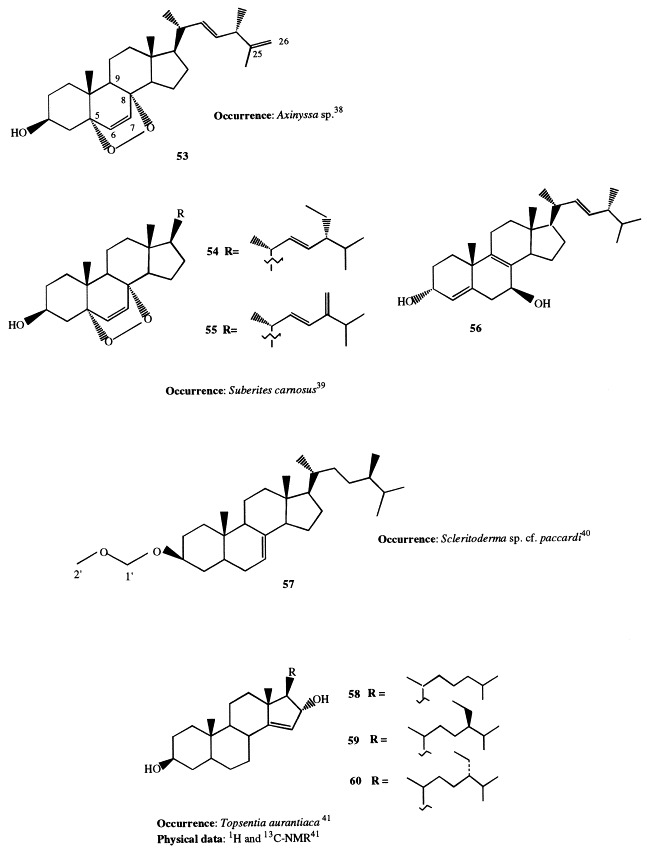
Figure 7.
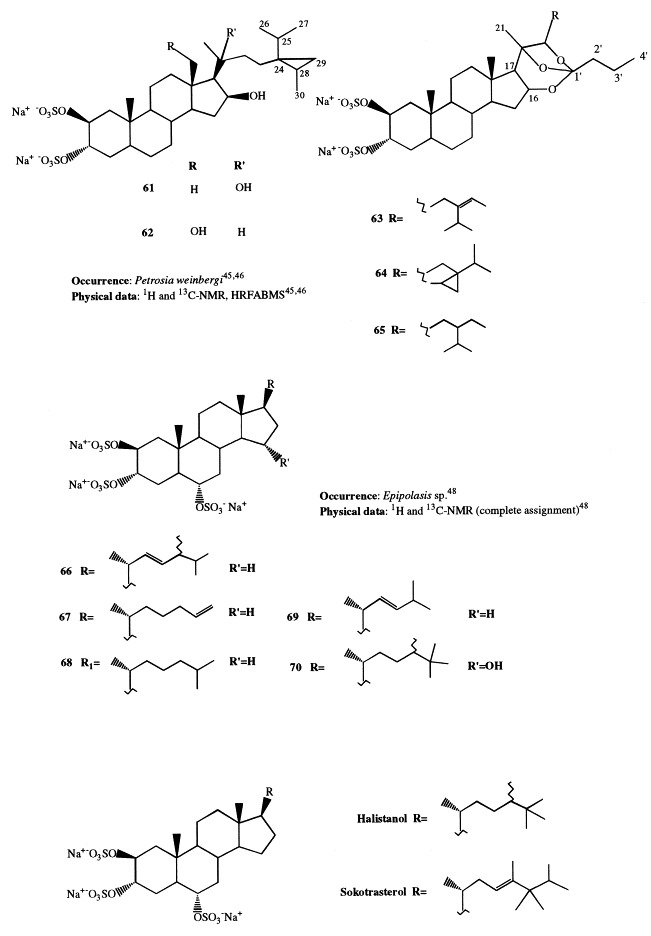
Figure 8.
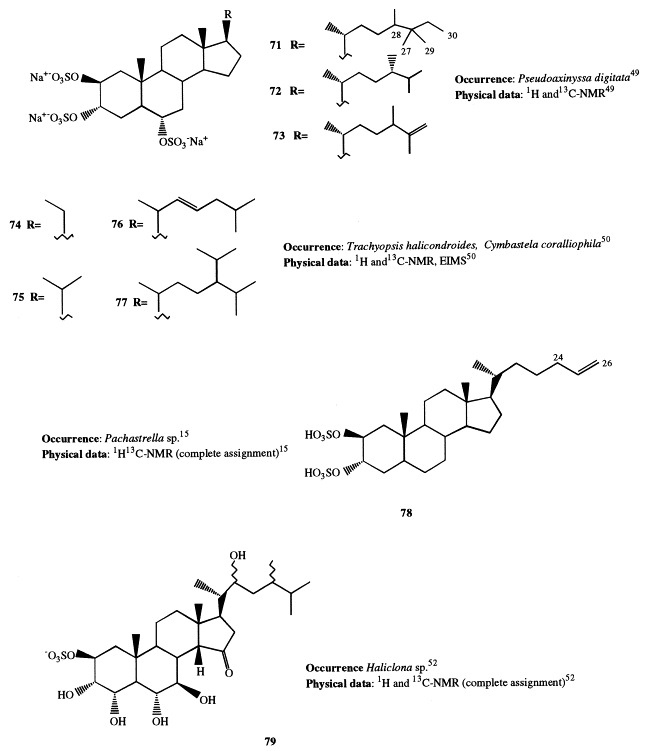
Figure 9.
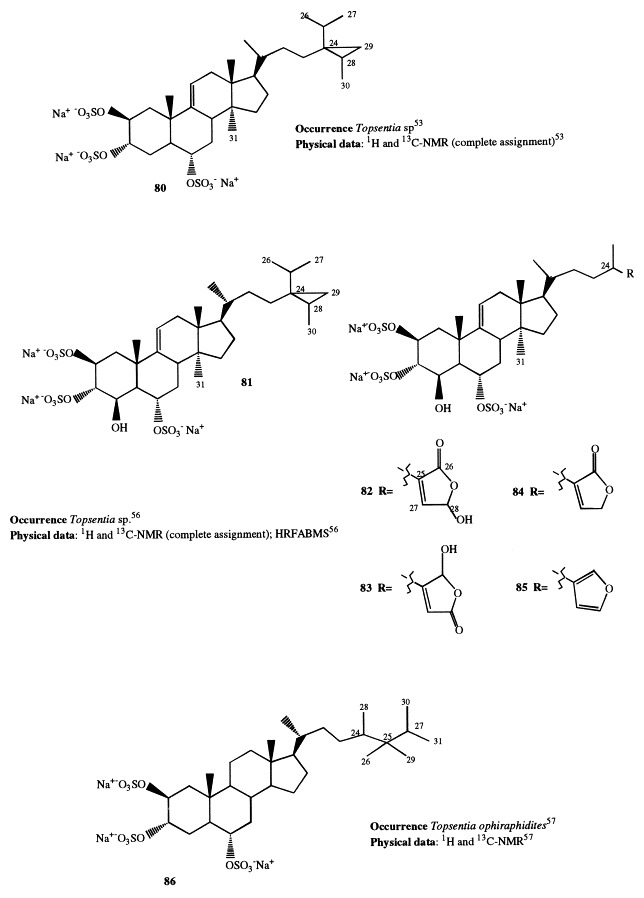
Figure 10.
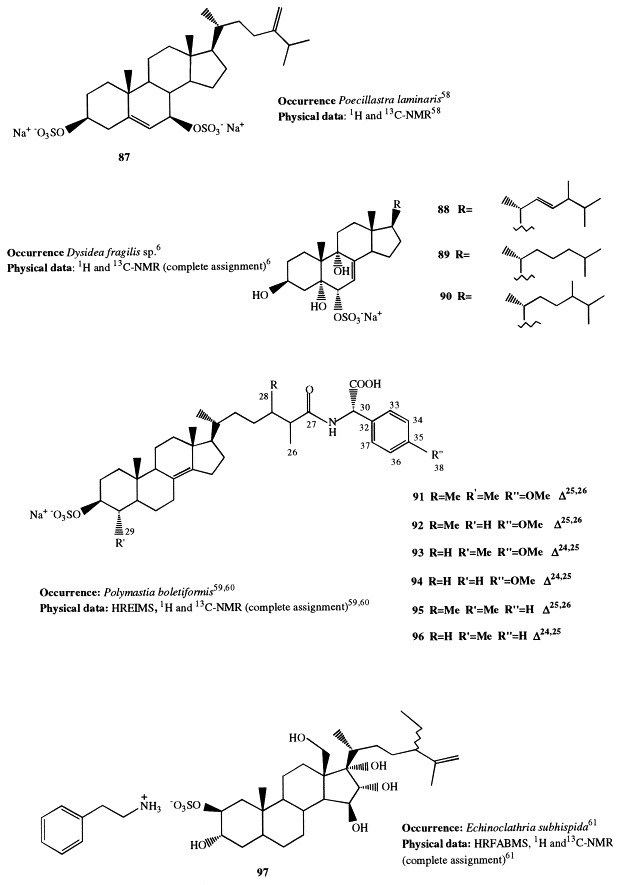
Figure 11.
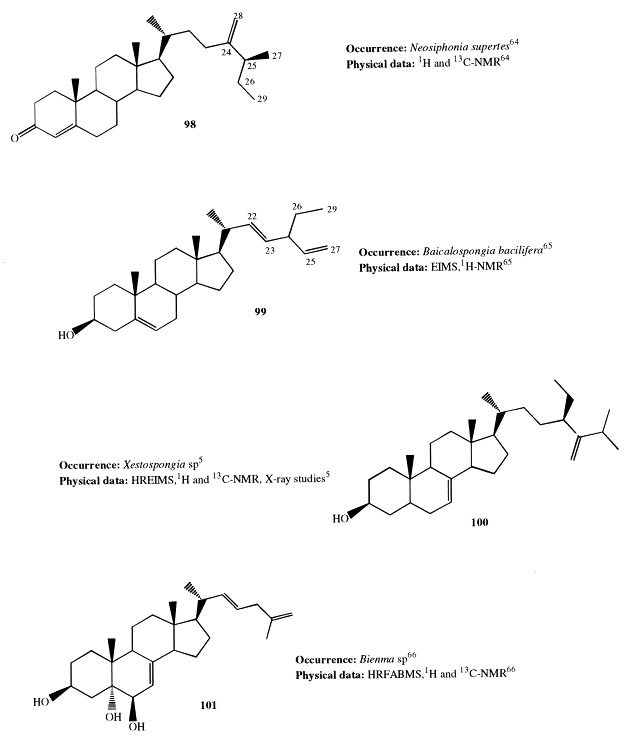
Figure 12.
