Abstract
The antiproliferative effects of bestatin and actinonin on U937 and K562 cells have been compared with their inhibitory activity on cell surface aminopeptidases. The results strongly suggest that the inhibition of cell surface aminopeptidases cannot be the main reason for the inhibition of cell proliferation. This was confirmed by studying the effect of buthionine sulfoximine (BSO), MK-571 (3-([{3-(2-[7-chloro-2-quinolinyl]-ethenyl)-phenyl}-{(3-dimethyl-amino-3-oxopropyl)-thio}-methyl]thio)propanoic acid) and verapamil on the inhibition of cell proliferation by bestatin and actinonin. BSO and MK-571, which inhibit the efflux of drugs mediated by multidrug resistance-associated protein (MRP), increased the action of both inhibitors, indicating that the latter enter the cells and that their export is mediated by MRP in both cell lines. Verapamil significantly increased the inhibitory activity of bestatin on K562 cells, indicating that the intracellular concentration of bestatin can be mediated also by P-glycoprotein.
Keywords: Bestatin, Actinonin, Cell proliferation, U937, K562, Multidrug resistance-associated protein
1. Introduction
Bestatin, a dipeptide synthesized by Streptomyces olivoreti [1], has diverse antitumor and immunomodulatory activities [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. It has been used in clinical trials for the treatment of leukemia [13], [14] and was proposed to be useful as an immunological adjuvant for DNA vaccination against HIV-1 [15].
The mechanism of action of bestatin is not known. Muller and co-workers [16] were the first to prove that it binds to cell surface proteins and suggested that its biological activity is the consequence of its inhibition of cell surface leucine aminopeptidase [17]. Aminopeptidase W [18] and aminopeptidase N (APN) [5] were proposed to be the key aminopeptidases responsible for the biological effects of aminopeptidase inhibitors. Additional support for the idea that APN is involved in regulating cell proliferation is provided by the facts that increased expression of APN is associated with increased proliferation of activated T cells [19] and CD34+ cord blood cells [20], and that APN antisense RNA inhibits cell proliferation of H9 and U937 cells [21]. On the other hand the sensitivity of different cell lines to bestatin does not correlate with the expression of APN [11]. Furthermore, amastatin, an inhibitor of APN [8], and antibodies against APN that inhibit its enzyme activity did not inhibit cell proliferation, and anti-APN antibodies also did not hinder the inhibitory effect of another inhibitor of APN, actinonin, on cell proliferation [22]. These findings, supported by the fact that bestatin methyl ester, a hydrophobic derivative of bestatin, induces apoptosis at lower concentrations than bestatin [11], suggest that aminopeptidase inhibitors inhibit cell proliferation by intracellular activity.
In our study we sought to elucidate the role of inhibition of cell surface aminopeptidases in the antiproliferative activity of bestatin, by quantitative comparison of these two activities on U937 and K562 cells. For comparison we used actinonin, another inhibitor of aminopeptidase N [23], [24] that has also been shown to inhibit cell proliferation [5], [19], [20], [22].
Since we found here that aminopeptidase inhibitors could not inhibit cell proliferation solely by inhibiting cell surface aminopeptidases we tested the hypothesis that actinonin and bestatin act intracellularly. We measured the antiproliferative effect of these inhibitors on two leukemia cell lines in the presence of drug efflux modifiers. K562 and U937 cells have been shown to express multidrug resistance-associated protein (MRP) and P-glycoprotein (Pgp) [25], [26], [27], both of which belong to the group of transporter proteins known to decrease intracellular drug accumulation and to cause drug resistance of cancer cells (reviewed in Ref. [28]). As drug efflux modifiers we used buthionine sulfoximine (BSO), an inhibitor of glutathione synthesis, that impairs the action of MRP, a glutathione-dependent drug efflux pump [29], [30], MK-571 (3-([{3-(2-[7-chloro-2-quinolinyl]-ethenyl)-phenyl}-{(3-dimethyl-amino-3-oxopropyl)-thio}-methyl]thio)propanoic acid), a leukotriene receptor antagonist that also inhibits MRP-dependent transport [31] and verapamil, a calcium channel blocker that impairs the activity of Pgp [32]. If bestatin and actinonin inhibit cell proliferation by intracellular activity, inhibitors of their efflux would be expected to increase their effect on K562 and U937 cells.
