Abstract
Background
A number of antiviral therapies have evolved that may be effectively administered to treat respiratory viral diseases. But these therapies are very often of limited efficacy or have severe side effects. Therefore there is great interest in developing new efficacious and safe antiviral compounds e.g. based on the identification of compounds of herbal origin.
Hypothesis
Since an aqueous extract of Aloe arborescens Mill. shows antiviral activity against viruses causing infections of the upper respiratory tract in vitro we hypothesised that a product containing it such as Biaron C® could have an antiviral activity too.
Study design
Antiviral activity of Bioaron C®, an herbal medicinal product consisting of an aqueous extract of Aloe arborescens Mill., Vitamin C, and Aronia melanocarpa Elliot. succus, added as an excipient, was tested in vitro against a broad panel of viruses involved in upper respiratory tract infections.
Methods
These studies included human adenovirus and several RNA viruses and were performed either with plaque reduction assays or with tests for the detection of a virus-caused cytopathic effect.
Results
Our studies demonstrated an impressive activity of Bioaron C® against members of the orthomyxoviridae – influenza A and influenza B viruses. Replication of both analysed influenza A virus strains – H1N1 and H3N2 – as well as replication of two analysed influenza B viruses – strains Yamagatal and Beiying – was significantly reduced after addition of Bioaron C® to the infected cell cultures. In contrast antiviral activity of Bioaron C® against other RNA viruses showed a heterogeneous pattern. Bioaron C® inhibited the replication of human rhinovirus and coxsackievirus, both viruses belonging to the family of picornaviridae and both representing non-enveloped RNA viruses. In vitro infections with respiratory syncytial virus and parainfluenza virus, both belonging to the paramyxoviridae, were only poorly blocked by the test substance. No antiviral activity of Bioaron C® was detected against adenovirus – a non-enveloped DNA virus.
Conclusions
These results represent the first proof of a selective antiviral activity of Bioaron C® against influenza viruses and create basis for further analyses of type and molecular mechanisms of the antiviral activity of this herbal medicine.
Keywords: Antiviral activity, Plant extract, Aloe arborescens Mill., Xanthorrhoeaceae
Abbreviations: Adeno 5, adenovirus C subtype 5; BGM, buffalo-green-monkey cells; CA9, coxsackievirus subtype 9; CPE, cytopathogenic effect; EC50, effective concentration 50; FluA, influenza A virus; FluB, influenza B virus; HEp-2, human epithelial cells; HRV14, human rhinovirus B subtype 14; IC50, inhibitory concentration 50; MDCK, Madin–Darby–Canine–Kidney cells; M.O.I., multiplicity of infection; Para 3, parainfluenza virus type 3; PFU, plaque-forming units; RSV, respiratory syncytial virus
Graphical abstract
Introduction
A number of antiviral therapies are effectively administered to treat respiratory viral diseases, thus providing the physician with a range of compounds including amantadine (Hay et al. 1985), neuraminidase inhibitors (Calfee and Hayden 1998) and nucleoside analogues (Fyfe et al., 1978, Hruska et al., 1990). Therapies with these compounds are very often of limited efficacy and on the other hand, side-effects and systemic toxicity may limit their application, particularly in paediatric, geriatric and immunocompromised patients (Bacon et al. 2003, Cassady and Whitley 1997, Englund et al., 1990, Hayden et al., 1983, Janai et al., 1990, Reusser, 1996). As a result, there is great interest in developing new efficacious and safe antiviral agents. Plant extracts have been widely used in traditional medicine (DeClercq 2004) due to their antimicrobial and antiviral activities.
Interestingly enough, about 10% of more than 4000 species studied showed a significant antiviral efficacy in vitro (Che 1991), although in most studies a systematic investigation of their activity against a broad panel of viruses still remains to be done.
Summerfield et al. (1997) described the activity of Acanthospermum hispidum DC. (Asteraceae) against animal pathogenic herpes viruses, pseudorabiesvirus (PRV) and bovine herpesvirus 1 (BHV-1). In 2001 we showed (Glatthaar-Saalmüller et al. 2001) the antiviral activity of an extract from Eleutherococcus senticosus Maxim. (Araliaceae), against human rhinovirus (HRV), human respiratory syncytial virus (RSV) and influenza A virus, which was discussed as RNA-virus specific reactivity. Michaelis et al. (2011) investigated the influence of a standardised extract of Pelargonium sidoides DC. (Geraniaceae), for the treatment of acute bronchitis, on replication of a panel of respiratory viruses. The authors were able to show that concentrations up to 100 µg/ml interfered with replication of seasonal influenza A virus strains (H1N1, H3N2), RSV, human coronavirus, parainfluenza virus, and coxsackie virus but did not affect replication of highly pathogenic avian influenza A virus (H5N1), adenovirus, or HRV.
Antiviral potential has also been demonstrated for a combination of Gentian root, Primula flower, Elder flower, Sorrel herb, and Verbena herb, which reduced in vitro the spreading of influenza A, RSV and parainfluenza type 1 virus (Para 1) (Glatthaar et al. 2012).
In the present study the antiviral effect of an herbal medicine used for the prevention and treatment of upper respiratory tract infections consisting of Aloe arborescens Mill. (Xanthorrhoeaceae), Vitamin C and as excipient Aronia melanocarpa Elliot. (Rosaceae) succus, named Bioaron C® has been investigated.
A. arborescens has been used in the treatment of upper respiratory tract infections in Central and Eastern European countries for many decades. Recent pre-clinical studies with Bioaron C® showed in vitro a clear dose-dependent antiviral activity against human rhinovirus 14 (HRV 14) (Glatthaar-Saalmüller et al. 2012). In addition clinical studies showed that anti-inflammatory and antiviral activities contribute to its therapeutic efficacy against viral infections of the upper respiratory tract (Bastian et al. 2013). Last published data about Bioaron C® refer to observational studies involving children characterised by susceptibility to upper respiratory tract infections. The results of this study suggest that Bioaron C® can be successfully used in the treatment of viral infections and as an adjuvant during antibiotic therapy because of shortening duration of infection and causing milder course of the infection (Fal and Michalak 2013).
The following study focuses on the antiviral activity of Bioaron C® against some viruses causing infections of the upper respiratory tract to get more detailed information about the specificity of the plant extract and to get some hints for a possible mode of action. Therefore the antiviral activity of Bioaron C® was tested on a DNA virus (adenovirus 5, Adeno 5) as well as on a broad panel of enveloped or non-enveloped RNA viruses. For non-enveloped viruses HRV14 (responsible for the majority of acute respiratory infections in both children and adults) and coxsackievirus type 9 (CA9 – tend to infect the skin and mucous membranes, causing herpangina, acute haemorrhagic conjunctivitis, and hand-foot-and-mouth (HFM) disease) were included in the analyses. Among the enveloped RNA viruses we chose parainfluenzavirus 3 (Para 3) and RSV – both associated with bronchiolitis and pneumonia. But the main focus of these studies was set on the reactivity of the test substance against different strains of influenza viruses belonging either to Type A influenza viruses (influenza H1N1 and H3N2) or to type B influenza viruses (influenza B Yamagatal and influenza B Beiying).
