Abstract
In the last half century, significant attention has been given to animal diseases; however, our understanding of disease processes and how to manage them at the livestock–wildlife interface remains limited. In this study, we conduct a systematic review of the scientific literature to evaluate the status of diseases at the livestock–wildlife interface in the United States. Specifically, the goals of the literature review were three fold: first to evaluate domestic animal diseases currently found in the United States where wildlife may play a role; second to identify critical issues faced in managing these diseases at the livestock–wildlife interface; and third to identify potential technical and policy strategies for addressing these issues. We found that of the 86 avian, ruminant, swine, poultry, and lagomorph diseases that are reportable to the World Organization for Animal Health (OIE), 53 are present in the United States; 42 (79%) of these have a putative wildlife component associated with the transmission, maintenance, or life cycle of the pathogen; and 21 (40%) are known to be zoonotic. At least six of these reportable diseases—bovine tuberculosis, paratuberculosis, brucellosis, avian influenza, rabies, and cattle fever tick (vector control)—have a wildlife reservoir that is a recognized impediment to eradication in domestic populations. The complex nature of these systems highlights the need to understand the role of wildlife in the epidemiology, transmission, and maintenance of infectious diseases of livestock. Successful management or eradication of these diseases will require the development of cross-discipline and institutional collaborations. Despite social and policy challenges, there remain opportunities to develop new collaborations and new technologies to mitigate the risks posed at the livestock–wildlife interface.
Keywords: Livestock–wildlife interface, Agriculture, Adaptive management, Mutual transmission, Wildlife diseases, Zoonoses
1. Introduction
Despite significant attention given to animal diseases in the last half-century, our understanding of disease processes, and how to manage them at the livestock–wildlife interface, remains limited (Rhyan and Spraker, 2010). The increasing role of wildlife in the emergence of livestock and human diseases is due to multiple changes occurring within wildlife, livestock, and human populations, as well as at the livestock–wildlife interface (Jones et al., 2008). Human driven land use change—which frequently includes encroachment into wildlife habitat—continues to increase along with more intensified livestock production practices (Daszak et al., 2001, Patz et al., 2004). Alteration of wildlife population demographics, such as larger deer populations, increases the potential for contact and pathogen transmission at the livestock–wildlife interface (Rhyan and Spraker, 2010).
All of these changes work to create new interfaces between livestock and wildlife (Gortázar et al., 2007, Decker et al., 2010, Rhyan and Spraker, 2010), potentially exacerbating pathogen transmission processes between them. Globally, the role of wildlife in livestock diseases is expected to increase (Siembieda et al., 2011) in conjunction with human population growth, which is expected to reach 9 billion by 2030. This will create increased demand for animal protein thereby increasing livestock populations (Anonymous, 2004). The demand will further increase potentially infectious contacts between livestock and wildlife leading to an increased potential for new zoonotic diseases to emerge. All of these challenges will require an improved understanding of the ecology of pathogens at the livestock–wildlife interface along with development of tools and mitigations to manage these pathogens.
Historically, managing diseases affecting both livestock and wildlife as a single, linked system in North America, has presented several obstacles. Conflicting agency and institutional missions, program goals, and cultural differences that limit the potential for developing comprehensive mitigation of pathogen transmission contribute to hampering efforts in this area. Nevertheless, research and policy at the livestock–wildlife interface has received increased attention in recent years with the number of scientific publications in English journals addressing this topic rising dramatically (Fig. 1 ). This is driven, though not exclusively, by a rapid increase in the number of zoonotic disease events associated with wildlife in the latter part of the 20th century (Dobson and Foufopoulos, 2001, Ostfeld and Holt, 2004, Decker et al., 2010). Three-fourths of all emerging infectious diseases (EIDs) of humans are zoonotic with most originating in wildlife (Taylor and Latham, 2001, Jones et al., 2008). A large proportion (77%) of livestock pathogens—and an even higher proportion (91%) of carnivore pathogens—infect multiple hosts including wildlife (Cleaveland et al., 2001). Therefore, diseases that arise from the livestock–wildlife interface are of paramount importance and must be an area of focus for animal health authorities (Siembieda et al., 2011).
Fig. 1.
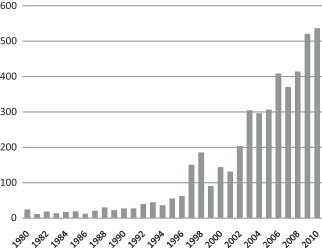
The number of publications in English language journals identified in Scopus database with the words “wildlife” and “parasite” or “disease” in the title, abstract, or key words.
One example, Nipah virus—classified as an emerging infectious pathogen—recently moved from its natural host (fruit bats) to domestic swine, causing disease and mortality in both swine and local agricultural workers and resulting in economic losses (Epstein et al., 2006a). The 1999 Nipah virus outbreak in Malaysia destroyed the Malaysian swine industry while the associated human fatalities simultaneously created massive public panic (Epstein et al., 2006a). This newly recognized virus was carried by fruit bats for decades and emerged as a result of newly occurring habitat destruction, climatic changes, and the encroachment of food–animal production into wildlife domains (Epstein et al., 2006b).
Although little discussed, pathogen transmission at the livestock–wildlife interface is frequently bi-directional (Bengis et al., 2002). In contrast to conventional thinking, livestock have introduced several pathogens, such as bovine brucellosis and tuberculosis bacterium, to naïve wildlife populations in North America. These two pathogens are found in at least five wildlife populations (Tessaro, 1986, Sweeney and Miller, 2010) and create significant challenges for disease control at the livestock–wildlife interface. In some instances, spillover events from livestock into wildlife impact conservation of species of concern (Dobson and Foufopoulos, 2001, Nishi et al., 2002, Joly and Messier, 2005, Cross et al., 2007). An example is the transmission and introduction of bovine brucellosis and tuberculosis from livestock into native wood bison (Bison bisonathabascae) populations in Canada, which has created a conservation challenge for the species (Tessaro et al., 1990, Nishi et al., 2002). Another well-publicized example is the introduction of brucellosis into native bison and elk populations of the Yellowstone ecosystem in 1917 (Meagher and Meyer, 1994, Meyer and Meagher, 1995). This resulted in a wildlife management challenge due to conflicts between livestock and bison preservation.
The presence of brucellosis poses continued risk for transmission back into livestock creating biological, social, and policy challenges (Cross et al., 2007, Cross et al., 2010). Obstacles faced by wildlife managers and livestock authorities for mitigating contact between wildlife and livestock has resulted in significant efforts to develop technology that reduces contact and is economically feasible. However, development of effective tools that can be readily deployed has been met with a host of challenges. Many devices prove to be ineffective or only effective for a short duration (VerCauteren et al., 2005, VerCauteren et al., 2006a). The most successful tools have involved fencing technology (e.g. high fence, wire mesh, electrified high-tensile steel wire, or polytape) that reduces contact between wildlife and livestock feed (VerCauteren et al., 2006b, VerCauteren et al., 2007). However, fencing suffers from limitations such as the need for relatively frequent maintenance. More recently, research has focused on the use of historic tools such as livestock protection dogs to prevent contact between livestock and wildlife. In some cases these traditional tools have proven to be the most effective (VerCauteren et al., 2008, VerCauteren et al., 2010). In addition to the challenges faced in developing effective mitigation tools is gaining social acceptance of their use by farmers, which is fundamental in successfully using these tools (Brook and McLachlan, 2006). However, there remains a need for identifying new economically feasible tools that wildlife and livestock managers can deploy to reduce contact at the livestock–wildlife interface.
Improving our understanding of the biological and anthropogenic processes that promote contact between wildlife and livestock is critical for limiting pathogen transmission at this interface. Given the frequently bi-directional nature of pathogen transmission, cooperation is required between livestock owners, animal health officials, and wildlife managers if control efforts are to be successful. Conflicts will undoubtedly continue to challenge wildlife managers and livestock authorities seeking solutions, which can only be found through the creation of new partnerships and the strengthening of existing ones that bridge the gap between wildlife and livestock agencies at all levels.
Here we conduct a systematic review of the English scientific literature to evaluate the status of diseases and pathogens at the livestock–wildlife interface in the United States. Specifically, the goals of the literature review were three fold: first, to evaluate domestic animal diseases currently found in the United States where wildlife may play a role; second, to identify critical issues faced in managing these diseases at the livestock–wildlife interface; and third, to identify potential technical and policy strategies for addressing these issues. We highlight two examples of emerging diseases at the livestock–wildlife interface in North America, which pose management challenges and offer an opportunity for comprehensive disease management by facilitating cross-agency and state-federal partnering.
2. Status of diseases at the livestock–wildlife interface in the United States
In the United States, there are currently 86 avian, ruminant, swine, and lagomorph diseases reportable to the OIE. Of those, 53 are listed as present in the United States (Anonymous, 2011b). Our review of these pathogens identified 42 (79%) which have a potential wildlife component associated with the transmission, maintenance, or life cycle of the pathogen, and 21 (40%) are known to be zoonotic (Table 1, Table 2 ). Of these 42 pathogens, 12 (29%) have an arthropod vector involved in the transmission while the remaining 71% involve direct or indirect transmission. Sixteen (38%) of the diseases present in the United States affect multiple species of livestock, all of these have a wildlife component, and 81% are zoonotic. Of the OIE reportable diseases affecting cattle, 6 out of 8 are present in the United States and have a wildlife component; 3 have zoonotic potential. A wildlife component has been identified for 10 out of 11 (91%) OIE reportable avian diseases with 3 of these recognized as zoonotic.
Table 1.
Number of OIE reportable diseases present in the United States and number with a known potential wildlife component associated with the transmission, maintenance, or life cycle of the causative agent.
| Established free (absent) | Known present (sporadic or limited distribution) | Total | Wildlife component | Zoonotic | |||
|---|---|---|---|---|---|---|---|
| Avian | 3 (1) | 9 (2) | 15 | 10 | 91% | 3 | 27% |
| Cattle | 4 (2*) | 8 | 14 | 6 | 75% | 3 | 38% |
| Equine | 4 | 6 (1) | 11 | 3 | 43% | 1 | 14% |
| Lagomorphs | 1 (1) | 2 | 2 | 100% | |||
| Multiple | 10 | 14 (2) | 26 | 16 | 100% | 13 | 81% |
| Sheep and goat | 4 | 6 (1) | 11 | 3 | 43% | 1 | 14% |
| Swine | 4 (1) | 2 | 7 | 2 | 100% | ||
| Total OIE diseases | 29 (4) | 53 | 86 | 42 | 79% | 21 | 40% |
Bovine spongiform encephalopathy (BSE) is not considered free or present in the United States but rather a controlled risk.
Table 2.
