Abstract
Background
Leukotriene B4 (LTB4) recruits and activates neutrophils. Accordingly, this leukotriene is involved in innate defense actions.
Objective
To examine if nasal LTB4 can produce neutrophil activity and to explore whether or not LTB4 can condition neutrophils to exert virucidal effects in vitro and in vivo.
Methods
1. Twenty-three healthy subjects received nasal LTB4 in a randomized and sham-controlled design. Symptoms were scored and nasal lavages carried out. Myeloperoxidase (MPO) and α-defensins were monitored as indices of neutrophil activity. IL-8, eosinophil cationic protein (ECP) and α2-macroglobulin were measured as indices of pro-inflammatory cytokine production, eosinophil activity, and plasma exudation. 2. Supernatants from neutrophils activated by LTB4 in vitro were assayed for virucidal activity against respiratory viruses. 3. In 38 healthy individuals, nasal inoculation with human rhinovirus-16 (HRV-16) was performed. In a preliminary study, intervention with LTB4 was given in a randomized and controlled design. Symptoms, virus replication, and antibody-titres were monitored.
Results
1. LTB4 produced statistically significant increases in MPO and α-defensins, whereas IL-8, ECP, and α2-macroglobulin were unaffected. 2. The supernatants efficiently killed human coronavirus, respiratory syncytial virus, and influenza B virus. 3. HRV-16 replication was lower in subjects receiving LTB4, but this difference failed to reach statistical significance. Common cold symptoms and incidence of seroconversion were unaffected.
Conclusion
Nasal LTB4 induces a selective recruitment/activation of neutrophils. LTB4 can condition neutrophils to exert virucidal effects in vitro and may reduce virus replication in vivo. We suggest that the condition induced by LTB4 reflects an enhanced state of innate defense.
Keywords: Leukotriene B4, Neutrophils, Innate defense, Rhinitis, Rhinovirus
Introduction
Leukotriene B4 (LTB4) is a metabolite of the 5-lipoxygenase pathway. Original findings on the biology of this leukotriene comprise demonstrations of its biosynthesis by, and its potent chemotactic and activating effects on, polymorpho-nuclear leukocytes .1, 2, 3 Accordingly, and substantiated by a series of subsequent observations,4, 5, 6, 7, 8, 9, 10, 11 LTB4 is regarded as a key mediator of the innate immune system.
Information on the role of LTB4 in human airways can be obtained through experiments involving airway challenges with this leukotriene. Administration of LTB4 to the bronchial airways has been shown to increase neutrophil recruitment,12, 13 without producing exudative inflammation12 or bronchial hyperresponsiveness.14 In contrast, there are no reports on effects of LTB4 administered to the human nasal airway. If LTB4 selectively increases human nasal neutrophil activity, topical administration of this leukotriene may be employed to enhance mucosal innate immune defense against infection.
Defensins are cationic antimicrobial peptides grouped into α- and β-defensin subfamilies.15 α-Defensins 1-4 are major components of neutrophil granules, whereas β-defensins 1-4 are found in epithelial cells.16 Flamand et al.10 recently demonstrated that intravenous administration of LTB4 to monkeys produced increased plasma levels of α-defensins and that these levels could exert antimicrobial effects ex vivo. Whether or not topical administration of LTB4 affects the nasal mucosal output of α-defensins is unknown.
In this study, we examined dose- and time-dependent effects of nasal administration of LTB4 in healthy subjects on symptoms, nasal peak inspiratory flow (PIF), and select nasal lavage fluid indices. Accordingly, we monitored the neutrophil granule protein myeloperoxidase (MPO) and α-defensins as indices of neutrophil activity. Interleukin-8 (IL-8) was analyzed in order to explore whether or not any neutrophil active effect of LTB4 involved this pro-inflammatory cytokine. Eosinophil cationic protein (ECP) and α2-macroglobulin were monitored as indices of eosinophil activity and plasma exudation, in order to explore whether or not LTB4, or any LTB4-produced neutrophil activity, had any consequence to the nasal mucosa in terms of producing eosinophil, exudative inflammation. In this study, we also studied whether or not exposure of neutrophils to LTB4 in vitro induced virucidal effects against respiratory viruses: human coronavirus, human respiratory syncytial virus (RSV), and human influenza B virus. Finally, in a preliminary experiment involving healthy subjects, we examined effects of LTB4 on human rhinovirus-16 (HRV-16) induced virus replication, seroconversion, and symptoms.
Methods
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of Lund University (Reference numbers 522/06 and 198/09). All patients provided written informed consent for the collection of samples and subsequent analysis.
Study design
-
1.
In healthy subjects, nasal challenges with LTB4 were carried out in a double-blinded, randomized, sham-controlled, and crossover design. Nasal lavages were carried out and IL-8, α-defensins, MPO, ECP, and α2-macroglobulin were measured.
-
2.
In experiments in vitro, possible virucidal effects of supernatants of neutrophils conditioned by LTB4 were examined on human coronavirus, RSV, and influenza B virus.
-
3.
In healthy individuals, nasal inoculation with HRV-16 was carried out. Intervention with LTB4 was administered in a double-blinded, randomized, controlled, and parallel group design. Nasal symptoms, virus replication, and neutralizing antibody-titres were monitored.
Effects of LTB4 on the human nasal mucosa in vivo
Subjects
Twenty-three subjects (11 female, 12 male, aged 21–29 years) were recruited. Inclusion criteria were a negative skin-prick test to relevant aeroallergens. Exclusion criteria were allergic rhinitis, other nasal disease (structural abnormalities, rhinosinusitis, and polyposis), chronic disease and/or on-going drug treatment, and pregnancy or lactation. The subjects were without medication and had been so for at least four weeks prior to the study. No medication except the study drug and occasional use over-the-counter pain relievers were allowed during the course of the study.