2. Materials and methods
2.1. Materials
Bestatin, actinonin, verapamil and BSO were purchased from Sigma Chemical Co. (USA), substrates l-alanine-4-methyl-coumaryl-7-amide (AlaMCA), l-leucine-4-methyl-coumaryl-7-amide (LeuMCA), and l-arginine-4-methyl-coumaryl-7-amide (ArgMCA) from Bachem (Switzerland), and MK-571 from Biomol Research Laboratories, USA.
Human histiocytic lymphoma cell line U937 [33] and human myelogenous leukemia cell line K562 were obtained from ATCC, Rockville, MD, USA. Cells were cultured in RPMI 1640 medium (Flow Laboratories Inc.,) supplemented with 10% fetal bovine serum (South HyClone Road, Logan, UT, USA) and with 2 mM glutamine (Gibco Ltd, UK) and incubated at 37 °C in humidified 5% CO2 atmosphere.
2.2. Cell growth assay
Cells were suspended at a final concentration of 5×104 cells/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum and with 2 mM glutamine, and seeded in 24-well culture plates. Various concentrations of aminopeptidase inhibitors were then added to each well and the plates were incubated for 5 days at 37 °C in a humidified 5% CO2 atmosphere. Cells were counted on the 3rd, 4th and 5th days with a hemocytometer. Cell viability was estimated by staining cells in trypan blue solution. The concentrations of inhibitors in culture media were monitored by measuring the inhibitory activity of the medium on aminopeptidase N. The inhibitory activities remained constant throughout the experiments. The concentrations of inhibitors that caused 50% inhibition of cell proliferation (IC50p) were determined from a plot of inhibition of cell proliferation against inhibitor concentration.
The effect of BSO on the antiproliferative activity of bestatin and actinonin was tested by pre-incubating the cells in the presence of 25 μM BSO for 20 h before adding inhibitors. MK-571 and verapamil were added to cell suspensions at the same time as aminopeptidase inhibitors. The final concentrations of inhibitors in cell cultures were 145 μM bestatin and 13 μM actinonin in the experiment with U937 cells and 580 μM bestatin and 26 μM actinonin in the experiment with K562 cells. The cells were counted on the third, fourth and fifth days after the addition of aminopeptidase inhibitors.
2.3. Determination of extracellular aminopeptidase activity
Cells were rinsed twice with ice cold phosphate-buffered saline (PBS) and suspended in PBS with 0.001 M Ca2+ and 0.001M Mg2+ ions. Cell suspensions were preincubated with and without each inhibitor for 1 h at room temperature. After the addition of substrate to a final concentration of 10−5 M and incubating at 37 °C for 10 min, the reaction was stopped by centrifugation at 200×g for 10 min. The concentration of released products was measured in the supernatants at 370/460 nm with a fluorescence spectrometer. The concentration of inhibitors that caused 50% inhibition of aminopeptidase activity (IC50i) was determined from a plot of the enzyme activity against concentration of inhibitor.
Aminopeptidase activity in the culture media was determined on 0.1 ml of media obtained after the centrifugation of cell cultures with densities 8×105 cells/ml.
3. Results and discussion
3.1. IC50 for the inhibition of proliferation by bestatin and actinonin (IC50p)
IC50p values for bestatin on U937 cells reported in the literature are 58 μM [3], about 150 μM [6], [12] and 8 μM [11]. Because of these large differences we determined IC50p values for bestatin and actinonin in our experimental system. IC50p values determined on the 3rd day of the experiment are listed in Table 1 . IC50p for actinonin is similar to values determined for other leukemia cell lines [22]. IC50p for bestatin on U937 cells is within the range of the values obtained previously, but that for bestatin on K562 cells is ten times higher than the value reported by Sekine and co-workers [11].
Table 1.