OIE reportable livestock diseases present in the United States with a known wildlife component.
| Disease | Affected livestock | Wildlife host | Citation | Transmission mode |
|---|---|---|---|---|
| Anthrax | Ruminantsc, horsesc-sc, swinec-sc | All mammals susceptible, environmental reservoirs | (Hugh-Jones and De Vos, 2002) | Direct |
| Aujeszky's disease | Domestic swinec, cattlec, sheepc, goatsc, horseso | Feral Swiner, wild mammalss | (Hahn et al., 1997, Corn et al., 2004, Kirkpatrick et al., 1980, Spickler et al., 2010) | Direct, indirect |
| Avian chlamydiosis | Ducksc, turkeysc, chickenso | Gullsr, ducksr, heronsr, egretsr, pigeonsr, blackbirdsr, gracklesr, house sparrowsr, killdeerr, raptorss, shorebirdss, migratory birdss | (Vanrompay et al., 1995, Thomas et al., 2007, Spickler et al., 2010) | Direct, indirect |
| Avian infectious bronchitis | Chickensc | Wild birdsu | (Jonassen et al., 2005, Muradrasoli et al., 2010) | Direct, indirect |
| Avian influenza | Chickensc, turkeysc, ducksc, geesec, game birdsc | Numerous wild birdsr, many mammals susceptiblea | (Cook, 2005, Olsen et al., 2006) | Direct, indirect |
| Avian mycoplasmosis (Mycoplasma gallisepticum, Mycoplasma synoviae) | Chickensc, turkeysc, game birdsc, ducksc, geesec | House finchesa, American goldfinchesa, purple finchesa, eastern tufted titmicea, pine grosbeaksa, evening grosbeaksa, othersa | (Thomas et al., 2007, Luttrell et al., 2001, Ley et al., 1996, Spickler et al., 2010) | Direct, indirect |
| Bluetongue | Sheepc, goatsc, cattlesc | Wild ovine speciesa, cervidsa, water buffaloa, pronghorna, | (Williams and Barker, 2001, Stallknecht et al., 1991, Robinson et al., 1967, Spickler et al., 2010, Hoff and Trainer, 1978) | Arthropod-borne |
| Bovine anaplasmosis | Cattlec | Cervidsr | (Woldehiwet, 2010, Kuttler, 1984) | Arthropod-borne |
| Bovine babesiosis* | Cattlec | White-tailed deers, water buffalos, African buffalos, reindeers | (Spickler et al., 2010, Schoelkopf et al., 2005, Cantu-C et al., 2009) | Arthropod-borne |
| Bovine genital campylobacteriosis | Cattlec | Numerousr | (Williams and Barker, 2001) | Direct, indirect |
| Bovine tuberculosis | Primarily cattlec | White-tailed deerr, feral swiner, numerous spillover hosts | (O’Brien et al., 2006, Buddle et al., 2000, Williams and Barker, 2001, Spickler et al., 2010) | Direct, indirect |
| Bovine viral diarrhea | Cattlec, camelidsc, bisono | White-tailed deerr, mule deers, caribous, pronghorns, elks, mooses, bisons | (Passler et al., 2007, Zarnke, 1983, Williams and Barker, 2001, Duncan et al., 2008) | Direct, indirect |
| Brucellosis (Brucella abortus) | Cattlec, sheepc, horseso | Bisonr, water buffalor, elkr, feral swineu, numerous spillover hosts | (Olsen, 2010b, Zarnke, 1983, Williams and Barker, 2001, Spickler et al., 2010) | Direct, indirect |
| Brucellosis (Brucella ovis)** | Sheepc | Red deera | (Ridler and West, 2002, Spickler et al., 2010) | Direct, indirect |
| Brucellosis (Brucella suis) | Domestic swinec, horseso | Feral swiner, European harer, caribour, reindeerr, rodentsr, numerous spillover hosts | (Galindo et al., 2010, Olsen, 2010b, Williams and Barker, 2001, Corn et al., 2009, Spickler et al., 2010) | Direct, indirect |
| Contagious agalactia | Sheepc, goatsc, cattleo, camelidso | Spanish ibexu, roe deeru, red deeru | (Verbisck-Bucker et al., 2008), (Spickler et al., 2010) | Direct, indirect |
| Echinococcosis/hydatidosis | Sheepc, cattlec | Carnivore sp. including canidsr, and felidsr, cervidsu, rodentsu, lagomorphsu, muskratsu | (Leiby et al., 1970, Storandt et al., 2002, Storandt and Kazacos, 1993, Thompson et al., 2006, Spickler et al., 2010) | Indirect |
| Epizootic hemorrhagic disease | Cattlec-sc, sheepsc,o | White-tailed deerr, mule deers, pronghorns, other wild ruminant speciess | (Anonymous, 2006) | Arthropod-borne |
| Equine encephalomyelitis (Eastern and Western) | Equidsc, occasional reports in cattle, sheep, camelids and pigs | Birdsr, rodentsr, jackrabbitsr, white-tailed deers, numerous speciess | (Reisen et al., 2000, Emord and Morris, 1984, Komar et al., 1999, Tate et al., 2005, Schmitt et al., 2007, Spickler et al., 2010) | Arthropod-borne |
| Equine influenza | Equidsc | Wild birdsr, numerous other speciess | (Munster et al., 2007) | Direct, indirect |
| Equine piroplasmosis | Equidsc | Uncertain | (Kellogg et al., 1971, Spickler et al., 2010) | Arthropod-borne |
| Equine rhinopneumonitis | Equidsc | Numerous speciesu | (Kinyili and Thorsen, 1979) | Direct, indirect |
| Fowl cholera | Poultryc | Wild birdsr | (Thomas et al., 2007, Petersen et al., 2001, Botzler, 1991, Blanchong et al., 2006) | Direct, indirect |
| Infectious bovine rhinotracheitis/infectious pustularvulvovaginitis | Cattlec | Several implicatedu | (Zarnke, 1983, Kinyili and Thorsen, 1979) | Direct, indirect |
| Infectious bursal disease | Chickensc, turkeyssc, duckssc, guinea fowlsc, ostrichessc | Game birdsr, Waterfowlr | (Thomas et al., 2007, Candelora et al., 2010, Anonymous, 2008) | Direct, indirect |
| Leptospirosis | Cattlec-sc, sheepc-sc, goatsc-sc, pigsc-sc, horsesc-sc, all mammalsc-sc | Rodentsr, raccoonsr, skunksr, opossumr, nutriar, otherss | (Zarnke, 1983, Williams and Barker, 2001) | Direct, indirect |
| Maedi-visna | Sheepc, goatsc | Wild ruminantsu | (Valas et al., 1997, Spickler et al., 2010) | Direct, indirect is rare |
| Marek's disease | Chickensc, Turkeyso, Quailo | Galliformesr | (Cho and Kenzy, 1975) | Direct, indirect |
| Myxomatosis | Lagomorphsc | Lagomorphsr | (Williams and Barker, 2001, Dwyer et al., 1990) | Arthropod-borne |
| Newcastle disease*** | Chickensc, turkeysc-sc, game birdsc-sc, duckssc, geesesc, pigeonsc-sc | Wild birdsr, exotic birdsr | (Thomas et al., 2007, Brugh and Beard, 1984, Seal et al., 2000, Clubb and Hinsch, 1982, Spickler et al., 2010) | Direct, indirect |
| Paratuberculosis | Cattlec, sheepc, goatsc | Wild ruminantsr, rabbitsr, numerous wild mammalsu | (Williams and Barker, 2001, Corn et al., 2005, Greig et al., 1997, Spickler et al., 2010, Ayele et al., 2001) | Direct, indirect |
| Porcine reproductive and respiratory syndrome | Swinec | Feral swiner | (Williams and Barker, 2001, Corn et al., 2009) | Direct, indirect |
| Pullorum disease | Chickensc, turkeysc, pheasantsc, other poultryo | Waterfowlr, numerous wild bird speciesu | (Thomas et al., 2007, Shivaprasad, 2000) | Direct, indirect |
| Q fever | Cattlec-sc, sheepc-sc, goatsc-sc | Numerous species including mammalsr, birdsr, and reptilesr | (Zarnke, 1983, Spickler et al., 2010) | Direct, indirect, arthropod borne |
| Rabbit hemorrhagic disease | Domestic Oryctolaguscuniculusc | Wild Oryctolaguscuniculusc | (Williams and Barker, 2001, Spickler et al., 2010) | Direct, indirect |
| Rabies | All mammals susceptible | Raccoonsr, coyotesr, foxr, batsr, skunksr, mongooser, bobcatsu, otherss | (Sterner and Smith, 2006, Krebs et al., 2003, Spickler et al., 2010) | Direct |
| Transmissible gastroenteritis | Swinec | Feral swiner | (Williams and Barker, 2001) | Direct, indirect |
| Trichinellosis | Swinec-sc | Carnivoresa, feral swinea, rodentsa, bearsa, othersa | (Murrell et al., 1987) | Direct, indirect |
| Tularemia | Sheepc, horseso, pigso | Lagomorphsr, muskratsr, rodentsr, minks, prairie dogss, otherss | (Al Dahouk et al., 2005, Williams and Barker, 2001, Spickler et al., 2010, Jellison and Parker, 1945, Morner, 1992) | Direct, indirect, arthropod-borne |
| Vesicular stomatitis | Cattlec, swinec, equidsc, camelidssc, sheepsc, goatssc | Numerous wildlife species susceptible including mammals and birds, reservoir hosts unknown | (Williams and Barker, 2001, Spickler et al., 2010, Webb et al., 1987) | Direct, indirect, arthropod-borne |
| West Nile | Equidsc, domestic geesec | Wild birdsr, other speciesu | (Daszak et al., 2001, Thomas et al., 2007, Spickler et al., 2010) | Arthropod-borne |
| Diseases of importance that are not OIE listed. | ||||
| Chronic Wasting Disease | Domestic cervidsc | Wild cervidsr | (Williams and Barker, 2001, Williams et al., 2002, Baeten et al., 2007, Williams, 2005, Hamir et al., 2001) | Direct, indirect |
| Malignant Catarrhal Fever | Cattlec, bisonc, swinec, sheepsc, goatssc | Wildebeestr, oryxs, ibexs, cervidss, wild ovine and caprinespeciesr | (Williams and Barker, 2001, Spickler et al., 2010) | Direct |
| Plague | Domestic mammalsc-sc | Prairie dogsr, chipmunksr, groundrsquirrelsr, other rodentsr, carnivoress, numerous other speciesa | (Salkeld and Stapp, 2006) | Direct, indirect, arthropod-borne |
| Trichomoniasis (Trichomonasgallinae) | Poultryc, dovesc, pigeonsc | Pigeonsr, dovesr, falconsa, hawksa, othersa | (Thomas et al., 2007) | Direct, indirect |
Bovine babesiosis is not present in cattle in the United States however the causative agent has been reported in wildlife and a vector eradication program exists.
B. ovis has been found to cause poor semen quality in red deer but abortions have not been reported. The role potential role of red deer is still in doubt.
The United States is considered free from new castle disease in poultry however new castle disease is present in free ranging species and is included here for completeness.
c = clinical
sc = subclinical
c-sc = may be clinical or subclinical
o = occasional reports
r = reservoir
s = spillover
a = affected species (not a true reservoir, nor a spillover host)
u = uncertain
Of the avian, ruminant, and swine diseases, 21 are currently actively managed in the United States with 11 of these having a Federal eradication or control program (Table 3 ). Thirteen (62%) of these actively managed diseases have a wildlife component and at least 6 (bovine tuberculosis, paratuberculosis, brucellosis, avian influenza, rabies, and cattle fever tick [vector control]) have a wildlife reservoir that is a recognized impediment to eradication due to continued spillover to domestic populations. Of these diseases, 2 (bovine tuberculosis and brucellosis) have foci of infection in wildlife as a result of spillover from livestock—further complicating eradication programs.