Material and methods
Test substance
Bioaron C® syrup is a commercial available herbal medicinal product of an aqueous extract of A. arborescens plus Vitamin C from Phytopharm Klęka S.A., Klęka 1, 63-040 Nowe Miasto nad Wartą, Poland. As excipients sucrose, concentrated chokeberry juice (A. melanocarpa), sodium benzoate, and purified water are used. The aloe extract of A. arborescens leaves is the key ingredient of the syrup which contains 1920 mg of the extract and 51 mg Vitamin C per 5 ml. In the present investigation the original product Bioaron C® has been investigated without sugar.
A preparation with all ingredients with the exception of A. arborescens and sugar served in all assays as a negative (solvent) control.
Medicinal products based on A. arborescens aqueous extracts are widely used in Poland, Russia, and Ukraine with a focus on upper respiratory tract infections in children and lack of appetite during or after long-lasting illness (Bastian et al. 2013).
Aloe arborescens Mill.
In the European Pharmacopoeia and HMPC monographs different Aloe species, especially Aloe ferox Mill. and Aloe barbadensis Mill., are described for their laxative effects based on their high content in hydroxyanthraquinones. However, A. arborescens is characterised by a very low anthranoid content and therefore without laxative effects (Phytopharm Klęka S.A., Klęka, personal communication). The natural habitats of A. arborescens are mountainous regions of Southern Africa. A. arborescens is also grown as a source material for medicinal, cosmetic, and food uses in various countries (China, Israel, Italy, Japan, Poland (green houses), and in Ukraine (Crimea peninsula)) (Jambor 2012). Plants used for this study were cultivated and harvested in a greenhouse plantation by Phytopharm Klęka S.A., in Poland.
Chromatograms of Bioaron C®
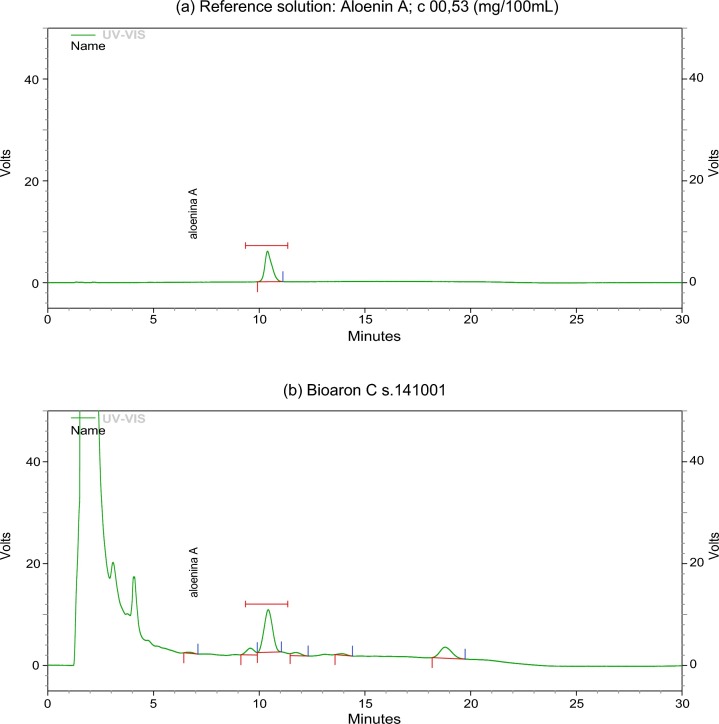
Aloenin A is an analytical marker specified for Bioaron C® and Aloe extractum fluidum as an active substance of this medicinal product. Reproducibility of the manufacturing process was confirmed by checking Aolenin A level in every batch of the active substance and the medicinal product (Phytopharm Klęka S.A., Klęka) Both analytical procedures were validated according to ICH guidance (CPMP/ICH/381/95 and CPMP/ICH/281/95). The range derived from linearity studies of the assay method is covering concentration from 2 to 12 mg of Aloenin A per 100 ml of Aloe extractum fluidum, whereas the average content of the marker is 9 mg/100 ml. Aloenin A content in Bioaron C® varied between 2.2 and 3.8 mg/100 ml, the average value was 3.0 mg/100 ml. The identity of Bioaron C® has been confirmed by the detection of Aloenin A. The chromatogram profile of Bioaron C® together with Aloenin A is presented in Fig. 1 (a: Aloenin A and b: Bioaron C®).
Fig. 1.
Chromatogram profile of Bioaron C®: Representative HPLC chromatograms of Aloenin A (a) and Bioaron C® (b). Signals of the reference solution correspond to 0.53 mg/100 ml.
Determination of Vitamin C in Bioaron C® was conducted by a direct redox titration method in acidic condition with an iodine solution (c = 0.05 mol/l). One millilitre of 0.05 mol/l iodine solution corresponded to 8.81 mg of Vitamin C. Molality of the titrant was determined by direct titration by means of a 0.1 mol/l sodium thiosulphate solution.
For the in vitro assays the test substance was diluted as described with the respective cell culture media. As control served a “solvent control” without the active component A. arborescens prepared under the same conditions.
Reference drugs
To verify the test systems, Ribavirin® (1-beta- d -ribofuranosyl-1,2,4-triazole-carboxamide, Sigma–Aldrich, Germany) was included in most of the assays as a positive control with known antiviral activity against RNA viruses (Hruska et al. 1990). Further Amantadine® (1-aminoadamantanehydrochloride, Sigma–Aldrich, Germany) a cyclic amine active against e.g. early steps in the influenza A replication was also used as a positive control (Richman et al. 1986). The efficacy of the reference substances was confirmed in the antiviral assays.
Cells and viruses
Human rhinovirus B subtype 14 (HRV 14) was obtained from the Institute for Virology of the Friedrich-Schiller-University, Jena, Germany. The human influenza A virus strains, influenza A Chile 1/83 (H1N1) virus (FluA H1N1), influenza A Shanghai/9/93/H3N2 (FluA H3N2) as well as both influenza B strains, influenza B Yamagatal/16/88 (FluB Yamagatal) and influenza B Beijing/184/93 (FluB Beiying) are seasonal WHO virus strains. Together with the respiratory syncytial virus, strain Long (RSV), parainfluenza type 3 virus (Para 3), coxsackievirus subtype A9 (CA9), and adenovirus C subtype 5 (Adeno 5) all virus strains were obtained from the former Department of Medical Virology and Epidemiology of Virus Diseases of the Hygiene Institute of the University of Tübingen, Germany.
RSV, Para 3 and Adeno 5 were propagated on human epithelial cells (HEp-2); HRV 14 on HeLa cells and CA9 on buffalo-green-monkey (BGM) cells, in Hank's/Earle's minimal essential medium (MEM) containing 2% (v/v) foetal calf serum, 25 mM MgCl2, 2 mM of l -glutamine, 100 U/ml of penicillin, and 0.1 mg/ml of streptomycin.