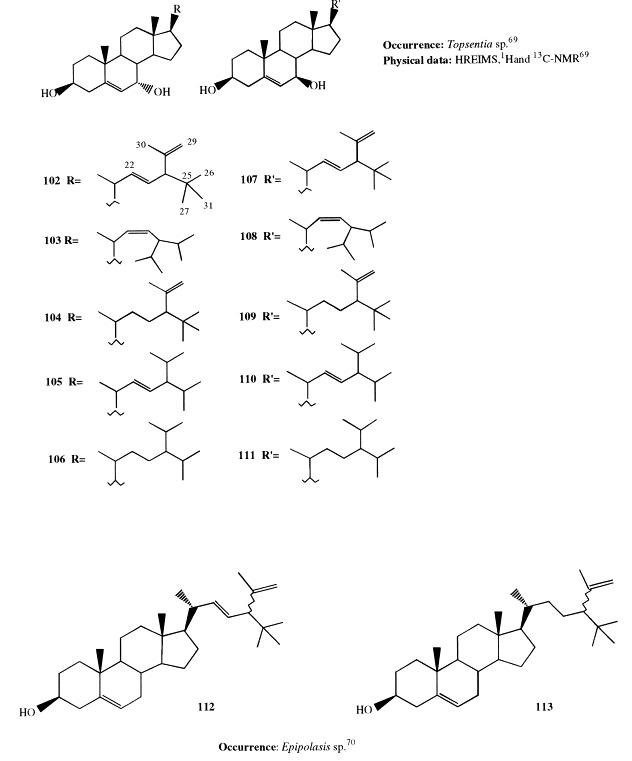
Figure 13.
The lipid fraction of the Pacific sponge Jereicopsis graphidiophora yielded several 3β-methoxysteroids, whereas the conventional 3β-hydroxysterols were totally absent; five of them (41–45) were novel compounds containing an additional oxygenated function such as an epoxy, hydroxy, or ketone group. Compound 42 is the first finding of a Δ9(11)-8,14-epoxide function in a naturally occurring steroid [32]. Fig. 14 , Fig. 15 , Fig. 16 , Fig. 17 , Fig. 18 , Fig. 19 , Fig. 20 , Fig. 21 , Fig. 22 , Fig. 23 , and Fig. 24
Figure 14.
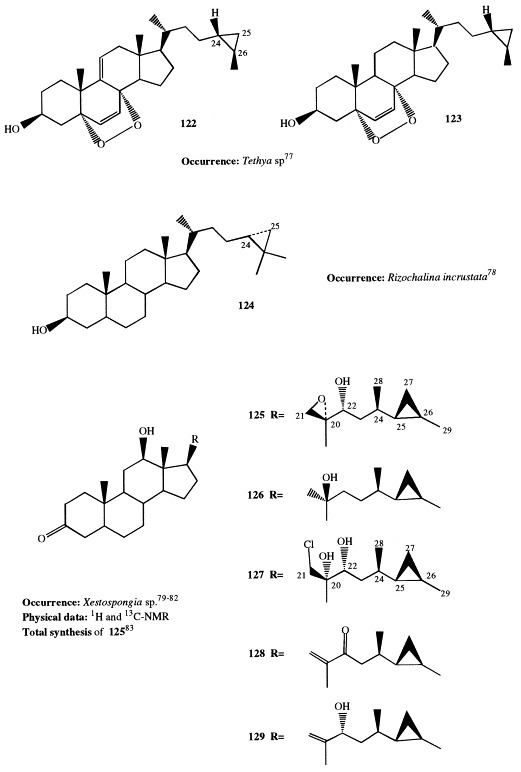
Figure 15.
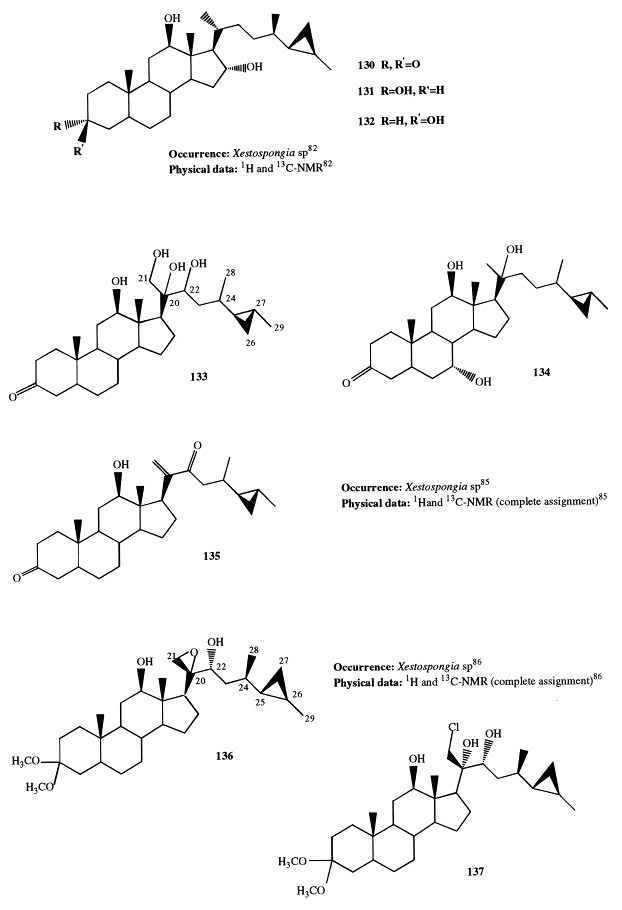
Figure 16.
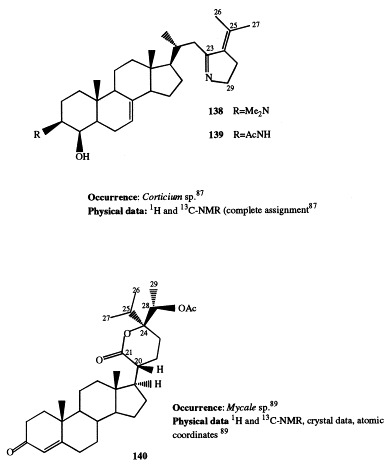
Figure 17.
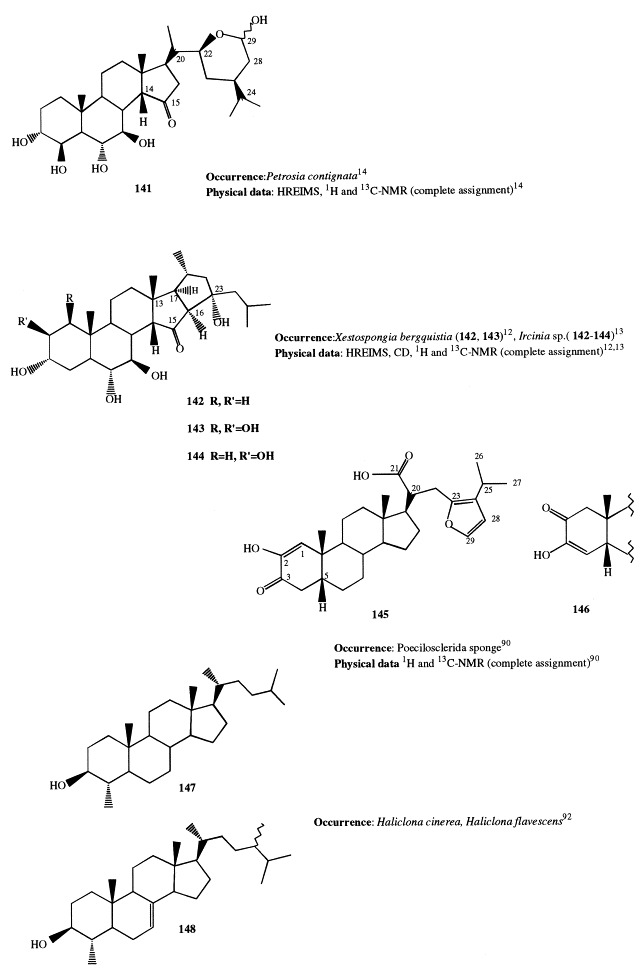
Figure 18.
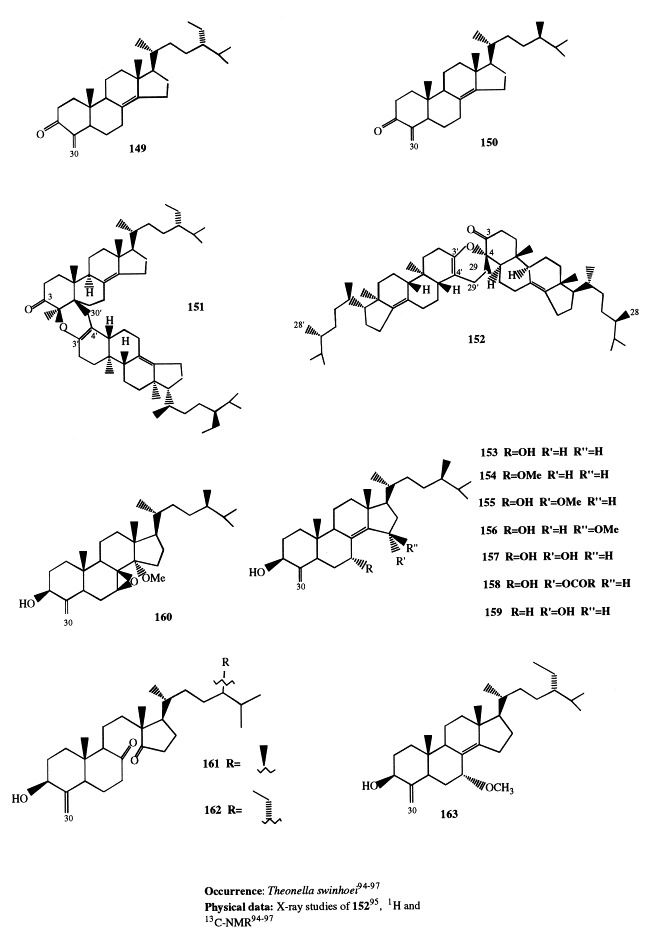
Figure 19.
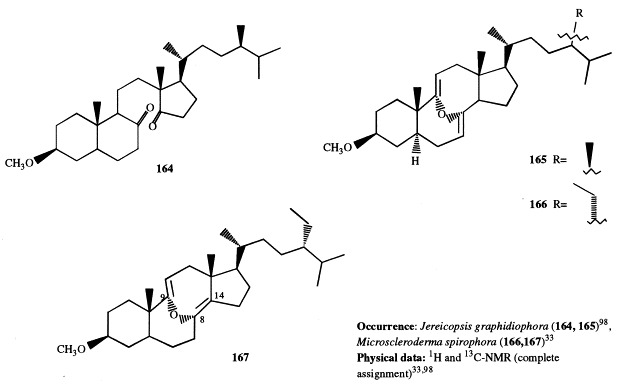
Figure 20.