Table 3.
Diseases actively managed in the United States and corresponding wildlife component.
| Disease | National or agency program | Primary domestic species | Wildlife component |
|---|---|---|---|
| Avian influenza | Control | Poultry | Yes |
| Bluetongue | Multiple | Yes (Arthopod-borne) | |
| Bovine spongiform encephalopathy | Cattle | ||
| Bovine Tuberculosis | Eradication | Multiple | Yes |
| Brucellosis (Brucella abortus) | Eradication | Multiple | Yes |
| Brucellosis (Brucella suis) | Eradication | Multiple | Yes |
| Cattle Fever Tick (vector only) | Eradication | Cattle | Yes (Arthopod-borne) |
| Chronic wasting disease | Eradication | Cervids | Yes |
| Classical swine fever | Swine | Yes | |
| Contagious equine metritis | Equine | ||
| Equine herpesvirus | Equine | ||
| Equine infectious anemia | Eradication | Equine | |
| Equine piroplasmosis | Equine | Uncertain (Arthopod-borne) | |
| Equine viral arteritis | Equine | ||
| Paratuberculosis (Johnes) | Control | Multiple | Yes (Arthopod-borne) |
| Pseudorabies (Aujeszky's disease) | Eradication | Multiple | Yes |
| Rabies | Eradication | Multiple | Yes |
| Scrapie | Eradication | Sheep, Goats | |
| Vesicular stomatitis | Multiple | Yes (Arthopod-borne) | |
| West Nile | Multiple | Yes (Arthopod-borne) |
Specific estimates of direct and indirect costs to livestock and recreational hunting industries, and to governmental agencies resulting from pathogen transmission at the livestock–wildlife interface, are elusive; however, some estimates are available for specific diseases. Reestablishment of bovine Babesia sp. to its historic range in North America via adaptation of Babesia sp. vectors to white-tailed deer would cost approximately $1.2 billion to the cattle industry (Anderson et al., 2010). In Michigan, the loss of bovine tuberculosis accredited-free status is estimated to result in total agriculture and livestock losses of approximately $12 million per year (Horan and Wolf, 2005). Furthermore, the Michigan Department of Natural Resources spent an estimated $15 million on defining the extent of the disease in wildlife and initial management steps alone (O’Brien et al., 2006) and to date has spent an estimated $23 million (O’Brien et al., 2011) on control, surveillance, and management of the disease.
Rabies—an important zoonotic disease with significant public health, agricultural, and ecological impacts—is known to impose a financial burden on countries around the world. The Centers for Disease Control and Prevention estimates that the United States spends in excess of $300 million annually on rabies prevention, detection, and control (Anonymous, 2011a) with more than $130 million spent on wildlife vaccination alone (Sterner et al., 2009). Avian influenza, which has a well-documented wild waterfowl reservoir, continues to plague the domestic poultry industry in the United States with estimated outbreak associated losses ranging from $5 to $212 million (Capua and Alexander, 2004, Saif and Barnes, 2008). Estimated impacts to the United States in the event of an epizootic avian influenza pandemic are at least $71 billion (Meltzer et al., 1999, Arnold et al., 2006). Other livestock diseases with wildlife reservoirs including brucellosis, bovine viral diarrhea, and several poultry diseases are associated with significant losses in livestock production.
3. Structured approach to livestock–wildlife disease management
Concepts for integrated and adaptive management systems for EIDs at the livestock–wildlife interface are proposed by multiple authors (Thirgood, 2009, Wasserberg et al., 2009). Many countries, have developed passive and active surveillance systems for EID events in wildlife. Some of the earliest systems were developed in Denmark (1930s) and Sweden (1940s) however surveillance systems are established in Norway, Finland, France, United Kingdom, Italy, Spain, Switzerland, and the United States (Morner et al., 2002, Pedersen et al., 2012). The United Kingdom (Sainsbury et al., 2001, Lysons et al., 2007, Hartley and Gill, 2010, Hartley and Lysons, 2011) has developed a program to implement integrated risk management and EID monitoring systems for wildlife. These nascent emerging systems have common themes, which may be adaptable to the United States. In the existing literature, five interdependent aspects of disease management are suggested as being necessary for successfully addressing disease issues at the livestock–wildlife interface: (1) horizon scanning (issue identification); (2) risk analysis and assessment; (3) risk mitigation; (4) surveillance and monitoring; and (5) disease control and management. These components, described as integrating sequentially with feedback loops, incorporate learning about the system. As information about the disease agent is improved, management is adapted thereby improving actions performed in the other components (Fig. 2 ). This process of adaptive management has been well described in the ecological and wildlife management literature (Kendall, 2001, McCarthy and Possingham, 2007), but concepts related to adaptive management have only recently been proposed as a method for managing disease systems (Thirgood, 2009, Wasserberg et al., 2009).
Fig. 2.
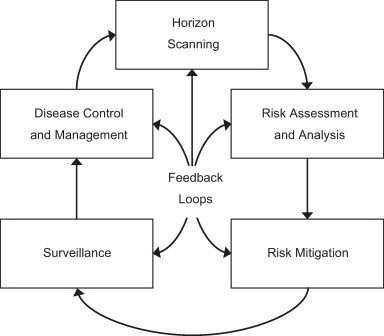
Conceptual model of adaptive disease management at the livestock–wildlife interface.
4. Horizon scanning
Rapid identification of new and emerging infectious diseases (horizon scanning) in wildlife is critical to protecting animal agriculture and human health. There is mounting concern over the zoonotic potential, and subsequent wide-ranging socioeconomic impacts, associated with wildlife-borne EIDs (Jones et al., 2008). Recent examples of EIDs emerging from wildlife include Nipah virus in swine (Chua et al., 2000), severe acute respiratory syndrome (SARS) in humans (Riley et al., 2003), and H5N1 HPAI in domestic poultry, wild birds, and humans (Ferguson et al., 2004). In addition, new issues continue to emerge with well-documented disease systems, such as bovine tuberculosis and brucellosis influencing agricultural systems and wildlife management in North America (Olsen, 2010a, O’Brien et al., 2011). Other EID's of concern to agriculture are certain to emerge in the future, some of which may disperse rapidly across broad geographic scales (Cleaveland et al., 2001, Siembieda et al., 2011). The risks of existing and new EID's to disperse rapidly highlight the need for robust systems for early identification of pathogens, which may have important health, social, economic, or other management consequences.
5. Risk analysis
Risk analysis is an often broadly used term referring to risk characterization, risk communication, and risk management, which provides support for decision making and policy in the face of uncertainty (Suter, 2007). In the case of animal disease, risk analysis is an important tool used to identify and characterize the potential risks posed by implementation of policy or by specific events such as importation of livestock. Risk analyses form the foundation from which animal health policy is established. However, for diseases at the livestock–wildlife interface, quantitative risk assessments are often difficult. Challenges for conducting quantitative risk assessments often result from incomplete information related to the disease status of wildlife or limited understanding of the potential contact between wildlife and livestock leading to pathogen transmission. In addition accurate quantitative data describing the spatial distribution, movement, population structure, and population density are typically unavailable limiting inference to the population or understanding population level risk factors.
Data quantifying important epidemiologic parameters necessary for describing disease risk such as contact rates, disease status of wildlife, wildlife population size, or biological process of the pathogen in wildlife are often unstudied or poorly understood. Risk assessments often assess the risk of pathogen transmission from wildlife to livestock (Daniels et al., 2003). However, for many diseases of livestock in North America (e.g. bovine tuberculosis, brucellosis) the initial transmission event is from livestock to wildlife, which in some cases results in the establishment of a wildlife reservoir for the pathogen posing continued risks to livestock (Tessaro, 1986, Sweeney and Miller, 2010). For these reasons the most successful and useful risk analyses consider the bi-directional nature of transmission and address questions of risk using statistical methods to explicitly incorporate uncertainty. In addition, studies that estimate contact between livestock and wildlife to understand potential for pathogen transmission are needed.
6. Risk mitigation
Mitigating transmission risk between livestock and wildlife has received considerable attention (VerCauteren et al., 2006b, VerCauteren et al., 2010, Wasserberg et al., 2009). The ability to eliminate livestock pathogens from North American wildlife populations has been rare and when successful required extensive culling of wildlife. An example is the eradication of foot-and-mouth disease from the United States in 1925 which required the culling of 22,000 deer from the Stanislaus National Forest in California (Williams and Barker, 2001). Wildlife removal strategies can have unintended consequence, which was exemplified in the United Kingdom where wildlife behavior was changed as a result of culling increasing the risk of bovine tuberculosis transmission to cattle (Woodroffe et al., 2009). In addition protected wildlife can complicate control or eradication efforts to control disease (Meyer and Meagher, 1995). Eradication efforts requiring the culling of large numbers of wildlife are likely untenable in the United States today, thus preventing establishment of livestock diseases in wildlife populations is a central pillar of long-term risk mitigation strategies.
Implementing risk mitigations may offer the greatest potential for reducing economic and social impacts resulting from shared diseases. This often involves modifying animal husbandry practices to reduce contact between livestock and wildlife—including modified livestock housing, which reduces contact with peri-domestic wildlife or altered feeding practices, which reduces available forage for wildlife. Other risk mitigations include tools that prevent direct contact between wildlife such as frightening devices, fencing, or livestock protection dogs (VerCauteren et al., 2005, VerCauteren et al., 2006b, VerCauteren et al., 2010). However, the development and implementation of these tools comes with their own set of challenges. Successful implementation often includes changing social behaviors of livestock producers and developing new tools to manage risk mitigation which are cost effective and efficacious over the long term. Other risk mitigations may include identifying and reducing or eliminating risky management practices—such as allowing contact between livestock and wildlife which may foster the emergence of new pathogens in the United States. These may include translocation of wildlife or domestic and international wildlife trade.
7. Surveillance and monitoring
The need to develop comprehensive surveillance systems that integrate livestock, wildlife, and human components has been suggested (Mörner et al., 2002). Robust surveillance systems in wildlife and at the livestock–wildlife interface to provide early detection of newly emerging EIDs or spillover and spillback of pathogens between livestock and wildlife is essential. Developing a comprehensive national monitoring system for EIDs in wildlife that is logistically and fiscally sustainable could yield economic benefits for livestock health management as a whole by reducing indemnity costs associated with spillover of disease from wildlife to livestock or by helping prevent spillover from livestock to wildlife through early detection. The objectives of such a system could be enhanced by close integration with existing livestock and wildlife health programs to guide “when”, “where”, and “how” surveillance is conducted. In addition, existing programs would benefit from closer working relationships between wildlife biologists, ecologists, epidemiologists, and veterinarians to improve efforts focused on reducing pathogen transmission (Boadella et al., 2011). One obstacle to developing long-term, comprehensive surveillance efforts at the livestock–wildlife interface is inconsistent funding for these activities (Leighton et al., 1997, Stitt et al., 2007). Funding has typically been in response to emergency directives (e.g. HPAI H5N1 surveillance) and focused for a short period until the threat is perceived to no longer exist. This has, predictably, generated problems for developing a comprehensive national infrastructure that can be maintained over the long-term. In addition, there are disease systems that have plagued agriculture for decades, such as bovine tuberculosis, that do not obtain sufficient levels of funding to fully address risks for introduction into new wildlife hosts such as feral swine (Sweeney and Miller, 2010). Another challenge faced by wildlife surveillance systems is that they often rely on hunter observations and reports, which are focused on game species. This increases the difficulty of identifying emergence of disease in non-game species. Finally, due to challenges associated with working across agency departmental boundaries, such as reduced communication, differing priorities, perceived competition in missions, and cultural differences wildlife surveillance efforts often remain less than fully coordinated which reduces their overall benefit.