FluA H1N1 and FluA H3N2 as well as FluB Yamagatal and FluB Beiying were grown on Madin–Darby-–Canine–Kidney (MDCK) cells with serum-free MEM containing 1 µg/ml of trypsin, 2 mM of l -glutamine, 100 U/ml of penicillin, and 0.1 mg/ml of streptomycin.
In order to determine the virus titres, the respective cells were incubated with serially diluted serum-free virus stock solutions for 1 h at 34 °C. After removal of the virus inoculum, cell cultures were overlaid with the respective virus-specific medium containing agarose. The analyses of the plaques (plaque-forming unit, PFU) and the cytopathogenic effect (CPE) were performed 3–6 days later. The respective virus titres were calculated as PFU per millilitre with the method of Cavalli–Sforza or with the Spearman–Kaerber method by the mean tissue culture infectious dose (log10 TCID50) per millilitre (Adeno 5).
Virus assays
Plaque-reduction assays or assays for the CPE were performed with MDCK, HEp-2, BGM and HeLa-cell cultures using standard procedures for the detection of infectious particles. Virus plaques and CPE were quantified employing an optical evaluation system (ELISpot reader, AID Diagnostika GmbH, Straßberg, Germany). For quantification Adeno 5 antigens, enzyme immunoassays (Merlin Diagnostika GmbH, Bornheim-Hersel, Germany) were used.
Cytotoxicity tests
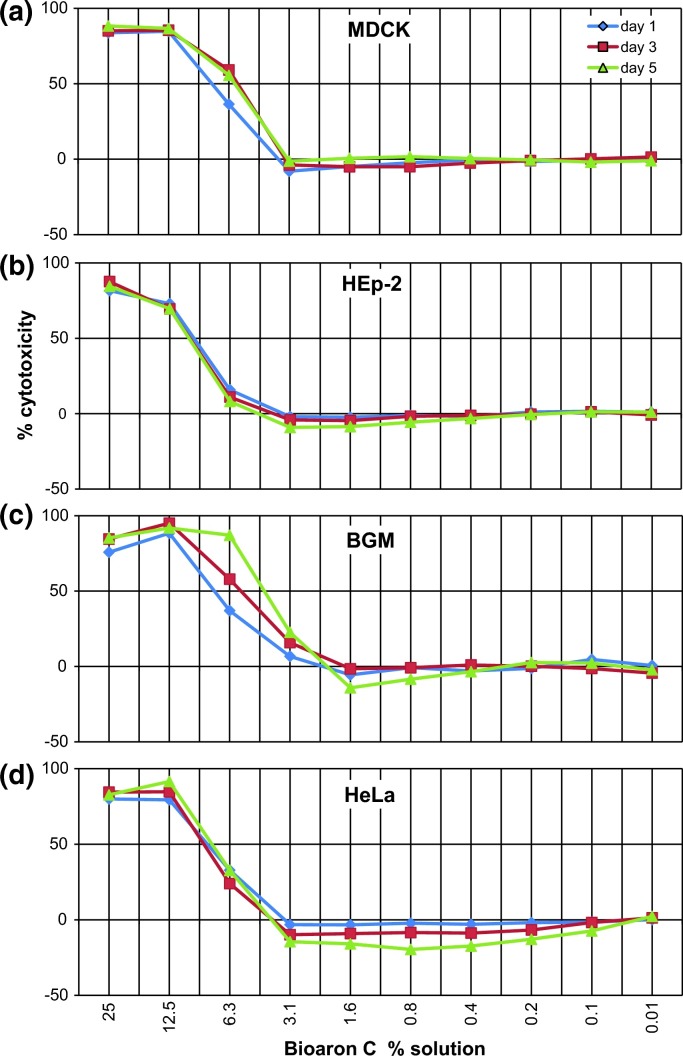
Analyses of the in vitro cytotoxicity of Bioaron C® were performed with physiologically active cells and an enzymatic assay (MTT-assay; Mosmann 1983) which is capable of quantifying the activity of mitochondrial enzymes in active and dividing cells showing a direct correlation between viability and enzyme activity. Additionally, the cytotoxicity of the test substance on the respective cells was monitored by microscopic examination (200 × magnification) of the cell cultures for altered cell morphology. For the determination of the limits of the toxic concentrations MDCK (Fig. 2 a), HEp-2 (Fig. 2b), BGM (Fig. 2c), and HeLa (Fig. 2d) cells were cultivated in their growth period together with different dilutions (log2-dilutions ranging from 25% to 0.01% (v/v)) of the test substance and the solvent control at 37 °C and 5% CO2 for at least 5 days. Respective cell culture media without any test component were used as control (medium control).
Fig. 2.
In vitro cytotoxicity of Bioaron C®: In vitro cytotoxicity of Bioaron C® was tested on cells used for propagation of viruses: MDCK (a) HEp-2 (b), BGM (c), and HeLa (d) over a period of several days. The titration curves show the dose-dependent cytotoxicity for day 1 (blue), day 3 (red), and day 5 (green) for each of the cell line. IC50 values were determined graphically and are presented in Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Assays for antiviral activity
Plaque-reduction assay (PFU), cytopathogenic effect (CPE), immunoassay (ELISA): The antiviral activity of Bioaron C® was measured with plaque-reduction assays (Cooper 1955) in plaque forming units (PFU) for FluA (H1N1, H3N2), FluB (Yamagatal, Beiying) and Para 3, RSV, HRV 14, CA9 or with analyses of a cytopathogenic effect (CPE) for Adeno 5. Cell monolayers were infected with a multiplicity of infection (M.O.I.) of about 0.0004 (FluA H1N1, FluA H2N3, FluB Yamagatal, FluB Beiying, Para 3, RSV, HRV 14, CA9), and 0.008 (Adeno 5) for 1 h at 34 °C. The cell monolayers were then washed and overlaid with agarose (0.1–0.3% final concentration) containing different concentrations (2–0.006%) of the test substance. Subsequently, the infected cell cultures were cultivated for 3 days (MDCK: FluA H1N1, FluA H2N3; HeLa: HRV 14; BGM: CA9), 4 days (MDCK: FluB Yamagatal), 5 days (MDCK: FluB Beiying, Para 3), 6 days (HEp-2: RSV, Adeno 5) until lesions were visible in the cell monolayer (plaques or CPE) of the virus infected control group cultivated in medium alone. At this point, the cells were fixed with paraformaldehyde and the remaining cell monolayers were stained with a crystal violet solution. Non-stained lesions in the cell monolayer (plaques, CPE) were quantified by employing an optical evaluation system (AID Diagnostika GmbH). In case of Adeno 5, in addition to the analysis of the CPE, the amount or newly synthesised virus was determined in virus-specific enzyme-linked immunosorbent assays (ELISA, Merlin Diagnostika GmbH, Bornheim-Hersel, Germany).
Calculation of antiviral activity
The quantification of the antiviral activity was either carried out by analysing the number of plaques (PFU: FluA, FluB, Para 3, HRV 14, CA9, RSV), the lesions of viral CPE (Adeno 5) and the amount of viral proteins detected in ELISA (Adeno 5). The calculation of the antiviral effect was based on mean values of four (CPE: Adeno 5; PFU: Beiying) or six (PFU: FluA, FluB, Para 3, HRV 14, CA9, RSV) replicates derived from at least two or three independent experiments. A solvent control which did not differ in its cytotoxicity from the medium control was included in all assays. The results of the non-treated virus control groups were defined as 100% infection (0% inhibition) and in vitro effects of the test substance were standardised as relative inhibitory effects.