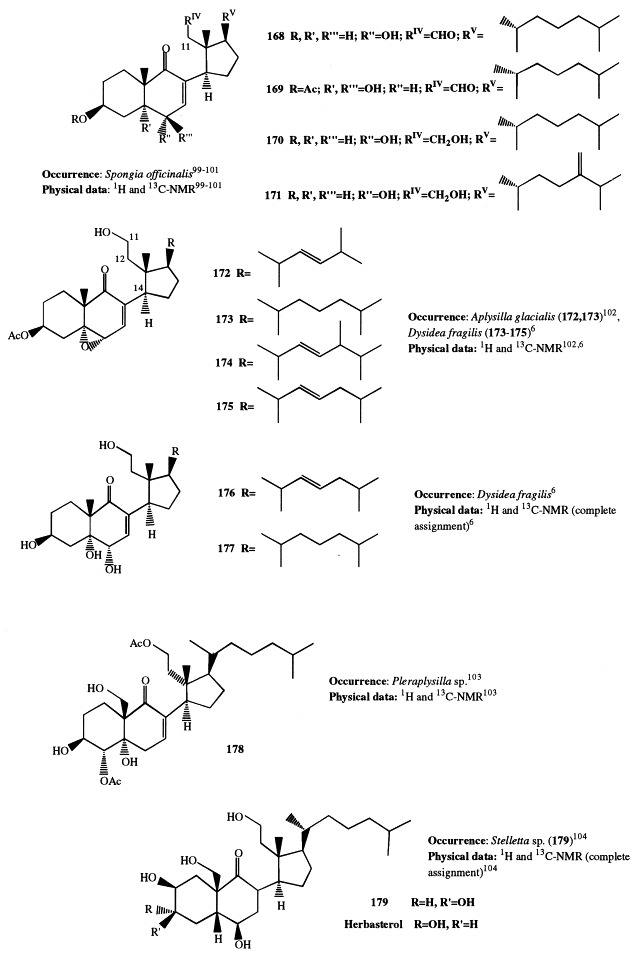
Figure 21.
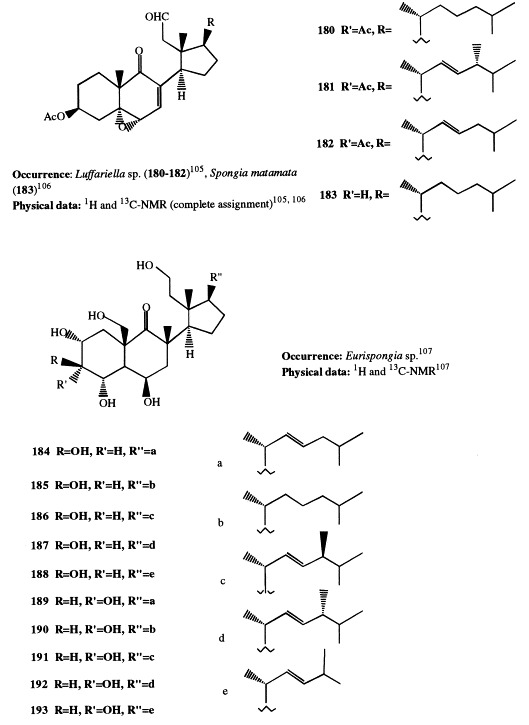
Figure 22.
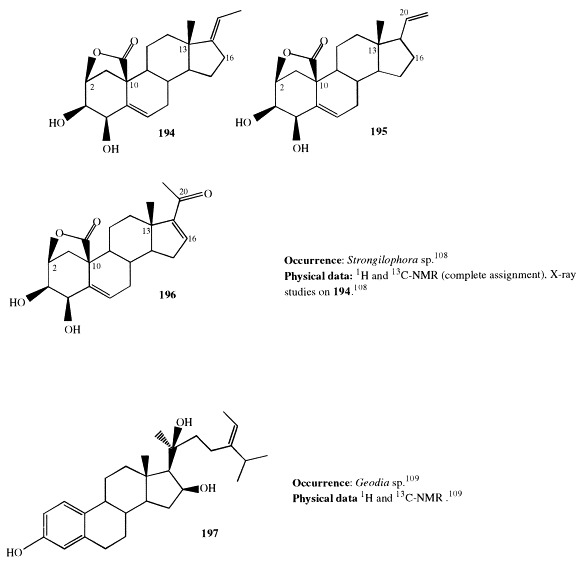
Figure 23.
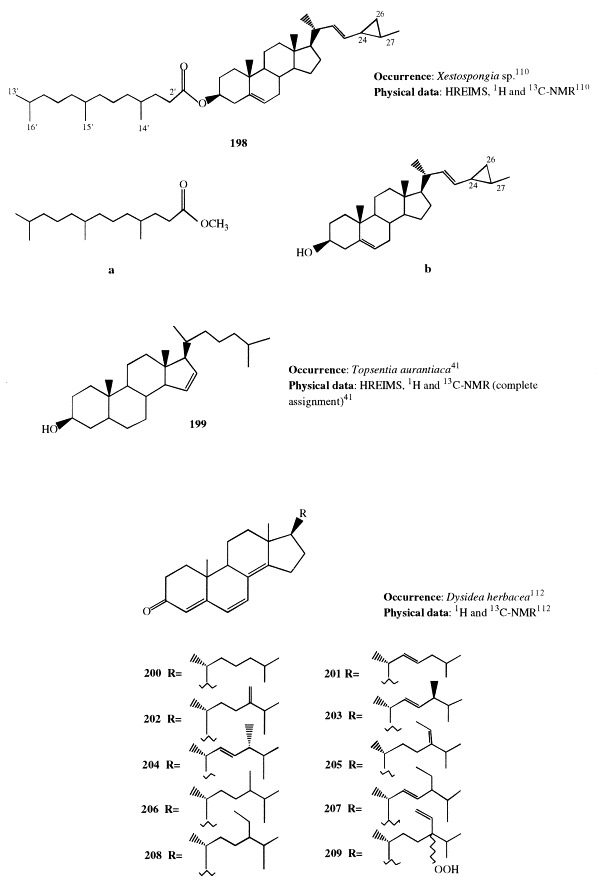
Figure 24.
Analogously to J. graphidiophora, the Senegalese sponge Microscleroderma spirophora elaborates large quantities of 3β-methoxysteroids, whereas no significant amounts of 3β-hydroxysteroids are present in its extract. Six different methoxysteroids have been isolated and three of them (46, 166, and 167) were novel compounds [33]. Compounds 167 and 168 are described in section 5; 46 contains a double bond at position 14 and a tetrasubstituted oxirane ring located at position 8,9. The occurrence of 3β-methoxysteroids has an interesting chemotaxonomic significance. Recently, Levi suggested to assign the genera Microscleroderma and Jereicopsis to the same order (Rhizomorina), based on interpretation of their morphologic attributes [34]. In addition to M. spirophora and J. graphidiophora, only one species of this order, Aciculites pulchra, has been analyzed for steroids and resulted to elaborate usual 3β-hydroxysterols [35]. So, the ability of producing methoxysteroids seems not to be a general feature of the order, but appears limited to some species. Therefore, the presence of methoxysterols could be a useful chemical marker for an improved classification of Rhizomorina sponges.
Two groups of new 5α,6α-epoxysterols with an unsaturation either at position 8(14) (47–49) or 8(9) (50–52) occur in Spongia officinalis, which is a rich source of diverse steroidal metabolites. The new products have been isolated as diacetates and their structures determined by spectral analysis; an X-ray diffraction experiment, performed on compound 47, gave conclusive information on its stereochemistry at C-7 [36]. Successively, compound 47 has been found in the sponge Ircinia fasciculata [37].
Axinysterol (53), occurring in an Okinawan sponge of the Axinyssa genus, is the first example of a 5α,8α-epidioxy sterol with a 24-methyl-22,25-diene system in the side-chain [38]. Its structure has been determined on the basis of spectroscopic evidences and chemical transformation; particularly, the catalytic hydrogenation of axinysterol and ergosterol peroxide gave the same 3β,5α-diol, allowing to confirm the full structure of 53 including the stereochemical details. Two axinysterol analogues, differing only in the nature of the side-chain (54 and 55), have been isolated from Suberites carnosus along with (22E,24R)-24-methylcholest-4,8(9),22-triene-3α,7β-diol (56) [39].
(24R)-24-Methyl-5α-cholest-7-enyl-3β-methoxymethyl ether (57), isolated from a deep-water marine sponge Scleritoderma sp. cf. paccardi, is the first report of a methoxymethyl ether sterol from a natural source. It was shown to be cytotoxic against P-388 tumor cell lines [40].
Three novel sterols with a rare D-ring unsaturation and bearing a 16α-hydroxyl group (58–60) have been isolated from the Mediterranean sponge Topsentia aurantiaca [41]. Previously, the only marine sterols possessing this unique unsaturation had been isolated from three taxonomically very different species, the Pacific sponge Homaxinella trachys [42], the starfish Echinaster sepositus [43], and some cultured Dinoflagellates [44].
3. Steroid sulfates
Several sulfated polyhydroxysterols have been recently isolated from sponges. Most of these natural products are characterized by the 2β,3α,6α-tri-O-sulfate functions together with additional alkylation in the side-chain. These steroids are of interest not only because of their structures but also because of their physiological activities, which include an anti-human immunodeficiency virus (HIV) effect and a high inhibitory action on some enzymes.
Weinbersterols A and B (61 and 62) and orthoesterol disulfates A, B, and C (63–65) are new antiviral steroid sulfates isolated from Petrosia weinbergi [45], [46]. They exhibited in vitro activity against the feline leukemia virus (FeLV), mouse influenza virus (PR8), and mouse coronavirus (A59). Weinbersterol sulfates A and B are the second group of steroids found to contain this unusual cyclopropane-containing side-chain and the first from a sponge. Compound 61 is also active in vitro against HIV.
Halistanol sulfate, present in Halichondriidae sponges and characterized by the 2β,3α,6α-trisulfoxy functionalities, is the first example of sulfated sterol isolated from Porifera [47]. Successively, several new halistanol sulfate analogues, differing only in their side-chains, have been reported. Halistanol sulfates A through E (66–70) have been isolated from Epipolasis sp. together with halistanol sulfate, whose absolute stereochemistry was determined by application of the modified Mosher method on the 2,3-diol system generated by acid hydrolysis [48]. Halistanol sulfates F through H (71–73) have been isolated from Pseudoaxinissa digitata [49]; they were proved to be cytoprotective against HIV. Four new steroids (74–77), all possessing identical nuclei containing the 2β,3α,6α-trisulfoxy substitution, have been isolated from Trachyopsis halichondroides and Cymbastela coralliophila [50]. Besides, halistanol sulfate was found to be the main steroid constituent of C. coralliophila, whereas the known sokotrasterol sulfate was predominant in T. halichondroides [51].
Halistanol disulfate B (78), present in Pachastrella sp., was found to be active at a micromolar concentration in the endothelin-converting enzyme assay [15].
Haliclostanone sulfate (79) and, once more, halistanol sulfate have been isolated from a Haliclona sponge [52]. Compound 79 is unique in that it is a member of the rare class of naturally occurring sterols sharing a cis C/day ring junction, a 14β proton, and a C-15 ketone. This is the first case of isolation of halistanol sulfate from a Haposclerida sponge rather than a Halichondrida or Axinellida sponge.
A further member of the halistanol series is ibisterol sulfate (80), discovered from a Topsentia species [53]. It combines a Δ9(11) olefin and a methyl group at C-14 with the 2β,3α,6α-trisulfoxy functionalities and a cyclopropane-containing side-chain. The presence of a Δ9(11) double bond with methyl substitution at position 14 is interesting from a biosynthetic point of view. This pattern is quite rare, having been observed among the marine organisms only in sterols from olothurians [54]. It, according to Cordeiro and Djerassi [55], is biosynthesized in sea cucumber directly from parkenol (Δ9(11) isomer of lanosterol), rather than from lanosterol or cycloartenol. However, this pathway has not been observed in sponges and, therefore, ibisterol sulfate could be a target for new biosynthetic studies [53].