Challenges
All challenges (and all lavages) were given to the right hand side of the nasal cavity. Sham solution (isotonic saline containing 10 mM glycine/sodium hydroxide buffer, pH 10.5), used as control challenge, and two doses of LTB4 (2.0 and 20 μg) dissolved in sham solution were given in a randomized order. The challenges were given as single actuations using a spray-device delivering 100 μl per actuation. The washout time between the administrations was at least one week.
Clinical measurements
Symptoms, nasal peak inspiratory flow (PIF), and nasal lavages were carried out before each challenge as well as 1 and 4 h thereafter. The symptoms blocked nose, runny nose, and irritation were scored by the subjects on a four-graded scale: 0 = no symptoms, 1 = mild symptoms, 2 = moderate symptoms, 3 = severe symptoms. The number of sneezes were counted and transformed into a score: 0 = 0 sneezes, 1 = 1–4 sneezes, 2 = 5–8 sneezes, 3 = more than 9 sneezes.17 The four scores of blocked nose, runny nose, irritation, and sneezes were then added to a total nasal symptom score (TNSS).17 Nasal PIF were measured using a PIF-meter (Clement-Clarke, Harlow, U.K.). On each occasion, the best of three PIF-recordings were used in the analysis.
Nasal lavages
A pool-device was used for isotonic saline lavage of the nasal mucosa as described previously.18 The volume of the pool-fluid was 14 ml and the dwell time 5 min (This high-volume lavage retrieves nasal mucosal surface liquids that are diluted in the process. The degree of dilution is likely similar at baseline and post challenge observations.) The lavages were carried out before each challenge as well as 1 and 4 h thereafter.
Analyses
LTB4 (Cayman Chemicals, Ann Arbour, MI; detection limit 13 pg/ml), MPO (Diagnostic Development, Uppsala, Sweden; detection limit 1.6 ng/ml), IL-8 (Invitrogen, Burlington, Ontario, Canada; detection limit 5 pg/ml), and α-defensins (HyCult Biotechnology, Uden, The Netherlands; detection limit 50 pg/ml) were measured by ELISA. ECP was measured using a fluoroimmunoassay (Pharmacia Diagnostics, Uppsala, Sweden; detection limit 2.0 ng/ml). α2-Macroglobulin was measured using a radioimmunoassay (detection limit 7.8 ng/ml).18
Effects of LTB4-conditioned neutrophils on respiratory viruses in vitro
Isolation of human polymorphonuclear leukocytes (PMN)
From a separate group of healthy volunteers, venous blood was collected in tubes containing heparin and PMNs were isolated as described previously.19 The PMN-suspension contained mainly neutrophils (95%) with eosinophils as the major contaminant. Cell viability, as measured by trypan blue exclusion, was always greater than 98%. PMN were re-suspended to 10x106 cells/ml in M199 medium without serum or antibiotics.
Stimulation of human PMNs and virucidal assays
Freshly isolated human PMNs were pre-incubated (10x106/ml) in M199 medium containing 10 μM of cytochalasin B for 10 min at 37 °C after which LTB4 was added to each tube to various concentrations (0, 1, 10, and 100 nM). Incubations were stopped after 5 min by transferring tubes into an ice/water bath. Cell suspensions were centrifuged (250 g, 10 min, 4 °C) and cell-free supernatants were collected and assayed for virucidal activity as follows. Preparations of infectious viruses (100 μl), including human Coronavirus (strain 229E, provided by Dr. P. Talbot, INRS-Institut Armand Frappier, Laval, Canada), human RSV (strain A2, obtained from the American Type Culture Collection [Manassas, VA]), and Influenza B virus (Harbin strain, provided by Dr. G. Boivin, CHUQ Research Center, Laval, Canada), were added to 100 μl of PMN-supernatants and the mixtures were incubated at 37 °C for 1 h. The volumes were adjusted to 1.0 ml with culture medium and the mixtures were added to cell monolayers to determine infectivity by standard assays 3–6 days later.20 Culture supernatants containing 10 μM cytochalasin B and 100 nM LTB4 without PMNs or supernatants from PMNs stimulated with a control solution (ethanol) were used as negative controls. Results were expressed as mean TCID50 + SD from one experiment representative of at least two independent experiments.
Effects of nasal LTB4 on HRV-16 infectivity
Subjects
Seventy-seven healthy subjects (45 female, 32 male, aged 19–31 years) were enrolled for screening. Inclusion and exclusion criteria were identical to those of the LTB4 challenge study described above. In addition, individuals with detectable levels of serum neutralizing antibodies to HRV-16 were excluded. Accordingly, 40 subjects (21 female, 19 male, aged 19–30 years) were randomized. Of these subjects, one was excluded from the analysis due to wild type HRV-16 infection at the day of the inoculation (i.e., a positive qPCR prior to inoculation) and one due to symptomatic upper respiratory tract infection of unknown cause on the same day. Ultimately, the control group comprised ten females and ten males (aged 21–30 years) and the LTB4-group nine females and nine males (aged 19–28 years).
LTB4 intervention
LTB4 (dissolved in 10 mM glycine/sodium hydroxide buffer, pH 10.5) was administered as intervention in a double-blinded, randomized, controlled, and parallel group design. The dose of LTB4 was 2.0 μg per nasal cavity and it was given twice daily for four days. The intervention started 2 h prior the HRV-16 inoculation. Control solution (“placebo”) was isotonic saline containing 10 mM glycine/sodium hydroxide buffer, pH 10.5. A nasal spray-device delivering 100 μl per actuation was used for the administration. In order to assure compliance, the investigators performed the administrations at visits to the clinic.