Results
Determination of the in vitro cytotoxicity of Bioaron C® and its solvent control
For a determination of optimal concentrations of substances in the assays for antiviral activities cytotoxic effects of the test substance and its solvent control onto virus-susceptible cells (HEp-2, MDCK, HeLa and BGM) used for the in vitro propagation of the viruses had to be excluded. Therefore Bioaron C® and its solvent control were tested for their in vitro cytotoxicity and metabolic effects onto the respective cell cultures. Both preparations were diluted with cell culture medium starting from a 25% solution. To determine the limits of toxic concentrations of the respective preparations, cells were cultivated without (controls) or with descending concentrations in log 2/log10-dilutions of the test substance and its solvent control ranging from 25% to 0.01% for at least 5 days. Analyses of the cytotoxicity were performed with the MTT-assay (Mosmann 1983). Additionally the cytotoxicity of the test substance to the respective cells was monitored by microscopic examination as explained above. The results of the analyses are presented in Table 1 and Fig. 2. The MTT-assay demonstrated a low cytotoxicity of the plant extract onto all cell lines. The 50% inhibitory concentration (IC50) for the aqueous A. arborescens extract Bioaron C® was defined for a solution of less than 4.5% for BGM cells (Fig. 2c), 5.9% for MDCK cells (Fig. 2a), 7.85% for HeLa cells (Fig. 2d) and 10.43% for HEp-2 cells (Fig. 2b). All assays showed during the measurements at different time points only minor differences (Fig. 2). The solvent control showed IC50 values exceeding 25%, which means that no cytotoxicity could be detected. All data from the assays for cytotoxicity are summarised in Table 1 and Fig. 2. Following these data the aqueous Bioaron C® A. arborescens was diluted to a solution of 2% for the determination of its antiviral activity.
Table 1.
Determination of the cytotoxicity of Bioaron C®
| Bioaron C® | Summary table:aIC50-concentrations/% solution |
|||
|---|---|---|---|---|
| Cells/days | MDCK | HEp-2 | BGM | HeLa |
| Day 1 | 7.54 | 10.02 | 7.85 | 8.64 |
| Day 3 | 5.76 | 10.18 | 5.76 | 8.90 |
| Day 5 | 5.90 | 10.43 | 4.50 | 7.85 |
| Solvent control mean of day 1, day 3, day 5 | >25 | >25 | >25 | >25 |
| Highest concentration used in the antiviral studies | Bioaron C® 2% solution | |||
The cytotoxicity of Bioaron C® on the respective cells (MDCK, HEp-2, BGM, HeLa) cultivated with different concentrations of aqueous Bioaron C® in the dilution range of 25–0.01% and the corresponding solvent control were quantified using an MTT-test. The relative cytotoxicity of preparations was standardised by the medium control representing 100% viability. The table shows the dose-dependent intoxication of Bioaron C® and its solvent control calculated as a 50% inhibitory concentration (IC50). All data represent at least six replicates derived from two independent experiments. Standard deviations were less than 10%.
Inhibitory concentrations showing 50% cytotoxicity (IC50, % solution).
Determination of the antiviral activity of Bioaron C®
To test the antiviral activity of Bioaron C® during virus replication (therapeutic protocol), cells (MDCK, HEp-2, HeLa, BGM) were infected with a multiplicity of infection (M.O.I.) as described above. One hour after infection cells were washed and Bioaron C® was added into the semi-solid overlay of the cell cultures starting with a 2% solution and followed by five log 2 dilution steps (1%, 0.5%, 0.25%, 0.12%, 0.06%) and a final log10 dilution step (0.006%). Thereafter cells were cultivated for 3–6 days as described (section “Material and methods”) until lesions were visible in the respective cell monolayers (plaques or CPE) of the virus-infected control group cultivated in medium alone. The antiviral activity of the Bioaron C® dilutions was determined as PFU in plaque-reduction assays (Cooper 1955) or as CPE. Therefore cell monolayers were fixed with paraformaldehyde and the remaining cells stained with a crystal violet solution. Non-stained lesions in the cell monolayer (plaques, CPE) were quantified employing an optical evaluation system (ELISpot reader, AID Diagnostika GmbH). The results of the analyses are presented in Fig. 3, Fig. 4, Fig. 5 showing a dose-dependent antiviral activity of Bioaron C® against the respective viruses. All results (including the IC50 values) which determine the 50% inhibitory concentrations correlating with the cytotoxicity as well as the EC50 values describing the effective concentrations of the antiviral activity are in addition summarised in Table 2.
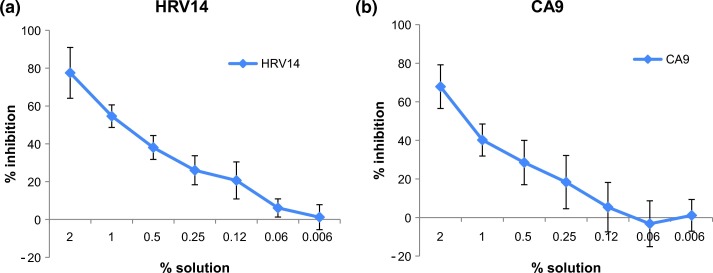
Fig. 3.
Antiviral activity of Bioaron C® against uncoated RNA viruses: Antiviral activity of Bioaron C® was determined against members of the family of picornaviridae: HRV14 (a) and CA9 (b). Plaque-forming units per ml (PFU/ml) were determined as readout for quantification of the antiviral activity. The figures present the % inhibition of the infectivity of the substance-treated cell cultures in comparison with the non-treated virus control. Data derived from four replicates of at least two independent studies, respectively, and are presented as mean values.
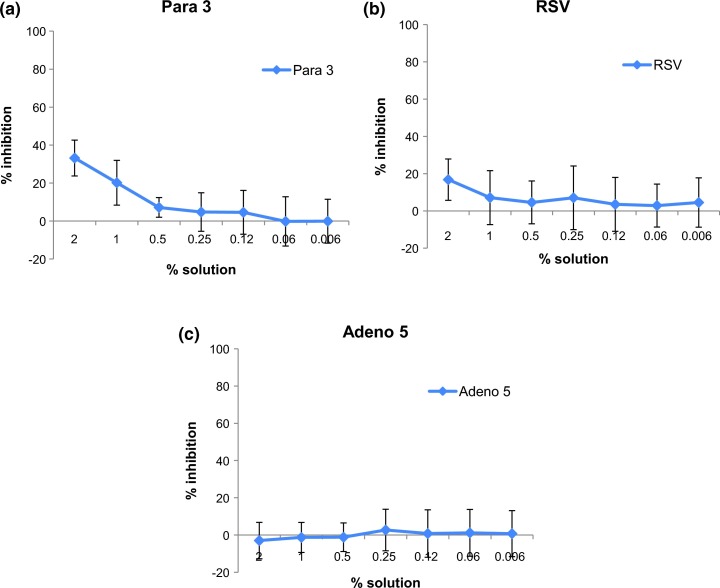
Fig. 4.