The 4β-hydroxy derivative of ibisterol (topsentiasterol E; 81) has been isolated from another Topsentia species together with four compounds (topsentiasterol sulfates A–D; 82–85) that represent the first isolation of polysulfated steroids possessing a butenolide or a furan functionality at the end of the side-chain [56]. Topsentiasterols A–E were shown to be antibacterial against Pseudomonas aeruginosa and Escherichia coli, but only topsentiasterols D and E exhibited antifungal activity against Mortierella ramannianus and Candida albicans. Ophirapstanol trisulfate (86), isolated from Topsentia ophiraphidites, is the 23,24-dihydro derivative of sokotrasterol. It exhibited significant inhibition in the guanidine diphosphate/G protein RAS exchange assay [57].
Annasterol sulfate (87) is the first representative of a new structural series of spongal steroid sulfates. It has been isolated from the sponge Poecillastra laminaris, collected at a depth of 750 m, and is the first naturally occurring derivative of 3β,7β-O-disulfated diols of the ergostane series [58]. This compound inhibits the enzyme endo-β-1,3-glucanase activity.
A 6α-sulfoxy function characterizes the steroids 88through 90, isolated from Dysidea fragilis collected in the lagoon of Venice, which were proved to be cytotoxic on two different tumor cell lines in vitro [6].
Polymastiamides A through F (91–96) are 3β-O-sulfated sterols occurring in the sponge Polymastia boletiformis [59], [60]. The minor polymastiamides B through F were initially obtained as an inseparable mixture because of the extreme polarity of these molecules; once the structure of the first isolated polymastiamide A was determined and the presence of the sulfate and carboxylic acid functionalities revealed, they were isolated as their desulfated methyl esters after acidic methanolysis. During this treatment, polymastiamides were found to undergo double-bond migration to give the Δ14 artifacts. The interesting side-chain modification in the polymastiamide series, which involves the linkage to a nonprotein amino acid via an amide bond, is without literature precedent.
Sulfated steroids are usually isolated as sodium or potassium salts. Bioassay-guided fractionation of the water-soluble portion of the MeOH extract of Echinoclathria subhispida, collected in South Australia, afforded a unique salt of a novel polyoxygenated sterol sulfate, echinoclasterol sulfate (97) [61]. It contains a phenethylammonium ion as a counter ion, which is the first example for marine natural products. Echinoclasterol sulfate is a hexahydroxy sterol, rarely encountered among sponge steroidal metabolites; the OH group at C-17 is particularly noteworthy. The compound exhibited antifungal activity against Mortierella ramannianus and moderate cytotoxicity against PC-9 human lung cancer cells.
4. Steroids with unconventional side-chain
Steroids isolated from sponges frequently contain substantially modified side-chains, such as those with high degrees of alkylation and/or other unusual functionalization. Biosynthetic studies have been performed on mechanism and scope of sterol side-chain dealkylation in sponges [62], and a detailed review has been published as a comprehensive summary of biosynthesis of sterol side-chain in marine organisms [63].
(25S)-26-Methyl-24-methylenecholest-4-en-3-one (98) has been isolated from the marine fossil sponge Neosiphonia supertes [64]. The unambiguous confirmation of this structure was obtained through an Oppenauer oxidation of the known 24(28)-dehydroaplysterol, also isolated from the sponge, which led to a keto compound whose spectroscopic properties were identical to those of compound 98.
Baikalosterol (99) is a novel steroid with a 24-ethyl-26-nor-22,25-diene group in the side-chain isolated from the sponge Baicalospongia bacilifera, a common species of the Baikal lake [65]. This unusual side-chain suggests interesting questions with respect to the biosynthesis of 99, which is probably a product of dealkylation of a C29 sterol. This is supported by the composition of the total sterol fraction of B. bacilifera, whose 30% is represented by C29 sterols. In particular, (22E,24S)-methylcholesta-5,22,25-trien-3β-ol, a minor 25-unsaturated C29 sterol also isolated from the sponge, could be the precursor of 99.
Sutinasterol (100) contains a C12 side-chain that is presumably the product of quadruple bioalkylation. It represents the 94% of the steroid fraction of a Xestospongia sponge. An X-ray crystallographic structure study of sutinasterol was performed to confirm its structure and to determine the stereochemistry at C-24 [5].
A new cytotoxic sterol, bienmasterol (101), possessing the rare 22,25-diene side-chain, has been isolated from the Okinawan sponge Bienma sp. [66]. This feature, previously found in the major component of the sterol mixture of the Hawaiian sponge Ciocalypta sp. [67], provided strong evidence for the proposed intermediacy of a 22,25-diene in the biosynthesis of sterols [68]. Bienmasterol exhibited cytotoxicity against murine lymphoma L1210 and human epidermoid carcinoma KB cells in vitro.
Topsentinols A through J (102–111), 10 new 7-hydroxysterols with unusual polyalkylated side-chains, have been isolated from the Okinawan marine sponge Topsentia sp. [69]. Side-chains containing the 24-isopropenyl-25-methyl-22E-ene group (in 102 and 107) and the 24-isopropyl-22Z-ene group (in 103 and 108) are unprecedented. Topsentinol B (103) showed antifungal activity against Trichophyton mentagrophytes.
Two new sterols, epipolasterol and 22,23-dihydroepipolasterol (112 and 113), have been found in Epipolasis sp. [70]. These are unusual metabolites, as they both contain a t-butyl group as well as two degrees of unsaturation in the side-chain.
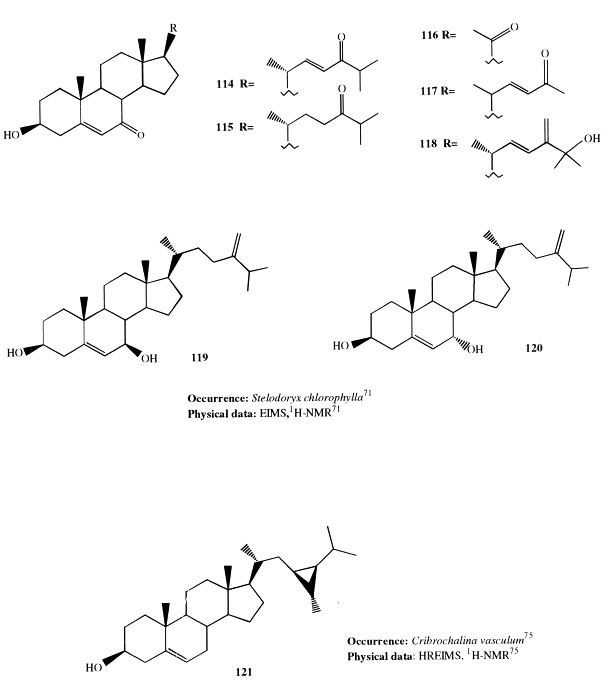
Twenty-two steroid components present in the deep-water sponge Stelodoryx chlorophylla, from New Caledonia, have been identified; among them, seven products (114–120) were new compounds [71]. With the exception of 119 and 120, they are steroids with oxygenated or short oxygenated side-chains. These compounds appear to be autoxidation products of the corresponding Δ5 sterols. Their presence in the steroid mixture could be the consequence of the storage of the sponge for a long time, even if the quantities of some steroids are much higher than expected for autoxidation products.
The cyclopropane and cyclopropene sterols found in marine sponges of the order Haposclerida are very interesting and, recently, a biosynthetic pathway leading to spongal cyclopropyl sterols has been shown starting from clionasterol through a novel biochemical desaturation reaction [72], [73], [74]. Several new examples of this class of sterols have been recently reported. A new cyclopropane sterol, 121, has been isolated from the sponge Cribrochalina vasculum [75]; it differs from the known (23S,24S,28R)-dihydrocalysterol only in the configuration at C-23 [76]. Its discovery points to new biosynthetic implications; in addition to the mechanistic interest of this compound in the biosynthetic cyclopropane-forming reaction, it could serve as precursor to calysterol via cis desaturation.
A 5α,8α-epidioxy functionality characterizes the two sterols, 122 and 123, isolated from a sponge of the genus Tethya possessing a cyclopropyl ring at C-24 [26] of the side-chain [77].
(24R)-24,25-Methylene-5α-cholestan-3β-ol (124) has been isolated from Rhizochalina incrustata and its structure has been determined on the basis of spectral data and chemical transformations [78].
Aragusterols A through H (125–132) are additional cyclopropane-containing sterols isolated from Okinawan Xestospongia species; they are characterized by the rare 26,27-cyclopropane group [79], [80], [81], [82]. Moreover, aragusterol C is the first marine steroid found to have a chlorinated side-chain. Its structure was determined by X-ray analysis. Aragusterols are important not only for their unique structures but also for their biological activity. Aragusterols A and C very strongly inhibit the proliferation of KB cells at IC50 0.042 and 0.041 μg/ml, respectively, and express potent in vivo antitumor activity against L1210 leukemia in mice. To have these compounds available in greater amounts for more detailed pharmacological research, the synthesis of aragusterols A, B, C, and D has been successfully performed. The synthesis involves the enantioselective formation of a side-chain segment and its stereoselective coupling with a 20-keto steroid obtained from (+)-hecogenin [83]. The synthesis of 5-epiaragusterol A from deoxycholic acid, whose natural abundance permits synthesis on a large scale, has also been achieved; 5-epiaragusterol A showed an antiproliferative activity comparable with that of aragusterol A [84].
Xestosterols A–C (133–135) [85] and aragusteroketals A and C (136 and 137, respectively) [86], strictly related to aragusterols, have also been reported from an Okinawan Xestospongia. Xestokerols A and B are rare C-20 oxidized steroids from marine origin; biogenetically, they may have been generated through oxidation of C-20/C-21 double bond of xestokerol C. Compounds 133 through 135 also exhibited in vitro cytotoxicity and antimicrobial activity against Gram-positive bacteria. Xestokerol C has a structure similar to that of aragusterol D, but the absolute configurations at C-24, C-25, and C-27 were undetermined.
Lokysterolamines A and B (138 and 139, respectively) are two steroidal alkaloids isolated from an undescribed Corticium species [87]. The compounds bear a skeletal relationship to the previously described plakinamine A [88]; compound 138 is N,N-dimethyl-4β-hydroxy-3-epi-plakinamine A and compound 139 is N-acetyl-4β-hydroxy-3-epi-plakinamine A. They possess antimicrobial and antifungal activity and in vitro activity in P-388, A-549, HT-29, and MEL-28 cell assays.
Mycalone (140), isolated from a southern Australian Mycale species, is a new steroid possessing a six-membered lactone side-chain [89]. Its stereostructure has been determined by spectroscopic methods and X-ray crystallographic structure analysis.
5. Unconventional nuclei
One of the significant features of steroids as a class of natural products is the almost universal occurrence of one particular set of configurations at the ring junction carbon atoms of the nucleus. Contignasterol (141) is the first marine steroid found to have a cis C/day ring junction as well as a cyclic hemiacetal functionality at C-29 in the side-chain, which is without precedent. It has been isolated from Petrosia contignata [14]; because of a slow spontaneous epimerization observed in contignasterol, a result of the presence of the hemiacetal functionality, the structural analysis was better performed on contignasterol tetraacetate and on the pentaacetate of its reduction product. This sterol represents the first example of a naturally occurring steroid with the ‘unnatural’ 14β proton orientation, although steroids with a 14β-hydroxyl functionality (i.e. digitoxin) are well known from nature. Because inverted ring junction configurations at any center in the steroidal nucleus are rare, biogenetic origin of the unnatural H-14 orientation in contignasterol is of considerable interest.