Inhibitory activity of bestatin and actinonin on cell proliferation and cell surface aminopeptidases
| IC50 (μM) | Substrate | Inhibitor and cell line |
|||
|---|---|---|---|---|---|
| Bestatin |
Bestatin |
Actinonin |
Actinonin |
||
| U937 | K562 | U937 | K562 | ||
| IC50p | 108 | 466 | 11 | 19 | |
| IC50i | AlaMCA | 2.47 | 2.2 | 0.16 | 1.46 |
| IC50p/IC50i | AlaMCA | 44 | 212 | 68 | 13 |
| IC50i | LeuMCA | 6.27 | 1.08 | 0.86 | 1.19 |
| IC50p/IC50i | LeuMCA | 17 | 432 | 13 | 16 |
| IC50i | ArgMCA | 0.03 | 0.06 | >25 | >50 |
| IC50p/IC50i | ArgMCA | 3597 | 7768 | – | – |
3.2. Cell surface aminopeptidase activity
Since bestatin is a competitive inhibitor of APN, of other leucine aminopeptidases and of aminopeptidase B [1], and since actinonin competitively inhibits APN [24], we assayed the cell surface aminopeptidase activity on substrates for these enzymes. U937 cells showed stronger activity against AlaMCA while K562 cells were more active against ArgMCA and LeuMCA (Table 2). These results agree with the findings of Bauvois and co-workers [34] who showed that U937 cells exhibit the highest activities on substrates with hydrophobic amino acid residues, and with those of Yamada and co-workers [35] who showed that K562 cells express high levels of aminopeptidase B. Both cell lines have also been shown to express APN [11].
Table 2.
Cell surface aminopeptidase activity
| Substrate | Product (MCA) concentration (nM/103 cells/10 min) |
|
|---|---|---|
| Cell line U937 | Cell line K562 | |
| AlaMCA | 4.54±0.49 | 2.16±0.17 |
| LeuMCA | 3.46±0.47 | 5.08±0.33 |
| ArgMCA | 2.18±0.36 | 6.30±1.04 |
3.3. IC50 values for the inhibition of cell surface aminopeptidases (IC50i)
IC50i values (Table 1) are in the micromolar range and are comparable to the K i constants for bestatin that are 1.4×10−6 M, 6×10−8 M and 2×10−8 M for APN [36], aminopeptidase B and leucine aminopeptidase [1], respectively. K i for the inhibition of APN by actinonin is 1.7×10−7 M [24]. If bestatin and actinonin inhibited cell proliferation only through the inhibition of cell surface aminopeptidases, IC50p values would be similar to the corresponding IC50i. The IC50p values are seen to be much higher and no correlation exists between IC50p and IC50i values for the two inhibitors or the two cell lines (Table 1).
3.4. Soluble aminopeptidase activity
The reason for the high IC50p/IC50i ratios could be a high concentration of soluble aminopeptidases in the cell culture medium. The results shown in Table 3 indicate that most of the activity against AlaMCA and LeuMCA in the culture medium originated from fetal bovine serum, while some of the activity against ArgMCA was released from the cells. The total soluble aminopeptidase activity represents 50–70% of the total extracellular aminopeptidase activity in the cell cultures at the beginning of the experiment and only 10–13% after 4 days. This is not high enough to account for the high IC50p values. Besides, according to the equation IC50=K i(1+[S]/K m)+[E]/2 [37], IC50i would be dependent on enzyme concentration only in the case where the latter was in the region of K i, in our case the μM range, which cannot be expected in cell cultures.
Table 3.
Soluble aminopeptidase activity in cell culture media
| Substrate | Product (MCA) concentration (nM/100 μl/10 min) |
||
|---|---|---|---|
| RPMI+10% FBS | Conditioned medium U937 | Conditioned medium K562 | |
| AlaMCA | 20.9±2.1 | 20.9±1.5 | 22.1±1.5 |
| LeuMCA | 15.4±0.6 | 20.2±1.5 | 27.6±0.6 |
| ArgMCA | 14.9±0.1 | 24.2±0.4 | 46.2±2.0 |
3.5. Effect of BSO, MK-571 and verapamil on the antiproliferative effect of bestatin and actinonin
The results in Table 4 show that BSO, which inhibits MRP-mediated efflux of drugs, increased the antiproliferative effects of both aminopeptidase inhibitors on both cell lines, indicating that both bestatin and actinonin must enter the cells and that their interactions inside the cells are involved in the inhibition of cell proliferation. Zaman and co-workers [30] observed a 50 to 80% decrease of IC50p values for anthracyclines, VP-16 and vincristine in presence of 25 μM BSO, which means a 25–40% higher inhibition of cell proliferation than the value obtained at concentrations corresponding to IC50p. Our results (Table 4) confirm this effect, leading to the conclusion that most of the antiproliferative effect of bestatin and actinonin results from their intracellular action.