HRV-16 inoculation
On study day 1, 10 TCID50 (tissue culture infective dose 50% in 0.5 ml isotonic saline) of HRV-16, initially harvested and cultivated by Bardin et al.21 was mixed with 0.5 ml isotonic saline and administered to each nasal passage (0.5 ml per side) using a mucosal Atomization Device (No. 40-0124, Wolfe Tory Medical, Salt Lake City, UT) attached to a 2 ml syringe. The inoculation took place 2 h after the first administration of LTB4/control solution.
Symptom scores
Symptoms were registered in the morning on study days 1–6, before the lavage procedure (below) and before administration of LTB4/control solution. Nasal secretion, blockage, nasal irritation, headache, sinus ache, sore throat, and hoarseness were scored by the subjects on a four-graded scale: 0 = no symptoms, 1 = mild symptoms, 2 = moderate symptoms, 3 = severe symptoms. These seven scores were added and divided by seven to a mean symptom score (range 0–3).
Nasal lavages
Nasal lavages were performed every morning on study days 1–6, between the symptom registration and LTB4/control administration. The subject tilted the head back 90°, closed the soft palate (in order to avoid displacement of saline to the throat), and 1.25 ml of sterile saline was instilled into each nasal cavity using a sterile syringe. After three seconds, the head was tilted forward and the lavage fluid was collected into a sterile test tube via a funnel. The fluid was centrifuged and the supernatant, except the part bound for qPCR analysis (∼200 μl), was mechanically homogenized (Yellow Line DI 18 Basic [IKA, Staufen, Germany]). Finally, the fluid was aliquoted and stored at −70 °C.
Analyses
HRV-16 in the nasal lavage fluid was detected through qPCR according to Message et al.21 Analysis of neutralizing HRV-16 antibodies in serum was also performed as described by Message et al.22 Seroconversion was defined as a four-fold (or more) increase in HRV-16 neutralizing antibodies titer between study day 1 and an observation point 6–8 weeks later. IL-8 was measured in the nasal lavage fluid as a surrogate marker for inflammation associated with the HRV-16 infection.
Statistics
For the LTB4 challenge study, differences in symptoms and levels of analytes between the LTB4 challenges and sham were analyzed using the Friedman test and the Wilcoxon signed rank test. For the in vitro study, the T-test with Welch’s correction was used. For the hrv-16-inoculation study, differences between the intervention groups were analyzed with Fisher’s exact test (for serology data) and the Mann Whitney U-test. Paired comparisons within each group were analyzed using the Friedman test and the Wilcoxon signed rank test. P-values < 0.05 were considered statistically significant. No power calculations were carried out for these studies as they were exploratory in nature and since no similar studies have been reported to provide data on which to perform such calculations. Nonparametric methods for comparison were for the LTB4 challenge study and in the hrv-16-inoculation study because the data were not normally distributed.
Results
Effects of LTB4 on the human nasal mucosa in vivo
Nasal administration of LTB4, in the dose-range of 2.0–20 μg, did not produce any nasal symptoms during the 4-h follow-up period (Table 1 ). Moreover, nasal PIF was unaffected by the LTB4 exposure (Table 2 ).
Table 1.
Nasal symptoms after LTB4 challenge.
| LTB4 (μg) | Sneezes (0–3) |
Secretion (0–3) |
Blockage (0–3) |
Irritation (0–3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior | 1 h | 4 h | Prior | 1 h | 4 h | Prior | 1 h | 4 h | Prior | 1 h | 4 h | |
| 0 (Sham) | 0 (0) | 0 (0) | 0 (0) | 0 (1.0) | 0 (1.0) | 0 (1.0) | 0 (1.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2.0 | 0 (0) | 0 (0) | 0 (0) | 0 (1.0) | 0 (1.0) | 0 (0) | 0 (1.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20 | 0 (0) | 0 (0) | 0 (0) | 0 (1.0) | 0 (0) | 0 (1.0) | 0 (1.0) | 0 (0) | 0 (1.0) | 0 (0) | 0 (0) | 0 (0) |
Median levels (interquartile ranges) of symptoms recorded prior to nasal challenge with sham and two doses of LTB4 as well as 1 and 4 h thereafter. LTB4 did not produce any rhinitis symptoms or any nasal irritation. Comparisons are made with sham challenge at each time-point (all statistically non-significant).
Table 2.
Nasal PIF after LTB4 challenge.
| LTB4 (μg) | Nasal PIF (l/min) |
||
|---|---|---|---|
| Prior | 1 h | 4 h | |
| 0 (Sham) | 150 (100) | 120 (65) | 140 (70) |
| 2.0 | 140 (80) | 130 (50) | 140 (70) |
| 20 | 140 (60) | 140 (80) | 130 (80) |
Median levels (interquartile ranges) of nasal PIF recorded prior to nasal challenge with sham and two doses of LTB4 as well as 1 and 4 h thereafter. LTB4 produced a minor but non-significant improvement in nasal PIF 1 h post challenge. Comparisons are made with sham challenge at each time-point (all statistically non-significant).
LTB4 was measured in nasal lavage fluids in order to estimate its retention in the nose (Table 3 ). LTB4 was measurable in lavages of sham-challenged subjects at a median level of 0.46 ng/ml and did not vary much in subsequent lavages. Levels of LTB4 increased at 1 h following challenge with 2.0 and 20 μg compared with sham (both p < 0.001). After 4 h, levels had returned to near baseline levels.
Table 3.