The same cis C/day ring junction has been found in (23S)-16β,23-cyclo-3α,6α,7β,23-tetrahydroxy-5α,14β-cholestan-15-one (xestobergsterol A; 142) and (23S)-16β,23-cyclo-1β,2β,3α,6α,7β,23-hexahydroxy-5α,14β-cholestan-15-one (xestobergsterol B; 143) isolated from the methanol/toluene extract of the Okinawan Xestospongia bergquistia [12]. They are the first report of steroids possessing five carbocyclic rings and the cis C/day ring junction. Biogenetically, they are considered most likely to be the products of an intramolecular aldol-type reaction in the organism. Xestobergsterols A and B strongly inhibited histamine release from rat peritoneal mast cells induced by anti-IgE in a dose-dependent manner. Successively, xestobergsterols A and B have been reisolated from an Ircinia species together with another analogous sterol, xestobergsterol C (144) [13]. In this study, the stereochemistry at C-23 (β-OH rather than α-OH, as initially assigned) and the conformation of the ring C (chair form) for xestobergsterol A and B have been revised on the basis of reexamination of the nuclear magnetic resonance data; furthermore, the CD exciton chirality method was applied to determine the absolute stereochemistries at C-6 and C-7 in xestobergsterol A. Xestobergsterols B and C were assumed to possess the same absolute configurations (both R) because these sterols may be generated through the same biosynthetic pathway. Xestobergsterol C and A were shown to be cytotoxic against murine leukemia cells L1210, whereas xestobergsterol B was not significantly cytotoxic.
Kiheisterones A and B (145 and 146, respectively) were isolated from a sponge of the order Poecilosclerida, collected along the coast of the island of Maui (Hawaii) [90]. Each sterol contained an α,β-disubstituted furan in the side-chain, cis-fused A/B ring, a monoenolized α-diketone in the A ring, and a C-21 carboxyl group. 145 and 146 are stable to acid, but both equilibrate to a 1:1 mixture in base. Oxidation of C-21 to an alcohol is not uncommon in marine sterols, but this is the first case of sponge sterols with a C-21 carboxylic acid. The occurrence of a furan in the side-chain of a sterol is also unprecedented; the heterocyclic was supposed to be biogenetically derivable from oxidation of C-23 and C-29 to carbonyl groups followed by condensation. Kiheisterones A and B exhibit mild cytotoxicity against several human tumor cell lines (A-549 lung carcinoma and HT-29 colon adenocarcinoma) and against the P-388 murine lymphocytic leukemia cell line. Both compounds are more cytotoxic against nontumorous monkey kidney cells.
4-Methyl sterols, mainly distributed in plants and dinoflagellates [91], have also been found sometimes in gorgonians and tunicates, but such compounds are rare in sponges. Thirteen 4α-methyl sterols have been identified from Haliclona cinerea (two samples) and Haliclona flavescens in the frame of a study aimed to establish whether these sterols are derived from the diet or are synthesized by the sponges [92]. Among these, two sterols (147 and 148) resulted in new compounds.
Two biosynthetically unusual 4-methylene steroids, theonellasterone and conicasterone (149 and 150, respectively), along with the previously described corresponding alcohols theonellasterol and conicasterol [93], have been isolated from the Okinawan Theonella swinhoei. A dimeric steroid, bistheonellasterone (151), considered to be biosynthesized through a Diels-Alder cycloaddition of theonellasterone (149) and its Δ4-isomer, has also been found in the same sponge [94]. It is noteworthy that theonellasterone and conicasterone were seen under an optical microscope as crystals deposited in the tissue of fresh marine sponge. The analysis of a T. swinhoei specimen, collected off the Hachijo-jima island, afforded conicasterol and a new dimeric steroid, bisconicasterone (152). Its structure was established as a Diels-Alder-type dimer of conicasterone [95]. It is noteworthy that no trace of theonellasterol, previously reported as the major component of the same species collected at Okinawa, has been found in this sample of T. swinhoei, which suggests a site-depending biochemistry of this species. A further investigation of the Hachijo specimen yielded seven oxygenated conicasterol derivatives (153–159) along with (24R)-7β,8β-epoxy-24-methyl-4-methylene-14α-methoxy-cholestan-3β-ol (160) and (234R)-3β-hydroxy-24-methyl-4-methylene-8,14-secocholestane-8,14-dione (161) [96]. Compound 161 has been isolated successively from an Okinawan T. swinhoei (swinhosterol B) along with swinhosterol A (162), which had an ethyl group at C-24 instead of a methyl group, and the new methoxysterol (24S)-24-ethyl-7-methoxy-4-methylene-5α-cholest-8(14)-en-3β-ol (swinosterol C; 163) [97].
Swinosterols A and B (162 and 161, respectively) are the second example of the unique 8,14-seco structure. This feature has been first encountered in jereisterol B (164) isolated from Jereicopsis graphidiophora [98]. Compound 164 combine the rare 3β-methoxy and seco features; its structural determination was confirmed by the synthesis of the model 3β-acetoxy-8,14-secoergostane-8,14-dione by oxidation with ruthenium tetroxide of 3β-acetoxy-ergost-8(14)-ene. Jereisterol A, (165), also isolated from J. graphidiophora, had the unique 8α,9α-oxido-8,9-seco-5α-cholesta-7,9(11)-diene feature [98]. This structure is without precedent in the steroid literature; the 8α,9α-oxido stereochemistry has been suggested by the chemical shift of the H-5 proton, downfield shifted, implying the location of the oxygen function and H-5 on the same face of the molecule.
A homologue of jereisterol A (165), possessing the unique 8α,9α-oxido-8,9-secocholesta-7,9(11)-diene feature and a 24-ethyl side-chain (166) has been isolated from Microscleroderma spirophora along with its Δ8(14),9(11) isomer (167) [33]. The 24S stereochemistry for compounds 166 and 167 has been proposed on the basis of the chemical shift values of H-28a and H-28b, which showed a diagnostic dependence on the configuration at C-24 when compared with those of sitosterol (24R-ethylcholesterol) and clionasterol (24S-ethylcholesterol). This analysis has been proposed as a new method for the determination of the configuration at C-24 of saturated 24-ethyl side-chains.
A large number of new 9,11-secosterols have been recently isolated; structurally, all have a keto group at C-9 and a side-chain like those usually found in ‘normal’ sterols, differences residing in the A/B ring (cis or trans junction or Δ5) and in the type and degree of oxygenation.
Four novel 9,11-secosterol (168–171) have been isolated from Spongia officinalis [99], [100], [101]. They could be derived from a 5,7,9(11)-triene sterol through oxidation at the C-5 and C-6 carbons, to give 169, or only at C-6, to give 168, 170, and 171, with concomitant oxidative cleavage of the 9(11) double bond. Partial synthesis confirmed the proposed structures of the sterols 168through 171.
Glaciasterols A and B (172 and 173, respectively) have been found in the Northeastern Pacific sponge Aplysilla glacialis [102]. Their structures have been clarified by a combination of spectroscopic analysis and chemical conversions on the native steroid 172 and the diacetate of 173. Glaciasterols A and B showed an interesting in vitro cytotoxic activity in murine leukemia L1210 and human breast cancer cell line assays.
Two glaciasterol analogues (174 and 175), present in a specimen of Dysidea fragilis coming from the lagoon of Venice, possess the same nuclear structure of 172 and 173, differing only in the nature of their side-chains. In addition, the sponge has been also found to produce the new 9,11-secosterols, 176 and 177; compound 177 exhibited cytotoxic activity in vitro on two different cell lines [6].
A cytotoxic 9,11-secosteroid, blancasterol (178), bearing two acetate functionalities located at C-4 and C-11, has been isolated from a Northeastern Pacific Pleraplysilla species [103]. Blancasterol showed in vitro cytotoxicity against L1210 murine leukemia, drug-sensitive MCF-7 human breast cancer, and drug-resistant MCF-7 Adr human breast cancer cell lines. The cytotoxicity of blancasterol parallels that of the glaciasterol diacetates, demonstrating that the epoxide functionality in the B ring of glaciasterols is not required for the toxicity.
Stellettasterol (179), a new antifungal 9,11-secosterol isolated from Stelletta sp. [104], differs from herbasterol, an ichthyotoxic secosterol previously isolated from Dysidea herbacea [11], only in the absolute configuration at C-3. It is noteworthy that two species taxonomically remote, belonging to the genera Dysidea (Dyctioceratida) and Stelletta (Christida), contained secosterols differing only in the stereochemistry at C-3.
Luffasterols A through C (180–182) are 9,11-secosterols elaborated from the Palauan sponge Luffariella [105] species; they contain a 3β-acetoxy-5α,6α-epoxy-9-oxo-9,11-secocholest-7-en-11-al ring system joined to three different side-chains. The 3-deacetyl derivative of luffasterol A (183) has been isolated from Spongia matamata [106].
Euryspongiols A1 through A5 and B1 through B5 (184–188 and 189–193, respectively) have been isolated from Euryspongia sp. [107]. Euryspongiols A1 through A5 differ in their side-chain but have the same 2α,3β,4α,6β,11,19-hexahydroxy-9,11-secocholestane skeleton. Euryspongiols B1 through B5 are the corresponding 3α-epimers. They are the most highly hydroxylated secosteroids isolated so far from sponges and are the first hydroxylated at C-4. Compounds 184 and 185 strongly inhibit the release of histamine from rat mastocysts.
Three new pregnanes (194–196) with the unusual 10,2-carbolactone structural feature have been found in Strongylophora species [108]. Their structures, confirmed by X-ray crystallography, derive from 2,3,4-trihydroxy-5-pregnenes differing in D ring and/or their side-chain.
Geodisterol (197), the major component of the polar extract of the sponge Geodia sp. [109], represents the first polyoxygenated sterol with an aromatic A ring isolated from marine organisms. Steroids with aromatic A ring have been obtained from terrestrial plants and animals as hormones (e.g. estradiol), but all those steroids have a small side-chain in comparison with 197.
6. Miscellaneous
(22E)-24,26-Cyclo-5α-cholest-22-en-3β-yl 4′,8′,12′-trimethyltridecanoate (198) is a sterol ester isolated from a deep water marine sponge, Xestospongia sp. [110]. The sterol ester 198 was hydrolyzed and successively methylated with diazomethane to give the methyl ester a and the sterol b, which were identified by analysis of their spectroscopic data. The sterol b was previously reported from the soft coral Sarcophyton glaucum [111].
5α-Cholest-15-en-3β-ol (199), isolated from Topsentia aurantiaca along with compounds 58through 60 (see section 2), contains an uncommon unsaturation in the D ring. This is the first report of 199 as a naturally occurring compound; it was previously described as a synthetic product [41].