Table 4.
Effect of BSO, MK-571 and verapamil on the antiproliferative activities of bestatin and actinonina
| Drug efflux modifier (M) | % of inhibition of cell proliferation |
|||
|---|---|---|---|---|
| MK-571 (10 μM) | MK-571 (30 μM) | BSO (25 μM) | Verapamil (30 μM) | |
| U937+bestatin | 71.0±8.6 | n.d. | 39.8±5.0 | n.d. |
| U937+bestatin+M | 87.4±4.1 | 53.4±4.8 | ||
| P<0.001 | P<0.05 | |||
| U937+actinonin | 69.3±5.1 | n.d. | 32.3±24.3 | n.d. |
| U937+actinonin+M | 82.1±3.4 | 48.4±25.7 | ||
| P<0.0005 | P<0.005 | |||
| K562+bestatin | 47.0±5.4 | 47.0±5.4 | 19.6±16.0 | 44.3±13.5 |
| K562+bestatin+M | 66.0±3.7 | 73.6±3.4 | 41.5±17.0 | 70.5±5.7 |
| P<0.0001 | P<0.0001 | P<0.05 | P<0.005 | |
| K562+actinonin | 76.5±4.2 | 76.5±4.2 | 55.4±7.8 | 71.0±4.2 |
| K562+actinonin+M | 76.7±2.5 | 86.9±3.3 | 77.9±17.5 | 78.0±6.6 |
| P<0.0005 | P<0.05 | P>0.1 | ||
The final concentrations of inhibitors in cell cultures were 145 μM bestatin and 13 μM actinonin in the experiment with U937 cells, and 580 μM bestatin and 26 μM actinonin in the experiment with K562 cells. The antiproliferative effect of BSO, MK-571 and verapamil at the concentrations used were not statistically significant (P>0.05). The measurements were made on the 4th day after the addition of drug efflux modifier. Results are the average of two experiments each performed in quadruplicate. Results were analyzed by paired t-test.
MK-571, an inhibitor of MRP, at 10 μM concentration increased the antiproliferative effects of both aminopeptidase inhibitors on U937 cells and of bestatin on K562 cells. At 30 μM concentration it also increased significantly the effect of actinonin on K562 cells (Table 4). These results further confirm the conclusion that bestatin and actinonin enter the cells and that their intracellular concentration can be modified by MRP-mediated drug efflux.
Verapamil, an inhibitor of drug efflux mediated by Pgp, exhibits an antiproliferative effect on U937 cells at 10 μM concentrations, precluding its use to study any effect on the action of bestatin and actinonin on U937 cells. However, it showed a significant effect on the action of bestatin on K562 cells (Table 4). Furthermore, the IC50p value of bestatin on K562 cells increased significantly with time of incubation of cells in the presence of bestatin (Fig. 1), which can be explained by the induction of Pgp, an effect that has been shown for different anticancer drugs on K562 cells by Chaudhary and Roninson [27]. These results suggest that the intracellular concentration of bestatin can be mediated by Pgp. Verapamil, on the other hand, showed no effect on the action of actinonin (Table 4), suggesting that actinonin is not a good ligand for Pgp-mediated efflux.
Fig. 1.