Antiviral activity of Bioaron C® against enveloped RNA viruses and a non-enveloped DNA virus: Antiviral activity of Bioaron C® was determined against enveloped RNA-viruses: Para 3 (a) and RSV (b). Furthermore the reactivity was tested against a non-enveloped DNA virus: Adeno 5 (c). For the RNA viruses plaque-forming units per millilitre (PFU/ml) were determined as readout for the antiviral activity. The effect against Adeno 5 was determined by the detection of the cytopathogenic effect (CPE). The figures present the % inhibition of the infectivity of the substance-treated cell cultures in comparison with the non-treated virus control (100% infection). Data derived from at least four replicates and two independent experiments.
Fig. 5.
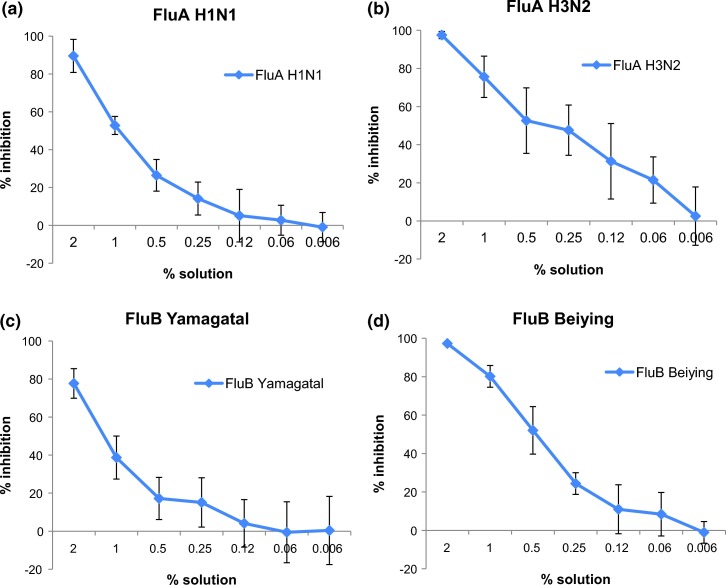
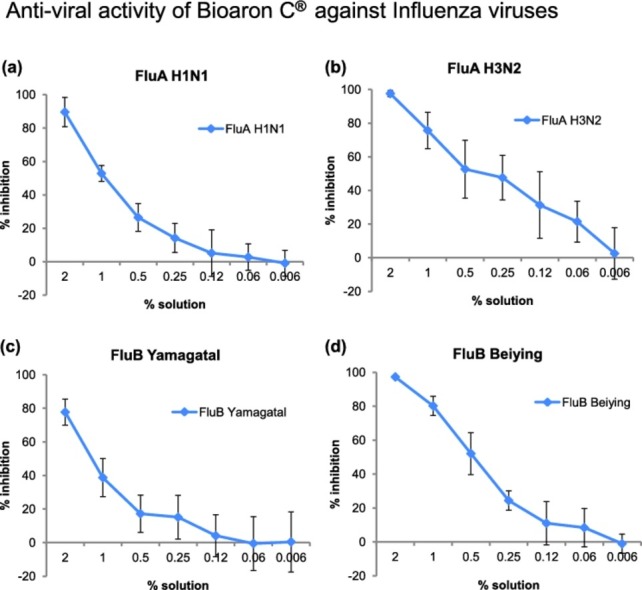
Antiviral activity of Bioaron C® against orthomyxoviridae: Antiviral activity of Bioaron C® against FluA H1N1 (a), FluA H3N2 (b), FluB Yamagatal (c) and FluB Beiying (d) was performed with plaque reduction assays. For FluA H1N1, FluA H3N2, and FluB Yamagatal data derived from six replicates from three independent studies, for FluB Beiying from four replicates (two studies).
Table 2.
Summary of antiviral activity of Bioaron C®
| Virus | Overview | % solution |
|
|---|---|---|---|
| # of study | EC50 | IC50 | |
| FluA H1N1 | Study 1 | 0.94 | 6.40 |
| Study 2 | 0.83 | ||
| Study 3 | 1.13 | ||
| FluA H3N2 | Study 1 | 0.22 | 6.40 |
| Study 2 | 0.38 | ||
| Study 3 | 0.49 | ||
| FluB Yamagatal | Study 1 | 1.25 | 6.40 |
| Study 2 | 1.45 | ||
| Study 3 | 1.08 | ||
| FluB Beiying | Study 1 | 0.69 | 6.40 |
| Study 2 | 0.38 | ||
| Para 3 | Study 1 | >2 | 10.21 |
| Study 2 | >2 | ||
| Study 3 | >2 | ||
| RSV | Study 1 | >3.3 | 10.21 |
| Study 2 | >3.3 | ||
| HRV 14 | Study 1 | 1.08 | 8.46 |
| Study 2 | 1.16 | ||
| Study 3 | 0.5 | ||
| CA9 | Study 1 | 1.75 | 6.03 |
| Study 2 | 1.0 | ||
| Adeno 5 | Study 1 | Negative | 10.21 |
| Study 2 | Negative | ||
EC50: Effective concentration (antiviral activity); IC50: inhibitory concentration (cytotoxicity, day 5); negative: no antiviral effect.
Overview and summary of the studies for detection of the antiviral activity of Bioaron C® against different virus strains (first column). Number of studies are shown in the second column. EC50 values (determining 50% inhibition of viral activity) are shown for each study in column three and can be directly compared with IC50 values (indicating 50% cytotoxicity, mean of day 1, day 3, day 5) in column four.
Grey highlighted lines: The original picture of the plaque reduction assays presented in the authentical 6-well plates was shown in Fig. 6 (FluA H1N1) and in supplementary Figs. 1 (FluA H3N2), 2 (FluB Yamagatal) and 3 (FluB Beiying).
Bioaron C® used together with HRV 14-infected HeLa cells (Fig. 3a) showed, in its highest dose (2%(v/v) solution) in the mean of three independent studies a 77.5% (72.1% (study 1), 75.6% (study 2), 84.9% (study 3, Fig. 3a) inhibition of the virus replication with an obvious dose-dependent activity resulting in a 20–30% reduction of the virus-caused plaques even ranged between a 0.25 and 0.1% Bioaron C® solution. The EC50 concentrations ranged from 0.5 to 1.16% when used in three independent studies (mean EC50 0.91% study 1–3, Table 2). Besides its antiviral effect against HRV 14 Bioaron C® was tested against another member of the picornaviridae, the non-enveloped plus strand RNA virus: coxsackievirus subtype A9 (CA9). Bioaron C® was a little bit less effective on CA9-infected cells compared to HRV 14 infections but its activity still resulted in a 67.9% (mean of two studies, 56.4% (study 1), 79.3% (study 2), Fig. 3b) plaque-reduction at its highest concentration and a clear dose-dependent reactivity with an EC50 ranging between 1.0% and 1.75% in two different experiments (Table 2).