Ten 3-oxo-4,6,8(14)-triunsaturated steroids with cholestane (200and 201), ergostane (202–204 and 206), and stigmastane skeletons (205 and 207–209) were found in the lipid extract of Dysidea herbacea [112]. Of these, 206 through 209 were obtained as C-24 epimeric mixtures. Compounds 200 through 209 are the second example of steroids isolated from a marine source having the conjugated 3-oxo-4,6,8(14)-triene system. Previously, compound 201 had been isolated from Dyctionella incisa [113]. The hydroperoxide 209 was shown to be an artifact derived from 205 during storage.
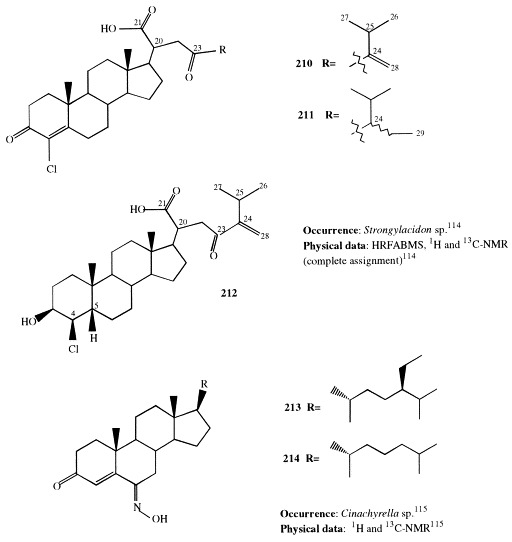
Halogenated steroids are very rare in nature. Kiheisterones C, D, and E (210–212) are three unprecedented halogenated marine steroids found in the 2-propanol/CH2Cl2 extract of the sponge Strongylacidon sp. [114], whose major cytotoxic constituents are kiheisterones A and B (145 and 146, respectively; see section 5). The existence in many organisms of reductase capable of converting 4-en-3-one steroids to the corresponding 3β-hydroxy-5β(H) compounds is well documented and kiheisterone D with its cis-chlorohydrin functionality may have arisen from enzymatic reduction of kiheisterone C.
Steroids 213 and 214, isolated from Cinachyrella sp. [115], are the first natural 6-hydroximino-4-en-3-one steroids occurring in nature. This new class of steroids, which has been recently synthesized, shows a high affinity for human placental aromatase and functions as a competitive inhibitor of this enzyme [116].
References
- 1.Djerassi C., Silva C.J. Sponge sterols: origin and biosynthesis. Acc Chem Res. 1991;24:371–378. [Google Scholar]
- 2.Kerr R.G., Baker B. Marine sterols. J Nat Prod Rep. 1991;8:465–497. [Google Scholar]
- 3.D’Auria M.V., Minale L., Riccio R. Polyoxygenated steroids of marine origin. Chem Rev. 1993;93:1839–1895. [Google Scholar]
- 4.Fattorusso E., Magno S., Mayol L., Santacroce C., Sica D. Calysterol: a C29 cyclopropene-containing marine sterol from the sponge Calyx nicaensis. Tetrahedron. 1975;31:1715–1716. [Google Scholar]
- 5.Kerr R.G., Kerr S.L., Pettit G.R., Herald D.L., Groy T.L., Djerassi C. Sterols of marine invertebrates. 63. Isolation and structure elucidation of sutinasterol, the major sterol of the marine sponge Xestospongia sp. J Org Chem. 1991;52:58–62. [Google Scholar]
- 6.Aiello A., Fattorusso E., Menna M., Carnuccio R., Iuvone T. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the lagoon of Venice. Steroids. 1995;60:666–673. doi: 10.1016/0039-128x(95)00055-u. [DOI] [PubMed] [Google Scholar]
- 7.Carballera N., Thomson J.E., Ayanoglu E., Djerassi C. Biosynthetic studies of marine lipids: the biosynthesis of long-chain branched fatty acids in marine sponge. J Org Chem. 1986;51:2751–2756. [Google Scholar]
- 8.Hahn S., Stoilov I.L., Tam Ha T.B., Raedersdorff D., Doss G.A., Li H.T., Djerassi C. Biosynthetic studies of marine lipids: the course of chain elongation and desaturation in long-chain fatty acids of marine sponge. J Am Chem Soc. 1988;110:8117–8124. [Google Scholar]
- 9.Lam W.K., Hahn S., Ayanoglu E., Djerassi C. Phospholipids studies of marine organisms: structure and biosynthesis of a novel brominated fatty acid from a Hymeniacidon sponge. J Org Chem. 1989;54:3428–3432. [Google Scholar]
- 10.Djerassi C., Lam W.K. Sponge phospholipids. Acc Chem Res. 1991;24:69–75. [Google Scholar]
- 11.Capon R.J., Faulkner D.J. Herbasterol, an ichthyotoxic 9,11-secosterol from the sponge Dysidea erbacea. J Org Chem. 1985;50:4771–4773. [Google Scholar]
- 12.Shoji N., Umeyama A., Shin K., Takeda K., Arihara S., Kobayashi J., Takei M. Two unique pentacyclic steroids with cis C/D ring junction from Xestospongia berguistia Fromont, powerful inhibitors of histamine release. J Org Chem. 1992;57:2996–2997. [Google Scholar]
- 13.Kobayashi J., Shinonaga H., Shigemori H., Umeyama A., Shoji N., Arihara S. Xestobergsterol C, a new pentacyclicsteroid from the Okinawan marine sponge Ircinia sp. and absolute stereochemistry of xestobergsterol A. J Nat Prod. 1995;58:312–318. doi: 10.1021/np50116a029. [DOI] [PubMed] [Google Scholar]
- 14.Burgoyne D.L., Andersen R.J., Allen T.M. Contignasterol, a highly oxygenated steroid with the “unnatural” 14β configuration from the marine sponge Petrosia contignata Thiele, 1899. J Org Chem. 1992;57:525–528. [Google Scholar]
- 15.Patil A.D., Freyer A.J., Breen A., Carte B., Johnson R.K. Halistanol disulfate B, a novel sulfated sterol from the sponge Pachastrella sp.: inhibitor of endothelin converting enzyme. J Nat Prod. 1996;59:606–608. doi: 10.1021/np9601770. [DOI] [PubMed] [Google Scholar]
- 16.Lopp A., Pihlak A., Paves H., Samuel K., Koljak R., Samel N. The effect of 9,11-secosterol, a newly discovered compound from the soft coral Gersemia fruticosa, on the growth and cell cycle progression of various tumor cells in culture. Steroids. 1994;59:274–281. doi: 10.1016/0039-128x(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 17.McKee T.C., Cardellina J.H., Riccio R., D’Auria M.V., Iorizzi M., Minale L., Moran R.A., Gulakowski R.J., McMahon J.B., Buckheit R.W., Snader K.M., Boyd M.R. HIV-inhibitory natural products. 11. Comparative studies of sulfated sterols from marine invertebrates. J Med Chem. 1994;37:793–797. doi: 10.1021/jm00032a012. [DOI] [PubMed] [Google Scholar]
- 18.Notaro G., Piccialli V., Sica D., Corriero G. 3β,5α,6β-Trihydroxylated sterols with saturated nucleus from two populations of the marine sponge Cliona copiosa. J Nat Prod. 1991;54:1570–1575. [Google Scholar]
- 19.Sica D., Notaro G., Piccialli V. New steroidal hydroxyketones and closely related diols from the marine sponge Cliona copiosa. J Nat Prod. 1992;55:1588–1594. [Google Scholar]
- 20.Aiello A., Fattorusso E., Magno S., Menna M. Steroids of the marine sponge Cinachyra tarentina: isolation of cholest-4-ene-3,6-dione and (24R)-24-ethylcholest-4-ene-3,6-dione. J Nat Prod. 1991;54:281–285. [Google Scholar]
- 21.Aiello A., Fattorusso E., Magno S., Menna M. Isolation of five new 5α-hydroxy-6-keto-Δ7 sterols from the marine sponge Oscarella lobularis. Steroids. 1991;56:337–340. doi: 10.1016/0039-128x(91)90057-3. [DOI] [PubMed] [Google Scholar]
- 22.Ciminiello P., Fattorusso E., Magno S., Mangoni A., Pansini M. Incisterols, a new class of highly degraded sterols from the marine sponge Dictyonella incisa. J Am Chem Soc. 1990;112:3505–3509. [Google Scholar]
- 23.Notaro G., Piccialli V., Sica D., Pronzato R. New Δ8(14)-3β:7α-dihydroxysterols from the marine sponge Pellina semitubulosa. J Nat Prod. 1992;55:773–779. [Google Scholar]
- 24.Das B, Srinivas KVNS. Studies on marine chemicals, part IV. Isolation of cholesterol derivatives from the marine sponge Spirastrella incostans. J Nat Prod 1992;55:1310–2.
- 25.Das B, Rao SP, Srinivas KVNS. Studies on marine chemicals, part VI. A new clionasterol derivative from the marine sponge Spirastrella incostans. J Nat Prod 1993;56:2210–1.