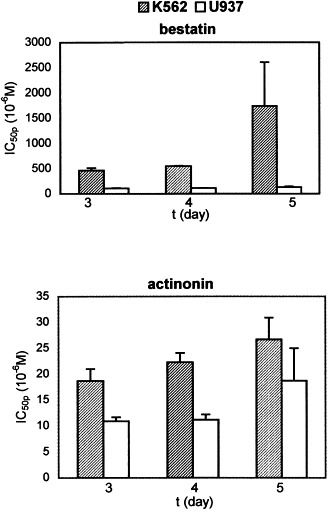
IC50 values for the inhibition of cell proliferation by bestatin and actinonin. K562 cells (shaded) and U937 cells (open) were grown in presence of various concentrations of bestatin or actinonin and were counted on the 3rd, 4th and 5th days. IC50p values were determined from a plot of inhibition of cell proliferation against inhibitor concentration. Results are averages of two independent experiments and standard errors are indicated.
The conclusion that aminopeptidase inhibitors bestatin and actinonin act intracellularly arises the question as to how they enter the cells. Possible explanations could be passive transport through the lipid bilayer, receptor-mediated endocytosis, or transport mediated by special proteins expressed on cell surface. Passive transport is implied by the results of Sekine et al. [11], which showed that a hydrophobic derivative of bestatin induces apoptosis at lower concentrations than bestatin, and also by the long incubation time necessary to detect the antiproliferative effect of aminopeptidase inhibitors. Receptor-mediated endocytosis is indicated by the IC50p values (Table 1), which are lower for both aminopeptidase inhibitors on U937 cells which exhibit higher expression of APN, suggesting that endocytosis of bestatin and actinonin could be mediated by APN. It has already been shown that APN serves as a receptor for coronavirus TGEV [38]. Finally, bestatin and actinonin could enter the cells also by special transporter proteins, as has been shown for HeLa cells transfected with PepT1 [39].
The synergy between inhibitors of drug efflux and bestatin constitutes the first report that leukemia cells can develop resistance to bestatin. The finding that the biological effects of bestatin and actinonin are the result of their intra- rather than extracellular activity points to the need to search for their intracellular targets and to the possibility of designing better therapeutic agents. Another important conclusion of this study is that bestatin and actinonin are not suitable for investigations of the biological role of cell surface aminopeptidases.
Acknowledgments
We thank Dr. Janko Kos and Dr. Igor Križaj for helpful discussions, and Professor Dr. Roger H. Pain for thoughtful comments on the manuscript. This work was supported by Ministry of Education, Science and Sport of Slovenia (106-504).
References
- 1.Umezawa H., Aoyagi T., Suda H., Hamada M., Takeuchi T.J. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J. Antibiot. 1976;29:97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 2.Mathe G. Bestatin, an aminopeptidase inhibitor with a multi-pharmacological function. Biomed. Pharmacother. 1991;45:49–54. doi: 10.1016/0753-3322(91)90122-a. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya K., Chiba S., Hino M., Kitamura T., Miyagawa K., Takaku F., Miyazano K. Enhancing effect of ubenimex (Bestatin) on proliferation and differentiation of hematopoietic progenitor cells, and the suppressive effect on proliferation of leukemic cell lines via peptidase regulation. Biomed. Pharmacother. 1991;45:71–80. doi: 10.1016/0753-3322(91)90125-d. [DOI] [PubMed] [Google Scholar]
- 4.Yoneda J., Saiki I., Fujii H., Abe F., Kojima Y., Azuma I. Inhibition of tumor invasion and extracellular matrix degradation by ubenimex (bestatin) Clin. Exp. Metastasis. 1992;10:49–59. doi: 10.1007/BF00163576. [DOI] [PubMed] [Google Scholar]
- 5.Ino K., Goto S., Okamoto T., Nomura S., Nawa A., Isobe K., Mizutani S., Tomoda Y. Expression of aminopeptidase N on human choriocarcinoma cells and cell growth suppression by the inhibition of aminopeptidase N activity. Jpn. J. Cancer Res. 1994;85:927–933. doi: 10.1111/j.1349-7006.1994.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata M., Kubota Y., Tanaka T., Iida-Tanaka K., Takahara J., Irino S. Effect of Ubenimex on the proliferation and differentiation of U937 human histiocytic lymphoma cells. Leukemia. 1994;8:2188–2193. [PubMed] [Google Scholar]