Besides the reactivity of Bioaron C® against non-enveloped RNA viruses of further interest was the reactivity against other RNA as well as DNA viruses. Therefore the reactivity of Bioaron C® was studied against Para 3 and RSV; both viruses belonging to enveloped RNA viruses of the paramyxoviridae family (Fig. 4a and b, respectively). Against both viruses Bioaron C® showed only a very moderate antiviral activity. Its effect against Para 3 in his highest concentration ranged in three independent experiments from 24.3% to 41.7% (Fig. 4a), but was obviously reduced with further dilutions, and a 0.5% Bioaron C® solution did not show any antiviral activity. Therefore the EC50 could be only estimated for solutions with more than 2% (Table 2). Less pronounced antiviral activity could be demonstrated against RSV (Fig. 4b). In two independent studies the highest concentration of Bioaron C® (2%) showed a very weak antiviral activity ranging between 15.8% and 17.7% which was completely lost in more diluted concentrations (Fig. 4b). An EC50 value of Bioaron C® against RSV could only be estimated and was more than 3.3% (Table 2). No antiviral effect of Bioaron C® was demonstrated against the non-enveloped DNA virus Adeno 5, a member of the adenoviridae family (Fig. 4c, Table 2).
Ribavirin®, with known reactivity against RNA viruses, was used as positive control. It was included in the assays in final concentrations of 5–50 µg/ml and could confirm its antiviral activity with 69.7–79.4% inhibition of HRV 14 replication, with 43.7–48.5% inhibition of CA9, with 59.3–61.6% inhibition of Para 3 and with 71.2–83.6% of RSV replication (data not shown).
Besides these viruses with less sensitivity for a Bioaron C® treatment, another group of viruses was of interest: influenza viruses. Influenza viruses are enveloped RNA viruses belonging to the orthomyxoviridae. To detect an antiviral activity of Bioaron C® against orthomyxoviridae four different members were chosen: two members belonging to the genus of influenza A viruses: influenza A H1N1 (FluA H1N1) and FluA H3N2 and in addition two members belonging to the genus of influenza B viruses: FluB Yamagatal and FluB Beiying.
In the following assays Bioaron C® showed an outstanding dose-dependent reactivity against all members of the orthomyxoviridae (Fig. 5). Replication of FluA H1N1 in MDCK cells was nearly completely blocked by the highest solution and ranged in three independent experiments from 82.9 (study 1), 93.6 (study 2) and 92.2 (study 3)% inhibition with a mean value of 88.6% (Fig. 5a). This dose-dependent antiviral activity resulted in EC50 values between 0.83% and 1.13% with a mean value of 0.96% (Table 2). Amantadine included as a positive control in a final concentration of 10 µg/ml confirmed its antiviral activity with a 54.3% inhibition of FluA H1N1 replication.
Even more efficacious was the effect of Bioaron C® against FluA H3N2 with a suppression of 95.9–98.4% with a mean value of 95.6% in three independent experiments (Fig. 5b). This is comparable to the effect of Ribavirin® which showed in a concentration of 10 µg/ml a nearly 100% inhibition. This antiviral effect against Flu H3N2 was dose-dependent and solutions of 0.25–1% still gave a reduction of about 50–80% (Fig. 5b). The EC50 value could be located between 0.22% and 0.49% with a mean value of 0.36% (Table 2).
Compared to FluA H3N2 a slightly decreased antiviral activity of Bioaron C® was observed against members of the FluB genus and in regard to FluB Yamagatal a reduction between 70.3%, 77.7% and 85.1% (study 1–3) was visible for the highest solution of Bioaron C® (Fig. 5c, mean value 77.7%). The antiviral effect declined very rapidly and the EC50 values ranged between 1.08 and 1.45% with a mean value of 1.26% (Table 2). More efficacious was the reactivity against FluB Beiying determined in two studies with a 95.8 (study 1) and a 98.7% inhibition (study 2) with a mean value of 97.3% (Fig. 5d). The EC50 values ranged between 0.38 and 0.69% (Table 2). As a positive control with both FluB virus strains served Ribavirin® and demonstrated in a concentration of 10 µg/ml a nearly 100% (strain Yamagatal) or a 40–50% inhibition against strain Beiying (5 µg/ml).
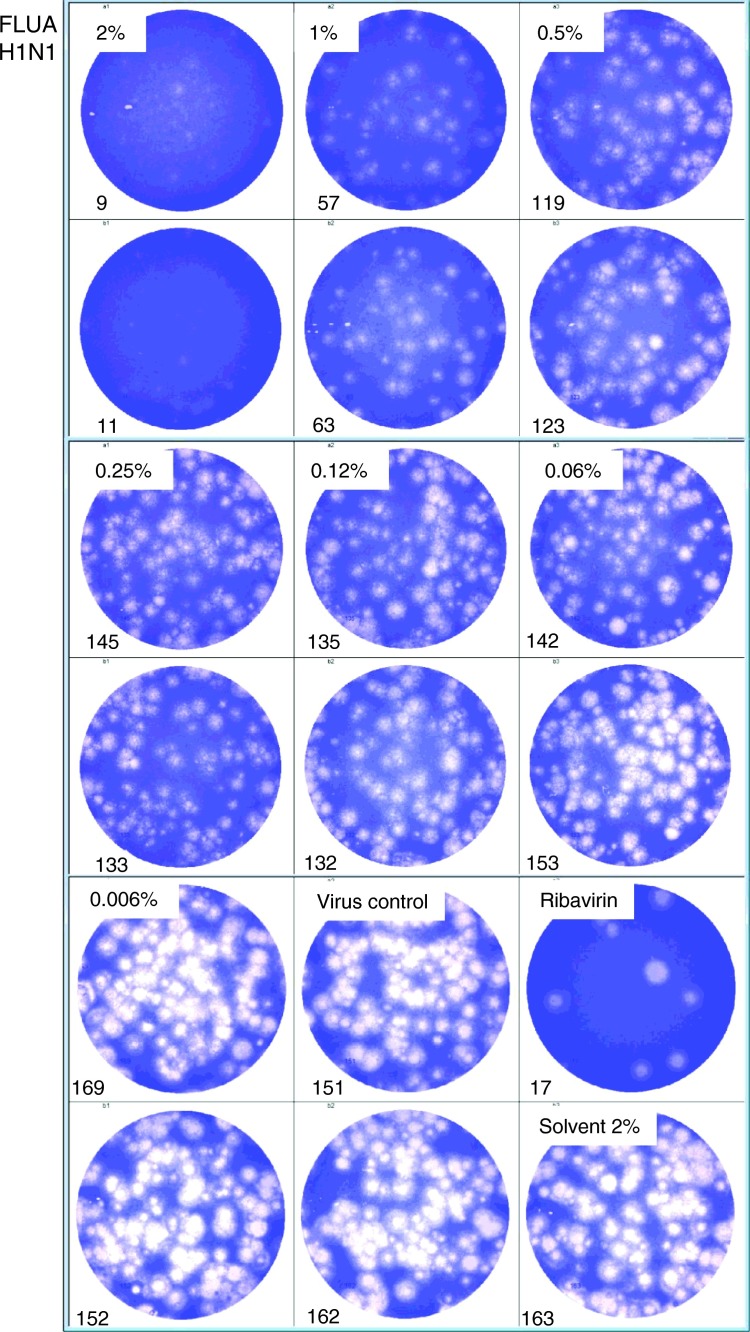
Furthermore in Fig. 6 and supplementary Fig. 1, Fig. 2, Fig. 3 original pictures of the plaque reduction assays with the influenza A and influenza B virus strains demonstrate the virus plaque numbers analysed and counted with a computer-based image processing system corresponding of study 2 for H1N1, study 3 for H3N2, study 1 for Yamagatal and study 2 for Beiying (grey highlighted in Table 2). The respective treatment scenario is presented in the authentic 6-well plates used for the analyses, respectively.