Lavage levels of LTB4 after LTB4 challenge.
| LTB4 (μg) | LTB4 (ng/ml) |
||
|---|---|---|---|
| Prior | 1 h | 4 h | |
| 0 (Sham) | 0.46 (0.18) | 0.48 (0.19) | 0.42 (0.21) |
| 2.0 | 0.50 (0.25) | 1.16 (2.23)∗∗∗ | 0.42 (0.28) |
| 20 | 0.47 (0.59) | 17.45 (23.15)∗∗∗ | 0.69 (1.28)∗∗∗ |
Median levels (interquartile ranges) of LTB4 in nasal lavages obtained prior to nasal challenge with sham and two doses of LTB4 as well as 1 and 4 h thereafter. Levels of LTB4 significantly increased 1 h following challenges with 2 and 20 μg LTB4. Approximately 1% of the administered dose of LTB4 was retrievable by lavage at 4 h post challenge, approaching baseline values. Comparisons are made with sham challenge at each time-point (∗∗∗ denotes p < 0.001.)
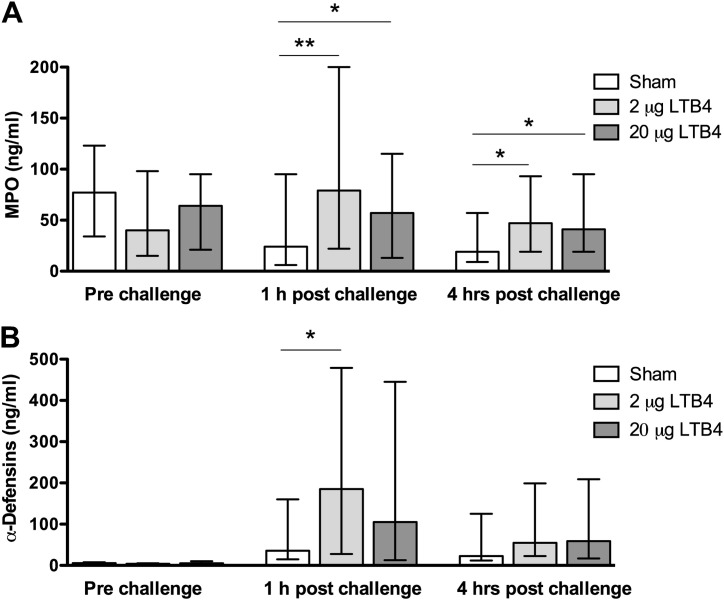
MPO was detected in all nasal lavage fluid samples (i.e., 207/207 observations). Levels of MPO increased following challenge with 2.0 μg LTB4, but the subsequent greater dose of 20 μg did not further elevate the levels (Fig. 1 A). The increases were statistically significant for both doses of LTB4 at 1 h post challenge (p < 0.01 and 0.05) as well as at 4 h (both p < 0.05) (c.f. sham).
Figure 1.
LTB4 produces increased nasal lavage levels of MPO and α-defensins; Levels of MPO (A) and α-defensins (B) in nasal lavages obtained prior to challenge with sham and two doses of LTB4 as well as 1 and 4 h thereafter. LTB4 increased levels of MPO and α-defensins: these changes reached statistical significance for MPO at 1 as well as 4 h post challenge for both doses and for α-defensins at 1 h post challenge for LTB4 (2.0 μg). Comparisons were made with sham challenge at each time-point. Note that the repeated lavages produced gradually lower levels of MPO following sham challenge (as for IL-8, ECP, and α2-macroglobulin: Table 4), whereas the sham challenge increased the levels of α-defensins. Data are expressed as median ± IQR (n = 23). ∗ Denotes p < 0.05 and ∗∗ p < 0.01, paired comparisons.
A five-fold increase in levels of α-defensins by LTB4 (2.0 μg) was recorded at 1 h following its administration (p < 0.05) (Fig. 1B). Similarly, the levels were increased at 4 h post challenge as well as at both observation points following challenge with LTB4 (20 μg), but these effects failed to reach statistical significance. In contrast to other analytes, where gradually lower levels were recorded over time following sham challenge (see below), increased levels of α-defensins were observed 1 and 4 h post sham challenge (c.f. prior to challenge) (p < 0.0001 and p = 0.0003). This baseline drift produced by the alkaline buffer itself suggests that the sensitivity to detect LTB4-induced changes in α-defensins was poor.
Levels of IL-8 were not affected by LTB4 (c.f. sham) (Table 4 ). ECP and α2-macroglobulin were low prior to challenge and LTB4 did not increase the levels of these analytes at any of the observation points (c.f. sham) (Table 4). Rather, the repeated lavages produced gradually lower levels of ECP and α2-macroglobulin, which was reflected by the fact that these markers were undetectable in 123/207 and 120/207 observations, respectively.
Table 4.
Lavage levels of IL-8, ECP, and α2-macroglobulin after LTB4 challenge.
| LTB4 (μg) | IL-8 (pg/ml) |
ECP (ng/ml) |
α2-macroglobulin (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Prior | 1 h | 4 h | Prior | 1 h | 4 h | Prior | 1 h | 4 h | |
| Sham | 171 (244) | 79 (130) | 50 (88) | 0 (4.5) | 0 (3.9) | 0 (2.2) | 0.2 (0.2) | 0 (0.1) | 0 (0.1) |
| 2.0 | 136 (188) | 94 (103) | 67 (82) | 0 (6.2) | 2.0 (5.0) | 0 (3.0) | 0 (0.2) | 0 (0.2) | 0 (0.1) |
| 20 | 148 (202) | 81 (106) | 65 (86) | 0 (3.5) | 0 (6.2) | 0 (3.8) | 0 (0.3) | 0 (0.2) | 0 (0.1) |
Median levels (interquartile ranges) of analytes in nasal lavages obtained prior to nasal challenge with sham and two doses of LTB4 as well as 1 and 4 h thereafter. LTB4 failed to affect the levels of IL-8, ECP, and α2-macroglobulin. Comparisons are made with sham challenge at each time-point (all statistically non-significant).
Focusing on the nasal lavage series conducted following sham challenge, it was evident that the repeated lavages gradually lowered the analyte levels (except for α-defensins). For example, MPO featured a 75% reduction between the observation pre- and 4 h post challenge. This finding emphasizes the necessity of the sham-controlled design of the experiment.