- 7.Ino K., Goto S., Nomura S., Isobe K., Nawa A., Okamoto T., Tomoda Y. Aminopeptidase inhibitor ubenimex (Bestatin) inhibits the growth of human choriocarcinoma in nude mice through its direct cytostatic activity. Anticancer Res. 1995;15:2081–2088. [PubMed] [Google Scholar]
- 8.Fujioka S., Kohno N., Hiwada K. Ubenimex activates the E-cadherin-mediated adhesion of a breast cancer cell line YMB-S. Jpn. J. Cancer Res. 1995;86:368–373. doi: 10.1111/j.1349-7006.1995.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezawa K., Minato K., Dobashi K. Induction of apoptosis by ubenimex (Bestatin) in human non-small-cell lung cancer cell lines. Biomed. Pharmacother. 1996;50:283–289. doi: 10.1016/0753-3322(96)84827-x. [DOI] [PubMed] [Google Scholar]
- 10.Ueda M., Ueki M., Fujii H., Yoshizawa K., Nakajima M. Inhibitory effects of ubenimex (Bestatin) on the invasion of uterine cervical carcinoma cells and their production and activation of gelatinase. Am. J. Med. 1997;28:175–190. [PubMed] [Google Scholar]
- 11.Sekine K., Fujii H., Abe F. Induction of apoptosis by bestatin (ubenimex) in human leukemic cell lines. Leukemia. 1999;13:729–734. doi: 10.1038/sj.leu.2401388. [DOI] [PubMed] [Google Scholar]
- 12.Sakuraya M., Tamura J., Itoh K., Kubota K., Naruse T. Aminopeptidase inhibitor ubenimex inhibits the growth of leukaemic cell lines and myeloma cells through its cytotoxicity. J. Int. Med. Res. 2000;28:214–221. doi: 10.1177/147323000002800503. [DOI] [PubMed] [Google Scholar]
- 13.Ota K. Review of ubenimex (Bestatin): clinical research. Biomed. Pharmacother. 1991;45:55–60. doi: 10.1016/0753-3322(91)90123-b. [DOI] [PubMed] [Google Scholar]
- 14.Urabe A., Mutoh Y., Mizoguchi H., Takaku F., Ogawa N. Ubenimex in the treatment of acute nonlymphocytic lekemia in adults. Ann. Hematol. 1993;67:63–66. doi: 10.1007/BF01788128. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki S., Fukushima J., Hamajima K., Ishii N., Tsuji T., Xin K.Q., Mohri I., Okuda K. Adjuvant effect o Ubenimex on a DNA vaccine for HIV-1. Clin. Exp. Immunol. 1998;111:30–35. doi: 10.1046/j.1365-2249.1998.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller W.E., Schuster D.K., Zahn R.K., Maidhof A., Leyhausen G., Falke D., Koren R., Umezawa H. Properties and specificity of binding sites for the immunomodulator bestatin on the surface of mammalian cells. Int. J. Immunopharmacol. 1982;4:393–400. doi: 10.1016/0192-0561(82)90012-1. [DOI] [PubMed] [Google Scholar]
- 17.Leyhausen G., Schuster D.K., Vaith P., Zahn R.K., Umezawa H., Falke D., Muller W.E.G. Identification and properties of cell membrane bound leucine aminopeptidase interacting with the potential immunostimulant and chemotherapeutic agent bestatin. Biochem. Pharmacol. 1983;32:1051–1057. doi: 10.1016/0006-2952(83)90624-x. [DOI] [PubMed] [Google Scholar]
- 18.Tieku S., Hooper N.M. Inhibition of aminopeptidases N, A and W, a re-evaluation of the actions of bestatin and inhibitors of angiotensin converting enzyme. Biochem. Pharmacol. 1992;44:1725–1730. doi: 10.1016/0006-2952(92)90065-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lendeckel U., Wex T., Reinhold D., Kähne T., Frank K., Faust J., Neubert K., Ansorge S. Induction of the membrane alanyl aminopeptidase gene and surface expression in human T-cells by mitogenic activation. Biochem. J. 1996;319:817–821. doi: 10.1042/bj3190817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenzwajg M., Tailleux L., Gluckman J.C. CD13/N-aminopeptidase is involved in the development of dendritic cells and macrophages from cord blood CD34+ cells. Blood. 2000;95:453–460. [PubMed] [Google Scholar]