Fig. 6.
Antiviral activity of Bioaron C® against FluA H1N1: 6-well plates with authentic plaque reduction assays are presented. MDCK cells were infected with FluA H1N1 and incubated for 3 days with an agarose overlay containing different dilutions of Bioaron C®. A solvent control (2%), a non-treated virus control, and a positive control with Ribavirin (5 µg/ml) were included in the test. After a 3 day incubation period cells were fixed and stained with crystal-violet. Plates were then analysed with an ELISpot analysing system (AID Diagnostika GmbH). Numbers of plaques are indicated in the lower left corners of the respective wells.
Taken together these data clearly demonstrate a very strong antiviral activity of Bioaron C® against all members of the orthomyxoviridae family tested. These data also indicate significant activity of Bioaron C® against other members of this family as well as other influenza viruses but this has to be precisely proven in further experiments.
Discussion
Taken together our results from the in vitro tests of an antiviral activity of Bioaron C® against various viruses responsible for infections of the upper respiratory tract demonstrate its clear and selective activity against non-enveloped RNA viruses belonging to the picornaviridae and against enveloped RNA viruses belonging to the orthomyxoviridae. Interestingly, only a reduced reactivity was visible against members of the paramyxoviridae representing also enveloped RNA viruses which are closely related to the orthomyxoviridae. This is somehow unexpected because most of the antiviral extracts analysed so far showed similar activities against these two virus families. This was shown for extracts from E. senticosus (Glatthaar-Saalmüller et al. 2001), P. sidoides (Michaelis et al. 2011) and a composition of different plant extracts derived from Gentian root, Primula flower, Elder flower, Sorrel herb, and Verbena herb (Glatthaar-Saalmüller et al. 2011). The active substance of Bioaron C®: the aqueous extract of A. arborecens is therefore unique in its orthomyxovirus-specific reactivity. These results might give a hint for a possible mode of action but to date one can only speculate and further tests including studies of infection kinetics and analyses of other members of the respective virus families are needed for final reasoning.
The lack of reactivity against DNA viruses seems also interesting. It might indicate a common attribute of RNA viruses differing from the DNA ones. But to date our results are based on experiments with only one non-enveloped DNA virus – adenovirus type 5 and have to be confirmed in trials with other DNA viruses.
Interesting further Bioaron C® research might be checking of a potential activity against herpes viruses. This has described for other plant-derived extracts e.g. extracts from black tea containing theaflavins (Cantatore et al. 2013). The theaflavins showed a clear reactivity against herpes-simplex type 1 virus (HSV-1), the same did extracts derived from Origanum vulgare L. which demonstrated activity against HSV-1, RSV and coxsackie virus B3 (Zhang et al. 2014).
Analysing the outstanding efficacy of Bioaron C® against influenza viruses we may conclude that a major gap in antiviral treatment can be covered after further research. So far two classes of synthetic anti-influenza drugs have been described: inhibitors of the M2 ion channel (e.g., amantadine and rimantadine) and neuraminidase inhibitors (e.g., osteltamivir and zanamivir). Since increasing resistances to these drugs are reported while the clinical efficacy of the neuraminidase inhibitors has been seriously questioned only recently by a Cochrane review (Jefferson et al. 2014) and also discussed (Leibovici and Paul 2014) there is an urgent need to introduce additional, reliable anti-influenza-virus agents. Won et al. (2013) described some plant extracts derived from Thuja orientalis Franco., Cuppresaceae, Aster spathulifolius Maxim., Asteraceae, and Pinus thunbergii Parl., Pinaceae, with antiviral activity against influenza A viruses in vitro but did not further characterise these extracts. An extract from Ribes nigrum L., Grossulariaceae, leaves was described recently (Ehrhardt et al. 2013) with a high anti-influenza potential which seems to interfere with virus internalisation. In regard to the functional mechanism of Bioaron C® we can only speculate that we are dealing with a similar antiviral mechanism.
Rajasekaaran et al. (2013) made an impressive study with 50 extracts from plants of the tropical rain forest of Borneo. The authors were able to identify eleven crude extracts inhibiting the enzymatic activity of the viral neuraminidase and four of them were also able to show inhibition of haemagglutination. These extracts have to be further investigated, standardised and purified, however.
Our study as well as the studies described above represent a first step in the characterisation of plant extracts as well as provide substantial support for the use of plant-derived substances as novel anti-influenza drug candidates. Such a development seems quite possible. Already other plant-derived substances e.g. polyphenols (e.g., resveratrol and epigallocatechin gallate) are characterised on the molecular level. They also showed a significant antiviral activity against influenza in vitro and/or in vivo. Especially isoquercetin influenced in an in vivo mouse model the virus replication. Interestingly, in vitro only synergistic effects together with amantadine were determined (Kim et al. 2010).
Compared to other test substances Bioaron C® showed in regard to its very low cytotoxicity and a big difference between IC50 and EC50 values, a very interesting reactivity pattern. But nothing is known to date about the active substances responsible for this effect. A fractionation of the extract for the identification of the active component(s) might be an appropriate approach for future research.
Another target for future research might be determination of Aloe constituents with strong affinity to a highly specific virus-derived target molecule; as it has been described e.g. for the strong viral protease 3C inhibitor, chrysin, a 5,7-dihydroxyflavone, which can be found in many plants (Wanget al. 2014).
Finally, Bioaron C® shows an obvious and resounding in vitro efficacy which is in accordance with its indication and known therapeutic effects. To discover the underlying antiviral mechanisms against influenza viruses remains a subject of future research.
Conflict of interest
The authors declare that they have no competing interest.
Contributors
This study was supported by Phytopharm Kleka S.A., Nowe Miasto nad Warta, Poland. The sponsor provided the test substances. Karina Schönknecht is employee of Phytopharm Kleka. Andrzej Fal is a medical consultant to Phytopharm Klęka. Frank Conrad and Hartwig Sievers are employees of PhytoLab, a pharmaceutical contract laboratory for Phytopharm Kleka. Bernadette Glatthaar-Saalmüller and Armin Saalmüller have performed the study and provided scientific advice to the sponsor. All authors contributed to and have approved the final manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2015.06.006.