8. Disease control and management
Once a pathogen is identified at the livestock–wildlife interface, active management and control of the disease agent is often the only method for reducing impacts to human health, agriculture, and recreational hunting industries (Boadella et al., 2011). Integrated strategies that bring wildlife, human, and agricultural agencies together offer the greatest opportunity for success. Management of diseases at the livestock–wildlife interface often requires long-term engagement using a combination of altered livestock husbandry practices, active disease suppression in wildlife, and prevention of transmission using mitigation techniques.
9. Inter-agency and cross-sector collaborations and partnership
If surveillance and risk management activities at the livestock–wildlife interface are to be successful, we must recognize the complex nature of current and emerging diseases. These diseases can involve different health jurisdictions, socio-economic dimensions, and a wide range of stakeholders (i.e. livestock industry, conservation organizations, recreational hunters, etc.). We must promote strategic collaboration and partnerships across various disciplines, sectors, departments, ministries, institutions, and organizations at country, regional, and international levels (Binder et al., 1999, FAO et al., 2008). With The recent focus on “One Health”, which recognizes that human, animal (both domestic and wild) and ecosystems are tightly linked, successful management of disease requires an integrated approach where efforts are focused in concert across these domains (King et al., 2008, Welburn, 2011). In response to the One Health focus several countries have developed specific plans to address wildlife health as it relates to human and domestic animal health (Sainsbury et al., 2001, Hartley and Lysons, 2011). However, obstacles still remain in developing robust systems which integrate across the domestic, wild animal, and human domains.
In most countries, sector-specific institutions have clear roles, responsibilities, and budgets—but mechanisms for cross-sector collaboration typically do not exist. Developing collaborations often proves difficult even mandated from the highest levels of government, as exemplified by continued outbreaks of highly pathogenic avian influenza in several countries (FAO et al., 2008). The United States suffers from similar limitations, due in part to the bicameral regulatory and legal authority for oversight of livestock and wildlife. States have clear ownership of wildlife; Federal regulatory authorities do not always extend to control disease in livestock to manage the disease in wildlife. Thus, the effective control of disease incursions from wildlife to livestock requires State and Federal livestock management agencies to foster positive working relationships with wildlife agencies. Unfortunately, such relationships frequently have not been developed resulting in a decision making process on livestock disease management in which wildlife appear as an afterthought, when often they are integral to disease maintenance and spread. Involving all relevant stakeholders (i.e. livestock industry, wildlife conservation groups, wildlife health authorities, livestock health authorities, etc.) in the development of regional or ecosystem-level livestock disease management planning, from the beginning of the process, increases the likelihood of success (Loomis, 2002).
One example of ongoing challenges animal health authorities face is found in the management of brucellosis in the Yellowstone ecosystem. Controversy has surrounded the management of brucellosis in the bison and elk within the ecosystem. These issues have often pitted Federal, State, agricultural, and wildlife agencies against one another. Several have noted that one of the most important constraints to managing brucellosis in Yellowstone is jurisdictional inertia, or the unwillingness of agencies to relinquish their existing domains of territorial control (Lavigne, 2002, McBeth and Shanahan, 2004). This example underscores the need for wildlife, human, and agricultural agencies to develop strong working relationships prior to emergence of disease. Integrated approaches to prevention before observing outbreaks in both wildlife and livestock may offer an opportunity for agencies to foster working relationships prior to a crisis.
Development of clear mechanisms and agreements will enhance collaboration and interaction at all levels and should include incorporation of the roles and mandates of the various institutions and agencies involved. Often the agreements and working relationships that are established occur only at the highest levels of the organization resulting in little benefit to those working to implement program objectives. Opportunities for professional interactions and working relationships needs to be created and supported at the field level in addition to the administrative level (FAO et al., 2008).
10. Opportunities for success
Historically, integrated cross-disciplinary collaboration between livestock and wildlife agencies has been a challenge. However, many programs managing animal health diseases could benefit significantly from increased communication and collaborations that combine program objectives and activities across agency jurisdictions. While challenging from a political and cultural perspective, the outcome could be beneficial and would enhance the ability to quickly identify and respond to new and emerging disease issues. Integrating State and Federal livestock and wildlife agencies into the disease program planning process could reap future rewards.
Below we illustrate the potential for cross-sector collaboration using two disease eradication programs—Cattle fever tick eradication and bovine tuberculosis eradication—facing challenges presented by the livestock–wildlife interface. Other disease eradication and management programs that address issues associated with the diseases and pathogens listed in Table 3 would also likely benefit from increased collaboration across livestock, wildlife, and human agencies at both State and Federal levels.
11. Discussion
11.1. Cattle fever tick eradication program
Bovine babesiosies, caused by hematoprotozoan parasites of the genus Babesia, is globally among the most significant tick-borne disease of cattle (White et al., 2003, Martinez et al., 2006). In North America, the most important vectors of bovine babesiosis are Rhipicephalus microplus and R. annulatus—collectively known as cattle fever ticks. Cattle fever ticks were extirpated from the United States in 1960 after a nearly 60-year eradication campaign (Graham and Hourrigan, 1977, Bram et al., 2002). The eradication campaign exploited the perceived narrow host range of cattle fever ticks in combination with highly effective and now banned acaricides, which allowed the program to focus almost exclusively on the treatment of cattle (Bram et al., 2002). Reestablishment of cattle fever ticks and bovine Babesia to their historic range in North America is estimated to cost $1.2 billion in control efforts and cattle production losses (Anderson et al., 2010). As a result, animal health authorities and livestock producers consider mitigating this risk a priority.
In recent years, there have been increasing infestations of cattle fever ticks on cattle along the Texas–Mexico border (de León Adalberto et al., 2010). Historically considered to be highly host specific for cattle, there is increasing evidence that white-tailed deer and other ungulates are suitable hosts for cattle fever ticks (Pound et al., 2010) with infested deer found in locations absent of cattle (Cantu et al., 2007). In Texas, cattle fever ticks have been recovered from free-ranging and captive-exotic ungulates including axis deer, fallow deer, elk, red deer, aoudad sheep, and nilgai antelope (Mertins et al., 1992). Due to the potential ineffectiveness of treating tick infestations in cattle with currently approved methods, such as mandatory removal of cattle from affected pastures for a period of time (i.e. pasture vacation) and treatment of cattle with acaricides the treatment of white-tailed deer and other wildlife has become necessary.
A recent study indicates that cattle fever ticks have a high degree of genetic fluidity, which may allow them to adapt to new host species and therefore provide a potential pathway for reestablishment in the United States via wildlife hosts (De Meeus et al., 2010). White-tailed deer are also increasingly being recognized as a potential reservoir for the Babesia species (B. bigemina, B. divergens, and B. bovis) which cause clinical disease in cattle (de León Adalberto et al., 2010). Surveys for Babesia in northern Mexico and Texas have identified molecular and serological evidence for the presence of B. bigemina and B. bovis in white-tailed deer and in nilgai antelope populations (Cantu et al., 2007, Cardenas-Canales et al., 2011). These changes in the host–pathogen system, and gaps in the understanding of cattle fever tick ecology and the host range of Babesia, require the formulation of more effective control strategies that include both wildlife and livestock.
To effectively address these challenges, State and Federal Agencies representing both livestock and wildlife authorities need to partner to develop policy that integrates surveillance and risk mitigations across both cattle and wildlife populations. An historic limitation of the program has been the nearly exclusive focus on controlling cattle fever ticks on cattle (León et al., 2010). Recently the program has begun to deploy mitigations to control ticks on wildlife; however, the program is limited by a lack of operational tools to mitigate infestations on wildlife and a regulatory framework that would integrate management of the disease across wildlife and livestock authorities. While challenging, this offers an exciting opportunity to develop effective strategies and methods to address surveillance at the livestock–wildlife interface and to develop new mitigations that reduce the risk of infestation.
11.2. Bovine tuberculosis eradication program
Bovine tuberculosis (bTB), identified in nine geographically distinct wildlife populations in North America and Hawaii, is endemic in at least four populations, including members of the Bovidae, Cervidae, and Suidaefamilies (Sweeney and Miller, 2010). The emergence of bTB in North American wildlife poses a serious and growing risk for livestock and human health and for the recreational hunting industry. Experience in many countries, including the United States and Canada, has shown that while bTB can be controlled when restricted to livestock species, it is almost impossible to eradicate this disease once it has spread into ecosystems with free-ranging maintenance hosts. Recent epidemiological models suggest that once bTB is introduced, the probability of becoming established in a wildlife population once introduced is at least 10% (Ramsey et al., 2011). Spillover into wildlife—and establishment of new foci of infection in wildlife—would be costly to the cattle industry and animal health authorities. In addition, new foci of wildlife infection would complicate eradication efforts. Therefore, preventing spillover of Mycobacterium bovis into wildlife may be the most effective way to mitigate economic costs of bTB.
Historically, wildlife control efforts for bTB have focused solely on potential spillover into wild cervid species. However, M. bovis has been isolated from free-ranging swine (i.e. wild boar and feral swine) in at least 15 countries (Letts, 1964, Corner et al., 1981, Essey et al., 1981, O’Reilly and Daborn, 1995, Aranaz et al., 1996, Serraino et al., 1999, Palmer, 2007). New evidence from Mediterranean ecosystems supports the role of wild swine as maintenance hosts of bTB—sustaining infection and transmitting the pathogen to other species (Aranaz et al., 1996, Naranjo et al., 2008). Circumstances favoring bTB transmission between wildlife and livestock in the Mediterranean include artificial increases in wild game populations stimulated by a robust hunting industry, feeding and baiting of wildlife, and intensive cattle grazing in proximity to wild swine (Hermoso de Mendoza et al., 2006). All of these characteristics likely apply to conditions in North America. Particularly worrisome is the recent appearance of feral swine in the state of Michigan where the potential exists for interaction with bTB-infected white-tailed deer and cattle. Regions of the southern United States also pose a risk where high densities of feral swine, an established hunting industry, significant baiting and feeding of wildlife, and introductions of bTB infected cattle from Mexico continue to occur (Sweeney and Miller, 2010). Furthermore recent evidence indicates that M. bovis may be present in free ranging white-tailed deer in northern Mexico. One study report the presence of M. tuberculosis complex identified using amplification of DNA from a tissue by PCR (Barrios-García et al., 2012). The authors also report histopathology consistent with M. bovis infection observed in white-tailed deer. Another study reported the frequent detection of antibodies against mycobacterium antigens in a cross-sectional survey of white-tailed deer in Northern Mexico (Medrano et al., 2012).