- 21.Wex T., Lendeckel U., Reinhold D., Kähne T., Arndt M., Frank K., Ansorge S. Antisense-mediated inhibition of aminopeptidase N (CD13) markedly decreases growth rates of hematopoietic tumour cells. Adv. Exp. Med. Biol. 1997;421:67–73. doi: 10.1007/978-1-4757-9613-1_9. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y., Lai L.T., Gabrilove J.L., Scheinberg D.A. Antitumor activity of actinonin in vitro and in vivo. Clin. Cancer Res. 1998;4:171–176. [PubMed] [Google Scholar]
- 23.Gordon J.J., Kelly B.K., Miller G.A. Actinonin and antibiotic substance produced by an Actinomycete. Nature. 1962;195:701–702. doi: 10.1038/195701b0. [DOI] [PubMed] [Google Scholar]
- 24.Umezawa H., Aoyagi T., Tanaka T., Suda H., Okuyama A., Naganawa H., Hamada M., Takeuchi T. Production of actinonin, an inhibitor of aminopeptidase M, by actinomycetes. J. Antibiot. 1985;38:1629–1630. doi: 10.7164/antibiotics.38.1629. [DOI] [PubMed] [Google Scholar]
- 25.Benderra Z., Morjani H., Trussardi A., Manfait M. Characterization of H+-ATPase-dependent activity of multidrug resistance-associated protein in homoharringtonine-resistant human leukemic K562 cells. Leukemia. 1998;12:1539–1544. doi: 10.1038/sj.leu.2401166. [DOI] [PubMed] [Google Scholar]
- 26.Liang B.C., Ullyatt E. Increased sensitivity to cis-diamminedichloroplatinum induced apoptosis with mitochondrial DNA depletion. Cell Death Differ. 1998;5:694–701. doi: 10.1038/sj.cdd.4400401. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary P.M., Roninson I.B. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J. Natl. Cancer Inst. 1993;85:632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- 28.Ross D.D. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14:467–473. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- 29.Zaman G.J.R., Flens M.J., Van Leusden M.R., de Haas M., Mülder H.S., Lankelma J., Pinedo H.M., Scheper R.J., Baas F., Broxterman H.J., Borst P. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc. Natl. Acad. Sci. USA. 1994;91:8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaman G.J.R., Lankelma J., van Tellingen O., Beijnen J., Dekker H., Paulusma C., Oude Elferink R.P., Baas F., Borst P. Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc. Natl. Acad. Sci. USA. 1995;92:7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gekeler V., Ise W., Sanders K.H., Ulrich W.R., Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995;108:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- 32.Ford J.M., Hait W.H. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42:155–198. [PubMed] [Google Scholar]
- 33.Sundstrom C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int. J. Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 34.Bauvois B., Sancéau J., Wietzerbin J. Human U937 cell surface peptidase activities: characterization and degradative effect on tumor necrosis factor-α. Eur. J. Immunol. 1992;22:923–930. doi: 10.1002/eji.1830220407. [DOI] [PubMed] [Google Scholar]
- 35.Yamada M., Sukenaga Y., Fujii H., Abe F., Takeuchi T. Purification and characterization of a ubenimex (Bestatin)-sensitive aminopeptidase B-like enzyme from K562 human chronic myeloid leukemia cells. FEBS Lett. 1994;342:53–56. doi: 10.1016/0014-5793(94)80583-0. [DOI] [PubMed] [Google Scholar]
- 36.Wilkes S.H., Prescott J.M. The slow, tight binding of bestatin and amastatin to aminopeptidases. J. Biol. Chem. 1985;260:13154–13162. [PubMed] [Google Scholar]
- 37.Szedlacsek S.E., Duggleby R.G. Kinetics of slow and tight binding inhibitors. Methods Enzymol. 1995;249:144–180. doi: 10.1016/0076-6879(95)49034-5. [DOI] [PubMed] [Google Scholar]
- 38.Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakanishi T., Tamai I., Takaki A., Tsuji A. Cancer cell-targeted drug delivery utilizing oligopeptide transport activity. Int. J. Cancer. 2000;88:274–280. [PubMed] [Google Scholar]