Effects of LTB4-conditioned neutrophils on respiratory viruses in vitro
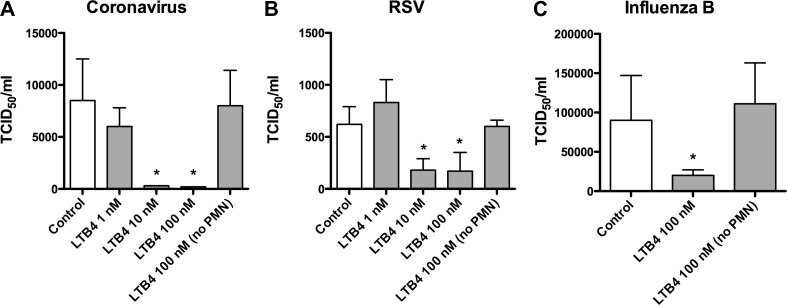
In the in vitro experiments, isolated PMNs were exposed to varying concentrations of LTB4 and cell-free supernatants were collected and incubated with human coronavirus (Fig. 2 A), human RSV (Fig. 2B), and human influenza B virus (Fig. 2C). The results indicated that the supernatants significantly reduced the infectivity of these viruses when concentrations of LTB4 of 10 nM and 100 nM were used to condition neutrophils, but not at 1 nM.
Figure 2.
LTB4 conditions neutrophils to exert antiviral effects; Supernatants from neutrophils conditioned with LTB4 produced virucidal effects against human coronavirus (A), RSV (B), and influenza B virus (C). Control experiments indicated that the effect was specific for the interaction between LTB4 and neutrophils. Data are expressed as mean TCID50 + S.D. from one experiment representative of at least two independent experiments. ∗ Denotes p < 0.05.
Effects of nasal LTB4 on HRV-16 infectivity
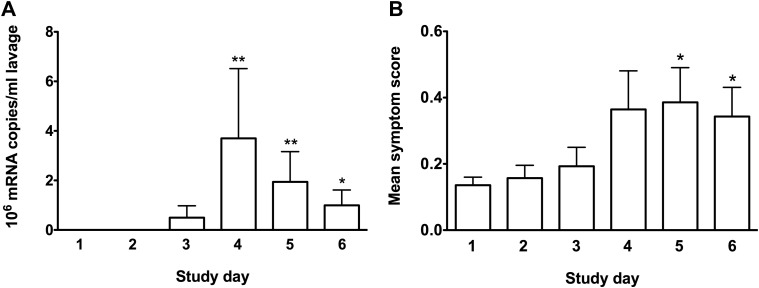
Focusing on the control group as a whole, significant virus replication was observed three to five days following HRV-16 inoculation (Fig. 3 A). Furthermore, this group presented mild, yet statistically significant, nasal symptoms of common cold (Fig. 3A). Twelve of the 20 control subjects seroconverted. Six subjects had very convincing evidence of virus replication (i.e., more than 100,000 copies/ml lavage on three consecutive days) and the response peaked three days after inoculation. This group also featured greater levels of IL-8 in nasal lavage fluids on day four following inoculation compared with the remainder of the group (median levels 7393 pg/ml and 649 pg/ml, respectively, p < 0.05). Eight of the 20 control subjects were negative for virus on all tested days. One control subject who was positive for virus did not seroconvert and, conversely, one subject who were negative for virus did seroconvert.
Figure 3.
The control subjects presented viral replication and common cold symptoms; Data from control subjects inoculated with HRV-16. These subjects presented HRV-16 replication (peaking three days following inoculation), as indicated by qPCR (A), and mild nasal symptoms of the common cold (B). Data are expressed as mean ± SEM (n = 20). ∗ Denotes p < 0.05 and ∗∗ denotes p < 0.01, paired comparisons with baseline levels prior to inoculation.
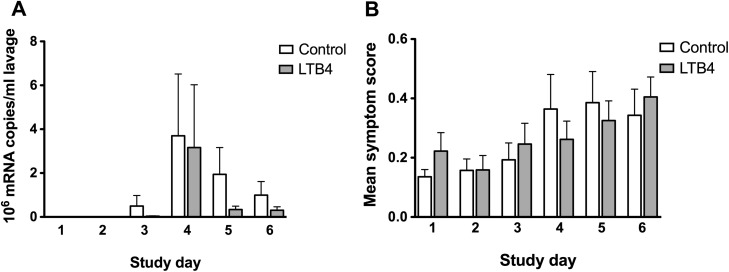
LTB4 intervention was associated with 46% lower viral shedding compared with controls (over the entire post-inoculation period), but this change failed to reach statistical significance (Fig. 4 A). However, of the top five virus-shedding individuals, four were found in the control group; LTB4 reduced clearly positive infections by 27% (defined as substantial virus replication: >100,000 copies/ml lavage fluid over three consecutive days in combination with seroconversion); LTB4 reduced high-grade viral shedding (>106 copies/ml lavage) by 35%. Symptom scores did not differ between the groups (Fig. 4B). Seroconversion occurred in eleven of the 18 subjects receiving LTB4. Nasal lavage fluid levels of IL-8 did not differ between the groups (data not shown).
Figure 4.
Comparison of viral replication and common cold symptoms between the two groups; Virus replication (A) and nasal symptoms (B) from the LTB4 and control groups. Virus replication was lower in the LTB4-group, but this difference (c.f. control group) failed to reach statistical significance overall as well as at all time-points. Nasal symptoms of the common cold were mild an unaffected by LTB4. Data are expressed as mean ± SEM (n = 20: control and 18: LTB4).