While the risks posed by wildlife have been recognized, current investigations and response to potential spillover events from cattle to wildlife (cervid or swine), where disease is exceedingly more difficult to control or eradicate is inconsistently managed. Few standards are in existence which establish best practices for investigating potential spillover into wildlife hosts. Developing national policies and working relationships across agencies responsible for domestic and wildlife health at the State and Federal level would have long-term benefits for preventing the risk of introduction of bTB into new wildlife host populations.
12. Conclusions
Nearly 80% of the pathogens present in the United States have a potential wildlife component. To successfully manage and control these pathogens at the livestock–wildlife interface will require the development of cross-discipline collaborations and establishing common goals between agencies and organizations that in some cases have rarely worked together. We believe the principles of adaptive management offer the greatest opportunities to formulate a framework from which collaborations can be developed to manage diseases at the livestock–wildlife interface. EID monitoring systems for wildlife that incorporate and implement integrated risk management in an adaptive management framework offer the best opportunity for success. In addition, new and creative funding mechanisms that bring livestock and wildlife animal health authorities along with livestock industry and wildlife stakeholders together will need to be created. Despite these social and policy challenges, there remain opportunities to develop new collaborations—along with the development of new technologies—to mitigate disease risks at the livestock–wildlife interface. We believe that two diseases eradication programs—bovine tuberculosis and cattle fever tick—offer the best opportunity to apply these principles and demonstrate success.
Acknowledgements
We would like to acknowledge the insightful comments and critical review of early versions of this manuscript by Dr. Steve Sweeney, Dr. Tom DeLiberto, Dr. Reginald Johnson, Dr. Kathe Bjork, Dr. Tracey Lynn, and Mr. Allan Nelson. We also would like to recognize the diligent efforts of Ms. Mary Foley for supporting our continued literature and library science inquiries and Ms. Carol LoSapio for her editing contribution. We also thank two anonymous reviewers for their critical and insightful comments on this manuscript.
References
- Al Dahouk S., Nöckler K., Tomaso H., Splettstoesser W.D., Jungersen G., Riber U., Petry T., Hoffmann D., Scholz H.C., Hensel A., Neubauer H. Seroprevalence of brucellosis, tularemia, and yersiniosis in wild boars (Sus scrofa) from North-Eastern Germany. J. Vet. Med. Ser. B. 2005;52:444–455. doi: 10.1111/j.1439-0450.2005.00898.x. [DOI] [PubMed] [Google Scholar]
- Anderson D.P., Hagerman A.D., Teal P.D., Wagner G.G., Outlaw J.L., Herbst B.K. Agricultural & Food Policy Center, Texas A&M University; College Station, TX: 2010. Economic Impact of Expanded Fever Tick Range. 28 pp. [Google Scholar]
- Anonymous . United Nations; New York: 2004. World Population to 2300. 254 pp. [Google Scholar]
- Anonymous . The Center for Food Security and Public Health; Ames, IA: 2006. Diseases Caused by the Epizootic Hemorrhagic Disease Virus Serogroup. 4 pp. [Google Scholar]
- Anonymous . World Organisation for Animal Health; 2008. Infectious Bursal Disease (Gumboro Disease). OIE Terrestrial Manual. pp. 549–565. [Google Scholar]
- Anonymous . Centers for Disease Control and Prevention; Atlanta, GA: 2011. Cost of Rabies Prevention. [Google Scholar]
- Anonymous . Animal Plant Health Inspection Service; Fort Collins, CO: 2011. United States Animal Health Report, 2011. Status of Reportable Diseases in the United States United States Department of Agriculture. [Google Scholar]
- Aranaz A., Liebana E., Mateos A., Dominguez L., Vidal D., Domingo M., Gonzolez O., Rodriguez-Ferri E.F., Bunschoten A.E., Embden J.D.V., Cousins D. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J. Clin. Microbiol. 1996;34:2734–2740. doi: 10.1128/jcm.34.11.2734-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R., De Sa J., Gronniger T., Percy A., Somers J. The Congress of the United States, Congressional Budget Office; 2006. A Potential Influenza Pandemic: Possible Macroeconomic Effects and Policy Issues. 44 pp. [Google Scholar]
- Ayele W.Y., Machackova M., Pavlik I. The transmission and impact of paratuberculosis infection in domestic and wild ruminants. Vet. Med.: Czech. 2001;46:205–224. [Google Scholar]
- Baeten L.A., Powers B.E., Jewell J.E., Spraker T.R., Miller M.W. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi) J. Wildl. Dis. 2007;43:309–314. doi: 10.7589/0090-3558-43.2.309. [DOI] [PubMed] [Google Scholar]
- Barrios-García H., Guizarnotegui-Blanco J., Zapata-Campos C., Almazán-García C., González-Alanís P., Villareal-Peña R., Hernandez-Jarguin A., Miranda-Hernandez D., Martinez-Burnes J. Identification of Mycobacterium tuberculosis complex by histopathology and PCR in white-tailed deer (Odocoileus virginianus) in Tamaulipas, Mexico. J. Anim. Vet. Adv. 2012;11:1036–1040. [Google Scholar]
- Bengis R.G., Kock R.A., Fischer J. Infectious animal diseases: the wildlife/livestock interface. Rev. Sci. Tech. (Off. Int. Épizoot.) 2002;21:53–66. doi: 10.20506/rst.21.1.1322. [DOI] [PubMed] [Google Scholar]
- Binder S., Levitt A.M., Sacks J.J., Hughes J.M. Emerging infectious diseases: public health issues for the 21st century. Science. 1999;284:1311–1313. doi: 10.1126/science.284.5418.1311. [DOI] [PubMed] [Google Scholar]
- Blanchong J.A., Samuel M.D., Goldberg D.R., Shadduck D.J., Lehr M.A. Persistence of Pasteurella multocida in wetlands following avian cholera outbreaks. J. Wildl. Dis. 2006;42:33–39. doi: 10.7589/0090-3558-42.1.33. [DOI] [PubMed] [Google Scholar]
- Boadella M., Gortazar C., Acevedo P., Carta T., Martín-Hernando M.P., de la Fuente J., Vicente J. Six recommendations for improving monitoring of diseases shared with wildlife: examples regarding mycobacterial infections in Spain. Eur. J. Wildl. Res. 2011;57:1–10. [Google Scholar]
- Botzler R. Epizootiology of avian cholera in wildfowl. J. Wildl. Dis. 1991;27:367–395. doi: 10.7589/0090-3558-27.3.367. [DOI] [PubMed] [Google Scholar]
- Bram R.A., George J.E., Reichard R.E., Tabachnick W.J. Threat of foreign arthropod-borne pathogens to livestock in the United States. J. Med. Entomol. 2002;39:405–416. doi: 10.1603/0022-2585-39.3.405. [DOI] [PubMed] [Google Scholar]
- Brook R.K., McLachlan S.M. Factors influencing farmers’ concerns regarding bovine tuberculosis in wildlife and livestock around Riding Mountain National Park. J. Environ. Manage. 2006;80:156–166. doi: 10.1016/j.jenvman.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Brugh M., Beard C.W. Atypical disease produced in chickens by Newcastle disease virus isolated from exotic birds. Avian Dis. 1984;28:482–488. [PubMed] [Google Scholar]
- Buddle B.M., Skinner M.A., Chambers M.A. Immunological approaches to the control of tuberculosis in wildlife reservoirs. Vet. Immunol. Immunopathol. 2000;74:1–16. doi: 10.1016/s0165-2427(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Candelora K.L., Spalding M.G., Sellers H.S. Survey for antibodies to infectious bursal disease virus serotype 2 in wild Turkeys and Sandhill cranes of Florida, USA. J. Wildl. Dis. 2010;46:742–752. doi: 10.7589/0090-3558-46.3.742. [DOI] [PubMed] [Google Scholar]
- Cantu-C A., Ortega-S J.A., García-Vázquez Z., Mosqueda J., Henke S.E., George J.E. Epizootiology of Babesia bovis and Babesia bigemina in free-ranging white-tailed deer in Northeastern México. J. Parasitol. 2009;95:536–542. doi: 10.1645/GE-1648.1. [DOI] [PubMed] [Google Scholar]
- Cantu A., Ortega-S J.A., Mosqueda J., Garcia-Vazquez Z., Henke S.E., George J.E. Immunologic and molecular identification of Babesia bovis and Babesia bigemina in free-ranging white-tailed deer in Northern Mexico. J. Wildl. Dis. 2007;43:504–507. doi: 10.7589/0090-3558-43.3.504. [DOI] [PubMed] [Google Scholar]
- Capua I., Alexander D.J. Avian influenza: recent developments. Avian Pathol. 2004;33:393–404. doi: 10.1080/03079450410001724085. [DOI] [PubMed] [Google Scholar]
- Cardenas-Canales E.M., Ortega-Santos J.A., Campbell T.A., Garcıa-Vazquez Z., Cantu-Covarrubias A., Figueroa-Millan J.V., DeYoung R.W., Hewitt D.G., Bryant F.C. Nilgai antelope as a possible carrier of Babesia spp. and cattle fever ticks in northern Mexico. J. Wildl. Dis. 2011;47:777–779. doi: 10.7589/0090-3558-47.3.777. [DOI] [PubMed] [Google Scholar]
- Cho B.R., Kenzy S.G. Virologic and serologic studies of zoo birds for Marek's disease virus infection. Infect. Immun. 1975;11:809–814. doi: 10.1128/iai.11.4.809-814.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K., Bellini W., Rota P., Harcourt B., Tamin A., Lam S., Ksiazek T., Rollin P., Zaki S., Shieh W.J. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- Cleaveland S., Laurenson M., Taylor L. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 2001;356:991–1000. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clubb S., Hinsch H. Infectious Disease Committee American Association of Zoo Veterinarians: report of the Subcommittee on Viscerotropic Velogenic Newcastle Disease. J. Zoo Anim. Med. 1982;13:43–45. [Google Scholar]