- 26.Isaacs S., Berman R., Kashman Y., Gebreyesus T., Yosief T. New polyhydroxy sterols, dysidamides, and a dideoxyhexose from the sponge Dysidea herbacea. J Nat Prod. 1991;54:83–91. [Google Scholar]
- 27.Zhong Y.L., Su J.Y., Zeng L.M., Shen W., Wang Q.W. Isolation and structure elucidation of a novel sterol from the south China sponge Dysidea sp. Chin Chem Lett. 1992;3:981–982. [Google Scholar]
- 28.Casapullo A., Minale L., Zollo F. New cytotoxic polyoxygenated steroids from the sponge Dysidea incrustans. Tetrahedron Lett. 1995;36:2669–2672. [Google Scholar]
- 29.Izzo I., De Riccardis F., Massa A., Sodano G. Synthesis of incrustasterols, two cytotoxic polyoxygenated sponge sterols. Tetrahedron Lett. 1996;37:4775–4776. [Google Scholar]
- 30.Milkova T.S., Mikhova B.P., Nikolov N.M., Popov S.S., Andreev S.N. Two new polyhydroxylated sterols from the sponge Dysidea fragilis. J Nat Prod. 1992;55:974–978. [Google Scholar]
- 31.Zhong Y.L., Su J.Y., Zeng L.M., Shen W., Wang Q.W. Structure of a new sterol from the South China sponge Dysidea fragilis. Chin J Chem. 1993;11:560–564. [Google Scholar]
- 32.D’Auria M.V., Gomez Paloma L., Minale L., Riccio R. Unique 3β-O-methylsterols from the Pacific sponge Jereicopsis graphidiophora. J Nat Prod. 1992;55:311–320. [Google Scholar]
- 33.Costantino V., Fattorusso E., Mangoni A., Aknin M., Gaydou E.M. Novel 3β-methoxysteroids from the Senegalese sponge Microscleroderma spirophora. Steroids. 1994;59:181–184. doi: 10.1016/0039-128x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 34.Levi C. Lithistid sponges from the Norfolt rise: recent and Mesozoic genera. In: Reitner J., Keupp H., editors. Fossil and recent sponges. Springer Verlag; Berlin: 1991. pp. 72–82. [Google Scholar]
- 35.Crist B.V., Li X., Bergquist P.R., Djerassi C. Sterols of marine invertebrates. 44. Isolation, structure elucidation, partial synthesis, and determination of absolute configuration of pulchrasterol: the first example of double bioalkylation of the sterol side-chain at position 26. J Org Chem. 1983;48:4472–4479. [Google Scholar]
- 36.Migliuolo A., Piccialli V., Sica D., Giordano F. New Δ8-and Δ8(14)-5α,6α-epoxysterols from the marine sponge Spongia officinalis. Steroids. 1993;58:134–140. doi: 10.1016/0039-128x(93)90050-w. [DOI] [PubMed] [Google Scholar]
- 37.Venkateswarlu Y., Venkata Rami Reddy M., Rama Rao M. A new epoxy sterol from the sponge Ircinia fasciculata. J Nat Prod. 1996;59:878–887. [Google Scholar]
- 38.Iguchi K., Shimura H., Yang Z., Yamada Y. A new 5α,8α-epidioxy sterol from the Okinawan marine sponge of the Axinyssa genus. Steroids. 1993;58:410–413. doi: 10.1016/0039-128x(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 39.Mishra P.D., Wahidulla S., Dsouza L., Kamat S.Y. Lipid constituents of marine sponge Suberites carnosus. Ind J Chem Sect B Org Chem Incl Med Chem. 1996;35:806–809. [Google Scholar]
- 40.Gunasekera S.P., Kelly–Borges M., Longley R.E. A new cytotoxic sterol methoxymethyl ether from a deep water marine sponge Scleritoderma sp cf. paccardi. J Nat Prod. 1996;59:161–162. doi: 10.1021/np9600311. [DOI] [PubMed] [Google Scholar]
- 41.Ciminiello P., Fattorusso E., Magno S., Mangoni A., Pansini M. Three new D-ring unsaturated sterols from the Mediterranean sponge Topsentia aurantiaca: structure determination and complete nuclear magnetic resonance assignment. Steroids. 1992;57:62–66. doi: 10.1016/0039-128x(92)90030-d. [DOI] [PubMed] [Google Scholar]
- 42.Eggerdorfer M.L., Kokke W.C.M.C., Crandell C.W., Hochlowsky J.E., Djerassi C. Sterol in marine invertebrates. 32. Isolation of 3β-(hydroxymethyl)-A-nor-5α-cholest-15-ene, the first naturally occurring sterol with a 15–16 double bond. J Org Chem. 1982;48:5304–5309. [Google Scholar]
- 43.Minale L., Riccio R., De Simone F., Dini A., Pizza C., Ramundo E. Starfish saponins. II. 3b-Hydroxy-5a-cholest-8,14-dien-23-one, the major sapogenin from the starfish Echinaster sepositus. Tetrahedron Lett. 1978:2609–2612. [Google Scholar]
- 44.Kokke W.C.M.C., Fenical W., Djerassi C. Sterol with unusual nuclear unsaturation from three cultured marine dinoflagellates. Phytochemistry. 1981;20:127–134. [Google Scholar]
- 45.Sun H.H., Cross S.S., Gunasekera M., Koehn F.E. Weinbersterol disulfates A and B, antiviral steroid sulfates from the sponge Petrosia weinbergi. Tetrahedron. 1991;47:1185–1190. [Google Scholar]
- 46.Kohenn F.E., Gunasekera M., Cross S.S. New antiviral sterol disulfate ortho esters from the marine sponge Petrosia weinbergi. J Org Chem. 1991;56:1322–1325. [Google Scholar]
- 47.Fusetani N., Matsunaga S., Konosu S. Bioactive marine metabolites. II. Halistanol sulfate, an antimicrobial novel steroid sulfate from the sponge Halichondria cf. moorei Bergquist. Tetrahedron Lett. 1981;21:1985–1988. [Google Scholar]
- 48.Kanazawa S., Fusetani N., Matsunaga S. Halistanol sulfates A-E, new steroid sulfates, from a marine sponge, Epipolasis sp. Tetrahedron. 1992;48:5467–5472. [Google Scholar]
- 49.Bifulco G., Bruno I., Minale L., Riccio R. Novel HIV-inhibitory halistanol sulfates F-H from a marine sponge, Pseudoaxinissa digitata. J Nat Prod. 1994;37:164–167. doi: 10.1021/np50103a026. [DOI] [PubMed] [Google Scholar]
- 50.Makarieva T.N., Stonik V.A., Dmitrenok A.S., Krasokhin V.B., Svetashev V.I., Vysotskii M.V. New polar steroids from the sponges Trachyopsis halichondroides and Cymbastela coralliophila. Steroids. 1995;60:316–320. doi: 10.1016/0039-128x(94)00056-i. [DOI] [PubMed] [Google Scholar]
- 51.Makarieva T.N., Shubina L.K., Kalinovsky A.I., Stonik V.A., Elyakov G.B. Steroids in Porifera. II. Steroid derivatives from two sponges of the family Halichondriidae: sokotrasterol sulfate, a marine steroid with a new pattern of side chain alkylation. Steroids. 1983;42:267–281. doi: 10.1016/0039-128x(83)90039-9. [DOI] [PubMed] [Google Scholar]
- 52.Sperry S., Crews P. Haliclostanone sulfate and halistanol sulfate from an Indo-Pacific Haliclona sponge. J Nat Prod. 1997;60:29–32. doi: 10.1021/np960592s. [DOI] [PubMed] [Google Scholar]
- 53.McKee T.C., Cardellina J.H., II, Tischler M., Snader K.M., Boyd M.R. Ibisterol sulfate, a novel HIV-inhibitory sulfated sterol from the deep water sponge Topsentia sp. Tetrahedron Lett. 1993;34:389–392. [Google Scholar]
- 54.Goad L.J., Garneau F.X., Simard J.L., Apsimon J.W., Girard M. Isolation of Δ9(11) sterols from the sea cucumber Psolus fabric II: implications for holothurin biosynthesis. Tetrahedron Lett. 1985;26:3513–3516. [Google Scholar]
- 55.Cordeiro M.L., Djerassi C. Biosynthetic studies of marine lipids. 25. Biosynthesis of Δ9(11) and Δ7 sterols and saponins in sea cucumbers. J Org Chem. 1990;55:2806–2813. [Google Scholar]
- 56.Fusetani N., Takahashi M., Matsunaga S. Topsentiasterol sulfates, antimicrobial sterol sulfates possessing novel side chains, from a marine sponge, Topsentia sp. Tetrahedron. 1994;50:7765–7770. [Google Scholar]
- 57.Gunasekera S.P., Sennet S.H., Kelly–Borges M., Bryant R.W. Ophirapstanol trisulfate, a new biologically active steroid sulfate from the deep water marine sponge Topsentia ophiraphidites. J Nat Prod. 1994;57:1751–1754. doi: 10.1021/np50114a024. [DOI] [PubMed] [Google Scholar]
- 58.Makarieva T.N., Stonik V.A., D’Yachuk O.G., Dmitrenok A.S. Annasterol sulfate, a novel marine sulfated steroid, inhibitor of glucanase activity from the deep water sponge Poecillastra laminaris. Tetrahedron Lett. 1995;36:129–132. [Google Scholar]
- 59.Kong F., Andersen R.J. Polymastiamide A, a novel steroid/amino acid conjugate isolated from the Norwegian marine sponge Polymastia boletiformis (Lamarck,1815) J Org Chem. 1993;58:6924–6927. [Google Scholar]
- 60.Kong F., Andersen R.J. Polymastiamide B-F, novel steroid/aminoacid conjugates isolated from the Norwegian marine sponge Polymastia boletiformis. J Nat Prod. 1996;59:379–385. [Google Scholar]
- 61.Li H., Matsunaga S., Fusetani N., Fujiki H., Murphy P.T., Willis R.H., Baker J.T. Echinoclasterol sulfate phenethylammonium salt, a unique steroid sulfate from the marine sponge Echinoclathria subhispida. Tetrahedron Lett. 1993;34:5733–5736. [Google Scholar]
- 62.Kerr R.G., Kerr S.L., Malik S., Djerassi C. Biosynthetic studies of marine lipids. 38. Mechanism and scope of sterol side chain dealkylation in sponges: evidence for concurrent alkylation and dealkylation. J Am Chem Soc. 1992;114:299–303. [Google Scholar]
- 63.Giner J.L. Biosynthesis of marine sterols side chains. Chem Rev. 1993;93:1735–1752. [Google Scholar]
- 64.Oger J.M., Richomme P., Bruneton J., Guinaudeau H., Sevenet T., Debitus C. Steroids from Neosiphonia supertes, a marine fossil sponge. J Nat Prod. 1991;54:273–275. [Google Scholar]
- 65.Makarieva T.N., Bondarenko I.A., Dmitrenok A.S., Boguslavsky V.M., Stonik V.A., Chernih V.I., Efremova S.M. Natural products from the lake Baikal organisms, I. Baikalosterol, a novel steroid with an unusual side chain, and other metabolites from the sponge Baicalospongia bacilifera. J Nat Prod. 1991;54:953–958. [Google Scholar]
- 66.Zeng C., Ishibashi M., Kobayashi J. Bienmasterol, a new cytotoxic sterol with the rare 22,25-diene side chain, isolated from the marine sponge Bienma sp. J Nat Prod. 1993;56:2016–2018. doi: 10.1021/np50101a027. [DOI] [PubMed] [Google Scholar]
- 67.John V., Stoilov I.L., Djerassi C., Karuso P., Poiner A., Scheuer P.J. Biosynthetic studies of marine lipids. 20. Sequence of double bond: introduction in the sponge sterol 24β-methylcholesta-5,7,22,25-tetraen-3β-ol. J Org Chem. 1989;54:1642–1647. [Google Scholar]
- 68.Stoilov I.L., Moreau M.B., Thompson S.E., Djerassi C. Biosynthetic studies of marine lipids. 12. Biosynthesis in marine sponges of sterols possessing the Δ5(7) nucleus typical of fungi and the 24-alkyl side chain characteristic of plants. Tetrahedron. 1987;43:2213–2222. [Google Scholar]
- 69.Ishibashi M., Yamagishi E., Kobayashi J. Topsentinols A-J, new sterols with highly branched side chains from marine sponge Topsentia sp. Chem Pharm Bull. 1997;45:1435–1438. [Google Scholar]
- 70.Kerr RG, Foss C, Matsunaga S, Fusetani N. Isolation and structure elucidation of epipolasterol and 22,23-dihydroepipolasterol from the marine sponge Epipolasis sp. Comp Biochem Physiol B Biochem Mol Biol 1997;117B:561–3.