Discussion
This study demonstrates that nasal administration of LTB4 selectively increases local neutrophil activity as reflected by increased levels of MPO and α-defensins in nasal mucosal surface liquids. Furthermore, it shows that LTB4 can condition neutrophils to produce factors that exert virucidal effects towards key respiratory viruses: human coronavirus, RSV, and influenza B virus. Finally, in a preliminary experiment involving healthy humans, the study explores whether or not nasal administration of LTB4 affects the outcome of a low-dose HRV-16 inoculation. The observations are of interest with regard to how LTB4 amplifies innate immune responses.
Nasal administration of LTB4 increased the lavage fluid levels of MPO (this study), indicating increased neutrophil activity. A statistically significant response was detected already at the first of the employed doses (i.e., 2.0 μg). The second dose produced a response of similar magnitude despite the fact that it was ten times greater, suggesting a flat response curve in this particular dose-range. The effect was numerically greater 1 h post challenge than at 4 h, indicating a rapid and transient response. Our finding that LTB4 was rapidly cleared from the nasal mucosa (∼1% retrievable by lavage within 1 h of nasal administration) might account for this effect profile. In agreement with the present findings, Martin et al.12 in a study involving healthy subjects, showed that bronchial instillation of 10 ml of a 5x10−7 M solution of LTB4 (∼1.7 μg) increased neutrophil numbers in BAL-fluids. Taken together, the above observations indicate that LTB4 can recruit and activate neutrophils in human airways, including the nasal airway.
A heightened neutrophil activity was also suggested by the present observations on nasal lavage fluid levels of α-defensins. The levels were increased by LTB4 by a median 5-fold at 1 h following challenge with 2.0 μg LTB4. α-Defensins were consistently increased also at the other observation points (c.f. sham), but these changes failed to reach statistical significance. The wide distribution of data for α-defensins, and the fact that the alkaline buffer (sham) appeared to increase the baseline, might have contributed to our failure to detect a specific LTB4-induced effect more convincingly. However, observations by Flamand et al.10, 23 indicated increased plasma levels of α-defensins in monkeys and humans following intravenous administration of LTB4. Furthermore, in the present study, neutrophils activated in vitro by LTB4 released factors with virucidal activities against human coronavirus, RSV, and influenza B virus: these results extend previous observations demonstrating the release of antimicrobial compounds (including α-defensins) following stimulation of neutrophils with LTB4.10 Taken together, the present observations suggest that α-defensins are a part of the innate defense system of the upper respiratory tract and that this feature can be enhanced by nasal administrations of LTB4.
IL-8 is a pro-inflammatory cytokine that attracts neutrophils: e.g., intranasal administration of recombinant human IL-8 increases neutrophil numbers in nasal smears.24 In this study, nasal lavage fluid levels of IL-8 were unaffected by topical administration of LTB4. Accordingly, the recorded increase in neutrophil activity (i.e., elevated levels of MPO) might not involve an IL-8-dependent mechanism. This is in contrast to conditions characterized by increased neutrophil activity, e.g., viral infections, where IL-8 is thought to be central in mediating a neutrophil response.18 Conversely, the present observations are in keeping with the notion that neutrophil activity mediated by LTB4 may not be a disease-like mechanism, but rather a feature of the innate immune defense system.
In vitro observations suggest the possibility that LTB4 can act as chemoattractant for IL-5-primed eosinophils25 and activate eosinophils.26 Also, eosinophils entering human bronchial airways following allergen challenge are chemotactically desensitized to LTB4,27 suggesting exposure to LTB4 in vivo. Together, these observations suggest an association between LTB4 and eosinophil activity. In contrast, in the present study, while LTB4 increased the generation of MPO, lavage fluid levels of ECP were unaffected, indicating that nasal granulocyte activity mediated by LTB4 in the human nasal airway does not involve eosinophils. We cannot exclude the possibility that LTB4 may increase eosinophil activity if elevated numbers of eosinophils would be present at the time of challenge, e.g., as in on-going allergic rhinitis or asthma (LTB4 has been administered to bronchial airways of patients with mild asthma, but without focus on airway eosinophil activity14). However, so far, available human in vivo observations with LTB4 argue against a role for LTB4 as a pro-eosinophil factor. For example, a LTB4 receptor antagonist had no effects on allergen-induced eosinophilia in asthma.28
Plasma exudation is a key feature of airway inflammation. The process comprises extravasation and luminal entry of bulk plasma, including high molecular weight proteins such as α2-macroglobulin (725 kDa). It can be monitored through analysis of plasma proteins in mucosal surface liquids.29 Accordingly, in airway diseases characterized by inflammation, levels of plasma proteins in nasal lavage- and BAL-fluids may reflect the degree of on-going inflammation.29 In this, lavage fluid levels of α2-macroglobulin were unaffected following LTB4 challenge, indicating that this leukotriene does not exert plasma exudation producing effects in human nasal airways at the dose used. In contrast, Bende et al.30 in a study on anesthetized rabbits, reported increased vascular permeability following nasal challenge with LTB4. We have no specific explanation for the discrepant findings, but species differences may be one reason.29 The present observation is in agreement with observations in man by Martin et al.12: unaffected levels of albumin as well as total protein in BAL-fluids were observed in healthy subjects following segmental LTB4 challenge. Available observations in man thus suggest that LTB4, in doses that produce increased neutrophil activity, does not produce exudative inflammation.