- Cook R.A. Emerging diseases at the interface of people, domestic animals and wildlife: the role of wildlife in our understanding of highly pathogenic avian influenza. Yale J. Biol. Med. 2005;78:343–353. [PMC free article] [PubMed] [Google Scholar]
- Corn J.L., Cumbee J.C., Barfoot R., Erickson G.A. Pathogen exposure in feral swine populations geographically associated with high densities of transitional swine premises and commercial swine production. J. Wildl. Dis. 2009;45:713–721. doi: 10.7589/0090-3558-45.3.713. [DOI] [PubMed] [Google Scholar]
- Corn J.L., Manning E.J.B., Sreevatsan S., Fischer J.R. Isolation of Mycobacterium avium subsp. paratuberculosis from free-ranging birds and mammals on livestock premises. Appl. Environ. Microbiol. 2005;71:6963–6967. doi: 10.1128/AEM.71.11.6963-6967.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn J.L., Stallknecht D.E., Mechlin N.M., Luttrell M.P., Fischer J.R. Persistence of pseudorabies virus in feral swine populations. J. Wildl. Dis. 2004;40:307–310. doi: 10.7589/0090-3558-40.2.307. [DOI] [PubMed] [Google Scholar]
- Corner L., Banrrett R., Lepper A., Lewis V., Pearson C. A survey of mycobacteriosis of feral pigs in the Northern Territory. Aust. Vet. J. 1981;57:537–542. doi: 10.1111/j.1751-0813.1981.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Cross P., Cole E., Dobson A., Edwards W., Hamlin K., Luikart G., Middleton A., Scurlock B., White P. Probable causes of increasing brucellosis in free-ranging elk of the Greater Yellowstone Ecosystem. Ecol. Appl. 2010;20:278–288. doi: 10.1890/08-2062.1. [DOI] [PubMed] [Google Scholar]
- Cross P., Edwards W., Scurlock B., Maichak E., Rogerson J. Effects of management and climate on elk brucellosis in the Greater Yellowstone Ecosystem. Ecol. Appl. 2007;17:957–964. doi: 10.1890/06-1603. [DOI] [PubMed] [Google Scholar]
- Daniels M., Hutchings M., Greig A. The risk of disease transmission to livestock posed by contamination of farm stored feed by wildlife excreta. Epidemiol. Infect. 2003;130:561–568. [PMC free article] [PubMed] [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78:103–116. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- de León Adalberto P., Daniel S., Donald K., Durland F., Eileen T. 2010. One Health Approach to Identify Research Needs in Bovine and Human Babesioses: Workshop Report. 12 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meeus T., Koffi B.B., Barré N., De Garine-Wichatitsky M., Chevillon C. Swift sympatric adaptation of a species of cattle tick to a new deer host in New Caledonia. Infect. Genet. Evol. 2010;10:976–983. doi: 10.1016/j.meegid.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Decker D., Evensen D., Siemer W., Leong K., Riley S., Wild M., Castle K., Higgins C. Understanding risk perceptions to enhance communication about human–wildlife interactions and the impacts of zoonotic disease. ILAR J. 2010;51:255–261. doi: 10.1093/ilar.51.3.255. [DOI] [PubMed] [Google Scholar]
- Dobson A., Foufopoulos J. Emerging infectious pathogens of wildlife. Philos. Trans. B. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C., Van Campen H., Soto S., LeVan I.K., Baeten L.A., Miller M.W. Persistent bovine viral diarrhea virus infection in wild cervids of Colorado. J. Vet. Diagn. Invest. 2008;20:650–653. doi: 10.1177/104063870802000521. [DOI] [PubMed] [Google Scholar]
- Dwyer G., Simon A.L., Buttel L. A Simulation model of the population dynamics and evolution of myxomatosis. Ecol. Monogr. 1990;60:423–447. [Google Scholar]
- Emord D.E., Morris C.D. Epizootiology of Eastern equine encephalomyelitis virus in update New York, USA. VI: Antibody prevalence in wild birds during an interepizootic period. J. Med. Entomol. 1984;21:395–404. doi: 10.1093/jmedent/21.4.395. [DOI] [PubMed] [Google Scholar]
- Epstein J., Field H., Luby S., Pulliam J., Daszak P. Nipah virus: impact, origins, and causes of emergence. Curr. Infect. Dis. Rep. 2006;8:59–65. doi: 10.1007/s11908-006-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J.H., Field H.E., Luby S., Pulliam J.R.C., Daszak P. Nipah virus: impact, origins, and causes of emergence. Curr. Infect. Dis. Rep. 2006;8:59–65. doi: 10.1007/s11908-006-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essey M.A., Payne R.L., Himes E.M., Luchsinger D. Bovine tuberculosis surveys of axis deer and feral swine on the Hawaiian island of Molokai. Proc. U.S. Anim. Health Assoc. 1981;85:538–549. [Google Scholar]
- FAO, OIE, WHO, UNICEF, The World Bank . 2008. Contributing to One World, One Health. A Strategic Framework for Reducing Risks of Infectious Diseases at the Animal–Human–Ecosystem Interface. 68 pp. [Google Scholar]
- Ferguson N.M., Fraser C., Donnelly C.A., Ghani A.C., Anderson R.M. Public health risk from the avian H5N1 influenza epidemic. Science. 2004;304:968–969. doi: 10.1126/science.1096898. [DOI] [PubMed] [Google Scholar]
- Galindo R.C., Muñoz P.M., de Miguel M.J., Marin C.M., Labairu J., Revilla M., Blasco J.M., Gortazar C., de la Fuente J. Gene expression changes in spleens of the wildlife reservoir species, Eurasian wild boar (Sus scrofa), naturally infected with Brucella suis biovar 2. J. Genet. Genom. 2010;37:725–736. doi: 10.1016/S1673-8527(09)60090-4. [DOI] [PubMed] [Google Scholar]
- Gortázar C., Ferroglio E., Höfle U., Frölich K., Vicente J. Diseases shared between wildlife and livestock: a European perspective. Eur. J. Wildl. Res. 2007;53:241–256. [Google Scholar]
- Graham O., Hourrigan J. Eradication programs for the arthropod parasites of livestock. J. Med. Entomol. 1977;13:629–658. doi: 10.1093/jmedent/13.6.629. [DOI] [PubMed] [Google Scholar]
- Greig A., Stevenson K., Perez V., Pirie A.A., Grant J.M., Sharp J.M. Paratuberculosis in wild rabbits (Oryctolagus cuniculus) Vet. Rec. 1997;140:141–143. doi: 10.1136/vr.140.6.141. [DOI] [PubMed] [Google Scholar]
- Hahn E.C., Page G.R., Hahn P.S., Gillis K.D., Romero C., Annelli J.A., Gibbs E.P.J. Mechanisms of transmission of Aujeszky's disease virus originating from feral swine in the United States. Vet. Microbiol. 1997;55:123–130. doi: 10.1016/s0378-1135(96)01309-0. [DOI] [PubMed] [Google Scholar]
- Hamir A.N., Cutlip R.C., Miller J.M., Williams E.S., Stack M.J., Miller M.W., O’Rourke K.I., Chaplin M.J. Preliminary findings on the experimental transmission of chronic wasting disease agent of mule deer to cattle. J. Vet. Diagn. Invest. 2001;13:91–96. doi: 10.1177/104063870101300121. [DOI] [PubMed] [Google Scholar]
- Hartley M., Gill E. Assessment and mitigation processes for disease risks associated with wildlife management and conservation interventions. Vet. Rec. 2010;166:487–490. doi: 10.1136/vr.c2051. [DOI] [PubMed] [Google Scholar]
- Hartley M., Lysons R. Development of the England Wildlife Health Strategy—a framework for decision makers. Vet. Rec. 2011;168:158–164. doi: 10.1136/vr.c4401. [DOI] [PubMed] [Google Scholar]
- Hermoso de Mendoza J.P.A., Tato A., Alonso J.M., Rey J.M., Peña J., García-Sánchez A., Larrasa J., Teixidó J., Manzano G., Cerrato R., Pereira G., Fernández-Llario P., Hermoso de Mendoza M. Bovine tuberculosis in wild boar (Sus scrofa), red deer (Cervus elaphus) and cattle (Bos taurus) in a Mediterranean ecosystem (1992–2004) Prev. Vet. Med. 2006;74:239–247. doi: 10.1016/j.prevetmed.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Hoff G.L., Trainer D.O. Bluetongue and epizootic hemorrhagic disease viruses: their relationship to wildlife species. Adv. Vet. Sci. Comp. Med. 1978;22:111–132. [PubMed] [Google Scholar]
- Horan R.D., Wolf C.A. The economics of managing infectious wildlife disease. Am. J. Agric Econ. 2005;87:537–551. [Google Scholar]
- Hugh-Jones M., De Vos V. Anthrax and wildlife. Rev. Sci. Tech. (Int. Off. Epizoot.) 2002;21:359. doi: 10.20506/rst.21.2.1336. [DOI] [PubMed] [Google Scholar]
- Jellison W.L., Parker R.R. Rodents, rabbits and tularemia in North America: some zoological and epidemiological considerations. Am. J. Trop. Med. Hygiene. 1945;s1–25:349–362. [Google Scholar]
- Joly D.O., Messier F. The effect of bovine tuberculosis and brucellosis on reproduction and survival of wood bison in Wood Buffalo National Park. J. Anim. Ecol. 2005;74:543–551. [Google Scholar]
- Jonassen C.M., Kofstad T., Larsen I.-L., Lovland A., Handeland K., Follestad A., Lillehaug A. Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos) J. Gen. Virol. 2005;86:1597–1607. doi: 10.1099/vir.0.80927-0. [DOI] [PubMed] [Google Scholar]
- Jones K., Patel N., Levy M., Storeygard A., Balk D., Gittleman J., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg F.E., Kistner T.P., Strickland R.K., Gerrish R.R. Arthropod parasites collected from white-tailed deer. J. Med. Entomol. 1971;8:495–498. doi: 10.1093/jmedent/8.5.495. [DOI] [PubMed] [Google Scholar]
- Kendall W.L. Island Press; Washington, DC: 2001. Using Models to Facilitate Complex Decisions. Modeling in Natural Resource Management. pp. 147–170. [Google Scholar]
- King L.J., Anderson L.R., Blackmore C.G., Blackwell M.J., Lautner E.A., Marcus L.C., Meyer T.E., Monath T.P., Nave J.E., Ohle J. Executive summary of the AVMA One Health Initiative Task Force report. J. Am. Vet. Med. Assoc. 2008;233:259–261. doi: 10.2460/javma.233.2.259. [DOI] [PubMed] [Google Scholar]