- 71.De Riccardis F., Minale L., Iorizzi M., Debitus C., Levi C. Marine sterols: side chain-oxygenated sterols, possibly of abiotic origin, from the new Caledonian sponge Stelodoryx chlorophylla. J Nat Prod. 1993;56:282–287. [Google Scholar]
- 72.Djerassi C. Structure and biosynthesis of cyclopropane-containing sterols of marine origin. New J Chem. 1987;14:713–719. [Google Scholar]
- 73.Proudfoot J.R., Djerassi C. Synthesis and stereochemistry of 23,24-dihydrocalysterol: implications for marine sterols of a unified biosynthetic scheme involving protonated cyclopropanes. J Chem Soc Perkin Trans 1. 1987:1283–1290. [Google Scholar]
- 74.Giner J.L., Silva C.J., Djerassi C. The missing step in sterol cyclopropyl biosynthesis: enzymatic desaturation of 24(S)-ethylcholesterol. J Am Chem Soc. 1990;112:9626–9627. [Google Scholar]
- 75.Giner J.L., Djerassi C. Minor and trace sterols in marine invertebrates 65. 23-Epidihydrocalysterol: a new cyclopropane-containing sponge sterol. Steroids. 1992;57:258–261. doi: 10.1016/0039-128x(92)90057-g. [DOI] [PubMed] [Google Scholar]
- 76.Doss G.A., Proudfoot J.R., Silva C.J., Djerassi C. Experimental demonstration of an unprecedented cyclopropane to cyclopropane rearrangement in the biosynthesis of the sponge sterol petrosterol. J Am Chem Soc. 1990;112:305–310. [Google Scholar]
- 77.Seo Y., Rho J., Cho K., Sim C.J., Shin J. Isolation of epidioxysteroids from a sponge of the genus Tethya. Bull Korean Chem Soc. 1997;18:631–635. [Google Scholar]
- 78.Makarieva T., Stonik V.A., Ponomarenko L.P., Kalinovsky A.I. Isolation of (24R)-24,25-methylene-5α-cholestan-3β-ol, a new cyclopropane-containing sponge sterol. J Chem Res Synop. 1996;10:468–469. [Google Scholar]
- 79.Iguchi K., Fujita M., Nagaoka H., Mitome H., Yamada Y. Aragusterol A: a potent antitumor marine steroid from the Okinawan sponge of the genus, Xestospongia. Tetrahedron Lett. 1993;34:6277–6280. [Google Scholar]
- 80.Iguchi K., Shimura H., Taira S., Yokoo C., Matsumoto K., Yamada Y. Aragusterol B and D, new 26,27-cyclosterols from the Okinawan marine sponge of the genus Xestospongia. J Org Chem. 1994;59:7499–7502. [Google Scholar]
- 81.Shimura H., Iguchi K., Yamada Y., Nakaike S., Yamagishi T., Matsumoto K., Yokoo C. Aragusterol C: a novel halogenated marine steroid from an Okinawan sponge, Xestospongia sp., possessing potent antitumor activity. Experientia. 1994;50:134–136. doi: 10.1007/BF01984951. [DOI] [PubMed] [Google Scholar]
- 82.Miyaoka H., Shinihara M., Shimomura M., Mitome H., Yano A., Iguchi K., Yamada Y. Aragusterols E-H, new 26,27-cyclosterols from the Okinawan marine sponge of the genus Xestospongia and absolute configuration of xestokerols A and B. Tetrahedron. 1997;53:5403–5412. [Google Scholar]
- 83.Mitome H., Miyaoka H., Nakano M., Yamada Y. Synthesis of antitumor marine steroid aragusterols. Tetrahedron Lett. 1995;36:8231–8234. [Google Scholar]
- 84.Mitome H., Miyaoka H., Nakano M., Yamada Y. Synthesis of 5-epiaragusterol A. Bioorg Med Lett. 1997;7:691–692. [Google Scholar]
- 85.Kobayashi J., Ishida K., Naitoh K., Shigemori H., Mikami Y., Sasaki T. Xestokerols A, B, and C, new C29 steroids with a cyclopropane ring from the Okinawan marine sponge Xestospongia sp. J Nat Prod. 1993;56:1350–1355. [Google Scholar]
- 86.Kobayashi M., Chen Y., Higuchi K., Aoki S., Kitagawa I. Marine natural products. XXXVII. Aragusteroketals A and C, two novel cytotoxic steroids from a marine sponge of Xestospongia sp. Chem Pharm Bull. 1996;44:1840–1842. [Google Scholar]
- 87.Jurek J., Scheuer P.J., Kelly–Borges M. Two steroidal alkaloids from a sponge, Corticium sp. J Nat Prod. 1994;57:1004–1007. doi: 10.1021/np50109a022. [DOI] [PubMed] [Google Scholar]
- 88.Rosser R.M., Faulkner D.J. Two steroidal alkaloids from a marine sponge Plakina sp. J Org Chem. 1984;49:5157–5160. [Google Scholar]
- 89.Rochfort S.J., Gable R.W., Capon R.J. Mycalone: a new steroidal lactone from a Southern Australian marine sponge, Mycale sp. Aust J Chem. 1996;49:715–718. [Google Scholar]
- 90.Carney J.R., Yoshida W.Y., Scheuer P.J. Kiheisterones, new cytotoxic steroids from a Maui sponge. J Org Chem. 1992;57:6637–6640. [Google Scholar]
- 91.Withers N. Dinoflagellate sterols. In: Scheuer P.J., editor. Marine Natural Products, V. Academic Press; New York: 1983. pp. 876–930. [Google Scholar]
- 92.Elenkov I, Dragova B, Andreev S, Popov S. 4α-Methyl sterols from the sponges Haliclona cinerea and Haliclona flacescens. Comp Biochem Physiol B Biochem Mol Biol 1997;118B:155–7.
- 93.Kho E., Imagawa K., Rohmer M., Kashman Y., Djerassi C. Sterols in marine invertebrates. 22. Isolation and structure elucidation of conicasterol and theonellasterol, two new 4-methylene sterols from the Red Sea sponges Theonella conica and Theonella swinhoei. J Org Chem. 1981;46:1836–1840. [Google Scholar]
- 94.Kobayashi M., Kawazoe K., Katori T., Kitagawa I. Marine natural products. XXX. Two new 3-keto-4-methylene steroids, Theonellasterone and Conicasterone, and a Diels-alder type dimeric steroid Bistheonellasterone, from the Okinawan marine sponge Theonella swinhoei. Chem Pharm Bull. 1992;40:1773–1778. [Google Scholar]
- 95.Inouye Y., Sugo Y., Kusumi T., Fusetani N. Structure and absolute stereochemistry of bisconicasterone from the marine sponge Theonella swinhoei. Chem Lett. 1994:419–420. [Google Scholar]
- 96.Sugo Y., Inouye Y., Nakayama N. Structures of nine oxygenated 4-methylene sterols from Hachijo marine sponge Theonella swinhoei. Steroids. 1995;60:738–742. doi: 10.1016/0039-128x(95)00108-3. [DOI] [PubMed] [Google Scholar]
- 97.Umeyama A., Shoji N., Enoki M., Shigenobu A. Swinhosterols A-C, 4-methilene secosteroids from the marine sponge Theonella swinhoei. J Nat Prod. 1997;60:296–298. [Google Scholar]
- 98.D’Auria M.V., Gomez Paloma L., Minale L., Riccio R., Debitus C. Jereisterol A and B: two 3β-methoxy-secosteroids from the pacific sponge Jereicopsis graphidiophora. Tetrahedron Lett. 1991;32:2149–2152. [Google Scholar]
- 99.Migliuolo A., Piccialli V., Sica D. Structure elucidation and synthesis of 3β,6α-dihydroxy-9-oxo-9,11-seco-5α-cholest-7-en-11-al, a novel 9,11-secosterol from the sponge Spongia officinalis. Tetrahedron. 1991;47:7937–7950. [Google Scholar]
- 100.Migliuolo A., Piccialli V., Sica D. Two new 9,11-secosterols from the marine sponge Spongia officinalis: synthesis of 9,11-seco-3β,6α,11-trihydroxy-5α-cholest-7-en-9-one. Steroids. 1992;57:344–347. doi: 10.1016/0039-128x(92)90054-d. [DOI] [PubMed] [Google Scholar]
- 101.Adinolfi R., Migliuolo A., Piccialli V., Sica D. Isolation and synthesis of a new 9,11-secosterol from the sponge Spongia officinalis. J Nat Prod. 1994;57:1200–1226. [Google Scholar]
- 102.Pika J., Tischler M., Andersen R.J. Glaciasterols A and B, 9,11-secosteroids from the marine sponge Aplysilla glacialis. Can J Chem. 1992;70:1506–1510. [Google Scholar]
- 103.Pika J., Andersen R.J. Blancasterol, a cytotoxic 9,11-secosteroid isolated from the Northeastern Pacific Marine Sponge Pleraplysilla sp. Tetrahedron. 1993;49:8757–8760. [Google Scholar]
- 104.Li H., Matsunaga S., Fusetani N. A new 9,11-secosterol, stellettasterol from a marine sponge Stelletta sp. Experientia. 1994;50:771–773. [Google Scholar]
- 105.Reddy V.R.M., Harper M.K., Faulkner D.J. Luffasterols A-C, 9,11-secosterols from the Palauan sponge Luffariella sp. J Nat Prod. 1997;60:41–43. doi: 10.1021/np9606207. [DOI] [PubMed] [Google Scholar]
- 106.Lu Q., Faulkner D.J. Two new sesterterpenoids and a new 9,11-secosterol from Spongia matamata. J Nat Prod. 1997;60:195–198. doi: 10.1021/np9606411. [DOI] [PubMed] [Google Scholar]
- 107.Dopeso J., Quinoa E., Riguera R., Debitus C., Bergquist P.R. Euryspongiols: ten new highly hydroxylated 9,11-secosteroids with antihistaminic activity from the sponge Euryspongia sp.: stereochemistry and reduction. Tetrahedron. 1994;50:3813–3828. [Google Scholar]
- 108.Corgiat J.M., Scheuer P.J., Rios Steiner J.L., Clardy J. Three pregnane-10,2-carbolactones from a sponge, Strongylophorus sp. Tetrahedron. 1993;49:1557–1562. [Google Scholar]
- 109.Wang G., Crews P. Geodisterol, a novel polyoxygenated sterol with an aromatic A ring from the tropical marine sponge Geodia sp. Tetrahedron Lett. 1996;37:8145–8146. [Google Scholar]
- 110.Gunasekera S.P., Cranick S., Pomponi S.A. New sterol ester from a deep water marine sponge, Xestospongia sp. J Nat Prod. 1991;54:1119–1122. [Google Scholar]
- 111.Kobayashi M., Ishizaka T., Mitsuhashi H. Isolation of 5α,6-dihydroglaucasterol, a new marine C-27 sterol with a 24,26-cyclized side chain, from the soft coral Sarcophyton glaucum. Chem Pharm Bull. 1983;31:1803–1805. [Google Scholar]
- 112.Kobayashi M., Krishna M.M., Ishida K., Anjaneyulu V. Marine sterols. XXII. Occurrence of 3-oxo-4,6,8(14)-triunsaturated steroids in the sponge Dysidea herbacea. Chem Pharm Bull. 1992;40:72–74. [Google Scholar]
- 113.Ciminiello P., Fattorusso E., Magno S., Mangoni A. A novel conjugated ketosteroid from the marine sponge Dictyonella incisa. J Nat Prod. 1989;52:1331–1333. [Google Scholar]
- 114.Carney J.R., Scheuer P.J., Kelly–Borges M. Three unprecedented chloro steroids from the Maui sponge Strongylacidon sp.: kiheisterones C, D, and E. J Org Chem. 1993;58:3460–3462. [Google Scholar]
- 115.Rodriguez J., Nunez L., Peixinho S., Jimenez C. Isolation and synthesis of the first natural 6-hydroximino 4-en-3-one-steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997;38:1833–1836. [Google Scholar]
- 116.Holland H.L., Kumaresan S., Tan L., Njar V.C.O. Synthesis of 6-hydroximino-3-oxo steroids, a new class of aromatase inhibitor. J Chem Soc Perkin Trans I. 1992:585–587. [Google Scholar]