Bachert et al.31 reported an interesting temporal relationship between increased levels of MPO in nasal secretions and a fall in virus replication in naturally acquired infections. The finding suggests that a heightened neutrophil activity is beneficial in the context of viral exposure of the upper respiratory tract. This is indirectly supported by the present in vitro observations that LTB4-activated neutrophils produce factors active against respiratory viruses. Moreover, previous studies have implicated neutrophils as an important player in the response to such infections,32, 33, 34, 35 and Gaudreault et al. demonstrated that i.v. LTB4 upregulated antimicrobial peptides and reduced viral load in influenza infected mice in vivo.36 In line with this, we employed HRV-16 inoculations and explored the effect of repeated nasal administration of LTB4 on rhinovirus infections in a preliminary study. The dose investigated (2.0 μg) was chosen based on the present dose-response experiments involving LTB4, whereby increased neutrophil activity was induced without producing exudative inflammation or symptoms. We elected to use a low-dose (10 TCID50) virus challenge as this has been shown to be effective in inducing colds in COPD-subjects.37 Furthermore, we believe this to be a better model of natural transmission of virus infections (where inoculation occurs with very small amount of virus followed by replication up to high titres with resultant symptom induction) than would be the case with high-dose challenge model where inoculation with doses as high as 10,000 TCID50 is performed.22
In this study significant virus replication was observed three through five days following HRV-16 inoculation in the control group as a whole. Furthermore, this group presented mild, yet statistically significant, nasal symptoms of common cold and a minor increase in nasal mucosal output of IL-8. However, unlike high-dose challenge where ∼90% of subjects become infected,22 only 12/20 control subjects seroconverted and 12/20 had evidence of virus replication with 6/20 having high virus titres. These lower than expected numbers of infected subjects likely hampered our ability to detect statistically significant effects on viral outcomes. Nonetheless, several of the present observations suggested that LTB4 might have affected virus replication. For example, the degree of viral shedding was reduced by nearly 50% in the LTB4-group compared with the control group. Also, of the top five virus-shedding individuals, four were found in the control group. However, likely in part reflecting an inferior power of the parallel group design, as well as the issue with numbers of infected subjects, the changes failed to reach statistical significance. While these data, together with our observations in vitro, were promising, symptoms and incidence of seroconversion were not affected by LTB4. Accordingly, LTB4 did not provide convincing evidence of a preventive treatment for common cold infections caused by HRV-16. Further studies with greater numbers of infected subjects, and properly powered using data from the present study, are warranted to explore whether or not nasal administration of LTB4 can be useful to prevent or treat upper respiratory tract infections. Also, it would be of interest to explore effects of LTB4 in bacterial infections, where neutrophil phagocytosis is of key importance to clear infections.37
We conclude that nasal administration of LTB4 in the present dose-range produces increased nasal neutrophil activity, potentially reflecting an enhanced state of innate immune defense, without being associated with any apparent untoward effects. Furthermore, LTB4 has a potential to exert antimicrobial effects on respiratory tract infections. However, further studies in which LTB4 intervention is undertaken and where effects on respiratory infections are studied in vivo are warranted to confirm this hypothesis.
Conflict of interest statement
Pierre Borgeat, Louis Flamand, Lennart Greiff and Sebastian Johnston recieved temporary grants from LTB4 Sweden AB, which partly financed the study. LTB4 Sweden AB took part in deciding the study design but had no role in data collection and analysis, decision to publish, or preparation of the manuscript. Otherwise, the authors report no competing interest.
Acknowledgments
Tatiana Kebadze, Lena Glantz-Larsson, and Charlotte Cervin-Hoberg for excellent laboratory assistance.
References
- 1.Ford-Hutchinson A.W., Bray M.A., Doig M.V., Shipley M.E., Smith M.J. Leukotriene B4, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 2.Malmsten C.L., Palmblad J., Uden A.M., Rådmark O., Engstedt L. Leukotriene B4: a highly potent and stereospecific factor stimulating migration of polymorphonuclear leukocytes. Acta Physiol Scand. 1980;110:449–451. doi: 10.1111/j.1748-1716.1980.tb06696.x. [DOI] [PubMed] [Google Scholar]
- 3.Dahlén S.E., Björk J., Hedqvist P., Arfors K.E., Hammarström S. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci USA. 1981;78:3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rola-Pleszczynski M., Gagnon L., Sirois P. Leukotriene B4 augments human natural cytotoxic cell activity. Biochem Biophys Red Commun. 1983;113:531–537. doi: 10.1016/0006-291x(83)91758-8. [DOI] [PubMed] [Google Scholar]
- 5.Doerfler M.E., Danner R.L., Shelhamer J.H., Parillo J.E. Bacterial lipopolysaccharides prime human neutrophils for enhanced production of leukotriene B4. J Clin Invest. 1989;83:970–977. doi: 10.1172/JCI113983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailie M.B., Standiford T.J., Laichalk L.L., Coffey M.J., Strieter R. Leukotriene-deficient mice manifest enhanced lethality from Kleibsiella pneumoniae in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- 7.Mancuso P., Nana-Sinkam P., Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Kleibsiella pneumoniae. Infect Immun. 2001;69:2011–2016. doi: 10.1128/IAI.69.4.2011-2016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardell L.O., Agustí C., Nadel J.A. Nasal secretion in ragweed-sensitized dogs: effect of leukotriene synthesis inhibition. Acta Otolaryngol. 2000;120:757–760. doi: 10.1080/000164800750000306. [DOI] [PubMed] [Google Scholar]