- Kinyili J., Thorsen J. Antigenic comparisons between herpesviruses isolated from fallow deer in Alberta and the viruses of infectious bovine rhinotracheitis, equine rhinopneumonitis and DN-599, a non-IBR bovine herpesvirus. J. Wildl. Dis. 1979;15:339–341. doi: 10.7589/0090-3558-15.2.339. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C., Kanitz C., McCrocklin S. Possible role of wild mammals in transmission of pseudorabies to swine. J. Wildl. Dis. 1980;16:601–614. doi: 10.7589/0090-3558-16.4.601. [DOI] [PubMed] [Google Scholar]
- Komar N., Dohm D.J., Turell M.J., Spielman A. Eastern equine encephalitis virus in birds: relative competence of European starlings (Sturnus vulgaris) Am. J. Trop. Med. Hygiene. 1999;60:387–391. doi: 10.4269/ajtmh.1999.60.387. [DOI] [PubMed] [Google Scholar]
- Krebs J., Williams S., Smith J., Rupprecht C., Childs J. Rabies among infrequently reported mammalian carnivores in the United States, 1960–2000. J. Wildl. Dis. 2003;39:253–261. doi: 10.7589/0090-3558-39.2.253. [DOI] [PubMed] [Google Scholar]
- Kuttler K. Anaplasma infections in wild and domestic ruminants: a review. J. Wildl. Dis. 1984;20:12–20. doi: 10.7589/0090-3558-20.1.12. [DOI] [PubMed] [Google Scholar]
- Lavigne J. Where the buffalo roam: boundaries and the politics of scale in the yellowstone region. GeoJournal. 2002;58:285–292. [Google Scholar]
- Leiby P.D., Carney W.P., Woods C.E. Studies on Sylvatic echinococcosis. III. Host occurrence and geographic distribution of Echinococcus multilocularis in the North Central United States. J. Parasitol. 1970;56:1141–1150. [PubMed] [Google Scholar]
- Leighton F.A., Wobeser G.A., Barker I.K., Daoust P.Y., Martineau D. The Canadian Cooperative Wildlife Health Centre and surveillance of wild animal diseases in Canada. Can. Vet. J. 1997;38:279–284. [PMC free article] [PubMed] [Google Scholar]
- León A.A.P.d., Strickman D.A., Knowles D.P., Fish D., Thacker E., Fuente J.d.l., Krause P.J., Wikel S.K., Miller R.S., Wagner G.G., Almazán C., Hillman R., Messenger M.T., Ugstad P.O., Duhaime R.A., Teel P.D., Ortega-Santos A., Hewitt D.G., Bowers E.J., Bent S.J., Cochran M.H., McElwain T.F., Scoles G.A., Suarez C.E., Davey R., Freeman J.M.H., Lohmeyer K., Li A.Y., Guerrero F.D., Kammlah D.M., Phillips P., Pound J.M. One Health approach to identify research needs in bovine and human babesioses: workshop report. Parasites Vectors. 2010;3:12. doi: 10.1186/1756-3305-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts G.A. Feral animals in the Northern Territory. Aust. Vet. J. 1964;40:84–88. [Google Scholar]
- Ley D.H., Berkhoff J.E., McLaren J.M. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis. 1996;40:480–483. [PubMed] [Google Scholar]
- Loomis J. Columbia University Press; New York: 2002. Integrated Public Lands Management: Principles and Applications to National Forests, Parks, Wildlife Refuges, and BLM Lands. [Google Scholar]
- Luttrell M.P., Stallknecht D.E., Kleven S.H., Kavanaugh D.M., Corn J.L., Fischer J.R. Mycoplasma gallisepticum in house finches (Carpodacus mexicanus) and other wild birds associated with poultry production facilities. Avian Dis. 2001;45:321–329. [PubMed] [Google Scholar]
- Lysons R., Gibbens J., Smith L. Progress with enhancing veterinary surveillance in the United Kingdom. Vet. Rec. 2007;160:105–112. doi: 10.1136/vr.160.4.105. [DOI] [PubMed] [Google Scholar]
- Martinez M., Machado M., Nascimento C., Silva M., Teodoro R., Furlong J., Prata M., Campos A., Guimaraes M., Azevedo A. Association of BoLA-DRB3. 2 alleles with tick (Boophilus microplus) resistance in cattle. Genet. Mol. Res. 2006;5:513–524. [PubMed] [Google Scholar]
- McBeth M.K., Shanahan E.A. Public opinion for sale: the role of policy marketers in Greater Yellowstone policy conflict. Policy Sci. 2004;37:319–338. [Google Scholar]
- McCarthy M.A., Possingham H.P. Active adaptive management for conservation. Conserv. Biol. 2007;21:956–963. doi: 10.1111/j.1523-1739.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- Meagher M., Meyer M. On the origin of brucellosis in bison of Yellowstone National Park: a review. Conserv. Biol. 1994;8:645–653. [Google Scholar]
- Medrano C., Boadella M., Barrios H., Cantú A., García Z., de la Fuente J., Gortazar C. Zoonotic pathogens among white-tailed deer, Northern Mexico, 2004–2009. Emerg. Infect. Dis. 2012;18:1372–1374. doi: 10.3201/eid1808.111902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M.I., Cox N.J., Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg. Infect. Dis. 1999;5:659–671. doi: 10.3201/eid0505.990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins J., Schlater J.L., Corn J.L. Ectoparasites of the blackbuck antelope (Antilope cervicapra) J. Wildl. Dis. 1992;28:481–484. doi: 10.7589/0090-3558-28.3.481. [DOI] [PubMed] [Google Scholar]
- Meyer M.E., Meagher M. Brucellosis in free-ranging bison (Bison bison) in Yellowstone, Grand Teton, and Wood Buffalo National Parks: a review. J. Wildl. Dis. 1995;31:579–598. doi: 10.7589/0090-3558-31.4.579. [DOI] [PubMed] [Google Scholar]
- Morner T. The ecology of tularemia. Rev. Sci. Tech. 1992;11:1123–1130. [PubMed] [Google Scholar]
- Morner T., Obendorf D., Artois M., Woodford M. Surveillance and monitoring of wildlife diseases. Rev. Sci. Tech. (Off. Int. Épizoot.) 2002;21:67–76. doi: 10.20506/rst.21.1.1321. [DOI] [PubMed] [Google Scholar]
- Mörner T., Obendorf D., Artois M., Woodford M. Surveillance and monitoring of wildlife diseases. Rev. Sci. Tech. (Off. Int. Epizoot.) 2002;21:67–76. doi: 10.20506/rst.21.1.1321. [DOI] [PubMed] [Google Scholar]
- Munster V.J., Baas C., Lexmond P., Waldenström J., Wallensten A., Fransson T., Rimmelzwaan G.F., Beyer W.E.P., Schutten M., Olsen B., Osterhaus A.D.M.E., Fouchier R.A.M. Spatial, temporal, and species variation in prevalence of influenza a viruses in wild migratory birds. PLoS Pathog. 2007;3:e61. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradrasoli S., Bálint Á., Wahlgren J., Waldenström J., Belák S., Blomberg J., Olsen B. Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait Area (Beringia) PLoS ONE. 2010;5:e13640. doi: 10.1371/journal.pone.0013640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell K.D., Stringfellow F., Dame J.B., Leiby D.A., Duffy C., Schad G.A. Trichinella spiralis in an agricultural ecosystem. II. Evidence for natural transmission of Trichinella spiralis spiralis from domestic swine to wildlife. J. Parasitol. 1987;73:103–109. [PubMed] [Google Scholar]
- Naranjo V., Gortazar C., Vicente J., Fuente J.d.l. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008;127:1–9. doi: 10.1016/j.vetmic.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nishi J., Stephen C., Elkin B. Implications of agricultural and wildlife policy on management and eradication of bovine tuberculosis and brucellosis in free-ranging wood bison of northern Canada. Ann. N. Y. Acad. Sci. 2002;969:236–244. doi: 10.1111/j.1749-6632.2002.tb04385.x. [DOI] [PubMed] [Google Scholar]
- O’Brien D.J., Schmitt S.M., Fitzgerald S.D., Berry D.E. Management of bovine tuberculosis in Michigan wildlife: current status and near term prospects. Vet. Microbiol. 2011;151:179–187. doi: 10.1016/j.vetmic.2011.02.042. [DOI] [PubMed] [Google Scholar]
- O’Brien D.J., Schmitt S.M., Fitzgerald S.D., Berry D.E., Hickling G.J. Managing the wildlife reservoir of Mycobacterium bovis: the Michigan, USA, experience. Vet. Microbiol. 2006;112:313–323. doi: 10.1016/j.vetmic.2005.11.014. [DOI] [PubMed] [Google Scholar]
- O’Reilly L.M., Daborn C.J. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber. Lung Dis. 1995;76:1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- Olsen B., Munster V.J., Wallensten A., Waldenström J., Osterhaus A.D.M.E., Fouchier R.A.M. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Olsen S. Brucellosis in the United States: role and significance of wildlife reservoirs. Vaccine. 2010;28:F73–F76. doi: 10.1016/j.vaccine.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Olsen S.C. Brucellosis in the United States: role and significance of wildlife reservoirs. Vaccine. 2010;28:F73–F76. doi: 10.1016/j.vaccine.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Ostfeld R., Holt R. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front. Ecol. Environ. 2004;2:13–20. [Google Scholar]
- Palmer M.V. Tuberculosis: a reemerging disease at the interface of domestic animals and wildlife. In: Childs J.E., Machenzie J.S., Richt J.A., editors. Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. Springer; New York: 2007. pp. 195–215. [Google Scholar]
- Passler T., Walz P.H., Ditchkoff S.S., Givens M.D., Maxwell H.S., Brock K.V. Experimental persistent infection with bovine viral diarrhea virus in white-tailed deer. Vet. Microbiol. 2007;122:350–356. doi: 10.1016/j.vetmic.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Patz J.A., Daszak P., Tabor G.M., Aguirre A.A., Pearl M., Epstein J., Wolfe N.D., Kilpatrick A.M., Foufopoulos J., Molyneux D. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K., John A. Baroch, Dale L. Nolte, Thomas Gidlewski, Thomas. J. Deliberto, 2012. The role of the National Wildlife Disease Program in wildlife disease surveillance and emergency response. Proceedings of the 14th Wildlife Damage Management Conference, 74-80.