Appendix. Supplementary Materials
References
- Bacon T.H., Levin M.J., Leary J.J., Sarisky R.T., Sutton D. Herpes simplex virus resistance to acyclovir and pencyclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian P., Fal A.M., Jambor J., Michalak A., Noster B., Sievers H., Steuber A., Walas-Marcinek N. Candelabra aloe (Aloe arborescens) in the therapy and prophylaxis of upper respiratory tract infections: traditional use and recent research results. Wien. Med. Wochenschr. 2013;163:73–79. doi: 10.1007/s10354-012-0171-3. [DOI] [PubMed] [Google Scholar]
- Calfee D.P., Hayden F.G. New approaches to influenza chemotherapy. Neuraminidase inhibitors. Drugs. 1998;56:537–553. doi: 10.2165/00003495-199856040-00003. [DOI] [PubMed] [Google Scholar]
- Cantatore A., Randall S.D., Traum D., Adams S.D. Effect of black tea extract on herpes simplex virus-1 infection of cultured cells. BMC Complement. Altern. Med. 2013;13:139. doi: 10.1186/1472-6882-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K.A., Whitley R.J. New therapeutic approaches to the alphaherpesvirus infections. J. Antimicrob. Agents Chemother. 1997;39:119–128. doi: 10.1093/jac/39.2.119. [DOI] [PubMed] [Google Scholar]
- Che C.-T. Plants as a source of potential antiviral agents. Econ. Med. Plant Res. 1991;5:197–237. [Google Scholar]
- Cooper P.D. A method for producing plaques in agar suspensions of animal cells. Virology. 1955;1:397–409. doi: 10.1016/0042-6822(55)90033-7. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral drugs in current clinical use. J. Clin. Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Englund J.A., Zimmermann M.E., Swierkosz E.M., Goodman J.L., Scholl D.R., Balfour H.H. Herpes simplex virus resistant to acyclovir. Ann. Intern. Med. 1990;112:416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C., Dudek S.E., Holzberg M., Urban S., Hrincius E.R., Haasbach E., Seyer R., Lapuse J., Planz O., Ludwig S. A plant extract of Ribes nigrum folium possesses anti-influenza virus activity in vitro and in vivo by preventing virus entry to host cells. PLoS One. 2013;8:e63657. doi: 10.1371/journal.pone.0063657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fal A.M., Michalak A. Bioaron C® w leczeniu zakażeń górnych dróg oddechowych u dzieci: wpływ na przebieg infekcji (Bioaron C® in the treatment of upper respiratory tract infections in children: effects on the course of infection) Wiad. Lek. 2013. 2013;66:340–345. [Google Scholar]
- Fyfe J.A., Keller P.M., Furman P.P.A., Miller R.L., Elion G.B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound 9-(2-hydroxyethoxymethyl) guanine. J. Biol. Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- Glatthaar-Saalmüller B., Sacher F., Esperester A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antiviral Res. 2001;50:223–228. doi: 10.1016/s0166-3542(01)00143-7. [DOI] [PubMed] [Google Scholar]
- Glatthaar-Saalmüller B., Rauchhaus U., Rode U., Haunschild J., Saalmüller A. Antiviral activity in vitro of two preparations of the herbal medicinal product Sinupret® against viruses causing respiratory infections. Phytomedicine. 2011;19:1–7. doi: 10.1016/j.phymed.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatthaar-Saalmüller B., Michalak A., Bastian P., Fal A.M. Ocena aktywności przeciwwirusowej in vitro preparatów Biostymina i Bioaron C względem ludzkiego rinowirusa (HRV14) Postępy Fitoterapii. 2012;3:156–161. [Google Scholar]
- Hay A.J., Wolstenholme A.J., Skehal J., Smith M.H. The molecular basis of the specific anti-influenza action of amandatine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G., Hoffman H.E., Spyker D.A. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrob. Agents Chemother. 1983;23:58–464. doi: 10.1128/aac.23.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska J.F., Bernstein J.M., Douglas R.G., Hall C.B. Effects of ribavirin on respiratory syncytial virus in vitro. Antimicrob. Agents Chemother. 1990;17:770–775. doi: 10.1128/aac.17.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambor J. Handbuch des Arznei- und Gewürzpflanzenanbaus, Band 4, Arznei und Gewürzpflanzen A–K. Verein für Arznei und Gewürzpflanzen SALUPLANTA e.V; Bernburg: 2012. Baumaloe (Aloe arborescens Mill.) pp. 219–228. [Google Scholar]
- Janai H.K., Marks M.I., Zaleska M., Stutman H.R. Ribavirin: adverse drug reactions. Rediatr. Infect Dis. J. 1990;9:209–211. [PubMed] [Google Scholar]
- Jefferson T., Jones M.A., Doshi P. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst. Rev. 2014;4:CD008965. doi: 10.1002/14651858.CD008965.pub3. [DOI] [PubMed] [Google Scholar]
- Kim Y., Narayanan S., Chang K.O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res. 2010;88:227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Leibovici L., Paul M. Should we recommend neuroaminidase inhibitors for influenza? Clin. Microbiol. Infect. 2014;20:979–980. doi: 10.1111/1469-0691.12708. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Doerr H.W., Cinatl Jr., J. Investigation of the influence of EPs® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18:384–386. doi: 10.1016/j.phymed.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Rajasekaran D., Palombo E.A., Chia Yeo T., Lim Siok Ley D., Lee Tu C., Malherbe F., Grollo L. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLos One. 2013;8:e79293. doi: 10.1371/journal.pone.0079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusser P. Herpesvirus resistance to antiviral drugs: a review of the mechanisms, clinical importance and therapeutic options. J. Hosp. Inf. 1996;3:235–248. doi: 10.1016/s0195-6701(96)90010-9. [DOI] [PubMed] [Google Scholar]
- Richman D.D., Hostetler K.Y., Yazaki P.J., Clark S. Fate of influenza A virion proteins after entry into subcellular fractions of LLC cells and the effect of amantadine. Virology. 1986;151:200–210. doi: 10.1016/0042-6822(86)90042-5. [DOI] [PubMed] [Google Scholar]
- Summerfield A., Keil G.M., Mettenleiter T.C., Rziha H.-J., Saalmüler A. Antiviral activity of an extract from leaves of the tropical plant Acanthospermum hispidum. Antiviral Res. 1997;36:55–62. doi: 10.1016/s0166-3542(97)00035-1. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang T., Du J., Cui S., Yang F., Jin Q. Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLos One. 2014;9:e89668. doi: 10.1371/journal.pone.0089668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J.N., Lee S.Y., Song D.S., Poo H. Antiviral activity of the plant extracts from Thuja orientalis, Aster spathulifolius, and Pinus thunbergii against Influenza virus A/PR/8/34. J. Microbiol. Biotechnol. 2013;23:125–130. doi: 10.4014/jmb.1210.10074. [DOI] [PubMed] [Google Scholar]
- Zhang X.L., Guo Y.S., Wang C.H., Li G.Q., Xu J.J., Chung H.Y., Ye W.C., Li Y.L., Wang G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014;152:300–306. doi: 10.1016/j.foodchem.2013.11.153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.