- 9.Qadri F., Raqib R., Ahmed F., Rahman T., Wenneras C. Increased levels of inflammatory mediators in children and adults infected with vibrio cholerae O1 and O139. Clin Diagn Lab Immunol. 2002;9:221–229. doi: 10.1128/CDLI.9.2.221-229.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flamand L., Tremblay M.J., Borgeat P. Leukotriene B4 triggers the in vitro and in vivo release of potent antimicrobial agents. J Immunol. 2007;178:8036–8045. doi: 10.4049/jimmunol.178.12.8036. [DOI] [PubMed] [Google Scholar]
- 11.Wan M., Sabirsh A., Wetterholm A., Agerberth B., Haeggström J.Z. Leukotriene B4 triggers release of cathelicidin LL-37 from human neutrophils: novel lipid-peptide interactions in innate immune responses. FASEB J. 2007;21:2897–2905. doi: 10.1096/fj.06-7974com. [DOI] [PubMed] [Google Scholar]
- 12.Martin T.R., Pistorese B.P., Chi E.Y., Goodman R.B., Matthay M.A. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh Y.Y., Dupuis R., Pollice M., Albertine K.H., Fish J.E. Neutrophils recruited to the lung by segmental antigen challenge display a reduced chemotactic response to leukotriene B4. Am J Respir Cell Mol Biol. 1993;8:493–499. doi: 10.1165/ajrcmb/8.5.493. [DOI] [PubMed] [Google Scholar]
- 14.Sampson S.E., Costello J.F., Sampson A.P. The effect of inhaled leukotriene B4 in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1789–1792. doi: 10.1164/ajrccm.155.5.9154893. [DOI] [PubMed] [Google Scholar]
- 15.Ganz T., Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 16.van Wetering S., Sterk P.J., Rabe K.F., Hiemstra P.S. Defensins: key players or bystanders in infection, injury and repair in the lung. J Allergy Clin Immunol. 1999;104:1131–1138. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- 17.Greiff L., Ahlström-Emanuelsson C., Bahl A., Bengtsson T., Dahlström K. Effects of a dual CCR3 and H1-antagonist on symptoms and eosinophilic inflammation in allergic rhinitis. Respir Res. 2010;11:17. doi: 10.1186/1465-9921-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greiff L., Andersson M., Svensson C., Linden M., Myint S. Allergen challenge-induced acute exudation of IL-8, ECP, and α2-macroglobulin in human rhinovirus-induced common colds. Eur Respir J. 1999;13:41–47. doi: 10.1034/j.1399-3003.1999.13a09.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- 20.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoint. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 21.Bardin P.G., Sanderson G., Robinson B.S., Holgate S.T., Tyrell D.A. Experimental rhinovirus infection in volunteers. Eur Respir Dis. 1996;11:2250–2255. doi: 10.1183/09031936.96.09112250. [DOI] [PubMed] [Google Scholar]
- 22.Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J. Rhinovirus-induced lower respiratory illness is incaresed in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flamand L., Borgeat P., Lalonde R., Gosselin J. Release of anti-HIV mediators after administration of leukotriene B4 to humans. J Infect Dis. 2004;189:2001–2209. doi: 10.1086/386374. [DOI] [PubMed] [Google Scholar]
- 24.Douglass J.A., Dhami D., Gurr C.E., Bulpitt M., Shute J.K. Influence of interleukin-8 challenge in the nasal mucosa in atopic and nonatopic subjects. Am J Respir Crit Care Med. 1994;150:1108–1113. doi: 10.1164/ajrccm.150.4.7921444. [DOI] [PubMed] [Google Scholar]
- 25.Sehmi R., Wardlaw A.J., Cromwell O., Kurihara K., Waltmann P. Interleukin-5 selectively enhances the chemotactic response to eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992;79:2952–2959. [PubMed] [Google Scholar]
- 26.Takafuji S., Tadokoro K., Ito K., Nakagawa T. Release of granule proteins from human eosinophils stimulated with mast-cell mediators. Allergy. 1998;53:951–956. doi: 10.1111/j.1398-9995.1998.tb03795.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim C.J., Kane G.C., Zangrilli J.G., Cho S.K., Koh Y.Y. Eosinophils recruited to the lung by segmental antigen challenge show a reduced chemotactic response to leukotriene B4. Prostaglandins. 1994;47:393–403. doi: 10.1016/0090-6980(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 28.Evans D.J., Barnes P.J., Spaethe S.M., van Alstyne E.L., Mitchell M.I. Effects of a leukotriene B4 receptor antagonist, LY293111, on allergen induced responses in asthma. Thorax. 1996;51:1178–1184. doi: 10.1136/thx.51.12.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson C.G.A., Erjefält J.S., Greiff L., Andersson M., Erjefält I. Plasma-derived proteins in airway defense, disease and repair of epithelial injury. Eur Respir J. 1998;11:958–970. doi: 10.1183/09031936.98.11040958. [DOI] [PubMed] [Google Scholar]
- 30.Bende M., Hansell P., Intaglietta M., Arfors K.E. Effects of oxymetazoline nose drops on vascular permeability of the nasal mucosa in the rabbit after provocation with leukotriene B4. ORL J Otorhinolaryngol Relat Spec. 1992;54:270–274. doi: 10.1159/000276313. [DOI] [PubMed] [Google Scholar]
- 31.Bachert C., van Kempen M.J., Hopken K., Holtappels G., Wagenmann M. Elevated levels of myeloperoxidase, pro-inflammatory cytokines and chemokines in naturally acquired upper respiratory tract infections. Eur Arch Otorhinolaryngol. 2001;258:406–412. doi: 10.1007/s004050100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol. 2008;82:2772–2783. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujisawa H. Inhibitory role of neutrophils on influenza virus multiplication in the lungs of mice. Microbiol Immunol. 2001;45:679–688. doi: 10.1111/j.1348-0421.2001.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 34.Tumpey T.M., García-Sastre A., Taubenberger J.K., Palese P., Swayne D.E. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujisawa H., Tsuru S., Taniguchi M., Zinnaka Y., Nomoto K. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J Gen Virol. 1987;68:425–432. doi: 10.1099/0022-1317-68-2-425. [DOI] [PubMed] [Google Scholar]
- 36.Gaudreault E., Gosselin J. Leukotriene B4 induces release of antimicrobial peptides in lungs of virally infected mice. J Immunol. 2008;180:6211–6221. doi: 10.4049/jimmunol.180.9.6211. [DOI] [PubMed] [Google Scholar]
- 37.Mallia P., Message S.D., Kebadze T., Parker H.L., Kon O.M. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]