- Petersen K.D., Christensen J.P., Permin A., Bisgaard M. Virulence of Pasteurella multocida subsp. multocida isolated from outbreaks of fowl cholera in wild birds for domestic poultry and game birds. Avian Pathol. 2001;30:27–31. doi: 10.1080/03079450020023168. [DOI] [PubMed] [Google Scholar]
- Pound J., George J.E., Kammlah D.M., Lohmeyer K.H., Davey R.B. Evidence for role of white-tailed deer (Artiodactyla: Cervidae) in epizootology of cattle ticks and southern cattle ticks (Acari: Ixodidae) in reinfestations along the Texas/Mexico border in South Texas: a review and update. J. Econ. Entomol. 2010;103:211–218. doi: 10.1603/EC09359. [DOI] [PubMed] [Google Scholar]
- Ramsey D.S., O’Brien D.J., Cosgrove M.K., Schmitt S.S., Rudolph B.A. Management of bovine tuberculosis in free-ranging Michigan white-tailed deer: predictions from a new spatially-explicit model. 60th Annual International Conference of the Wildlife Disease Association; The Wildlife Disease Association, Quebec City, Canada; 2011. [Google Scholar]
- Reisen W.K., Lundstrom J.O., Scott T.W., Eldridge B.F., Chiles R.E., Cusack R., Martinez V.M., Lothrop H.D., Gutierrez D., Wright S.E., Boyce K., Hill B.R. Patterns of avian seroprevalence to western equine encephalomyelitis and Saint Louis encephalitis viruses in California, USA. J. Med. Entomol. 2000;37:507–527. doi: 10.1603/0022-2585-37.4.507. [DOI] [PubMed] [Google Scholar]
- Rhyan J., Spraker T. Emergence of diseases from wildlife reservoirs. Vet. Pathol. Online. 2010;47:34–39. doi: 10.1177/0300985809354466. [DOI] [PubMed] [Google Scholar]
- Ridler A.L., West D.M. Effects of Brucella ovis infection on semen characteristics of 16-month-old red deer stags. N. Z. Vet. J. 2002;50:19–22. doi: 10.1080/00480169.2002.36244. [DOI] [PubMed] [Google Scholar]
- Riley S., Fraser C., Donnelly C.A., Ghani A.C., Abu-Raddad L.J., Hedley A.J., Leung G.M., Ho L.M., Lam T.H., Thach T.Q. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–1966. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- Robinson R.M., Hailey T.L., Livingston C.W., Thomas J.W. Bluetongue in the desert bighorn sheep. J. Wildl. Manage. 1967;31:165–168. [Google Scholar]
- Saif Y., Barnes H. Blackwell Pub.; Ames, IA: 2008. Diseases of Poultry. pp. 452–514. [Google Scholar]
- Sainsbury A., Bennett P., Cunningham A., Kirkwood J. Status of wildlife health monitoring in the United Kingdom. Vet. Rec. 2001;148:558–563. doi: 10.1136/vr.148.18.558. [DOI] [PubMed] [Google Scholar]
- Salkeld D., Stapp P. Seroprevalence rates and transmission of plague (Yersinia pestis) in mammalian carnivores. Vector-Borne Zoonot. Dis. 2006;6:231–239. doi: 10.1089/vbz.2006.6.231. [DOI] [PubMed] [Google Scholar]
- Schmitt S.M., Cooley T.M., Fitzgerald S.D., Bolin S.R., Lim A., Schaefer S.M., Kiupel M., Maes R.K., Hogle S.A., O’Brien D.J. An outbreak of eastern equine encephalitis virus in free-ranging white-tailed deer in Michigan. J. Wildl. Dis. 2007;43:635–644. doi: 10.7589/0090-3558-43.4.635. [DOI] [PubMed] [Google Scholar]
- Schoelkopf L., Hutchinson C.E., Bendele K.G., Goff W.L., Willette M., Rasmussen J.M., Holman P.J. New ruminant hosts and wider geographic range identified for Babesia odocoilei. J. Wildl. Dis. 2005;41:683–690. doi: 10.7589/0090-3558-41.4.683. [DOI] [PubMed] [Google Scholar]
- Seal B.S., King D.J., Sellers H.S. The avian response to Newcastle disease virus. Dev. Comp. Immunol. 2000;24:257–268. doi: 10.1016/s0145-305x(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Serraino A.M.G., Sanguinetti V., Ross M.C., Zanoni R.G., Catozzi L., Bandera A., Dini W., Mignone W., Franzetti F., Gori A. Monitoring transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. J. Clin. Microbiol. 1999;37:2766–2771. doi: 10.1128/jcm.37.9.2766-2771.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad H.L. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 2000;2:405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- Siembieda J., Kock R., McCracken T., Newman S. The role of wildlife in transboundary animal diseases. Anim. Health Res. Rev. 2011;12:95–111. doi: 10.1017/S1466252311000041. [DOI] [PubMed] [Google Scholar]
- Spickler A.R., Roth J.A., Galyon J., Lofstedt J., editors. Emerging and Exotic Diseases of Animals. Center for Food Security and Public Health and the Institute for International Cooperation in Animal Biologics, Iowa State University, College of Veterinary Medicine; Ames, IA: 2010. [Google Scholar]
- Stallknecht D., Blue J., Rollor E.A., 3rd, Nettles V., Davidson W., Pearson J. Precipitating antibodies to epizootic hemorrhagic disease and bluetongue viruses in white-tailed deer in the southeastern United States. J. Wildl. Dis. 1991;27:238–247. doi: 10.7589/0090-3558-27.2.238. [DOI] [PubMed] [Google Scholar]
- Sterner R.T., Meltzer M.I., Shwiff S.A., Slate D. Tactics and economics of wildlife oral rabies vaccination, Canada and the United States. Emerg. Infect. Dis. 2009;15:1176–1184. doi: 10.3201/eid1508.081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner R.T., Smith G.C. Modelling wildlife rabies: transmission, economics, and conservation. Biol. Conserv. 2006;131:163–179. [Google Scholar]
- Stitt T., Mountifield J., Stephen C. Opportunities and obstacles to collecting wildlife disease data for public health purposes: results of a pilot study on Vancouver Island, British Columbia. Can. Vet. J. 2007;48:83–90. doi: 10.4141/cjas68-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt S.T., Kazacos K.R. Echinococcus multilocularis identified in Indiana, Ohio, and East-central Illinois. J. Parasitol. 1993;79:301–305. [PubMed] [Google Scholar]
- Storandt S.T., Virchow D.R., Dryden M.W., Hygnstrom S.E., Kazacos K.R. Distribution and prevalence of Echinococcus multilocularis in Wild predators in Nebraska, Kansas, and Wyoming. J. Parasitol. 2002;88:420–422. doi: 10.1645/0022-3395(2002)088[0420:DAPOEM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Suter G.W. CRC Press; Boca Raton, FL: 2007. Ecological Risk Assessment. [Google Scholar]
- Sweeney S.J., Miller R.S. Free-ranging wildlife. In: Portacci K., Lombard J., editors. Assessment of Pathways for the Introduction and Spread of Mycobacterium bovis in the United States. United States Department of Agriculture, Animal Plant Health Inspections Service; Fort Collins, CO: 2010. pp. 94–123. [Google Scholar]
- Tate C.M., Howerth E.W., Stallknecht D.E., Allison A.B., Fischer J.R., Mead D.G. Eastern equine encephalitis in a free-ranging white-tailed deer (Odocoileus virginianus) J. Wildl. Dis. 2005;41:241–245. doi: 10.7589/0090-3558-41.1.241. [DOI] [PubMed] [Google Scholar]
- Taylor L., Latham S. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessaro S., Forbes L., Turcotte C. A survey of brucellosis and tuberculosis in bison in and around Wood Buffalo National Park, Canada. Can. Vet. J. 1990;31:174–180. [PMC free article] [PubMed] [Google Scholar]
- Tessaro S.V. The existing and potential importance of brucellosis and tuberculosis in Canadian wildlife: a review. Can. Vet. J. 1986;27:119–122. [PMC free article] [PubMed] [Google Scholar]
- Thirgood S. New perspectives on managing wildlife diseases. J. Appl. Ecol. 2009;46:454–456. [Google Scholar]
- Thomas N.J., Hunter D.B., Atkinson C.T. Blackwell Pub.; 2007. Infectious Diseases of Wild Birds. [Google Scholar]
- Thompson R.C.A., Boxell A.C., Ralston B.J., Constantine C.C., Hobbs R.P., Shury T., Olson M.E. Molecular and morphological characterization of Echinococcus in cervids from North America. Parasitology. 2006;132:439–447. doi: 10.1017/S0031182005009170. [DOI] [PubMed] [Google Scholar]
- Valas S., Benoit C., Guionaud C., Perrin G., Mamoun R.Z. North American and French Caprine arthritis-encephalitis viruses emerge from ovine Maedi-Visna viruses. Virology. 1997;237:307–318. doi: 10.1006/viro.1997.8800. [DOI] [PubMed] [Google Scholar]
- Vanrompay D., Ducatelle R., Haesebrouck F. Chlamydia psittaci infections: a review with emphasis on avian chlamydiosis. Vet. Microbiol. 1995;45:93–119. doi: 10.1016/0378-1135(95)00033-7. [DOI] [PubMed] [Google Scholar]
- Verbisck-Bucker G., Gonzalez-Candela M., Galian J., Cubero-Pablo M.J., Martin-Atance P., Leon-Vizcaino L. Epidemiology of Mycoplasma agalactiae infection in free-ranging Spanish ibex (Capra Pyrenaica) in Andalusia, Southern Spain. J. Wildl. Dis. 2008;44:369–380. doi: 10.7589/0090-3558-44.2.369. [DOI] [PubMed] [Google Scholar]
- VerCauteren K., Gehring T., Landry J. The dynamic role of livestock protection dogs in a changing world. J. Vet. Behav.: Clin. Appl. Res. 2010;6:73–74. [Google Scholar]
- VerCauteren K., Gilsdorf J., Hygnstrom S., Fioranelli P., Wilson J., Barras S. Green and blue lasers are ineffective for dispersing deer at night. Wildl. Soc. Bull. 2006;34:371–374. [Google Scholar]
- VerCauteren K., Lavelle M., Hygnstrom S. Fences and deer-damage management: a review of designs and efficacy. Wildl. Soc. Bull. 2006;34:191–200. [Google Scholar]
- VerCauteren K., Lavelle M., Phillips G. Livestock protection dogs for deterring deer from cattle and feed. J. Wildl. Manage. 2008;72:1443–1448. [Google Scholar]
- VerCauteren K., Seward N., Lavelle M., Fischer J., Phillips G. A fence design for excluding elk without impeding other wildlife. Rangeland Ecol. Manage. 2007;60:529–532. [Google Scholar]
- VerCauteren K., Shivik J., Lavelle M. Efficacy of an animal-activated frightening device on urban elk and mule deer. Wildl. Soc. Bull. 2005;33:1282–1287. [Google Scholar]
- Wasserberg G., Osnas E.E., Rolley R.E., Samuel M.D. Host culling as an adaptive management tool for chronic wasting disease in white tailed deer: a modelling study. J. Appl. Ecol. 2009;46:457–466. doi: 10.1111/j.1365-2664.2008.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P., McLean R., Smith G., Ellenberger J., Francy D., Walton T., Monath T. Epizootic vesicular stomatitis in Colorado, 1982: some observations on the possible role of wildlife populations in an enzootic maintenance cycle. J. Wildl. Dis. 1987;23:192–198. doi: 10.7589/0090-3558-23.2.192. [DOI] [PubMed] [Google Scholar]
- Welburn S. One Health: the 21st century challenge. Vet. Rec. 2011;168:614–615. doi: 10.1136/vr.d3528. [DOI] [PubMed] [Google Scholar]
- White N., Sutherst R.W., Hall N., Whish-Wilson P. The vulnerability of the Australian beef industry to impacts of the cattle tick (Boophilus microplus) under climate change. Clim. Change. 2003;61:157–190. [Google Scholar]
- Williams E.S. Chronic wasting disease. Vet. Pathol. Online. 2005;42:530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- Williams E.S., Barker I.K. Iowa State University Press; Ames, IA: 2001. Infectious Diseases of Wild Mammals. [Google Scholar]
- Williams E.S., Miller M.W., Kreeger T.J., Kahn R.H., Thorne E.T. Chronic wasting disease of deer and elk: a review with recommendations for management. J. Wildl. Manage. 2002;66:551–563. [Google Scholar]
- Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010;167:108–122. doi: 10.1016/j.vetpar.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Woodroffe R., Donnelly C.A., Cox D., Gilks P., Jenkins H.E., Johnston W.T., Le Fevre A.M., Bourne F.J., Cheeseman C., Clifton-Hadley R.S. Bovine tuberculosis in cattle and badgers in localized culling areas. J. Wildl. Dis. 2009;45:128–143. doi: 10.7589/0090-3558-45.1.128. [DOI] [PubMed] [Google Scholar]
- Zarnke R. Serologic survey for selected microbial pathogens in Alaskan wildlife. J. Wildl. Dis. 1983;19:324–329. doi: 10.7589/0090-3558-19.4.324. [DOI] [PubMed] [Google Scholar]


